Abstract
The ubiquitous gas, carbon monoxide (CO), is of substantial biological importance, but apart from its affinity for reduced transition metals, particularly heme-iron, it is surprisingly non-reactive—as is the ferrous-carbonyl—in living systems. CO does form strong complexes with heme proteins for which molecular O2 is the preferred ligand and to which are attributed diverse physiological, adaptive, and toxic effects. Lately, it has become apparent that both exogenous and endogenous CO produced by heme oxygenase engender a pro-oxidant milieu in aerobic mammalian cells which initiates signaling related to reactive oxygen species (ROS) generation. ROS signaling contingent on CO can be segregated by CO concentration-time effects on cellular function, by the location of heme proteins, e.g. mitochondrial or non-mitochondrial sites, or by specific oxidation-reduction (redox) reactions. The fundamental responses to CO involve overt physiological regulatory events, such as activation of redox-sensitive transcription factors or stress-activated kinases, which institute compensatory expression of anti-oxidant enzymes and other adaptations to oxidative stress. In contrast, responses originating from highly elevated or protracted CO exposures tend to be non-specific, produce untoward biological oxidations, and interfere with homeostasis. This brief overview provides a conceptual framework for understanding CO biology in terms of this physiological-pathological hierarchy.
Keywords: heme oxygenase, reactive oxygen species, nitric oxide, mitochondria, heme proteins
Introduction
Carbon monoxide (CO) is a primordial gas that people have long equated with incomplete combustion and silent asphyxiation. However, since the 1950's CO also has been recognized as an endogenous product of heme metabolism 1 and today its importance is appreciated more prevalently in biology than even a decade ago.2 It is known, for instance, that CO is produced and metabolized by organisms from prokaryotes to mammals and serves as an important intrinsic signaling molecule predominantly via its propensity to bind to reduced transition-metal centers in heme proteins 3-5.
In the bacterial world, CO is a central metabolite in the anaerobic carbon cycle, and some aerobic chemotrophs even utilize it as their sole source of energy.6,7 In these microbes, as well as in the endosymbiotic mitochondria of eukaryotes, CO undergoes oxidation to CO2.8 In higher animals, especially vertebrates, CO is recognized as a by-product of the normal enzymatic degradation of heme by the heme oxygenases (HO). 9 The excess CO is carried by hemoglobin to the lungs for excretion, while that in the cell services physiological processes from platelet aggregation10 to vasodilation11 to apoptosis—including both pro- and anti-apoptotic effects.12-18 Moreover, the HO/CO system is part of the integrated defense against cell stress including heat shock, heavy metals, ROS, lipopolysaccharide (LPS), and other inflammatory processes. 19-23
The gaps in our understanding CO biology exist mainly in the biochemical mechanisms of CO signaling in integrated systems—specifically when and how CO's effects are conveyed as physiological signals based on interactions with iron- or other metallo-proteins that contain active-site transition metals. Perhaps most widely appreciated in this regard is CO's activation of guanylate cyclase (GC), a pivotal heme enzyme whose product, guanosine 3,5-cyclic monophosphate GMP (cGMP), is involved in vascular tone, gene regulation, neurotransmission, and many other cellular processes.24-27 CO's capacity to activate GC however is much weaker than is its gaseous alternative, nitric oxide (NO), which dominates cGMP-dependent cell signaling.28
Apart from its high affinity for reduced transition metals such as Fe2+, CO is notably un-reactive in biological systems, as is the ferrous-carbonyl, which is dissipated slowly by displacement of CO with molecular O2 or by oxygenation of CO to CO2.8 Nevertheless, CO does not go unattended physiologically, for its binding to reduced transition metal centers is relatively tight, especially among heme proteins for which molecular O2 is the preferred ligand. 29 The formation of metal-CO complexes generates diverse biological effects ranging from those where a specific enzyme is inhibited and the substrate accumulates to those that redirect electrons and lead to reactive oxygen and nitrogen species (RNS) formation. Indeed, NO does induce CO production through up-regulation of HO-1 mRNA and protein, thereby increasing the potential for the interaction of CO with reduced iron and influencing NO signaling independently of GC 30-34. Moreover, it has been proposed that CO and NO may in some cases bind different sides of active heme moieties. 35
Although modest in number, each CO reaction is potentially important in both cell signaling and oxidative stress, and thus contributes to the theme of this overview, which also places special emphasis on how CO induces adaptive responses to assist mammalian systems in the tolerance of oxidative stress. A discussion of the basic physiological chemistry of CO, O2 and transition metal centers however is not intended, and the reader is referred to earlier publications for that information. 4,36-38
The Realm of CO— Physiology, Adaptation, and Toxicity
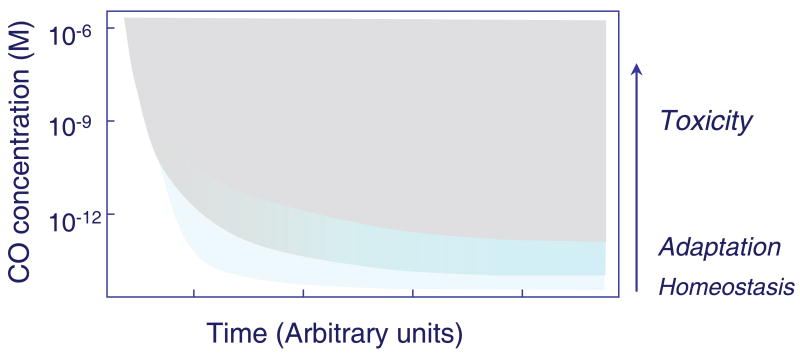
From the outset, it is important to place the responses to CO in vivo in line with archetypal physiology. In other words, in order to understand CO biology, core physiological processes that are tightly-regulated spatially and temporally need to be differentiated from secondary or adaptive responses to CO arising from interference with physiological reactions or due to secondary ROS or RNS generation. As CO concentration rises, specificity of effect is dissipated, analogous to the progressive cellular effects of oxidative and nitrosative stress. It is also imperative to identify CO toxicity, and in particular, the cellular and tissue responses that compensate for it. Customarily, the inputs and outputs for these contrasting processes are determined by studies of molecular localization, for instance, by measuring the sensitivity and specificity of an exact reaction or pathway to a particular CO concentration-time integral. An approach to stratification-of-effect is illustrated schematically in Figure 1, which conceptualizes the presumed relationships among the physiological, adaptive, and toxic effects of CO.
Figure 1.
A plot of the putative concentration-time relationships involved in the physiological, adaptive, and toxic effects of carbon monoxide (CO) in cells and tissues. As intracellular CO concentration increases from the picomolar into the nanomolar range, adaptive responses to the cell stress overlay the physiological homeostatic effects and the period for overt toxicity to supervene becomes progressively shorter.
The general concept of effect-stratification is straightforward, but as usual, the devil is in the details. Simply put, it is rarely easy to decide when an effect is homeostatic or constitutes physiological adaptation, whether such effects should be lumped or split, or how they merge into pathological responses. For instance, a CO exposure that causes widespread apoptosis in the hippocampus is clearly pathological 39 while an ROS-dependent conditioning effect produced by CO against ischemia or hypoxia 40 would usually be interpreted as adaptive. On the other hand, an arterial system that fails to dilate in response to CO after heme oxidation associated with large-conductance calcium-activated potassium channels implicates a physiological mechanism 41 42 that may also have relevance to pre-conditioning, while CO-dependent blockade of the elaboration of pro-inflammatory cytokines after exposure of leukocytes to lipopolysaccharide (LPS) 43, could be viewed alternatively as adaptation or along the lines of inhibition of normal chemokine or adherence mechanisms. Loss of an innate inflammatory response, however, would be difficult to interpret as immune counter-regulation because the endogenous CO signal is driven strictly by the degradation of heme substrate, which represents the clearance of pro-oxidant or pro-inflammatory substances with intrinsic pathogenic chemistry.
As CO concentration rises, the gas assumes a decided pleiotropy; hence, diverse, overlapping biological processes confound the interpretation of brute-force experiments. For instance, the multifarious literature on CO is frequently inscrutable with respect to when mimicry by exogenous CO can be taken as proof-of-signaling by the heme oxygenase (HO/CO) system, particularly when experimental manipulations involve massive over-expression of HO-1 or the prolonged or administration of excessive CO or CO-donating molecules to cells, tissues, or animals. Close attention to differences in CO effect due to concentration, timing, molecular specificity, and sub-cellular localization are the proximate steps for unraveling the implications of activating HO/CO in intricate injury-response paradigms.
Tissue and Cellular CO Concentrations
A fundamental factor in understanding the effects of CO from the perspective of oxidation-reduction (redox) chemistry is to know how much CO cells and tissues contain, produce normally, and take up exogenously, as well as the extent to which CO concentration varies under different types of stress. Because endogenous HO activity and heme turnover rates are difficult to quantify, it is useful to measure quasi-steady-state CO concentrations in cells and tissues using sensitive analytical techniques. Although most available techniques are destructive or lack sensitivity at the cellular level, the pioneering work of Vreman and Stephenson cannot be undervalued, as it provides an indication of just how low physiological CO levels are normally. 44-46 Even so, the dynamic range can be substantial, although the amount of endogenous CO generated to service physiological or even pathological responses is restricted on the one hand by the aforementioned need for heme substrate, and bounded on the other by the capacity of exogenous CO to exceed physiological stringency and overwhelm the cell. Moreover, in vivo, the importance of perfusion cannot be ignored, as blood flow optimizes the cellular PO2 for heme catalysis, and perfusion eliminates CO from cells and tissues via hemoglobin in the circulation.
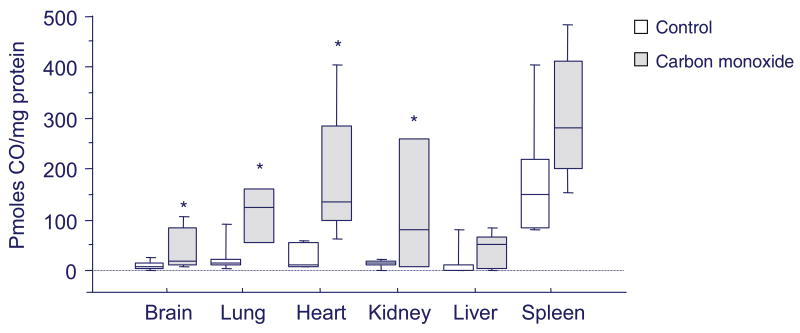
A key experimental concern is the use of excessive CO to mimic physiological homeostatic or adaptive mechanisms that instead produce compensatory responses to injury. Although tested guidelines have yet to be developed, cellular standards call for CO exposures that avoid macromolecular damage, and also in vivo, CO concentration-times that account for the effects of secondary cellular hypoxia. Adherence to these principles allows a range of tissue CO concentrations to be explored for physiological effects that are below those produced at 20-25% carboxyhemoglobin, the conventional threshold in mammals for serious poisoning (Figure 2). As a rule, the use of slow CO-releasing agents at concentrations of 50 micromolar or less in cells is advisable, and in rodents, inhaled CO concentrations that acutely do not exceed 500 ppm for one hour or 50 ppm continuously. In humans, the OSHA permissible exposure limit (PEL) of 50 ppm for 8 hours and the NIOSH recommended exposure ceiling of 200 ppm appear safe from overt toxicity while engendering physiological effects.
Figure 2.
Box plot of average cellular CO concentrations in different organs in mice. The mice were either air controls or had received one hour of CO inhalation at 500 ppm to achieve blood carboxyhemoglobin levels of ∼25%. One hour later, after euthanasia, tissues were harvested after perfusion of the vascular system with phosphate buffered saline (pH 7.4) to remove the hemoglobin. CO measurements were made in tissue homogenates using reduction gas chromatography as reported in references 44-46. The boxes represent the middle quartiles and the bars are the data ranges for 5 to 10 mice per tissue. Basal physiological CO levels are low, except for the spleen, but demonstrate significant intra- and inter-organ variability. CO exposure at the mouse's injury threshold increases cellular CO concentration by 4 to 10 fold; significant by ANOVA in all organs except the liver and the spleen (asterisks indicate P<0.05).
Heme Protein Interference by CO
The lugubrious biology of CO involves functional interference with various heme proteins, most importantly, binding ferrous heme centers on a competitive basis with molecular oxygen (O2). The binding is defined by the Warburg partition coefficient—the ratio of CO to O2 at which half the reactive sites are occupied by CO.4 It is widely known that CO avidly binds hemoglobin's ferrous heme moieties with an affinity of some 200 times that of molecular O2, thereby interfering with O2 carriage by hemoglobin. The upshot of this so-called high M value is a decrease in the O2 content of arterial blood and a change in hemoglobin allostery that hinders O2 release from the tetramer (the oxyhemoglobin dissociation curve shifts to the left). For decades, these features have been known to account for the asphyxiating actions of CO because they lower the PO2 in the tissues. 37
Once it became apparent that CO binds to a range of intracellular heme proteins, including the ferrous heme a3 of cytochrome c oxidase—the terminal electron acceptor of the mitochondrial respiratory chain—an array of new effects were revealed about the cell's handling of molecular O2.47-49 Paramount to living cells, however, is the CO inhibition of mitochondrial electron transport and increased reduction levels of the upstream electron carriers,50 most conspicuously in vivo in the cytochrome bc1 region of the chain.51 The result of CO binding to cytochrome oxidase is a slower rate of cellular respiration, an increase in the Complex III peroxide leak rate, and more limited turnover of adenosine diphosphate (ADP). On the other hand, in defense of its main energy-producing function, as noted earlier, the oxidase oxygenates CO to CO2 reflected by the slow burning of CO observed in living tissues. 52
Another site of CO binding is the cytoplasmic family of mixed function oxidases, i.e. the P450 cytochromes, originally named for the strong absorption peak of the CO complex. 53 Indeed, cytochrome P450 was first demonstrated to be a monooxygenase by means of the photochemical action spectrum technique to reverse CO inhibition of the 21-hydroxylation of adrenal 17α-OH progesterone.54 Other sites of CO-complex formation include myoglobin, 55 as well as the more recently discovered and related monomeric globins, neuroglobin and cytoglobin.56-60 In each case, CO binds only the ferrous heme, encumbering its capacity to handle O2 in the usual way. The presence of the CO-bound form, however, does keep iron from participating in redox cycling, a potentially important anti-oxidant mechanism that may limit superoxide-driven Fenton chemistry in the cell. Such a mechanism, for instance, has recently been proposed for the protective effects of CO in cerebral malaria. 61
Endogenous CO Production
As noted earlier, CO is an important endogenous gas synthesized by the heme oxygenases (HO)—enzymes that catalyze the rate-limiting step in heme degradation. Two main isoforms, HO-1 and HO-2, are fairly well understood while the status of a putative third (HO-3) is uncertain.2,62 HO breaks the alpha-methene carbon bond of the porphyrin ring using NADPH and molecular O2 in a reaction that releases equimolar amounts of biliverdin, iron, and CO. In mammalian cells, biliverdin is rapidly converted to bilirubin, which have anti-oxidant and anti-inflammatory properties, while the converting enzyme—biliverdin reductase—also has kinase activity.63 The central role of HO in the heme turnover process is illustrated in Figure 3.
Figure 3.
Diagram of the heme oxygenase (HO) enzyme system in the context of heme synthesis and heme protein turnover showing endogenous CO production and sites in the pathways of pro-oxidant and anti-oxidant effects.
Gene transcription for HO-1 (Hmox1) is inducible by a range of factors while HO-2 (Hmox2) is expressed constitutively. HO-1 responds to conditions that increase oxidative stress, and Hmox1 is induced by transcription factors, such as the NF-E2-related factor-2 (Nrf2), which binds anti-oxidant response elements (ARE) in the gene promoter region. 64,65 The ARE is critical to enzyme induction by LPS, cytokines, heat shock, hyperoxia, oxidants, hypoxia-ischemia, NO, and UV. 2,62 Also, negative regulators of HO-1 expression, especially ROS scavengers, are well described. HO induction and endogenous CO is protective in numerous experimental models of vascular, cardiac, and pulmonary injury, and against damage from certain inflammatory conditions.33,66-70 In some cases, exogenous CO replicates cell protection, which has been interpreted as cell-specific anti-inflammatory, anti-proliferative, or anti-apoptotic effects, although the biochemical mechanisms are mostly unknown.
The normal rate of endogenous CO production results in very low cellular CO concentrations—picomolar to nanomolar—that can increase several fold by heme turnover, whereas exogenous CO can raise cellular CO content by perhaps 20 to 50 fold. 44,45,71,72 At high CO levels, the physiology is superseded by the emergence of myriad adaptive and oxidative stress-related responses, overlapping hypoxia, and cellular toxicity, which are difficult to organize and categorize. Moreover, CO in the cell modulates not only heme biochemistry, but the metal-carbonyl influences the signaling and noxious activities of NO.73,74 It has already been said that CO can induce NO production but highly elevated CO concentrations may interfere with the NO synthases, especially in the absence of adequate arginine or tetrahydrobiopterin. 75-81
In spite of the requirement of all cells for heme, excess free heme is reactive and causes cellular damage by generating oxidative stress.82 Because free heme is lipophilic and naturally interacts with plasma and organelle membranes, its clearance by HO controls untoward membrane oxidations. The prevention of ROS generation by heme is facilitated by the enzyme's requirement for molecular O2, which reduces PO2 at the sites of the substrate pool and the free iron release, so the latter can be safely sequestered by ferritin.83
The extent to which endogenous CO protects against iron-mediated biological stress directly through iron-carbonyl formation is not well understood, but it is clear that HO activity does induce the iron defenses.5 Moreover, Hmox1 deficient mice develop iron-overload and a pro-inflammatory state84 and HO-1 over-expression in fibroblasts increases iron export and decreases free intracellular iron content, which imparts resistance to apoptosis.85 Despite some direct anti-oxidant effects of CO, it has become increasingly clear that exogenous and endogenous CO promulgate a pro-oxidant state in most mammalian systems that engages ROS-mediated signaling and the induction of vital anti-oxidant enzyme systems. These anti-oxidant responses include, among others, the mitochondrial superoxide dismutase (SOD2; MnSOD) as well as HO-1 itself16,17, in part via CO stimulation of heme release from mitochondria.72 And protection by HO-1 in PC12 cells in response to peroxynitrite involves induction of the catalytic subunit of glutamate-cysteine ligase (GCLC), the rate-limiting enzyme in GSH biosynthesis, via the Nrf2 transcription factor and activation of PI3-kinase. 86 This pro/anti-oxidant duality fits the proclivity of CO both to facilitate adaptation and to cause cell injury, illustrated, for instance, by discrete pro- and anti-apoptotic events during inflammation.87
CO and signaling through mitochondrial H2O2 production
For most of the 80 plus years since CO was first identified as a respiratory inhibitor37 and the nearly 20 years since it was shown to bind to cytochrome c oxidase in vivo88 mitochondrial CO effects in living tissues were considered minor because the a3 heme is primarily in the oxidized state. However, even at physiological PO2, CO does bind cytochrome oxidase, as some a3 does remain in the reduced state, especially in metabolically-active tissues like the brain and the heart. Moreover, this CO binding produces unique reduction responses in the cytochrome bc1 region of the chain,89 as well as mitochondrial ROS production sufficient to deplete mitochondrial glutathione stores, 90 which is independent of hypoxia.91 CO's penchant to generate mitochondrial ROS also has been demonstrated for endogenous CO 92-94.
Today, the area of CO signaling through mitochondrial ROS production is still in its early stages, and the issues of how much CO binding occurs physiologically and what the role of H2O2 egress from mitochondria is in cell signaling are not fully understood. Mitochondrial ROS have been implicated in a number of cell regulatory processes including stabilization of hypoxia-inducible factor-1 (HIF-1),95 cell proliferation,96 angiogenesis factor expression,97 metalloproteinase expression in tumor cells, 98,99 and mitochondrial biogenesis. 92
The HIF-1 example is instructive from the standpoint of intricacy and controversy, for instance in macrophages, where CO reportedly increases HIF-1 stability through mitochondrial ROS production.100 Such stability is clearly conditional101 as any mitochondrial ROS mechanism would require a high peroxide leak rate and therefore substantial CO binding, for instance, as occurs at hundreds of mmHg in the carotid body where photochemical reversibility of HIF-1 effect has actually been demonstrated by the a3-CO action spectrum.102 Frank inhibition of the respiratory chain by CO causes chemical hypoxia, which invokes non-ROS mechanisms by slowing ATP turnover and cell growth.103 Inhibition of respiration also increases PO2 and Krebs' cycle metabolite accumulation while the abrogation of the HIF-1 effect in respiration-deficient cells sidesteps the issue of regulation since a high PO2 opposes iron-carbonyl formation and suppresses HIF-1α in the classical way. Therefore, in the interest of clarity, I will use activation of mitochondrial biogenesis, which is sensitive to low CO levels, to illustrate the principles of H2O2-mediated cell signaling by mitochondria.
Mitochondrial biogenesis is a specialized bi-genomic program that maintains the cell's mitochondrial mass and allows it to compensate stresses associated with high energy demand or which involve mitochondrial damage.104 The regulation of mitochondrial volume density and functional capacity through mitochondrial biogenesis requires close coordination between nucleus and mitochondrion at the level of both genomes. Communication of nucleus with mitochondria—anterograde—and mitochondria with nucleus—retrograde—appear to involve two endogenous gases, NO and CO, and the retrograde CO signal clearly engages mitochondrial H2O2 production as it is blocked by mitochondrial targeting of catalase.92
The main source of H2O2 production by mitochondria is Complex III, regulated specifically by single electron production of superoxide in the Q cycle.105 The rate of superoxide production is enhanced by slowing the terminal transfer rate of electrons to molecular O2 by CO binding at cytochrome c oxidase (and NO in some cases) for two reasons. First, the delay in electron transfer allows the upstream carriers to become more reduced and fill the Q cycle electron pool. Second, the rate of mitochondrial electron transfer from semi-reduced Q to molecular O2 to form superoxide is proportional to the product of the concentrations of semi-reduced Q and O2, and the O2 concentration increases with inhibition of the oxidase. Another factor is SOD2, which vectors H2O2 out of the mitochondrion.98 In this respect, SOD2 induction by CO serves a dual purpose: the first is as a classical superoxide scavenger and the second as a ROS-signaling molecule. These concepts are illustrated in Figure 4.
Figure 4.
Schematic diagram of the effects of cytochrome a,a3-CO binding on mitochondrial electron transport and superoxide (O2-) and H2O2 production by Complex III. The increase in the reduction state of the cytochrome bc1 region of the respiratory chain has been demonstrated in vivo.92 The reduced, oxidized, and semi-quinone forms of ubiqinone are indicated by QH2, Q, and Q. respectively.
CO-mediated mitochondrial H2O2 production serves mitochondrial biogenesis in the following way. Mitochondrial H2O2 oxidizes functional thiol groups on counter-regulatory phosphatases, like PTEN and PTP1B, inactivating them.96,106,107 This phosphatase inactivation permits unopposed activity of the pro-survival kinase Akt (PKB)—as observed after CO.40,92,108 In the mitochondrial biogenesis program, Akt is regulatory, for example, in the phosphorylation of nuclear respiratory factor-1 (NRF-1), a key transcription factor for some 100 genes involved in oxidative phosphorylation, heme synthesis, and mitochondrial biogenesis. 109 An example of how such CO regulation operates is illustrated in Figure 5 using the nuclear-encoded mitochondrial transcription factor–A (Tfam) as the readout. Tfam is essential for mitochondrial DNA (mtDNA) transcription, replication, and the maintenance of mtDNA copy number.110 CO also stimulates the NRF-2 transcription factor as well as the co-activator PGC-1α, also required for the expression of mitochondrial transcriptosome proteins needed in mitochondrial biogenesis.111
Figure 5.
Role of CO-dependent mitochondrial H2O2 signaling in mitochondrial biogenesis. The egress of H2O2 from mitochondria allows unopposed Akt kinase activity via inactivation of thiol moieties in counter-regulatory phosphatases. Akt phosphorylates NRF-1, which translocates to the nucleus and transactivates the gene for mitochondrial transcription factor-A (Tfam), required by the bi-genomic mitochondrial biogenesis program for mtDNA transcription and replication.
An important factor in the active mitochondrial biogenesis program initiated by CO is HO-1 induction, supported in part by mitochondrial heme release.72 This would provide substrate for the continued production of endogenous CO to stabilize the retrograde signal in the on-position.92 In this case, endogenous CO, like NO, may also activate GC, which has an important gene regulatory role in mitochondrial biogenesis.112 Although the importance of mitochondrial biogenesis to homeostasis is self-evident, the program is also essential for cells and tissues to survive and recover from mitochondrial damage, for example, during sepsis,113 as well as from other damage that activates energy-requiring repair processes. The HO/CO system also protects the heart from doxorubicin-induced cardiotoxicity by restoring mitochondrial biogenesis, which opposes necrosis and apoptosis 114, and HO/CO has been implicated in the adjustment of mitochondrial phenotype to oxidative stress.16
CO and non-mitochondrial ROS signaling
ROS-dependent signaling, of course, also occurs via non-mitochondrial sources, perhaps most notably via the family of NADPH oxidase (Nox) and Duox enzymes that generate ROS in various tissues under physiological circumstances.115 The Nox enzymes are di-heme, flavin-adenine dinucleotide-containing membrane proteins that catalyze the NAD(P)H-dependent single electron reduction of O2 to superoxide anion. CO binding to Nox cytochrome b558 moiety has been demonstrated, although the affinity is low and the reaction is slow. 116,117 This effect of CO would of course decrease ROS production and signaling by Nox, but simultaneously increase ROS production by mitochondria.103 In any event, suppression of ROS production by Nox has been invoked to explain inhibition of toll-like receptor (TLR) targeting to lipid rafts in macrophages 118 as well as in the anti-apoptotic effects of CO during hyperoxia.119 The theory that CO inhibits pro-inflammatory signaling by “differentially-inhibiting” TLR trafficking to lipid rafts, thereby blocking access to ligands at the plasma membrane, is said to involve caveolin-1, but how ROS inhibition participates is unknown.
The search for the mechanisms of the anti-inflammatory effects of CO through blocking pro-inflammatory cytokine production while augmenting several anti-inflammatory cytokines stems from observations of CO and cGMP-independent, p38 mitogen-activated protein kinase (MAPK) signaling. 13,66 The topic of p38 MAPK activation by CO is distinguished from mitochondrial ROS production because p38 isoforms in mammalian cells are broadly sensitive to oxidative, environmental, and inflammatory stress, e.g. cytokines.120 The p38 MAP kinases (α, β, γ and δ) act in a cascade in which the membrane-proximal components activate upstream kinases—primarily MEK3 and MEK6—in response to physical and chemical stressors including oxidative stress (most notably H2O2), UV, hypoxia, ischemia, and early-phase cytokines, especially tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1). Therefore, in considering CO an “anti-oxidant” and “anti-inflammatory” molecule because of p38 MAPK activation one should keep in mind that CO likely activates the kinase by generating oxidants and that p38 activation is a component of an integrated adaptive response to oxidants.
The precise details of p38 MAPK activation by oxidative stress (as well as many other protein kinases) are still in process in most cell types, but as noted earlier, significant data for Akt activation by exogenous and endogenous CO already indicates that mitochondrial H2O2 production92 serves multiple adaptive signaling and protective functions. The sources of ROS for p38 MAPK activation may be non-mitochondrial, e.g. from the vascular NAD(P)H oxidases, or in tissue or vascular injury, stem from ROS production by the influx of inflammatory cells. This point requires better resolution, but it is foreseeable that the predominant sources of ROS production under the influence of CO or the CO/HO system will depend on cell type and injury model as well as on the CO dose and the duration of dispensation. At the same time, CO binding to multiple NAD(P)H-requiring heme proteins in the cell could expand the cytosolic NAD(P)H pool and produce an assortment of secondary redox effects.
The role of endogenous CO in modulating the innate immune response is also far from settled, and data on interactions with the early-phase cytokine response, i.e. mediated by the NF-kB transcription factor, are conflicting. One study supports HO-1 transcriptional induction by LPS through NF-kB and p38 MAPK 121 and another that heme induction of HO-1 opposes neutrophil apoptosis, but requires nuclear translocation of the NF-kB p65 subunit and ERK2 along with Akt phosphorylation. 122 On the other hand, heme-driven HO-1 induction inhibits NF-kB and TNF-α mediated E-selectin and VCAM-1 expression in endothelial cells, but this does not require p38 MAPK and cannot be replicated with CO.123 In other situations, cytokines induce HO-1 and endogenous CO production; for instance IL-10 induces HO-1 expression through activation of STAT-3 (signal transducer and activator of transcription) as well as activation of the PI-3 kinase pathway, yet this does not appear to play a role in the anti-inflammatory activities of the cytokine.124 HO-1 gene expression is also induced in hepatocytes by IL-6, and activation of the JAK/STAT pathway by the IL-6 receptor is necessary.125
Finally, CO has been implicated in the regulation of heme-based transcription factors stemming from observations some years ago that CO might serve as a gaseous neurotransmitter. 126 More recently, a role of CO is emerging in the regulation of so-called gas-sensitive transcription factors for which CO binding to the heme moiety clearly enhances DNA promoter binding and activates gene transcription. At least two heme-based transcription factors have been reported to be CO-sensitive, NPAS2-BMAL1 and the insect nuclear receptor, e75.127-129 The regulation of NPAS2, which helps control the hypothalamic circadian clock in the mammalian brain, involves PAS domains that bind heme as a prosthetic group, forming a gas-regulated sensor that exerts heme-control of DNA binding. A model of the pathway involves light-induction of heme- and iron homeostasis-related transcripts including Hmox2 and cytochrome P450 oxidoreductase (Por). HO-2 thus generates CO as a signal, and the redox state of the cell is influenced by the NADPH/NADP ratio. 127 Presently however, the involvement of ROS in this type of CO signaling is unknown.
CO and NO
Earlier it was mentioned that both CO and NO activate the GC system by binding the active heme and enhancing cGMP production. Although NO is decidedly more potent than CO in activating GC, for instance in vasodilation, 130 CO does operate in the system under specific physiological conditions; for instance, by regulating vascular smooth muscle cell proliferation in hypoxia. 131 This is an example of a GC-mediated physiological process apart from vasodilation that may be activated by CO, particularly if NO production is restricted, 132 for instance, by limited availability of O2, tetrahydrobiopterin, or L-arginine. On the other hand, CO does interfere as a function of dose with cell growth by inhibiting respiration103 as well as by stimulating the cellular egress of iron.85,133 Therefore, similar to NO, the elucidation of physiological effects of CO on cell proliferation is difficult, and like apoptosis, both pro- and anti-proliferative effects of CO have been reported. 131,134-137
NO is the more reactive of the two gases, having the same effective size and polarity as the O2 molecule, and unlike CO, binds to both Fe2+ and Fe3+ heme. Moreover, low CO concentrations stimulate NO release and are associated with peroxynitrite generation in vascular cells.73,138 The chemistry of CO-mediated NO release depends on the initial distributions of NO and CO in the cell and according to the differing equilibrium constants for transition-metal binding of the two gases.4,34,139
The association constants for NO binding to Fe2+ heme proteins such as myoglobin and hemoglobin are greater than those for CO (see 4 for references). For hemoglobin, the affinity of Fe2+ for NO is at least a thousand times greater than for CO; however, CO dissociation is much slower than for NO or O2. 140 Therefore, CO gradually displaces NO from Fe2+ heme proteins, requiring minutes or longer for equilibrium even at CO excess. NO displacement from Fe2+ by CO may also be enhanced in proximity to reduced thiols that serve as a sink for SNO-protein formation. The kinetics of CO displacement of NO from heme at physiological concentrations of NO and O2 are not obvious because CO is usually introduced at far above the relevant concentrations.
Although this brief review has focused on exogenous CO and the endogenous HO/CO system, it is also known that tiny amounts of CO are produced by lipid peroxidation during periods of oxidative stress.141,142 CO formation also occurs during the P450 metabolism of the halogenated methanes,143 some of which have proven quite useful as CO donating molecules. The physiological significance of such alternative sources of endogenous CO remains unknown, and their interactions with the mechanisms discussed here remain to be investigated.
Conclusions
The biological significance of and interest in carbon monoxide is mounting, but apart from its high affinity for reduced transition metals, especially heme-iron, CO is surprisingly un-reactive—as is the ferrous-carbonyl moiety. CO does form relatively tight complexes with heme proteins for which molecular O2 is the preferred ligand, and this chemistry is responsible for the diverse physiological, adaptive, and toxic effects of the gas. Over the past few years, it has become increasingly apparent that exogenous CO exposure and the endogenous production of CO by HO favors a pro-oxidant milieu in aerobic mammalian cells that promotes ROS-dependent signaling, including the activation of redox-sensitive transcription factors and protein kinases, which induce certain anti-oxidant enzyme systems, including HO-1 itself. Early CO-dependent ROS events can be partitioned on the basis of site of origin—mitochondrial or non-mitochondrial—of heme proteins that play unique roles in the cellular adaptation to oxidative stress, while the cell's responses to exposure to high or protracted CO concentrations lack specificity and lead to interference with homeostasis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sjostrand T. Early studies of CO production. Ann N Y Acad Sci. 1970;174:5–10. doi: 10.1111/j.1749-6632.1970.tb49767.x. [DOI] [PubMed] [Google Scholar]
- 2.Maines MD. The heme oxygenase system: update 2005. Antioxid Redox Signal. 2005;7:1761–6. doi: 10.1089/ars.2005.7.1761. [DOI] [PubMed] [Google Scholar]
- 3.Caughey WS. Carbon monoxide bonding in hemeproteins. Ann N Y Acad Sci. 1970;174:148–53. doi: 10.1111/j.1749-6632.1970.tb49781.x. [DOI] [PubMed] [Google Scholar]
- 4.Piantadosi CA. Biological chemistry of carbon monoxide. Antioxid Redox Signal. 2002;4:259–70. doi: 10.1089/152308602753666316. [DOI] [PubMed] [Google Scholar]
- 5.Boczkowski J, Poderoso JJ, Motterlini R. CO-metal interaction: Vital signaling from a lethal gas. Trends Biochem Sci. 2006;31:614–21. doi: 10.1016/j.tibs.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Roberts GP, Youn H, Kerby RL. CO-sensing mechanisms. Microbiol Mol Biol Rev. 2004;68:453–73. doi: 10.1128/MMBR.68.3.453-473.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts GP, Kerby RL, Youn H, Conrad M. CooA, a paradigm for gas sensing regulatory proteins. J Inorg Biochem. 2005;99:280–92. doi: 10.1016/j.jinorgbio.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 8.Young LJ, Caughey WS. Oxygenation of carbon monoxide by bovine heart cytochrome c oxidase. Biochemistry. 1986;25:152–61. doi: 10.1021/bi00349a022. [DOI] [PubMed] [Google Scholar]
- 9.Tenhunen R, Marver HS, Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969;244:6388–94. [PubMed] [Google Scholar]
- 10.Brune B, Ullrich V. Inhibition of platelet aggregation by carbon monoxide is mediated by activation of guanylate cyclase. Mol Pharmacol. 1987;32:497–504. [PubMed] [Google Scholar]
- 11.Ramos KS, Lin H, McGrath JJ. Modulation of cyclic guanosine monophosphate levels in cultured aortic smooth muscle cells by carbon monoxide. Biochem Pharmacol. 1989;38:1368–70. doi: 10.1016/0006-2952(89)90347-x. [DOI] [PubMed] [Google Scholar]
- 12.Fang J, Akaike T, Maeda H. Antiapoptotic role of heme oxygenase (HO) and the potential of HO as a target in anticancer treatment. Apoptosis. 2004;9:27–35. doi: 10.1023/B:APPT.0000012119.83734.4e. [DOI] [PubMed] [Google Scholar]
- 13.Brouard S, et al. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med. 2000;192:1015–26. doi: 10.1084/jem.192.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu XM, Chapman GB, Peyton KJ, Schafer AI, Durante W. Carbon monoxide inhibits apoptosis in vascular smooth muscle cells. Cardiovasc Res. 2002;55:396–405. doi: 10.1016/s0008-6363(02)00410-8. [DOI] [PubMed] [Google Scholar]
- 15.Liu XM, Chapman GB, Wang H, Durante W. Adenovirus-mediated heme oxygenase-1 gene expression stimulates apoptosis in vascular smooth muscle cells. Circulation. 2002;105:79–84. doi: 10.1161/hc0102.101369. [DOI] [PubMed] [Google Scholar]
- 16.Piantadosi CA, Carraway MS, Suliman HB. Carbon monoxide, oxidative stress, and mitochondrial permeability pore transition. Free Radic Biol Med. 2006;40:1332–9. doi: 10.1016/j.freeradbiomed.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 17.Thom SR, Fisher D, Xu YA, Notarfrancesco K, Ischiropoulos H. Adaptive responses and apoptosis in endothelial cells exposed to carbon monoxide. Proc Natl Acad Sci U S A. 2000;97:1305–10. doi: 10.1073/pnas.97.3.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song R, et al. Carbon monoxide promotes Fas/CD95-induced apoptosis in Jurkat cells. J Biol Chem. 2004;279:44327–34. doi: 10.1074/jbc.M406105200. [DOI] [PubMed] [Google Scholar]
- 19.Calabrese V, Stella AM, Butterfield DA, Scapagnini G. Redox regulation in neurodegeneration and longevity: role of the heme oxygenase and HSP70 systems in brain stress tolerance. Antioxid Redox Signal. 2004;6:895–913. doi: 10.1089/ars.2004.6.895. [DOI] [PubMed] [Google Scholar]
- 20.Siow RC, Sato H, Mann GE. Heme oxygenase-carbon monoxide signalling pathway in atherosclerosis: anti-atherogenic actions of bilirubin and carbon monoxide? Cardiovasc Res. 1999;41:385–94. doi: 10.1016/s0008-6363(98)00278-8. [DOI] [PubMed] [Google Scholar]
- 21.Caudill TK, Resta TC, Kanagy NL, Walker BR. Role of endothelial carbon monoxide in attenuated vasoreactivity following chronic hypoxia. Am J Physiol. 1998;275:R1025–30. doi: 10.1152/ajpregu.1998.275.4.R1025. [DOI] [PubMed] [Google Scholar]
- 22.Fujita T, et al. Paradoxical rescue from ischemic lung injury by inhaled carbon monoxide driven by derepression of fibrinolysis. Nat Med. 2001;7:598–604. doi: 10.1038/87929. [DOI] [PubMed] [Google Scholar]
- 23.Otterbein LE. Carbon monoxide: innovative anti-inflammatory properties of an age-old gas molecule. Antioxid Redox Signal. 2002;4:309–19. doi: 10.1089/152308602753666361. [DOI] [PubMed] [Google Scholar]
- 24.Arias-Diaz J, Vara E, Garcia C, Villa N, Balibrea JL. Evidence for a cyclic guanosine monophosphate-dependent, carbon monoxide-mediated, signaling system in the regulation of TNF-alpha production by human pulmonary macrophages. Arch Surg. 1995;130:1287–93. doi: 10.1001/archsurg.1995.01430120041006. [DOI] [PubMed] [Google Scholar]
- 25.Brune B, Schmidt KU, Ullrich V. Activation of soluble guanylate cyclase by carbon monoxide and inhibition by superoxide anion. Eur J Biochem. 1990;192:683–8. doi: 10.1111/j.1432-1033.1990.tb19276.x. [DOI] [PubMed] [Google Scholar]
- 26.Christodoulides N, Durante W, Kroll MH, Schafer AI. Vascular smooth muscle cell heme oxygenases generate guanylyl cyclase-stimulatory carbon monoxide. Circulation. 1995;91:2306–9. doi: 10.1161/01.cir.91.9.2306. [DOI] [PubMed] [Google Scholar]
- 27.Durante W, Kroll MH, Christodoulides N, Peyton KJ, Schafer AI. Nitric oxide induces heme oxygenase-1 gene expression and carbon monoxide production in vascular smooth muscle cells. Circ Res. 1997;80:557–64. doi: 10.1161/01.res.80.4.557. [DOI] [PubMed] [Google Scholar]
- 28.Martin E, Berka V, Bogatenkova E, Murad F, Tsai AL. Ligand selectivity of soluble guanylyl cyclase: effect of the hydrogen-bonding tyrosine in the distal heme pocket on binding of oxygen, nitric oxide, and carbon monoxide. J Biol Chem. 2006;281:27836–45. doi: 10.1074/jbc.M601078200. [DOI] [PubMed] [Google Scholar]
- 29.Caughey WS, Barlow CH, Maxwell JC, Volpe JA, Wallace WJ. Reactions of oxygen with hemoglobin, cytochrome c oxidase and other hemeproteins. Ann N Y Acad Sci. 1975;244:1–9. doi: 10.1111/j.1749-6632.1975.tb41517.x. [DOI] [PubMed] [Google Scholar]
- 30.Choi BM, Pae HO, Chung HT. Nitric oxide priming protects nitric oxide-mediated apoptosis via heme oxygenase-1 induction. Free Radic Biol Med. 2003;34:1136–45. doi: 10.1016/s0891-5849(03)00064-9. [DOI] [PubMed] [Google Scholar]
- 31.Brune B, Mohr S, Messmer UK. Protein thiol modification and apoptotic cell death as cGMP-independent nitric oxide (NO) signaling pathways. Rev Physiol Biochem Pharmacol. 1996;127:1–30. doi: 10.1007/BFb0048263. [DOI] [PubMed] [Google Scholar]
- 32.Foresti R, Motterlini R. The heme oxygenase pathway and its interaction with nitric oxide in the control of cellular homeostasis. Free Radic Res. 1999;31:459–75. doi: 10.1080/10715769900301031. [DOI] [PubMed] [Google Scholar]
- 33.Zuckerbraun BS, et al. Carbon monoxide protects against liver failure through nitric oxide-induced heme oxygenase 1. J Exp Med. 2003;198:1707–16. doi: 10.1084/jem.20031003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cooper CE. Nitric oxide and iron proteins. Biochim Biophys Acta. 1999;1411:290–309. doi: 10.1016/s0005-2728(99)00021-3. [DOI] [PubMed] [Google Scholar]
- 35.Andrew CR, Green EL, Lawson DM, Eady RR. Resonance Raman studies of cytochrome c′ support the binding of NO and CO to opposite sides of the heme: implications for ligand discrimination in heme-based sensors. Biochemistry. 2001;40:4115–22. doi: 10.1021/bi0023652. [DOI] [PubMed] [Google Scholar]
- 36.Chan MK. Recent advances in heme-protein sensors. Curr Opin Chem Biol. 2001;5:216–22. doi: 10.1016/s1367-5931(00)00193-9. [DOI] [PubMed] [Google Scholar]
- 37.Coburn RF. Mechanisms of carbon monoxide toxicity. Prev Med. 1979;8:310–22. doi: 10.1016/0091-7435(79)90008-2. [DOI] [PubMed] [Google Scholar]
- 38.Gilles-Gonzalez MA, Gonzalez G. Heme-based sensors: defining characteristics, recent developments, and regulatory hypotheses. J Inorg Biochem. 2005;99:1–22. doi: 10.1016/j.jinorgbio.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Piantadosi CA, Zhang J, Levin ED, Folz RJ, Schmechel DE. Apoptosis and delayed neuronal damage after carbon monoxide poisoning in the rat. Exp Neurol. 1997;147:103–14. doi: 10.1006/exnr.1997.6584. [DOI] [PubMed] [Google Scholar]
- 40.Fujimoto H, et al. Carbon monoxide protects against cardiac ischemia--reperfusion injury in vivo via MAPK and Akt--eNOS pathways. Arterioscler Thromb Vasc Biol. 2004;24:1848–53. doi: 10.1161/01.ATV.0000142364.85911.0e. [DOI] [PubMed] [Google Scholar]
- 41.Williams SE, et al. Hemoxygenase-2 is an oxygen sensor for a calcium-sensitive potassium channel. Science. 2004;306:2093–7. doi: 10.1126/science.1105010. [DOI] [PubMed] [Google Scholar]
- 42.Jaggar JH, et al. Heme is a carbon monoxide receptor for large-conductance Ca2+-activated K+ channels. Circ Res. 2005;97:805–12. doi: 10.1161/01.RES.0000186180.47148.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morse D, et al. Suppression of inflammatory cytokine production by carbon monoxide involves the JNK pathway and AP-1. J Biol Chem. 2003;278:36993–8. doi: 10.1074/jbc.M302942200. [DOI] [PubMed] [Google Scholar]
- 44.Vreman HJ, et al. Concentration of carbon monoxide (CO) in postmortem human tissues: effect of environmental CO exposure. J Forensic Sci. 2006;51:1182–90. doi: 10.1111/j.1556-4029.2006.00212.x. [DOI] [PubMed] [Google Scholar]
- 45.Marks GS, Vreman HJ, McLaughlin BE, Brien JF, Nakatsu K. Measurement of endogenous carbon monoxide formation in biological systems. Antioxid Redox Signal. 2002;4:271–7. doi: 10.1089/152308602753666325. [DOI] [PubMed] [Google Scholar]
- 46.Vreman HJ, Stevenson DK. Heme oxygenase activity as measured by carbon monoxide production. Anal Biochem. 1988;168:31–8. doi: 10.1016/0003-2697(88)90006-1. [DOI] [PubMed] [Google Scholar]
- 47.Basu A, Yin M, Waterland RA, Chance B. Kinetics of ligand binding of cytochrome oxidases: a comparative study. Biochim Biophys Acta. 1994;1184:291–5. doi: 10.1016/0005-2728(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 48.Nicholls P, Chanady GA. Reactivity of photoreduced cytochrome aa3 complexes with molecular oxygen. Biochem J. 1981;194:713–20. doi: 10.1042/bj1940713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wikstrom KF, Harmon HJ, Ingledew WJ, Chance B. A re-evaluation of the spectral, potentiometric and energy-linked properties of cytochrome c oxidase in mitochondria. FEBS Lett. 1976;65:259–77. doi: 10.1016/0014-5793(76)80127-5. [DOI] [PubMed] [Google Scholar]
- 50.Vanneste WH. The stoichiometry and absorption spectra of components a and a-3 in cytochrome c oxidase. Biochemistry. 1966;5:838–48. doi: 10.1021/bi00867a005. [DOI] [PubMed] [Google Scholar]
- 51.Piantadosi CA, Sylvia AL, Saltzman HA, Jobsis-Vandervliet FF. Carbon monoxide-cytochrome interactions in the brain of the fluorocarbon-perfused rat. J Appl Physiol. 1985;58:665–72. doi: 10.1152/jappl.1985.58.2.665. [DOI] [PubMed] [Google Scholar]
- 52.Fenn WO. The burning of CO in tissues. Ann N Y Acad Sci. 1970;174:64–71. doi: 10.1111/j.1749-6632.1970.tb49772.x. [DOI] [PubMed] [Google Scholar]
- 53.Omura T, Sato R, Cooper DY, Rosenthal O, Estabrook RW. Function of cytochrome P-450 of microsomes. Fed Proc. 1965;24:1181–9. [PubMed] [Google Scholar]
- 54.Estabrook RW, Cooper DY, Rosenthal O. The Light Reversible Carbon Monoxide Inhibition of the Steroid C21-Hydroxylase System of the Adrenal Cortex. Biochem Z. 1963;338:741–55. [PubMed] [Google Scholar]
- 55.Coburn RF, Mayers LB. Myoglobin O2 tension determined from measurement of carboxymyoglobin in skeletal muscle. Am J Physiol. 1971;220:66–74. doi: 10.1152/ajplegacy.1971.220.1.66. [DOI] [PubMed] [Google Scholar]
- 56.Couture M, Burmester T, Hankeln T, Rousseau DL. The heme environment of mouse neuroglobin. Evidence for the presence of two conformations of the heme pocket. J Biol Chem. 2001;276:36377–82. doi: 10.1074/jbc.M103907200. [DOI] [PubMed] [Google Scholar]
- 57.Dewilde S, et al. Biochemical characterization and ligand binding properties of neuroglobin, a novel member of the globin family. J Biol Chem. 2001;276:38949–55. doi: 10.1074/jbc.M106438200. [DOI] [PubMed] [Google Scholar]
- 58.Fago A, et al. Allosteric regulation and temperature dependence of oxygen binding in human neuroglobin and cytoglobin. Molecular mechanisms and physiological significance. J Biol Chem. 2004;279:44417–26. doi: 10.1074/jbc.M407126200. [DOI] [PubMed] [Google Scholar]
- 59.Sawai H, et al. Structural characterization of the proximal and distal histidine environment of cytoglobin and neuroglobin. Biochemistry. 2005;44:13257–65. doi: 10.1021/bi050997o. [DOI] [PubMed] [Google Scholar]
- 60.Vallone B, Nienhaus K, Matthes A, Brunori M, Nienhaus GU. The structure of carbonmonoxy neuroglobin reveals a heme-sliding mechanism for control of ligand affinity. Proc Natl Acad Sci U S A. 2004;101:17351–6. doi: 10.1073/pnas.0407633101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pamplona A, et al. Heme oxygenase-1 and carbon monoxide suppress the pathogenesis of experimental cerebral malaria. Nat Med. 2007;13:703–10. doi: 10.1038/nm1586. [DOI] [PubMed] [Google Scholar]
- 62.Abraham NG, Kappas A. Heme oxygenase and the cardiovascular-renal system. Free Radic Biol Med. 2005;39:1–25. doi: 10.1016/j.freeradbiomed.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 63.Maines MD, Miralem T, Lerner-Marmarosh N, Shen J, Gibbs PE. Human biliverdin reductase, a previously unknown activator of protein kinase C betaII. J Biol Chem. 2007;282:8110–22. doi: 10.1074/jbc.M513427200. [DOI] [PubMed] [Google Scholar]
- 64.Liu XM, et al. Endoplasmic reticulum stress stimulates heme oxygenase-1 gene expression in vascular smooth muscle. Role in cell survival. J Biol Chem. 2005;280:872–7. doi: 10.1074/jbc.M410413200. [DOI] [PubMed] [Google Scholar]
- 65.Lee BS, et al. Carbon monoxide mediates heme oxygenase 1 induction via Nrf2 activation in hepatoma cells. Biochem Biophys Res Commun. 2006;343:965–72. doi: 10.1016/j.bbrc.2006.03.058. [DOI] [PubMed] [Google Scholar]
- 66.Otterbein LE, et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–8. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 67.Akamatsu Y, et al. Heme oxygenase-1-derived carbon monoxide protects hearts from transplant associated ischemia reperfusion injury. Faseb J. 2004;18:771–2. doi: 10.1096/fj.03-0921fje. [DOI] [PubMed] [Google Scholar]
- 68.Chapman JT, Otterbein LE, Elias JA, Choi AM. Carbon monoxide attenuates aeroallergen-induced inflammation in mice. Am J Physiol Lung Cell Mol Physiol. 2001;281:L209–16. doi: 10.1152/ajplung.2001.281.1.L209. [DOI] [PubMed] [Google Scholar]
- 69.Kim HP, et al. Caveolin-1 expression by means of p38beta mitogen-activated protein kinase mediates the antiproliferative effect of carbon monoxide. Proc Natl Acad Sci U S A. 2005;102:11319–24. doi: 10.1073/pnas.0501345102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou Z, et al. Carbon monoxide suppresses bleomycin-induced lung fibrosis. Am J Pathol. 2005;166:27–37. doi: 10.1016/S0002-9440(10)62229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vreman HJ, Stevenson DK, Henton D, Rosenthal P. Correlation of carbon monoxide and bilirubin production by tissue homogenates. J Chromatogr. 1988;427:315–9. doi: 10.1016/0378-4347(88)80134-8. [DOI] [PubMed] [Google Scholar]
- 72.Cronje FJ, Carraway MS, Freiberger JJ, Suliman HB, Piantadosi CA. Carbon monoxide actuates O(2)-limited heme degradation in the rat brain. Free Radic Biol Med. 2004;37:1802–12. doi: 10.1016/j.freeradbiomed.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 73.Thom SR, Ohnishi ST, Fisher D, Xu YA, Ischiropoulos H. Pulmonary vascular stress from carbon monoxide. Toxicol Appl Pharmacol. 1999;154:12–9. doi: 10.1006/taap.1998.8553. [DOI] [PubMed] [Google Scholar]
- 74.Thom SR, Xu YA, Ischiropoulos H. Vascular endothelial cells generate peroxynitrite in response to carbon monoxide exposure. Chem Res Toxicol. 1997;10:1023–31. doi: 10.1021/tx970041h. [DOI] [PubMed] [Google Scholar]
- 75.Bengea S, et al. Analysis of the kinetics of CO binding to neuronal nitric oxide synthase by flash photolysis: dual effects of substrates, inhibitors, and tetrahydrobiopterin. J Inorg Biochem. 2004;98:1210–6. doi: 10.1016/j.jinorgbio.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 76.Zemojtel T, et al. Role of the interdomain linker probed by kinetics of CO ligation to an endothelial nitric oxide synthase mutant lacking the calmodulin binding peptide (residues 503-517 in bovine) Biochemistry. 2003;42:6500–6. doi: 10.1021/bi026886w. [DOI] [PubMed] [Google Scholar]
- 77.Fan B, Wang J, Stuehr DJ, Rousseau DL. NO synthase isozymes have distinct substrate binding sites. Biochemistry. 1997;36:12660–5. doi: 10.1021/bi9715369. [DOI] [PubMed] [Google Scholar]
- 78.Matsuoka A, Stuehr DJ, Olson JS, Clark P, Ikeda-Saito M. L-arginine and calmodulin regulation of the heme iron reactivity in neuronal nitric oxide synthase. J Biol Chem. 1994;269:20335–9. [PubMed] [Google Scholar]
- 79.Sato H, et al. CO binding studies of nitric oxide synthase: effects of the substrate, inhibitors and tetrahydrobiopterin. FEBS Lett. 1998;430:377–80. doi: 10.1016/s0014-5793(98)00699-1. [DOI] [PubMed] [Google Scholar]
- 80.Stevenson TH, Gutierrez AF, Alderton WK, Lian L, Scrutton NS. Kinetics of CO binding to the haem domain of murine inducible nitric oxide synthase: differential effects of haem domain ligands. Biochem J. 2001;358:201–8. doi: 10.1042/0264-6021:3580201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abu-Soud HM, Wu C, Ghosh DK, Stuehr DJ. Stopped-flow analysis of CO and NO binding to inducible nitric oxide synthase. Biochemistry. 1998;37:3777–86. doi: 10.1021/bi972398q. [DOI] [PubMed] [Google Scholar]
- 82.Ryter SW, Tyrrell RM. The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med. 2000;28:289–309. doi: 10.1016/s0891-5849(99)00223-3. [DOI] [PubMed] [Google Scholar]
- 83.Vogt BA, Alam J, Croatt AJ, Vercellotti GM, Nath KA. Acquired resistance to acute oxidative stress. Possible role of heme oxygenase and ferritin. Lab Invest. 1995;72:474–83. [PubMed] [Google Scholar]
- 84.Kapturczak MH, et al. Heme oxygenase-1 modulates early inflammatory responses: evidence from the heme oxygenase-1-deficient mouse. Am J Pathol. 2004;165:1045–53. doi: 10.1016/S0002-9440(10)63365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ferris CD, et al. Haem oxygenase-1 prevents cell death by regulating cellular iron. Nat Cell Biol. 1999;1:152–7. doi: 10.1038/11072. [DOI] [PubMed] [Google Scholar]
- 86.Li MH, Jang JH, Na HK, Cha YN, Surh YJ. Carbon monoxide produced by heme oxygenase-1 in response to nitrosative stress induces expression of glutamate-cysteine ligase in PC12 cells via activation of phosphatidylinositol 3-kinase and Nrf2 signaling. J Biol Chem. 2007;282:28577–86. doi: 10.1074/jbc.M701916200. [DOI] [PubMed] [Google Scholar]
- 87.Tanaka H, et al. E2F1 and c-Myc potentiate apoptosis through inhibition of NF-kappaB activity that facilitates MnSOD-mediated ROS elimination. Mol Cell. 2002;9:1017–29. doi: 10.1016/s1097-2765(02)00522-1. [DOI] [PubMed] [Google Scholar]
- 88.Brown SD, Piantadosi CA. In vivo binding of carbon monoxide to cytochrome c oxidase in rat brain. J Appl Physiol. 1990;68:604–10. doi: 10.1152/jappl.1990.68.2.604. [DOI] [PubMed] [Google Scholar]
- 89.Piantadosi CA, Sylvia AL, Jobsis-Vandervliet FF. Differences in brain cytochrome responses to carbon monoxide and cyanide in vivo. J Appl Physiol. 1987;62:1277–84. doi: 10.1152/jappl.1987.62.3.1277. [DOI] [PubMed] [Google Scholar]
- 90.Zhang J, Piantadosi CA. Mitochondrial oxidative stress after carbon monoxide hypoxia in the rat brain. J Clin Invest. 1992;90:1193–9. doi: 10.1172/JCI115980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Piantadosi CA, Zhang J, Demchenko IT. Production of hydroxyl radical in the hippocampus after CO hypoxia or hypoxic hypoxia in the rat. Free Radic Biol Med. 1997;22:725–32. doi: 10.1016/s0891-5849(96)00423-6. [DOI] [PubMed] [Google Scholar]
- 92.Suliman HB, Carraway MS, Tatro LG, Piantadosi CA. A new activating role for CO in cardiac mitochondrial biogenesis. J Cell Sci. 2007;120:299–308. doi: 10.1242/jcs.03318. [DOI] [PubMed] [Google Scholar]
- 93.D'Amico G, Lam F, Hagen T, Moncada S. Inhibition of cellular respiration by endogenously produced carbon monoxide. J Cell Sci. 2006;119:2291–8. doi: 10.1242/jcs.02914. [DOI] [PubMed] [Google Scholar]
- 94.Zuckerbraun BS, et al. Carbon monoxide signals via inhibition of cytochrome c oxidase and generation of mitochondrial reactive oxygen species. Faseb J. 2007;21:1099–106. doi: 10.1096/fj.06-6644com. [DOI] [PubMed] [Google Scholar]
- 95.Chandel NS, et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–8. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 96.Lee SR, et al. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277:20336–42. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- 97.Connor KM, et al. Mitochondrial H2O2 regulates the angiogenic phenotype via PTEN oxidation. J Biol Chem. 2005;280:16916–24. doi: 10.1074/jbc.M410690200. [DOI] [PubMed] [Google Scholar]
- 98.Zhang HJ, et al. Activation of matrix metalloproteinase-2 by overexpression of manganese superoxide dismutase in human breast cancer MCF-7 cells involves reactive oxygen species. J Biol Chem. 2002;277:20919–26. doi: 10.1074/jbc.M109801200. [DOI] [PubMed] [Google Scholar]
- 99.Nelson KK, et al. Redox-dependent matrix metalloproteinase-1 expression is regulated by JNK through Ets and AP-1 promoter motifs. J Biol Chem. 2006;281:14100–10. doi: 10.1074/jbc.M601820200. [DOI] [PubMed] [Google Scholar]
- 100.Chin BY, et al. Hypoxia-inducible factor 1alpha stabilization by carbon monoxide results in cytoprotective preconditioning. Proc Natl Acad Sci U S A. 2007;104:5109–14. doi: 10.1073/pnas.0609611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang LE, Willmore WG, Gu J, Goldberg MA, Bunn HF. Inhibition of hypoxia-inducible factor 1 activation by carbon monoxide and nitric oxide. Implications for oxygen sensing and signaling. J Biol Chem. 1999;274:9038–44. doi: 10.1074/jbc.274.13.9038. [DOI] [PubMed] [Google Scholar]
- 102.Roy A, Baby SM, Wilson DF, Lahiri S. Rat carotid body chemosensory discharge and glomus cell HIF-1 alpha expression in vitro: regulation by a common oxygen sensor. Am J Physiol Regul Integr Comp Physiol. 2007;293:R829–36. doi: 10.1152/ajpregu.00882.2006. [DOI] [PubMed] [Google Scholar]
- 103.Taille C, El-Benna J, Lanone S, Boczkowski J, Motterlini R. Mitochondrial respiratory chain and NAD(P)H oxidase are targets for the antiproliferative effect of carbon monoxide in human airway smooth muscle. J Biol Chem. 2005;280:25350–60. doi: 10.1074/jbc.M503512200. [DOI] [PubMed] [Google Scholar]
- 104.Kelly DP, Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004;18:357–68. doi: 10.1101/gad.1177604. [DOI] [PubMed] [Google Scholar]
- 105.Trumpower BL. The protonmotive Q cycle. Energy transduction by coupling of proton translocation to electron transfer by the cytochrome bc1 complex. J Biol Chem. 1990;265:11409–12. [PubMed] [Google Scholar]
- 106.Chen K, Thomas SR, Albano A, Murphy MP, Keaney JF., Jr Mitochondrial function is required for hydrogen peroxide-induced growth factor receptor transactivation and downstream signaling. J Biol Chem. 2004;279:35079–86. doi: 10.1074/jbc.M404859200. [DOI] [PubMed] [Google Scholar]
- 107.Leslie NR, et al. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. Embo J. 2003;22:5501–10. doi: 10.1093/emboj/cdg513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang X, Shan P, Alam J, Fu XY, Lee PJ. Carbon monoxide differentially modulates STAT1 and STAT3 and inhibits apoptosis via a phosphatidylinositol 3-kinase/Akt and p38 kinase-dependent STAT3 pathway during anoxia-reoxygenation injury. J Biol Chem. 2005;280:8714–21. doi: 10.1074/jbc.M408092200. [DOI] [PubMed] [Google Scholar]
- 109.Virbasius JV, Scarpulla RC. Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: a potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc Natl Acad Sci U S A. 1994;91:1309–13. doi: 10.1073/pnas.91.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Piantadosi CA, Suliman HB. Mitochondrial transcription factor A induction by redox activation of nuclear respiratory factor 1. J Biol Chem. 2006;281:324–33. doi: 10.1074/jbc.M508805200. [DOI] [PubMed] [Google Scholar]
- 111.Wu Z, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–24. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 112.Nisoli E, et al. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299:896–9. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- 113.Haden DW, et al. Mitochondrial Biogenesis Restores Oxidative Metabolism during Staphylococcus aureus Sepsis. Am J Respir Crit Care Med. 2007;176:768–77. doi: 10.1164/rccm.200701-161OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Suliman HB, et al. The CO/HO system reverses inhibition of mitochondrial biogenesis and prevents murine doxorubicin cardiomyopathy. J Clin Invest. 2007;117:3730–41. doi: 10.1172/JCI32967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lambeth JD, Kawahara T, Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic Biol Med. 2007;43:319–31. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nisimoto Y, Otsuka-Murakami H, Iwata S. NADPH-cytochrome c reductase from human neutrophil membranes: purification, characterization and localization. Biochem J. 1994;297(Pt 3):585–93. doi: 10.1042/bj2970585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lutter R, et al. Purification and partial characterization of the b-type cytochrome from human polymorphonuclear leukocytes. J Biol Chem. 1985;260:2237–44. [PubMed] [Google Scholar]
- 118.Nakahira K, et al. Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts. J Exp Med. 2006;203:2377–89. doi: 10.1084/jem.20060845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang X, et al. Carbon monoxide protects against hyperoxia-induced endothelial cell apoptosis by inhibiting reactive oxygen species formation. J Biol Chem. 2007;282:1718–26. doi: 10.1074/jbc.M607610200. [DOI] [PubMed] [Google Scholar]
- 120.Brancho D, et al. Mechanism of p38 MAP kinase activation in vivo. Genes Dev. 2003;17:1969–78. doi: 10.1101/gad.1107303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wijayanti N, Huber S, Samoylenko A, Kietzmann T, Immenschuh S. Role of NF-kappaB and p38 MAP kinase signaling pathways in the lipopolysaccharide-dependent activation of heme oxygenase-1 gene expression. Antioxid Redox Signal. 2004;6:802–10. doi: 10.1089/ars.2004.6.802. [DOI] [PubMed] [Google Scholar]
- 122.Arruda MA, Rossi AG, de Freitas MS, Barja-Fidalgo C, Graca-Souza AV. Heme inhibits human neutrophil apoptosis: involvement of phosphoinositide 3-kinase, MAPK, and NF-kappaB. J Immunol. 2004;173:2023–30. doi: 10.4049/jimmunol.173.3.2023. [DOI] [PubMed] [Google Scholar]
- 123.Soares MP, et al. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J Immunol. 2004;172:3553–63. doi: 10.4049/jimmunol.172.6.3553. [DOI] [PubMed] [Google Scholar]
- 124.Ricchetti GA, Williams LM, Foxwell BM. Heme oxygenase 1 expression induced by IL-10 requires STAT-3 and phosphoinositol-3 kinase and is inhibited by lipopolysaccharide. J Leukoc Biol. 2004;76:719–26. doi: 10.1189/jlb.0104046. [DOI] [PubMed] [Google Scholar]
- 125.Tron K, et al. Regulation of rat heme oxygenase-1 expression by interleukin-6 via the Jak/STAT pathway in hepatocytes. J Hepatol. 2006;45:72–80. doi: 10.1016/j.jhep.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 126.Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH. Carbon monoxide: a putative neural messenger. Science. 1993;259:381–4. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- 127.Ben-Shlomo R, et al. Light pulse-induced heme and iron-associated transcripts in mouse brain: a microarray analysis. Chronobiol Int. 2005;22:455–71. doi: 10.1081/CBI-200062353. [DOI] [PubMed] [Google Scholar]
- 128.Dioum EM, et al. NPAS2: a gas-responsive transcription factor. Science. 2002;298:2385–7. doi: 10.1126/science.1078456. [DOI] [PubMed] [Google Scholar]
- 129.Reinking J, et al. The Drosophila nuclear receptor e75 contains heme and is gas responsive. Cell. 2005;122:195–207. doi: 10.1016/j.cell.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 130.Furchgott RF, Jothianandan D. Endothelium-dependent and -independent vasodilation involving cyclic GMP: relaxation induced by nitric oxide, carbon monoxide and light. Blood Vessels. 1991;28:52–61. doi: 10.1159/000158843. [DOI] [PubMed] [Google Scholar]
- 131.Morita T, Mitsialis SA, Koike H, Liu Y, Kourembanas S. Carbon monoxide controls the proliferation of hypoxic vascular smooth muscle cells. J Biol Chem. 1997;272:32804–9. doi: 10.1074/jbc.272.52.32804. [DOI] [PubMed] [Google Scholar]
- 132.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–54. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 133.Ghio AJ, et al. Carbon Monoxide Reversibly Alters Iron Homeostasis and Respiratory Epithelial Cell Function. Am J Respir Cell Mol Biol. 2008 doi: 10.1165/rcmb.2007-0179OC. [DOI] [PubMed] [Google Scholar]
- 134.Liu Y, et al. Carbon monoxide and nitric oxide suppress the hypoxic induction of vascular endothelial growth factor gene via the 5′ enhancer. J Biol Chem. 1998;273:15257–62. doi: 10.1074/jbc.273.24.15257. [DOI] [PubMed] [Google Scholar]
- 135.Carraway MS, et al. Carbon monoxide promotes hypoxic pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol. 2002;282:L693–702. doi: 10.1152/ajplung.00211.2001. [DOI] [PubMed] [Google Scholar]
- 136.Peyton KJ, et al. Heme oxygenase-1-derived carbon monoxide is an autocrine inhibitor of vascular smooth muscle cell growth. Blood. 2002;99:4443–8. doi: 10.1182/blood.v99.12.4443. [DOI] [PubMed] [Google Scholar]
- 137.Li Volti G, et al. Carbon monoxide signaling in promoting angiogenesis in human microvessel endothelial cells. Antioxid Redox Signal. 2005;7:704–10. doi: 10.1089/ars.2005.7.704. [DOI] [PubMed] [Google Scholar]
- 138.Thom SR, Ischiropoulos H. Mechanism of oxidative stress from low levels of carbon monoxide. Res Rep Health Eff Inst. 1997:1–19. discussion 21-7. [PubMed] [Google Scholar]
- 139.Kinobe R, Ji Y, Nakatsu K. Peroxynitrite-mediated inactivation of heme oxygenases. BMC Pharmacol. 2004;4:26. doi: 10.1186/1471-2210-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gibson QH, Olson JS, McKinnie RE, Rohlfs RJ. A kinetic description of ligand binding to sperm whale myoglobin. J Biol Chem. 1986;261:10228–39. [PubMed] [Google Scholar]
- 141.Wolff DG. The formation of carbon monoxide during peroxidation of microsomal lipids. Biochem Biophys Res Commun. 1976;73:850–7. doi: 10.1016/0006-291x(76)90199-6. [DOI] [PubMed] [Google Scholar]
- 142.Vreman HJ, Wong RJ, Sanesi CA, Dennery PA, Stevenson DK. Simultaneous production of carbon monoxide and thiobarbituric acid reactive substances in rat tissue preparations by an iron-ascorbate system. Can J Physiol Pharmacol. 1998;76:1057–65. doi: 10.1139/cjpp-76-12-1057. [DOI] [PubMed] [Google Scholar]
- 143.Ahmed AE, Kubic VL, Stevens JL, Anders MW. Halogenated methanes: metabolism and toxicity. Fed Proc. 1980;39:3150–5. [PubMed] [Google Scholar]