During the past several years, substantial progress has been described in the development of palladium- and nickel-catalyzed methods for cross-coupling a range of unactivated alkyl electrophiles.1-4 Initial work focused on reactions of primary alkyl electrophiles, but recent efforts have addressed the more difficult challenge of coupling secondary electrophiles. To date, the only alkylmetal species that have been effectively cross-coupled with unactivated secondary halides are organozinc reagents.5
The Suzuki reaction is a particularly widely used carbon-carbon bond-forming process.6 Thus far, the only reports of Suzuki cross-couplings of unactivated secondary electrophiles have involved aryl- and vinylboron compounds.7 The tremendous potential of coupling reactions of alkyl halides would be enhanced considerably if alkylboranes could be employed as partners. In this report, we establish that a 1,2-diamine provides an active nickel-based catalyst for Suzuki cross-couplings of unactivated secondary alkyl electrophiles with alkylboron reagents at room temperature (eq 1).
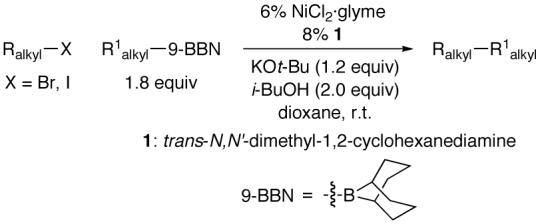 |
(1) |
Unfortunately, the methods that had previously been described for achieving Suzuki reactions of unactivated secondary halides with aryl- and vinylboron compounds are not useful for alkylboron species (eq 2).7 A variety of other bipyridine and aminoalcohol ligands afforded similarly disappointing results.
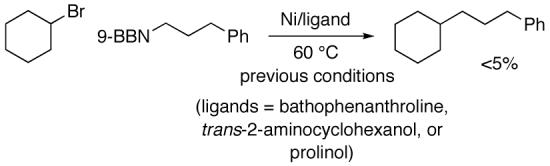 |
(2) |
We therefore decided to pursue the use of new families of ligands to accomplish our objective of Suzuki cross-couplings of secondary alkyl electrophiles with alkylboranes. Upon examining a wide range of structures, we determined that certain 1,2-diamines furnish efficient catalysts for the desired alkyl-alkyl coupling. For example, in the presence of NiCl2·glyme and commercially available trans-N,N’-dimethyl-1,2-cyclohexanediamine (1), bromocyclohexane couples with an alkylborane at room temperature in good yield (Table 1, entry 1).
Table 1.
Alkyl-Alkyl Suzuki Cross-Coupling of an Unactivated Secondary Alkyl Bromide: Effect of Reaction Parameters 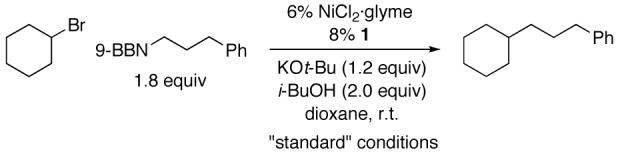
| entry | variation from the “standard” conditions | yield (%) a |
|---|---|---|
| 1 | none | 83 |
| 2 | bathophenanthroline, instead of 1 | 25 |
| 3 | trans-2-aminocyclohexanol, instead of 1 | <5 |
| 4 | prolinol, instead of 1 | <5 |
| 5 | s-Bu-Pybox, b instead of 1 | 7 |
| 6 | trans-1,2-cyclohexanediamine, instead of 1 | 53 |
| 7 | trans-N,N,N’,N’-tetramethyl-1,2-cyclohexanediamine, instead of 1 | <5 |
| 8 | cis isomer of 1, instead of 1 | 46 |
| 9 | N,N’-dimethylethylenediamine, instead of 1 | 7 |
| 10 | no 1 | <5 |
| 11 | no NiCl2·glyme | <5 |
| 12 | NiBr2·diglyme, instead of NiCl2·glyme | 81 |
| 13 | Ni(cod)2, instead of NiCl2·glyme | 75 |
| 14 | NiCl2, instead of NiCl2·glyme | <5 |
| 15 | THF, instead of dioxane | 80 |
| 16 | toluene, instead of dioxane | 47 |
| 17 | Et2O, instead of dioxane | <5 |
| 18 | 3% NiCl2·glyme and 4% 1, instead of 6% NiCl2·glyme and 8% 1 | 56 |
| 19 | no KOt-Bu | <5 |
| 20 | no i-BuOH | <5 |
Determined by GC analysis versus a calibrated internal standard (average of two experiments).
s-Bu-Pybox = (S,S)-2,6-bis(4-(2- butyl)-2-oxazolin-2-yl)pyridine.
Under these conditions, bathophenanthroline, trans-2-aminocyclohexanol, and prolinol are still ineffective (Table 1, entries 2-4), as is s-Bu-Pybox5 (entry 5). trans-1,2-Cyclohexanediamine provides a significant amount of the cross-coupling product (53%; entry 6), whereas trans-N,N,N’,N’-tetramethyl-1,2-cyclohexanediamine does not (entry 7). The cis isomer of 1 is moderately useful (46%; entry 8). An acyclic 1,2-diamine, N,N’-dimethylethylenediamine, furnishes very little of the target compound (entry 9).
In the absence of ligand 1 or of NiCl2·glyme, essentially none of the desired alkyl-alkyl bond formation is observed (Table 1, entries 10 and 11). Nickel complexes such as NiBr2·diglyme and Ni(cod)2 can be used in place of NiCl2·glyme, but NiCl2, which has low solubility in dioxane, cannot (entries 12-14). The coupling proceeds with comparable efficiency in THF as in dioxane (entry 15), whereas toluene is less suitable and Et2O is unsuitable as a solvent (entries 16 and 17). Use of a lower catalyst loading leads to a lower yield (entry 18).
If KOt-Bu or i-BuOH is omitted, essentially none of the target alkyl-alkyl cross-coupling product is generated (Table 1, entries 19 and 20).8 We believe that the role of these species is to activate the alkylborane for transmetalation with nickel.9 Indeed, when we mix an organoborane, KOt-Bu, and i-BuOH, we observe quantitative formation of a tetravalent -ate complex by 11B NMR spectroscopy (eq 3).
This new method can be applied to a range of alkyl-alkyl cross-couplings of unactivated secondary bromides with
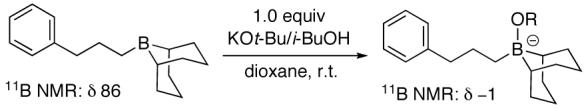 |
(3) |
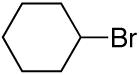
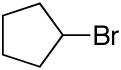
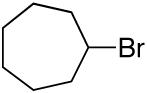
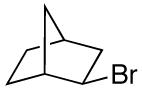
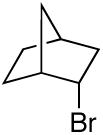
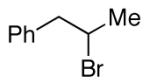
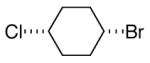
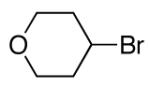
alkylboranes (Table 2). Not only cyclohexyl (entry 1), but also cyclopentyl and cycloheptyl (entries 2 and 3), bromide are suitable reaction partners. Bicyclic (entries 4 and 5), as well as acyclic (entry 6), alkyl halides can be employed. Secondary bromides cross-couple in preference to chlorides (entry 7).10 In addition to chlorides, other functional groups can be present in the electrophile and in the organoborane (e.g., ethers, carbamates, and esters; entries 8-13).11,12
Table 2.
Alkyl-Alkyl Suzuki Cross-Couplings of Unactivated Secondary Alkyl Bromides (for conditions, see eq 1)
| entry | Ralkyl—Br | R1alkyl—9-BBN | yield (%)a |
|---|---|---|---|
| 1 |
|
|
75 |
| 2 |
|
|
77 |
| 3 |
|
|
80 |
| 4 |
|
|
75b |
| 5 |
|
|
65b |
| 6 |
|
|
82 |
| 7 |
|
|
82c |
| 8 |
|
|
87 |
| 9 |
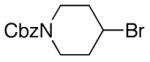
|
|
64 |
| 10d |
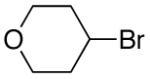
|
|
79 |
| 11 |
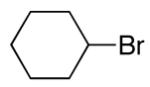
|
|
81 |
| 12 |
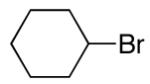
|
|
29 |
| 13 |
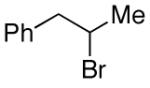
|
|
93 |
Isolated yield (average of two experiments).
Diastereoselectivity: >20:1 (exo:endo).
Ratio of trans/cis = 65/35.
Eight equivalents of i-BuOH were used.
The utility of this Ni/diamine catalyst is not limited to alkyl-alkyl Suzuki reactions of secondary bromides. Thus, without modification, the method can be applied to couplings of unactivated secondary alkyl iodides, primary bromides, and primary iodides (Table 3).13
Table 3.
Alkyl-Alkyl Suzuki Cross-Couplings of Unactivated Secondary and Primary Alkyl Electrophiles (for conditions, see eq 1)
| entry | Ralkyl—X | R1alkyl—9-BBN | yield (%)a |
|---|---|---|---|
| 1 |
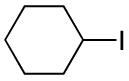
|
|
68 |
| 2 |
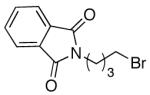
|
|
77 |
| 3 |
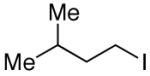
|
|
94 |
Isolated yield (average of two experiments).
In summary, we have established that a commercially available 1,2-diamine serves as an effective ligand for metal-catalyzed cross-couplings of unactivated alkyl electrophiles at room temperature. In particular, Ni/trans-N,N’-dimethyl-1,2-cyclohexanediamine provides the first method for achieving alkyl-alkyl Suzuki reactions of unactivated secondary alkyl halides with alkylboranes; earlier success in Suzuki couplings of such electrophiles had been restricted to reactions with aryl- and vinylboron reagents at elevated temperature. We anticipate that the observation that simple, readily available diamines can furnish active catalysts will greatly facilitate the development of powerful new methods for cross-coupling alkyl electrophiles. Efforts to substantiate this hypothesis are underway.
Supplementary Material
Acknowledgments
This paper is dedicated to the memory of Prof. Yoshihiko Ito. Dr. Francisco González-Bobes is acknowledged for preliminary studies. Support has been provided by the National Institutes of Health (National Institute of General Medical Sciences, R01-GM62871), the Japan Society for the Promotion of Science (postdoctoral fellowship to B.S.), Merck Research Laboratories, Novartis, and Boehringer Ingelheim.
References
- (1).(a) For general reviews of metal-catalyzed cross-coupling reactions, see: de Meijere A, Diederich F, editors. Metal-Catalyzed Cross-Coupling Reactions. Wiley-VCH; New York: 2004. Negishi E.-i., editor. Handbook of Organopalladium Chemistry for Organic Synthesis. Wiley Interscience; New York: 2002.
- (2).(a) For reviews of cross-coupling reactions of alkyl electrophiles, see: Frisch AC, Beller M. Angew. Chem., Int. Ed. 2005;44:674–688. doi: 10.1002/anie.200461432. Netherton MR, Fu GC. In: Topics in Organometallic Chemistry: Palladium in Organic Synthesis. Tsuji J, editor. Springer; New York: 2005. Netherton MR, Fu GC. Adv. Synth. Catal. 2004;346:1525–1532.
- (3).(a) For two pioneering investigations of palladium- and nickel-catalyzed cross-couplings of alkyl electrophiles, see: Ishiyama T, Abe S, Miyaura N, Suzuki A. Chem. Lett. 1992:691–694. Devasagayaraj A, Stüdemann T, Knochel P. Angew. Chem., Int. Ed. Engl. 1995;34:2723–2725.
- (4).For additional leading references, see: Terao J, Kambe N. Bull. Chem. Soc. Jpn. 2006;79:663–672.
- (5).Zhou J, Fu GC. J. Am. Chem. Soc. 2003;125:14726–14727. doi: 10.1021/ja0389366. [DOI] [PubMed] [Google Scholar]
- (6).(a) For reviews of the Suzuki reaction, see: Reference 1. Miyaura N. Top. Curr. Chem. 2002;219:11–59.
- (7)(a).Zhou J, Fu GC. J. Am. Chem. Soc. 2004;126:1340–1341. doi: 10.1021/ja039889k. [DOI] [PubMed] [Google Scholar]; (b) González-Bobes F, Fu GC. J. Am. Chem. Soc. 2006;128:5360–5361. doi: 10.1021/ja0613761. [DOI] [PubMed] [Google Scholar]
- (8).Notes: (a) Certain other alcohols (2,2-dimethyl-1-propanol, 2-ethyl-1-butanol, and s-BuOH) furnish comparable or slightly diminished yields. (b) In the presence of KOi-Bu (1.2 equiv), rather than KOt-Bu/i-BuOH, the desired product is generated in 74% yield.
- (9).(a) For discussions of transmetalation in the context of palladium-catalyzed Suzuki reactions, see: Matos K, Soderquist JA. J. Org. Chem. 1998;63:461–470. doi: 10.1021/jo971681s. Miyaura N. J. Organomet. Chem. 2002;653:54–57.
- (10).A competition experiment among secondary alkyl halides revealed the following reactivity order: I>Br≫Cl.
- (11).Preliminary experiments indicate that, under our standard reaction conditions, alkylboranes derived from the hydroboration of more highly substituted olefins, as well as alkylboron reagents that bear certain nitrogen-containing functional groups, are not suitable cross-coupling partners. Furthermore, couplings of activated secondary alkyl halides (i.e., a benzylic bromide and an α-bromoester) have not proceeded cleanly. We intend to explore the use of different ligands and reactions conditions to address these limitations.
- (12).In the case of the coupling depicted in entry 9 of Table 2, we have determined that undesired products from elimination, reduction, or homocoupling of the alkyl bromide are not formed in significant yields.
- (13).Notes: (a) We have not yet undertaken optimization studies for cross-couplings of these families of electrophiles. (b) A competition experiment among alkyl bromides revealed the following reactivity order: secondary>primary≫tertiary (under our standard conditions, the cross-coupling of t-butyl bromide with PhCH2CH2CH2(9-BBN) proceeds in <5% yield).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


