Abstract
Classical Menkes disease is an X-linked recessive neurodegenerative disorder caused by mutations in a P-type ATPase (ATP7A) that normally delivers copper to the developing central nervous system. Infants with large deletions, or other mutations in ATP7A that incapacitate copper transport to the brain, show poor clinical outcomes and subnormal brain copper despite early subcutaneous copper histidine (CuHis) injections. These findings suggest a need for direct central nervous system approaches in such patients. To begin to evaluate an aggressive but potentially useful new strategy for metabolic improvement of this disorder, we studied the acute and chronic effects of CuHis administered by intracerebroventricular (ICV) injection in healthy adult rats. Magnetic resonance imaging (MRI) after ICV CuHis showed diffuse T1-signal enhancement, indicating wide brain distribution of copper after ICV administration, and implying the utility of this paramagnetic metal as a MRI contrast agent. The maximum tolerated dose (MTD) of CuHis, defined as the highest dose that did not induce overt toxicity, growth retardation, or reduce lifespan, was 0.5 mcg. Animals receiving multiple infusions of this MTD showed increased brain copper concentrations, but no significant differences in activity, behavior, and somatic growth, or brain histology compared to saline-injected controls. Based on estimates of the brain copper deficit in Menkes disease patients, CuHis doses 10-fold lower than the MTD found in this study may restore proper brain copper concentration. Our results suggest that ICV CuHis administration have potential as a novel treatment approach in Menkes disease infants with severe mutations. Future trials of direct CNS copper administration in mouse models of Menkes disease will be informative.
Keywords: Copper histidine, Intracerebral administration, Maximum tolerated dose, Menkes disease, Copper transport
Introduction
Menkes disease (MD) is an X-linked recessive disorder of copper transport caused by defects in a gene that encodes an evolutionarily conserved copper-transporting ATPase [1-3]. In mammals, this gene product functions as an intracellular pump to transport copper into the trans-Golgi network for incorporation by copper-dependent enzymes and also mediates copper exodus from cells [4]. The disorder presents in infancy with delayed development, failure to thrive, neurodegeneration, and premature death (typically by 3 years of age) [5].
Daily subcutaneous injections of copper histidine (CuHis) can significantly modify this dismal natural history when commenced in early infancy and in the context of certain ATP7A mutations that allow partial copper transport to the developing brain [6-8]. However, the blood-brain barrier poses a challenging obstacle in MD patients whose mutations do not permit any residual copper transport [9,10]. Consequently, we are exploring alternative therapeutic approaches—including intracerebroventricular (ICV) CuHis administration—to bypass the blood-brain barrier.
There are several important caveats related to the potential efficacy of this approach. First, copper uptake by neuronal cells via high affinity copper uptake genes (e.g., hCTR1) and expression of copper chaperones (e.g., Cox17 and CCS1) in human neuronal cells are necessary, both of which have strong experimental support [11-14]. A second requirement is that the distribution and cerebrospinal fluid clearance of copper administered by ICV injection be adequate for generalized neuronal uptake. ICV injection of other trace metals, e.g., zinc chloride (65ZnCl2), zinc histidine (65Zn-His) and manganese chloride (MnCl2), results in wide distribution and sustained presence within brain as documented by autoradiography or magnetic resonance imaging [15-19]. These latter results augur well for neuronal delivery of ICV CuHis. As small molecules, (e.g., atomic mass 55 Da for manganese, 63.5 Da for copper, and 65.4 Da for zinc), the distribution and brain penetration of trace metal ions, especially paramagnetic ions such as Mn and Cu administered by ICV injection, are superior compared to larger drug molecules [20].
To begin to evaluate the safety of this aggressive treatment approach and to determine a maximum tolerated dose, we performed acute and chronic toxicology assessments of ICV CuHis in adult male rats.
Materials and methods
The study was approved by the Animal Care and Use Committee of the National Institute of Child Health and Human Development.
ICV catheter placement
Thirty-six Sprague-Dawley (Taconic, Germantown, NY) rats were used. Rats were anesthetized by intraperitoneal injection of 50 mg/kg pentobarbital (Abbott Laboratories, Chicago, IL) and placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA) with the incisor bar 3.1 mm below the interaural line. The skin was incised and a 0.9 mm diameter hole was drilled through the skull for placement of a 33 g cannula (Plastics One, Roanoke, VA) into the left lateral ventricle of the brain via stereotaxic guidance. Stereotaxic coordinates were 1.6 mm left lateral and 0.8 mm posterior to bregma, and catheter insertion from the skull surface was to a depth of 3.2 mm [21]. Aseptic technique was used throughout the procedure. A 26 Ga guide cannula was secured with acrylic dental cement and two bone screws. Between infusions, the internal cannula was replaced with a 0.2 mm diameter dummy cannula.
ICV infusions of CuHis and normal saline
CuHis (prepared by the National Institute of Health Pharmaceutical Development Service) was diluted in normal saline to deliver the desired dose in a volume of 20 μL. Controls received 20 μL of normal saline alone. Doses were administered at a rate of 2 μL per minute for 10 min. For animals receiving subsequent infusions, the protocol was repeated (without surgery) with each respective dose.
Brain MRI
Rodent brain MRI was performed as previously described [18,19]. Following injection of Cu contrast agent, or saline, the animals were transferred to a custom made holder for imaging and anesthesia was maintained with 1-3% isoflurane in a medical air/oxygen mix (60/40) via a mask. Body core temperature was monitored and maintained at 35 °C with a recirculating hot water pad. During scanning, the breath rate was monitored and the anesthetic gas adjusted to maintain a rate of 30-40 breaths per minute.
All MR Imaging was performed at 4.7T using a Bruker Avance MRI system (Bruker-Biospin, Billerica, MA). A 4 cm surface coil was placed over the head and the animal was positioned in the isocenter of the magnet. After initial setup and locator scans, a 3D data set was acquired with a gradient echo pulse sequence. The imaging parameters were: TR/TE D150/6ms, FOV=40 × 30× 30 mm, acquisition matrix = 256× 128 ×64, and the flip angle was Ernst-optimized. The images were reconstructed to a 256× 128× 128 matrix size.
CuHis dosing
To assess acute toxicologic effects, rats were administered single doses of either 100, 20, 10, 5, or 1 mcg CuHis and were euthanized after 6 h. To assess chronic effects, rats were administered single doses of 7.5 or 2.5 mcg CuHis, or two to four doses of 0.5 mcg CuHis at 1 week intervals and were euthanized within 1 or 2 weeks of the final infusion. In acute and chronic studies, an equivalent number of normal saline ICV infusions were administered to control rats.
Animal monitoring
Daily health checks were conducted for all animals to assess eating, drinking, as well as general activity and behavior. When indicated, animals with clinical evidence of untoward effects from the experimental treatment were euthanized immediately.
Histopathology
The animals were euthanized with CO2 and their brains harvested by the NIH Division of Veterinary Resources. After fixation in 10% buffered formalin, brains were sectioned, trimmed, and embedded in paraffin. Five micron sections were mounted on slides and stained with hematoxylin and eosin (Histoserv, Inc., Germantown, MD). Slides from animals receiving CuHis doses of 5 or 0.5 μg, and saline-treated controls were scored by one of the authors (LRB) in five categories of brain histopathology: spongiosis, ventricular dilation, cavitation, encephalitis, and meningitis. Scoring was from 0 to 4 using the following scale: 0=no change or within normal limits; 1=minimal; 2=mild; 3=moderate; and 4=marked.
Brain copper levels
Brain copper levels were determined from paraffin-embedded tissues. Paraffin surrounding the tissue was dissolved with n-hexane and the tissue dried overnight (at 50-55 °C). Brain and control (e.g., National Institute of Standards and Technology, Gaithersburg, MD; liver specimen SRM#1577b) tissues were microwave digested (CEM Corporation, North Carolina; model MARS-CEM at 600 W power) in 1 mL nitric acid (HNO3), 1 mL of 30% hydrogen peroxide (H2O2), and 1 mL distilled water. Digestion was performed using a closed vessel system at a controlled temperature and pressure using with the following program: 20 psi for 10 min; 40 psi for 10 min; 60 psi for 10 min; 90 psi for 10 min; and 120 psi for 30 min. Digested samples were brought to a final volume of 4 mL using distilled deionized water (Millipore Corporation System). The tissue extracts were then analyzed, employing an inductively coupled plasma optical emission spectrometer, ICP-OES, (Perkin-Elmer Corporation, New Jersey) instrument. To control for matrix interferences and changes in rate of ionization, we used an internal standard based on a 2 ppm gallium (Ga) solution. The instrument was calibrated with a five-point calibration curve including blank standard, 0.050, 0.2, 1, 2 and 5 ppm Cu standards. All standards, samples, blanks, and controls contained 2 ppm gallium. Samples were analyzed in duplicates and each measurement is the result of three replicates. The relative standard deviation (%RSD) between measurements was less than 0.2%. Samples with %RSD higher than 2% were re-digested and re-analyzed. Results are reported in micrograms (mcg) of Cu per gram of dry tissue.
Statistical analysis
Mean brain copper concentrations, quantitative histopathology scores, and weight gain were compared between groups of rats given a single 5 mcg dose of copper histidine, multiple doses of 0.5 mcg CuHis at one week intervals, and multiple doses of saline at one week intervals. Group means were compared using nonparametric Wilcoxon rank-sum tests [22]. A two-tailed P-value less than 0.05 was considered to denote statistical significance.
Results
Dose-related effects of ICV CuHis
Animals that received single doses of 100, 20, and 15 mcg doses CuHis, respectively, died within 6 h of study drug administration. The ICV dose of 100 mcg produced acute massive edema and spongiosis within the left lateral ventricle and over the dorsal and ventral surfaces of the brain bilaterally (Fig. 1b). A normal saline-treated control showed minimal spongiosis and no other significant pathology (Fig. 1a). The 20 mcg ICV dose was associated with enhanced T1 signal on MRI (Fig. 2b). Early euthanasia was performed due to weakness, depression, or other neurological abnormalities in three of three animals that received a 10 mcg dose and one of five that received a 7.5 mcg dose.
Fig. 1.
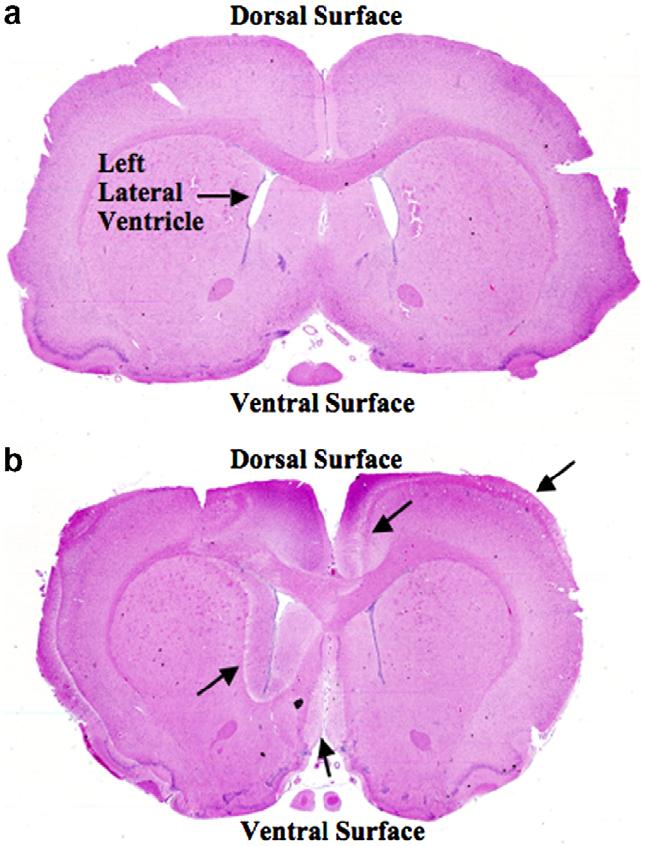
Evidence that ICV CuHis infusion distributes to dorsal and ventral brain surfaces. Whole brain coronal sections stained with hematoxylin and eosin. (a) Saline-treated control. (b) After 100 mcg CuHis infusion. Note marked spongiosis (intercellular edema) in left periventricular region, and at dorsal and ventral brain surfaces (arrows). The saline-treated control was euthanized 6 h post-infusion. The CuHis-treated animal died 4 h post-infusion. 5× magnification.
Fig. 2.
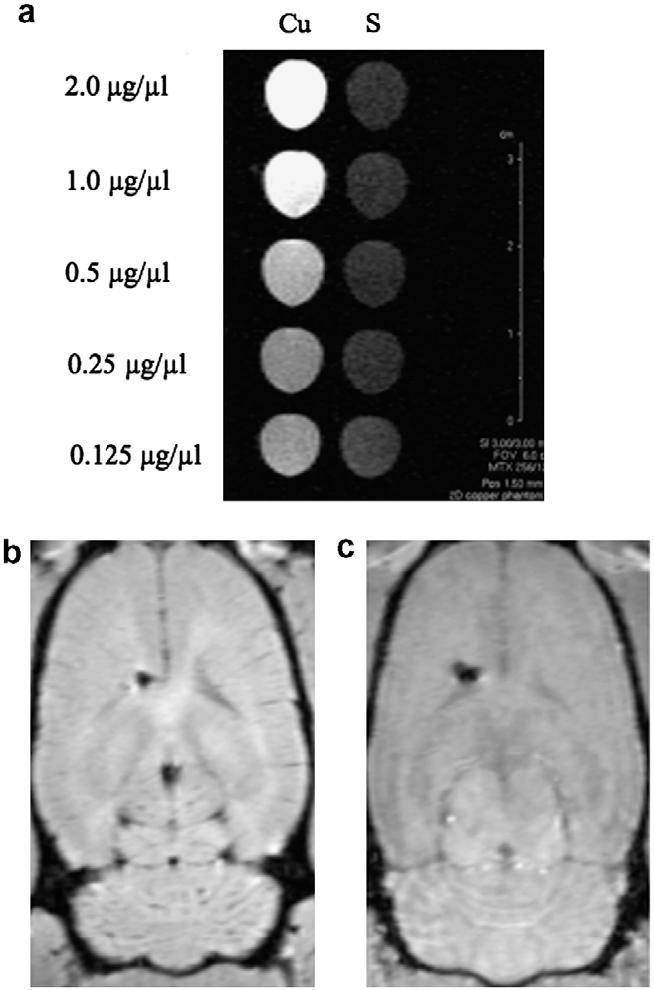
CuHis enhances T1 signal. (a) CuHis solutions (Cu) show increased enhancement compared to saline (S) on T1-weighted imaging in a phantom magnetic resonance imaging experiment. From top to bottom, [CuHis] is 2.0, 1.0, 0.5, 0.25 and 0.125 mcg/μl. (b) Coronal magnetic resonance imaging section of adult rat brain immediately after 20 μg ICV CuHis administration. (c) Adult rat brain immediately after 20 μl saline injection. Increased T1 signal is evident after ICV CuHis administration, in comparison to saline. In both brains, a left hemispheric cavity marks the site of catheter placement in the left lateral ventricle.
Acute histologic effects were demonstrable with an ICV CuHis dose of 10 or 5 mcg (Fig. 3, top two panels). These included spongiosis (intercellular edema) and periventricular necrotic cells. At a dose of 1 mcg CuHis, the histopathologic picture was not easily distinguishable from that of a saline-treated control (Fig. 4, bottom two panels). Chronic histologic effects after a single CuHis dose of either 7.5 or 2.5 mcg involved encephalitis, meningitis and spongiosis (Fig. 4, top two panels). In contrast, a dose of 0.5 mcg CuHis produced minimal changes compared to a saline injected control (Fig. 4, bottom two panels).
Fig. 3.
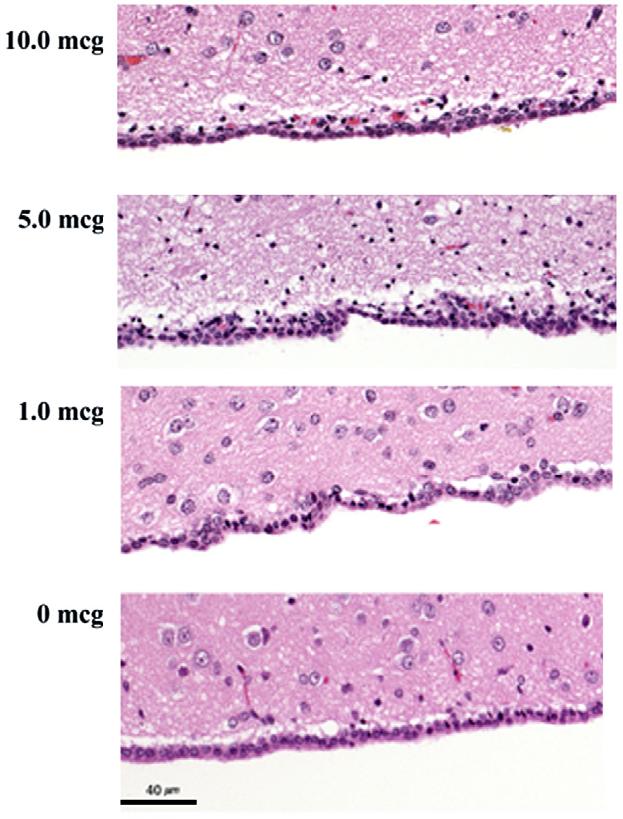
Dose-dependent acute effects of ICV CuHis. Ten and 5 mcg CuHis doses are associated with spongiosis (intercellular edema reflected in multifocal vacuoles and clear areas within the neuropil, the brain tissue that lies between cell bodies), and multifocal necrotic cells showing round, dark-staining nuclei. Minimal histologic differences are present between 1.0 mcg CuHis and 0 mcg (saline). Animals were euthanized 6 h post-infusion. 10× magnification.
Fig. 4.
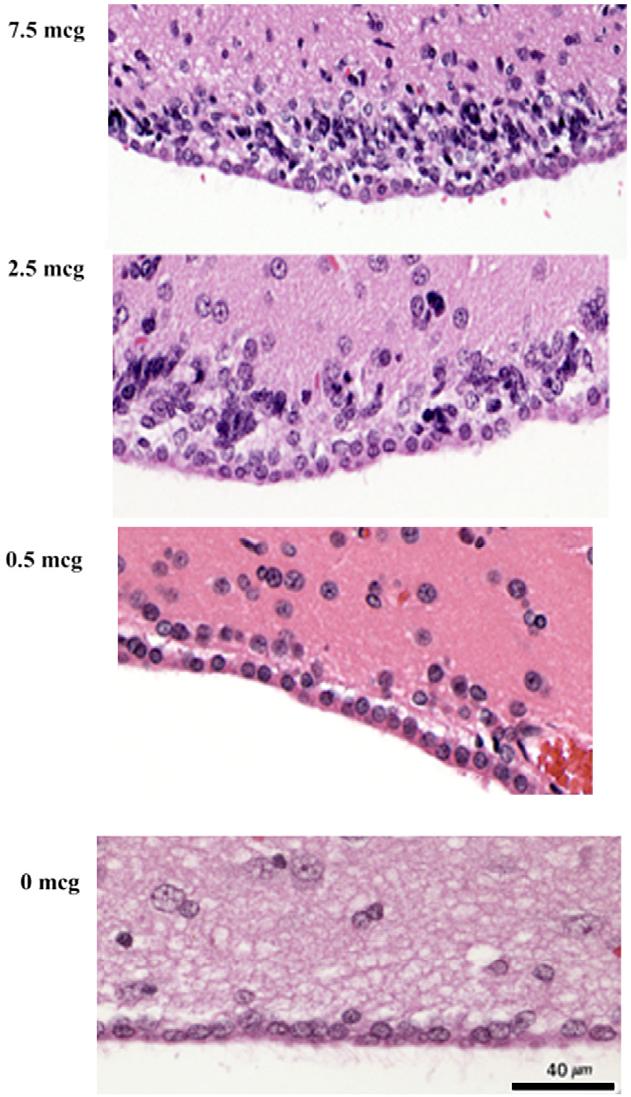
Dose-dependent chronic effects of ICV CuHis. Spongiosis (multifocal vacuoles) and inflammatory cells are evident at doses of 7.5 and 2.5 mcg copper histidine. In contrast, no significant pathology is evident with a dose of 0.5 mcg, or with saline treatment. Animals received a single dose, as indicated, and were euthanized 2 weeks post-infusion. 10× magnification.
Statistical analyses documented significantly more brain pathology in rats that received a single ICV dose of 5.0 mcg CuHis compared to a single saline infusion (Table 1, top half). In contrast, there were no statistically significant differences between rats that received multiple doses of 0.5 mcg CuHis and rats that received multiple saline infusions (Table 1, bottom half).
Table 1.
Brain histopathology in relation to ICV CuHis dose
| Dose | Spongiosis | Ventricular dilation | Cavitation | Encephalitis | Meningitis |
|---|---|---|---|---|---|
| 5 μg, 1 dose (n = 7) | 2.14 | 3.14 | 3.0 | 3.14 | 2.0 |
| Saline controls; 1 dose only (n = 6) | 0.33 | 0.5 | 0 | 0.67 | 0.33 |
| P-value | <0.01* | <0.001* | <0.001* | <0.001* | <0.001* |
| 0.5 μg, 3 or 4 doses at 1 week intervals (n = 4) | 2.25 | 1.25 | 0.75 | 2.25 | 1.0 |
| Saline controls; 4 doses at 1 week intervals (n = 3) | 1.67 | 0 | 0 | 1.67 | 1.0 |
| P-value | N.S. | N.S. | N.S. | N.S. | N.S. |
Scoring was from 0 to 4 using the following scale: 0 = within normal limits; 1 = minimal; 2 = mild; 3 = moderate; and 4 = marked. Mean values shown.
N.S. = non-significant (P > 0.10).
Statistically significant.
Post-infusion brain copper levels were determined by atomic absorption spectrometry in three groups of animals: rats that received a single dose of 5 mcg CuHis, two to four doses of normal saline, or four doses of 0.5 mcg CuHis. Mean brain copper was 22.2±2.5 ppm in the 5 mcg group, 10.9±0.3 ppm in the 0.5 mcg group, and 8.6±1.2 ppm in the saline-treated group. Brain copper level in each CuHis treatment group differed significantly from the saline-treated controls (p < 0.01, and p < 0.025, respectively).
Weekly weight was measured in two groups of animals: rats that received up to four doses of 0.5 mcg, and controls that received two to four infusions of saline. No differences in growth were evident between these two groups (Fig. 5), nor were any differences in their activity or behavior (ambulation, grooming, drinking, eating) observed, based on daily health checks. In contrast, rats that received multiple 5.0 mcg doses of CuHis lost approximately 3% body weight over a comparable time frame (data not shown).
Fig. 5.
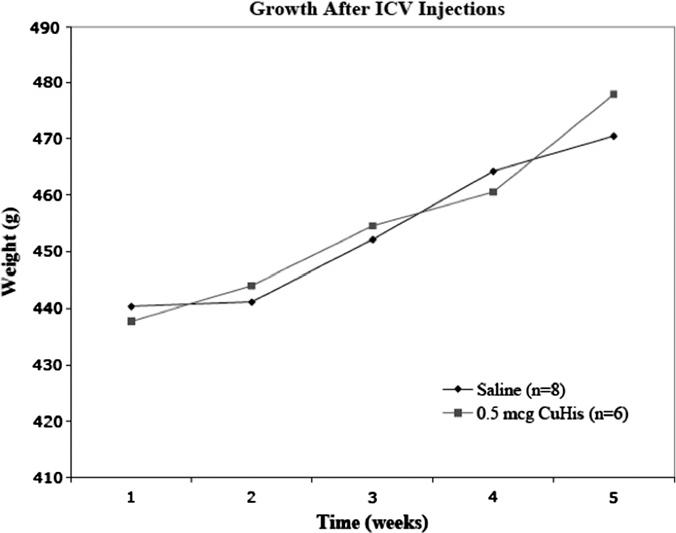
Animals receiving multiple doses of the MTD (0.5 mcg CuHis) grow at the same rate as saline-treated controls. Animals received from 2 to 4 doses of 0.5 mcg CuHis or saline, and were weighed weekly.
Discussion
In light of copper chaperones that deliver copper to various cuproenzymes [23], provision of copper directly to the central nervous system in Menkes disease patients may significantly enhance copper bioavailability within the brain. We hypothesize that when copper crosses the blood-brain barrier normally, specific uptake and transport proteins mediate delivery of copper to neuronal cells, and its incorporation into superoxide dismutase and cytochrome c oxidase. For example, while the Menkes gene product (ATP7A) is necessary to convey copper from the blood stream into cerebrospinal fluid [4,5,10], the copper uptake gene hCTR1 actually transports the metal into neuronal cell bodies [8]. The copper chaperone COX17 is involved in the delivery of copper to neuronal cell mitochondria where cytochrome c oxidase is activated [4,14]. Whether copper uptake by the neuropil (unmyelinated neuronal processes within gray matter) occurs, and the mechanism thereof, is not known.
Since entry of copper at the blood-brain barrier is blocked in Menkes patients with severe mutations [10], copper uptake by neuronal cells is greatly reduced. In such patients, the intraneuronal copper chaperones do not receive copper, resulting in a deficiency of cytochrome c oxidase, a likely critical factor in the neurodegeneration associated with this disease [4,5]. Therefore, by providing copper in a fashion that allows it to reach the brain (i.e., direct administration into the cerebrospinal fluid) at an early postnatal stage, prevention of neurodegeneration in any Menkes patient, regardless of mutation severity, may be possible.
Dopamine-β-hydroxylase (DBH) and peptidyl-amidating mono-oxygenase are copper-dependent enzymes in the CNS that are processed within the secretory pathway of neuronal cells and presumably require ATP7A for activation [5] rather than a copper chaperone. These enzymes would therefore not be fully functional in the context of null ATP7A mutations, even after successful ICV delivery of copper. However, the degree to which deficiencies of these enzymes contribute to neurodevelopmental problems in Menkes disease is not clear. Perinatal lethality is associated with a murine DBH knockout [24], whereas humans with congenital absence of DBH do not manifest the Menkes disease phenotype and only show symptoms of general autonomic dysfunction [25].
The present results establish a maximum tolerated dose of ICV CuHis in adult rats as 0.5 mcg. Animals in this study that received up to 4 weekly infusions of this dose showed no behavioral abnormalities and grew normally in comparison to saline-infused controls, while brain copper levels increased. No significant differences were identified for any of the five scored histopathological criteria. However, the number of animals in these comparisons was small and it is conceivable that histopathologic differences between animals receiving the maximum tolerated dose versus saline might be statistically significant for larger groups.
As demonstrated in Fig. 1, ICV-administered copper reaches both the dorsal and ventral surfaces of the brain, implying rapid distribution throughout the cerebrospinal fluid. The MRI data showing T1 signal enhancement also suggests wide distribution and penetration throughout the brain (Fig. 2b and c). For other trace metals, wide distribution and sustained presence within brain have been documented by autoradiography or T1 signal enhancement on magnetic resonance imaging after ICV injection [15-19].
The effect of variable CuHis dosages was evident in terms of both acute and chronic histopathology. (Figs. 3 and 4, Table 1) which showed a graded response to doses from 0.5 to 10 mcg CuHis. Since 0.5 mcg CuHis was identified as the highest ICV dose that did not induce overt toxicity, growth retardation, or reduce lifespan, we consider this the maximum tolerated dose (MTD).
The mortality and morbidity associated with the higher doses of ICV copper clearly indicate the potential toxicity of this prospective treatment approach for Menkes disease infants. We note, however, that even the MTD for ICV CuHis as defined in this study is much higher than that proposed for human infants affected with severe Menkes disease. This conclusion is based both on cerebrospinal fluid copper levels in normal and affected infants, as well as the differences in cerebrospinal fluid (CSF) volume between adult rats and human infants. We previously noted that the CSF copper level in a normal 2-month-old infant may be as high as 15 parts per billion, whereas in Menkes disease, cerebrospinal fluid copper is approximately 30% of normal (reference [10] and unpublished results). Since concentration in parts per billion converts to nanograms per milliliter (ng/mL) and the normal volume of cerebrospinal fluid in human infants is approximately 30 mL [26], the total cerebrospinal fluid copper in normal infants is approximately 450 ng, in comparison to approximately 150 ng in Menkes disease infants, a deficit of approximately 300 ng. Thus, repletion of cerebrospinal fluid copper in Menkes patients with severe mutations might be accomplished via six monthly ICV infusions of CuHis with a unit dose of 50 ng, 10-fold lower than the rodent MTD. Taken together with the inter-species cerebrospinal fluid volume difference (60-fold greater in human infants versus adult rats), this dose would present a CuHis concentration to the human infant brain 600-fold lower than the MTD for adult rat brain.
The current study addresses tolerance of ICV CuHis in adult rats which express normal quantities of both ATP7A and ATP7B (the copper ATPase associated with Wilson disease). As such, we acknowledge that extrapolation of these results to consideration of the effects of ICV CuHis in younger animals or higher species with mutations in these genes is precarious. A recent study of the developmental expression pattern of ATP7A in mouse suggested that in ependymal cells that line the ventricles in postnatal and adult CNS, ATP7A could mediate copper exodus from the neuropil, as well as copper entry into the brain [27]. Thus, future studies of ICV copper administration in animal models of Menkes and Wilson diseases, e.g., the mottled-brindled mouse or Long-Evans Cinnamon (LEC) rat will contribute further useful information and provide closer approximations to the treatment of human infants with Menkes disease.
In summary, a maximum tolerated dose of 0.5 mcg ICV CuHis was established in adult rats. Multiple infusions of this dose were associated with normal growth and behavior over a 5 week period and no significant differences in brain histopathology in comparison to saline-treated controls. These results suggest that ICV CuHis administration in human infants with Menkes disease has potential as a treatment approach in instances of severe ATP7A mutations for which death or profoundly impaired neurodevelopmental outcomes are otherwise anticipated. Evaluation of this proposed treatment approach in murine models of Menkes disease is an appropriate next consideration.
References
- [1].Vulpe C, Levinson B, Whitney S, Packman S, Gitschier J. Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase. Nat. Genet. 1993;3:7–13. doi: 10.1038/ng0193-7. [DOI] [PubMed] [Google Scholar]
- [2].Chelly J, Tumer Z, Tonnesen T, Petterson A, Ishikawa-Brush Y, Tommerup N, Horn N, Monaco AP. Isolation of a candidate gene for Menkes disease that encodes a potential heavy metal binding protein. Nat. Genet. 1993;3:14–19. doi: 10.1038/ng0193-14. [DOI] [PubMed] [Google Scholar]
- [3].Mercer JFB, Livingston J, Hall B, Paynter JA, Begy C, Chandrasekharappa S, Lockhart P, Grimes A, Bhave M, Siemieniak D, Glover TW. Isolation of a partial candidate gene for Menkes disease by positional cloning. Nat. Genet. 1993;3:20–25. doi: 10.1038/ng0193-20. [DOI] [PubMed] [Google Scholar]
- [4].Cullota VC, Gitlin JD. Disorders of copper transport. In: Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular basis of inherited disease. eighth ed. Vol. 2. McGraw-Hill; New York: 2001. pp. 3105–3126. [Google Scholar]
- [5].Kaler SG. Menkes disease. In: Barnes LA, editor. Adv. Pediatr. Vol. 41. C.V. Mosby; St. Louis: 1994. pp. 263–304. [PubMed] [Google Scholar]
- [6].Kaler SG. Menkes disease mutations and response to early copper histidine treatment. Nat. Genet. 1996;13:21–22. doi: 10.1038/ng0596-21. [DOI] [PubMed] [Google Scholar]
- [7].Kaler SG, Das S, Levinson B, Goldstein DS, Holmes CS, Patronas NJ, Packman S, Gahl WA. Successful early copper therapy in Menkes disease associated with a mutant transcript containing a small in-frame deletion. Biochem. Mol. Med. 1996;57:37–46. doi: 10.1006/bmme.1996.0007. [DOI] [PubMed] [Google Scholar]
- [8].Kim BE, Smith K, Petris MJ. A copper treatable Menkes disease mutation associated with defective trafficking of a functional Menkes copper ATPase. J. Med. Genet. 2003;40:290–295. doi: 10.1136/jmg.40.4.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kaler SG, Buist NRM, Holmes CS, Goldstein DS, Miller RC, Gahl WA. Early copper therapy in classic Menkes disease patients with a novel splicing mutation. Ann. Neurol. 1995;38:921–928. doi: 10.1002/ana.410380613. [DOI] [PubMed] [Google Scholar]
- [10].Liu P-C, Chen Y-W, Centeno J, Quezado M, Lem K, Kaler S. Downregulation of myelination, energy, and translational genes in Menkes disease brain. Mol. Genet. Metab. 2005;85:291–300. doi: 10.1016/j.ymgme.2005.04.007. [DOI] [PubMed] [Google Scholar]
- [11].Hartter DE, Barnea A. Brain tissue accumulates 67copper by two ligand-dependent saturable processes. J. Biol. Chem. 1988;263:799–805. [PubMed] [Google Scholar]
- [12].Qian Y, Zheng Y, Abraham L, Ramos KS, Tiffany-Castiglioni E. Differential profiles of copper-induced ROS generation in human neuroblastoma and astrocytoma cells. Mol. Brain Res. 2005;134:323–332. doi: 10.1016/j.molbrainres.2004.11.004. [DOI] [PubMed] [Google Scholar]
- [13].Rothstein JD, Dykes-Hoberg M, Corson LB, Becker M, Cleveland DW, Price DL, Culotta VC, Wong PC. The copper chaperones CCS is abundant in neurons and astrocytes in human and rodent brain. J. Neurochem. 1999;72:422–429. doi: 10.1046/j.1471-4159.1999.0720422.x. [DOI] [PubMed] [Google Scholar]
- [14].Takahashi Y, Kako K, Kashiwabara S, Takehara A, Inada Y, Arai H, Nakada K, Kodama H, Hayashi J, Baba T, Munekata E. Mammalian copper chaperones COX17p has an essential role in activation of cytochrome c oxidase and embryonic development. Mol. Cell. Biol. 2002;22:7614–7621. doi: 10.1128/MCB.22.21.7614-7621.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Takeda A, Sawashita J, Okada S. Biological half-lives of zinc and manganese in rat brain. Brain Res. 1995;695:53–58. doi: 10.1016/0006-8993(95)00916-e. [DOI] [PubMed] [Google Scholar]
- [16].Takeda A, Suzuki M, Okada S, Oku N. 65Zn localization in rat brain after ICV injection of 65Zn-histidine. Brain Res. 2000;863:241–244. doi: 10.1016/s0006-8993(00)02168-5. [DOI] [PubMed] [Google Scholar]
- [17].Liu C, D’ Arceuil H, de Crespigny A. Direct cerebrospinal fluid injection of MnCl2 for dynamic manganese-enhanced MRI. Magn. Reson. Med. 2004;51:978–987. doi: 10.1002/mrm.20047. [DOI] [PubMed] [Google Scholar]
- [18].Aoki I, Lin Wu Y-J, Silva A, Lynch R, Koretsky A. In vivo detection of neuroarchitecture in the rodent brain using manganese-enhanced MRI. NeuroImage. 2004;22:1046–1059. doi: 10.1016/j.neuroimage.2004.03.031. [DOI] [PubMed] [Google Scholar]
- [19].Lee JH, Silva AC, Merkle H, Koretsky AP. Manganese-enhanced magnetic resonance imaging of mouse brain after systemic administration of MnCl2: dose-dependent and temporal evolution of T1 contrast. Magn. Reson. Med. 2005;53:640–648. doi: 10.1002/mrm.20368. [DOI] [PubMed] [Google Scholar]
- [20].Yan Q, Matheson C, Sun J, Radeke MJ, Feinstein SC, Miller JA. Distribution of intracerebral ventricularly administered neurotrophins in rat brain and its correlation with trk receptor expression. Exp. Neurol. 1994;127:23–36. doi: 10.1006/exnr.1994.1076. [DOI] [PubMed] [Google Scholar]
- [21].Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1982. [Google Scholar]
- [22].Dawson-Saunders B, Trapp RG. Basic and Clinical Biostatistics. second ed Appleton and Lange; Norwalk, Connecticut: 1994. pp. 117–119. [Google Scholar]
- [23].O’Halloran TV, Culotta VC. Metallochaperones, an intracellular shuttle service for metal ions. J. Biol. Chem. 2000;275:25057–25060. doi: 10.1074/jbc.R000006200. [DOI] [PubMed] [Google Scholar]
- [24].Thomas SA, Matsumoto AM, Palmiter RD. Noradrenaline is essential for mouse fetal development. Nature. 1995;374:643–646. doi: 10.1038/374643a0. [DOI] [PubMed] [Google Scholar]
- [25].Biaggioni I, Hollister AS, Robertson D. Dopamine in dopamine-beta-hydroxylase deficiency. N. Engl. J. Med. 1987;317:1415–1416. doi: 10.1056/NEJM198711263172212. [DOI] [PubMed] [Google Scholar]
- [26].Inder TE, Warfield SK, Wang H, ppi P.S. Hü, Volpe JJ. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115:286–294. doi: 10.1542/peds.2004-0326. [DOI] [PubMed] [Google Scholar]
- [27].Niciu MJ, Ma XM, El Meskini R, Ronnett GV, Mains RE, Eipper BA. Developmental changes in the expression of ATP7A during a critical period in postnatal neurodevelopment. Neuroscience. 2006;139:947–964. doi: 10.1016/j.neuroscience.2006.01.044. [DOI] [PubMed] [Google Scholar]


