Abstract
The goal of this study was to engineer cardiac tissue constructs with uniformly anisotropic architecture, and to evaluate their electrical function using multi-site optical mapping of cell membrane potentials. Anisotropic polymer scaffolds made by leaching of aligned sucrose templates were seeded with neonatal rat cardiac cells and cultured in rotating bioreactors for 6-14 days. Cells aligned and interconnected inside the scaffolds and when stimulated by a point electrode, supported macroscopically continuous, anisotropic impulse propagation. By culture day 14, the ratio of conduction velocities along vs. across cardiac fibers reached a value of 2, similar to that in native neonatal ventricles, while action potential duration and maximum capture rate respectively decreased to 120 ms and increased to ~5 Hz. The shorter culture time and larger scaffold thickness were associated with increased incidence of sustained reentrant arrhythmias. In summary, this study is the first successful attempt to engineer a cm2-size, functional anisotropic cardiac tissue patch.
Keywords: Cardiac tissue engineering, polymer scaffold, electrophysiology, arrhythmia, cardiomyoplasty, anisotropic, bioreactor
INTRODUCTION
One promising approach for preventing the development of heart failure after myocardial infarction is the implantation of an engineered tissue patch at the site of cardiac injury. In recent proof-of-principle studies, engineered cardiac constructs composed of cardiac cells [1, 2], skeletal myoblasts [3], or mesenchymal stem cells [4] have been shown to slightly, yet significantly, improve heart function after myocardial infarction in rats. Although programmable electrical stimulation was not used to systematically assess potential electrical instabilities before or after implantation, the apparent absence of arrhythmias in these studies offers the hope that proarrhythmic risks may not be the limiting factor in future cardiac tissue engineering therapies.
While the optimal donor cell type for cardiac repair is still unknown, it is expected that 3-D cell alignment (anisotropy) inside an engineered cardiac patch, as present in native heart, will facilitate structural and functional integration of the patch into the host tissue, yielding a safer and more efficient therapy. A number of methods exist to align cardiac cells on a 2-D substrate including the use of unidirectional stretch [5], oriented chemical cues [6, 7] or aligned surface topography [6, 8]. The current methods to align cardiac cells in 3-D are however limited to the use of pseudo-1-D geometry of thin, cylindrical-[9] or ring-shaped [10] tissue constructs that constrain the cell growth in one direction. The method to engineer a 3-D cardiac patch with a relatively large area (>1cm2) and uniform 3-D cell alignment is still non-existent, even for patches as thin as 50 μm.
In previous work by Bursac and co-workers [11-13], neonatal rat ventricular cells formed cardiac tissue-like constructs when cultured on fibrous, laminin-coated poly(glycolic) acid (PGA) scaffolds inside high-aspect-ratio vessel bioreactors (HARVs). In these constructs, cardiac cells align along randomly oriented polymer fibers, interconnect through gap junctions, and support isotropic electrical propagation with velocities 90% as high as average velocities measured in neonatal rat ventricles [13]. Cell action potentials exhibit maximum depolarization upstrokes, amplitudes, and resting potentials similar to those in neonatal ventricles, while action potential duration is prolonged due to down regulation of the transient outward potassium current [12]. In addition, cardiomyocytes in this 3-D setting maintain a more differentiated phenotype (i.e. higher expression of connexin-43, creatine kinase MM, and myosin heavy chain-α) than those cultured in 2-D monolayers [12]. As a continuation of this work, in the current study we have: 1) manufactured novel, aligned poly(lactic-co-glycolic) acid (PLGA) scaffolds and used them to engineer cm2-size cardiac tissue constructs with anisotropic structure and function and 2) systematically characterized electrical anisotropy and arrhythmogenic potential of the obtained tissue constructs as a function of time in culture using multi-site optical mapping of electrical propagation.
MATERIALS AND METHODS
All animals were treated according to protocols approved from the Animal Use and Care Committee at the Johns Hopkins University. All materials were obtained from Sigma-Aldrich unless stated otherwise.
Preparation of aligned scaffolds
Twenty grams of sucrose were melted in the rotating head of a cotton candy machine (Gold Medal, Supplemental Fig. 1) at a temperature that was varied between 160 and 200°C. The rotating speed of the head was controlled by varying the voltage supply between 70 and 120 V using a conventional potentiometer. The extruded sucrose fibers were humidified 0-10 min in a tissue culture incubator, manually stretched, pressed to form thin sheets, and baked overnight in a vacuum-dry oven (Fisher Sci.) at 30°C. Obtained sucrose templates were coated with 0.5-6% solution of PLGA (75%/25%) in methylene chloride and left to evaporate overnight under vacuum (Supplemental Fig. 1). Sucrose was subsequently leached by multiple washes of ethanol and de-ionized water. The resulting PLGA scaffolds were then cut into 14 mm diameter disks, surface hydrolyzed with 0.05 M NaOH for 10 min [14], and coated with fibronectin solution (25 μg/ml) overnight at 4°C. Sucrose residue in the scaffold was estimated using Benedict’s reduction test [15]. The scaffolds were produced at thicknesses of 0.7±0.1 mm (termed “thin”) and 2±0.2 mm (termed “thick) and used for tissue culture.
Tissue culture
Cardiac cells were dissociated from the ventricles of 2-3 day old neonatal Sprague-Dawley rats (Charles River) using trypsin and collagenase, as previously described [6]. Polymer scaffolds were inoculated with a dense cell suspension (8×106 cells in 80 μl medium per scaffold) and cultured for 6-14 days in a rotating tissue culture bioreactor (HARV, Synthecon) [12]. Tissue constructs were assessed for their structure and function at culture days 6, 10 and 14. Isotropic cardiac monolayers [6] on fibronectin-coated polyvynil chloride coverslips (VWR) served as controls.
Scanning electron microscopy
As previously described [6], samples were frozen in liquid nitrogen, dried under high vacuum, and coated with a 20 nm layer of gold. Images were acquired using a scanning electron microscope (LEO 1550 FESEM).
Histology
As previously described [11], constructs were fixed in neutral-buffered formalin, embedded in paraffin, sliced into 5 μm thick sections, and stained with hematoxylin and eosin (H&E).
Electrophysiological assessment
Action potentials were optically recorded from 61 sites using previously described methods [6]. Briefly, tissue constructs were placed in a custom designed chamber and stained with voltage-sensitive dye RH-237 (8 μM, Molecular Probes). To minimize contraction artifacts, an optically clear, thin (0.2 mm) perforated silicone membrane was used to gently cover-press the tissue construct against the bottom of the chamber. Stable electrical properties throughout the experiment confirmed that no tissue ischemia was induced by this procedure. Cultures were transilluminated with excitation light (530±25nm) and optical responses recorded by a fiber optic bundle arranged in a 17 mm diameter hexagonal array [6].
Pacing protocol
Cathodal unipolar point stimuli (1.2× threshold) were applied at a rate of 1.5 Hz using a 100 μm diameter platinum wire placed ~0.5 mm above the center of the tissue construct. The pacing rate was increased every minute in steps of 0.5 Hz. At the maximum capture rate (MCR) (i.e. the maximum rate at which each stimulus evoked a tissue response in at least 90% of recording sites) pacing was stopped. If stable reentrant arrhythmia did not spontaneously evolve, short pacing bursts at 1-1.2× MCR were subsequently applied up to 5 times to induce reentrant arrhythmias. Arrhythmia was defined stable if it lasted for more than 30 sec.
Data analysis
The activation time was defined as the instant of maximum positive slope of the optical action potential. Velocity fields were calculated from inverse gradients of isochrone maps [6]. Longitudinal and transverse conduction velocities during point stimulation at 1.5 Hz were measured along the major and minor axes of elliptical isochrones, respectively, at sites at least 3 mm away from the pacing electrode. The same sites were used to measure the longitudinal and transverse action potential duration defined as the interval from activation time to time of 80% repolarization. Anisotropy ratio was defined as the ratio of longitudinal to transverse conduction velocity. The percentage of arrhythmia induction was obtained by dividing the number of constructs with induced arrhythmias by the total number of constructs in which the induction was attempted.
Statistical analysis
Data were expressed as mean ± SD and analyzed for different culture days using one-way ANOVA followed by a posthoc Student’s t test. Differences were considered to be significant when p<0.05.
RESULTS
Polymer scaffolds
Increasing the temperature of melted sucrose increased fiber extrusion rate, but yielded non-uniform thickness and density of the extruded sucrose layers. The temperature of 180°C resulted in the most efficient and uniform fiber extrusion. Increasing the speed of the rotating head decreased the average diameter of sucrose fibers (Supplemental Fig. 2). The rotational speed produced at 80-90V was adopted as optimal, since fibers thinner than 6 μm often broke and collapsed when coated with the PLGA solution. The PLGA concentration of 3.5%, which appeared to induce the highest degree of macroscopic fiber orientation (as assessed by light microscopy), was used in all experiments. Increasing the humidification time between 0 and 10 min increased both sucrose fiber diameter and the number of bonds between the fibers, yielding a variety of aligned scaffolds with fibrous (Fig. 1A1&2, short humidification) or foamy (Fig. 1B1&2, long humidification) architecture. The fibrous scaffolds humidified for 1 min were used for tissue culture since they exhibited the highest porosity (assessed by scanning electron microscopy). The sucrose residue inside the scaffold was estimated by Benedict’s test to be less than 0.1% of the total polymer mass.
Figure 1. Structure-function relationships in cardiac tissue constructs.

Fibrous (A) and foamy (B) polymer scaffolds produced by the leaching of a sucrose template (see text for details). A&B1, light micrographs; A&B2 scanning electron micrographs. C, H&E staining of a 10 day old tissue construct made from a fibrous scaffold. Note that cardiac cells spread along and bridge between the polymer fibers (denoted by black arrows). Low (D1) and high (D2) magnification scanning electron micrographs reveal aligned and interconnected cells inside the construct. White arrows in D2 denote anastomosing junctions. E, Action potential propagation in a 10 day old cardiac tissue construct. Frames are isovoltage snapshots at 10 ms intervals. Blue and red denote rest and peak of the action potential, respectively. Pulse symbol denotes stimulus site while “+” symbols denote recording sites. Double-headed arrow denotes direction of polymer fibers in the scaffold. Short arrows show direction of the elliptical propagating wave. F, Isochrone maps in cardiac constructs and control monolayers. Numbers denote time in ms; “*” symbols denote recording sites. Note that propagation in tissue constructs is anisotropic with largest velocity in the direction of scaffold fibers. In contrast, isotropic monolayers yield circular isochrones.
Structure and morphology of tissue constructs
Similar to our previous studies with non-aligned PGA scaffolds [11-13], cardiac cells attached and spread on fibronectin-coated PLGA fibers (Fig. 1C) to form a 3-D cell network. Scanning electron micrographs revealed numerous areas consisting of bundled (Fig. 1D1), co-aligned (in the direction of polymer fibers), and interconnected (Fig. 1D2) cardiac cells. Spontaneous contractions at rates of 2.5-3 Hz, observed 2 days after seeding, ceased with time in culture within both monolayers and tissue constructs, and by culture day 6, only irregular cell contractions at rates of less than 1.5 Hz could be observed. In contrast to monolayer cultures which deteriorated by culture day 9, the tissue constructs could be electrophysiologically assessed for at least 2 weeks with no signs of cell death or significant scaffold breakdown.
Electrophysiological properties of tissue constructs
To assess if the presence of numerous cardiac bundles in tissue constructs yielded functional anisotropy at the macroscopic scale, we optically mapped electrical activity in constructs using the voltage-sensitive dye, RH-237. Specifically, pacing with a thin electrode at the center of the tissue construct resulted in an elliptical, macroscopically continuous spread of electrical activity throughout the entire construct, i.e. anisotropic impulse propagation (Fig. 1E, 1F, left). In contrast, randomly oriented cells in control cardiac monolayers supported only isotropic propagation associated with circular isochrone maps (Fig. 1F, right). As expected, the presence of multiple cardiac cell layers in tissue constructs yielded significantly higher optical action potential amplitudes than those recorded in monolayers (Fig. 2A). Furthermore, the transverse conduction velocities in tissue constructs (measured at 1.5 Hz pacing rate) remained constant with time in culture (averaging 10.9±2.9 cm/s, Fig. 2B), while longitudinal velocities increased to reach steady values (20.8±3.2 cm/s) after culture day 10. Consequently, longitudinal-to-transverse conduction velocity ratio increased with time of culture (Fig. 2C). The isotropic conduction velocity in 7 day old control monolayers (16.8±2.1 cm/s) ranged between the longitudinal and transverse velocities of 10 and 14 day old tissue constructs. Construct action potential duration decreased after day 6 (Fig. 2D) reaching values (122±26 ms) comparable to those in 7 day monolayer controls (117±27 ms). Similarly, the maximum steady state capture rates in constructs increased after day 6 (Fig. 2E) to levels (4.7±1.0 Hz) slightly but insignificantly lower than those in monolayer cultures (5.4±0.7 Hz; p=0.1).
Figure 2. Electrophysiological properties of cardiac tissue constructs as a function of time in culture.
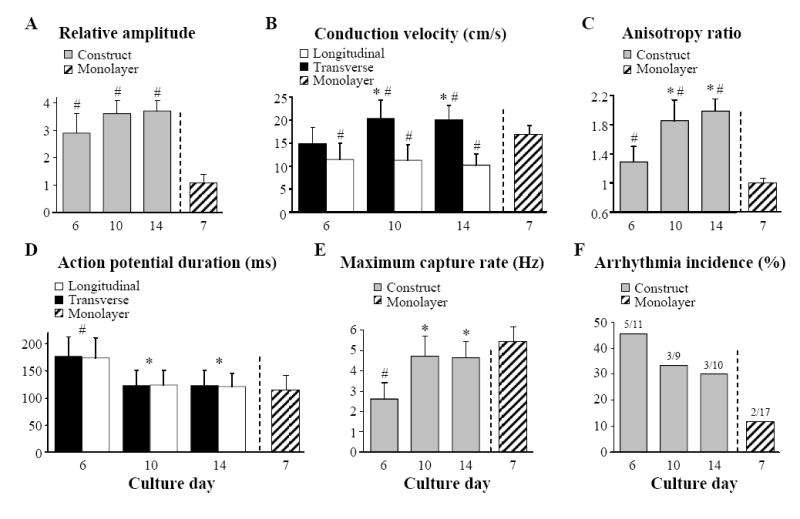
A, Signal amplitude relative to that in 7 day old monolayers. B, Velocity of actionpotential propagation along (longitudinal) and across (transverse) aligned polymer fibers. Note that velocity in monolayers is isotropic. C, Longitudinal-to-transverse velocity anisotropy ratio. D, Action potential duration at 80% repolarization. E, Maximum point pacing steady-state capture rate. F, Percent incidence of arrhythmia induction. Ratio denotes number of constructs with induced arrhythmia / number of construct with attempted inductions. Parameters in A-D are 17 measured at pacing rate of 1.5 Hz. #, statistically different from monolayers; *, statistically different from day 6 constructs.
Reentrant arrhythmias in tissue constructs
Short burst point pacing at rates slightly higher than the maximum capture rate initiated sustained reentry in 45%, 33%, and 30% of tissue constructs at days 6, 10, and 14, respectively (Fig. 2F), while in control monolayers, the reentry incidence under the same pacing protocol was only 12%. In thin constructs, cardiac cells were electrically connected throughout the construct depth, effectively supporting 2-dimensional impulse propagation. Consequently, in thin constructs (and monolayers), the most common mode of sustained arrhythmia was planar single-loop reentry (Fig. 3A), followed by figure-eight reentry (Fig. 3B). The thick constructs on the other hand contained an acellular (not shown) and likely ischemic inner region which acted as a barrier to electrical conduction, particularly at high pacing rates. As a result, electrical waves were able to propagate around the cellular outer “shell” of the construct and independently activate its top and bottom surfaces. In the transillumination mode of optical mapping, this bi-planar propagation was evident by the presence of double-humped action potentials (Fig. 4, right), where each hump represented activation at one of the construct surfaces (top or bottom). Therefore, the thick constructs contained a larger effective area for electrical propagation than the thin constructs and could also support reentrant waves that “wrapped” around the construct, alternately activating its top and bottom surfaces (Fig. 4, left).
Figure 3. Reentrant arrhythmias in thin cardiac tissue constructs.
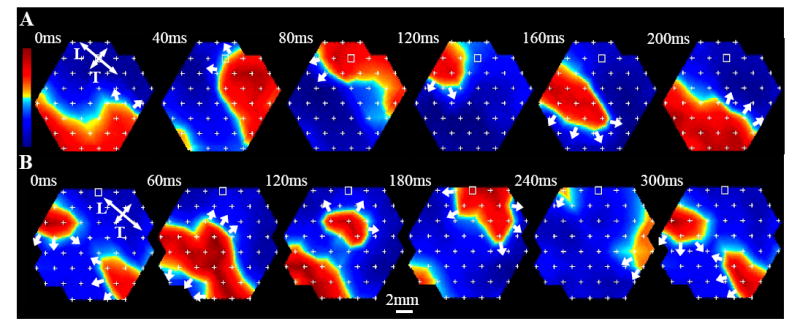
A, single-loop reentry in a 10 day old construct. Isovoltage snapshots are shown during one reentry cycle. A single propagating wave rotates counterclockwise to repeatedly activate construct at a period of 220 ms. B, figure-eight reentry in a 6 day old construct. Two counter-rotating waves activate construct at a period of 295 ms. Double headed arrows in A and B denote longitudinal (L) and transverse (T) directions of propagation. The rest of the nomenclature is the same as in Fig. 1E.
Figure 4. Bi-planar reentrant arrhythmia in a 10 day old thick cardiac tissue construct.
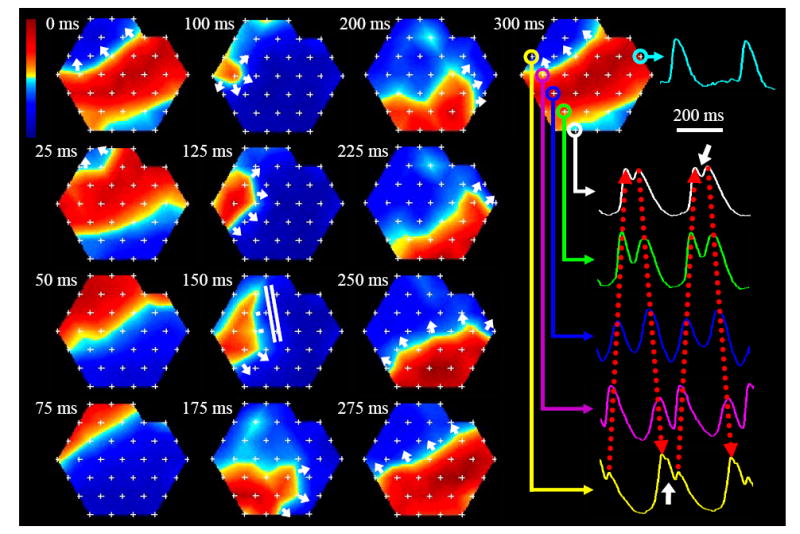
Isovoltage frames show wave propagation at 25 ms intervals. In the first 4 frames (0-75 ms) the wave propagates in one plane, followed by propagation in the opposite direction in the other plane (frames 100-225 ms). At 250 ms the plane and direction of propagation are switched back to original (frames 250-300 ms). Bi-planar propagation in transillumination mode of optical mapping is characterized by double-humped signals (shown in color at different recording sites). Signals with closely spaced humps (shown by white arrows) represent the sites where the propagation plane is changed. Red dotted arrows show activations that belong to one plane. Regular action potential morphology (cyan trace) occurs where propagation in one plane is blocked or both planes are simultaneously activated. In general, particular spatial sequence of propagation during bi-planar reentry is determined by the distribution of sites where two propagating planes are electrically connected. White parallel lines in the 150 ms frame denote site of conduction block. The rest of the nomenclature is the same as in Fig. 3.
DISCUSSION
In this study, we have presented a novel method for the production of aligned 3-D polymer scaffolds based on the leaching of baked sucrose templates. Particulate leaching of sucrose or salt crystals has been widely used in production of porous non-aligned polymer scaffolds for tissue engineering applications [16]. Zhang et al. [17] was the first to utilize the glassy properties of sucrose to manually extrude single, relatively large diameter (100-500 μm) fibers and layer them into a template that was leached to obtain macroporous polymer scaffolds. In the current study, we employed a conventional cotton candy machine to rapidly produce significantly thinner sucrose fibers (<20 μm, supplemental Fig. 2) and manufacture aligned scaffolds with microscopic pores that supported the 3-D growth and confluence of neonatal cardiac cells. Controlling the duration of the humidification step in the manufacturing process enabled us to also vary the scaffold architecture between fibrous and foamy configurations (Fig. 1A&B).
When seeded on the scaffolds and cultured in HARV bioreactors, neonatal rat cardiac cells aligned and interconnected to form multiple bundles (Fig. 1C&D) and yielded anisotropic electrophysiological properties (Fig. 1E) that stabilized after 10 days of culture (Fig. 2). To our knowledge, this is the first successful attempt to engineer a relatively large 3-D cardiac tissue construct (>1 cm2 area) with macroscopically anisotropic structure and function. Average velocity anisotropy ratios of 2 obtained after 2 weeks of culture (Fig. 2C) are in agreement with those previously measured in neonatal ventricles [18, 19], but lower than those measured in adult ventricles (due to the differences in shape and gap junction distribution of adult vs. neonatal cardiomyocytes [19]).
It is important to note that previous cardiac tissue engineering studies have not attempted a detailed in vitro evaluation of impulse propagation and arrhythmogenicity in tissue constructs. Instead, functionality of the constructs was assessed by measuring force [9, 10] or motion [20, 21] amplitude generated in response to strong electric shocks applied by field electrodes. The use of field shocks to assess functionality of cardiac tissue constructs may be misleading because the strong electric shock can induce the simultaneous contraction of most if not all of the cardiomyocytes within the construct, even if the cells are not coupled or able to propagate action potentials. Therefore, in this study, a near-threshold, point stimulus was used to locally excite the cardiac cells. Simultaneously, the spread of electrical activity was monitored throughout the tissue construct which provided a direct assessment of functional coupling among the cells in a physiologically relevant setting.
We believe that a thorough assessment of electrical propagation should be routinely performed in cardiac tissue constructs, and together with mechanical tests, used as one of the main quality control criteria in functional cardiac tissue engineering. From our experience, it is important to assess the electrical properties of engineered cardiac tissues in challenging regimes such as fast or premature stimulation, similar to standard clinical tests of arrhythmia vulnerability [22]. For example, a cardiac tissue construct may support macroscopically continuous propagation at low pacing rates, but yield conduction blocks and reentrant arrhythmias during rapid pacing (Fig. 3&4), possibly due to the abundance of microscopic anatomic heterogeneities. Engineering relatively thick constructs with an ischemic or acellular core may pose additional arrhythmogenic risks (Fig. 4) due to an increase in the effective tissue area which is likely to facilitate the induction of reentrant activity.
Currently, a limitation of the described methodology is the inability to achieve a wide range of structural and functional anisotropies in tissue constructs, a goal that we previously accomplished in cardiac monolayers using methods of micropatterning and microabrasion [6]. The ability to precisely engineer cardiac patch anisotropy to match that of the surrounding host heart tissue would significantly increase the versatility of future therapies. Scaffolds with reproducible structure made by rapid prototyping [16] may provide the means to achieve this goal.
Supplementary Material
Sucrose was melted in the rotating head of a cotton candy machine. Thin layers of aligned sucrose fibers were extruded, humidified, stretched, pressed, and baked overnight. Baked sucrose templates were coated with PLGA in methylene chloride and dried overnight under vacuum. Sucrose was leached out, and the scaffolds were cut into disks, dried, coated with fibronectin, seeded with cardiac cells, and cultured in HARV bioreactors (see text for additional details).
Acknowledgments
The authors acknowledge Melissa E. Irby, Evangeline Ng, and Sean Sheehy for technical help. This work was supported by NIH grant HL66239 to L.T. and an American Heart Association, Mid-Atlantic Affiliate fellowship to N.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Furuta A, Miyoshi S, Itabashi Y, Shimizu T, Kira S, Hayakawa K, Nishiyama N, Tanimoto K, Hagiwara Y, Satoh T, Fukuda K, Okano T, Ogawa S. Pulsatile cardiac tissue grafts using a novel three-dimensional cell sheet manipulation technique functionally integrates with the host heart, in vivo. Circ Res. 2006;98:705–712. doi: 10.1161/01.RES.0000209515.59115.70. [DOI] [PubMed] [Google Scholar]
- 2.Zimmermann WH, Melnychenko I, Wasmeier G, Didie M, Naito H, Nixdorff U, Hess A, Budinsky L, Brune K, Michaelis B, Dhein S, Schwoerer A, Ehmke H, Eschenhagen T. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452–458. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 3.Memon IA, Sawa Y, Fukushima N, Matsumiya G, Miyagawa S, Taketani S, Sakakida SK, Kondoh H, Aleshin AN, Shimizu T, Okano T, Matsuda H. Repair of impaired myocardium by means of implantation of engineered autologous myoblast sheets. J Thorac Cardiovasc Surg. 2005;130:1333–1341. doi: 10.1016/j.jtcvs.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 4.Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H, Ishino K, Ishida H, Shimizu T, Kangawa K, Sano S, Okano T, Kitamura S, Mori H. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–465. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 5.Terracio L, Miller B, Borg TK. Effects of cyclic mechanical stimulation of the cellular components of the heart: in vitro. In Vitro Cell Dev Biol. 1988;24:53–58. doi: 10.1007/BF02623815. [DOI] [PubMed] [Google Scholar]
- 6.Bursac N, Parker KK, Iravanian S, Tung L. Cardiomyocyte cultures with controlled macroscopic anisotropy: a model for functional electrophysiological studies of cardiac muscle. Circ Res. 2002;91:e45–54. doi: 10.1161/01.res.0000047530.88338.eb. [DOI] [PubMed] [Google Scholar]
- 7.McDevitt TC, Angello JC, Whitney ML, Reinecke H, Hauschka SD, Murry CE, Stayton PS. In vitro generation of differentiated cardiac myofibers on micropatterned laminin surfaces. J Biomed Mater Res. 2002;60:472–479. doi: 10.1002/jbm.1292. [DOI] [PubMed] [Google Scholar]
- 8.Bien H, Yin L, Entcheva E. Cardiac cell networks on elastic microgrooved scaffolds. IEEE Eng Med Biol Mag. 2003;22:108–112. doi: 10.1109/memb.2003.1256279. [DOI] [PubMed] [Google Scholar]
- 9.Baar K, Birla R, Boluyt MO, Borschel GH, Arruda EM, Dennis RG. Self-organization of rat cardiac cells into contractile 3-D cardiac tissue. Faseb J. 2005;19:275–277. doi: 10.1096/fj.04-2034fje. [DOI] [PubMed] [Google Scholar]
- 10.Zimmermann W, Schneiderbanger K, Schubert P, Didie M, Munzel F, Heubach J, Kostin S, Nehuber W, Eschenhagen T. Tissue engineering of a differentiated cardiac muscle construct. Circ Res. 2002;90:223–230. doi: 10.1161/hh0202.103644. [DOI] [PubMed] [Google Scholar]
- 11.Bursac N, Papadaki M, Cohen RJ, Schoen FJ, Eisenberg SR, Carrier R, Vunjak-Novakovic G, Freed LE. Cardiac muscle tissue engineering: toward an in vitro model for electrophysiological studies. Am J Physiol. 1999;277:H433–444. doi: 10.1152/ajpheart.1999.277.2.H433. [DOI] [PubMed] [Google Scholar]
- 12.Bursac N, Papadaki M, White JA, Eisenberg SR, Vunjak-Novakovic G, Freed LE. Cultivation in rotating bioreactors promotes maintenance of cardiac myocyte electrophysiology and molecular properties. Tissue Eng. 2003;9:1243–1253. doi: 10.1089/10763270360728152. [DOI] [PubMed] [Google Scholar]
- 13.Papadaki M, Bursac N, Langer R, Merok J, Vunjak-Novakovic G, Freed LE. Tissue engineering of functional cardiac muscle: molecular, structural, and electrophysiological studies. Am J Physiol Heart Circ Physiol. 2001;280:H168–178. doi: 10.1152/ajpheart.2001.280.1.H168. [DOI] [PubMed] [Google Scholar]
- 14.Gao J, Niklason L, Langer R. Surface hydrolisis of poly(glycolic acid) meshes increases the seeding density of vascular smooth muscle cells. J Biomed Mat Res. 1998;42:417–424. doi: 10.1002/(sici)1097-4636(19981205)42:3<417::aid-jbm11>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 15.Gupta RC, Goyal A, Singh PP. How good is the good old Benedict’s test? J Assoc Physicians India. 1983;31:507–508. [PubMed] [Google Scholar]
- 16.Weigel T, Schinkel G, Lendlein A. Design and preparation of polymeric scaffolds for tissue engineering. Expert Rev Med Devices. 2006;3:835–851. doi: 10.1586/17434440.3.6.835. [DOI] [PubMed] [Google Scholar]
- 17.Zhang R, Ma PX. Synthetic nano-fibrillar extracellular matrices with predesigned macroporous architectures. J Biomed Mater Res. 2000;52:430–438. doi: 10.1002/1097-4636(200011)52:2<430::aid-jbm25>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 18.Litchenberg W, Norman L, Holwell A, Martin K, Hewett K, Gourdie R. The rate and anisotropy of impulse propagation in the postnatal terminal crest are correlated with remodeling of Cx43 gap junction pattern. Cardiovasc Res. 2000;45:379–387. doi: 10.1016/s0008-6363(99)00363-6. [DOI] [PubMed] [Google Scholar]
- 19.Spach MS, Heidlage JF, Dolber PC, Barr RC. Electrophysiological effects of remodeling cardiac gap junctions and cell size: experimental and model studies of normal cardiac growth. Circ Res. 2000;86:302–311. doi: 10.1161/01.res.86.3.302. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu T, Yamato M, Isoi T, Akutsu T, Setomaru T, Abe K, Kikuchi A, Umezu M, Okano T. Fabrication of pulsatile cardiac tissue grafts using a novel 3-dimensional cell sheet manipulation technique and temperature-responsive cell culture surfaces. Circ Res. 2002;90:e40–48. doi: 10.1161/hh0302.105722. [DOI] [PubMed] [Google Scholar]
- 21.Radisic M, Park H, Shing H, Consi T, Schoen FJ, Langer R, Freed LE, Vunjak-Novakovic G. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci U S A. 2004;101:18129–18134. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrick KJ, Maher M, Roth JA, Kim SG, Fisher JD. Reproducibility of electrophysiological testing during antiarrhythmic therapy for ventricular arrhythmias unrelated to coronary artery disease. Pacing Clin Electrophysiol. 1995;18:1395–1400. doi: 10.1111/j.1540-8159.1995.tb02601.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sucrose was melted in the rotating head of a cotton candy machine. Thin layers of aligned sucrose fibers were extruded, humidified, stretched, pressed, and baked overnight. Baked sucrose templates were coated with PLGA in methylene chloride and dried overnight under vacuum. Sucrose was leached out, and the scaffolds were cut into disks, dried, coated with fibronectin, seeded with cardiac cells, and cultured in HARV bioreactors (see text for additional details).


