Abstract
The growth characteristics and influence of glucose and glutamine on the growth and maintenance of channel catfish ovary (CCO) cells were investigated. Besides glutamine, amino acids threonine, arginine, methionine and serine were found to be essential for CCO cell growth. In the glucose-free medium, glutamine is utilized as energy source and no cell growth limitation was observed. However, the lack of glutamine in culture medium did not stimulate CCO cells to efficient glucose consumption. When both glucose and glutamine were deficient, cell growth was also observed suggesting no rigorous nutritional requirements. Obtained results are useful for further understanding of culture processes using CCO cells.
Keywords: CCO cells, Cell metabolism, Glucose, Glutamine
Introduction
Channel catfish ovary (CCO) cells have been used in channel-catfish virus production (Colyer and Doyle 1985) but are also applied for research in virology (Stingley and Gray 2000) and aquatic toxicology (Tan et al. 2008). However, their metabolic characteristics, especially the energy metabolism and its regulation modes have not been studied yet. Studies reported by Bols et al. (1994) found that glutamine requirement for the in vitro proliferation of fish cells was conditional and dependent on the choice of basal medium and serum supplementation.
Glucose and glutamine are main nutrients of mammalian cells in culture. Glucose is utilized either through the pentose phosphate pathway or through the glycolysis pathway to provide metabolic intermediates and energy. Glutamine can be used as protein and peptide constituent and a large portion of glutamine may undergo deamination and transamination reactions. Glutamine metabolism can provide 30–65% of the energy required for mammalian cell growth (Reitzer et al. 1979; Zielke et al. 1984). Most of mammalian cell lines die immediately once the glucose is depleted (Mercille and Massie 1994; Gaurina Srček et al. 2004). However, if glutamine is depleted, some cell lines can not survive but others can adapt to conditions lacking of glutamine (McDermott and Butler 1993).
In this work, CCO cells were cultivated in order to determine basic growth characteristics as well as the influence of different glucose and glutamine concentrations on cell proliferation and maintenance.
Materials and methods
Cell line and culture conditions
CCO cell line was obtained from ATCC (CRL-2772). Cells growing in monolayer were cultured in tissue culture flasks in DME medium (ATCC) supplemented with 10% fetal bovine serum (FBS) or in DME medium without glucose and glutamine (Sigma) supplemented with 10% FBS (GIBCO) and, when necessary, 5.56 mM glucose and 4.0 mM glutamine were added. For the study of cell growth and metabolic parameters, cells were inoculated in 12-well plates in 1 mL of DME (ATCC) culture medium or in 1 mL of culture media with different glucose and glutamine concentrations at an initial cell density of about 5 × 104 cells mL−1. Trypan blue exclusion method was used for estimation of viable cells in haemocytometer after detaching cells from the well surface with trypsin. Antibiotics were not used during the cultivation. The CCO cells were maintained at 30 °C in an atmosphere of humidified air (95%) and CO2 (5%).
Analysis of metabolites
Glucose, lactate and ammonia in culture medium were quantified enzymatically. The glucose concentration was determined using Glucose-PAP colourenzymatic assay kit (Herbos Sisak, Croatia). Lactate and ammonia were assayed by UV-test (Boehringer Mannheim/R-Biopharm, Darmstadt, Germany). Amino acids were assayed by ion exchange liquid chromatography with an Amersham Biochrom 20 plus analyzer (Laboratory for Diagnosis of Metabolic Diseases, Clinical Hospital Center Zagreb, Zagreb, Croatia).
Metabolic parameter calculations
Specific rates of substrate consumption and metabolite production were calculated using the following formula:
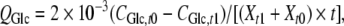 |
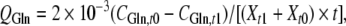 |
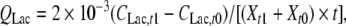 |
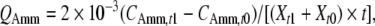 |
where Q is specific rate, mmol cell−1 day−1; C is the metabolite concentration, mmol cell−1 day−1; t0 and t1 are the seeding and harvest times, d; Xt1 and Xt0 are the viable cell number at t0 and t1, cells mL−1. Subscripts Glc, Gln, Lac and Amm indicate glucose, glutamine, lactate and ammonia, respectively.
The yield coefficients of lactate to glucose and ammonia to glutamine (mmol mmol−1) were calculated from the formula:
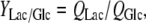 |
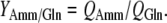 |
Results and discussion
Cell growth and metabolism
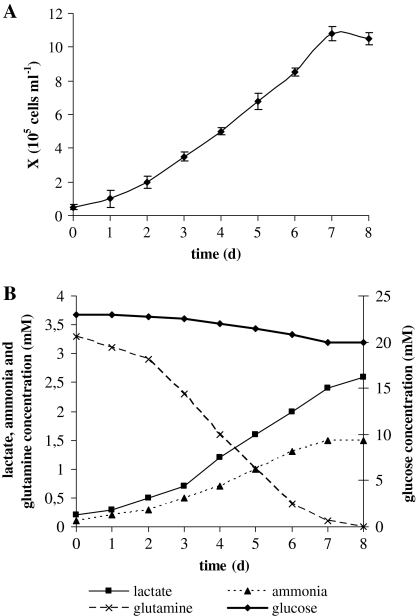
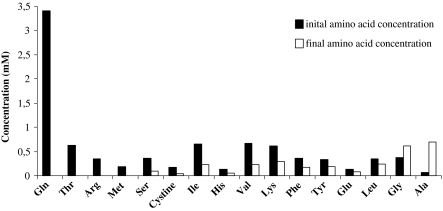
The growth and survival of cell cultures, among different parameters, depends on the availability of nutrients in culture medium (Zielke et al. 1984). In this study, we focused on the nutrient consumption and metabolite production of CCO cells with specific interest on the influence of different glucose and glutamine concentrations. The growth curve of CCO cells in standard DME medium during 8 days in culture is shown in Fig. 1a. After 8 days of cultivation, the cell density reached 10.5 × 105 cells mL−1 with specific growth rate of 0.024 h−1 during the exponential phase. Glucose and glutamine consumption together with lactate and ammonia production during the cell growth period is shown in Fig. 1b. Glucose consumption was not a limiting factor in the cell growth phase since residual glucose concentration was almost 20 mM. It is reported that glucose turnover rates for fish species are in general 20–100 times lower than values reported in mammals consistent with their lower body temperatures and metabolic rates (Weber and Zwingelstein 1995). Furthermore, Wright et al. (2000) reported that the metabolic role of glucose in some tilapia tissues may be much less significant than in mammalian tissues. Produced lactate concentration reached 2.8 mM. Because glucose utilization is very low, it seems that lactate in culture medium originates from pyruvate converted from small nonessential amino acids like serine, glycine, alanine and cysteine. Furthermore, lactate can be derived from glutamine via intermediates of TCA cycle. In terms of energetic efficiency, the TCA cycle is more favored than glycolysis and glutamine transamination pathway (Häggström 1991). Glutamine was completely depleted in culture medium indicating that this amino acid is essential for CCO cell growth as for mammalian cell cultures. Ammonia concentration at the end of cultivation period reached 1.6 mM and together with exhausted glutamine probably caused arrest in cell growth. Although glutamine is the most relevant amino acid in cell culture, it is also important to consider metabolism of other amino acids in culture medium. The main cause of amino acid consumption is their integration in peptides during protein synthesis as well as their participation in cell metabolism by sustaining the TCA cycle. Figure 2 presents the initial amino acid concentration and the concentration detected in eight days old culture. Besides glutamine, the amino acids depleted completely or almost completely were: threonine, arginine, methionine and serine. Arginine is one of the most versatile amino acids in animal cells, serving as a precursor for the synthesis of proteins as well as nitric oxide, urea, polyamines, proline and glutamate (Wu and Morris 1998). Serine can be converted to pyruvate to enter TCA cycle and supply energy for cell growth. Glycine and alanine were produced by CCO cells. Glycine derives from serine metabolism while alanine derives from glutamic acid and pyruvate; at high glucose concentrations, the increase in pyruvate production leads to an increase in alanine production.
Fig. 1.
Growth kinetics of CCO cells in DME medium (panel a). Glucose (♦), lactate (■), glutamine (×) and ammonia (▲) concentration (panel b)
Fig. 2.
Amino acids consumed and produced during the cell growth; initial medium concentration (■) and amino acid concentration on the last day of cultivation (□)
Presented results showed that the nutrient requirements of CCO cells are mainly dependent on glutamine and other amino acid metabolism rather than glucose.
Effects of glucose and glutamine on CCO cell growth
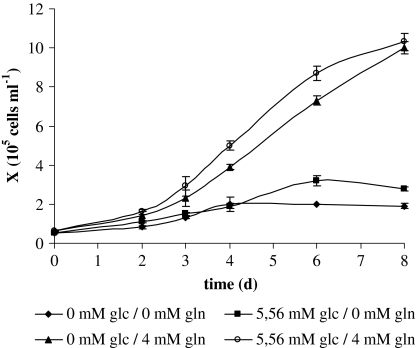
In animal cell culture both glucose and glutamine can be utilized as the carbon and energy sources to provide energy for cell growth. Thus, the significance of the two major nutrients in two metabolic directions must be studied in each cell line. The growth of CCO cells at different initial glucose and glutamine concentrations is shown in Fig. 3. The maximum viable cell densities on the eighth day in glutamine-free medium (with 5.56 mM glucose) and glucose-free medium (with 4 mM glutamine) were 2.8 × 105 and 10.0 × 105 cells mL−1, respectively. At the same time in complete medium maximum cell density reached 10.3 × 105 cells mL−1, which is similar to the cell density achieved in the medium with only glutamine added. These results show that glutamine is critical for growth and maintenance of CCO cells and can substitute glucose as sole energy substrate which is also reported for HeLa (Reitzer et al. 1979) and NCTC 21071 cell lines (Wice et al. 1981). Glucose deficiency did not result in cellular growth limitation. In the medium lacking both glucose and glutamine, CCO cells grew more slowly than cells in complete medium reaching density of 2 × 105 cells mL−1. Cell proliferation in the same medium condition was also reported for chinook salmon embryo (CHSE) cells (Chen et al. 2005) and this phenomenon could be specific for some established fish cell lines.
Fig. 3.
Growth kinetics of CCO cells with different initial glucose and glutamine concentrations. 0 mM Glc/0 mM Gln; 0 mM Glc/4 mM Gln; 5.56 mM Glc/0 mM Gln; 5.56 mM Glc/4 mM Gln
The metabolic parameters of glucose and glutamine during eight days of batch culture with different glucose and glutamine concentrations are shown in Table 1. In glutamine-free culture the specific consumption rate of glucose (QGlc) is slightly decreased. This result is in agreement with data obtained in pulse culture of BHK cells, reporting about reduction of QGlc with the decrease of glutamine concentration (Martinelle et al. 1998). Furthermore, specific production rate of lactate (Qlac) and yield of lactate from glucose (YLac/Glc) in glutamine-free medium were increased for 13.2 and 20 %, respectively, compared to complete medium.
Table 1.
Metabolic parameters of glucose and glutamine in CCO cell culture at different glucose and glutamine concentration
| Medium | QGlc (10−10 mmol cell−1 d−1) | QLac (10−10 mmol cell−1 d−1) | YLac/Glc (mmol mmol−1) | QGln (10−10 mmol cell−1 d−1) | QAmm (10−10 mmol cell−1 d−1) | YAmm/Gln (mmol mmol−1) |
|---|---|---|---|---|---|---|
| 0 mM Glc/0 mM Gln | – | 6.4 | – | – | 4.0 | |
| 0 mM Glc/4 mM Gln | – | 5.6 | – | 7.0 | 6.0 | 0.86 |
| 5.56 mM Glc/0 mM Gln | 10.2 | 10.6 | 1.0 | – | 4.0 | |
| 5.56 mM Glc/4 mM Gln | 11.6 | 9.2 | 0.8 | 7.4 | 6.8 | 0.92 |
QGlc, QGln, QLac and QAmm are specific consumption or production rates of glucose, glutamine, lactate and ammonia, respectively (in mmol cell−1 day−1). YLac/Glc and YAmm/Gln are the yield coefficients of lactate to glucose and ammonia to glutamine, respectively (in mmol mmol−1)
The increase of YLac/Glc demonstrates that glucose is not utilized for energetically efficient pathway indicating a metabolic shift towards the pathway to lactate production. Since oxidation of glucose is evidently minimal, it is probable that metabolism of glucose is related to ribose formation for nucleotide biosynthesis and for the synthesis of nonessential amino acids. Because some of these amino acids could produce pyruvate, as discussed previously, it appears that some portion of pyruvate could end up in lactate.
In glucose-free medium and in medium with added glucose, cells consumed glutamine with almost identical rates indicating that the lack of glucose did not stimulate utilization of glutamine. It was reported that addition of glutamine inhibits glycolysis in the myeloma cell culture (Martinelle et al. 1998) while in the cultures of BHK cells glutamine cannot be substituted by glucose but glucose can be substituted by glutamine (Cruz et al. 1999). The consumption of glutamine and other amino acids brought about an accumulation of ammonium ions. Relatively constant specific production rate of ammonia and YAmm/Gln confirmed similar glutamine consumption in glucose-free and glucose added culture medium.
Assessment of cell growth and metabolic activities are essential for the successful control and improvement of a CCO cell culture processes especially for the study of channel catfish virus (CCV) and related vaccine production. It is possible that viral infection results in profound changes in cell metabolism. Infection of PER.C6® cells with recombinant adenovirus increased their glucose consumption and lactate production (Xie et al. 2002). Genzel et al. (2004) reported about post-infectious changes in glutamate and aspartate metabolism of MDCK cells and put this in correlation with cell disruption and apoptosis. Furthermore, metabolic waste products of these cells can inhibit replication and decrease infection ability of equine influenza virus (Genzel et al. 2004; Whittaker et al. 1996). Therefore the influence of medium components and/or cell metabolic activity could be of interest for further investigation, especially when looking at high density CCO cell cultivation and CCV production.
Conclusions
The growth characteristics as well as metabolic parameters of CCO cells at different glucose and glutamine concentrations have been examined. Glucose and most of the amino acids were not depleted by CCO cells while glutamine, threonine, arginine, methionine and serine were completely depleted showing that CCO cell growth in complete medium is mainly dependent on glutamine and some of these amino acids. Glucose deficiency did not result in cell growth arrest, suggesting that glutamine is the major energy source. However, the lack of glutamine in culture medium did not stimulate cells to efficient glucose consumption but made them grow at significantly lower rates. Results obtained in this study are useful for designing optimal culture medium and successful cell processes using CCO cells.
Acknowledgement
This work was supported by the Ministry of Science, Education and Sports of the Republic of Croatia (Grant No. 058-0582184-2141).
References
- Bols NC, Ganassin RC, Tom DJ, Lee IEJ (1994) Growth of fish cell lines in glutamine-free medium. Cytotechnology 16:159–166 [DOI] [PubMed]
- Chen J, Sun X, Zhang Y (2005) Growth and metabolism of marine fish Chinook salmon embryo cells: response to lack of glucose and glutamine. Biotechnol Lett 27:395–401 [DOI] [PubMed]
- Colyer T, Doyle JA (1985) Optimization of conditions for production of channel catfish ovary cells and channel catfish virus DNA. Appl Environ Microbiol 49:1025–1028 [DOI] [PMC free article] [PubMed]
- Cruz HJ, Ferreira AS, Freitas CM, Moreira JL, Carrond MJT (1999) metabolic responses to different glucose and glutamine levels in baby hamster kidney cell cultures. Appl Microbiol Biotechnol 51:579–585 [DOI] [PubMed]
- Gaurina Srček V, Čajavec S, Sladić D, Kniewald Z (2004) BHK 21 C13 cells for Aujeszky’s disease virus production with multiple harvest process. Cytotechnology 45:101–106 [DOI] [PMC free article] [PubMed]
- Genzel Y, Behrendt I, König S, Sann H, Reichl U (2004) Metabolism of MDCK cells during cell growth and influenza virus production in large-scale microcarrier culture. Vaccine 22:2202–2208 [DOI] [PubMed]
- Häggström L (1991) Energetics of glutaminolysis—a theoretical evaluation. In: Spier RE, Griffiths JB, Meigner B (eds) Production of biologicals from animal cells in culture. Butterworth-Heinemann, Oxford, pp 79–81
- Martinelle K, Doverskog M, Jacobsson U, Chapman BE, Kuchel PW, Häggström L (1998) Elevated glutamate dehydrogenase flux in glucose deprived hybridoma and myeloma cells: evidence from 1H/15N NMR. Biotechnol Bioeng 60:508–517 [DOI] [PubMed]
- McDermott RH, Butler M (1993) Uptake of glutamate, not glutamine synthetase, regulates adaptation of mammalian cells to glutamine-free medium. J Cell Sci 104:51–58 [DOI] [PubMed]
- Mercille S, Massie B (1994) Induction of apoptosis in nutrient deprived cultures hybridoma of and myeloma cells. Biotechnol Bioeng 44:1140–1154 [DOI] [PubMed]
- Reitzer LJ, Wice BM, Kennel D (1979) Evidence that glutamine, not sugar is the major energy source for cultured HeLa cells. J Biol Chem 254:2569–2676 [PubMed]
- Stingley RL, Gray WL (2000) Transcriptional regulation of the channel catfish virus genome direct repeat region. J Gen Virol 81:2005–2010 [DOI] [PubMed]
- Tan F, Wang M, Wang W, Lu Y (2008) Comparative evaluation of the cytotoxicity sensitivity of six fish cell lines to four metals in vitro. Toxicol In Vitro 22:164–170 [DOI] [PubMed]
- Weber JM, Zwingelstein G (1995) Circulatory substrate fluxes and their regulation. In: Hochachka PW, Mommesen TP (eds) Biochemistry and molecular biology of fishes—Metabolic biochemistry. Elsevier, Amsterdam
- Whittaker G, Bui M, Helenius A (1996) The role of nuclear import and export in influenza virus infection. Trends Cell Biol 6:67–71 [DOI] [PubMed]
- Wice BM, Reitzer LJ, Kennel D (1981) The continuous growth of vertebrate cells in the absence of sugar. J Biol Chem 256:7812–7819 [PubMed]
- Wright JR Jr, Bonen A, Conlon JM, Pohajdak B (2000) Glucose homeostasis in the teleost fish tilapia: insights from Brockmann body xenotransplantation studies. Am Zool 40:234–245 [DOI]
- Wu G, Morris SM Jr (1998) Arginine metabolism: nitric oxide and beyond. Biochem J 336:1–17 [DOI] [PMC free article] [PubMed]
- Xie L, Pilbrough W, Metallo C, Zhong T, Pikus L, Leung J, Auniņš JG, Zhou W (2002) Serum-free suspension cultivation of PER.C6® cells and recombinant adenovirus production under different pH conditions. Biotechnol Bioeng 80:569–579 [DOI] [PubMed]
- Zielke HR, Zielke CR, Ozand PT (1984) Glutamine: a major energy source for cultured mammalian cells. Fed Proc 43:121–125 [PubMed]