Abstract
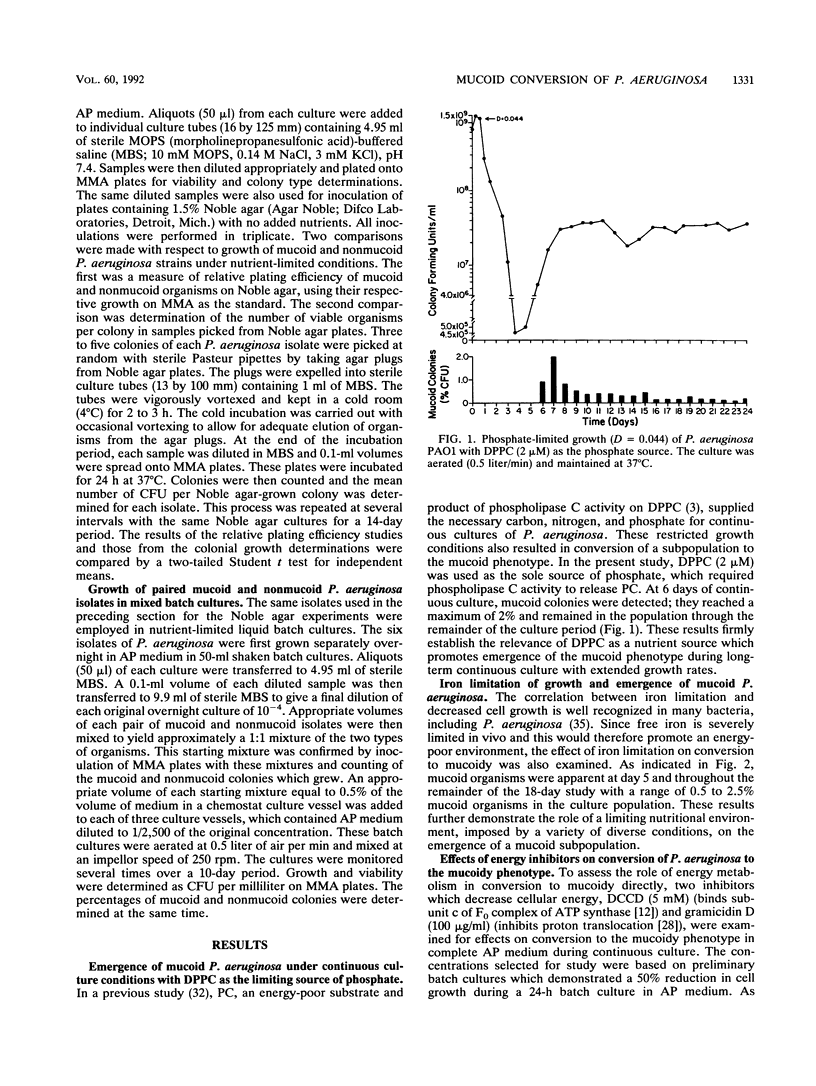
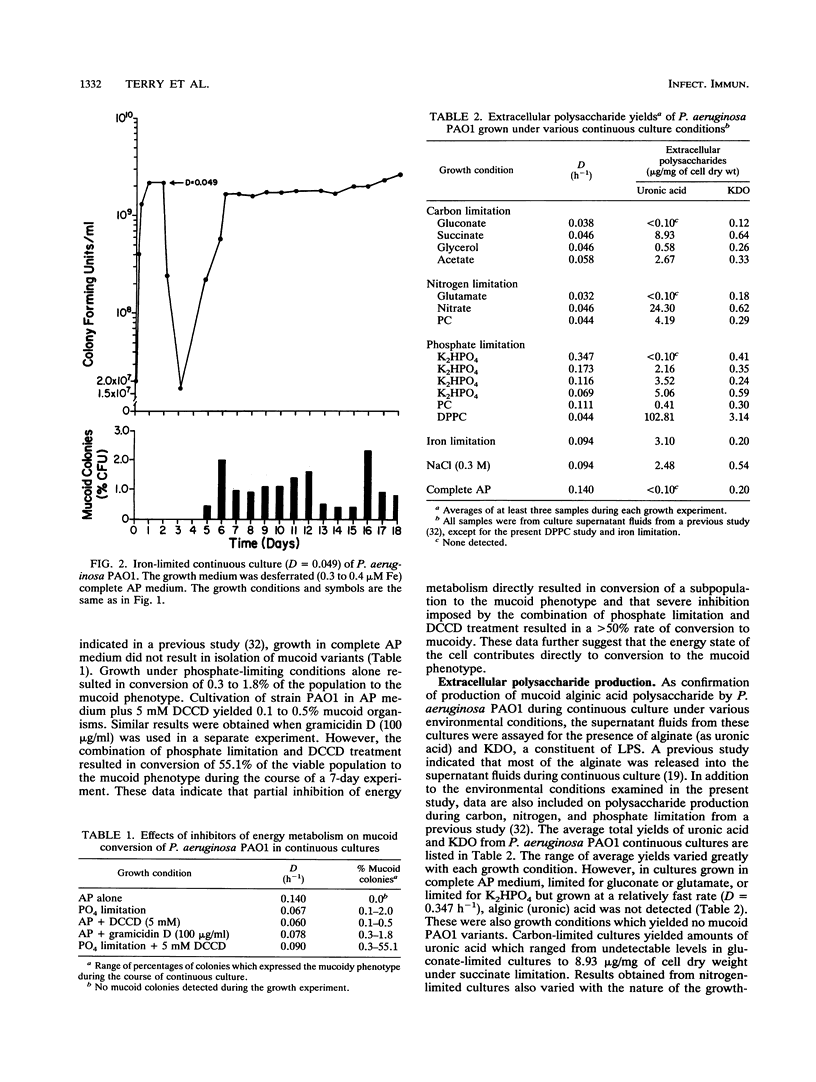
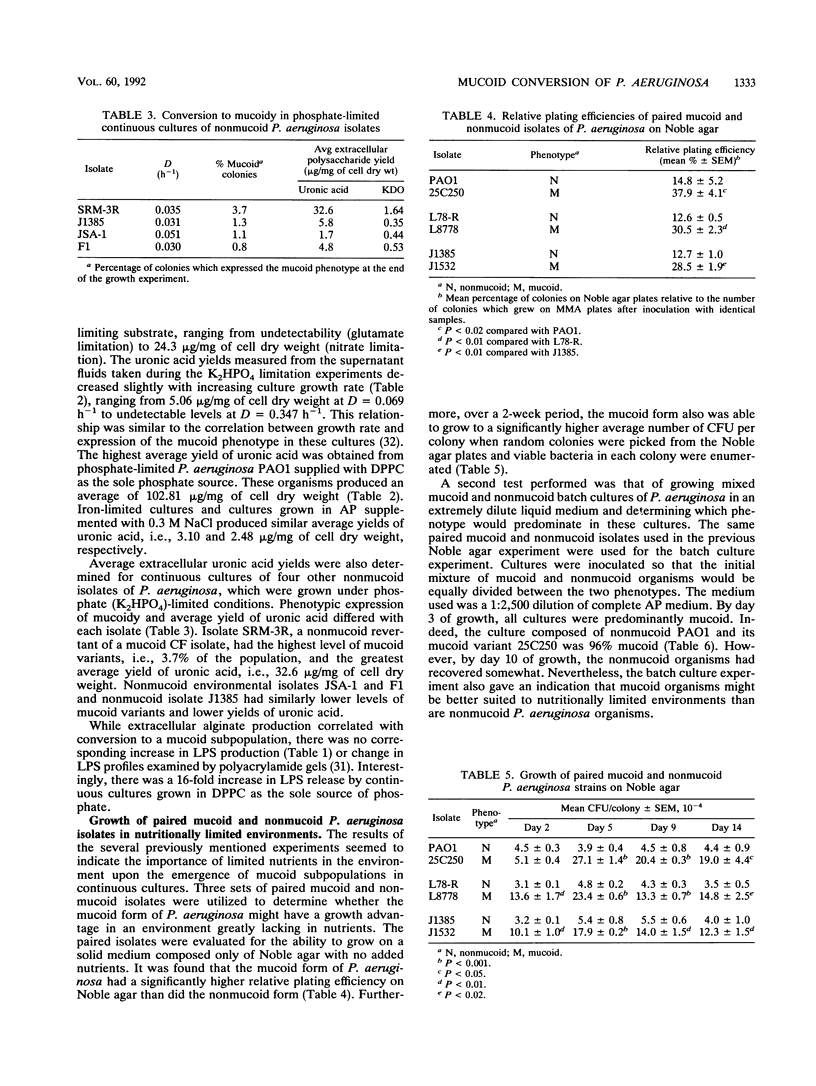
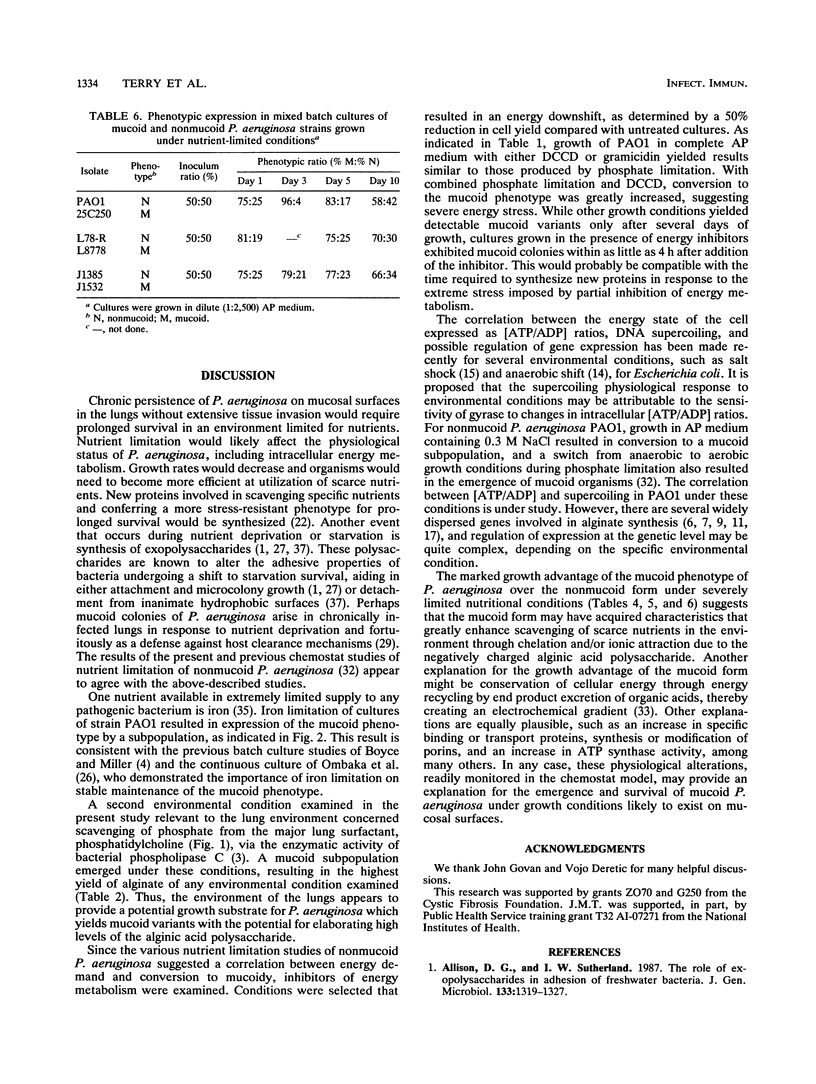
Phosphatidylcholine, the major component of lung surfactant, when supplied as the sole source of phosphate for Pseudomonas aeruginosa PAO1, resulted in conversion of as much as 2% of the population to the mucoid phenotype under continuous culture conditions over a 24-day culture period. In addition, growth in phosphatidylcholine resulted in the highest yields of extracellular alginate compared with other environmental conditions. Iron limitation, another environmental condition relevant to the lungs of patients with cystic fibrosis, also resulted in conversion to mucoid. Since both conditions suggested the likelihood of an energy-deprived growth environment as a common variable, the effect of direct inhibition of energy generation by N,N'-dicyclohexylcarbodiimide or gramicidin on the conversion of nonmucoid P. aeruginosa to the mucoid phenotype was examined. Both inhibitors resulted in mucoid subpopulations (0.5 and 0.8%, respectively). Severe energy stress imposed by the combination of phosphate limitation and N,N'-dicyclohexylcarbodiimide treatment resulted in conversion of 55% of the population to mucoidy during a 7-day growth period. A growth advantage of the mucoid over the nonmucoid phenotype was observed under severe nutrient deprivation by growth on unsupplemented Noble agar or in a 1/2,500 dilution of a chemically defined medium. These results clearly demonstrate a significant role for the energy state of the cell in conversion to mucoid and in selection for the mucoid phenotype.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berka R. M., Gray G. L., Vasil M. L. Studies of phospholipase C (heat-labile hemolysin) in Pseudomonas aeruginosa. Infect Immun. 1981 Dec;34(3):1071–1074. doi: 10.1128/iai.34.3.1071-1074.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce J. R., Miller R. V. Selection of nonmucoid derivatives of mucoid Pseudomonas aeruginosa is strongly influenced by the level of iron in the culture medium. Infect Immun. 1982 Aug;37(2):695–701. doi: 10.1128/iai.37.2.695-701.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V., Gill J. F., Chakrabarty A. M. Gene algD coding for GDPmannose dehydrogenase is transcriptionally activated in mucoid Pseudomonas aeruginosa. J Bacteriol. 1987 Jan;169(1):351–358. doi: 10.1128/jb.169.1.351-358.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V., Konyecsni W. M. Control of mucoidy in Pseudomonas aeruginosa: transcriptional regulation of algR and identification of the second regulatory gene, algQ. J Bacteriol. 1989 Jul;171(7):3680–3688. doi: 10.1128/jb.171.7.3680-3688.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L. R., Linker A. Production and characterization of the slime polysaccharide of Pseudomonas aeruginosa. J Bacteriol. 1973 Nov;116(2):915–924. doi: 10.1128/jb.116.2.915-924.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn J. L., Ohman D. E. Cloning of genes from mucoid Pseudomonas aeruginosa which control spontaneous conversion to the alginate production phenotype. J Bacteriol. 1988 Apr;170(4):1452–1460. doi: 10.1128/jb.170.4.1452-1460.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George R. H. Pseudomonas infections in cystic fibrosis. Arch Dis Child. 1987 May;62(5):438–439. doi: 10.1136/adc.62.5.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J. B., Ohman D. E. Construction and characterization of Pseudomonas aeruginosa algB mutants: role of algB in high-level production of alginate. J Bacteriol. 1987 Apr;169(4):1593–1602. doi: 10.1128/jb.169.4.1593-1602.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermolin J., Fillingame R. H. H+-ATPase activity of Escherichia coli F1F0 is blocked after reaction of dicyclohexylcarbodiimide with a single proteolipid (subunit c) of the F0 complex. J Biol Chem. 1989 Mar 5;264(7):3896–3903. [PubMed] [Google Scholar]
- Hsieh L. S., Burger R. M., Drlica K. Bacterial DNA supercoiling and [ATP]/[ADP]. Changes associated with a transition to anaerobic growth. J Mol Biol. 1991 Jun 5;219(3):443–450. doi: 10.1016/0022-2836(91)90185-9. [DOI] [PubMed] [Google Scholar]
- Hsieh L. S., Rouviere-Yaniv J., Drlica K. Bacterial DNA supercoiling and [ATP]/[ADP] ratio: changes associated with salt shock. J Bacteriol. 1991 Jun;173(12):3914–3917. doi: 10.1128/jb.173.12.3914-3917.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høiby N., Döring G., Schiøtz P. O. The role of immune complexes in the pathogenesis of bacterial infections. Annu Rev Microbiol. 1986;40:29–53. doi: 10.1146/annurev.mi.40.100186.000333. [DOI] [PubMed] [Google Scholar]
- Kerem E., Corey M., Gold R., Levison H. Pulmonary function and clinical course in patients with cystic fibrosis after pulmonary colonization with Pseudomonas aeruginosa. J Pediatr. 1990 May;116(5):714–719. doi: 10.1016/s0022-3476(05)82653-8. [DOI] [PubMed] [Google Scholar]
- Konyecsni W. M., Deretic V. DNA sequence and expression analysis of algP and algQ, components of the multigene system transcriptionally regulating mucoidy in Pseudomonas aeruginosa: algP contains multiple direct repeats. J Bacteriol. 1990 May;172(5):2511–2520. doi: 10.1128/jb.172.5.2511-2520.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg D. P., Bass J. A., Mattingly S. J. Aeration selects for mucoid phenotype of Pseudomonas aeruginosa. J Clin Microbiol. 1986 Dec;24(6):986–990. doi: 10.1128/jcm.24.6.986-990.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg D. P., Bass J. A., Mattingly S. J. Phosphorylcholine stimulates capsule formation of phosphate-limited mucoid Pseudomonas aeruginosa. Infect Immun. 1988 Apr;56(4):864–873. doi: 10.1128/iai.56.4.864-873.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg D. P., Helmke R. J., German V. F., Mangos J. A. Resistance of mucoid Pseudomonas aeruginosa to nonopsonic phagocytosis by alveolar macrophages in vitro. Infect Immun. 1988 Dec;56(12):3173–3179. doi: 10.1128/iai.56.12.3173-3179.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A., Auger E. A., Blum P. H., Schultz J. E. Genetic basis of starvation survival in nondifferentiating bacteria. Annu Rev Microbiol. 1989;43:293–316. doi: 10.1146/annurev.mi.43.100189.001453. [DOI] [PubMed] [Google Scholar]
- Mian F. A., Jarman T. R., Righelato R. C. Biosynthesis of exopolysaccharide by Pseudomonas aeruginosa. J Bacteriol. 1978 May;134(2):418–422. doi: 10.1128/jb.134.2.418-422.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols W. W., Evans M. J., Slack M. P., Walmsley H. L. The penetration of antibiotics into aggregates of mucoid and non-mucoid Pseudomonas aeruginosa. J Gen Microbiol. 1989 May;135(5):1291–1303. doi: 10.1099/00221287-135-5-1291. [DOI] [PubMed] [Google Scholar]
- Ohman D. E., Sadoff J. C., Iglewski B. H. Toxin A-deficient mutants of Pseudomonas aeruginosa PA103: isolation and characterization. Infect Immun. 1980 Jun;28(3):899–908. doi: 10.1128/iai.28.3.899-908.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ombaka E. A., Cozens R. M., Brown M. R. Influence of nutrient limitation of growth on stability and production of virulence factors of mucoid and nonmucoid strains of Pseudomonas aeruginosa. Rev Infect Dis. 1983 Nov-Dec;5 (Suppl 5):S880–S888. doi: 10.1093/clinids/5.supplement_5.s880. [DOI] [PubMed] [Google Scholar]
- Read R. R., Costerton J. W. Purification and characterization of adhesive exopolysaccharides from Pseudomonas putida and Pseudomonas fluorescens. Can J Microbiol. 1987 Dec;33(12):1080–1090. doi: 10.1139/m87-189. [DOI] [PubMed] [Google Scholar]
- Rottenberg H., Koeppe R. E., 2nd Mechanism of uncoupling of oxidative phosphorylation by gramicidin. Biochemistry. 1989 May 16;28(10):4355–4360. doi: 10.1021/bi00436a035. [DOI] [PubMed] [Google Scholar]
- Simpson J. A., Smith S. E., Dean R. T. Alginate inhibition of the uptake of Pseudomonas aeruginosa by macrophages. J Gen Microbiol. 1988 Jan;134(1):29–36. doi: 10.1099/00221287-134-1-29. [DOI] [PubMed] [Google Scholar]
- Speert D. P., Farmer S. W., Campbell M. E., Musser J. M., Selander R. K., Kuo S. Conversion of Pseudomonas aeruginosa to the phenotype characteristic of strains from patients with cystic fibrosis. J Clin Microbiol. 1990 Feb;28(2):188–194. doi: 10.1128/jcm.28.2.188-194.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry J. M., Piña S. E., Mattingly S. J. Environmental conditions which influence mucoid conversion Pseudomonas aeruginosa PAO1. Infect Immun. 1991 Feb;59(2):471–477. doi: 10.1128/iai.59.2.471-477.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoni N., Aon M. A., Lebeault J. M., Thomas D. Proton motive force, energy recycling by end product excretion, and metabolic uncoupling during anaerobic growth of Pseudomonas mendocina. J Bacteriol. 1990 Dec;172(12):6673–6681. doi: 10.1128/jb.172.12.6673-6681.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardi A. H., Allen W. S., Varma R. A simple method for the detection and quantitative determination of hexuronic acids and pentoses. Anal Biochem. 1974 Jan;57(1):268–273. doi: 10.1016/0003-2697(74)90072-4. [DOI] [PubMed] [Google Scholar]
- Weinberg E. D. Cellular regulation of iron assimilation. Q Rev Biol. 1989 Sep;64(3):261–290. doi: 10.1086/416359. [DOI] [PubMed] [Google Scholar]
- Wrangstadh M., Conway P. L., Kjelleberg S. The production and release of an extracellular polysaccharide during starvation of a marine Pseudomonas sp. and the effect thereof on adhesion. Arch Microbiol. 1986 Aug;145(3):220–227. doi: 10.1007/BF00443649. [DOI] [PubMed] [Google Scholar]


