Abstract
The brain has been known to be a sensitive target organ for the permanent organisational effects of gonadal steroids for close to 50 years. Recent advances have revealed a variety of unexpected cellular mechanisms by which steroids impact on the synaptic profile of hypothalamic nuclei critical to the control of reproduction. This review focuses on three in particular: 1) prostaglandins in the masculinisation of the preoptic area and control of male sexual behaviour; 2) GABA in the arcuate nucleus and potential control of the anterior pituitary; and 3) non-genomic activation of phosphotydolinositol 3 (PI3) kinase and glutamate in the ventromedial nucleus, which is relevant to the control of female reproductive behaviour. The importance of cell-to-cell communication, be it between neurones or between neurones and astrocytes, is highlighted as an essential principle for expanding the impact of steroids beyond those cells that express nuclear receptors.
Keywords: preoptic area, hypothalamus, prostaglandins, astrocytes, GABA, glutamate
Brain development is impacted by a multitude of endogenous and exogenous factors ranging from genetics to the environment. Variations as subtle as the phenotype of neighbouring cells, the intensity of sensory input and the amount of maternal attention received shortly after birth can permanently imprint upon on the developing neuronal network. Gonadal steroids constitute a particularly unique signal for regulating brain development in that levels differ between males and females, are endogenously derived but can be exogenously mimicked, and act during a perinatal sensitive period in specific parts of the brain to exert epigenetic and largely permanent modifications that direct adult brain function. The focus of this review is on one gonadal steroid in particular, oestradiol. The reason for this emphasis is twofold; (i) in the rodent brain oestradiol is a primary determinant of masculinisation following the aromatisation of testicular androgens, and (ii) accumulating evidence suggests that oestradiol can be synthesised entirely within the brain. We shall highlight emerging principles of oestrogen action both for purposes of achieving sex differences in the brain and as a general trophic and possibly endogenous neuroprotective factor.
Brief historical overview
One of the first principles learned by students of behavioural neuroendocrinology is the organisational/activational hypothesis of steroid action on the developing brain [see (1, 2)]. Codified in a seminal paper published 50 years ago (3), the organisational/activational hypothesis demonstrates that early hormone actions permanently organise the neural substrate so that it is appropriately activated by adult steroid hormone profiles derived from the mature gonads to achieve ultimate reproductive success both behaviourally and physiologically. In males, a brain phenotype that matches the gonadal phenotype is achieved by the onset of androgen production from the fetal testis during the last few days of gestation and into the first days of life. An unexpected component of this process was the discovery of a requisite conversion of testosterone to oestradiol within the brain, and that it was in fact oestradiol that was the dominant factor mediating masculinisation (4, 5). In females the brain is, by default, programmed to provide for adult sexual receptivity and gonadotrophin secretion patterns that optimise ovulation and fertilisation. This is largely considered a hormone-independent process, similar to ovarian development, although recent evidence has challenged this view (6).
The ability of oestradiol to masculinise the brain is restricted to a sensitive period, which in the rat begins in late embryonic life and extends postnatally for the first week to 10 days of life, depending upon the end-point. This convenient fact provides an excellent empirical tool for the study of the oestradiol mechanism of action by allowing investigators to administer oestradiol to newborn female pups and then monitor the impact in real time. This approach has been the primary tool of our research group and has provided the opportunity to dissect specific cellular events initiated by oestradiol followed by downstream sequelae that ultimately differentiate the brain into a male phenotype, or provide trophic support.
Oestradiol effects on developing neurones are not cell-autologous
When considering how steroids act on the developing brain, an easy and logical assumption is that only those cells expressing receptors for a given steroid will respond with a change in morphology and/or physiology. This seemingly simple statement not only belies the complexity of actually determining which cells have receptors for steroids, but is also inconsistent with experimental observation. Oestradiol binds to two receptor isoforms, ERalpha and ERbeta, both of which are members of a superfamily of nuclear transcription factors (7). Many atlases of steroid receptor-containing brain regions were updated, and the brain regions considered to be oestradiol-responsive were considerably expanded, following the discovery of ERbeta in 1996 (8). The additional discovery of a novel G-protein coupled oestrogen receptor, GPR30, in 2007 (9) further expanded the potential number of cells capable of directly responding to oestradiol. The inability to prove a negative notwithstanding, there is still an urge to distinguish one cell from another by whether or not it has an ER. This is not a futile exercise as it has certainly proved pivotal to the understanding of oestrogen-responsive gonadotrophin-releasing hormone (GnRH) neurones in the hypothalamus which definitively do not express any appreciable quantity of ERalpha, but do have some ERbeta (10, 11), and may still prove useful in elucidating sources of individual variation in physiology and behaviour (12). However, if we generalise to the developing brain the principle that only a subset of neurones express ER, and that only those neurones will change in response to oestradiol, we face the dilemma of searching for a needle in a haystack when trying to identify individual neurones that have been sexually differentiated. Empirically this would predict that any quantification method that either mixes ER-containing and -non-containing cells (i.e. western blot and PCR) or randomly identifies cells for morphometry (i.e. electron microscopy (EM), Golgi-Cox impregnation and immunocytochemistry) should produce low-magnitude effects with high variability. If, however, the entire population of cells within a given region changes in response to oestradiol in a co-ordinated fashion, then high-magnitude low-variability effects would be predicted. The latter scenario has proven to be true on multiple occasions for the neurones and astrocytes of the preoptic area (13, 14), the ventromedial nucleus (VMN) of the hypothalamus (15) and the arcuate nucleus (16, 17). Thus, either all the neurones and astrocytes express ERs, which have gone undetected, or there is some form of cell-to-cell communication that orchestrates morphological changes across an entire region. Careful elucidation of the mechanism of oestradiol action in various regions strongly supports the latter possibility.
Immature astrocytes are responsive to oestradiol and important to establishment of sex differences in the synaptic profile
Astrocytes in the arucate nucleus of the adult female are fundamental to synaptic changes occurring across the oestrous cycle and control of gonadotrophin secretion from the anterior pituitary (18). We reported in this journal in 2002 that arcuate astrocytes also undergo a process of oestradiol-mediated sexual differentiation such that in males they are more complex with a greater number of primary, secondary and tertiary processes (19). Multiple attempts to identify oestrogen receptors in arcuate astrocytes failed, but this became irrelevant in light of the observation that the signalling molecule mediating the effects of oestradiol on astrocyte morphology was GABA, an amino acid transmitter synthesised exclusively in neurones by the rate-limiting enzyme glutamic acid decarboxylase (GAD). Oestradiol up-regulates GAD in the developing arcuate, thereby increasing the synthesis of GABA (20), which then acts on neighbouring astrocytes to induce growth and branching of processes (Fig. 1). The increased stellation of astrocytes is strongly inversely correlated with the development of dendritic spine synapses, which are at significantly higher density on the neurones of female rat pups than on those of males (21). The changed morphology of astrocytes in the male arcuate is maintained throughout development and into adulthood (22) and is presumed to assist in, if not directly determine, the enduring sex difference in the synaptic profile observed in this region.
Fig. 1.
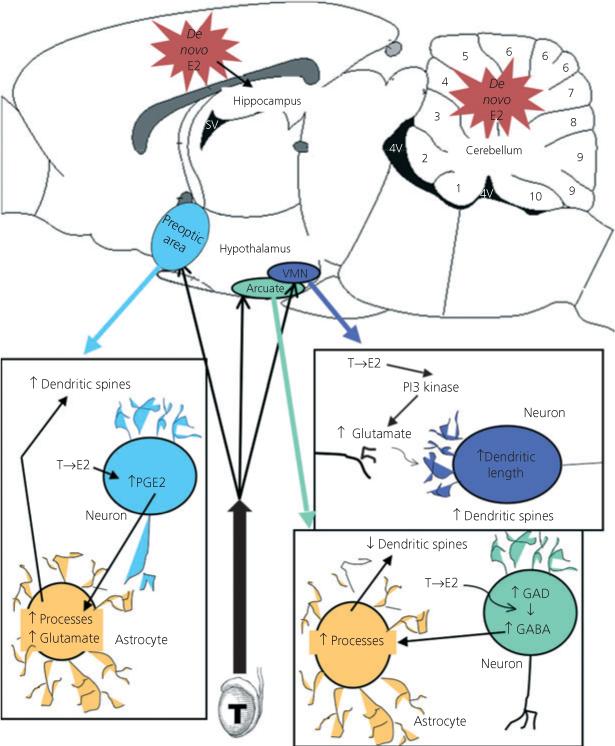
Mechanisms of oestradiol-mediated organisation of sexually dimorphic synaptic patterning. Three distinct hypothalamic nuclei show profound sex differences in the number and/or density of dendritic spines and attendant excitatory synapses. In each of these regions, oestradiol (E2) is locally aromatised from testicularly derived testosterone (T). The preoptic area is a critical brain region for control of male sexual behaviour. Early in life, males have two- to threefold more dendritic spines along a given length of dendrite than females. Oestradiol increases the synthesis of prostaglandin E2 (PGE2) by up-regulating the rate-limiting cyclooxygenase 2 (COX-2) enzyme (13). PGE2 is then believed to act on neighbouring astrocytes, increasing the stellate morphology of this cell type and inducing the release of glutamate, which in turn promotes the formation of dendritic spines on the originating or neighbouring neurones. In the ventromedial nucleus (VMN), a critical brain region controlling female sexual behaviour, oestradiol directly promotes the release of glutamate from neurones via a non-genomic activation of PI3 kinase. This presynaptic glutamate then promotes dendritic growth and formation of dendritic spines on the postsynaptic neurone (31). The arcuate nucleus contributes to control of the anterior pituitary and to female reproductive behaviour. In this nucleus oestradiol acts in neurones to increase GABA synthesis by up-regulating the rate-limiting enzyme glutamic acid decarboxylase (GAD). GABA then acts on neighbouring astrocytes, increasing process growth and branching (19). The end result is a decrease in the density of dendritic spine synapses, the opposite of the pattern achieved in the other two brain regions. There is also emerging evidence for de novo, from cholesterol, synthesis of oestradiol in the developing hippocampus, cortex and cerebellum, although the mechanisms by which oestradiol might be acting in these brain regions are as yet unknown.
Astrocytes in the nearby preoptic area (POA) also undergo oestradiol-mediated sexual differentiation (14) and, while a potential role for GABA has not been explored, there is at least partial involvement of glutamate acting at AMPA receptors. Glutamate is also a mediator of a sex difference in dendritic spine density in this region, but with an effect opposite to that observed in the arcuate nucleus, with males having a greater spine density compared with females (13). The POA is the primary brain region controlling male sex behaviour, and the permanent organisation of more spines per length of dendrite has been directly correlated with masculinisation of behaviour (23). The complexity of astrocytes is positively correlated with more synapses and is again presumed, although not proven, to be a principal determinant.
Oestradiol-induced masculinisation is mediated by a prostaglandin
Shortly after the discovery that oestradiol derived from testicular androgens was a principal determinant of masculinisation or defeminisation of the brain, there were many attempts to identify the neurotransmitter system(s) mediating those effects. The usual suspects were rounded up and slowly eliminated, beginning with the catecholamines, progressing through the biogenic amines and indolamines and on through most peptides and amino acid transmitters (24). Our own work on GABA as a determinant of behavioural masculinisation was largely ambiguous (25). This is not to say that there is no role for other transmitters in sex differentiation; there certainly is and there are many interesting sex differences, but the final common pathway of oestradiol action for masculinisation was not found, leading to the conclusion that perhaps multiple systems were modified in concert to achieve the male phenotype. This made it all the more surprising when an unexpected cell signalling system was identified as both necessary and sufficient for masculinisation of the POA synaptic profile and adult sexual behaviour, namely prostaglandin E2 (PGE2). Prostaglandins are synthesised from arachadonic acid derived from lipid membranes by the rate-limiting enzyme cyclooxygenase (COX), which comes in two forms, a constitutive (COX-1) and inducible (COX-2) form. Oestradiol up-regulates COX-2 mRNA and protein, leading to a sevenfold increase in PGE2 in the POA of developing males compared with females. Administering PGE2 to females or a COX-2 inhibitor to males will effectively masculinise brain and behaviour or completely prevent this process, respectively (23). PGE2 induces calcium-dependent glutamate release from astrocytes in other brain regions (26, 27), and its effects on the development of dendritic spines on POA neurones are partially blocked by antagonism of AMPA receptors, suggesting a similar action in this brain region. This conclusion is further supported by the ability of PGE2 to induce calcium flux in cultured POA astrocytes (Fig. 2). Taken together, these observations have led to a working model in which oestradiol up-regulates PGE2 synthesis in neurones, from which it is released to act on neigh-bouring astrocytes to induce differentiation and glutamate release which then acts back on neurones to promote the formation of dendritic spines [reviewed in Ref. (28)]. Via this route, oestradiol can act trans-cellularly to alter the morphology of neurones throughout the POA in a co-ordinated fashion.
Fig. 2.
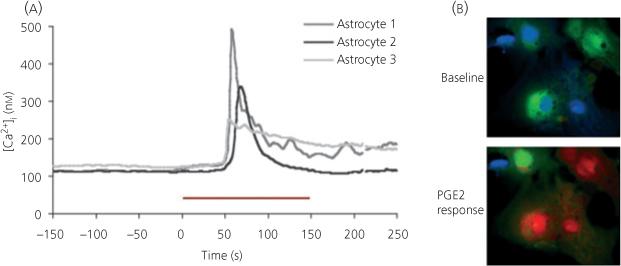
Prostaglandin E2 (PGE2) induces a calcium transient in preoptic area astrocytes. (a) The free intracellular calcium concentration was measured with the ratiometric fluorescent dye FURA-2AM in preoptic area (POA) culture astrocytes before and after exposure to bath-applied PGE2 (20 μm). The red bar represents 150 s of PGE2 exposure. A response is considered a 20% increase in the internal Ca2+ concentration ([Ca2+]i) above baseline. Traces are from three representative cells. (b) Psuedocoloured images of POA cultured astrocytes prior to (above) and during (below) PGE2 exposure. Cooler to warmer colours represent increasing [Ca2+]i.
Oestradiol non-genomically promotes glutamate release to promote dendritic spine growth in the immature mediobasal hypothalamus
The VMN is located within the mediobasal hypothalamus and is considered a critical locus in the control of female sexual behaviour (29, 30). The morphology of dendrites in this region is also sexually dimorphic, with males having longer dendrites that branch more frequently, and as a consequence having overall more dendritic spine synapses (15, 16). Astrocytes in the mediobasal hypothalamus are notably delayed in development compared with the arcuate and POA, and there is no effect of prostaglandins on the dendritic spine profile (15, 16). However, oestradiol potently promotes the formation of dendritic spines on VMN dendrites, and can do so over a time-course of hours as opposed to days. The ability of oestradiol to promote dendritic spine development in the immature hypothalamus is entirely dependent upon glutamate. Antagonising either the AMPA or NMDA ionotropic glutamate receptor prevents new spine formation (31) (Fig. 3). However, our failure to find any evidence for oestradiol-mediated changes in either the AMPA or NMDA receptor directed our attention to the presynaptic side of the equation. Combined use of electrophysiological recordings and live imaging of fluorescent dye depletion from synaptic vesicles revealed that oestradiol promotes the release of glutamate from nerve terminals. Rapid effects of oestrogens can be transduced at the membrane by direct interactions between the classic ER and MAP kinases (32-34), as well as other kinases (35-37). In this instance, we have determined that oestradiol-activated ER induces activation of PI3 kinase, thereby promoting the phosphorylation of Akt, and, via a mechanism that remains to be determined, promotes synaptic vesicle fusion with the terminal membrane. The increased glutamate release activates post-synaptic AMPA and NMDA receptors, which secondarily activates MAP kinases and leads to the production of dendritic spines. The presynaptic activation of PI3 kinase does not require protein synthesis and provides a novel mechanism by which oestradiol permanently organises brain development during a sensitive period (31).
Fig. 3.
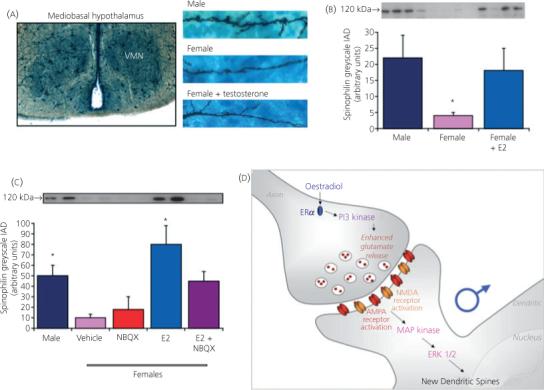
Oestradiol non-genomically induces glutamate release to promote synaptogenesis. (a) Golgi-Cox impregnation of neurones in the ventromedial nucleus (VMN) of 2-day-old rat pups reveals that dendrites in males are longer, branch more frequently (16) and have a greater number of dendritic spines (15), the site of excitatory synapses, than females. Treatment of females with testosterone for 48 h increases the growth of dendrites such that the morphology no longer differs from that in males. (b) Spinophilin has proved a reliable proxy marker for dendritic spines and is at two- to threefold higher levels in males than females. Treatment of females with oestradiol, the aromatised product of testosterone, again reverses the sex difference and this can be achieved in as little as 6 h. (c) The effects of oestradiol are prevented by antagonising glutamate receptors, implicating glutamate release in the downstream sequelae of oestradiol action. (d) A series of experiments have now established that oestradiol binds to oestradiol receptor ERalpha and subsequently activates PI3 kinase, promoting glutamate release. Glutamate binds to AMPA and NMDA receptors on the adjacent neurone, leading to activation of MAP kinase, phosphorylation of ERK, induction of spinophilin synthesis and the construction of new spines. This transneuronal communication is a novel mechanism for establishing sex differences in the brain and does not rely on ERs being present in the neurone in which synaptic changes are induced by oestradiol (31). NBQX, ampa receptor antagonist; IAD, integrated area density.
Oestradiol is synthesised locally by the developing brain
The aromatisation hypothesis, proposed over 30 years ago, neatly explained how testicular androgens could gain access to the brain by avoiding the alpha-foetoprotein buffering capacity of the foetal circulation, and then be locally aromatised within neurones in brain regions destined to be masculinised, i.e. the POA and mediobasal hypothalamus [reviewed in McCarthy (28)]. Essential support for the hypothesis included observations that aromatase activity was highest in the neonatal telencephalon and from there declined to adult levels. Over the intervening years there has been an eroding of the boundaries of the aromatisation hypothesis such that it was considered to encompass the entire brain and to imply that all regions were equally subject to the organisational/activational paradigm. Recently this slippage has been challenged with the observation that there are no sex differences in the amount of oestradiol in some telencephalic brain regions during the perinatal sensitive period (38) and that the cerebellum robustly synthesises oestradiol, as evident from higher tissue levels than those found in the circulation (39). The timing of oestradiol synthesis in the cerebellum appears to be delayed compared with other brain regions in that peak levels are found as late as the end of the first postnatal week, whereas levels seem to drop precipitously after birth in the few other areas that have been measured. The functional significance of locally synthesised oestradiol in the brain remains to be determined, but the lack of robust sex differences suggests that it may play an important trophic role that is independent of sexual differentiation.
Oestradiol is neuroprotective against glutamate-mediated excitoxicity
The advent of hormone replacement therapy in post-menopausal women has drawn substantial attention to the potentially beneficial effects of oestrogen(s) against catastrophic neuronal loss induced by sudden ischemia as well as aging-related gradual decline associated with Alzheimer's or other forms of dementia (40). Ischemia-induced neuronal loss is also a major risk factor for premature infants and full-term babies alike (41). Hippocampal neurones are particularly vulnerable to the glutamate-induced excitoxicity that occurs consequent to oxygen deprivation (42), and this is no less true for immature neurones. Physiologically relevant levels of oestradiol potently protect against glutamate-induced cell death of hippocampal neurones treated in vitro, but the mechanism by which this is achieved is fundamentally different from in mature neurones. The divergence from the adult begins with the source of calcium, which is extracellular in the adult but derives from internal stores released by activation of metabotropic glutamate receptors (mGluRs) in the neonate (43). Oestradiol down-regulates type I mGluRs and as a consequence dampens the amount of free calcium released into the cytoplasm. This raises the intriguing possibility that de novo oestradiol synthesis by the developing hippocampus is a pre-emptive step to reduce damage in the event of an ischemic event, including that associated with the natural birth process.
The mechanisms of oestradiol action are distinct and regionally specific
In this review we have highlighted at least four distinct mediators of oestradiol action in four separate brain regions: GABA in the arcuate, prostaglandins in the POA, glutamate release in the mediobasal hypothalamus and down-regulation of mGluR in the hippocampus. We have not included discussion of the well-characterised protective effect of oestradiol on naturally occurring cell death in the sexually dimorphic nucleus (SDN) or the opposite effect in the anteroventral periventricular (AVPV) nucleus of the POA (44). Oestradiol promotes the growth of axons from the principle nucleus of the bed nucleus of the stria terminalis (pBNST) via a target-derived diffusible factor (45), and down-regulates the growth-associated protein focal adhesion kinase (FAK) and paxillin in the hypothalamus (46). All of these events occur within the first few days of life and therefore at essentially the same time. Thus, instead of a unified field theory in which oestradiol acts through one common mechanism in multiple brain areas to promote masculinisation, there is a complex orchestra of distinct instruments which provides for an infinite variety of musical arrangements that produce a unique symphony of brain development in each individual.
References
- 1.Becker JB, Breedlove SM, Crews D, McCarthy MM. Behavioral Endocrinology. 2nd edn. MIT Press; Cambridge, MA: 2002. [Google Scholar]
- 2.Nelson RJ. Sinauer Assoc. Inc.; Sunderland, MA: 1995. An Introduction to Behavioral Endocrinology. [Google Scholar]
- 3.Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone proprionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- 4.McEwen BS, Lieberburg I, Chaptal C, Krey LC. Aromatization: important for sexual differentiation of the neonatal rat brain. Horm Behav. 1977;9:249–263. doi: 10.1016/0018-506x(77)90060-5. [DOI] [PubMed] [Google Scholar]
- 5.Naftolin F, Ryan KJ, Petro Z. Aromatization of Androstenedione by the Diencephalon. J Clin Endocrin Metab. 1971;33:368. doi: 10.1210/jcem-33-2-368. [DOI] [PubMed] [Google Scholar]
- 6.Bakker J, Honda S, Harada N, Balthazart J. The aromatase knockout (ArKO) mouse provides new evidence that estrogens are required for the development of the female brain. Ann N Y Acad Sci. 2003;1007:251–262. doi: 10.1196/annals.1286.024. [DOI] [PubMed] [Google Scholar]
- 7.Beato M, Klug J. Steroid hormone receptors: an update. Hum Reprod Update. 2000;6:225–236. doi: 10.1093/humupd/6.3.225. [DOI] [PubMed] [Google Scholar]
- 8.Kuiper GGJM, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson J-A. Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prossnitz ER, Arterburn JB, Sklar LA. GPR30: a G protein-coupled receptor for estrogen. Mol Cell Endocrinol. 2007;265:138–142. doi: 10.1016/j.mce.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abraham IM, Han SK, Todman MG, Korach KS, Herbison AE. Estrogen receptor beta mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J Neurosci. 2003;23:5771–5777. doi: 10.1523/JNEUROSCI.23-13-05771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shivers BD, Harlan RE, Morrell JI, Pfaff DW. Absence of oestradiol concentration in cell nuclei of LHRH-immunoreactive neurones. Nature. 1983;304:345–347. doi: 10.1038/304345a0. [DOI] [PubMed] [Google Scholar]
- 12.Ball GF, Balthazart J. Individual variation and the endocrine regulation of behaviour and physiology in birds: a cellular/molecular perspective. Philos Trans R Soc Lond B Biol Sci. 2007;363:1699–1710. doi: 10.1098/rstb.2007.0010. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amateau SK, McCarthy MM. A novel mechanism of dendritic spine plasticity involving estradiol induction of prostglandin-E2. J Neurosci. 2002;22:8586–8596. doi: 10.1523/JNEUROSCI.22-19-08586.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amateau SK, McCarthy MM. Sexual differentiation of astrocyte morphology in the developing rat preoptic area. J Neuroendo. 2002;14:904–910. doi: 10.1046/j.1365-2826.2002.00858.x. [DOI] [PubMed] [Google Scholar]
- 15.Todd BJ, Schwarz JM, Mong JA, McCarthy MM. Glutamate AMPA/kainate receptors, not GABAA receptors, mediate estradiol-induced sex differences in the hypothalamus. Dev Neurobiol. 2007;67:304–315. doi: 10.1002/dneu.20337. [DOI] [PubMed] [Google Scholar]
- 16.Mong JA, Glaser E, McCarthy MM. Gonadal steroids promote glial differentiation and alter neuronal morphology in the developing hypothalamus in a regionally specific manner. J Neurosci. 1999;19:1464–1472. doi: 10.1523/JNEUROSCI.19-04-01464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mong JA, Kurzweil RL, Davis AM, Rocca MS, McCarthy MM. Evidence for sexual differentiation of glia in rat brain. Horm Behav. 1996;30:553–562. doi: 10.1006/hbeh.1996.0058. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Segura LM, Chowen JA, Naftolin F. Endocrine glia: roles of glial cells in the brain actions of steroid and thyroid hormones and in the regulation of hormone secretion. Front Neuroendocrinol. 1996;17:180–211. doi: 10.1006/frne.1996.0005. [DOI] [PubMed] [Google Scholar]
- 19.Mong JA, Nunez JL, McCarthy MM. GABA mediates steroid-induced astrocyte differentiation in the neonatal rat hypothalamus. J Neuroendo. 2002;14:1–16. doi: 10.1046/j.1365-2826.2002.00737.x. [DOI] [PubMed] [Google Scholar]
- 20.Davis AM, Grattan DR, Selmanoff MK, McCarthy MM. Sex differences in glutamic acid decarboxylase mRNA in neonatal rat brain: Implications for sexual differentiation. Horm Behav. 1996;30:538–552. doi: 10.1006/hbeh.1996.0057. [DOI] [PubMed] [Google Scholar]
- 21.Mong JA, Roberts RC, Kelly JJ, McCarthy MM. Gonadal steroids reduce the density of axospinous synapses in the developing rat arcuate nucleus: an electron microscopy analysis. J Comp Neurol. 2001;432:259–267. doi: 10.1002/cne.1101. [DOI] [PubMed] [Google Scholar]
- 22.Mong JA, McCarthy MM. Ontogeny of sexually dimorphic astrocytes in the neonatal rat arcuate. Dev Brain Res. 2002;139:151–158. doi: 10.1016/s0165-3806(02)00541-2. [DOI] [PubMed] [Google Scholar]
- 23.Amateau SK, McCarthy MM. Induction of PGE(2) by estradiol mediates developmental masculinization of sex behavior. Nat Neurosci. 2004;7:643–650. doi: 10.1038/nn1254. [DOI] [PubMed] [Google Scholar]
- 24.De Vries GJ, Buijs RM, Van Leeuwen FW. Sex differences in vasopressin and other neurotransmitter systems in the brain. Prog Brain Res. 1984;61:185–203. doi: 10.1016/S0079-6123(08)64435-0. [DOI] [PubMed] [Google Scholar]
- 25.Davis AM, Grattan DR, McCarthy MM. Decreasing GAD neonatally attenuates steroid-induced sexual differentiation of the rat brain. Behav Neurosci. 2000;114:923–933. [PubMed] [Google Scholar]
- 26.Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- 27.Sanzgiri RP, Araque A, Haydon PG. Prostaglandin E(2) stimulates glutamate receptor-dependent astrocyte neuromodulation in cultured hippocampal cells. J Neurobiol. 1999;41:221–229. [PubMed] [Google Scholar]
- 28.McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88:91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfaff DW, Sakuma Y. Deficit in the lordosis reflex of female rats caused by lesions in the ventromedial nucleus of the hypothalamus. J Physiol. 1979;288:203–210. [PMC free article] [PubMed] [Google Scholar]
- 30.Pfaff DW, Sakuma Y. Facilitation of the lordosis reflex of female rats from the ventromedial nucleus of the hypothalamus. J Physiol. 1979;288:189–202. [PMC free article] [PubMed] [Google Scholar]
- 31.Schwarz JM, Liang S-L, Thompson SM, McCarthy MM. Estradiol induces dendritic spines on developing hypothalamic neurons by enhancing glutamate release independent of transcription: a mechanism for organizational sex differences. Neuron. 2008 doi: 10.1016/j.neuron.2008.03.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuroki Y, Fukushima K, Kanda Y, Mizuno K, Watanabe Y. Putative membrane-bound estrogen receptors possibly stimulate mitogen-activated protein kinase in the rat hippocampus. Eur J Pharmacol. 2000;400:205–209. doi: 10.1016/s0014-2999(00)00425-8. [DOI] [PubMed] [Google Scholar]
- 33.Singh M, Setalo G, Jr., Guan X, Warren M, Toran-Allerand CD. Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: convergence of estrogen and neurotrophin signaling pathways. J Neurosci. 1999;19:1179–1188. doi: 10.1523/JNEUROSCI.19-04-01179.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Y, Watters JJ, Dorsa DM. Estrogen rapidly induces the phosphorylation of the cAMP response element binding protein in rat brain. Endocrinology. 1996;137:2163–2166. doi: 10.1210/endo.137.5.8612562. [DOI] [PubMed] [Google Scholar]
- 35.Abraham IM, Herbison AE. Major sex differences in non-genomic estrogen actions on intracellular signaling in mouse brain in vivo. Neuroscience. 2005;131:945–951. doi: 10.1016/j.neuroscience.2004.10.046. [DOI] [PubMed] [Google Scholar]
- 36.Aronica SM, Kraus WL, Katzenellenbogen BS. Estrogen action via the cAMP signaling pathway: stimulation of adenylate cyclase and cAMP-regulated gene transcription. Proc Natl Acad Sci U S A. 1994;91:8517–8521. doi: 10.1073/pnas.91.18.8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Znamesnsky V, Akama KT, McEwen BS, Milner TA. Estrogen levels regulate the subcellular distribution of phosphorylated Akt in hippocampal CA1 dendrites. J Neurosci. 2003;23:2340–2347. doi: 10.1523/JNEUROSCI.23-06-02340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amateau SK, Alt JJ, Stamps CL, McCarthy MM. Brain estradiol content in newborn rats: sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology. 2004;145:2906–2917. doi: 10.1210/en.2003-1363. [DOI] [PubMed] [Google Scholar]
- 39.Lavaque E, Mayen A, Azcoitia I, Tena-Sempere M, Garcia-Segura LM. Sex differences, developmental changes, response to injury and cAMP regulation of the mRNA levels of steroidogenic acute regulatory protein, cytochrome p450scc, and aromatase in the olivocerebellar system. J Neurobiol. 2006;66:308–318. doi: 10.1002/neu.20221. [DOI] [PubMed] [Google Scholar]
- 40.Wise PM. Estrogens: protective or risk factors in brain function? Prog Neurobiol. 2003;69:181–191. doi: 10.1016/s0301-0082(03)00035-2. [DOI] [PubMed] [Google Scholar]
- 41.Thornberg E, Thiringer K, Odeback A, Milsom I. Birth asphyxia: incidence, clinical course and outcome in a Swedish population. Acta Paediatr. 1995;84:927–932. doi: 10.1111/j.1651-2227.1995.tb13794.x. [DOI] [PubMed] [Google Scholar]
- 42.Gozal E, Row BW, Schurr A, Gozal D. Developmental differences in cortical and hippocampal vulnerability to intermittent hypoxia in the rat. Neurosci Lett. 2001;305:197–201. doi: 10.1016/s0304-3940(01)01853-5. [DOI] [PubMed] [Google Scholar]
- 43.Hilton GD, Nunez JL, Bambrick L, Thompson SM, McCarthy MM. Glutamate-mediated excitotoxicity in neonatal hippocampal neurons is mediated by mGluR-induced release of Ca++ from intracellular stores and is prevented by estradiol. Eur J Neurosci. 2006;24:3008–3016. doi: 10.1111/j.1460-9568.2006.05189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Forger NG. Cell death and sexual differentiation of the nervous system. Neuroscience. 2006;138:929–938. doi: 10.1016/j.neuroscience.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 45.Ibanez MA, Gu G, Simerly RB. Target-dependent sexual differentiation of a limbic-hypothalamic neural pathway. J Neurosci. 2001;21:5652–5659. doi: 10.1523/JNEUROSCI.21-15-05652.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Speert DB, Konkle ATM, Zup SL, Schwarz JA, Shiroor C, Taylor M. Focal adhesion kinase and paxillin: novel regulators of brain sexual differentiation? Endocrinology. 2007;148:3391–3401. doi: 10.1210/en.2006-0845. [DOI] [PubMed] [Google Scholar]


