Abstract
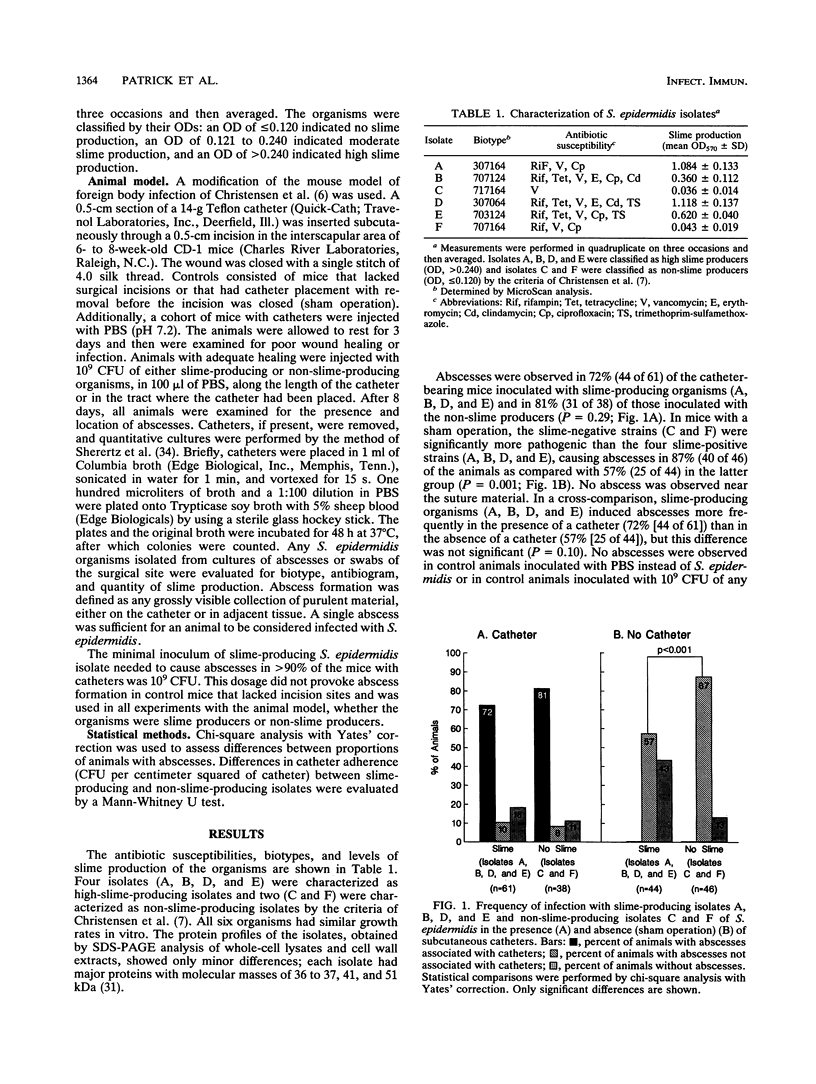
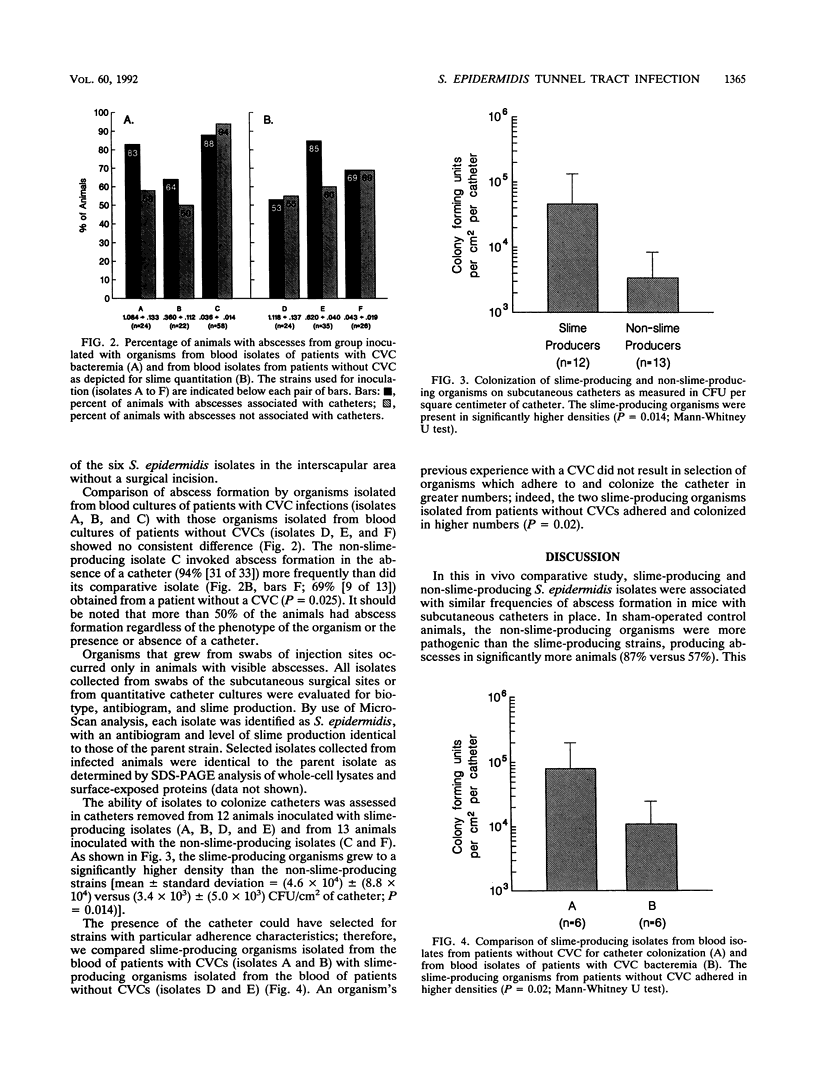
An experimental animal model was used to assess the slime layer of Staphylococcus epidermidis as a pathogenic factor in tunnel tract infections. Mice were inoculated with high-slime-producing or non-slime-producing strains of S. epidermidis, either along the length of a subcutaneous catheter or in the area where a catheter had been placed and immediately removed (controls). Among the catheter-bearing mice, the phenotypically distinct staphylococci produced similar, high frequencies of abscess formation (72% [44 of 61] versus 81% [31 of 38]; P = 0.29). In controls, the non-slime-producing organisms were significantly more pathogenic (87% [40 of 46] versus 57% [25 of 44] abscess formation; P = 0.001). No consistent difference was detected between blood isolates obtained from patients with central venous catheter bacteremia and those from neonates with bacteremia in the absence of a prosthetic medical device. Quantitative culture of removed catheters showed greater adherence by the slime-producing isolates (P = 0.014). In this mouse model, slime production by S. epidermidis did not increase the risk of catheter tunnel tract infection, despite the greater catheter adherence of the slime-producing organisms. These findings suggest that traumatized tissue may be a sufficient condition for the development of S. epidermidis catheter-associated infections.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayston R., Penny S. R. Excessive production of mucoid substance in staphylococcus SIIA: a possible factor in colonisation of Holter shunts. Dev Med Child Neurol Suppl. 1972;27:25–28. doi: 10.1111/j.1469-8749.1972.tb09769.x. [DOI] [PubMed] [Google Scholar]
- Bykowska K., Ludwicka A., Wegrzynowicz Z., Lopaciuk S., Kopeć M. Anticoagulant properties of extracellular slime substance produced by Staphylococcus epidermidis. Thromb Haemost. 1985 Dec 17;54(4):853–856. [PubMed] [Google Scholar]
- Christensen G. D., Baddour L. M., Simpson W. A. Phenotypic variation of Staphylococcus epidermidis slime production in vitro and in vivo. Infect Immun. 1987 Dec;55(12):2870–2877. doi: 10.1128/iai.55.12.2870-2877.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen G. D., Parisi J. T., Bisno A. L., Simpson W. A., Beachey E. H. Characterization of clinically significant strains of coagulase-negative staphylococci. J Clin Microbiol. 1983 Aug;18(2):258–269. doi: 10.1128/jcm.18.2.258-269.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen G. D., Simpson W. A., Bisno A. L., Beachey E. H. Experimental foreign body infections in mice challenged with slime-producing Staphylococcus epidermidis. Infect Immun. 1983 Apr;40(1):407–410. doi: 10.1128/iai.40.1.407-410.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen G. D., Simpson W. A., Younger J. J., Baddour L. M., Barrett F. F., Melton D. M., Beachey E. H. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. 1985 Dec;22(6):996–1006. doi: 10.1128/jcm.22.6.996-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport D. S., Massanari R. M., Pfaller M. A., Bale M. J., Streed S. A., Hierholzer W. J., Jr Usefulness of a test for slime production as a marker for clinically significant infections with coagulase-negative staphylococci. J Infect Dis. 1986 Feb;153(2):332–339. doi: 10.1093/infdis/153.2.332. [DOI] [PubMed] [Google Scholar]
- Dickinson G. M., Bisno A. L. Infections associated with indwelling devices: concepts of pathogenesis; infections associated with intravascular devices. Antimicrob Agents Chemother. 1989 May;33(5):597–601. doi: 10.1128/aac.33.5.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty S. H., Simmons R. L. Endogenous factors contributing to prosthetic device infections. Infect Dis Clin North Am. 1989 Jun;3(2):199–209. [PubMed] [Google Scholar]
- Drewry D. T., Galbraith L., Wilkinson B. J., Wilkinson S. G. Staphylococcal slime: a cautionary tale. J Clin Microbiol. 1990 Jun;28(6):1292–1296. doi: 10.1128/jcm.28.6.1292-1296.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne W. M., Jr Effects of subinhibitory concentrations of vancomycin or cefamandole on biofilm production by coagulase-negative staphylococci. Antimicrob Agents Chemother. 1990 Mar;34(3):390–393. doi: 10.1128/aac.34.3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M. S., Baker C. J. Median sternotomy wound infections in children. Pediatr Infect Dis. 1983 Mar-Apr;2(2):105–109. doi: 10.1097/00006454-198303000-00007. [DOI] [PubMed] [Google Scholar]
- Gray E. D., Peters G., Verstegen M., Regelmann W. E. Effect of extracellular slime substance from Staphylococcus epidermidis on the human cellular immune response. Lancet. 1984 Feb 18;1(8373):365–367. doi: 10.1016/s0140-6736(84)90413-6. [DOI] [PubMed] [Google Scholar]
- Grinnell F. Fibronectin and wound healing. J Cell Biochem. 1984;26(2):107–116. doi: 10.1002/jcb.240260206. [DOI] [PubMed] [Google Scholar]
- Grossi E. A., Culliford A. T., Krieger K. H., Kloth D., Press R., Baumann F. G., Spencer F. C. A survey of 77 major infectious complications of median sternotomy: a review of 7,949 consecutive operative procedures. Ann Thorac Surg. 1985 Sep;40(3):214–223. doi: 10.1016/s0003-4975(10)60030-6. [DOI] [PubMed] [Google Scholar]
- Hall R. T., Hall S. L., Barnes W. G., Izuegbu J., Rogolsky M., Zorbas I. Characteristics of coagulase-negative staphylococci from infants with bacteremia. Pediatr Infect Dis J. 1987 Apr;6(4):377–383. doi: 10.1097/00006454-198704000-00007. [DOI] [PubMed] [Google Scholar]
- Haslett T. M., Isenberg H. D., Hilton E., Tucci V., Kay B. G., Vellozzi E. M. Microbiology of indwelling central intravascular catheters. J Clin Microbiol. 1988 Apr;26(4):696–701. doi: 10.1128/jcm.26.4.696-701.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann M., Vaudaux P. E., Pittet D., Auckenthaler R., Lew P. D., Schumacher-Perdreau F., Peters G., Waldvogel F. A. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J Infect Dis. 1988 Oct;158(4):693–701. doi: 10.1093/infdis/158.4.693. [DOI] [PubMed] [Google Scholar]
- Ishak M. A., Gröschel D. H., Mandell G. L., Wenzel R. P. Association of slime with pathogenicity of coagulase-negative staphylococci causing nosocomial septicemia. J Clin Microbiol. 1985 Dec;22(6):1025–1029. doi: 10.1128/jcm.22.6.1025-1029.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. M., Lee D. A., Regelmann W. E., Gray E. D., Peters G., Quie P. G. Interference with granulocyte function by Staphylococcus epidermidis slime. Infect Immun. 1986 Oct;54(1):13–20. doi: 10.1128/iai.54.1.13-20.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristinsson K. G., Hastings J. G., Spencer R. C. The role of extracellular slime in opsonophagocytosis of Staphylococcus epidermidis. J Med Microbiol. 1988 Nov;27(3):207–213. doi: 10.1099/00222615-27-3-207. [DOI] [PubMed] [Google Scholar]
- Langley J., Gold R. Sepsis in febrile neutropenic children with cancer. Pediatr Infect Dis J. 1988 Jan;7(1):34–37. doi: 10.1097/00006454-198801000-00008. [DOI] [PubMed] [Google Scholar]
- Lew D. P. Physiopathology of foreign body infections. Eur J Cancer Clin Oncol. 1989 Sep;25(9):1379–1382. doi: 10.1016/0277-5379(89)90091-6. [DOI] [PubMed] [Google Scholar]
- Mirro J., Jr, Rao B. N., Stokes D. C., Austin B. A., Kumar M., Dahl G. V., Colten M., Balas L., Rafferty M., Hancock M. A prospective study of Hickman/Broviac catheters and implantable ports in pediatric oncology patients. J Clin Oncol. 1989 Feb;7(2):214–222. doi: 10.1200/JCO.1989.7.2.214. [DOI] [PubMed] [Google Scholar]
- Noel G. J., Edelson P. J. Staphylococcus epidermidis bacteremia in neonates: further observations and the occurrence of focal infection. Pediatrics. 1984 Nov;74(5):832–837. [PubMed] [Google Scholar]
- Pascual A., Fleer A., Westerdaal N. A., Verhoef J. Modulation of adherence of coagulase-negative staphylococci to Teflon catheters in vitro. Eur J Clin Microbiol. 1986 Oct;5(5):518–522. doi: 10.1007/BF02017694. [DOI] [PubMed] [Google Scholar]
- Patrick C. C. Coagulase-negative staphylococci: pathogens with increasing clinical significance. J Pediatr. 1990 Apr;116(4):497–507. doi: 10.1016/s0022-3476(05)81593-8. [DOI] [PubMed] [Google Scholar]
- Patrick C. C., Kaplan S. L., Baker C. J., Parisi J. T., Mason E. O., Jr Persistent bacteremia due to coagulase-negative staphylococci in low birth weight neonates. Pediatrics. 1989 Dec;84(6):977–985. [PubMed] [Google Scholar]
- Patrick C. C., Plaunt M. R., Sweet S. M., Patrick G. S. Defining Staphylococcus epidermidis cell wall proteins. J Clin Microbiol. 1990 Dec;28(12):2757–2760. doi: 10.1128/jcm.28.12.2757-2760.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G., Locci R., Pulverer G. Adherence and growth of coagulase-negative staphylococci on surfaces of intravenous catheters. J Infect Dis. 1982 Oct;146(4):479–482. doi: 10.1093/infdis/146.4.479. [DOI] [PubMed] [Google Scholar]
- Press O. W., Ramsey P. G., Larson E. B., Fefer A., Hickman R. O. Hickman catheter infections in patients with malignancies. Medicine (Baltimore) 1984 Jul;63(4):189–200. doi: 10.1097/00005792-198407000-00001. [DOI] [PubMed] [Google Scholar]
- Schmidt B. K., Kirpalani H. M., Corey M., Low D. E., Philip A. G., Ford-Jones E. L. Coagulase-negative staphylococci as true pathogens in newborn infants: a cohort study. Pediatr Infect Dis J. 1987 Nov;6(11):1026–1031. [PubMed] [Google Scholar]
- Sherertz R. J., Raad I. I., Belani A., Koo L. C., Rand K. H., Pickett D. L., Straub S. A., Fauerbach L. L. Three-year experience with sonicated vascular catheter cultures in a clinical microbiology laboratory. J Clin Microbiol. 1990 Jan;28(1):76–82. doi: 10.1128/jcm.28.1.76-82.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojo M., Yamashita N., Goldmann D. A., Pier G. B. Isolation and characterization of a capsular polysaccharide adhesin from Staphylococcus epidermidis. J Infect Dis. 1988 Apr;157(4):713–722. doi: 10.1093/infdis/157.4.713. [DOI] [PubMed] [Google Scholar]
- Venditti M., Santini C., Serra P., Micozzi A., Gentile G., Martino P. Comparative in vitro activities of new fluorinated quinolones and other antibiotics against coagulase-negative Staphylococcus blood isolates from neutropenic patients, and relationship between susceptibility and slime production. Antimicrob Agents Chemother. 1989 Feb;33(2):209–211. doi: 10.1128/aac.33.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscoli C., Garaventa A., Boni L., Melodia A., Dini G., Cuneo R., Rizzo A., Moroni C., Rogers D., De Bernardi B. Role of Broviac catheters in infections in children with cancer. Pediatr Infect Dis J. 1988 Aug;7(8):556–560. [PubMed] [Google Scholar]
- Wade J. C., Schimpff S. C., Newman K. A., Wiernik P. H. Staphylococcus epidermidis: an increasing cause of infection in patients with granulocytopenia. Ann Intern Med. 1982 Oct;97(4):503–508. doi: 10.7326/0003-4819-97-4-503. [DOI] [PubMed] [Google Scholar]
- Winston D. J., Dudnick D. V., Chapin M., Ho W. G., Gale R. P., Martin W. J. Coagulase-negative staphylococcal bacteremia in patients receiving immunosuppressive therapy. Arch Intern Med. 1983 Jan;143(1):32–36. [PubMed] [Google Scholar]
- van Bronswijk H., Verbrugh H. A., Heezius H. C., Renders N. H., Fleer A., van der Meulen J., Oe P. L., Verhoef J. Heterogeneity in opsonic requirements of Staphylococcus epidermidis: relative importance of surface hydrophobicity, capsules and slime. Immunology. 1989 May;67(1):81–86. [PMC free article] [PubMed] [Google Scholar]


