SUMMARY
The diverse vocal signals of songbirds are produced by highly coordinated motor patterns of syringeal and respiratory muscles. These muscles control separate sound generators on the right and left side of the duplex vocal organ, the syrinx. Whereas most song is under active neural control, there has been a growing interest in a different class of nonlinear vocalizations consisting of frequency jumps, subharmonics, biphonation and deterministic chaos that are also present in the vocal repertoires of many vertebrates, including many birds. These nonlinear phenomena may not require active neural control, depending instead on the intrinsic nonlinear dynamics of the oscillators housed within each side of the syrinx. This study investigates the occurrence of these phenomena in the vocalizations of intact northern mockingbirds (Mimus polyglottos). By monitoring respiratory pressure and airflow on each side of the syrinx, we provide the first analysis of the contribution each side of the syrinx makes to the production of nonlinear phenomena and are able to reliably discriminate two-voice vocalizations from potentially similar appearing unilaterally-produced nonlinear events. We present the first evidence of syringeal lateralization of nonlinear dynamics during bilaterally-produced chaotic calls. The occurrence of unilateral nonlinear events was not consistently cor elated with fluctuations in air sac pressure or the rate of syringeal airflow. Our data support previous hypotheses for mechanical and acoustic coupling between the two sides of the syrinx. These results help lay a foundation upon which to understand the com unicative functions of nonlinear phenomena.
INTRODUCTION
One of the fascinating aspects of songbirds is the diversity and complexity of their vocal signals. Two well-documented functions of birdsong are territory defense and mate attraction, and there is increasing evidence that natural and sexual selection may favour increased vocal complexity (Nowicki and Searcy, 2004). Certain features of vocal performance and acoustic complexity associated with the vocal motor skills of male songbirds may be used by females during mate selection (e.g. Ballentine et al., 2004; Forstmeier et al., 2002; Vallet et al., 1998). The ability for vocal learning and the production of increasingly complex vocal signals may have facilitated the radiation of the songbirds (oscine Passeriformes) into the largest and most diverse order of birds (Fitzpatrick, 1988; Vermeij, 1988), but its role in speciation is controversial (Baptista and Trail, 1992; Raikow, 1986).
Understanding the vocal mechanisms responsible for the spectral and temporal complexity of bird song can provide a valuable insight into the performance constraints and evolution of song diversity. A major source of acoustic complexity in songbirds comes from the versatility of motor control of their duplex vocal organ, but in some species the intrinsic biomechanical properties of the vibratory sound generating structures in the vocal organ may also contribute significantly to song complexity. In this paper, we attempt to asses the contribution each of these sources makes to the vocal diversity of song by the northern mockingbird.
Sources of acoustic complexity
The bipartite syrinx
The songbird’s vocal organ, the syrinx, is composed of modified cartilages at the cranial end of each primary bronchus and the caudal end of the trachea. Each bronchus contains a pair of fleshy pads, the medial and lateral labia (King, 1989), which vibrate when adducted into the respiratory airflow (Goller and Larsen, 2002) and provide the bird with two independently controlled sound sources, one in each bronchus (Suthers, 1990). The acoustic properties of song generated by each pair of labia are controlled by the activity of ipsilateral syringeal muscles innervated by the tracheosyringeal branch of the hypoglossal nerve (Goller and Suthers, 1996a; Goller and Suthers, 1996b).
Spectrographic analyses of birdsong (Borror and Reese, 1956; Greenewalt, 1968; Stein, 1968; Thorpe, 1961) revealed the presence in many species of two, simultaneous non-harmonically related frequencies. Greenewalt (1968) referred to these as “two-voice” phenomena and hypothesized the voices originated on opposite sides of the syrinx, a view supported by subsequent experiments showing varying degrees of song lateralization following unilateral section of the tracheosyringeal branch of the hypoglossal nerve (Floody and Arnold, 1997; Lemon, 1973; Nottebohm, 1971; Nottebohm and Nottebohm, 1976; Suthers, 1990; Suthers et al., 2004; Williams et al., 1992).
A more detailed understanding of the acoustic contribution each sound source makes to Oscine song has come from techniques for recording airflow through each side of the syrinx, together with syringeal and respiratory motor activity during spontaneous song with both sides of the syrinx intact (Suthers, 1990). These data show that songbirds exploit their dual sound source to increase vocal virtuosity in multiple ways, including the production of two-voice elements, switching phonation between sides to produce abrupt frequency steps between notes and taking advantage of lateralized functional specializations in the acoustic properties of each sound source (reviewed in Suthers, 1999; Suthers and Gol er, 1997; Suthers and Zollinger, 2004).
Syringeal nonlinear dynamics
In addition to vocal production by coordinated neuromuscular control of the syrinx and respiratory system, there is evidence that intrinsic, passive, biomechanical properties of syrinx, particularly the dynamic vibratory properties of the paired oscillators (the medial and lateral labia) comprising each sound source, can also result in the production of complex sounds (Fee, 2002; Fee et al., 1998; Mindlin and Laje, 2005). Recent experiments have demonstrated that the labia, much like the vocal folds in the mammalian larynx (Berry et al., 1996; Herzel et al., 1994), comprise a nonlinear physical system (Fee, 2002; Fe et al., 1998). As in any such system, the oscillating masses predictably exhibit certain traits or behaviors including abrupt bifurcations between different vibratory modes.
Four acoustic phenomena are associated with nonlinear systems (Wilden et al., 1998). These “nonlinear phenomena” (NLP) include frequency jumps, subharmonics, biphonation, and deterministic chaos. In addition to the NLP recorded from excised syrinxes of zebra finches (Fee et al., 1998), nonlinear phenomena have been identified in the natural vocalizations of a diverse set of species, including non-songbirds such as doves (Beckers and ten Cate, 2006) and parrots (Fletcher, 2000), frogs (Suthers et al., 2006), terrestrial and marine mammals (Herzel et al., 1995; Riede et al., 2007; Riede et al., 2000; Riede et al., 2004; Riede et al., 1997; Titze et al., 1993; Tokuda et al., 2002; Tyson et al., 2007; e.g. Wilden et al., 1998).
Identifying the source of acoustic complexity in a two-voice vocal system
Because their vocal organ contains two independent sound sources, spectrographic analyses alone in songbirds cannot always reliably distinguish between two voices and some kinds of NLP. Frequency jumps produced by rapidly switching phonation from one side of the syrinx to the other (e.g. as described in Allan and Suthers, 1994) may be indistinguishable from frequency jumps resulting from bifurcations due to the nonlinear dynamics of a single pair of oscillators.
In mammals, biphonation is traditionally defined as the simultaneous appearance of two independent frequencies (Berry et al., 1996; Wilden et al., 1998). Although, the same definition was applied by Greenewalt (1968) to describe standard two-voice phenomena in songbirds, it is now clear that though both examples contain simultaneous harmonically unrelated frequencies, their physical basis is quite different. In two-voice phenomena each voice is generated by a separate set of paired oscillators whereas in biphonation harmonically unrelated frequencies are generated by the nonlinear properties of a single set of oscillators (Suthers et al., 2005).
Additional problems in discerning NLP from two-voice phenomena based solely on emitted vocalizations were described by Laje, Mindlin and colleagues (Laje and Mindlin, 2005; Laje et al., 2008), whose models of source-source and source-tract interactions in the oscine syrinx demonstrate that interactions of the two sides of the syrinx can produce acoustic effects resembling those commonly associated with nonlinear theory, such as subharmonics and biphonation. Their models support earlier evidence of source-source coupling in the oscine syrinx (Nowicki and Capranica, 1986) and demonstrate that certain complex sounds typically associated with nonlinear dynamics, such as frequency jumps, subharmonics or biphonation in birdsong might also result from acoustic interactions within the syrinx and trachea of sounds produced on the two sides.
Here we investigate the occurrence of the nonlinear characteristics in spontaneous song of the northern mockingbird, a vocal mimic. By monitoring subsyringeal pressure and airflow through each side of the syrinx we can determine if these nonlinear features are lateralized to one side of the syrinx or produced bilaterally and distinguish them from superficially similar two-voice vocalizations in order to more accurately estimate their contribution to song complexity.
METHODS
Rearing and acoustic experience of birds
Mockingbirds were housed in individual cages as groups within a single sound attenuating chamber (Industrial Acoustics Company, Inc., Bronx, NY). Subjects were 4 adult (1-3 year old) male northern mockingbirds (Mimus polyglottos), that had been hand-reared in the laboratory. Each bird was tutored during their first year with a wide variety of sounds including recordings of heterospecific songs from several species and computer-synthesized sounds designed to replicate certain distinctive acoustic features of different song types (Table 1). Because mockingbirds are vocal mimics, the individual repertoires of each bird were dominated by mimicries of some subset of the tutor syllables they had heard. The repertoires of the birds in this study varied seasonally but contained between ∼ 90 and 120 syllable types at the time of the experiment. Tutor sounds were chosen or designed for an experiment on song production (see Zollinger and Suthers, 2004 for tutoring details). While the tutor experience of these mockingbirds was not specifically designed to investigate nonlinear phenomena, the sounds were intended to expose juvenile mockingbirds to a wide variety of complex sounds, including some possible NLP. All birds had been exposed to tutor sounds containing two simultaneous unrelated frequencies, either in recordings of heterospecific song or in computer-synthesized tutor sounds. Sounds containing two independent frequencies were of two general types. The first type consisted of two distinct tones (between 1-7 kHz) with independent rates of frequency modulation (FM), e.g. Fig 1A. The second type consisted of a single high f0 accompanied by a lower (<750 Hz) modulating frequency (m0), resulting in a periodic amplitude modulation (AM) of the waveform with sidebands (e.g. Fig 1B). Some tutor sounds contained frequency jumps, consisting of abrupt step-like changes of 0.5 and 3 kHz in f0, in the frequency range between 1 and 7 kHz. None of the tutor sound recordings contained subharmonics or chaos, however mockingbirds were occasionally exposed to the song of northern cardinals (Cardinalis cardinalis), eastern towhees (Pipilo erythrophthalmus), zebra finches (Taeniopygia guttata) and ring doves (Streptopelia risoria) that were housed in adjacent rooms in the laboratory, therefore we cannot rule out the possibility that mockingbirds heard NLP that may have been present in the songs of these other species.
Table 1. Tutor regimes for 4 mockingbirds in this study.
| Bird ID | Sound typea | Tutor type b | ||
|---|---|---|---|---|
| Recordingsc | Livec | Synthesizedd | ||
| m108 | 1f0 | NOMO, WOTH | NOCA | FM CF |
| FJ | WOTH tone pairs e | - | CF tone pairs | |
| 2f0-SB | NOMO -- | AM | ||
| 2f0-other | NOMO, WOTH | -- | FM “two-voice” | |
| m123 | 1f0 | NOCA, BRCO, canary | NOCA | - |
| FJ | BRCO tone pairs | -- | -- | |
| 2f0-SB | EATO | -- | -- | |
| 2f0-other | -- | -- | -- | |
| m130 | 1f0 | NOMO, NOCA, canary, WOTH | NOCA | FM CF |
| FJ | WOTH tone pairs | - | CF tone pairs | |
| 2f0-SB | EATO -- | AM | ||
| 2f0-other | NOMO, WOTH | -- | FM “two-voice” | |
| m152 | 1f0 | NOMO, NOCA, canary, WOTH, WAVI | NOCA | FM CF |
| FJ | BLJA, WOTH and HETH tone pairs | - | CF tone pairs | |
| 2f0-SB | EATO -- | AM | ||
| 2f0-other | NOMO, WOTH | -- | FM “two-voice” | |
1f0, single f0 varying over time (may be pure-tonal or include upper harmonics); FJ, tutor sound contains an abrupt jump up or down in frequency; 2 f0-SB, sound that contains prominent sidebands above and below a f0, visible in a narrowband spectrogram, cor esponding to a second, modulating frequency (m0); 2f0-other - sounds that contain two independent fundamental frequencies, in any other combination than the sideband relationship described above (such as two-voice phenomena).
“Recordings” refers to field recordings of naturally-produced bird songs; playback to mockingbird juveniles during tutoring was via compact disc. “Live” tutors, were birds housed in adjacent rooms, which mockingbirds may have heard. “Synthesized” tutors were computer-generated sounds, playback was via compact disc.
Recorded and Live tutor species, with the exception of “canary” (Waterschlager canary, Serinus canarius), are listed by standard American Ornithological Union 4-letter abbreviations as follows: NOMO - northern mockingbird (Mimus polyglottos), WOTH - wood thrush (Hylocichla mustelina), NOCA - northern cardinal (Cardinalis cardinalis), BRCO - brown-headed cowbird (Molothrus ater), EATO - eastern towhee (Pipilo erythrophthalmus), WAVI - warbling vireo (Vireo gilvus), BLJA - blue jay (Cyanocitta cristata), HETH - hermit thrush (Catharus gut atus).
FM, frequency modulated pure-tonal sounds; CF, constant frequency pure tones; CF “tone pairs”, tones of different frequency presented in step-wise pairs; AM, amplitude modulated FM or CF tones with sidebands corresponding to the modulation frequency; FM “two-voice”, two simultaneous, independent, synthesized FM sounds.
“Tone pairs” describes abrupt step-wise jumps in frequency, however since the production patterns for these recorded birdsong elements are not known, do not necessarily represent frequency jumps due to nonlinear dynamics.
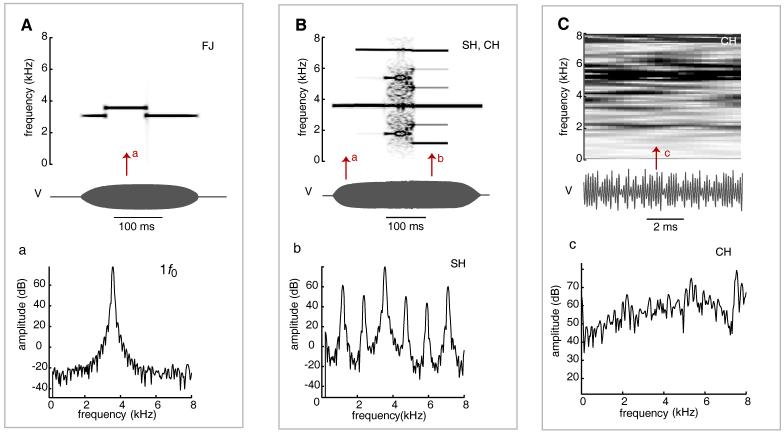
Figure 1.
Synthesized examples illustrating 4 types of biphonation and two-voice phenomena observed in mockingbird songs. The spectrogram (top) and spectrum (bottom) in box (A) illustrate the spectral properties typical of both “type A” biphonation and two-voice phenomena, consisting of two independent f0s. “Type B” biphonation (B) consists of a fundamental frequency and sidebands. In this synthesized example, f0 is a 1 kHz tone. The second frequency is a 250 Hz modulation frequency, m0, which appears spectrographically as sound energy 250 Hz above and below f0. (C) A synthesized example of “dual biphonation” similar to the type observed in mockingbird vocalizations. Each side of the syrinx produces “type B” biphonation simultaneously. In this example, two unrelated fundamental frequencies (f0 and g0), originate from opposite sides of the syrinx. The two “voices” are each modulated by an unrelated, lower modulating frequency (250 Hz, m0). (D) A synthesized example of “dual biphonation” similar to panel C, but in this case m0 is also frequency modulated, resulting in sidebands that are not parallel to f0 or g0. BP, biphonation 2VC, two-voice phenomena; SB, sidebands; 2SB, dual biphonation (two f0s, each with sidebands).
Surgery and Data Acquisition
Birds were anesthetized by injection of chloropent (4.1 μl /g body weight, recipe from Fort Dodge Animal Health, Overland Park, KS) into the pectoral muscle. A silastic cannula (Dow Corning Corp, Midland, MI; ID 1.02 mm, OD 2.16 mm) was inserted into a cranial thoracic air sac for measurement of subsyringeal pressure. The cannula was attached to a miniature piezoresistive pressure transducer (Fujikura FPM-02PG, Marietta, GA) mounted on a small backpack attached to an elastic belt fitted around the bird’s thorax.
A mid-ventral incision was made between the clavicles in order to expose the syrinx through an opening in the interclavicular membrane. The rate of airflow was recorded by a heated microbead thermistor (Thermometrics, Edison, NJ, BB05JA202) inserted into each bronchus a few semi-rings caudal to the syrinx. The interclavicular membrane was sealed around the thermistor leads, which were routed under the skin to the backpack. For more detailed surgical methods see Suthers et al. (1994) and Zollinger and Suthers (2004). Pressure and airflow signals were transmitted from the backpack on leads that exited through the top of the cage to signal conditioning instruments (Hector Engineering, Elletsville, IN) and a multi-channel digital data recorder (Metrum DataTape RSR512, Littleton, CO). Four signals (vocalization, rate of airflow through the right and left bronchi, and air sac pressure) were recorded digitally (40,000 samples -1 per channel) onto separate tracks on S-VHS ½” magnetic tape cassettes (Maxell ST-31BQ SVHS, Fair Lawn, NJ) using the Metrum recorder. Signals were transferred from tape to microcomputer using a Data Translation board (DT-2821G) and an antialiasing filter (TTE, J87, St Pete Beach, FL, 8 kHz high cut-off, stopband attenuation 60 dB per 1/3 octave). An experiment lasted 7-10 days, during which the bird could move freely about its cage. Vocalizations during experiments were recorded with a directional condenser microphone (Audio-technica AT835b, Stow, OH) positioned approximately 50 cm in front of the cage.
Two methods were used to determine what each side of the syrinx contributed to the song. The first method measured airflow through the syrinx; any air flowing through one side of the syrinx while the other side was closed, indicated that the sound was produced entirely with the open side. In some recordings, the thermistors responded to air oscillations up to ∼2 or 3 kHz produced by the acoustic signal from the ipsilateral side of the syrinx. In these cases it was possible to determine the sound generated by each side when both sides were phonating (Suthers, 1990). The low frequency components of bronchial signals related to respiratory or phonatory motor patterns were removed post-recording with a digital 100 Hz Hanning high-pas filter.
Signals were analyzed with Igor Pro v. 5 (WaveMetrics Inc., Lake Oswego, OR) and with Adobe Audition v. 1.5 (Adobe Systems Inc., San Jose, CA). Statistical analysis was conducted using Igor Pro v. 5 and SigmaStat v. 2.03 (SPSS Inc., Chicago, IL). Preoperative song (100-300 minutes per bird) was recorded from adult birds (>300 days post hatching) for comparison with pre- and post-surgery repertoires (Avisoft-Recorder v. 1.7, Avisoft Bioacoustics, Berlin, Germany).
For each syllable that spectrographically resembled one of the four NLP, we examined the airflow and pressure recordings, and noted the acoustic contribution of each side of the syrinx. A syllable was defined as a sound in which the air sac pressure was negative prior to the sound, positive during the sound production, and negative after completion of the sound (a single expiratory pulse). Vocalizations were first examined for the occurrence of NLP through visual inspection of narrowband spectrograms (1024 points at 40,000 samples s -1, frame duration 25.6 ms, window duration 75%, Hanning window type) and associated power spectra. Each syllable was scored for the presence of the four NLP described above. If a syllable contained more than one type of NLP it was counted in each category.
Identifying Nonlinear Phenomena
Spectrographic evaluation
We examined 1000 syllables each from 4 subjects for acoustic evidence of NLP (frequency jumps, subharmonics, biphonation or deterministic chaos). This initial sorting of sounds by visual spectrographic examination is not sufficient to determine the mechanism of production, but was done to identify potential NLP for further investigation.
Frequency jumps are sudden changes in fundamental frequency (f0) to a higher or lower f0 (Fig 2A). A frequency jump was defined as a visible, instantaneous (<5 ms silent interval between adjacent frequencies, as measured from the time waveform) step-change in f0.
Figure 2.
Spectral properties typical of three types of nonlinear phenomena; frequency jumps, subharmonics and deterministic chaos. (A) A 3500 Hz tone with two 250 Hz frequency jumps. A power spectrum (a) taken at arrow a shows a single peak of sound energy corresponding to f0. (B) A 3750 Hz tone, with a series of bifurcations, the first from a single f0 to a 1/2 f0 subharmonic regime, then an abrupt transition to deterministic chaos, and then to a 1/3 f0 subharmonic regime. Comparing spectra and b illustrates the increase in spectral complexity resulting from the addition of subharmonic values and their harmonics (b, Box B). (C) A 10 ms section of deterministic chaos (in this case, a low-dimensional noise, generated using a Rossler attractor equation). The aperiodicity of the sound waveform (V) and the sound energy fairly evenly distributed across the entire spectrum (spectrogram C, and power spectrum, c) are indicators used to identify potential chaos in mockingbird songs. Abbreviations the same as those in Tables 1 and 2; FJ, frequency jump; SH, subharmonics; CH, deterministic chaos; 1 f0, single frequency sounds; V, amplitude of sound waveform.
Subharmonics (Fig 2B) are additional spectral components at integer fractional values of f0 (e.g., f0/2, f0/3, etc.). They appear at evenly spaced intervals below f0 and between adjacent harmonics throughout the frequency spectrum.
Deterministic chaos (low-dimensional noise) is technically distinguishable from stochastic noise (high-dimensional chaos) by the number of dimensions needed to describe it (Tokuda et al., 2002). However, the distinction can also be made based on telltale characteristics visible in narrowband spectrograms (Wilden et al., 1998), including preceding subharmonics (e.g., Fig 2B) and the presence of harmonic “windows” in otherwise noisy segments. We classified sounds as “deterministic chaos” if we observed a broadband, noisy segment in the spectrogram (Fig 2C), plus at least two additional indications, such as a sudden onset of the noise, preceding subharmonics or harmonic windows within the noise.
Biphonation refers to the occurrence of two simultaneous but independent fundamentals that are generated by a single sound source (e.g. one pair of vibrating tissues). We include at least two types of phenomena under this term. We assume a primary oscillator producing a f0. In the first type of biphonation, a second independent frequency (g0) also exists, which shows no obvious interaction with f0 (Fig 1A). In the second type, we observe a primary fundamental frequency (f0) and an additional, much lower, modulating frequency (m0). Spectrographically, this type of biphonation is characterized by sidebands that are above and below f0 and its harmonics (Fig 1B). Sidebands are associated with the frequency of the cyclic amplitude fluctuations in the waveform. Because of the presence of two sound sources in the oscine syrinx, biphonation may be unilateral or bilateral. For example, bilateral or “dual biphonation” (biphonation produced by both “voices” simultaneously) might be characterized spectrographically by two independent frequencies (f0 and g0), each with sidebands corresponding to a low fundamental frequency (m0) (Fig 1C or 1D).
Mechanism of vocal production
For each putative instance of NLP observed spectrographically, we then examined concurrent airflow through the right and left sides of the syrinx, along with subsyringeal air sac pressure. This analysis allowed us to determine if the occurrence of such sounds could be explained by independent phonation on the two sides of the syrinx, or if they were produced by a single source.
In order to test whether the relationships between the occurrence of frequency jumps or subharmonics and changes in either air sac pressure or bronchial airflow were significant, we investigated changes in rates of air flow immediately prior to the bifurcation and at an earlier point in the same syllables. Temporal resolution of the time series was 25 μs. The signal was examined over two 5-ms time intervals (20 - 25 and 5 - 0 ms) prior to the bifurcation and 0-5 ms after the bifurcation. Air sac pressure and rate of air flow through the syrinx were normalized to a percentage of maximum flow rate or pressure. The normalized pressure or rate was then regressed against distance (time) to the point of bifurcation. Slopes of these regression lines were tested for dif erences in variance between groups using SigmaStat 3.11 (Systat Software Inc., San Jose, CA). Because most examples of chaos occurred either for the entire duration of the syllable, or immediately fol owing a period of subharmonics, similar analyses of flow and pressure prior to the onset of chaos were not conducted. Similarly, biphonation usually had a gradual onset or lasted the entire duration of the syllable, precluding a meaningful analysis of flow and pressure fluctuation associated with the bifurcation in these cases.
RESULTS
Unilaterally-produced acoustic phenomena typical of nonlinear systems, including frequency jumps, subharmonics, deterministic chaos and biphonation, were observed in 8.5% of syllables analyzed across individuals (1000 syllables each from 4 birds, individually: m108, 5.8%; m123, 4.3%; m130, 7.2%; m152, 16.5%) (Table 2). “Unilaterally-produced” means that there was airflow through only one side of the syrinx for the duration of the sound in question. The pre-operative occurrence of sounds resembling NLP was not different from the post-operative occurrence (Wilcoxin Signed-Rank test: W= 4.000, T+ = 7.000, T-= -3.000, P (exact) = 0.625). We did not find any vocalizations that were produced during inspiration.
Table 2. Number of nonlinear phenomena, two-voice phenomena and harmonic vocalizations present in the vocal repertoires of the mockingbirds in this studya.
| Bird ID | repertoire sizeb | Unilateral flow classification categoriesc | Bilateral flow classification categoriesd | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1f0 | FJ SH | CH | BP | 1f0 | FJ SH | CH | SB-2VC | 2SB-fixed | 2SB-changing | 2VC | ||||
| m108 | 121 | 171 | 8 | 4 | 34 | 12 | 38 | 33 | 30 | 29 | 28 | 26 | 64 | 566 |
| m123 | 60 | 258 | 12 | 10 | 5 | 16 | 2 | 30 | 9 | 45 | 35 | 13 | 21 | 536 |
| m130 | 73 | 166 | 15 | 18 | 8 | 31 | 0 | 68 | 75 | 59 | 96 | 113 | 38 | 666 |
| m152 | 102 | 309 | 13 | 18 | 21 | 113 | 13 | 65 | 10 | 132 | 40 | 6 | 2 | 297 |
| mean | 89 | 226 | 12 | 12.5 | 17 | 43 | 13.3 | 49 | 31 | 66.25 | 49.75 | 39.5 | 31.25 | 516.25 |
| Std dev | 27.63 | 69.62 | 2.94 | 6.81 | 13.29 | 47.38 | 17.46 | 20.28 | 30.89 | 45.51 | 31.2 | 49.70 | 26.32 | 156.38 |
Number of individual syllables assigned to each classification category (n= 1000 syllables per bird). If a syllable contained more than one type of NLP it was counted toward totals of each, but if it contained at least one type, it was not included in “harmonic” categories (1f0 and 2VC) regardles of the ratio of harmonic vs. nonlinear elements.
Total number of discrete syllable types within the 1000 syllable sample per bird.
Syl ables produced with flow through only one side of the syrinx. 1f0, single f0 sounds; FJ, frequency jumps; SH, subharmonics; CH, deterministic chaos; BP, biphonation (two independent fundamental frequencies, but air flow through only one side of the syrinx);
Syl ables produced with flow through both sides of the syrinx during the portion of the sound containing the NLP. SB-2VC, sidebands (single carrier frequency with sidebands, but flow through both sides of the syrinx) since there was flow through both sides of the syrinx during these sounds, we did not classify them as NLP; 2SB-fixed, two f0, each with sidebands with a constant modulation frequency (parallel to f0); 2SB-changing, two f0, each with sidebands with a changing modulation frequency (not parallel to f0); 2VC, two-voice phenomena (each side of the syrinx producing a distinct, unrelated f0).
Frequency jumps
Occur ences of Frequency Jumps
Unilaterally-produced frequency jumps (Fig 3) occurred in 1.2 % of syllables overall (mockingbird m108, 0.8%; m123, 1.2%; m130, 1.5%; m152, 1.3%) bilateral frequency jumps were observed in 4.9% of all syllables (m108, 3.3%; m123, 3.0%; m130, 6.8%; m152, 6.5%). In unilaterally-produced frequency jumps, the fundamental frequency shifts abruptly up or down with a silent interval <5 ms between adjacent frequencies. During the jumps there is airflow through only one side of the syrinx the other side being closed. The change in frequency due to the jumps ranged from ∼45-450 Hz. Both jumps from a higher to lower frequency and lower to higher frequency were observed (47.9% down-jumps, 52.1% up-jumps). Mimicked copies of tutored frequency jumps between tone-pairs could spectrographically resemble frequency jumps resulting from nonlinear dynamics, however these large steps in frequency (∼500-450 Hz) were always produced bilaterally, by alternating phonation between the two sides of the syrinx, rather than unilaterally (Zollinger and Suthers, 2004).
Figure 3.

Examples of frequency jumps and subharmonics in mockingbird song (bird m123). (A) Frequency jumps occurred with airflow through only the right (arrow a) or left (arrow c) side of the syrinx. Arrow b indicates a shift from a ½ f0 to ¼ f0 subharmonic regime. Arrow c indicates a shift between a 1/3 f0 and ½ f0 subharmonic regime. (B) Expanded views of the sound waveform at each arrow in spectrogram (A), showing the abrupt changes in oscillation patterns at bifurcation points. (C) Power spectrum taken at arrow b, showing spectral peaks at f0 and its harmonics (2 f0, 3f0, etc), as wel as at fractional integer values corresponding with a ¼ f0 subharmonic, and its harmonics. F L and FR, rate of airflow through left and right bronchus, respectively. Airflow associated with positive pressure is expiratory, and that associated with negative pressure is inspiratory. P, pressure in the cranial thoracic air sac; V, oscillogram of vocalization (sound waveform). Horizontal lines indicate ambient pressure or zero air flow.
Physiology and Production of Frequency Jumps
No significant differences in subsyringeal air sac pressure accompanied the production of frequency jumps. Airflow through the bronchus producing the frequency jump often, but not always, occurred concurrently with a transient (5-10 ms) change in rate of airflow (only those jumps produced with airflow through a single side of the syrinx were investigated). The slope of standardized rates of air flow regressed against time immediately before and after the bifurcation (Fig 4A) had greater variance immediately before the frequency jumps (0-5 ms prior to bifurcation, mean slope -0.28 ± 2.773 SD, variance 7.7) than at an earlier point in the syllable where no nonlinear phenomenon was observed (20-25 ms prior to bifurcation, mean slope -0.26 ± 0.896 SD, variance 0.8). While the mean slope of the normalized rate of airflow at 20-25 ms before bifurcations was not significantly different than that immediately before the bifurcation, the variance was significantly higher in the 5-0 ms before a frequency jump (Levene’s test of equal variances, P < 0.050), and thus we reject the nul hypothesis that the variances between the two groups are the same. Changes in the slope of the rate of air flow occurred immediately before a frequency jump more often than at times during the same syllable 25 ms before or after the jump. However, detectable changes in flow were not always found, and therefore cannot be considered a necessary condition for the production of this type of bifurcation. When airflow changed during a jump, the change was not consistent in either the absolute magnitude or in an increase or decrease in flow rate. Jumps were observed concurrently with increases, decreases or no detectable change in the rate of flow, regardless of the direction of the jump (increase or decrease in frequency). Frequency jumps also occurred when the flow rate was not changing (slope ≈ 0).
Figure 4.
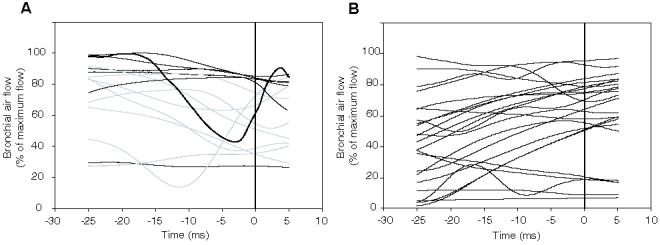
Normalized rates of bronchial airflow 25 ms before to 5 ms after a frequency jump (A) or the onset of subharmonics (B). Bifurcations occurred at time 0. Slopes of linear regressions over thre different 5 ms periods were measured (25-20 ms before, 5-0 ms before, and 0-5 ms after bifurcation points, indicated by vertical lines). Rates of bronchial airflow (A) show greater variance in their slope 0-5 ms before and 0-5 ms after a frequency jump than at an earlier point in the syllable, where no NLP were observed (failed Levene’s test of equal variances below the 5% level, P<0.05. Variance at 25-20 ms prior to bifurcation, σ2 = 0.8; 5-0 ms prior, σ2 = 7.7; 0-5 ms after, σ2 = 7.2). Airflow was normalized to percent of maximum flow rate during syllable. Grey lines = upward jump in frequency; black lines - downward jump. (B) Rate of bronchial airflow 25-20 ms prior, 5-0 ms prior and 0-5 ms after onset of subharmonics did not show significant differences in slope (passed Levene’s test for equal variances wel above the 5% level, P = 0.21).
Subharmonics
Occur ences of Subharmonics
Unilaterally-produced subharmonics were observed in 1.3% of syllables analyzed (m108, 0.4%; m123, 1.0%; m130, 1.8%; m152, 1.8%). Subharmonics occurred both alone (Fig 3), or preceding or following chaotic regimes (Fig 5). We found subharmonics in the f0/2 mode (Fig 3 arrow a, and Fig 5), with subharmonic frequency bands occurring at 0.5 f0, 1.5 f0, 2.5 f0, etc., and also in the f0/3 and f0/4 mode (Fig 3, arrows c and b, respectively). The example in Fig 3 shows shifts between three different subharmonic modes within two syllables. In the f0/2 mode (immediately to the right of arrow a, Fig 3), the fundamental frequency is 1720 Hz, with subharmonics at 860 Hz (0.5 f0), 2580 Hz (1.5 f0), 4300 Hz (2.5 f0). Another period doubling (Fig 3, arrow b), produces subharmonics in the f0/4 mode, with the fundamental frequency at 1763 Hz, and subharmonics visible at 2203 Hz (1.25 f0), 2645 Hz (1.5 f0), 3085 Hz (1.75 f0), etc.
Figure 5.
Unilaterally-produced subharmonics and deterministic chaos (mockingbird m152). (A) Arrows indicate abrupt transitions from a harmonic vocalization to a chaotic sound. The chaotic region is followed by a period of subharmonics (arrow b) after which the vocalization returns to a periodic state. (B) A 25 ms segment of the sound waveform showing the abrupt transition from periodic to aperiodic oscillation. (C) Power spectrum taken at arrow b (spectrogram, A). Spectral peaks show sound energy at f0 and associated harmonics (2 f0, 3f0, etc) as well as at 0.5 f0 and related harmonics (1.5 f0, 2.5f0, 3.5f0, etc.) Abbreviations as in Fig 3.
Physiology and Production of Subharmonics
The occurrence of subharmonics in mockingbird song did not correlate with predictable changes in rates of bronchial air flow or pressure (Fig 4B). Rates of airflow were not significantly different, either in their mean or variance, just prior to the onset of subharmonics, from these values 5 ms after onset or 25 ms prior to onset, (25 ms prior, mean 0.33 ± 0.929 SD, variance 0.86; 5 ms prior, mean 0.79 ± 1.287 SD, variance 1.66; 5 ms post, mean 0.41 ± 0.736 SD, variance 0.54). Differences between groups were not significant (one-way ANOVA, df = 2, S = 2.74, MS = 1.37, F = 1.34, P = 0.268). Airflow could either increase or decrease and the magnitude of these changes varied. Whereas many of the instances of subharmonics coincided with an increasing rate of airflow (43.5% of cases), subharmonics also occurred during periods of decreasing or constant flow rates (30.4% and 26.1% of cases, respectively).
Biphonation and two-voice phenomena
Occurrences of Biphonation and Two-Voice Phenomena
The simultaneous presence of two or more independent frequencies was very common in the acoustic signals of these mockingbirds (68.0 % of all syllables; individually: m108, 69.6%; m123, 62.1%; m130, 94.4%; m152, 45.8%). Sounds containing 2 independent frequencies were often produced during bilateral vocalization indicated by simultaneous airflow through both sides of the syrinx (63.7% of total syllables, 93.7% of sounds with ≥2 independent frequencies), and therefore meet the traditional definition of two-voice phenomena. When a single f0 with sidebands was observed concurrent with bilateral airflow, we did not count these as NLP since the contribution of the two voices to the sound was not clear (Table 2, SB-2VC column)
Mockingbirds also produced biphonic sounds, i.e., two harmonically-unrelated sounds using one side of the syrinx, the other side being closed (4.3% of total syllables, 6.3% of sounds with ≥2 independent frequencie s). We found unilateral biphonation of both types illustrated in the synthesized examples (Fig 1A and 1B). Examples of “type A” biphonation (Fig 6) were les common than “type B” biphonation (Fig 7, arrow a), but both were observed in mockingbird vocalizations.
Figure 6.
Biphonation in mockingbird song. (A) Two independent frequencies produced by a single side of the syrinx. Arrows a and b indicate biphonic sounds concurrent with airflow through only the left side of the syrinx. At arrow a, sound energy is present at 990 Hz (f0) and 1505 Hz (g0), and their harmonics, 2 f0 (1980 Hz) and 2 g0 (3010 Hz). (B) An expanded view of the sound waveform at arrow a (spectrogram, A), showing a change from the biphonation event to a single f0 tone. (C) Power spectrum taken at arrow b, in spectrogram (A), showing the two fundamental frequencies (f0, and g0), as wel as sound energy at various linear combinations of the two fundamentals. Abbreviations as in Fig 3.
Figure 7.
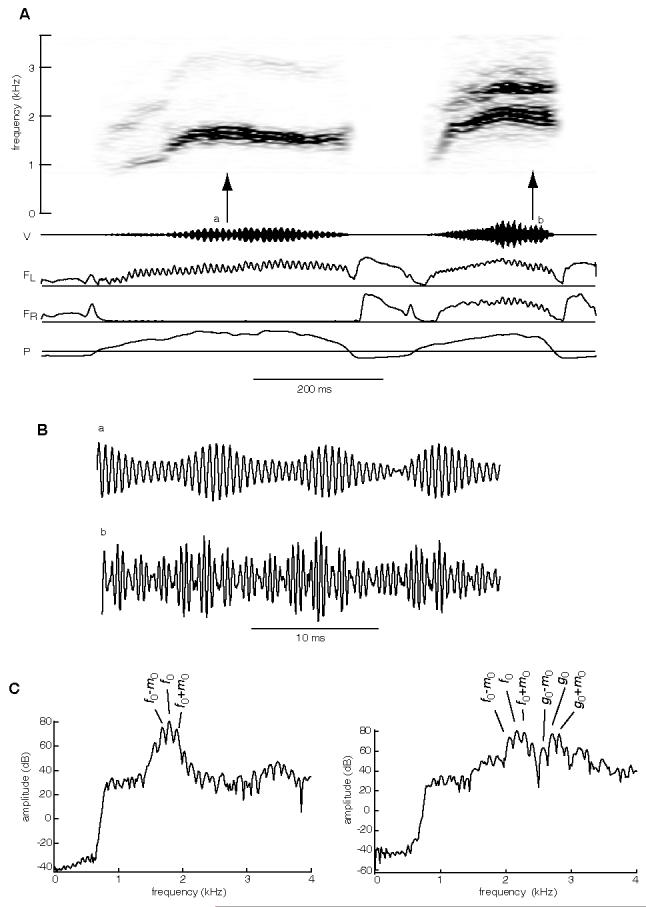
Unilateral biphonation and “dual biphonation” in mockingbird song. (A) Spectrogram of two syllables produced in sequence by mockingbird m108. The first syllable in the pair (a) is an example of unilateral biphonation. This syllable is produced with the left side only, but two independent frequencies are present, the fundamental frequency (f0) at ∼1800 Hz, and a lower, modulating frequency (m0) visible as sidebands ∼115 Hz above and below f0. The bird adds phonation from the right side of the syrinx in second syllable (b), and a second fundamental (g0) appears, also with sidebands 115 Hz above and below, indicating that g0 is also modulated by m0. This is a two-voice syllable in which each voice is biphonic. (B) Expanded view of the sound waveform at arrows a and b in spectrogram (A), showing the AM pattern on the waveforms at a rate of ∼115 Hz (period of one modulation cycle ∼11.5 ms) both during unilateral flow (a) and bilateral flow (b). In addition to the 115 Hz modulation pattern, the two-voiced sound exhibits a second pattern in the waveform which is likely the result of beating between f0 and g0. A beat frequency is equal to the difference between f0 and g0, in this example, the second modulation rate in b is approximately equal to 550, which corresponds with the difference frequency between g0 and f0 (2695 - 2135 Hz). (C) Power spectra taken at arrows a and b in panel (A). Sound was filtered with a digital 800 Hz Hanning shape high pass filter. Peaks labeled correspond with fundamental frequencies as well as the sidebands resulting from the interaction of modulating frequency (m0) with the carrier (f0) in a, and with the two carrier frequencies (f0 and g0) in b. Abbreviations as in Fig 3.
Since oscines have two theoretically independent sound sources (a pair of vibratory tissues within each side of the syrinx) we looked for examples of two simultaneous biphonic sounds, or “dual biphonation” such as illustrated in Fig 1C and 1D. Mockingbirds did produce dual biphonation, or two-voice syllables in which each “voice” was biphonic (Fig 7, arrow b and Fig 8). Type B “dual biphonation” (e.g. each side producing a different biphonic sound, each consisting of a fundamental frequency and an independent modulating frequency) represented between 0.8 and 15.1% of syllables examined per bird (mean 7.1% ±6.35 SD). Interestingly, in every case of bilateral biphonation, the modulating frequency (m0) of the two voices was the same for both right and left-produced carrier frequencies (i.e. voices), even when m0 was itself frequency modulated. We did not find any examples of simultaneous bilateral production of type A biphonation.
Figure 8.
(A) Dual biphonation (two-voice phenomenon with biphonation in both voices) The sound at the arrow consists of two fundamental frequencies (f0 and g0), both simultaneously modulated by a third frequency (m0). Although f0 and g0 are modulated in opposite patterns in the frequency domain (one downsweeping and the other upsweeping in frequency), m0, or the rate of amplitude modulation frequency is the same (approx 250 Hz) for both, as evidenced by evenly spaced sidebands the same distance from both f0 and g0. (B) An expanded view of the sound waveform showing the pattern of amplitude modulation. (C) Power spectrum taken at arrow a in spectrogram (A). Abbreviations as in Fig 3.
Physiology and Production of Biphonation and Two-Voice Phenomena
Figure 6 (arrows a and b) shows examples of type A biphonation produced with airflow through only a single side of the syrinx. A power spectrum of the sound at arrow b shows the first fundamental frequency (f0) at 946 Hz and a second fundamental frequency (g0) at 1505 Hz. Second harmonics of f0 and g0 are visible at 1892 Hz and 3010 Hz, respectively. Other peaks correspond to linear combination products of f0 and g0, as labelled. At arrow a, the lower frequency (f0) is 990 Hz, and the higher frequency is 1505 Hz.
An example of type B unilateral biphonation is shown in Fig 7 (first syllable). Across our sample, the modulating frequencies (m0) in type B biphonation ranged from 57 to 540 Hz. For type B unilateral biphonation, the spacing of the sidebands in the frequency domain corresponds to the rate of AM in the sound waveform. Examination of the waveform during the first syllable (top trace, Fig 7B) shows the period of the AM pattern is approximately 11.5 ms, and sidebands 115 Hz above and below f0 in a power spectrum (Fig 7C).
Examples of two-voice biphonation are shown in Fig 7 (second syllable) and Fig 8. At the start of the second syllable in Fig 7 the bird opens both sides of the syrinx to produce a two-voice syllable in which each side generates independent fundamental frequencies, and both fundamentals (f0 and g0) are flanked by sidebands. In this second syllable, the sidebands are equal distance from both f0 and g0, and the modulating frequency (m0) is the same for both. Comparing 35 ms segments from the center of each syllable in the pair (Fig 7B), a 115 Hz modulation pattern is apparent in both. However, compared with the waveform of the unilaterally-produced syllable (Fig 7B, top trace), addition of a second fundamental (g0), and airflow through the second side of the syrinx, results in an additional AM pattern on the sound waveform of 550 Hz (Fig 7B, bottom trace). This 550 Hz modulation during the two-voice syllable is likely the result of beating (sinusoidal oscillations in amplitude resulting from the linear interaction of two similar frequencies). The observed frequency of 550 Hz amplitude modulation is equal to the difference in frequency of g0 - f0 (2695-2145 Hz in Fig 7).
In another example of two-voice biphonation (Fig 8), the two carrier frequencies (f0 and g0,; representing the left and right voice, respectively) are frequency modulated (FM) in opposite directions. During the first 50 ms of the syllable in Fig 8A, both the right and left sides of the syrinx are open and the bird sings two converging FM sounds, each with sidebands. Unlike the second syllable in Fig 7, the two-voice biphonation during the first part of the syllable in Fig 8 does not result in the appearance of a pronounced beating pattern in the waveform. However, in all cases of two-voice biphonation, m0 was always the same on the two sides of the syrinx, even if the FM pattern of f0 and g0 were opposite (upsweeping or downsweeping, respectively). The source of m0 in mockingbirds is not known.
Deterministic chaos
Occurrences of Deterministic Chaos
Syllables containing apparent deterministic chaos were present in 8.3% of syllables (individually, m108, 6.3%; m123, 5.0%; m130, 6.7%, m152; 15.3%). Chaotic sounds occurred in vocalizations accompanied by unilateral airflow (Fig 5) as well as those produced during bilateral airflow (Fig 9). Most examples of chaos in the repertoires of these birds were produced with bilateral syringeal airflow (m108, 2.9%; m123, 4.5%; m130, 5.9%; m152, 13.2% of syllables sampled), however, examples of unilaterally-produced aperiodic sounds were also present (1.7% of all syllables sampled for all birds. Individually: m108, 3.4%; m123, 0.5%; m130, 0.8%; m152, 2.1% of syllables sampled). In each case, the apparent chaos took the form of broadband noise with a sudden onset. Chaotic segments were sometimes preceded by or followed by subharmonics (Fig 5), and harmonic windows within the “noisy” segment (e.g. Fig 9A, arrow b) were commonly observed.
Figure 9.
Unequal contribution of right and left sides to bilaterally-produced chaos. Chaotic sounds often contained harmonic windows, or brief moments of periodic oscillation, such as that seen around arrow b. Mockingbird “loud hew” calls (A) were always produced with flow through both sides of the syrinx for the entire duration of the call. In most cases (56 of 67 calls) one side alone appeared to contribute the majority of the aperiodicity in the call, while the contribution of the other side is more pure-tonal. (B) The difference in aperiodic behavior of the two sides is shown by examination of sound recorded inside the right and left bronchi and cranial thoracic air sac (obtained by high-pas filtering and amplifying thermistor and pressure transducer outputs). (C) 20 ms segment of the sound waveform, at the time indicated by arrow a in the spectrogram, illustrating the aperiodic nature of the oscillation. (D) Power spectrum of the sound taken at arrow a in the spectrogram (A). Additionally, the air flow in the right side (F R) shows an aperiodic, rapid modulation, which not present in the flow on the left (F L). Other abbreviations as in Fig 3.
Syringeal Lateralization of Chaotic Sound Production
At least three types of unlearned mockingbird calls contain chaotic sounds, including “loud hews” (Fig 9), “soft hews” and “chat” calls. Male mockingbirds are known to sometimes include calls in their song repertoire (Derrickson and Breitwisch, 1992). During a singing bout mockingbirds may include several repetitions of loud and soft hews and chat calls in addition to the mimicked syllables learned from tutors. Loud hew calls are broadband sounds with an abrupt onset and offset, and a duration of 214 - 600 ms (mean = 359.7 ±110.03 ms, N=61). Loud hew calls were always produced bilaterally, but quiet hew calls were typically produced unilaterally. Soft hews are not only quieter than loud hews, but are more restricted in bandwidth. Chat calls are short (ranging between 34 - 8 ms mean = 56.3 ±17.37 ms, N=30) broadband explosive sounds, produced bilaterally.
Al four of the birds in this study included “loud hew” calls in their song repertoires. We examined the rate of airflow and sound recorded in each bronchus for 67 loud hew calls from the 4 birds in this study (to increase sample size, we used an additional 50 loud hews that were not in the 400 syllables used for NLP count). Although always produced with airflow through both sides of the syrinx, the chaotic nature of the hew calls often appeared to be the result of chaotic oscillations from only a single side of the syrinx. Figure 9 shows the contribution to sound from the right and left sides during hew calls produced by mockingbird m130, as recorded in the right and left bronchus by the thermistors. The frequency response of the thermistors rolls off at about 3 or 4 kHz, so it is not possible to completely rule out a chaotic contribution at higher frequencies on the left side. However, the presence of rapid fluctuations in the air flow rate through the right side (F R in Fig 9A and B) and their absence in the flow rate on the left side (FL in Fig 9A and B) suggests that the two sides are behaving differently when producing these chaotic sounds. In most hew calls (51 of 67) the right side alone appeared responsible for most of the aperiodicity, while the left side’s contribution was more tonal. In these 51 cases, the rate of air flow in the right side shows an aperiodic, rapid modulation, which is not present in the flow on the left. In 5 of 67 calls, the left side appeared responsible for most of aperiodicity, and a strong aperiodic fluctuation in left flow rate throughout the duration of the call, while the flow rates on the right side were more constant. In 11 of 67 calls, the two sides appeared to contribute equally, or the relative contribution of the right and left sides could not be determined from the bronchial flow signals.
DISCUSSION
Nonlinear phenomena vs. two-voice complexity
Excised syrinx experiments predict that the tracheobronchial syrinx should spontaneously produce NLP in vocalizing songbirds (Fee et al., 1998), and NLP have been identified in the natural vocalizations of intact birds with only a single sound source (e.g. a tracheal syrinx), such as parrots and doves (Beckers et al., 2003; Fletcher, 2000; Lavenex, 1999). But in songbirds, the presence of the “two voices” makes it difficult to identify some kinds of NLP on the basis of vocalizations alone. By recording the respiratory dynamics of vocalization on each side of the syrinx of intact, singing mockingbirds, we found unilaterally produced NLP in about 8% of all syllables. This represents only about one-sixth of the sounds containing apparent biphonation, chaos, frequency jumps or subharmonics based on spectrographic analysis alone (48.7 %). The relatively smal ratio of NLP to harmonic sounds in our sample suggests that while the exploitation of the passive biomechanical properties of the syrinx is a possible strategy for production of complex sounds, mockingbirds rely primarily on their “two voices” for the rich diversity of complex sounds in their repertoires. Two-voice complexity may be easier to control than NLP and the availability of two sound sources might reduce the need for birds to rely on NLP, which are potentially les predictable, to increase vocal diversity. Nonetheless, our analysis of the peripheral vocal and respiratory dynamics associated with NLP provides insights into how songbirds might exploit the properties of each of its two sound sources, alone or in combination, to produce or avoid complex sounds. For example, a songbird might produce biphonation simultaneously with both “voices” resulting in sounds with up to four unrelated frequencies, such as those described by Thorpe (1961).
We found unilateral biphonation of two types in mockingbird song: as two harmonically-unrelated fundamental frequencies, each independently modulated (Fig 6), and as one f0 modulated by a second, lower frequency, appearing as sidebands parallel to the f0 in a narrowband spectrogram (Fig 7). It is important to note that while sidebands indicate the occurrence of two independent frequencies, there are several ways in which they may be produced. In biphonation, sidebands result from one f0 modulated by a second, lower frequency (Greenewalt, 1968; Lavenex, 1999). Experiments with an excised larynx and computer modelling have shown that the mammalian vocal folds, vibrating asymmetrically, can produce AM with accompanying sidebands that are the result of linear combinations of the two fundamentals (e.g. 2 g0 - f0, 2f0 - g0) (Giovanni et al., 1999; Herzel et al., 1995; Mergell and Herzel, 1997; Neubauer et al., 2001). In such cases, the two vocal folds oscillate at slightly different frequencies, each producing a separate sound pressure wave that is close to the other in fundamental frequency. If the same phenomenon can occur during oscillation of the medial and lateral labia in songbirds, biphonic vocalizations might be the result of either a higher f0 with a lower modulating frequency (e.g. Fig 1B), or the interaction of two higher tones, which are close in fundamental frequency (Fig 1A). In either case, neither unilateral biphonation nor dual (two-voice) biphonation, together with their syringeal motor correlates, has been previously described in intact songbirds.
Production and control of nonlinear phenomena
We hypothesize that although production of NLP is a passive biomechanical process, birds may sometimes exert voluntary control over the conditions under which it occurs. However, in other cases NLP might be “unintentional,” resulting from instabilities of the vocal system. The occurrence of NLP in mam alian vocalizations increases as sound level and frequency increase, suggesting it may be produced by driving the vocal system to its performance limit (Riede et al., 2007) and past threshold points that mark boundaries between stable vibratory modes.
Fee et al. (1998) demonstrated in a zebra finch excised syrinx preparation that a linear increase in subsyringeal pressure results in bifurcations between different vibratory modes. The absence of a consistent relationship between the occurrence of NLP and a detectable increase in rates of bronchial air flow, subsyringeal air sac pressure, or f0 in our mockingbirds may reflect the availability of respiratory, syringeal and upper vocal tract neuromuscular control in our live birds. Although we did not observe a consistent pattern in the direction or rate of bronchial airflow or in subsyringeal pressure associated with the occurrence of NLP, the fact that frequency jumps were significantly (but not always) correlated with increased variability in the rate of airflow is consistent with the hypothesis that at least some NLP reflects a failure in vocal motor control. Nonlinear theory suggests that even very small changes in control parameters such as subglottal pressure can result in production of NLP. There are many potential control parameters, such as syringeal resistance, the tension of the oscillating labia or the pressure and flow profiles acros their surface, which we did not measure.
Source-source and source-tract interactions
We found several cases of airflow through both sides of the syrinx generating thre independent frequencies (f0, g0, and m0). Nowicki and Capranica (1986) reported evidence for a source-vocal tract coupling in the amplitude-modulated calls of black-capped chickadees (Parus atricapillus), and speculated on possible source-source (acoustic or mechanical) coupling. Our observation that in each case of bilateral biphonation the frequency of the amplitude modulation (m0) was the same for both the left- and right-produced fundamentals supports their hypothesis of source-source coupling in the oscine syrinx.
Possible mechanisms of source-filter and source-source interactions were investigated recently with computational models (Laje and Mindlin, 2005; Laje et al., 2008), which demonstrate that such interactions could indeed produce the complex, multi-frequency sounds observed in birdsong. Laje and Mindlin’s results are relevant to our observations, because they demonstrate how sounds, such as subharmonics, previously presumed to be NLP in intact songbirds such as zebra finches (e.g. Fe et al., 1998), could alternatively be the result of acoustic interactions between the two sides. In addition, Nelson (2004) describes how rapid FM within a song element in eastern towhees can produce an AM-like sideband pattern in narrowband spectrograms, and further speculated on an as yet unidentified third independent modulator in the syrinx, but further evidence for the identity or existence of such a structure is lacking.
These simulations and models demonstrate that crosstalk between a fundamental frequency and resonance frequencies may affect the vibration of the source. The likelihood of an interaction of sound source and vocal tract filter increases if vocal tract impedance is adjusted to match the impedance of the source (Titze, 2004). A highly variable vocal tract system with complex motor patterns has been demonstrated in songbirds (Fletcher et al., 2006; Riede et al., 2006), and thus one may expect that songbirds, like humans, have a variety of vocal “tools” at their disposal to avoid or minimize the occurrence of involuntary NLP during song.
Deterministic Chaos in Calls and Song
Periods of deterministic chaos can theoretically occur in any system of coupled oscillators, such as the paired labia within each side of a songbird syrinx. Whereas chaos may or may not be a common feature of learned song, the unlearned calls of many songbirds, such as alarm calls, contact calls and aggressive calls, are often characterized by broadband, noisy, “buzzy” or “harsh” sounds (Marler, 2004).
Although our data show that mockingbirds may have some control over the respiratory and syringeal parameters necessary to allow the production of chaotic sounds, as evidenced by the chaotic nature of thre very common call types, chaotic vibratory modes are also presumably induced involuntarily at times. Broadband, aperiodic sounds were found within a small number of otherwise pure-tonal song elements. These erratic occurrences of chaos may be more akin to those described in the mam alian literature, which are often attributed to pathologies (Herzel et al., 1994; Mende et al., 1990), instabilities in the vocal system (Mergel et al., 2000), increasing, or maximizing frequency or amplitude levels (Berry, 2001; Brown et al., 2003; Riede et al., 2007) or abrupt desynchronization of the vibrating labia (Neubauer et al., 2001). Instances of chaotic sounds within more tonal vocalizations were likely involuntary, as they rarely occurred more than once in the same syllable type from the same bird.
Possible Communicative Roles of Nonlinear Phenomena
Several communicative functions of NLP have been hypothesized. For instance, it has been suggested that increased vocal “roughness” might be an honest indicator of poor reproductive fitness (Goller, 1998) or health status (Herzel et al., 1994; Riede et al., 1997). A preference for pure-tonal over harmonic or aperiodic vocalizations has been shown for some songbirds (Strote and Nowicki, 1996). An alternative, seemingly contradictory, hypothesis suggests that animals might exploit the nonlinear properties of their vocal systems to increase vocal complexity, and that NLP could aid in individual recognition in some species (Fe et al., 1998; Fitch et al., 2002; Volodina et al., 2006; Wilden et al., 1998). Additionally, NLP may function to increase the auditory impact of calls (Owren, 2003; Owren and Rendall, 2001), and so could be useful both for attracting allies or mates, as wel as for signalling status or physical condition. While it is still not clear what, if any, adaptive advantages or selective pressures are associated with NLP in animal vocalizations, the literature is becoming increasingly rich with examples of these phenomena in an ever widening range of taxa. The extent to which birds use nonlinear phenomena as salient features in vocal communication varies significantly between species. Species differences in the inclusion of NLP in their vocal signals may reflect the need, in part, to achieve an appropriate balance between syllable stereotypy and syllable diversity.
ACKNOWLEDGEMENTS
We are grateful to Drs. Kenneth K. Jensen, G. Troy Smith, and two anonymous reviewers for helpful comments on this manuscript. Dr. Brian S. Nelson wrote the sound analysis scripts used in Igor Pro, and provided many helpful suggestions on data analysis. Dr Isao Tokuda generously produced the synthesized chaos and subharmonic signals used in Fig 2. Thanks to Johanna Kolodziejski, Sandra Ronan and Rachel Davis for help collecting birds and technical assistance. The research was supported by NIH-NINDS grant R01 NS02467 to R.A.S.; training grant support by NIH_NIDCD DC00012 to S.Z. and a postdoctoral fellowship to T.R. from the Deutsche Akademie der Naturforscher Leopoldina (BMBF-LPD 9901/8-127). Al experiments were in compliance with the guidelines of the National Institutes of Health and were reviewed and approved by the Institutional Animal Care and Use Commit e of Indiana University.
REFERENCES
- Allan SE, Suthers RA. Lateralization and motor stereotypy of song production in the brown-headed cowbird. J. Neurobiol. 1994;25:1154–1166. doi: 10.1002/neu.480250910. [DOI] [PubMed] [Google Scholar]
- Ballentine B, Hyman J, Nowicki S. Vocal performance influences female response to male bird song: an experimental test. Behavioral Ecology. 2004;15:163–168. [Google Scholar]
- Baptista LF, Trail PW. The Role of Song in the Evolution of Passerine Diversity. Systematic Biology. 1992;41:242–247. [Google Scholar]
- Beckers GJL, Suthers RA, ten Cate C. Mechanisms of frequency and amplitude modulation in ring dove song. Journal of Experimental Biology. 2003;206:1833–1843. doi: 10.1242/jeb.00364. [DOI] [PubMed] [Google Scholar]
- Beckers GJL, ten Cate C. Nonlinear phenomena and song evolution in Streptopelia doves. Acta Zoologica Sinica. 2006;52:482–485. [Google Scholar]
- Berry DA. Mechanisms of modal and nonmodal phonation. Journal of Phonetics. 2001;29:431–450. [Google Scholar]
- Berry DA, Herzel H, Titze IR, Story BH. Bifurcations in excised larynx experiments. Journal of Voice. 1996;10:129–138. doi: 10.1016/s0892-1997(96)80039-7. [DOI] [PubMed] [Google Scholar]
- Borror DJ, Reese CR. Vocal gymnastics in wood thrush songs. The Ohio Journal of Science. 1956;56:177–182. [Google Scholar]
- Brown CH, Alipour F, Berry DA, Montequin D. Laryngeal biomechanics and vocal com unication in the squirrel monkey (Saimiri boliviensis) Journal of the Acoustical Society of America. 2003;113:2114–2126. doi: 10.1121/1.1528930. [DOI] [PubMed] [Google Scholar]
- Derrickson KC, Breitwisch R. Northern Mockingbird. In: Poole PSA, Gill F, editors. The Birds of North America. Vol. 7. Smith, Edwards, Dunlop, Inc.; Philadelphia, PA: 1992. [Google Scholar]
- Fee MS. Measurement of the linear and nonlinear mechanical properties of the oscine syrinx: Implications for function. Journal of Comparative Physiology a-Neuroethology Sensory Neural and Behavioral Physiology. 2002;188:829–839. doi: 10.1007/s00359-002-0349-z. [DOI] [PubMed] [Google Scholar]
- Fee MS, Shraiman B, Pesaran B, Mitra PP. The role of nonlinear dynamics of the syrinx in the vocalizations of a songbird. Nature. 1998;395:67–71. doi: 10.1038/25725. [DOI] [PubMed] [Google Scholar]
- Fitch WT, Neubauer J, Herzel H. Calls out of chaos: the adaptive significance of nonlinear phenomena in mammalian vocal production. Animal Behaviour. 2002;63:407–418. [Google Scholar]
- Fitzpatrick JW. Why so many passerine birds? A response to Raikow. Systematic Zoology. 1988;37:71–76. [Google Scholar]
- Fletcher NH. A clas of chaotic bird calls? Journal of the Acoustical Society of America. 2000;108:821–826. doi: 10.1121/1.429615. [DOI] [PubMed] [Google Scholar]
- Fletcher NH, Riede T, Suthers RA. Model for vocalization by a bird with distensible vocal cavity and open beak. Journal of the Acoustical Society of America. 2006;119:1005–1011. doi: 10.1121/1.2159434. [DOI] [PubMed] [Google Scholar]
- Floody OR, Arnold AP. Song lateralization in the zebra finch. Hormones and Behavior. 1997;31:25–34. doi: 10.1006/hbeh.1997.1368. [DOI] [PubMed] [Google Scholar]
- Forstmeier W, Kempenaers B, Meyer A, Leisler B. A novel song parameter correlates with extra-pair paternity and reflects male longevity. Proceedings of the Royal Society of London Series B-Biological Sciences. 2002;269:1479–1485. doi: 10.1098/rspb.2002.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanni A, Ouaknine M, Guelfucci B, Yu P, Zanaret M, Triglia JM. Nonlinear behavior of vocal fold vibration: The role of coupling between the vocal folds. Journal of Voice. 1999;13:465–476. doi: 10.1016/s0892-1997(99)80002-2. [DOI] [PubMed] [Google Scholar]
- Goller F. Vocal gymnastics and the bird brain. Nature. 1998;395:11–12. [Google Scholar]
- Goller F, Larsen ON. New perspectives on mechanisms of sound generation in songbirds. Journal of Comparative Physiology a-Neuroethology Sensory Neural and Behavioral Physiology. 2002;188:841–850. doi: 10.1007/s00359-002-0350-6. [DOI] [PubMed] [Google Scholar]
- Goller F, Suthers RA. Role of syringeal muscles in controlling the phonology of bird song. J. Neurophysiol. 1996a;76:287–300. doi: 10.1152/jn.1996.76.1.287. [DOI] [PubMed] [Google Scholar]
- Goller F, Suthers RA. Role of syringeal muscles in gating airflow and sound production in singing brown thrashers. J. Neurophysiol. 1996b;75:867–876. doi: 10.1152/jn.1996.75.2.867. [DOI] [PubMed] [Google Scholar]
- Greenewalt CH. Bird Song: Acoustics and Physiology. Smithsonian Institution Press; Washington, DC: 1968. [Google Scholar]
- Herzel H, Berry D, Titze IR, Saleh S. Analysis of vocal disorders with methods from nonlinear dynamics. Journal of Speech and Hearing Research. 1994;37:1008–1019. doi: 10.1044/jshr.3705.1008. [DOI] [PubMed] [Google Scholar]
- Herzel H, Berry D, Titze IR, Steinecke I. Nonlinear dynamics of the voice: Signal analysis and biomechanical modelling. Chaos. 1995;5:30–34. doi: 10.1063/1.166078. [DOI] [PubMed] [Google Scholar]
- King AS. Functional anatomy of the syrinx. In: King AS, McLelland J, editors. Form and Function in Birds. Vol. 4. Academic Press; London: 1989. pp. 105–192. [Google Scholar]
- Laje R, Mindlin GB. Modeling source-source and source-filter acoustic interaction in birdsong. Physical Review E. 2005;72 doi: 10.1103/PhysRevE.72.036218. [DOI] [PubMed] [Google Scholar]
- Laje R, Sciamarella D, Zanella J, Mindlin GB. Bilateral source acoustic interaction in a syrinx model of an oscine bird. Physical Review E. 2008;77 doi: 10.1103/PhysRevE.77.011912. [DOI] [PubMed] [Google Scholar]
- Lavenex PB. Vocal production mechanisms in the budgerigar (Melopsittacus undulatus): The presence and implications of amplitude modulation. Journal of the Acoustical Society of America. 1999;106:491–505. doi: 10.1121/1.427079. [DOI] [PubMed] [Google Scholar]
- Lemon RE. Nervous control of the syrinx in white-throated sparrows (Zonotrichia albicollis) J. Zool., Lond. 1973;171:131–140. [Google Scholar]
- Marler P. Bird calls - Their potential for behavioral neurobiology. Annals of the New York Academy of Sciences. 2004;1016:31–44. doi: 10.1196/annals.1298.034. [DOI] [PubMed] [Google Scholar]
- Mende W, Herzel H, Wermke K. Bifurcations and Chaos in Newborn-Infant Cries. Physics Letters A. 1990;145:418–424. [Google Scholar]
- Mergell P, Herzel H. Modelling biphonation - The role of the vocal tract. Speech Communication. 1997;22:141–154. [Google Scholar]
- Mergell P, Herzel H, Titze IR. Irregular vocal-fold vibration - High-speed observation and modeling. Journal of the Acoustical Society of America. 2000;108:2996–3002. doi: 10.1121/1.1314398. [DOI] [PubMed] [Google Scholar]
- Mindlin GB, Laje R. The Physics of Birdsong Berlin. Springer-Verlang; 2005. [Google Scholar]
- Nelson BS. Dynamics of frequency and amplitude modulations in vocalizations produced by eastern towhees, Pipilo erythrophthalmus. Journal of the Acoustical Society of America. 2004;115:1333–1344. doi: 10.1121/1.1648976. [DOI] [PubMed] [Google Scholar]
- Neubauer J, Mergell P, Eysholdt U, Herzel H. Patio-temporal analysis of irregular vocal fold oscil ations: Biphonation due to desynchronization of spatial modes. Journal of the Acoustical Society of America. 2001;110:3179–3192. doi: 10.1121/1.1406498. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. Neural lateralization of vocal control in a passerine bird I. song. J. Exp. Zool. 1971;177:229–262. doi: 10.1002/jez.1401770210. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Nottebohm ME. Left hypoglossal dominance in the control of canary and white-crowned sparrow song. J. Comp. Physiol. 1976;108:171–192. [Google Scholar]
- Nowicki S, Capranica RR. Bilateral syringeal coupling during phonation of a songbird. J. Neurosci. 1986;6:3595–3610. doi: 10.1523/JNEUROSCI.06-12-03595.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicki S, Searcy WA. Song function and the evolution of female preferences - Why birds sing, why brains matter. Annals of the New York Academy of Sciences. 2004;1016:704–723. doi: 10.1196/annals.1298.012. [DOI] [PubMed] [Google Scholar]
- Owren MJ. Vocal production and perception in nonhuman primates provide clues about early hominids and speech evolution. ATR Sympos HIS Series. 2003;1:1–19. [Google Scholar]
- Owren MJ, Rendall D. Sound on the rebound: Bringing form and function back to the forefront in understanding nonhuman primate vocal signaling. Evolutionary Anthropology. 2001;10:58–71. [Google Scholar]
- Raikow RJ. Why are there so many kinds of passerine birds? Systematic Zoology. 1986;35:255–259. [Google Scholar]
- Riede T, Arcadi AC, Owren MJ. Nonlinear acoustics in the pant hoots of com on chimpanzees (Pan troglodytes): Vocalizing at the edge. Journal of the Acoustical Society of America. 2007;121:1758–1767. doi: 10.1121/1.2427115. [DOI] [PubMed] [Google Scholar]
- Riede T, Herzel H, Mehwald D, Seidner W, Trumler E, Bohme G, Tembrock G. Nonlinear phenomena in the natural howling of a dog-wolf mix. Journal of the Acoustical Society of America. 2000;108:1435–1442. doi: 10.1121/1.1289208. [DOI] [PubMed] [Google Scholar]
- Riede T, Owren MJ, Arcadi AC. Nonlinear acoustics in pant hoots of com on chimpanzees (Pan troglodytes): Frequency jumps, subharmonics, biphonation, and deterministic chaos. American Journal of Primatology. 2004;64:277–291. doi: 10.1002/ajp.20078. [DOI] [PubMed] [Google Scholar]
- Riede T, Suthers RA, Fletcher NH, Blevins WE. Songbirds tune their vocal tract to the fundamental frequency of their song. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:5543–5548. doi: 10.1073/pnas.0601262103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riede T, Wilden I, Tembrock G. Subharmonics, biphonations, and frequency jumps - com on components of mammalian vocalization or indicators for disorders? Zeitschrift fur Saugetierkunde - International Journal of Mammalian Biology. 1997;62:198–203. [Google Scholar]
- Stein RC. Modulation in bird sound. Auk. 1968;94:229–243. [Google Scholar]
- Strote J, Nowicki S. Responses to songs with altered tonal quality by adult song sparrows (Melospiza melodia) 1996. pp. 161–172. [Google Scholar]
- Suthers RA. Contributions to birdsong from the left and right sides of the intact syrinx. Nature. 1990;347:473–477. [Google Scholar]
- Suthers RA. The motor basis of vocal performance in songbirds. In: Hauser M, Konishi M, editors. The Design of Animal Communication. MIT Press; Cambridge, MA: 1999. pp. 37–62. [Google Scholar]
- Suthers RA, Beckers GJL, Nelson BS, Kanwal JS, Ehret G. Behavior and Neurodynamics for Auditory Communication. Cambridge University Press; Cambridge: 2005. Vocal Mechanisms for Avian Com unication. [Google Scholar]
- Suthers RA, Goller F. Motor correlates of vocal diversity in songbirds. In: Nolan V Jr., Ket erson E, Thompson CF, editors. Current Ornithology. Vol. 14. Plenum Press; New York: 1997. pp. 235–288. [Google Scholar]
- Suthers RA, Goller F, Hartley RS. Motor dynamics of song production by mimic thrushes. J. Neurobiol. 1994;25:917–936. doi: 10.1002/neu.480250803. [DOI] [PubMed] [Google Scholar]
- Suthers RA, Narins PM, Lin WY, Schnitzler HU, Denzinger A, Xu CH, Feng AS. Voices of the dead: complex nonlinear vocal signals from the larynx of an ultrasonic frog. Journal of Experimental Biology. 2006;209:4984–4993. doi: 10.1242/jeb.02594. [DOI] [PubMed] [Google Scholar]
- Suthers RA, Vallet E, Tanvez A, Kreutzer M. Bilateral song production in domestic canaries. Journal of Neurobiology. 2004;60:381–393. doi: 10.1002/neu.20040. [DOI] [PubMed] [Google Scholar]
- Suthers RA, Zollinger SA. Producing Song: The Vocal Apparatus. Annals of the New York Academy of Science. 2004;1016:109–129. doi: 10.1196/annals.1298.041. [DOI] [PubMed] [Google Scholar]
- Thorpe WH. Bird-Song. Cambridge University Press; Cambridge: 1961. [Google Scholar]
- Titze IR. Theory of glottal airflow and source-filter interaction in speaking and singing. Acta Acustica United with Acustica. 2004;90:641–648. [Google Scholar]
- Titze IR, Baken R, Herzel H. Evidence of chaos in vocal fold vibration. In: Titze IR, editor. Vocal fold physiology. Singular Publishing Group; San Diego: 1993. pp. 143–188. [Google Scholar]
- Tokuda I, Riede T, Neubauer J, Owren MJ, Herzel H. Nonlinear analysis of irregular animal vocalizations. Journal of the Acoustical Society of America. 2002;111:2908–2919. doi: 10.1121/1.1474440. [DOI] [PubMed] [Google Scholar]
- Tyson RB, Nowacek DP, Mil er PJO. Nonlinear phenomena in the vocalizations of North Atlantic right whales (Eubalaena glacialis) and killer whales (Orcinus orca) Journal of the Acoustical Society of America. 2007;122:1365–1373. doi: 10.1121/1.2756263. [DOI] [PubMed] [Google Scholar]
- Vallet E, Beme I, Kreutzer M. Two-note syl ables in canary songs elicit high levels of sexual display. Animal Behaviour. 1998;55:291–297. doi: 10.1006/anbe.1997.0631. [DOI] [PubMed] [Google Scholar]
- Vermeij GJ. The evolutionary success of passerines: A question of semantics? Systematic Zoology. 1988;37:69–71. [Google Scholar]
- Volodina EV, Volodin IA, Isaeva IV, Unck C. Biphonation may function to enhance individual recognition in the dhole, Cuon alpinus. Ethology. 2006;112:815–825. [Google Scholar]
- Wilden I, Herzel H, Peters G, Tembrock G. Subharmonics, biphonation, and deterministic chaos in mammal vocalization. Bioacoustics. 1998;9:171–196. [Google Scholar]
- Williams H, Crane LA, Hale TK, Esposito MA, Nottebohm F. Right-side dominance for song control in the Zebra Finch. J. Neurobiol. 1992;23:1006–1020. doi: 10.1002/neu.480230807. [DOI] [PubMed] [Google Scholar]
- Zollinger SA, Suthers RA. Motor mechanisms of a vocal mimic: implications for birdsong production. Proceedings of the Royal Society of London Series B-Biological Sciences. 2004;271:483–491. doi: 10.1098/rspb.2003.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]