Abstract
Conditioned flavor-taste preference (CFTP) is a robust form of learning in which animals acquire a preference for a flavor (e.g. Kool-Aid) previously mixed with a highly preferred tastant (e.g. fructose) over a flavor previously mixed with a less preferred tastant (e.g. saccharin). Here, the role of the N-methyl-D-aspartate (NMDA) glutamate-glycine receptor (NR) was probed using systemic MK-801, a non-competitive antagonist, and D-cycloserine (DCS), a glycine agonist. Rats were injected with MK-801 (100 μg/kg) or vehicle 30 min prior to a daily 2-h conditioning session with 1-bottle access to a Kool-Aid flavor (grape or cherry) mixed with either 8% fructose (CS+/F) or 0.2% saccharin (CS−/S). CFTP expression was measured in 2-bottle preference tests between the Kool-Aid flavors mixed with 0.2% saccharin (CS+/S vs. CS−/S). While vehicle-treated rats acquired a preference for CS+/S over CS−/S, MK-801 prior to conditioning completely blocked CFTP learning. The effect of MK-801 was specific to CFTP acquisition, because follow-up experiments demonstrated that MK-801 did not induce a conditioned taste aversion, cause state-dependent learning, or affect CFTP expression. In a second approach, rats were injected with DCS (15 mg/kg) 60 min prior to daily conditioning. In contrast to MK-801, adminstration of DCS prior to conditioning enhanced CFTP learning (but not reversal conditioning). These results demonstrate that NR neurotransmission is critical for CFTP learning. Furthermore, enhancement of CFTP learning by DCS suggests that endogenous levels of glycine or D-serine may be a limiting factor in CFTP learning.
Keywords: fructose, saccharin, associative learning, olfactory conditioning, glutamate, glycine, serine
1. Introduction
Conditioned flavor-taste preference (CFTP) is a form of associative learning in which an animal comes to prefer a neutral flavor after it has been paired with a preferred taste. CFTP learning is mediated by orosensory stimuli (i.e. CFTP can be acquired by rats sham-drinking the flavors and tastants, in which the postingestive effects are minimized (Sclafani and Ackroff, 1994)), rapidly acquired after only a few trials, and very resistant to extinction (Baker et al., 2003; Baker et al., 2004). In one model of CFTP learning (Baker et al., 2003; Baker et al., 2004), one flavor (the conditioned stimulus or CS+; e.g., cherry or grape Kool-Aid) is paired in mixture with the sweet and highly preferred taste of fructose (F; the unconditioned stimulus or US) while a second flavor (the CS−) is paired in mixture with the less preferred taste of saccharin (S) on 1-bottle conditioning days (CS+/F or CS−/S). The acquisition of the learned preference is then assessed with a 2-bottle preference test in which both flavors mixed with saccharin are presented simultaneously (CS+/S vs. CS−/S).
Although CFTP learning is common and robust, its neural substrates are not well characterized. The specific olfactory and gustatory relays and the associative brain regions necessary for CFTP learning are unknown. While chemical mediators of reward properties of the CS and US have been identified (e.g. dopamine receptors; (Baker et al., 2003; Baker et al., 2004; Yu et al., 1999; Yu et al., 2000a, b)), the associative mechanisms underlying the formation of the CFTP itself have not been explored.
One candidate molecule is the N-methyl-D-aspartate (NMDA) glutamate-glycine receptor (NR). NR is widely distributed throughout the CNS, but is particularly dense in highly plastic regions involved in learning (e.g. cortex, limbic system, and cerebellum) (Monyer et al., 1994). NR activation and the resulting calcium influx play a critical role in neural plasticity in vitro as a synaptic coincidence detector (e.g. in LTP) (Cain, 1997).
NR has previously been identified as a participant in olfactory and taste learning (Barkai and Saar, 2001; Jimenez and Tapia, 2004). For example, during conditioned taste aversion learning (CTA), exposure to a novel taste (but not a familiar taste) results in increased phosphorylation of the NR2B subunit in the insular cortex, which parallels the high saliency of novel tastes in CTA learning (Rosenblum et al., 1997). NR activity is required in CTA learning, because antagonism of the NR in the insular cortex by injection of the competitive NR antagonist 2-amino-5-phosphonopentanoic acid (APV) impairs CTA learning (Rosenblum et al., 1997).
NR activity in the amygdala is also required for odor-potentiated CTA. Pretreatment with the competitive NR antagonist APV impaired potentiated taste-odor aversion learning (i.e., saccharin presented with an almond odor and paired with LiCl injection), although in odor-alone and taste-alone conditioning, aversion learning was not impaired (Willner et al., 1992).
The present study was conducted in order to establish a role for NR in CFTP learning. Two approaches were taken. First, a necessary role for the NR was established using systemic injections of MK-801, a non-competitive antagonist. Systemic MK-801 has been used to attenuate other forms of learning, such as olfactory discrimination learning for water reward in weanling (Griesbach et al., 1998) and adult rats (Quinlan et al., 2004). A complication of systemic MK-801 treatment that must be controlled for in behavioral experiments, however, is the induction of “non-specific” or aversive side effects at high doses (Sharp et al., 2001).
Second, the contribution of activity at the glycine-binding site of the NR was assessed by administration of D-cycloserine (DCS), a high-affinity glycine agonist. In addition to glutamate binding, NR channel opening requires agonist binding to a glycine-binding site. Endogenous ligands for the glycine-binding site of the NR include glycine of neural origin or D-serine synthesized by astrocytes. For example, neuronal migration in the developing cerebellum is dependent on serine racemase in glia to convert L-serine to D-serine, which together with glutamate potentiates NR activity in granule neurons (Kim et al., 2005).
By stimulating NR at the time of learning, exogenous DCS can potentiate learning in behavioral studies. For example, DCS has been shown to enhance spatial learning in a water maze (Riekkinen and Riekkinen, 1997) and to accelerate extinction in rats after fear conditioning with footshock (Walker et al., 2002). Potentiation of NR activity and learning by DCS treatment has been interpreted as indicating that endogenous levels of glycine or D-serine are less than optimal and thus limit NR activation; exogenous DCS can then raise NR activation to an optimal level for learning.
To assess the effects of MK-801 and DCS on CFTP, rats were given injections of the drugs prior to a daily, 2-h pairing of Kool-Aid flavors mixed with either highly preferred fructose (CS+/F) or less-preferred saccharin (CS−/S). CFTP was assessed after every 4 conditioning days in 2-h, 2-bottle preference tests with both Kool-Aid flavors containing saccharin (CS+/S vs. CS−/S). We found that MK-801 blocked acquisition of CFTP, but not expression of a previously learned CFTP. To rule out non-specific or aversive effects that might oppose or mask CFTP learning, control experiments demonstrated that MK-801 under our conditions did not reduce unconditioned intake, induce a conditioned taste aversion (CTA), or lead to state-dependent learning.
Conversely, DCS administered prior to conditioning potentiated the acquisition of CFTP, by both accelerating the rate of learning and increasing the magnitude of CFTP expression. Because CFTP learning is very resistant to extinction, we therefore tested the effects of DCS pretreatment on reversal conditioning rather than on unreinforced extinction trials. In contrast to its effects on acquisition, DCS did not enhance the rate or magnitude of reversal CFTP learning.
2. Methods
2.1 Subjects
Male albino Sprague –Dawley rats (290–375 g, Charles River Laboratories, Wilmington, MA, USA) were housed individually in polycarbonate cages under a 12:12-h light-dark cycle with Purina rat chow and water available ad libitum. All testing took place in the rat’s home cage during the first half of the lights-on phase. Six days before testing began, the rats were placed on a food restriction schedule (15–20g rat chow/day) that maintained their body weights at approximately 90% of their initial ad libitum weight through the entire experiment. Water was available ad libitum at all times. Rats were initially trained to drink 8% maltose dextrin (BioServ, Frenchtown, NJ, USA) during a 2-h one-bottle session. This conditioning procedure was repeated daily for 5 days until all rats approached the sipper tubes with short latency (< 1 min). The daily food ration was given after each conditioning session.
2.2 Conditioning solutions
The conditioning solutions were 8% fructose (BioServ, Frenchtown, NJ, USA) or 0.2% sodium saccharin (Sigma, St. Louis, MO, USA) flavored with 0.05% unsweetened grape or cherry Kool-Aid (Kraft Foods North America, Inc., Rye Brook, NY, USA). Half of the rats in each group received cherry-flavored fructose solution and grape-flavored saccharin solution; the flavors were reversed for the remaining rats. In the 2-bottle preference tests, the cherry and grape flavors were each presented in a 0.2% saccharin solution without fructose. The fructose-paired flavor is referred to as the CS+ and the saccharin-paired flavor as the CS− because 8% fructose is preferred to 0.2% saccharin (Sclafani and Ackroff, 1994). CS+/F refers to the flavored fructose solution used in conditioning, and CS+/S refers to the same flavor in saccharin solution used during 2-bottle preference tests. The CS−/S refers to the flavored saccharin solution used in conditioning and preference testing.
Intakes were measured by weighing the bottles before and after each session to the nearest 0.1 g. Significant differences were detected by ANOVA, using the Newman-Keuls test for post hoc comparisons. Data are presented as mean intake ± standard error of the mean.
2.3 Acquisition and expression of CFTP
Rats received 4 one-bottle conditioning sessions (2 h/day) with the CS+/F solution presented on days 1 and 3, and the CS−/S solution presented on days 2 and 4. This pattern of 4 days of one-bottle conditioning sessions was repeated a total of 3 times in experiment 1 and 4 times in experiments 4 and 5 to establish a CFTP. Thus rats received a total of 6 CS+/F sessions and 6 CS-/S sessions in experiment 1 and 8 CS+/F sessions and 8 CS−/S sessions in experiments 4 and 5. During one-bottle sessions, rats were presented with the solution bottle and an empty bottle; the positions of the solution bottle and empty bottle were alternated daily.
To measure the CFTP, rats were given a 2-bottle preference test (2 h/day) with the CS+/S and CS−/S solutions. The positions of the grape- and cherry-flavored solutions were also alternated with each 2-bottle test. During the 2-bottle preference test the CS+/S and CS−/S solutions differed only in flavor; therefore increased intake of CS+ is a measure of a conditioned response acquired from the prior association of the flavor with fructose.
2.4 Experiment 1: MK-801 and CFTP acquisition
Naive rats (n=16) were placed on food restriction and trained with 2-h maltose dextrin access as described above. Rats were divided into two groups. The rats in the first group (MK-801 group, n = 8) received a MK-801 injection (Sigma, St. Louis, MO, USA; 100 μg in 1ml/kg ip) while rats in the second group (vehicle group, n = 8) received a vehicle injection (0.15 M NaCl, 1 ml/kg ip). The dose of MK-801 was selected based on efficacy as an NR antagonist in other learning paradigms, without inducing noticeable motoric side effects (Griesbach et al., 1998; Stuchlik et al., 2004; Weldon et al., 1997). Thirty minutes after the injections, rats were given 2-h access to either the CS+/F or CS−/S solution in a one-bottle conditioning session.
Following four days of conditioning, both groups received vehicle injections; 30 min later, all rats were given a 2-h, 2-bottle preference test with the CS+/S and CS−/S solutions. This was repeated 3 times so that all rats received a total of 6 CS+/F and 6 CS−/S conditioning days interspersed with 3 CS+/S vs. CS−/S 2-bottle preference test days.
2.5 Experiment 2: MK-801 and CFTP expression
To examine the effects of MK-801 on the expression of preference and to determine if preference learning in the MK-801-treated rats was state-dependent, the rats (n= 16) from experiment 1 were administered MK-801 (100 μg in 1ml/kg ip) or vehicle (0.15 M NaCl, 1 ml/kg ip) 30-min prior to a 2-h, 2-bottle preference test of CS+/S vs. CS−/S. Rats were tested on 2 consecutive days. All rats received both MK-801 and vehicle injections, counterbalanced across days and groups.
2.5 Experiment 3: MK-801 and CTA
The rats (n=16) from experiment 1 and 2 were used to determine if treatment with MK-801 during CFTP conditioning had caused the acquisition of a CTA. Rats received no injections during this experiment, and were given ad libitum access to rodent chow. Rats were given 2-bottle preference tests (23 h/day) of Kool-Aid-flavored saccharin solutions vs. water for 4 consecutive days. All rats had two consecutive days of access to grape-flavored saccharin and two consecutive days of access to cherry-flavored saccharin (i.e. 2 days of CS+/S vs. water and 2 days of CS−/S vs. water.) The positions of the solution bottle and the water bottle were alternated daily. Both intake and flavored-saccharin preference (flavored saccharin intake/total intake) were analyzed.
2.6 Experiment 4: DCS and CFTP acquisition
Naive rats (n=23) were placed on food restriction and trained with 2-h maltose dextrin access as described above. Rats were divided into two groups. The rats in the first group (DCS group, n = 11) received a DCS injection (Sigma, St. Louis, MO, USA; 15mg in 1ml/kg ip) while rats in the second group (vehicle group, n = 12) group received a vehicle injection (0.15 M NaCl, 1 ml/kg ip). The dose of DCS was selected based on its efficacy at enhancing other forms of learning without adverse effects such as conditioned taste aversion (Andersen et al., 2002; Walker et al., 2002). Sixty minutes after the injections, rats were given 2-h access to either the CS+/F or CS−/S solution in a one-bottle conditioning session.
Following four days of conditioning, both groups received vehicle injections; 60 min later, all rats were given a 2-h, 2-bottle preference test with the CS+/S vs. CS−/S solutions presented simultaneously. Intake was measured after 2 h. This was repeated four times so that all rats received a total of 8 CS+/F and 8 CS−/S conditioning days interspersed with 4 CS+/S vs. CS−/S 2-bottle test days.
To examine extinction, rats were then given 2-bottle preference tests (2 h/day) of CS+/S vs. CS−/S for 7 consecutive days without injections.
2.7 Experiment 5: DCS and CFTP reversal conditioning
DCS has been shown to accelerate extinction in other models of learning. Because CFTP is very resistent to extinction, the effects of DCS on reversal of CFTP were examined. Naive rats (n=18) were placed on food restriction and trained with 2-h maltose dextrin access as described above. A preference for the flavor paired with fructose was established in all rats as described above with 8 CS+/F and 8 CS−/S sessions. Three 2-h, 2-bottle preference tests with CS+/S vs. CS−/S were interspersed on days 9, 14, and 19 to measure the expression of the CFTP during acquisition. On the last 2-bottle preference test day (after 8 CS+/F and 8 CS−/S conditioning days), rats showed a CFTP of 0.8 ± 0.1 (expressed as the ratio of CS+/S over total 2-h intake).
After the CFTP was established, rats underwent reversal conditioning by reversing the pairing of fructose and CS solutions. Rats were divided into two groups. The rats in the first group (DCS group, n = 9) received a DCS injection (Sigma, St. Louis, MO, USA; 15mg in 1ml/kg ip) while rats in the second group (vehicle group, n = 9) received a vehicle injection (0.15 M NaCl, 1 ml/kg ip). Sixty minutes after the injections, rats were given 2-h access to either the rCS+/F (i.e., their old CS− flavor now paired with 8% fructose) or rCS−/S (i.e., their old CS+ flavor now paired with 0.2% saccharin) in a one-bottle conditioning session. Following four days of conditioning, both groups received vehicle injections; 60 min later, all rats were given a 2-bottle preference test (2 h) with the rCS+/S and rCS−/S solutions. Intake was measured after 2 h. This was repeated four times so that all rats received a total of 8 rCS+/F and 8 rCS−/S conditioning days interspersed with 4 rCS+/S vs. rCS−/S 2-bottle test days.
3. Results
3.1 Experiment 1: MK-801 and CFTP acquisition
To determine if NMDA neurotransmission is required for CFTP learning, food-restricted rats were injected with either vehicle (0.15 M NaCl, 1 ml/kg i.p., n= 8) or MK-801 (100 μg/kg, n = 8) 30 min before a daily 2-h conditioning session with eitherCS+/F or CS−/S. Although CS+/F intake was relatively stable across the 12 days of conditioning, there was a significant interaction of drug treatment and days [F(4,56) = 4.0, p < 0.01; see Figure 1A]. (Intakes were not recorded on days 3 and 6 due to technical errors.) Vehicle-treated rats drank significantly more CS+/F than MK-801-treated rats only on conditioning day 7. From day 2 to day 12, CS−/S intake increased significantly for both the vehicle and MK-801 groups, with a significant effect of days [F(4,56) = 28.7, p < 0.001] but not drug treatment (see Figure 1B).
Figure 1.
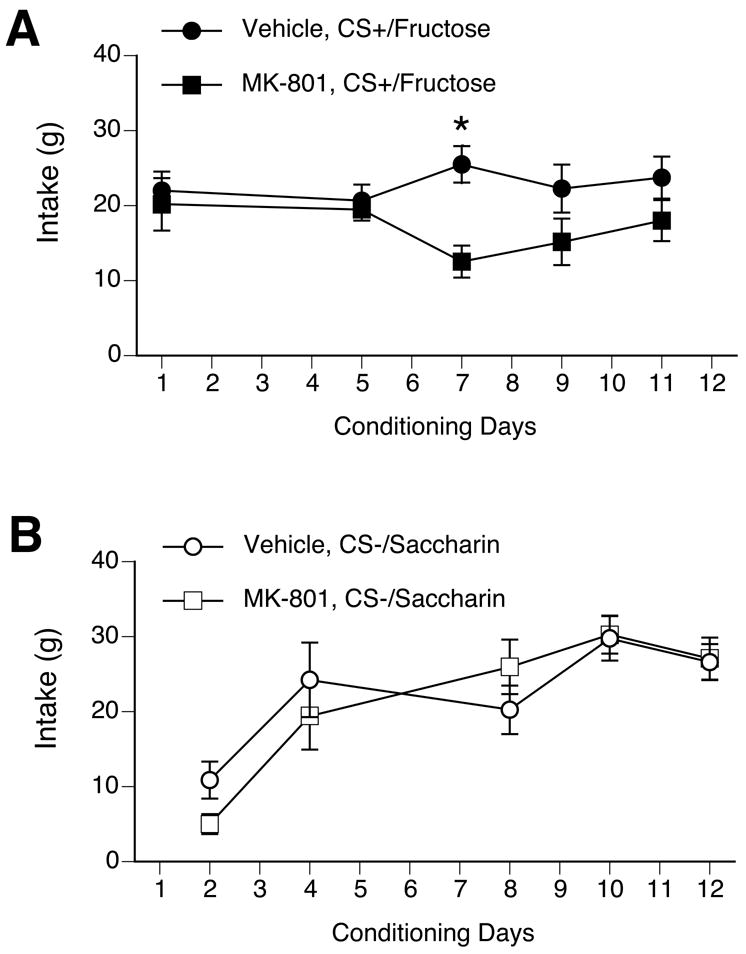
Systemic injections of MK-801 did not affect CS intake during conditioning. Intake was measured after a 2-h, 1-bottle presentation. A. With the exception of conditioning day 7, there was no difference in CS+/F intake between rats treated with MK-801 during conditioning (black squares) and vehicle-treated rats (black circles). * p < 0.05 vs. MK-801. B. There was no difference in CS−/S intake during conditioning between MK-801-treated rats (white squares) and vehicle-treated rats (white circles). There was an increase in CS−/S intake in both groups, however, such that intake on days 4 – 12 was significantly higher than intake on day 2.
The vehicle group acquired a CFTP after 8 conditioning sessions as shown by increased CS+ intake in the first 2-bottle preference test. Rats in the MK-801 group, however, did not acquire a CFTP after 12 conditioning sessions as shown by equal intake of the CS+/S and CS−/S in all three 2-bottle preference tests (see Figure 2A). Within the treatment groups, two-way ANOVAs on CS+/S vs. CS−/S intake across the three 2-bottle preference test days using test solution and test day as factors showed a significant interaction of test solution and days for the vehicle group (F[2,28]=14.39, p<0.05), but no significant effects for the MK-801 group. Thus, while vehicle-treated rats acquired a CFTP within 8 conditioning days, MK-801 treatment completely blocked CFTP acquisition.
Figure 2.
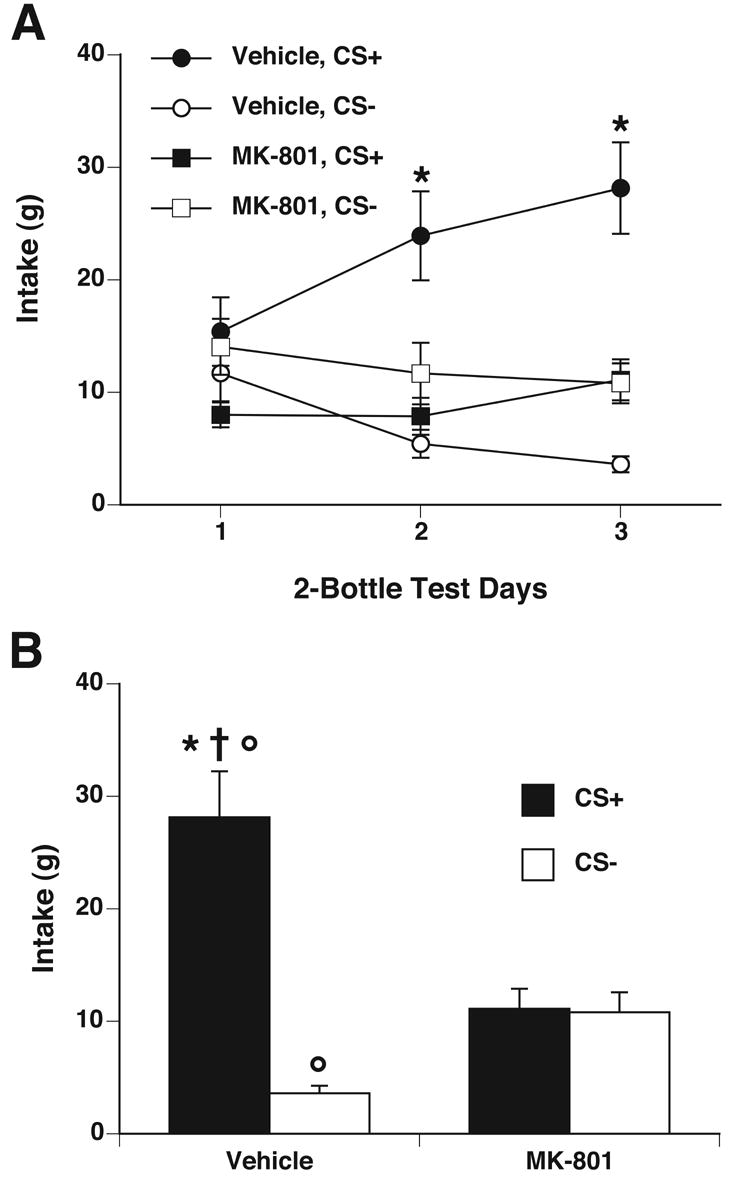
Systemic injections of MK-801 during conditioning blocked acquisition of CFTP. After every four conditioning days, CFTP expression was measured as intake during a 2-h, 2-bottle preference test. Rats received a saline injection prior to preference testing. A. Beginning with the second preference test after 8 conditioning days, rats treated with vehicle during conditioning (circles) consumed significantly more CS+/S (black symbols) than CS−/S (white symbols). Intake of CS+/S and CS−/S by the rats treated with MK-801 during conditioning (squares) were not different in any preference test. * p < 0.05 vs. own CS−. B. On the third preference test after 12 conditioning days, vehicle-treated rats consumed significantly more CS+/S (black bars) and significantly less CS−/S (white bars) than MK-801-treated rats. * p < 0.05 vs. own CS−; † p < 0.05 vs. MK-801 CS+; ° p < 0.05 vs. Mk-801 CS−.
When CS+/S and CS−/S intakes were compared between the MK-801 and vehicle groups on the final 2-bottle test day (see Figure 2B), there was a significant interaction of group and solution (F[1,28]= 25.02, p < 0.05). Post hoc comparisons showed that CS+/S intake was greater than CS−/S intake the vehicle group but not the MK-801 group; furthermore, the intake of CS+/S by the vehicle group was greater than CS+/S intake by the MK-801 group, and CS−/S intake by the vehicle group was less than CS−/S intake by the MK-801 group.
3.2 Experiment 2: MK-801 and CFTP expression
The effect of MK-801 on CFTP expression was tested on the rats from experiment 1. MK-801 injected 30 min prior to a 2-bottle preference test did not alter CS+/S or CS+/S intake in either MK-801 or vehicle groups (see Figure 3). A two-way ANOVA with drug pretreatment and solution as factors showed no effect of drug pretreatment, but a significant effect of solution (F[1,28] = 28.18, p<0.001). Whether treated with MK-801 or vehicle immediately prior to the 2-bottle preference tests, there was no significant difference between CS+/S and CS−/S intakes in the MK-801 group. Conversely, the vehicle group drank significantly more CS+/S than CS−/S, regardless of pretreatment with MK-801 or vehicle immediately prior to the 2-bottle preference tests.
Figure 3.
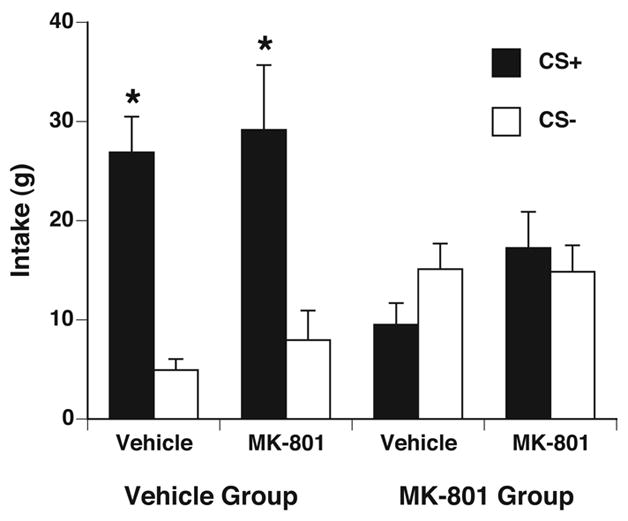
Effects on intake of the acute administration of MK-801 in previously conditioned rats. Acute MK-801 did not affect the CFTP expression in a 2-h, 2-bottle preference test in rats treated with vehicle during conditioning (left side): CS+/S intake (black bars) was significantly greater than CS−/S intake (white bars) regardless of pretreatment. Furthermore, acute MK801 prior to the preference test did not reveal any state-dependent effects in rats treated with MK-801 during conditioning (right side): no difference was seen between CS+/S and CS−/S intake regardless of pretreatment. * p < 0.05 vs. own CS−.
Thus CFTP learning in the MK-801 group was not dependent on MK-801 injection prior to expression testing (i.e. was not state-dependent learning). Furthermore, MK-801 had no effect on expression of a previously acquired CFTP in drug-naive rats, nor did it significantly affect overall intake. These results suggest a specific effect on CFTP acquisition of this dose of MK-801 (100 μg/kg); because only a single dose of MK801 was tested, however, we cannot completely rule out a role for NMDA receptors in intake or CFTP expression.
3.3 Experiment 3: MK-801 and CTA
Rats from Experiments 1 and 2 were given access to Kool-Aid/saccharin vs. water in 24-h, 2-bottle preference tests across 4 days ( 2 days of CS+/S vs. water and 2 days of CS−/S vs. water.) Kool-Aid/saccharin intake was higher than distilled water intake for both grape and cherry flavors in both the MK-801 group and vehicle group (see Figure 4). Two-way ANOVAs on Kool-Aid-saccharin preferences and intakes across the four 24-h, 2-bottle preference test days using group and solution (CS+/S and CS−/S) as factors showed no significant effects. (F[1,27] = 2.35, p = 0.13 for preference, F[1,27] = 2.55, p =0.12 for intake). Although the vehicle group showed a CFTP for CS+/S when the CS+/S and CS−/S were presented simultaneously in Experiments 1 and 2, both Kool-Aid/saccharin solutions were highly preferred to water when presented individually.
Figure 4.
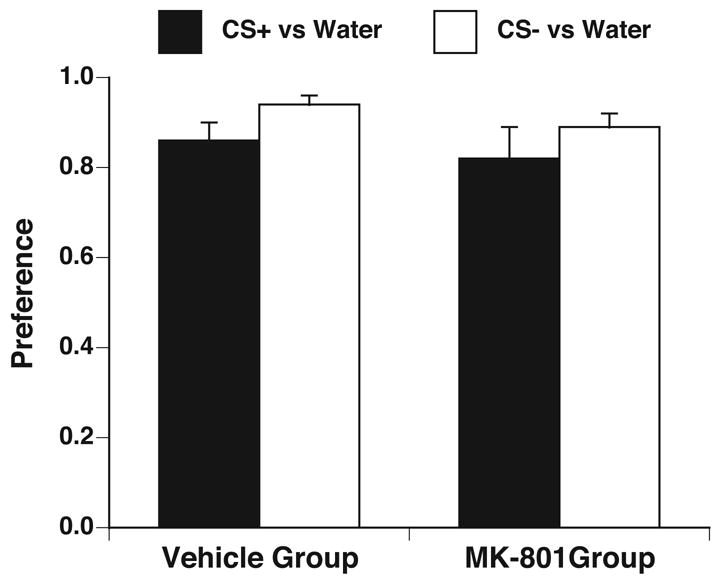
Systemic injections of MK-801 during conditioning did not induce a CTA against the CS+ and CS− flavors. In 48-h, 2-bottle preference tests of Kool-Aid/saccharin solutions vs. water, all rats treated with either vehicle or MK-801 during conditioning showed significantly higher intake of both CS+/S (black bars) and CS−/S (white bars) compared to water (hatched bars). There was no difference in the preference for the CS+ and CS-solutions. * p < 0.05 vs. water.
Thus, MK-801-treated rats did not show a CTA to the Kool-Aid flavors. Therefore the absence of a CFTP for CS+/S in the MK-801 group cannot be ascribed to an opposing CTA induced by the drug treatment.
3.4 Experiment 4: DCS and CFTP acquisition
To determine if the glycine-binding site of the NR contributes to CFTP learning, food-restricted rats were administered vehicle (0.15 M NaCl, 1 ml/kg i.p., n = 11) or DCS (15 mg/kg, i.p., n = 11) 60 minutes before a daily 2-h conditioning session with either CS+/F or CS−/S as in experiment 1. Across the 16 conditioning days, there was a significant increase in both CS+/F intake [main effect of days, F(7,147) = 14.27, p <0.001] and CS−/S intake [main effect of days, F(7,147) = 25.99, p <0.001]. There was no significant effect of drug treatment on either CS+/F or CS−/S intake during conditioning (see Figure 5).
Figure 5.
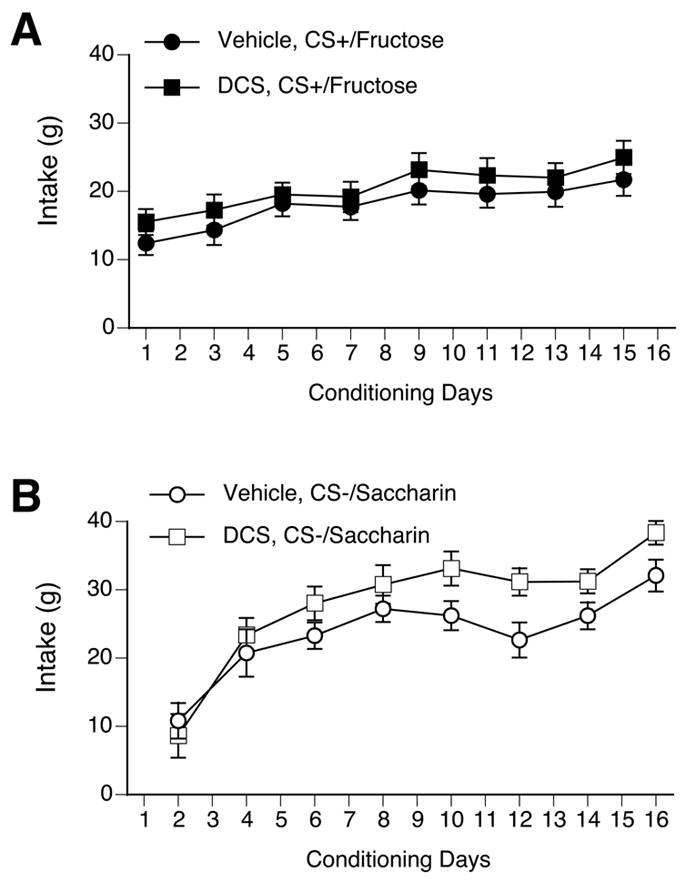
Systemic injections of DCS did not effect CS intake during conditioning. Intake was measured after a 2-h, 1-bottle presentation. A. There was no difference in CS+/F intake during conditioning between rats treated with DCS (black squares) and vehicle-treated rats (black circles). In both groups there was an increase in CS+/F over conditioning days, such that intake was significantly higher than day 1 intake on days 5–15 for vehicle-treated rats and days 9–15 for DCS-treated rats. B. There was no difference in CS−/S intake during conditioning between DCS-treated rats (white squares) and vehicle-treated rats (white circles). In both groups CS−/S intake increased such that intake was significantly higher on days 2–16 than on day 1.
DCS pretreatment during conditioning enhanced CFTP acquisition compared to vehicle-treated rats (see Figure 6). Both DCS and vehicle groups acquired a CFTP after 8–12 conditioning sessions as shown by increased CS+ intake in 2-bottle test sessions (see Figure 6A). Two-way ANOVAs on CS+ and CS− intake across the four 2-bottle test days during conditioning revealed a significant interaction of CS+/CS− treatment and days for both groups (F[3,63] = 23.8, p < 0.0001 for DCS group, F[3,63]=9.3, p < 0.0001 for vehicle group). Post hoc tests revealed that CS+ intake was higher than CS− intake for the DCS group on the second 2-bottle test (after 8 conditioning sessions) and for the vehicle group on the third 2-bottle test (after 12 conditioning sessions). This suggests that DCS-treated rats acquired the CFTP faster than the vehicle-treated rats.
Figure 6.
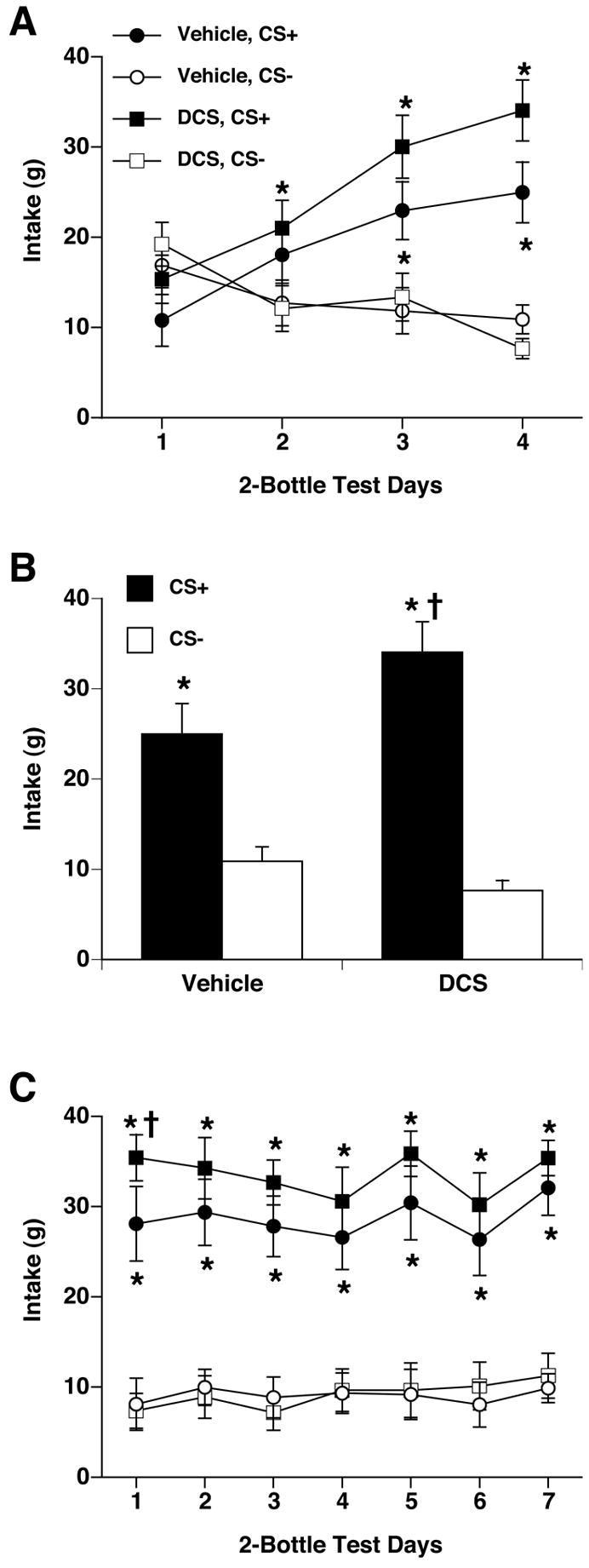
Systemic injections of DCS during conditioning enhanced CFTP learning in rats. A. Rats treated with vehicle during conditioning showed significantly greater intake of CS+/S (black circles) than CS−/S (white circles) on the third preference test (after 12 conditioning days). Rats treated with DCS during conditioning showed significantly greater intake of CS+/S (black squares) than CS−/S (white squares) on the second preference test (after 8 conditioning days). Thus the DCS-treated rats learned the CFTP faster than vehicle-treated rats. B. On the fourth preference test after 16 conditioning days, all rats consumed significantly more CS+/S (black bars) than CS−/S (white bars); DCS-treated rats consumed significantly more CS+/S than vehicle-treated rats. C. Across seven consecutive daily 2-h, 2-bottle preference tests of CS+/S vs. CS−/S, all rats showed little or no sign of extinction. On the first test day, CS+/S intake was significantly greater in DCS-treated rats (black squares) than vehicle-treated rats (black circles). In both groups, CS+/S intake (black symbols) was significantly greater than CS−/S intake (white symbols) on all days. * p < 0.05 vs. own CS-; †p < 0.05 vs. vehicle/CS+.
When CS+/S and CS−/S intakes were compared between the DCS and vehicle groups on the final 2-bottle test day (see Figure 6B), there was a significant interaction of group and solution (F[1,40]= 5.62, p < 0.05). Post hoc comparisons showed that CS+/S intake was greater than CS−/S intake in both groups, but the intake of CS+/S by the DCS group was greater than CS+/S intake by the vehicle group.
During unreinforced extinction testing with CS+/S and CS−/S in 2-bottle preference tests (2 h/day), both DCS-treated and vehicle-treated rats showed a higher intake of CS+ than CS− (See Figure 6C). Two-way ANOVAs on CS+ and CS− intake across the seven 2-bottle test days during extinction testing revealed a significant effect of solution but not days within each group (F[1,6]=62.15, p < 0.0001 for DCS group, F[1,6] = 23.32, p < 0.0001 for vehicle group), such that D-treated and vehicle-treated rats consumed more CS+ than CS− on all days with no apparent extinction. Thus a CFTP does not extinguish rapidly, if at all. A two-way ANOVA comparing CS+ intakes of DCS and vehicle groups across the seven 2-bottle test days revealed a significant effect of days (F[6,126] = 3.6, p < 0.002) but not groups, such that DCS-treated rats consumed more CS+ than vehicle-treated rats only in the first 2-bottle preference test. Thus there was a transient enhancement of CFTP by DCS that persisted briefly into extinction.
3.5 Experiment 5: DCS and CFTP reversal conditioning
CFTP learning is very resistant to extinction. Therefore, the effects of DCS on reversal conditioning, rather than extinction, were examined. Rats (n=18) were conditioned with CS+/F and CS+/S in daily 2-h conditioning sessions over 16 days as above.
Both DCS and vehicle groups acquired a CFTP after 16 conditioning sessions as shown by higher CS+ intake than CS− intake for both groups in a 2-bottle preference test prior to reverse conditioning. (t-test; p<0.001 for DCS, p<0.001 for vehicle; see Figure 7, test day 0).
Figure 7.
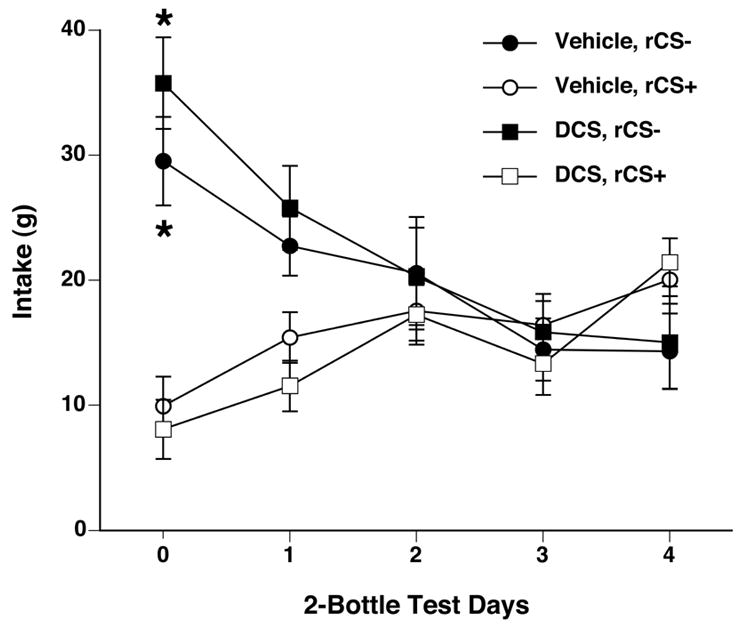
Systemic administration of DCS did not potentiate reversal learning in rats. After acquiring a CFTP (as shown on test day 0), rats were reverse conditioned with alternate daily presentations of the previous CS+ flavor now mixed with saccharin (rCS−, black symbols) and the previous CS− now mixed with fructose (rCS+, white symbols). Preferences were assessed after every 4 reverse conditioning days (test days 1 – 4). In both groups, preference for the rCS−/S decreased while preference for the rCS+/S increased during reverse conditioning. Pretreatment during reverse conditioning with either vehicle (circles) or DCS (squares) had no effect on the magnitude or time course of reversal learning. * p < 0.05 vs. own rCS+.
Reversal conditioning abolished the CFTP in both DCS and vehicle groups after 16 conditioning sessions as shown by decreased CS+ and increased rCS+ intake in 2-bottle preference tests (see Figure 7, test days 1–4). Two-way ANOVAs on rCS+ and rCS− intake across the 2-bottle preference test days revealed a significant interaction of rCS+/rCS− treatment and days for both groups (F[4,64]=6.29, p < 0.0005 for DCS group, F[4,64] = 9.14, p < 0.0001 for vehicle group). Pretreatment with DCS prior to reversal conditioning did not reverse the CFTP any faster than the vehicle pretreatment. A two-way ANOVA comparing rCS− intakes in DCS and vehicle-treated groups across the five 2-bottle preference test days revealed a significant effect of test day (F[4,64] = 7.58, p<0.0001) but not drug pretreatment. Thus DCS did not potentiate reversal learning.
4. Discussion
This study examined the role of NR in CFTP learning using systemic injections of MK-801, a noncompetitive NR antagonist, and DCS, a NR glycine-site agonist. MK-801 or DCS was administered prior to 1-bottle conditioning trials with Kool-Aid flavors in solution with either highly preferred fructose (CS+/F) or the less preferred saccharin (CS−/S). MK-801 blocked CFTP acquisition, while DCS accelerated CFTP acquisition. Thus, our results show that NR is necessary for CFTP learning and that the NR glycine-site contributes to CFTP learning.
As expected, rats reliably developed a preference for a flavor paired with 8% fructose over a flavor paired with 0.2% sodium saccharin (Baker et al., 2003; Baker et al., 2004; Sclafani and Ackroff, 1994). Although fructose has osmotic and caloric properties in addition to a preferred taste, the primary reinforcing effect of fructose is attributable to taste, with little or no postingestive contribution (Sclafani and Ackroff, 1994; Sclafani et al., 1993; Sclafani et al., 1999). Thus, gastric infusion of fructose is a weak US for conditioning a flavor preference (Ackroff et al., 2001; Sclafani et al., 1993; Sclafani et al., 1999), while sham-feeding of flavors in sweet solutions is sufficient to form flavor preferences (Yu et al., 1999; Yu et al., 2000a, b).
When administered 30 min prior to conditioning trials, systemic injections of MK-801 blocked the acquisition of a CFTP. Thus NR neurotransmission is necessary for CFTP learning. Follow-up experiments ruled out several alternate explanations for these findings: MK-801 pretreatment did not induce a CTA; MK801 did not have an acute effect on ingestion that might have compromised CFTP learning; and no evidence was found for state-dependent learning.
It has been shown that lower doses of MK-801 (50 – 100 μg/kg ip) do not induce CTA, although a higher dose of MK-801 (200 μg/kg ip) paired with a novel taste can cause CTA acquisition (Bienkowski et al., 1998). The failure of MK-801-treated rats to increase CS+ intake during conditioning, therefore, might have been due to CTA acquisition rather than CFTP blockade. In order to verify that MK-801 did not reduce CS+/S intake by inducing a CTA in the MK-801-treated rats, the conditioned rats were given 23-h, 2-bottle tests of water vs. CS+/S or CS−/S for 4 consecutive days (see Figure 4). Both MK-801- and vehicle-treated rats showed a 90% or higher preference for the Kool-Aid flavor mixed with saccharin over water. Thus the reduced CS+/S intake seen in the MK-801 group was not due to the acquisition of a CTA.
MK-801 can also have acute behavioral effects that might have altered intake during conditioning days, and thus confounded CFTP learning. For example, MK-801 can induce ataxia and other locomotor effects that might reduce intake (Frantz and Van Hartesveldt, 1999). Conversely, MK-801 has been shown to increase the intake of highly palatable foods in fed rats and regular chow in fasted rats (Burns and Ritter, 1997) or at dark onset (Jahng and Houpt, 2001). After MK-801 pretreatment, however, we did not observe any ataxia, and all rats showed a very short latency to start drinking (< 1 min). Furthermore, the volume of CS+/F and CS−/S intakes during CFTP conditioning did not differ between MK-801- and vehicle-treated rats (data not shown).
The effects of MK-801 were specific to acquisition, because MK-801 pretreatment of rats with a previously acquired CFTP did not alter CFTP expression during 2-bottle preference tests (see Figure 3). Thus, while the NR is necessary for CFTP acquisition, it is not necessary for CFTP expression.
The same data indicate that MK-801 had no state-dependent effects on CFTP learning. MK-801 has been shown to support state-dependent learning in other models. For example, rats treated with MK-801 (100 μg/kg ip) during place-footshock aversion conditioning appeared unable to express a place aversion when tested drug-free (Harrod et al., 2001). When tested immediately after MK-801 injection, however, the rats did express a place aversion. Our results contrast with the place aversion results: rats treated with MK-801 prior to CFTP acquisition did not express a CFTP during 2-bottle preference tests with or without MK-801 injection before the expression test.
The effect of MK-801 on CFTP learning are consistent with other reports that NR blockade impairs or inhibits olfactory learning in several paradigms. Systemic injections of MK-801 have impaired olfactory-water reward discrimination learning and its reversal (Griesbach et al. 1998), and olfactory-tactile preference learning in neonatal rats (Weldon et al., 1997). In addition, central administration of the competitive NR antagonist APV has identified critical sites for olfactory associations in different paradigms. APV infused into the prefrontal cortex (but not hippocampus) impaired memory of an odor-food reward association when infused in the prefrontal cortex (Tronel and Sara, 2003). APV infused into the basolateral amygdala disrupted learning in taste-potentiated odor-LiCl aversion (Ferry and Di Scala, 2000; Hatfield and Gallagher, 1995) and in odor-footshock fear conditioning (Walker et al., 2005).
In a second approach to exploring the role of NR, we found that systemic DCS enhanced CFTP learning. Our findings are in agreement with other studies in which DCS accelerated the rate of learning. For example, DCS has been shown in rats to enhance acquisition of spatial water-maze learning (Riekkinen and Riekkinen, 1997), inhibitory avoidance (Land and Riccio, 1999), and the extinction of fear-conditioning (Parnas et al., 2005) or fear-potentiated startle response (Walker et al., 2002). Relevant to gustatory learning, we have recently demonstrated that DCS potentiated acquisition of CTA (Houpt et al., 2005). DCS is also effective in the extinction of fear in phobic human subjects undergoing behavior modification (Ressler et al., 2004): phobic patients treated with DCS in combination with exposure therapy were shown to have greatly reduced symptoms of acrophobia that persisted for at least 3 months after treatment.
In this study, DCS−treated rats acquired a CFTP faster than the vehicle-treated rats. DCS also increased the magnitude of CFTP learning. The enhancement by DCS persisted transiently into extinction. Because the half-life of DCS is approximately 2 hours in rats (Baran et al., 1995), it is unlikely that the transient enhancement was due to any residual drug effect.
These results suggest that the NR glycine-binding site contributes to CFTP learning. Because exogenous DCS augments CFTP learning, endogenous glycine or D-serine may be a limiting factor in NR activation.
Unlike its enhancement of extinction in other models of learning (Ledgerwood et al., 2005; Richardson et al., 2004), DCS did not potentiate reversal learning in CFTP when the flavors paired with fructose and saccharin were reversed (i.e., the old CS+ was now paired with S and the old CS− was now paired with F). In both DCS− and vehicle-treated rats, intake of the previously established CS+ decreased and intake of the reversed CS+ increased in 2-bottle preference tests. There was no difference in intakes between the DCS and vehicle groups. It is important to note, however, that CFTP learning is very resistant to extinction even when the CS+/F contingency is removed (Sclafani and Ackroff, 1994). Therefore, the increase in reversed CS+ intake may reflect an increased preference for the reversed CS+ without any change in the rat’s evaluation of the old CS+; eventually the flavors become isopalatable again. Thus, while the rats underwent reversal conditioning, it is not clear that reversal learning per se occurred. More detailed microstructural comparisons would be required to establish this.
We hypothesize that NMDA receptors are involved specifically in the associative processes underlying CFTP acquisition. Conceptually, CFTP learning is mediated by gustatory, olfactory, preference (reward), and associative processes. Although NR is abundant in olfactory and gustatory pathways, NR blockade does not appear to compromise olfactory processing of the CS flavor cues as measured behaviorally. For example, conditioned rats were able to express a CFTP in a 2-bottle preference test after MK-801 pretreatment, showing that they could discriminate the CS+/S flavor from the CS−/S flavor. These findings parallel the observation that intraamygdalar infusion of the NR antagonist APV did not attenuate expression of odor- and taste-guided aversions in rats with a previously acquired taste-potentiated odor aversion (Hatfield and Gallagher, 1995).
NR might be involved in reward processing during CFTP acquisition, but there is strong evidence that dopaminergic pathways are active during CFTP learning. The reward basis for preference and CFTP learning has been probed pharmacologically (Baker et al., 2003; Baker et al., 2004; Yu et al., 1999; Yu et al., 2000a, b). In real-feeding rats, both the dopamine D1 receptor antagonist SCH23390 and dopamine D2 receptor antagonist raclopride blocked the acquisition and attenuated the expression of CFTP conditioned with fructose-paired flavors (Baker et al., 2003). Thus, DA antagonists not only blocked acquisition of a CFTP, but also reduced intake of the preferred US and blocked the expression of a previously acquired CFTP. Therefore, DA neurotransmission is apparently related to the unconditioned rewarding properties of the gustatory US and the acquisition of conditioned rewarding properties by the flavor CS+. Endogenous opioids do not play a critical role. In the identical paradigm, the general opioid antagonist, naltrexone, reduced the intake of sweet solutions, but did not significantly attenuate the acquisition and expression of CFTP in either sham-feeding or real-feeding rats (Baker et al., 2004; Yu et al., 1999).
In conclusion, the dopamine D1 and D2 receptors (Baker et al., 2003; Baker et al., 2004; Yu et al., 1999; Yu et al., 2000a, b) and NR are necessary for the acquisition of CFTP. While dopamine D1 and D2 receptors are necessary for the expression of CFTP (Baker et al., 2003), NR is not. Furthermore, the glycine binding site of the NR may contribute to activation during CFTP acquisition. Thus, the NR may specifically mediate the associative processes that link the flavor of the CS+ with the rewarding properties of the gustatory US, allowing the CS+ to acquire rewarding properties and become preferred in expression tests.
Acknowledgments
Supported by National Institute on Deafness and Other Communication Disorders grants DC03198 (TAH) and T32DC00044 (GJG) and an FSU Neuroscience Graduate Fellowship (GJG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackroff K, Touzani K, Peets TK, Sclafani A. Flavor preferences conditioned by intragastric fructose and glucose: differences in reinforcement potency. Physiol Behav. 2001;72:691–703. doi: 10.1016/s0031-9384(01)00442-5. [DOI] [PubMed] [Google Scholar]
- Andersen JM, Lindberg V, Myhrer T. Effects of scopolamine and D-cycloserine on non-spatial reference memory in rats. Behav Brain Res. 2002;129:211–216. doi: 10.1016/s0166-4328(01)00318-7. [DOI] [PubMed] [Google Scholar]
- Baker RM, Shah MJ, Sclafani A, Bodnar RJ. Dopamine D1 and D2 antagonists reduce the acquisition and expression of flavor-preferences conditioned by fructose in rats. Pharmacol Biochem Behav. 2003;75:55–65. doi: 10.1016/s0091-3057(03)00039-x. [DOI] [PubMed] [Google Scholar]
- Baker RW, Li Y, Lee MG, Sclafani A, Bodnar RJ. Naltrexone does not prevent acquisition or expression of flavor preferences conditioned by fructose in rats. Pharmacol Biochem Behav. 2004;78:239–246. doi: 10.1016/j.pbb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Baran H, Gramer M, Loscher W. Alterations in plasma and brain amino acids after administration of the glycine/NMDA receptor partial agonist, D-cycloserine, to mice and rats. Eur J Pharmacol. 1995;273:197–201. doi: 10.1016/0014-2999(94)00745-s. [DOI] [PubMed] [Google Scholar]
- Barkai E, Saar D. Cellular correlates of olfactory learning in the rat piriform cortex. Rev Neurosci. 2001;12:111–120. doi: 10.1515/revneuro.2001.12.2.111. [DOI] [PubMed] [Google Scholar]
- Bienkowski P, Koros E, Piasecki J, Kostowski W. Prior exposure to MK-801 sensitizes rats to ethanol-induced conditioned taste aversion. Alcohol Alcohol. 1998;33:116–120. doi: 10.1093/oxfordjournals.alcalc.a008366. [DOI] [PubMed] [Google Scholar]
- Burns GA, Ritter RC. The non-competitive NMDA antagonist MK-801 increases food intake in rats. Pharmacol Biochem Behav. 1997;56:145–149. doi: 10.1016/S0091-3057(96)00171-2. [DOI] [PubMed] [Google Scholar]
- Cain DP. LTP, NMDA, genes and learning. Curr Opin Neurobiol. 1997;7:235–242. doi: 10.1016/s0959-4388(97)80012-8. [DOI] [PubMed] [Google Scholar]
- Ferry B, Di Scala G. Basolateral amygdala NMDA receptors are selectively involved in the acquisition of taste-potentiated odor aversion in the rat. Behav Neurosci. 2000;114:1005–1010. [PubMed] [Google Scholar]
- Frantz K, Van Hartesveldt C. Locomotion elicited by MK801 in developing and adult rats: temporal, environmental, and gender effects. Eur J Pharmacol. 1999;369:145–157. doi: 10.1016/s0014-2999(99)00070-9. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, Hu D, Amsel A. Effects of MK-801 on vicarious trial-and-error and reversal of olfactory discrimination learning in weanling rats. Behav Brain Res. 1998;97:29–38. doi: 10.1016/s0166-4328(98)00015-1. [DOI] [PubMed] [Google Scholar]
- Harrod SB, Flint RW, Riccio DC. MK-801 induced retrieval, but not acquisition, deficits for passive avoidance conditioning. Pharmacol Biochem Behav. 2001;69:585–593. doi: 10.1016/s0091-3057(01)00565-2. [DOI] [PubMed] [Google Scholar]
- Hatfield T, Gallagher M. Taste-potentiated odor conditioning: impairment produced by infusion of an N-methyl-D-aspartate antagonist into basolateral amygdala. Behav Neurosci. 1995;109:663–668. doi: 10.1037//0735-7044.109.4.663. [DOI] [PubMed] [Google Scholar]
- Houpt TA, Davenport RA, Golden GJ, Lockwood DR. Learning & Memory. Cold Spring Harbor, New York: CSH Press; 2005. D-Cycloserine potentiates short-delay but not long-delay conditioned taste aversion learning: Implications for NMDA receptor-taste interactions; p. 126. [Google Scholar]
- Jahng JW, Houpt TA. MK801 increases feeding and decreases drinking in nondeprived, freely feeding rats. Pharmacol Biochem Behav. 2001;68:181–186. doi: 10.1016/s0091-3057(00)00434-2. [DOI] [PubMed] [Google Scholar]
- Jimenez B, Tapia R. Biochemical modulation of NMDA receptors: role in conditioned taste aversion. Neurochem Res. 2004;29:161–168. doi: 10.1023/b:nere.0000010445.27905.aa. [DOI] [PubMed] [Google Scholar]
- Kim PM, Aizawa H, Kim PS, Huang AS, Wickramasinghe SR, Kashani AH, Barrow RK, Huganir RL, Ghosh A, Snyder SH. Serine racemase: activation by glutamate neurotransmission via glutamate receptor interacting protein and mediation of neuronal migration. Proc Natl Acad Sci U S A. 2005;102:2105–2110. doi: 10.1073/pnas.0409723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land C, Riccio DC. d-Cycloserine: effects on long-term retention of a conditioned response and on memory for contextual attributes. Neurobiol Learn Mem. 1999;72:158–168. doi: 10.1006/nlme.1998.3897. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. d-cycloserine facilitates extinction of learned fear: Effects on reacquisition and generalized extinction. Biol Psychiatry. 2005;57:841–847. doi: 10.1016/j.biopsych.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Myers KP, Sclafani A. Conditioned enhancement of flavor evaluation reinforced by intragastric glucose: I. Intake acceptance and preference analysis. Physiol Behav. 2001;74:481–493. doi: 10.1016/s0031-9384(01)00595-9. [DOI] [PubMed] [Google Scholar]
- Parnas AS, Weber M, Richardson R. Effects of multiple exposures to d-cycloserine on extinction of conditioned fear in rats. Neurobiol Learn Mem. 2005;83:224–231. doi: 10.1016/j.nlm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Quinlan EM, Lebel D, Brosh I, Barkai E. A molecular mechanism for stabilization of learning-induced synaptic modifications. Neuron. 2004;41:185–192. doi: 10.1016/s0896-6273(03)00874-2. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- Richardson R, Ledgerwood L, Cranney J. Facilitation of fear extinction by D-cycloserine: theoretical and clinical implications. Learn Mem. 2004;11:510–516. doi: 10.1101/lm.78204. [DOI] [PubMed] [Google Scholar]
- Riekkinen M, Riekkinen P., Jr Nicotine and D-cycloserine enhance acquisition of water maze spatial navigation in aged rats. Neuroreport. 1997;8:699–703. doi: 10.1097/00001756-199702100-00024. [DOI] [PubMed] [Google Scholar]
- Rosenblum K, Berman DE, Hazvi S, Lamprecht R, Dudai Y. NMDA receptor and the tyrosine phosphorylation of its 2B subunit in taste learning in the rat insular cortex. J Neurosci. 1997;17:5129–5135. doi: 10.1523/JNEUROSCI.17-13-05129.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Ackroff K. Glucose- and fructose-conditioned flavor preferences in rats: taste versus postingestive conditioning. Physiol Behav. 1994;56:399–405. doi: 10.1016/0031-9384(94)90213-5. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Cardieri C, Tucker K, Blusk D, Ackroff K. Intragastric glucose but not fructose conditions robust flavor preferences in rats. Am J Physiol. 1993;265:R320–325. doi: 10.1152/ajpregu.1993.265.2.R320. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Fanizza LJ, Azzara AV. Conditioned flavor avoidance, preference, and indifference produced by intragastric infusions of galactose, glucose, and fructose in rats. Physiol Behav. 1999;67:227–234. doi: 10.1016/s0031-9384(99)00053-0. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Tomitaka M, Bernaudin M, Tomitaka S. Psychosis: pathological activation of limbic thalamocortical circuits by psychomimetics and schizophrenia? Trends Neurosci. 2001;24:330–334. doi: 10.1016/s0166-2236(00)01817-8. [DOI] [PubMed] [Google Scholar]
- Stuchlik A, Rezacova L, Vales K, Bubenikova V, Kubik S. Application of a novel Active Allothetic Place Avoidance task (AAPA) in testing a pharmacological model of psychosis in rats: comparison with the Morris Water Maze. Neurosci Lett. 2004;366:162–166. doi: 10.1016/j.neulet.2004.05.037. [DOI] [PubMed] [Google Scholar]
- Tronel S, Sara SJ. Blockade of NMDA receptors in prelimbic cortex induces an enduring amnesia for odor-reward associative learning. J Neurosci. 2003;23:5472–5476. doi: 10.1523/JNEUROSCI.23-13-05472.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Paschall GY, Davis M. Glutamate receptor antagonist infusions into the basolateral and medial amygdala reveal differential contributions to olfactory vs. context fear conditioning and expression. Learn Mem. 2005;12:120–129. doi: 10.1101/lm.87105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weldon DA, Fedorcik GG, LoRusso CM, Tiburzi MJ, Lenoci JM. Olfactory conditioning impairment following posttraining NMDA receptor blockade in neonatal rats. Neurobiol Learn Mem. 1997;67:34–42. doi: 10.1006/nlme.1996.3744. [DOI] [PubMed] [Google Scholar]
- Willner J, Gallagher M, Graham PW, Crooks GB., Jr N-methyl-D-aspartate antagonist D-APV selectively disrupts taste-potentiated odor aversion learning. Behav Neurosci. 1992;106:315–323. doi: 10.1037//0735-7044.106.2.315. [DOI] [PubMed] [Google Scholar]
- Yu WZ, Sclafani A, Delamater AR, Bodnar RJ. Pharmacology of flavor preference conditioning in sham-feeding rats: effects of naltrexone. Pharmacol Biochem Behav. 1999;64:573–584. doi: 10.1016/s0091-3057(99)00124-0. [DOI] [PubMed] [Google Scholar]
- Yu WZ, Silva RM, Sclafani A, Delamater AR, Bodnar RJ. Pharmacology of flavor preference conditioning in sham-feeding rats: effects of dopamine receptor antagonists. Pharmacol Biochem Behav. 2000a;65:635–647. doi: 10.1016/s0091-3057(99)00239-7. [DOI] [PubMed] [Google Scholar]
- Yu WZ, Silva RM, Sclafani A, Delamater AR, Bodnar RJ. Role of D(1) and D(2) dopamine receptors in the acquisition and expression of flavor-preference conditioning in sham-feeding rats. Pharmacol Biochem Behav. 2000b;67:537–544. doi: 10.1016/s0091-3057(00)00396-8. [DOI] [PubMed] [Google Scholar]


