Abstract
It is well known that the characteristics of cell lines possibly alter when cell lines are at high-passage number because of the environmental selection. We do not know whether non-permissive or low-permissive cell lines could become permissive or more permissive to virus infection after over-high passage. In the present studies, the alteration of the permissiveness of Spodoptera litura cell line Sl-zsu-1 to three baculovirus infection was investigated after over-high passage, and the possible mechanisms are also investigated. Vigorous apoptosis in Sl-zsu-1 cells was induced by both the recombinant Autographa californica multiple nucleopolyhedrovirus AcMNPV-GFP-actin and the celery looper Anagrapha falcifera multiple nucleopolyhedrovirus AfMNPV, suggesting the replication of the two viruses was blocked by apoptosis. However, the cells infected by S. litura multicapsid nucleopolyhedrovirus SpltMNPV did not undergo apoptosis, but the SpltMNPV titre of the supernatant was not detectable, suggesting this cell line was low-permissive for this virus infection and other factor(s) involved in blockage of the virus replication except apoptosis. However, when Sl-zsu-1 cells had been subcultured continuously for more than 4 years (high-passage cell), which was named as Sl-HP cell line afterwards, no significant apoptosis was induced by the three baculovirus in Sl-HP cells, and many replicated virions or nucleocapsids were observed in the cells. But the permissiveness of Sl-HP cells to the three viruses was very different according to the titre of viruses in the cell cultures. Interestingly, the DNA extracted from SpltMNPV could induce vigorous apoptosis of Sl-HP cells. Altogether, Sl-zsu-1 cell line becomes more permissive to baculovirus infection after over-high passage and multiple paths can block the baculovirus infectivity.
Keywords: Baculovirus, Spodoptera litura cell line, Permissiveness, Apoptosis, Cell bleb, Over-high passage
Introduction
The characteristics of cell lines possibly alter when cell lines are at high passage number because of the environmental selection (Briske-Anderson et al. 1997; Calles et al. 2006). A general experience is that long-term passaging increases the growth rate of cells (Peiser et al. 1993; Park et al. 2004). Donaldson and Shuler (1998) noted differences in size, shape, growth characteristics and protein expression when comparing two Trichoplusia ni cultures, one early Tn5B1-4 stock at 130 passages and one commercially available high five cell line at 360 passages. The low-passage cells were smaller in size but expressed up to ~20-fold more protein in total, whereas the glycosylation of the protein from the high-passage culture was more complex with a higher degree of sialylated glycans Joosten and Shuler (2003). Clemm (1992) stated that high-passage insect cells in serum-free media generally exhibit reduced yields and require higher m.o.i. to achieve the same level of protein production as recently adapted cells. In a word, it appears that the expression of baculovirus genes could down-regulate in high-passage cells, compared with that in low-passage cells.
Spodoptera litura (Lepidoptera: Noctuidae) cell line Sl-zsu-1 at low or middle passage number is non-permissive for the replication of some baculoviruses such as AcMNPV and AfMNPV because of apoptosis after the infection of these baculoviruses (Dai et al. 1998; Xiu et al. 2005; Liu et al. 2007a). The expression of AcMNPV ie-1 gene at an immediately early stage of infection also can induce Sl-zsu-1 cell apoptosis (Zhang et al. 2002). However, the expression of anti-apoptotic genes such as Bcl-2 and IAP of baculovirus and insect cell can inhibit apoptosis. The expression level of anti-apoptotic genes and apoptotic genes regulate insect cell apoptosis. In the present work, we have investigated the alteration of the permissiveness of S. litura cell line Sl-zsu-1 to three baculovirus replication after over-high passage and possible mechanisms of the changes. The results have shown that Sl-zsu-1cell line is more permissive at least to the tested virus infection after over-high passage and multiple paths can block virus infectivity.
Materials and methods
Insect cell lines
The Sl-zsu-1 cell line was derived from ovary of S. litura (Xie et al. 1988). This cell line has been cultured continuously in our laboratory since 2002. In 2005, we found that some characteristics of the cell line changed and named it as Sl-HP cell because of over-high passage. BTI-TN-5B1-4 cell line (Hi5 cell line) derived from embryo of T. ni and Sf9 cell line derived from pupal ovarian tissue of the fall armyworm Spodoptera frugiperda were donated by Granadios (Cornell University). These insect cell lines were cultured at 28 °C in GIBCO™ Grace’s insect medium supplemented with 10% GIBCO™ fetal bovine serum from Invitrogen.
Baculovirus
S. litura multiple nucleopolyhedrovirus SpltMNPV was originally isolated from infected larvae of S. litura in Guangzhou, China, in 1976 (Pang et al. 2001). Polyhedral inclusion bodies (PIB) were propagated in the third-instar S. litura larvae and purified as described previously (Simon et al. 2004). The haemolymph of the infected larvae at 72 h of p.i. identified by observation of optical microscope was collected (Feng et al. 2007). In the following, we briefly described the method. The infected larvae were chilled for 30 min at 4 °C before haemolymph extraction. Haemolymph was collected by cutting an anterior proleg and allowing the larvae to bleed into ice-cold Grace’s medium supplemented with 8 mM dithiothreitol. Samples were centrifuged at 800g for 10 min at 4 °C; supernatant samples were sterilized through a 0.45 μm Millipore filter and stored at −80 °C.
AcMNPV-GFP-actin was the recombinant baculovirus derived from Autographa californica multiple nucleopolyhedrovirus (AcMNPV), which was constructed in our laboratory, and the polyhedrin gene was destroyed by a fused gene of GFP-actin, which was under the control of polyhedrin promoter of AcMNPV (Li et al. 2004).
Celery looper Anagrapha falcifera multiple nucleopolyhedrovirus (AfMNPV) and AcMNPV were also gifts by Granadios (Cornell University). AcMNPV-GFP-actin, AfMNPV and AcMNPV were propagated in Hi5 cells, respectively, and the supernatant solutions were collected at 72 h of p.i. and steriled with 0.45 μm filter membrane after centrifugation at 1,000g for 10 min at 4 °C and stored at −20 or −80 °C.
Analysis of esterase isoenzyme
Sl-zsu-1 and Sl-HP cells were harvested by centrifugation at 1,000g for 5 min, respectively, and analysis of esterase isoenzyme was performed in order to identify cross-contamination of different cell lines according to the method of Liu et al. (2005).
Infection of baculoviruses
Five millilitre of Sl-zsu-1 or Sl-HP cell suspension at 5 × 105 cells/mL were seeded in each of 25-cm2 culture flask and cultured over night. Then they were infected with AcMNPV-GFP-actin, AfMNPV and SpltMNPV at an m.o.i. of 5 PFU per cell, respectively. The morphological changes of the infected cells were observed by optical, or fluorescence microscope, or analysed after the staining of DAPI (Liu et al. 2007b).
Production of baculoviruses
The infected cells were harvested by centrifugation at 1,000g for 10 min at 4 °C at 72 h of p.i., respectively, and the supernatant samples were sterilized through a 0.45 μm Millipore filter and stored at −80°C for determination of virus levels. Budded virus (BV) titres were assayed by a TCID50 end-point dilution assay on Hi5 cells (O’Reilly et al. 1992). We multiplied the TCID50 titre (per mL) by 0.69 to predict the mean number of PFU/mL for baculoviruses or PFU/cell. (Knudson and Tinsley 1974; Chang et al. 1998).
On the other hand, Sl-HP cells were infected with AcMNPV-GFP-actin, AfMNPV, SlptMNPV and wild-type AcMNPV, respectively. PIBs were extracted and determined at 120 h of p.i. with a hemocytometer. In the same time, the percent of cells with PIB or emitting green fluorescence under microscope was analysed at different periods of post-infection.
Transfection of SpltMNPV DNA into Sl-HP cells
SpltMNPV DNA was extracted from the PIBs propagated by the 3rd larvae and dissolved in sterile water. Before transfection, the cells were washed twice with Grace’s insect medium without antibiotics and serum. Then the cells were transfected with 1 μg SpltMNPV DNA or 1 μg plasmid (as a control) using Lipofetamine reagent according to the manufacturer’s protocol (GIBCO, BRL). A total of 1 μg DNA and 10 μL of Lipofetamine reagent were each diluted into 250 μL of Grace’s insect medium without antibiotics and serum. The diluted DNA and Lipofetamine reagent were mixed gently and incubated at room temperature for 30 min to allow DNA-liposome complexes formation. Cells were then overlaid with DNA–liposome complexes. The Grace’s medium containing antibiotics and serum was supplemented after 4 h of transfection.
Observation of electron microscope
In order to study the replication of the three viruses, the infected Sl-HP cells were harvested by centrifugation at 48 or 72 h of p.i. and used for observation under transmission electron microscope (TEM).
Analysis of caspase-3-like activity
At different time of post-infection, the cells were collected by centrifugation at 10,000g for 5 min at 4 °C and suspended in cell lysis buffer (50 mM Hepes-KOH, pH 7.5, 75 mM NaCl, 0.1%TritonX-100, 1 mM EDTA-Na2, 1 mM EGTA-Na2, 1 mM DTT, 1 mM PMSF) and incubated on ice for 30 min, then the cell lysates were centrifuged at 10,000g for 20 min at 4 °C. The resultant supernatant was collected. Caspase-3-like activities were determined by measuring the proteolytic cleavage of the fluorogenic substrate AC-DEVD-AFC. The reaction mixtures consisting of 25 μg of extracts and 100 mM substrate in 250 μL assay buffer (50 mM Hepes-KOH, pH 7.5, 75 mM NaCl, 1 mM EDTA-Na2, 2 mM DTT, 0.5% chaps, 10% sucrose) were incubated at 37 °C for 1 h and terminated by dilution with 1 mL ice-cold assay buffer and fluorescence was measured using a spectrofluorimeter at an excitation wavelength of 400 nm and an emission wavelength of 505 nm. Caspase-3-like activities were expressed in relative fluorescence intensity units per fraction.
Assay of DNA fragmentation
At different times of post-infection, the cells were collected by centrifugation at 10,000g for 5 min at 4 °C, washed with PBS (pH 7.2) twice. The pellets were then incubated in TES lysis buffer (10 mM Tris–HCl, pH 7.4, 1 mM EDTA, 0.4% Sodium dodecylsulphate) supplemented by proteinase K at final concentration of 50 μg/mL at 37 °C for 4 h. The DNA was extracted twice with an equal volume of phenol (saturated with 100 mM Tris–HCl, pH 8.0)/chloroform/isoamyl alcohol (25:24:1), then once with chloroform alone. The extracted DNA was precipitated with ethanol, dissolved in TE buffer (10 mM Tris–HCl, pH 7.4, 1 mM EDTA) with RNase (at final concentration of 50 μg/mL), then incubated at 37 °C for 2 h. The DNA samples were separated by electrophoresis in 1.5% agarose gels.
Statistics and others
All experiments were performed in triplicate and were repeated at least three times. Representative experiments or mean values ±SD are shown in figures or tables. Statistical differences were determined by Student’s t-test. A p-value of <0.05 was considered significant. Cell numbers and PIB were determined using a hemocytometer and cell viability was determined using trypan blue exclusion.
Results
Difference of morphology and profiles of esterase isoenzyme between Sl-zsu-1 and Sl-HP cell
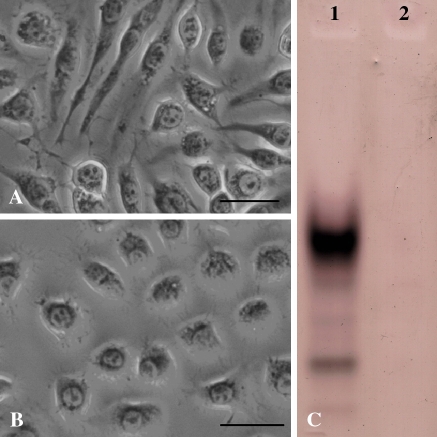
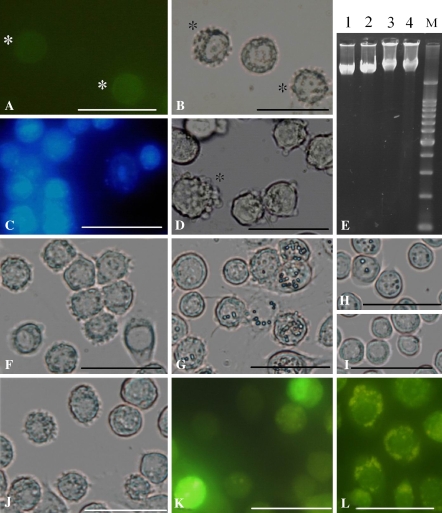
Although Sl-HP cells were derived from Sl-zsu-1 cells by over-time subcultures, there were differences in shape and the profile of the esterase isoenzyme between them. The morphology of most Sl-zsu-1 cells was a spindle-like fibroblastic appearance, but that of most Sl-HP cells was a polygonal shape (Fig. 1a, b). It could be that continuous subculture has selected the polygonal cells and the fibroblast-like cells have been lost. Four bands were shown in the profile of the esterase isoenzyme from Sl-zsu-1 cell sample, but no significant bands were shown in that of Sl-HP cell sample (Fig. 1c). The profile of esterase isoenzymes for Sl-HP cell sample was also different from that for the samples of other insect cell lines in our laboratory, suggesting Sl-HP cells were not accidently cross-contaminated by other insect cell lines (Liu et al. 2005).
Fig. 1.
Difference of morphology and esterase isoenzyme between Sl-zsu-1 and Sl-HP cells. (a) Sl-zsu-1 cells; (b) Sl-HP cells; (c) Profiles of esterase isoenzyme. Lane 1, Sl-zsu-1 cell sample; Lane 2, Sl-HP cell sample. Bars, 20 μm
Apoptosis induced by AfMNPV and AcMNPV-GFP-actin
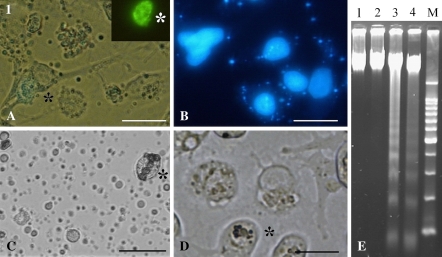
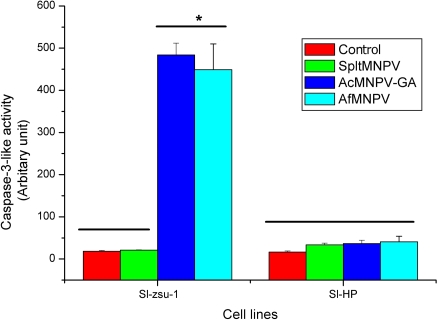
The apoptotic body formation and the chromatin condensation occurred in Sl-zsu-1 cells infected by AcMNPV-GFP-actin and AfMNPV (Fig. 2a, b, c) and the high activity of caspase-3-like was also demonstrated in the infected cells by both the viruses, shown in Fig. 3. As illustrated in Fig. 2e, DNA extracted from Sl-zsu-1 cells infected by AcMNPV-GFP-actin and AfMNPV showed DNA ladder resulting from inter-nucleosomal DNA degradation. In contrast, Sl-zsu-1 cells infected by SpltMNPV did not have similar characteristics (Figs. 2d, e, 3). Therefore, AfMNPV and AcMNPV-GFP-actin could induce Sl-zsu-1 cell apoptosis, but SpltMNPV could not (the DNA of SpltMNPV could. See below).
Fig. 2.
Apoptosis induced by AcMNPV-GFP-actin and AfMNPV (a) Cell apoptosis induced by AcMNPV-GFP-actin at 16 h of p.i.; * indicated the corresponding cell emitting green fluorescence. (b) Apoptotic cells induced by AcMNPV-GFP-actin and stained by DAPI at 16 h of p.i.; (c) Cell apoptosis induced by AcMNPV-GFP-actin at 48 of p.i.; * indicated the cell containing PIBs; (d) Cells infected by SpltMNPV at 72 h of p.i.; * indicated the cell containing PIBs; (e) Inter-nucleosome DNA degration induced by baculovirus. Lane 1, mock-infected cells; lane 2, SpltMNPV; Lane 3, AcMNPV-GFP-actin; Lane 4, AfMNPV; Lane M, 200 bp-DNA marker. Bars, 20 μm
Fig. 3.
Assay of caspase-3-like activity in Sl-zsu-1 and Sl-HP cells infected by different baculoviruses. The caspase-3-like activities in Sl-zsu-1 and Sl-HP cells were determined at 12 h of p.i. and 48 h of p.i., respectively. * indicated the significant difference at p < 0.05
GFP-actin gene was expressed or PIBs were formed only in few of cells (1–5) in each culture flask (Fig. 2a, b, c). Although SpltMNPV did not induce Sl-zsu-1 cell apoptosis (Figs. 2d, e, 3), it was difficult to detect the titre of BV in the supernatant of the SpltMNPV-infected cells (data not shown). Thus, the two baculovirus (AcMNPV-GFP-actin and AfMNPV) replication were blocked by apoptosis in Sl-zsu-1 cell line, but SpltMNPV replication was blocked by other factors.
Yields of three baculoviruses in Sl-HP cells
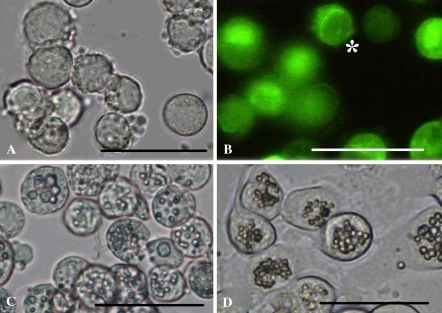
Although the titres of the three baculoviruses in Sl-zsu-1 cell cultures were not detectable, they could replicate in Sl-HP cells (Fig. 4a, b, c, d). The expression of GFP-actin gene of AcMNPV-GFP-actin was demonstrated in 67.8% cells with fluorescence microscope, but the virus titre of the supernatant of Sl-HP cells, determined by TCID50 method, was low (6.1 PFU/cell) at 72 h of p.i. (Table 1, 2). However, the titre of AcMNPV was much higher than that of AcMNPV-GFP-actin (Table 2). The data suggested that expression of GFP-actin reduced the replication of AcMNPV-GFP-actin. AfMNPV could replicate more vigorously than AcMNPV-GFP-actin in Sl-HP cells (Table 2). When Sl-HP cells were infected with SpltMNPV, the percent of the cells with PIBs increased by 28.5% at 72 h of p.i., and the yield of PIBs was 10.5 PIB/cells at 120 h of p.i., but the virus titre of the supernatant was very low at 72 h of p.i. (Table 1, 2). All these data suggested that Sl-HP cell line was more permissive to the baculovirus infection than Sl-zsu-1 cell line.
Fig. 4.
Analysis of the replication of three baculoviruses in Sl-HP cells using optical microscope. (a) Cells infected by AcMNPV-GFP-actin at 48 h of p.i.; (b) Cells infected by AcMNPV-GFP-actin and observed under fluorescence microscope, * indicated blebbing cell; (c) Cells infected by AfMNPV at 48 h of p.i.; (d) Cells infected by SpltMNPV at 72 h of p.i. Bars, 20 μm
Table 1.
Percent of Sl-HP cells with GFP-actin protein or PIBs at different periods of post-infection
| Virus | m.o.i. (PFU/cell) | Percent of cells with GFP-actin protein or inclusion bodies (%) | ||
|---|---|---|---|---|
| 24 h | 48 h | 72 h | ||
| AcMNPV-GFP-actin | 5.0 | 35.3 ± 2.3 | 66.1 ± 1.8 | 67.8 ± 2.0 |
| AfMNPV | 5.0 | 14.2 ± 2.7 | 49.8 ± 1.3 | 61.6 ± 3.6 |
| SpltMNPV | Na | 0 | 17.6 ± 0.4 | 28.5 ± 1.3 |
aIndicated that m.o.i. was not determined for the infection of SpltMNPV BV from haemolymph of the infected larvae on Sl-HP cells because the titre of virions from cell cultures was very low (see Table 2). Sl-HP cells infected by AcMNPV-GFP-actin did not show typical apoptotic characteristics (chromatin condensation, caspase-3-like activation and DNA ladder (Figs. 3, 5)
Table 2.
Yields of BVs and PIBs in Sl-HP cells infected by four baculoviruses, respectively
| Virus | m.o.i. (PFU/cell) | BV (PFU/cell) | Yield of inclusion bodies (PIB/cell) |
|---|---|---|---|
| AcMNPV-GFP-actin | 5 | 6.1 ± 2.1 | N |
| AcMNPV | 5 | 36.5 ± 3.0 | 16.5 ± 1.4 |
| AfMNPV | 5 | 77.5 ± 12.8 | 5.5 ± 0.15 |
| SpltMNPV | Na | (3.9 ± 0.4) × 10 −2 | 10.5 ± 0.3 |
aIndicated that m.o.i. was not determined for the infection of SpltMNPV BV from haemolymph of the infected larvae on Sl-HP cells because infectious virions from the cultured cells were fewer. There were significant statistical differences among the titres of BVs and the yields of PIBs of different baculoviruses per cell
Expression of GFP-actin gene reduced the BV titre in Sl-HP cells
The cells infected with the four baculovirus were observed under optical microscope and only Sl-HP cells infected by AcMNPV-GFP-actin blebbed significantly since 16 h of p.i. (Fig. 5b). However, the Sl-HP cells infected by AcMNPV-GFP-actin did not show typical apoptotic characteristics (chromatin condensation, caspase-3-like activation and DNA ladder (Figs. 5, 3). Thus, no apoptosis occurred in Sl-HP cell line infected by AcMNPV-GFP-actin. The expressed fused GFP-actin was able to be observed in the blebbing cells under fluorescence microscope (Figs. 5a, 4b). Since the titre of AcMNPV-GFP-actin was much lower than that of AcMNPV in the infected Sl-HP cell cultures, we suggested that it was the expression of GFP-actin not apoptosis reduced the titre of AcMNPV-GFP-actin.
Fig. 5.
Sl-HP cell blebbing induced by AcMNPV-GFP-actin. (a) and (b) Cell blebbing and green fluorescence emitted by GFP-actin under fluorescence microscope at 16 h of p.i.; * indicated the same cells in the same row, showing the blebbing cell emitting green fluorescence; (c) Cell nucleus stained by DAPI at 48 h of p.i., showing no significant chromatin condensation (See Fig. 1b); (d) Cell blebbing at 48 h of p.i.; * indicated the cell blebbing; (e) Electrophoresis of DNA extracted Sl-HP cells infected by different baculoviruses. Lane M, DNA ladder marker (200, 400, 600, 800, 1,000, 1,200, 1,400 bp); Lane 1, SpltMNPV; Lane 2, AcMNPV-GFP-actin; Lane 3, AfMNPV; Lane 4, mock-infected cells. (f) and (g) Sl-HP cells infected by AcMNPV-GFP-actin and wild-type of AcMNPV at 28 h of p.i., respectively; (h) and (i) Sf9 cells infected by wild-type AcMNPV and AcMNPV-GFP-actin at 28 h of p.i., respectively; (j) Cell blebbing induced by AcMNPV-GFP-actin at 30 h of p.i.; (k) Blebbing cells infected with AcMNPV-GFP-actin, showing acidic vesicular organelles using acridine orange; (l) Control (mock-infected) cells showing acidic vesicular organelles. Bars, 50 μm
In order to determine other pathway of the death for the Sl-HP cells infected by AcMNPV-GFP-actin, the staining of acridine orange was also used, which could be used to identify autophagic cell death. The result have shown that acidic vesicular organelles shown by the staining of acridine orange in the blebbed cells were at least no more than that in the mock-infected cells (Fig. 5i, k, l). This result demonstrated that autophagic death also did not occur in the blebbing Sl-HP cells. The cell death was most likely necrosis because apoptosis and autophagic death were excluded.
The percent of blebbing cells induced by AcMNPV-GFP-actin and wild-type AcMNPV was 63 ± 5% and 12.2 ± 0.5% at 48 h of p.i., respectively. Thus, the high expression of the fused GFP-actin gene of AcMNPV-GFP-actin enhanced cell blebbing. However, AcMNPV-GFP-actin and wild-type AcMNPV can not induce vigorous cell blebbing in Sf9 (Fig. 5h, j) and Hi5 cells (data not shown). Thus, the expression of GFP-actin gene seemed to be related to Sl-HP cell blebbing and the role of inducing cell blebbing was specific according to different cell lines.
Ultrastructure of the infected Sl-HP cells
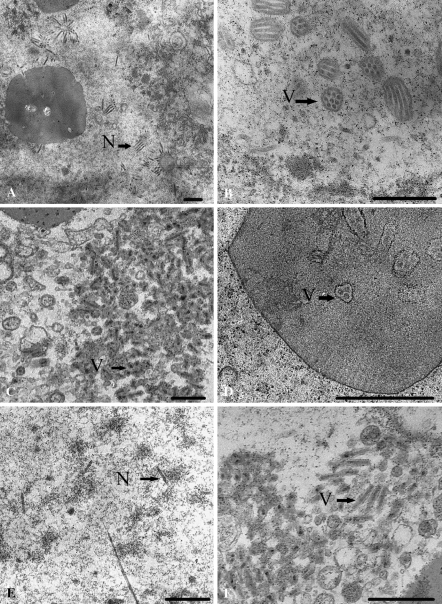
The replication of the three baculoviruses in the infected Sl-HP cells was analysed under TEM in order to explore the mechanisms of different permissiveness of the cells to different baculoviruses. Many PIBs, nucleocapsids and virions with envelopes in the Sl-HP cell nucleus were demonstrated after the cells were infected by the three baculoviruses, respectively, at 48 or 72 h of p.i. (Fig. 6). Although the titres of SpltMNPV or AcMNPV-GFP-actin in the cell cultures were very low (Table 1), a lot of virions or nucleocapsids were observed in the cells (Fig. 6c, d, e, f). These results have shown that the assembly of the virions or nucleocapsids is correct and the low titres may be caused by the blocking transportation and release of virions, which was needed to be confirmed in the future work.
Fig. 6.
Electron microscopic analysis of different baculovirus-infected Sl-HP cells (a) Image of the nucleus of a cell infected with AfMNPV, showing most of nucleocapsids without the envelope; (b) Image of the nucleus of a cell infected with AcMNPV-GFP-actin, showing virions containing multiple nucleocapsids and nucleocapsids without envelope; (c) Image of the nucleus of a cell infected with SpltMNPV, showing most of virions containing a single nucleocapsid; (d) SpltMNPV PIBs showing virions containing multiple nucleocapsids; (e) SpltMNPV nucleocapsids; (f) SpltMNPV virons, showing the envelope coated on the nucleocapsids. N, nucleocapsid; V, virion with envelope. Bars, 540 nm
Apoptosis induced by transfection of SpltMNPV DNA in Sl-HP cells
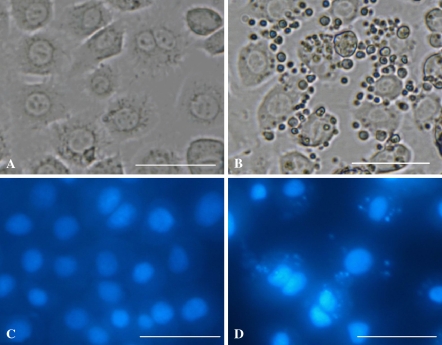
In order to know whether SpltMNPV DNA could induce apoptosis of Sl-HP cells, we performed the transfection with SpltMNPV DNA. SpltMNPV DNA extracted from PIBs propagated by the 3rd larvae was able to induce apoptosis in Sl-HP cells by transfection (Fig. 7). However, SpltMNPV BVs could not induce apoptosis in Sl-HP cells.
Fig. 7.
Apoptosis induced by transfection of SpltMNPV DNA into Sl-HP cells. (a) Cells transfected by plasmid at 24 h; (b) Cells transfected by SpltMNPV DNA at 24 h; (c) Cells transfected by plasmid and stained by DAPI at 24 h; (d) Cells transfected by SpltMNPV DNA and stained by DAPI at 24 h. Bars, 50 μm
A possible mechanism was that more DNA of this virus could enter Sl-HP cells by transfection than by infection with BV, and the sufficient virus DNA in a single cell can induce Sl-HP cell apoptosis by the high-level expression of ie-1 gene of SpltMNPV. Another possibility that the protein from the budded virions inhibited apoptosis induced by the expression of virus genes was excluded because many PIBs were observed in a few cells transfected by SpltMNPV DNA at 96 h of p.i. (data not shown). It was possible that less DNA of this virus that entered the cells and resulted in the formation of PIBs, but for many cells, apoptosis occurred because the sufficient virus DNA entered a single cell.
In a word, non-permissive or low-permissive Sl-zsu-1 cell line can become permissive to some baculovirus infection after over-high passage and multiple paths can block the infectivity of baculovirus.
Discussion
Why did the over-time subcultures result in the characteristic alteration of Sl-zsu-1 cell line? A few of cells expressing GFP-actin (1–5 cells), or forming AfMNPV PIBs (1–5 cells) and SpltMNPV PIBs (5% of cells) in Sl-zsu-1 cell cultures were observed in each culture flask containing 2.5 × 106 cells (Fig. 2a, c), suggesting the increase in the permissiveness of the Sl-HP cell line compared to the Sl-zsu-1 cell line may be due to selection of the permissive cell type over time.
Polyhedrin promoter could drive the expression of the fused GFP-actin gene of AcMNPV-GFP-actin and a lot of virions could be replicated in Sl-HP cell nucleus, but the titre of BVs was low. The mechanism possibly was that cell blebbing inhibited the replication containing release of this virus because the titre of wild-type AcMNPV in Sl-HP cell line, which induced fewer cell blebbing, was higher than that of AcMNPV-GFP-actin. Of course, the possibility that the over-expressed GFP-actin inhibited virus replication by blocking transportation of nucleocapsids from nucleus to cell plasm membrane in these cells can not be excluded.
Jia et al. (2005) reported that the expression of the fused GFP-actin gene from vAc-ph/70GA (AcMNPV-ph/70GFP-actin) had no influence on the virus titre of the supernatant of Sf9 cells, compared with wild-type AcMNPV. However, the titre of supernatant from Sl-HP cells infected by wild-type AcMNPV was much higher than that by AcMNPV-GFP-actin at 72 h of p.i. (Table 2). The PFU per cell for AcMNPV-GFP-actin decreased by 83.3%, compared with that for wild-type AcMNPV (Table 2). The mechanism for the different results will be elucidated in the future, and the possible mechanism is that the expression of GFP-actin induced more cell blebbing in Sl-HP cells than in Sf9 cells.
It was very interesting how cell blebbing was induced by infection of AcMNPV-GFP-actin in Sl-HP cells. Cell blebbing could occur when cells undergo apoptosis, necrosis or autophagy (Rosser and Gores 1995; Barros et al. 2003; Deschesnes et al. 2001; Hirata et al. 1998; Zheng et al.1998; Inbal et al. 2002). In our present studies, no caspase-3-like was activated, no DNA ladder was shown on agarose gel by electrophoresis, and no more acidic vesicular organelles were formed in the blebbing Sl-HP cells induced by AcMNPV-GFP-actin. Thus, the path of the blebbing cell death in our experiments was most likely necrosis.
Another interesting question is what the mechanisms for the change of the cell permissiveness. (1) The references indicated that the expression of baculovirus genes down-regulated in the high-passage cells, compared with that in low-passage cells (Joosten and Shuler 2003; Clemm 1992). (2) SpltMNPV DNA can induce cell apoptosis by transfection at high concentration, but the BV can not in the present studies. (3) A few of Sl-HP cells undergo apoptosis after AfMNPV or AcMNPV-GFP-actin infection at very high m.o.i. (over 20 or more), but no significant apoptosis occurs at low m.o.i. (data not shown). (4) The expression of ie-1 gene can induce apoptosis in Sl-zsu-1 cells at the early stage of infection (Zhang et al. 2002), and AcMNPV, AcMNPV-GFP-actin and AfMNPV also can induce apoptosis in this cell line at 6–8 h of p.i. in the present experiments. Based on the above, the down-regulation of ie gene of baculovirus in Sl-HP cells may be responsible for the change of the permissiveness. More work will be needed to confirm this opinion, and in the future, we will study the difference of ie gene expression between Sl-zsu-1 and Sl-HP cell line at the early stage of infection by baculoviruses.
Acknowledgement
This was supported by the Natural Science of Foundation of Hubei Province (Grant No: 2007ABA160), the National Natural Science Foundation of China (Grant No: 30470073) and the State Ministry of-Education College Students’ Innovative Projects (2007). Hong-Ye Zhang, Xin-Xue Cai and Yi-Chun Lin took part in some experimental work.
Abbreviation
- SpltMNPV
Spodoptera litura nucleopolyhedrovirus
- AfMNPV
Anagrapha falcifera multiple nucleopolyhedrosis virus
- AcMNPV
Autographa californica multiple nucleopolyhedrovirus
- AcMNPV-GFP-actin
A recombinant Autographa californica multiple nucleopolyhedrovirus with a fused GFP-actin gene
References
- Barros LF, Kanaseki T, Sabirov R et al (2003) Apoptotic and necrotic blebs in epithelial cells display similar neck diameters but different kinase dependency. Cell Death Differ 10(6):687–697. doi:10.1038/sj.cdd.4401236 [DOI] [PubMed]
- Briske-Anderson MJ, Finley JW, Newman SM (1997) Influence of culture time and passage number on morphological and physiological development of Caco-2 cells. Proc Soc Exp Biol Med 214(3):248–257 [DOI] [PubMed]
- Calles K, Svensson I, Lindskog E et al (2006) Effects of conditioned medium factors and passage number on Sf9 cell physiology and productivity. Biotechnol Prog 22(2):394–400. doi:10.1021/bp050297a [DOI] [PubMed]
- Chang S, Sun H, Li Z (1998) Effect of temperature oscillation on insect cell growth and baculovirus replication. Appl Environ Microbiol 64(6):2237–2239 [DOI] [PMC free article] [PubMed]
- Clemm DL (1992) Scale-up of protein production in a stirred bioreactor. In: O’Reilly DR, Miller LK, Luckow VA (eds) Baculovirus expression vectors: a laboratory manual. W. H. Freeman, New York, pp 241–248
- Dai XJ, Pang Y, Nong G et al (1998) Is baculovirus gp64 gene a primary factor inducing apoptosis of insect cells? Acta Sci Natur Univ Sunyatseni 37(3):7–12 In Chinese
- Deschesnes RG, Huot J, Valerie K et al (2001) Involvement of p38 in apoptosis-associated membrane blebbing and nuclear condensation. Mol Biol Cell 12(6):1569–1582 [DOI] [PMC free article] [PubMed]
- Donaldson MS, Shuler ML (1998) Effects on long-term passaging of BTI-Tn5B1-4 insect cells on growth and recombinant protein production. Biotechnol Prog 14(4):543–547. doi:10.1021/bp9800485 [DOI] [PubMed]
- Feng G, Yu Q, Hu C et al (2007) Apoptosis is induced in the haemolymph and fat body of Spodoptera exigua larvae upon oral inoculation with Spodoptera litura nucleopolyhedrovirus. J Gen Virol 88:2185–2193. doi:10.1099/vir.0.82919-0 [DOI] [PubMed]
- Hirata H, Hibasami H, Yoshida T et al (1998) Differentiation and apoptosis without DNA fragmentation in cultured Schwann cells derived from wallerian-degenerated nerve. Apoptosis 3(5):353–360. doi:10.1023/A:1009633205444 [DOI] [PubMed]
- Inbal B, Bialik S, Sabanay I et al (2002) DAP kinase and DRP-1 mediate membrane blebbing and the formation of autophagic vesicles during programmed cell death. J Cell Biochem 157(3):455–468 [DOI] [PMC free article] [PubMed]
- Jia Y, Yu Z, Chen X (2005) Actin and production of AcMNPV polyhedra. Prog Biochem Biophys 32(9):829–834
- Joosten CE, Shuler ML (2003) Effect of culture conditions on the degree of sialylation of a recombinant glycoprotein expressed in insect cells. Biotechnol Prog 19(3):739–749. doi:10.1021/bp0201049 [DOI] [PubMed]
- Knudson DL, Tinsley TW (1974) Replication of a nuclear polyhedrosis virus in a continuous cell culture of Spodoptera frugiperda: purification, assay of infectivity, and growth characteristics of the virus. J Virol 14(4):934–944 [DOI] [PMC free article] [PubMed]
- Li X, Zhou R, Jia Y et al (2004) Function of actin in transportation of the AcMNPV from nuclear to outside of cell. Virol Sin 19(6):630–635
- Liu K, Zheng J, Hong H et al (2005) Mechanisms for Bt toxin resistance and increased chemical pesticide susceptibility in Cry1Ac10-resistant cultured insect cells. Cytotechnology 49(2–3):153–160. doi:10.1007/s10616-006-6880-y [DOI]
- Liu L, Peng J, Liu K et al (2007a) Influence of cytochrome c on apoptosis induced by Anagrapha (Syngrapha) falcifera multiple nuclear polyhedrosis virus (AfMNPV) in insect Spodoptera litura cells. Cell Biol Int 31(9):996–1001. doi:10.1016/j.cellbi.2007.03.011 [DOI] [PubMed]
- Liu K, Tang Q, Fu C et al (2007b) Influence of glucose starvation on the pathway of death in insect cell line Sl: apoptosis follows autophagy. Cytotechnology 54(2):97–105. doi:10.1007/s10616-007-9080-5 [DOI] [PMC free article] [PubMed]
- O’Reilly DR, Miller LK, Luckow VA (1992) Baculovirus expression vectors: a laboratory manual. W. H. Freeman & Company, New York
- Pang Y, Yu J, Wang L et al (2001) Sequence analysis of the Spodoptera litura multicapsid nucleopolyhedrovirus genome. Virology 287(2):391–404. doi:10.1006/viro.2001.1056 [DOI] [PubMed]
- Park MT, Lee MS, Kim SH et al (2004) Influence of culture passages on growth kinetics and adenovirus vector production for gene therapy in monolayer and suspension cultures of HEK 293 cells. Appl Microbiol Biotechnol 65:553–558. doi:10.1007/s00253-004-1617-3 [DOI] [PubMed]
- Peiser C, Riebe-Imre M, Emura M et al (1993) Influence of culture passages on growth kinetics, xenobiotic metabolism, chromosomal stability and transformation in a clonal fetal hamster lung epithelial cell line. Mutat Res 289(2):281–290. doi:10.1016/0027-5107(93)90079-U [DOI] [PubMed]
- Rosser BG, Gores GJ (1995) Liver cell necrosis: cellular mechanisms and clinical implications. Gastroenterology 108(1):252–275. doi:10.1016/0016-5085(95)90032-2 [DOI] [PubMed]
- Simon O, Williams T, Lopez-Ferber M et al (2004) Virus entry or the primary infection cycle are not the principal determinants of host specificity of Spodoptera spp. nucleopolyhedroviruses. J Gen Virol 85:2845–2855. doi:10.1099/vir.0.80179-0 [DOI] [PubMed]
- Xie WD, Qu SR, Pang Y (1988) The establishment of an insect cell line from Spodoptera litura and the study of virus infection. Acta Sci Natur Univ Sunyatseni 45:113–116
- Xiu M, Peng J, Hong H (2005) Mitochondrial response and calcium ion change in apoptotic insect cells induced by SfaMNPV. Chin Sci Bull 50(12):1191–1198. doi:10.1360/04WC0275 [DOI]
- Zhang P, Yang B, Dai X et al (2002) Apoptosis of Spodoptera litura cells induced by AcMNPV ie-1 gene. Acta Biochim Biophys Sin (Shanghai) 34(6):707–711 [PubMed]
- Zheng TS, Schlosser SF, Dao T et al (1998) Caspase-3 controls both cytoplasmic and nuclear events associated with Fas-mediated apoptosis in vivo. Proc Natl Acad Sci USA 95(23):13618–13623. doi:10.1073/pnas.95.23.13618 [DOI] [PMC free article] [PubMed]