Abstract
The geometry of the semicircular canals has been used in evolutionary studies to predict the behaviors of extinct animals. These predictions have relied on an assumption that the responses of the canals can be determined from their dimensions, and that an organism’s behavior can be determined from these responses. However, the relationship between a canal’s sensitivity and its size is not well known. An intraspecies comparison among canal responses in each of three species (cat, squirrel monkey, and pigeon) was undertaken to evaluate various models of canal function and determine how their dimensions may be related to afferent physiology. All models predicted the responses of the cat afferents, but the models performed less well for squirrel monkey and pigeon. Possible causes for this discrepancy include incorrectly assuming that afferent responses accurately represent canal function, or errors in current biophysical models of the canals. These findings leave open the question as to how reliably canal anatomy can be used to estimate afferent responses and how closely afferent responses are related to behavior. Other labyrinthine features—such as orientation of the horizontal canal, which is reliably held near earth-horizontal across many species—may be better to use when extrapolating the posture and related behavior of extinct animals from labyrinthine morphology.
Changes in the geometry of the semicircular canals have offered insights into the evolution of species, including determining when ancestral humans adopted bipedal locomotion (Spoor et al., 1994), tracing the reentry of cetaceans (whales and dolphins) to an aquatic environment during the Eocene epoch approximately 35 million years ago (Spoor et al., 2002), evaluating when birds developed the ability to coordinate flight (Alonso et al., 2004), and assessing how dinosaurs might have held and moved their heads (Rogers, 1998; Clarke, 2005). These studies have assumed that the canals’ geometry can be matched to the responses of vestibular-nerve afferents, and that these responses can in turn be used to estimate head movements. Such assumptions, however, have been strongly disputed by vestibular anatomists and physiologists (Graf and Vidal, 1996; Spoor et al., 1996). In particular, the degree to which the bony anatomy of an animal’s semicircular canals may correlate with vestibular-afferent responses remains uncertain. This question is addressed here by comparing the responses of semicircular canal afferents arising from canals of different dimensions in three species: cat, squirrel monkey, and pigeon.
The peripheral vestibular system in higher vertebrates includes three semicircular canals responsible for collecting information regarding head rotations (Retzius, 1881, 1884; Wersäll and Bagger-Sjoback, 1974). Other vestibular end-organs, such as the otolith organs and the lagena, are not identifiable landmarks in bony or fossilized specimens so examination of the vestibular system in extinct animals has been limited to studying the shape and size of the bony semicircular canals. In living animals, these canals each enclose a membranous duct, which is filled with endolymphatic fluid and tethered by trabecular filaments to the surrounding bony canal, with the intervening space filled with perilymph (de Burlet, 1929). The radius of the semicircular arc of the bony canal is termed its radius of curvature (R), distinguished from the smaller radius of the membranous duct (r) obtained by measuring the cross-section of the duct. Occluding each duct across a widened section called the ampulla is the cupula. The cupula acts as a diaphragm, which is deformed as the endolymph’s inertia resists rotations of the head. Hair cells lie across the base of the cupula and are coupled to it by stereocilia; deflection of these structures by movement of the cupula causes a signal to be transduced by the hair cell and transmitted to primary vestibular afferents (Wersäll and Bagger-Sjoback, 1974). The afferent signal determined by cupular movement is modified by downstream processes inherent to the hair cell, hair cell-afferent synapse, and afferent itself (Highstein et al., 2005). Data collected by the vestibular end-organs on each side are transmitted to the central nervous system, where they are combined with visual, proprioceptive, and other stimuli to give a multisensory impression of the head and body’s orientation and movement in space (Leigh and Zee, 1999). This information is then provided to the vestibuloocular reflex, which stabilizes the eyes during head movements (Raphan and Cohen, 2002); the vestibulocollic reflex, which assists in stabilizing the head in space (Baker et al., 1985); autonomic reflexes, which maintain blood pressure and other homeostatic mechanisms during changes in body position (Balaban, 1999); and place cells, which provide a sense of direction during locomotion (Taube, 1998; Stackman et al., 2002).
Several hydrodynamic models of the semicircular canals’ responses (measured in terms of deflection of the cupula with respect to angular head velocity) have been published. Steinhausen (1933) proposed the classic torsion-pendulum model, which serves as the basis for several subsequent models of the semicircular canals (Van Egmond et al., 1949; Mayne, 1950). In its simplest form, the torsion-pendulum model consists of a second-order differential equation describing the response of a heavily damped system. Subsequent refinements of the model included adding an elastic representation of the cupula (Grant and van Buskirk, 1976; van Buskirk et al., 1976), accounting for the nonuniform shape of the canal (Oman et al., 1987), and including the coupling of mechanical effects among ipsilateral semicircular canals (Oman et al., 1987; Rabbitt, 1999).
All models suggest that the dynamic responses of the semicircular canals depend on the viscosity and density of the endolymph, the physical characteristics of the cupula, the radius of curvature of the canal, and the cross-sectional radius of the membranous duct. Within a species, where the properties of the endolymph and the cupula are likely to remain constant among the canals, the radius of curvature of the canal and the radius of the membranous duct are likely to be the major determinants of the responses of the semicircular canals to rotations (Jones and Spells, 1963). Because intraspecies comparisons allow many variables to be held constant among the canals being compared, these studies are particularly attractive for testing models of semicircular canal function. This approach has been used in two species to examine explicitly canal function in terms of its geometry. In neonatal rats (whose canals continue to grow during the first week of life), afferent sensitivity was found to be correlated to R (Curthoys, 1982) but in fish it was found that the threshold of the horizontal vestibuloocular reflex to head rotations did not change despite enlargement of the size of the horizontal canal with growth (ten Kate et al., 1970; ten Kate, 1973). This was explained as due to possible changes in the characteristics of the cupula with growth of the fish (ten Kate et al., 1970; Howland and Masci, 1973a) but central adaptation of the vestibulo-ocular reflex could also have been responsible.
No studies directly comparing head movements to afferent responses or canal geometry have been published (perhaps due to the difficulties inherent in measuring head movements accurately) but the question has been examined theoretically. Jones and Spells (1963) showed that the body mass of a wide variety of vertebrates was allometrically related to their canal size, interpreting this to mean that larger animals experience more sluggish head movements and therefore require enhanced sensitivity to low-amplitude motions, while smaller animals need canals that respond to higher angular amplitudes. Testing this hypothesis, an inertial model of fish was used to compare head motion indirectly to body mass and to canal size and orientation both within one species, the sunfish (Howland and Masci, 1973a), and across fish species (Howland and Masci, 1973b) with the results suggesting that the canal sizes were appropriately tuned to the estimated head movements.
Despite the paucity of data correlating canal geometry to afferent physiology or head movements, many authors have drawn conclusions about the behaviors of animals from the radius of curvature of their semicircular canals. The mole (Talpa europaea) has canals with a particularly large radius of curvature given its mass. This feature has been interpreted to represent an adaptation to increase vestibular sensitivity and aid in navigating the lightless habitat of these animals (Lindenlaub et al., 1995; McVean, 1999). If this is an important adaptation, however, it is somewhat surprising that other animals living without visual input, such as the blind fish Anoptichthes jordani, do not have similarly enlarged canals (Howland and Masci, 1973b). The prosimian Tarsius bancanus has been found to have larger semicircular canals than other primates, a finding hypothesized to be related to its particularly acrobatic behaviors (Matano et al., 1985). Among cetaceans (whales and dolphins), the semicircular canals have long been recognized as being much smaller than expected given the size of the body and cochlea (Hyrtl, 1845; Boenninghaus, 1903; Jones and Spells, 1963; Graf and Vidal, 1996; Spoor et al., 2002). The most recent of these reports indicated that the radius of curvature of the modern cetacean canal is approximately equal to that expected of an animal 1/1000 of its mass, with the size of ancestral cetacean canals forming an intermediate group (Spoor et al., 2002). The authors of that study theorized that the change in semicircular canal size occurred as the animals were re-acquiring an aquatic lifestyle during the Eocene epoch. Drawing on the observation that the cervical vertebrae of modern cetaceans are sometimes fused (DeSmet, 1977; Rommel, 1990), they hypothesized that the head movements of ancestral cetaceans increased in amplitude with increasing coupling of the head to the body, requiring reduced canals to avoid saturation of the vestibular signal due to increased head motion. Arguing against this theory is the fact that reduced semicircular canal size seems to be a universal finding among cetaceans despite the fact that many cetaceans do not have fused cervical vertebrae (Yablokov et al., 1972; Barnes and McLeod, 1984) and the observation that fish, which share an aquatic habitat with cetaceans and also have a tight coupling of the head to body, actually have larger radii of curvature than expected given their size (Jones and Spells, 1963; Howland and Masci, 1973a). In addition, recordings of head motion of freely swimming bottlenose dolphins (Tursiops truncatus) do not suggest that their head movements are unusually accentuated given their size (Hullar and Armand, 2004).
In many species, the anterior canal is relatively large compared to the other canals (Graf and Vidal, 1996) and therefore has been of particular interest to those correlating canal anatomy to head motion. Brachiosaur fossils indicate that this dinosaur had an elongated neck, leading to speculation that it would have had difficulty maintaining brain perfusion during high-amplitude, high-frequency vertical movements and might therefore have benefited from larger anterior canals to increase its sensitivity to allow accurate measurement of their presumably slow pitch movements (Clarke, 2005). Birds have also been recognized to have particularly large anterior canals, which has been explained as an adaptation to resolve fine head movements in the plane of the canal during flight (Gray, 1955); a notable exception is the woodpecker, whose distinctive percussive head motions may be the cause for its relatively smaller vertical canal (Turkewitsch, 1934; Hadziselimovic and Savkovic, 1964; Clarke, 2005). Modern humans and H. erectus have somewhat larger anterior canals than their quadrupedal ancestors, a finding interpreted as an adaptation to increase the sensitivity of the system to measure pitch movements of the head as human ancestors developed bipedal locomotion and hay have experienced increased rotational head movements in the sagittal plane (Spoor et al., 1994, 1996; Spoor and Zonneveld, 1998; Spoor et al., 2003). However, the difference in anterior canal size among hominids is relatively small and has not been shown to be statistically significant (Graf and Vidal, 1996). Furthermore, the argument that increased head movement would favor larger canals appears to be directly at odds with arguments made by Spoor et al. (2002) linking increased head movements to reduced canal size in cetaceans as described above.
The precise shape (in distinction to the size) of the canals has also been related to the behaviors of certain species. The horizontal canal in some reptiles is prominently bent into a triangular shape (Retzius, 1884; Baird, 1974; Lewis et al., 1985; Clarke, 2005), a finding which has been assumed to represent an adaptation lowering the sensitivity and preventing overstimulation of the system during rapid side-to-side headshaking associated with prey capture (Rogers, 1998, 1999, 2005). A qualitative study of semicircular canal shape in birds correlated “good fliers” such as pigeon, owl, thrush, peregrine, eagle, and raven with long, thin, arcing canals and “poor fliers” such as hen, duck, and goose with thick, short canals and relatively small ampullae but the subjective nature of these observations makes their interpretation difficult (Hadziselimovic and Savkovic, 1964). Furthermore, published canal models indicated that deviations from a smoothly arcing canal form may not change the responsiveness of the canals significantly (Howland and Masci, 1973a; McVean, 1999).
Materials and Methods
The scientific literature was surveyed for studies reporting the responses of afferents leading from two or three of the semicircular canals within a single species. Suitable data were found representing neuronal responses from cat, squirrel monkey, and pigeon as shown in Table 1. The literature was also reviewed for biophysical models of the labyrinth. Some models suggest a linear relationship between canal response and r2 (Jones and Spells, 1963; Peterka and Tomko, 1984) while others indicate a linear relationship between canal response and R.r4 (ten Kate et al., 1970; Oman et al., 1987; Muller, 1994; Rabbitt et al., 2004; Squires, 2004). A third possibility, that canal response is related to R, has also been suggested (Curthoys, 1982; Spoor et al., 2002). The predictions of each model (using each canal’s dimensions) were compared to the actual afferent sensitivity of that canal to determine the model’s validity.
TABLE 1.
Sources of afferent response and canal dimension data
| Animal | Source for afferent responses | Source for anatomy |
|---|---|---|
| Pigeon (Columba livia) | (Anastasio et al., 1985) | (Ramprashad et al., 1986) |
| Cat (Felis silvestris catus) | (Blanks et al., 1975) | (Curthoys et al., 1977) |
| Squirrel monkey (Saimiri sciureus) | (Goldberg and Fernandez, 1971) | (Ramprashad et al., 1984) |
Results
The sensitivity of the semicircular canals has been assumed to be related to the geometry of the canals. Testing the validity of this assumption requires eliminating as many other variables as possible by keeping other biophysical characteristics such as density and viscosity of the endolymph are held constant. This can be achieved by comparing responses from canals of different sizes from the same species.
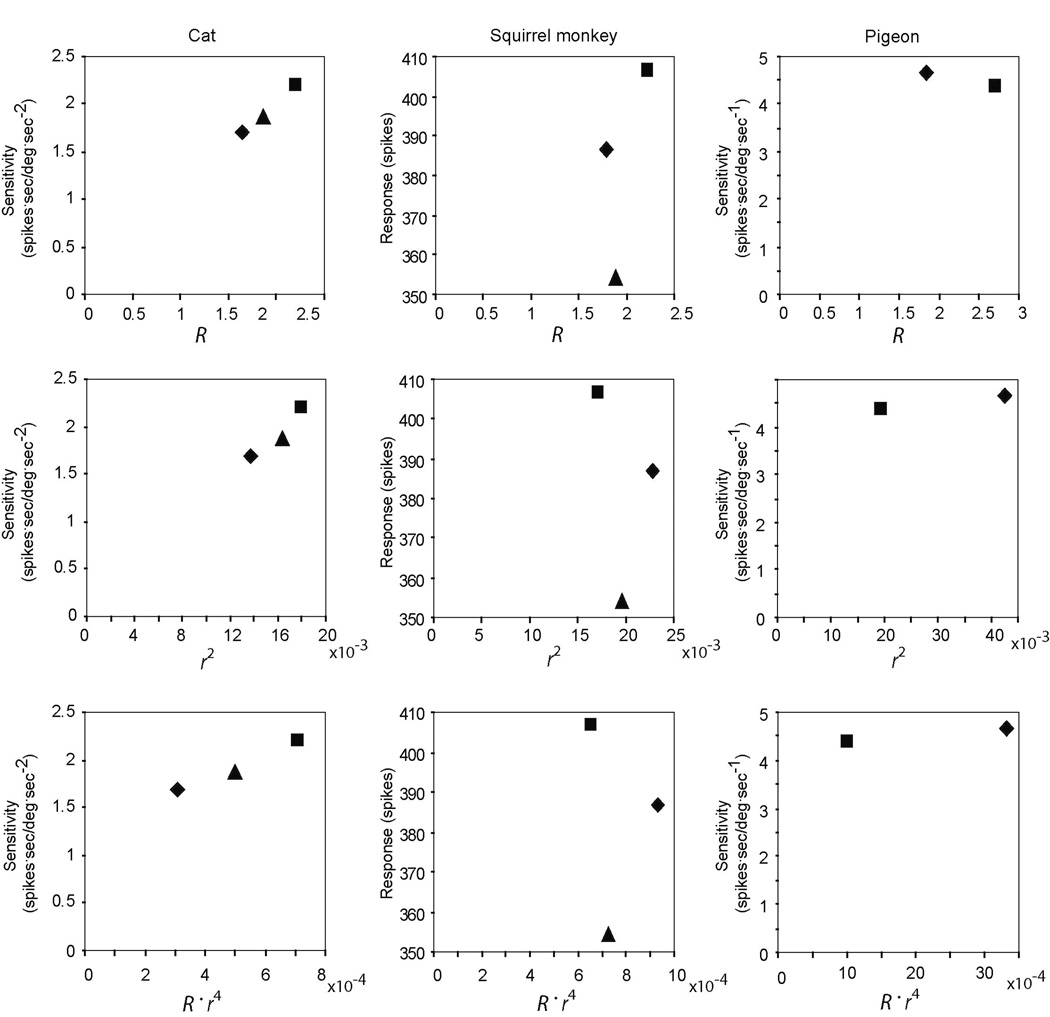
Shown in Fig. 1 are data relating the dimensions of the canals for each of three species to the actual sensitivity of afferents from individual canals in each species. Each model proposes a linear relationship between canal sensitivity and a particular geometric measurement or measurements (R, r2, or R.r4). It is apparent that all of the models adequately fit the data for the cat, while none of them fits the data for the squirrel monkey. Both the r2 and R.r4 model appear to fit the pigeon data, but the R model does not.
Figure 1.
Relationship between canal dimensions and sensitivity. Anterior canal, squares; horizontal canal, diamonds; posterior canal, triangles. Left column, cat; middle column, squirrel monkey; right column; pigeon. First row, relationship between radius of curvature (R) and afferent response. Second row, relationship between square of membranous duct radius (r2) and afferent response. Third row, relationship between product of the radius of curvature and the membranous duct radius to the fourth power (R.r4) and afferent response. Responses for cat measured during a 4–6 deg/sec2 constant-acceleration rotational stimulus and reported (re acceleration) as spikes.sec / degree.sec2; squirrel monkey responses measured during an excitatory rotation of 30 deg/sec2 over ten seconds and reported as the total number of spikes during this stimulus; pigeon responses measured during a sinusoidal stimulation of 0.293 Hz, 13.52 deg/sec peak amplitude and reported (re rotational velocity) as spikes.sec / degree.sec.
Discussion
Numerous studies have proposed models relating the responses of the semicircular canals to their biophysical characteristics. The cat data match the predictions of all three models examined here, while data from the pigeon fit two models and the squirrel monkey data do not fit any. Is this because the afferent data do not accurately represent canal function, or does the literature simply not contain a satisfactory model of the canals? One of the most important considerations regarding whether the afferent data truly reflect canal function is that none of the data presented here are separated into groups of regularly and irregularly discharging units. Irregularly firing afferents have been observed to have a high-frequency phase lead and gain increase in many species including fish (Hartmann and Klinke, 1980; Boyle and Highstein, 1990), amphibian (Blanks and Precht, 1976), bird (Correia and Landolt, 1973; Landolt and Correia, 1980), and mammalian species (Fernandez and Goldberg, 1971; Keller, 1976; Louie and Kimm, 1976; Anderson et al., 1978; Ezure et al., 1978; Hullar et al., 2005). These dynamics are related at least in part to where on the neuroepithelium the particular afferent’s peripheral terminals are located (O'Leary et al., 1974; Honrubia et al., 1981; Lysakowski et al., 1995; Brichta and Goldberg, 2000) with current data suggesting that the distribution of GABAergic hair cell-afferent synapses may be particularly important in determining this response (Holstein et al., 2004; Highstein et al., 2005). These fibers have been suggested to contribute to velocity storage, a centrally mediated low-pass filter (integrator) of vestibular information (Raphan et al., 1979; Angelaki and Perachio, 1993); to the ability of the vestibulo-ocular reflex to compensate for viewing distance (Chen-Huang and McCrea, 1998; Angelaki et al., 2000); to short-latency responses of the vestibulo-ocular reflex (Lewis and Parnas, 1994; Hullar et al., 2005); and to vestibulo-spinal reflexes (Bilotto et al., 1982; Highstein et al., 1987). Not knowing the contribution from each afferent group to the data from each canal compared here means that this variable could not be controlled for and may have prevented accurate comparisons of the responses of the canals from each species.
A related consideration is adaptation. The pigeon data were collected during sinusoidal stimulations, while the cat and squirrel monkey data were collected during steps of acceleration. Irregular fibers tend to be particularly subject to adaptation, changing their firing rate and apparent sensitivity to the rotation during long periods of stimulation (Lowenstein, 1955; Ledoux, 1961; Fernandez and Goldberg, 1971; Precht et al., 1971; Fernandez and Goldberg, 1976; Dickman and Correia, 1989; Boyle and Highstein, 1990). As different types of afferents adapt to a rotation variably, an uneven distribution of adaptation among afferents from each semicircular canal could have led to an erroneous calculation of afferent sensitivities compared here. Further examination of cat and squirrel monkey afferents recorded under different conditions—or corrected for adaptation before calculating sensitivity—is necessary to guarantee that adaptation did not affect the results shown in Fig. 1.
Another source of error, common to all afferent-recording experiments, is inappropriate positioning of the animal relative to the axis of rotation. The horizontal-canal pigeon data presented here were collected with the horizontal canal in the plane of rotation, but the anterior-canal data were not (Anastasio et al., 1985). Although the authors of that paper did not include a correction factor for this offset, a later study of the pigeon canal anatomy (Dickman, 1996) provides enough information to allow the actual sensitivity of the anterior canal afferents to be approximated using a simple cosine rule. Because the pigeon canal is dihedral instead of planar in shape, however, the accuracy of this approximation may be further reduced and errors introduced by it may affect the data presented in Fig. 1 (Dickman, 1996).
Errors in the models used may occur at several steps. Fluid flow through the semicircular canals is believed to be determined more by the cross-sectional area of the slender membranous duct than by the portion of the circuit bridging the vestibule itself; therefore, simply measuring the radius of curvature and the cross-sectional area of the membranous duct may not be adequate to model the canals accurately (Rabbitt et al., 2004). Other factors, including differences between the shape of the membranous duct and bony canal, departures from a strictly toroidal shape, nonplanarity of the canal, and fluid coupling among the canals during particular rotations may also affect the estimated response of a canal. (Oman et al., 1987; Muller and Verhagen, 1988b, 1988a; Ghanem et al., 1998; Rabbitt, 1999). The cupula itself may also have inherent dynamics altering the responses of vestibular-nerve afferents (Damiano and Rabbitt, 1996; Yamauchi et al., 2002; Highstein et al., 2005).
The concerns discussed above indicate that canal dimensions may provide only limited insight into afferent responses, but other anatomic observations may provide important clues to the behavior of extinct animals. It has been noted in several reports that, during normal animal activity, the horizontal semicircular canal is held close to earth-horizontal (Perez, 1922; Girard, 1923; Ledebkin, 1924; de Beer, 1947). A series of more recent studies have used photographic and radiographic methods for assessing this relationship. Mazza and Winterson (1984) found that at rest, the horizontal canals of rabbits are held angled anteriorly upward 15 degrees and laterally downward 7 degrees. Studies in other animals, including guinea pigs, rabbitr, cats, monkeys, and humans, made similar observations (Vidal et al., 1986; Graf et al., 1995a; Graf et al., 1995b). The guinea pig orients its canals within a range of about 20 degrees above and below the earth-horizontal plane, with an average slightly upward anteriorly; the cat ranges from 26 degrees downward to 54 degrees upward, with the average anterior upward inclination increasing to 60 degrees during normal activity such as locomotion, grooming, and feeding. Monkeys were reported to hold their horizontal canals tilted anteriorly upward by 18 degrees, while humans held their heads at rest with the horizontal canals tilted upward by 16 degrees. During activity, animals were found to maintain average horizontal canal positions that were approximately the same as those at rest. In the “startle position”, rats were found to orient themselves 14 degrees nose-down with respect to the horizontal plane, bringing it to an average position 21 degrees anteriorly upward relative to the earth horizontal (Blanks and Torigoe, 1989) while the startle position of rabbits orients their horizontal canals coplanar with earth horizontal (Soodak and Simpson, 1988). In non-mammalian species, a relatively close correspondence between the orientation of the horizontal canal and the earth horizontal has also been reported: in the turtle, the horizontal canal is pitched anteriorly upward by 3–4 degrees (Brichta et al., 1988) and in the pigeon by 10 degrees (Erichsen et al., 1989). Data relating the horizontal canal position and head orientation have been used to estimate a characteristic head position of the dinosaur Allosaurus fragilis, a carnivorous predator prevalent during the late Jurassic period, using a CT scan of the cranial endocast (Rogers, 1998). Although there is clearly a range of angles over which the head may be oriented, positioning of the horizontal canal tilted slightly anteriorly upward does appear to be a relatively consistent finding. Excepting particularly unusual species such as the bilaterally asymmetric adult flatfish, whose “horizontal” canals become vertical during metamorphosis from the larval stage (Graf and Baker, 1985), the remarkable consistency of this finding makes the orientation of the horizontal canal an attractive method for estimating the customary head position of animals.
ACKNOWLEDGMENTS
Thanks to Asim Haque, Daniel Calabrese, David Dickman, and Stephen Highstein for providing helpful comments on the manuscript.
Grant Sponsor: National Institute of Deafness and Other Communication Disorders
Grant Number: KO8 DC 006869
Grant Sponsor: McDonnell Center for Higher Brain Function.
REFERENCES
- Alonso PD, Milner AC, Ketcham RA, Cookson MJ, Rowe TB. The avian nature of the brain and inner ear of Archaeopteryx. Nature. 2004;430:666–669. doi: 10.1038/nature02706. [DOI] [PubMed] [Google Scholar]
- Anastasio TJ, Correia MJ, Perachio AA. Spontaneous and driven responses of semicircular canal primary afferents in the unanesthetized pigeon. J Neurophysiol. 1985;54:335–347. doi: 10.1152/jn.1985.54.2.335. [DOI] [PubMed] [Google Scholar]
- Anderson JH, Blanks RHI, Precht W. Response characteristics of semicircular canal and otolith systems in cat. I. Dynamic responses of primary vestibular nerve fibers. Exp Brain Res. 1978;32:491–507. doi: 10.1007/BF00239549. [DOI] [PubMed] [Google Scholar]
- Angelaki DE, McHenry MQ, Dickman JD, Perachio AA. Primate translational vestibuloocular reflexes. III. Effects of bilateral labyrinthine electrical stimulation. J Neurophysiol. 2000;83:1662–1676. doi: 10.1152/jn.2000.83.3.1662. [DOI] [PubMed] [Google Scholar]
- Angelaki DE, Perachio AA. Contribution of irregular semicircular canal afferents to the horizontal vestibuloocular response during constant velocity rotation. J Neurophysiol. 1993;69:996–999. doi: 10.1152/jn.1993.69.3.996. [DOI] [PubMed] [Google Scholar]
- Baird IL. Some aspects of the comparative anatomy and evolution of the inner ear in submammalian vertebrates. Brain Behav Evol. 1974;10:11–36. doi: 10.1159/000124300. [DOI] [PubMed] [Google Scholar]
- Baker J, Goldberg J, Peterson B. Spatial and temporal response properties of the vestibulocollic reflex in decerebrate cats. J Neurophysiol. 1985;54:735–756. doi: 10.1152/jn.1985.54.3.735. [DOI] [PubMed] [Google Scholar]
- Balaban CD. Vestibular autonomic regulation. Curr Opin Neurol. 1999;12:29–33. doi: 10.1097/00019052-199902000-00005. [DOI] [PubMed] [Google Scholar]
- Barnes LG, McLeod SA. The fossil record and phyletic relationships of gray whales. In: Jones ML, Swartz SL, editors. The gray whale. New York: Academic Press; 1984. pp. 3–28. [Google Scholar]
- Bilotto G, Goldberg J, Peterson BW, Wilson VJ. Dynamic properties of vestibular reflexes in the decerebrate cat. Exp Brain Res. 1982;47:343–352. doi: 10.1007/BF00239353. [DOI] [PubMed] [Google Scholar]
- Blanks RH, Torigoe Y. Orientation of the semicircular canals in rat. Brain Res. 1989;487:278–287. doi: 10.1016/0006-8993(89)90832-9. [DOI] [PubMed] [Google Scholar]
- Blanks RHI, Estes MS, Markham CH. Physiologic characteristics of vestibular first order canal neurons in the cat. II. Response to constant angular acceleration. J Neurophysiol. 1975;38:1250–1268. doi: 10.1152/jn.1975.38.5.1250. [DOI] [PubMed] [Google Scholar]
- Blanks RHI, Precht W. Functional characterization of primary vestibular afferents in the frog. Exp Brain Res. 1976;25:369–390. doi: 10.1007/BF00241728. [DOI] [PubMed] [Google Scholar]
- Boenninghaus G. Das Ohr des Zahnwales, zugleich ein Beitrag zur Theorie der Schalleitung. Zool Jahr (Anatomie) 1903;17:189–360. [Google Scholar]
- Boyle R, Highstein SM. Resting discharge and response dynamics of horizontal semicircular canal afferents of the toadfish, Opsanus tau. The Journal of Neuroscience. 1990;10:1557–1569. doi: 10.1523/JNEUROSCI.10-05-01557.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brichta AM, Acuna DL, Peterson EH. Planar relations of semicircular canals in awake, resting turtles, Pseudemys scripta. Brain Behav Evol. 1988;32:236–245. doi: 10.1159/000116551. [DOI] [PubMed] [Google Scholar]
- Brichta AM, Goldberg JM. Morphological identification of physiologically characterized afferents innervating the turtle posterior crista. J Neurophysiol. 2000;83:1202–1223. doi: 10.1152/jn.2000.83.3.1202. [DOI] [PubMed] [Google Scholar]
- Chen-Huang C, McCrea RA. Contribution of vestibular nerve irregular afferents to viewing distance-related changes in the vestibulo-ocular reflex. Exp Brain Res. 1998;119:116–130. doi: 10.1007/s002210050325. [DOI] [PubMed] [Google Scholar]
- Clarke AH. On the vestibular labyrinth of Brachiosaurus brancai. J Vestib Res. 2005;15:65–71. [PubMed] [Google Scholar]
- Correia MJ, Landolt JP. Spontaneous and driven responses from primary neurons of the anterior semicircular canal of the pigeon. Adv Otorhinolaryngol. 1973;19:134–148. doi: 10.1159/000393986. [DOI] [PubMed] [Google Scholar]
- Curthoys IS. Postnatal development changes in the response of rat primary horizontal semicircular canal neurons to sinusoidal angular accelerations. Exp Brain Res. 1982;47:295–300. doi: 10.1007/BF00239389. [DOI] [PubMed] [Google Scholar]
- Curthoys IS, Markham CH, Curthoys EJ. Semicircular duct and ampulla dimensions in cat, guinea pig and man. J Morphol. 1977;151:17–34. doi: 10.1002/jmor.1051510103. [DOI] [PubMed] [Google Scholar]
- Damiano ER, Rabbitt RD. A singular perturbation model of fluid dynamics in the vestibular semicircular canal and ampulla. J. Fluid Mech. 1996;307:333–372. [Google Scholar]
- de Beer GR. How animals hold their heads. Proc Linn Soc Lond. 1947;159:125–139. [Google Scholar]
- de Burlet HM. Zur vergleichenden Anatomie und Physiologie des perilymphatischen Raumes. Acta Otolaryngol. 1929;13:153–187. [Google Scholar]
- DeSmet WMA. The regions of the cetacean vertebral column. In: Harrison RJ, editor. Functional anatomy of marine mammals. London: Academic Press; 1977. pp. 59–79. [Google Scholar]
- Dickman JD. Spatial orientation of semicircular canals and afferent sensitivity vectors in pigeons. Exp Brain Res. 1996;111:8–20. doi: 10.1007/BF00229550. [DOI] [PubMed] [Google Scholar]
- Dickman JD, Correia MJ. Responses of pigeon horizontal semicircular canal afferent fibers I. Step, trapezoid, and low-frequency sinusoid mechanical and rotational stimulation. J Neurophysiol. 1989;62:1090–1101. doi: 10.1152/jn.1989.62.5.1090. [DOI] [PubMed] [Google Scholar]
- Erichsen JT, Hodos W, Evinger C, Bessette BB, Phillips SJ. Head orientation in pigeons: postural, locomotor and visual determinants. Brain Behav Evol. 1989;33:268–278. doi: 10.1159/000115935. [DOI] [PubMed] [Google Scholar]
- Ezure K, Schor RH, Yoshida K. The response of horizontal semicircular canal afferents to sinusoidal rotation in the cat. Exp Brain Res. 1978;33:27–39. doi: 10.1007/BF00238792. [DOI] [PubMed] [Google Scholar]
- Fernandez C, Goldberg JM. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey II. Response to sinusoidal stimulation and dynamics of peripheral vestibular system. J Neurophysiol. 1971;34:661–675. doi: 10.1152/jn.1971.34.4.661. [DOI] [PubMed] [Google Scholar]
- Fernandez C, Goldberg JM. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. I. Response to static tilts and to long duration centrifugal force. J Neurophysiol. 1976;39:970–984. doi: 10.1152/jn.1976.39.5.970. [DOI] [PubMed] [Google Scholar]
- Ghanem T, Rabbitt RD, Tresco PA. Three-dimensional reconstruction of the membranous vestibular labyrinth in the toadfish, Opsanus tau. Hear Res. 1998;124:27–43. doi: 10.1016/s0378-5955(98)00108-7. [DOI] [PubMed] [Google Scholar]
- Girard L. Le plan des canau semicirculaires horizontaux considere comme plan horizontal de la tete. Bull Mem Soc Anthrop (Paris) 1923;14:14. [Google Scholar]
- Goldberg JM, Fernandez C. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey I.Resting discharge and response to constant angular accelerations. J Neurophysiol. 1971;34:635–660. doi: 10.1152/jn.1971.34.4.635. [DOI] [PubMed] [Google Scholar]
- Graf W, Baker R. The vestibuloocular reflex of the adult flatfish I.Oculomotor organization. J Neurophysiol. 1985;54:887–899. doi: 10.1152/jn.1985.54.4.887. [DOI] [PubMed] [Google Scholar]
- Graf W, de Waele C, Vidal P-P. Functional anatomy of the head-neck movement system of quadrupedal and bipedal mammals. J Anat. 1995a;186:55–74. [PMC free article] [PubMed] [Google Scholar]
- Graf W, de Waele C, Vidal P-P, Wang DH, Evinger C. The orientation of the cervical vertebral column in unrestrained awake animals II. Movement strategies. Brain Behav Evol. 1995b;45:209–231. doi: 10.1159/000113551. [DOI] [PubMed] [Google Scholar]
- Graf W, Vidal P-P. Semicircular canal size and upright stance are not interrelated. J Hum Evol. 1996;30:175–181. [Google Scholar]
- Grant JW, van Buskirk WC. Experimental measurement of the stiffness of the cupula. Biophys J. 1976;16:669–678. doi: 10.1016/S0006-3495(76)85720-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray O. A brief survey of the phylogenesis of the labyrinth. J Laryngol Otol. 1955;69:151–179. doi: 10.1017/s0022215100050556. [DOI] [PubMed] [Google Scholar]
- Hadziselimovic H, Savkovic L. Appearance of semicircular canals in birds in relation to mode of life. Acta Anat (Basel) 1964;57:306–315. [PubMed] [Google Scholar]
- Hartmann R, Klinke R. Discharge properties of afferent fibres of the goldfish semicircular canal with high frequency stimulation. Pflugers Arch. 1980;388:111–121. doi: 10.1007/BF00584116. [DOI] [PubMed] [Google Scholar]
- Highstein SM, Goldberg JM, Moschovakis AK, Fernandez C. Inputs from regularly and irregularly discharging vestibular nerve afferents to secondary neurons in the vestibular nuclei of the squirrel monkey. II. Correlation with output pathways of secondary neurons. J Neurophysiol. 1987;58:719–738. doi: 10.1152/jn.1987.58.4.719. [DOI] [PubMed] [Google Scholar]
- Highstein SM, Rabbitt RD, Holstein GR, Boyle RD. Determinants of spatial and temporal coding by semicircular canal afferents. J Neurophysiol. 2005;93:2359–2370. doi: 10.1152/jn.00533.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstein GR, Rabbitt RD, Martinelli GP, Friedrich VL, Jr, Boyle RD, Highstein SM. Convergence of excitatory and inhibitory hair cell transmitters shapes vestibular afferent responses. Proc Natl Acad Sci U S A. 2004;101:15766–15771. doi: 10.1073/pnas.0402824101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honrubia V, Sitko S, Kimm J, Betts W, Schwartz I. Physiological and anatomical characteristics of primary vestibular afferent neurons in the bullfrog. Int J Neurosci. 1981;15:197–206. doi: 10.3109/00207458108985857. [DOI] [PubMed] [Google Scholar]
- Howland H, Masci J. The functional allometry of semicircular canals, fins, and body dimensions in the juvenile centrarchid fish, Lepomis gibbosus (L.) Journal of Embryol. exp. Morph. 1973a;29:721–743. [PubMed] [Google Scholar]
- Howland HC, Masci J. The phylogenetic allometry of the semicircular canals of small fishes. Z Morphol Tiere. 1973b;75:283–296. [Google Scholar]
- Hullar T, Armand M. Head motion of freely swimming dolphins. J Vestib Res. 2004;14:144–145. [Google Scholar]
- Hullar TE, Della Santina CC, Hirvonen TP, Lasker DM, Carey JP, Minor LB. Responses of irregularly discharging chinchilla semicircular canal vestibular-nerve afferents during high-frequency head rotations. J Neurophysiol. 2005;93:2777–2786. doi: 10.1152/jn.01002.2004. [DOI] [PubMed] [Google Scholar]
- Hyrtl J. Vergleichend-anatomische Untersuchungen über das innere Gehörorgan des Menschen und der Säugethiere. Prag: Friedrich Ehrlich. 1845 [Google Scholar]
- Jones GM, Spells KE. A theoretical and comparative study of the functional dependence of the semicircular canal upon its physical dimensions. Proc R Soc Lond B Biol Sci. 1963;157:403–419. doi: 10.1098/rspb.1963.0019. [DOI] [PubMed] [Google Scholar]
- Keller EL. Behavior of horizontal semicircular canal afferents in alert monkey during vestibular and optokinetic stimulation. Exp Brain Res. 1976;24:459–471. doi: 10.1007/BF00234963. [DOI] [PubMed] [Google Scholar]
- Landolt JP, Correia MJ. Neurodynamic response analysis of anterior semicircular canal afferents in the pigeon. J Neurophysiol. 1980;43:1746–1770. doi: 10.1152/jn.1980.43.6.1746. [DOI] [PubMed] [Google Scholar]
- Ledebkin S. Über die Lage des Canalis semicircularis lateralis bei Säugern. Anat Anz. 1924;58:447–460. [Google Scholar]
- Ledoux A. L'adaptation du systéme vestibulaire périphérique. Acta Otolaryngol (Stockh) 1961;53:307–316. [Google Scholar]
- Leigh RJ, Zee DS. The Neurology of Eye Movement. Third Edition. Oxford University Press; 1999. [Google Scholar]
- Lewis ER, Leverenz EL, Bialek WS. The Vertebrate Inner Ear. Boca Raton, FL: CRC Press; 1985. [Google Scholar]
- Lewis ER, Parnas BR. Theoretical bases of short-latency spike volleys in the peripheral vestibular system. J Vestib Res. 1994;4:189–202. [PubMed] [Google Scholar]
- Lindenlaub T, Burda H, Nevo E. Convergent evolution of the vestibular organ in the subterranean mole-rats Cryptomys and Spalax, as compared with the aboveground rat, Rattus. J Morphol. 1995;224:303–311. doi: 10.1002/jmor.1052240305. [DOI] [PubMed] [Google Scholar]
- Louie AW, Kimm J. The response of eighth nerve fibers to horizontal sinusoidal oscillation in the alert monkey. Exp Brain Res. 1976;24:447–457. doi: 10.1007/BF00234962. [DOI] [PubMed] [Google Scholar]
- Lowenstein O. The effect of galvanic polarization on the impulse discharge from sense endings on the isolated labyrinth o fhte thorn back ray (Raja clavata) J Physiol (Lond) 1955;127:104–117. doi: 10.1113/jphysiol.1955.sp005241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysakowski A, Minor LB, Fernandez C, Goldberg JM. Physiological identification of morphologically distinct afferent classes innervating the cristae ampullares of the squirrel monkey. J Neurophysiol. 1995;73:1270–1281. doi: 10.1152/jn.1995.73.3.1270. [DOI] [PubMed] [Google Scholar]
- Matano S, Kubo T, Niemitz C, Guenther M. Semicircular canal organ in three primate species and behavioral correlations. Fortschr Zool. 1985;30:677–680. [Google Scholar]
- Mayne R. The dynamic characteristics of the semicircular canals. J Comp Physiol Psychol. 1950;43:309–319. doi: 10.1037/h0054827. [DOI] [PubMed] [Google Scholar]
- McVean A. Are the semicircular canals of the European mole, Talpa europaea, adapted to a subterranean habitat? Comp Biochem Physiol A Mol Integr Physiol. 1999;123:173–178. doi: 10.1016/s1095-6433(99)00047-1. [DOI] [PubMed] [Google Scholar]
- Muller M. Semicircular duct dimensions and sensitivity of the vertebrate vestibular system. J Theor Biol. 1994;167:239–256. [Google Scholar]
- Muller M, Verhagen JHG. A mathematical approach enabling the calculation of the total endolymph flow in the semicircular ducts. J Theor Biol. 1988a;134:503–529. doi: 10.1016/s0022-5193(88)80054-7. [DOI] [PubMed] [Google Scholar]
- Muller M, Verhagen JHG. A new quantitative model of total endolymph flow in the system of semicircular ducts. J Theor Biol. 1988b;134:473–501. doi: 10.1016/s0022-5193(88)80053-5. [DOI] [PubMed] [Google Scholar]
- O'Leary DP, Dunn RF, Honrubia V. Functional and anatomical correlation of afferent responses from the isolated semicircular canal. Nature. 1974;251:225–227. doi: 10.1038/251225a0. [DOI] [PubMed] [Google Scholar]
- Oman CM, Marcus EN, Curthoys IS. The influence of semicircular canal morphology on endolymph flow dynamics. Acta Otolaryngol (Stockh) 1987;103:1–13. doi: 10.3109/00016488709134691. [DOI] [PubMed] [Google Scholar]
- Perez F. Craniologie vestibienne, ethnique et zoologique. Bull Mem Soc Anthrop (Paris) 1922;13:16. [Google Scholar]
- Peterka RJ, Tomko DL. Differences between cats in response properties of horizontal semicircular canal primary afferents. Exp Brain Res. 1984;56:162–166. doi: 10.1007/BF00237453. [DOI] [PubMed] [Google Scholar]
- Precht W, Llinas R, Clarke M. Physiological responses of frog vestibular fibers to horizontal angular rotation. Exp Brain Res. 1971;13:378–407. doi: 10.1007/BF00234338. [DOI] [PubMed] [Google Scholar]
- Rabbitt RD. Directional coding of three-dimensional movements by the vestibular semicircular canals. Biol Cybern. 1999;80:417–431. doi: 10.1007/s004220050536. [DOI] [PubMed] [Google Scholar]
- Rabbitt RD, Damiano ER, Grant JW. Biomechanics of the semicircular canals and otolith organs. In: Highstein S, Fay RR, Popper AN, editors. The vestibular system. New York: Springer; 2004. pp. 153–201. [Google Scholar]
- Ramprashad F, Landolt JP, Money KE, Laufer J. Dimensional analysis and dynamic response characterization of mammalian peripheral vestibular structures. Am J Anat. 1984;169:295–313. doi: 10.1002/aja.1001690306. [DOI] [PubMed] [Google Scholar]
- Ramprashad F, Landolt JP, Money KE, Laufer J. Comparative morphometric study of the vestibular system of the vertebrata: Reptilia, Aves, Amphibia, and Pisces. Acta Otolaryngol Suppl. 1986;427:1–42. [PubMed] [Google Scholar]
- Raphan T, Cohen B. The vestibulo-ocular reflex (VOR) in three dimensions. Exp Brain Res. 2002;145:1–27. doi: 10.1007/s00221-002-1067-z. [DOI] [PubMed] [Google Scholar]
- Raphan T, Matsuo V, Cohen B. Velocity storage in the vestibulo-ocular reflex arc (VOR) Exp Brain Res. 1979;35:229–248. doi: 10.1007/BF00236613. [DOI] [PubMed] [Google Scholar]
- Retzius G. Das Gehörorgan der der Fische und Amphibien. 1 ed. Stockholm: Samson & Wallin; 1881. Des Gehörorgan der Wirbelthiere: morphologisch-histologisch Studien. I. [Google Scholar]
- Retzius G. Das Gehörorgan der Reptilien, der Vögel under der Säugetiere. 1 ed. Stockholm: Samson & Wallin; 1884. Des Gehörorgan der Wirbelthiere: morphologisch-histologisch Studien. II. [Google Scholar]
- Rogers SW. Exploring dinosaur neuropaleobiology: viewpoint computed tomography scanning and analysis of an Allosaurus fragilis endocast. Neuron. 1998;21:673–679. doi: 10.1016/s0896-6273(00)80585-1. [DOI] [PubMed] [Google Scholar]
- Rogers SW. Allosaurus, crocodiles, and birds: evolutionary clues from spiral computed tomography of an endocast. Anat Rec. 1999;257:162–173. doi: 10.1002/(SICI)1097-0185(19991015)257:5<162::AID-AR5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Rogers SW. Reconstructing the behaviors of extinct species: an excursion into comparative paleoneurology. Am J Med Genet A. 2005;134:349–356. doi: 10.1002/ajmg.a.30538. [DOI] [PubMed] [Google Scholar]
- Rommel S. Osteology of the bottlenose dolphin. In: Leatherwood S, Reeves RR, editors. The Bottlenose Dolphin. San Diego: Academic Press; 1990. pp. 29–49. [Google Scholar]
- Soodak RE, Simpson JI. The accessory optic system of rabbit. I. Basic visual response properties. J Neurophysiol. 1988;60:2037–2054. doi: 10.1152/jn.1988.60.6.2037. [DOI] [PubMed] [Google Scholar]
- Spoor F, Bajpai S, Hussain ST, Kumar K, Thewissen JG. Vestibular evidence for the evolution of aquatic behaviour in early cetaceans. Nature. 2002;417:163–166. doi: 10.1038/417163a. [DOI] [PubMed] [Google Scholar]
- Spoor F, Hublin J, Braun M, Zonneveld F. The bony labyrinth of Neanderthals. J Hum Evol. 2003;44:141–165. doi: 10.1016/s0047-2484(02)00166-5. [DOI] [PubMed] [Google Scholar]
- Spoor F, Wood B, Zonneveld F. Implications of early hominid labyrinthine morphology for evolution of human bipedal locomotion. Nature. 1994;369:645–648. doi: 10.1038/369645a0. [DOI] [PubMed] [Google Scholar]
- Spoor F, Wood B, Zonneveld F. Evidence for a link between human semicircular canal size and bipedal behaviour. J Hum Evol. 1996;30:183–187. [Google Scholar]
- Spoor F, Zonneveld F. Comparative review of the human bony labyrinth. Am J Phys Anthropol. 1998:211–251. doi: 10.1002/(sici)1096-8644(1998)107:27+<211::aid-ajpa8>3.3.co;2-m. [DOI] [PubMed] [Google Scholar]
- Squires TM. Optimizing the vertebrate vestibular semicircular canal: could we balance any better? Phys Rev Lett. 2004;93:198106. doi: 10.1103/PhysRevLett.93.198106. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Clark AS, Taube JS. Hippocampal spatial representations require vestibular input. Hippocampus. 2002:291–303. doi: 10.1002/hipo.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS. Head direction cells and the neurophysiological basis for a sense of direction. Prog.Neurobiol. 1998;55:225–256. doi: 10.1016/s0301-0082(98)00004-5. [DOI] [PubMed] [Google Scholar]
- ten Kate JH. Angular acceleration detection by the growing pike. Adv Otorhinolaryngol. 1973;19:110–119. doi: 10.1159/000393983. [DOI] [PubMed] [Google Scholar]
- ten Kate JH, van Barneveld HH, Kuiper JW. The dimensions and sensitivities of semicircular canals. J Exp Biol. 1970;53:501–514. doi: 10.1242/jeb.53.2.501. [DOI] [PubMed] [Google Scholar]
- Turkewitsch BG. Zur Anatomie des Gehörorgans der Vögel (Canales semicirculares) Z Anat Entwicklungsgesch. 1934;103:551–608. [Google Scholar]
- van Buskirk WC, Watts RG, Liu YK. The fluid mechanics of the semicircular canals. J Fluid Mech. 1976;78:87–98. [Google Scholar]
- Van Egmond AAJ, van Groen JJ, Jongkees LBW. The mechanics of the semicircular canal. J Physiol (London) 1949;110:1–17. doi: 10.1113/jphysiol.1949.sp004416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal PP, Graf W, Berthoz A. The orientation of the cervical vertebral column in unrestrained awake animals. I. Resting position. Exp Brain Res. 1986;61:549–559. doi: 10.1007/BF00237580. [DOI] [PubMed] [Google Scholar]
- Wersäll J, Bagger-Sjoback D. Vestibular system, part I: Basic mechanisms. Berlin: Springer; 1974. Morphology of the vestibular sense organ; pp. 123–170. editor. [Google Scholar]
- Yablokov AV, Bel'kovish BM, Borisov VI. Whales and Dolphins. Jerusalem: Israel Programs for Scientific Translations; 1972. [Google Scholar]
- Yamauchi A, Rabbitt RD, Boyle R, Highstein SM. Relationship between inner-ear fluid pressure and semicircular canal afferent nerve discharge. JARO. 2002;3:26–44. doi: 10.1007/s101620010088. [DOI] [PMC free article] [PubMed] [Google Scholar]