Abstract
This article is a summary of a lecture on cellular mechanotransduction that was presented at a symposium on “Cardiac Mechano-Electric Feedback and Arrhythmias” that convened at Oxford, England in April 2007. Although critical mechanosensitive molecules and cellular components, such as integrins, stretch-activated ion channels, and cytoskeletal filaments, have been shown to contribute to the response by which cells convert mechanical signals into a biochemical response, little is known about how they function in the structural context of living cells, tissues and organs to produce orchestrated changes in cell behavior in response to stress. Here, studies are reviewed that suggest our bodies use structural hierarchies (systems within systems) composed of interconnected extracellular matrix and cytoskeletal networks that span from the macroscale to the nanoscale to focus stresses on specific mechanotransducer molecules. A key feature of these networks is that they are in a state of isometric tension (i.e., experience a tensile prestress), which ensures that various molecular-scale mechanochemical transduction mechanisms proceed simultaneously and produce a concerted response. These features of living architecture are the same principles that govern tensegrity (tensional integrity) architecture, and mathematical models based on tensegrity are beginning to provide new and useful descriptions of living materials, including mammalian cells. This article reviews how the use of tensegrity at multiple size scales in our bodies guides mechanical force transfer from the macro to the micro, as well as how it facilitates conversion of mechanical signals into changes in ion flux, molecular binding kinetics, signal transduction, gene transcription, cell fate switching and developmental patterning.
Introduction
My laboratory is interested in how living cells and tissues are constructed so that they exhibit their novel organic properties, including their ability to change shape, move and grow. We primarily work on angiogenesis – the development of capillary blood vessels; however, our goal is to identify fundamental design principles that apply to many, if not all, living tissues.
Most biologists tend to view vascular tissue development in a linear way: a growing tissue or an expanding tumor secretes a soluble growth factor, and then capillary endothelial cells in nearby preexisting vessels extend out and march in file towards the stimulus, thereby vascularizing the tissue. In reality, capillary development is a much more complex process in which new blood vessels grow by iterative rounds of sprouting and branching so that highly complex networks result, rather than many parallel linear tubes (Huang and Ingber, 1999). Furthermore, neighboring cells in the same angiogenic microenvironment can and must exhibit distinct behaviors to ensure that these functional networks form. Endothelial cells that are separated by only micrometers distance within the same growing microvessel may either growth, become quiescent and differentiate, or undergo apoptosis, even though the microenvironment often contains scores of soluble mitogens, and these spatial variations of cell behavior are critical for complex pattern formation (Clark, 1938). Thus, although soluble growth factors and hormones drive tissue development, there must be some other invisible mechanism by which capillary cell sensitivity to these cytokines is controlled locally in order for normal development to proceed.
Micromechanical Control of Tissue Development
Over a quarter of a century ago, I suggested the strange possibility that this local mechanism of switching between cell fates may be controlled mechanically (Ingber et al., 1981; Ingber and Jamieson, 1985). This seemed bizarre to many at the time; however, it has been long known that the sculpting of tissues and organs that occurs in the embryo is an extremely physical process. Cells within various regions of the growing embryo independently move, stretch, and pull against one another through the action of cell-generated forces. Studies with cultured cells similarly revealed that all living mammalian cells actively generate tensional forces through an actomyosin filament sliding mechanism in the ‘contractile microfilaments’ of their cytoskeleton that are composed of associated actin and myosin fillaments, and that they exert traction forces on their adhesions to extracellular matrix (ECM) and to neighboring cells.
These observations led me to suggest that pattern formation in developing tissues in the embryo may be governed by local changes in micromechanical forces (Fig. 1) (Ingber et al., 1981; Ingber and Jamieson, 1985; Huang and Ingber, 1999). In the late 1970s, local increases in cell proliferation that drive new bud formation during growth of epithelial glands were shown to be preceded by regional thinning of the basement membrane ECM that underlines these very same regions (Bernfield and Banerjee, 1978). A similar correlation between basement membrane thinning and onset of new capillary sprout formation was demonstrated during initiation of angiogenesis (Ausprunk and Folkman, 1977). If all cells actively generate tensional forces and apply them to their ECM adhesions, then these forces must be balanced by equal and opposite forces if the shape of the tissue is stable in form, as observed in living tissues (i.e., even embryonic tissues are stable at any instant in time because morphogenesis occurs over hours to days, not seconds to minutes). The cells and their linked ECMs that comprise these living tissues must therefore be in a state of isometric tension, and hence, they are tensionally ‘prestressed’ structures.
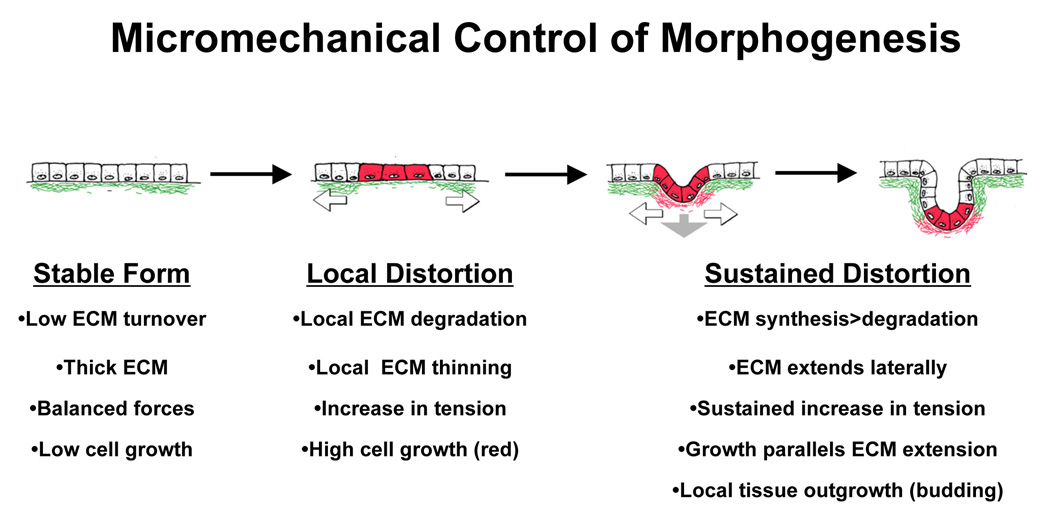
Fig. 1. Micromechanical Control of Tissue Morphogenesis.
Diagrams of a model for tension-driven tissue remodeling during normal epithelial morphogenesis. Local increases in ECM turnover result in formation of a focal defect in the basement membrane (green) that stretches and thins due to the contraction and pulling of neighboring adherent epithelium (white arrows) and underlying mesenchyme (gray arrow). Cells adherent to the basement membrane in this extending region will distort or experience changes in tension within the cytoskeleton and thus, become preferentially sensitive to growth stimuli. Cell division is accompanied by deposition of new basement membrane (red) and thus, cell mass expansion and ECM extension are tightly coupled leading to bud formation in this localized area of the developing tissue (modified from Huang and Ingber, 1999).
If the basement membrane beneath the epithelium is under tension, then local thinning of this cell anchoring scaffold due to changes in enzymatic activity should result in stretching of this small region, much like a ‘run’ in a woman’s stocking extends more than the rest of the fabric (Fig. 1). The micromechanical model of developmental control (Ingber and Jamieson, 1985; Huang and Ingber, 1999) therefore suggested that the cells that adhere to these stretched regions of the thinned basement membrane will distort more than their neighbors only a few micrometers away that remain adherent to intact ECM. If cell spreading promotes growth in mitogen-stimulated cells, as first suggested by studies in the 1970s (Folkman and Moscona, 1978), then this mechanical change could lead to differential cell growth, and hence localized budding and branching as observed in developing tissues (Fig. 1).
In summary, viewing development as a problem of material construction led me to suggest the following developmental control hypothesis (Fig. 1): 1) regional variations of ECM remodeling that occurs during embryogenesis will lead to local differentials in ECM structure and mechanics, 2) changes in matrix compliance (e.g., increased stiffness when the thinned basement membrane is stretched) will alter the mechanical force balance across membrane receptors that mediate cell-ECM adhesion, and 3) altering the level of forces that are transmitted to the internal cytoskeleton will produce cell distortion and change intracellular biochemistry, thereby switching cells between growth, differentiation and apoptosis.
Mechanical Control of Cell Fate Switching
Central to the mechanochemical control hypothesis is the concept that cell growth and function are controlled through physical distortion of the cell and cytoskeleton. To test this hypothesis, it was necessary to develop an experimental tool to make cell shape distortion an “independent variable” such that the degree of cell spreading can be altered separately from changes in the density of soluble growth factors (e.g., FGF) or insoluble ECM molecules, which can both elicit signals directly by binding and clustering of their respective cell surface receptors. We accomplished this by adapting a novel nanotechnology-based microfabrication technique developed earlier by our collaborator George Whitesides (Harvard U.) as an inexpensive way to manufacture microchips for the computer industry (Prime and Whitesides, 1991).
We used this technique to microfabricate adhesive islands coated with a saturating density of ECM molecules (e.g., fibronectin, laminin) that are on the same size scale of individual cultured cells. These ECM islands were surrounded by non-adhesive (polyethylene glycol-treated) regions to prevent ECM adsorption in these areas, thereby limiting cell spreading to the area of the adhesive island (Singhvi et al., 1994; Chen et al., 1997; Chen et al., 2000).
When mammalian cells are cultured on these planar substrates, they spread and take on the precise size and shape of the islands (Fig. 2). Capillary endothelial cells, liver epithelial cells, fibroblasts, smooth muscle cells, and skeletal muscle cells that are normally highly pleiotropic in form on standard culture substrates, appear perfectly round on circular adhesive islands, and exhibit ninety degree corners on square islands (Singhvi et al., 1994; Chen et al., 1997; Parker et al., 2002). Most importantly, in the presence of a saturating amount of soluble mitogen (e.g., FGF, EGF), cells that physically distort to the greatest degree exhibit the highest rates of cell cycle progression and growth (Singhvi et al., 1994; Chen et al., 1997; Huang et al., 1998), whereas cells that are prevented from spreading but still remain adherent undergo apoptosis in the same growth factor-containing medium (Chen et al., 1997). Thus, cells can be switched between growth and death solely by varying the degree to which they mechanically distend (Fig. 2).
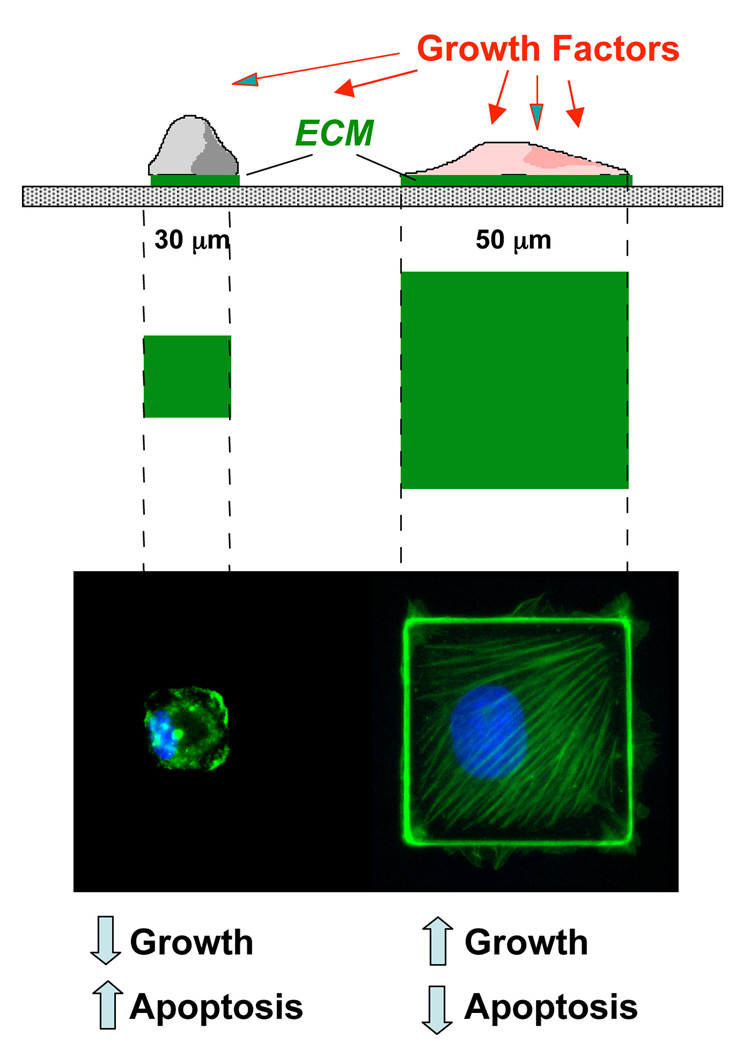
Fig. 2. Control of Cell Shape and Function using Micropatterned Adhesive Substrates containing Micrometer-Sized ECM Islands.
Top) Side view of a schematic design of a cell culture substrate containing adhesive islands of defined shape and size on the micron scale that were coated with a saturating density of fibronectin (green) and separated by intervening non-adhesive regions coated with polyethylene glycol using a self assembly-based microfabrication method. Middle) A view from above showing the same micropatterned adhesive islands. Bottom) Immunofluorescence micrographs of endothelial cells cultured on the corresponding islands shown above and stained for actin microfilaments with FITC-phalloidin (green) and DNA with DAPI (blue). Note that cells remain small and are devoid of large actin bundles on the small adhesive island, but spread and form well organized stress fibers oriented diagonally when cultured on a large island. Under these conditions, spread cells on large islands pass through the late G1 checkpoint when stimulated by growth factors, whereas endothelial cells that are restricted in their spreading never enter S phase, and instead undergo apoptosis.
When the same cells are cultured on intermediate size ECM islands that neither promoted growth nor apoptosis, they become quiescent and differentiate. For example, hepatocytes secrete liver-specific proteins and capillary endothelial cells organize into hollow capillary tubes (Singhvi et al., 1994; Dike et al., 1999). Furthermore, when endothelial cells, fibroblasts or muscle cells are cultured on square, hexagonal, pentagonal or other angulated polygonal islands and stimulated with motility factors, the cells extend new motile processes (lamellipodia, filopodia) preferentially from their corners, whereas cells on circular islands exhibit no bias (Parker et al., 2002; Brock et al., 2003). Thus, the direction of cell movement, which is critical for tissue development, is governed by physical interactions between cells and their ECM.
Importantly, the contractility of vascular smooth muscle cells and myofibrillogenesis in cardiac myocytes, also can be modulated by altering cell shape and orientation through modification of cell-ECM interactions using these microfabricated substrates or by altering the mechanical compliance of artificial ECM substrates (Lee et al., 1998; Bursac et al., 2002; Polte et al., 2004; Parker and Ingber, 2007). In the case of smooth muscle cells, changes of ECM mechanics alter cell contractility at the level of biochemical signal transduction. This mechanical control mechanism involves physical interplay between ECM and the cytoskeleton, such that cell spreading and generation of cytoskeletal tension feed back to promote actomyosin tension generation in the cell (Polte et al., 2004). In addition, recent studies show that the electrical conduction properties of heart cells and the electrical circuitry of neuronal networks can similarly be modulated by physically constraining cell-ECM interactions or altering ECM mechanics (Wilson et al., 2008), as can cell fate switching in human mesenchymal stem cells (McBeath et al., 2004; Engler et al., 2006).
Taken together, these studies confirm that cell shape distortion does indeed govern whether a cell will proliferate, differentiate, contract or die, as well as the direction it will move. This is important because it is the ability to establish local differentials in these behaviors that drive the fractal-like growth patterns in all tissues and all species. These findings also support the concept that mechanical distortion of cells plays a central role in sculpting tissue form during development of many organs, including heart.
Cellular Tensegrity
Given the central role of cell distortion in control of cell fate switching, it is critical to understand cellular mechanotransduction–the process by which cells sense mechanical forces and transduce them into changes in intracellular biochemistry and gene expression. For many years, people viewed living cells as small bits of viscous protoplasm surrounded by an elastic membrane, and thus they assumed that cells sense mechanical forces as a result of distortion of their surface that somehow altered membrane-associated signaling activities. But as there was no molecular specificity, this mechanism was essentially a ‘black box’.
Thus, I began to pursue the idea that we might gain better insight into this mechanotransduction mechanism, if we could understand how living cells are constructed at the nanometer scale. Specifically, I explored the possibility that instead of being built like balloons filled with molasses, cells might be constructed more like tents with tensed cables and membranes that are winched in against internal struts and external tethers (e.g., analogous to tent poles, ground pegs and ties to tree branches) in order to prestress, and thereby mechanically stabilize, the entire structure. This was based on the discovery in the mid 1970s that all nucleated cells (not just muscle cells) contain an internal molecular framework, known as the ‘cytoskeleton’, that actively generates tensile forces and distributes them to other components inside the cell, as well as to cell-cell and cell-ECM adhesions. Many biologists and biophysicists studied the cytoskeleton; however, they either analyzed its molecular components in isolation, or studied its ‘gel’ properties. In contrast, I viewed the cytoskeleton as an architectural structure.
Based on the importance of tensional prestress for cell and cytoskeletal shape stability, I proposed that living cells use tensegrity (tensional integrity) architecture to control their shape and structure. Tensegrity was first described by the architect Buckminster Fuller and the sculpture Kenneth Snelson (Fuller, 1961). It refers to network structures that mechanically stabilize themselves through use of a tensile prestress. They are composed of a network of tensed elements (e.g., cables) that tend to pull towards the center; however, they are balanced by a subset of other structural elements that resist being compressed. As a result, the whole structure is placed in a state of isometric tension that makes it strong, resilient and immediately responsive to external mechanical stresses.
In early studies, spherical tensegrity models composed of sticks and elastic strings were shown to mimic many behaviors of cultured cells including their ability to spread when adherent, and spontaneously round when their anchors are dislodged (e.g., during trypsinization) (Fig. 3). All geodesic structures are tensegrities (Fuller, 1961; Ingber, 1998), and the possibility that cells use tensegrity architecture is supported by the finding that actin ‘geodomes’ have been visualized in the cytoskeleton of living cells both in vitro (Lazarides, 1976; Rathke et al., 1979; Heuser and Kirschner, 1980) and in vivo (Rafferty and Scholz, 1985). Interestingly, tensegrity models composed straws connected by tensed elastic strings can predict how actomyosin filament nets can transform between linear bundles (stress fibers) and triangulated actin geodomes without losing structural integrity; and these models create forms that correspond to those observed in living cells by electron microscopy with nanometer resolution (Ingber, 1993; Ingber et al., 1994).
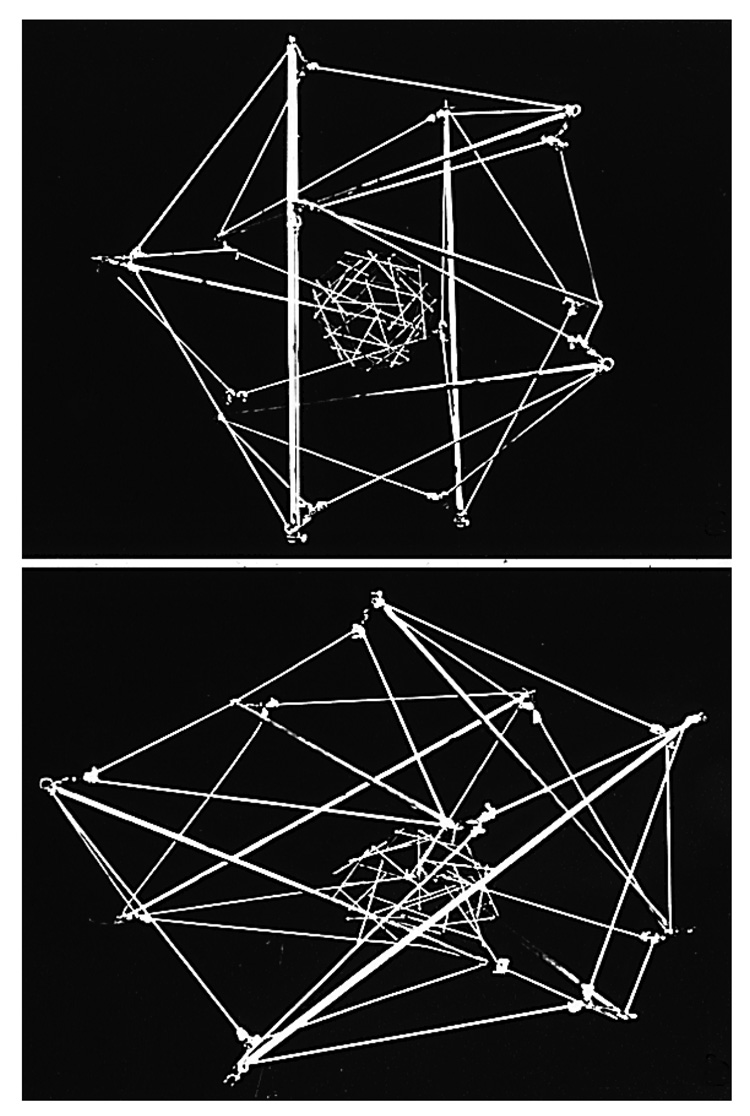
Fig. 3. Tensegrity Cell Model.
A large tensegrity structure built as a model of a nucleated mammalian cell that was constructed from aluminum struts and thick elastic cord, with a geodesic sphere composed of wooden sticks and thin white elastic thread at its center. The cell and nucleus are interconnected by thin black elastic thread that can not be seen due to the black background. Top) Cell and nuclear shape are both round in a symmetrical cell that generates internal tension and is unanchored. Bottom) The tensegrity cell and nucleus extend in a coordinated fashion when attached to a rigid substrate, and the nucleus polarizes (moves to the base) because of tensional continuity in the structure (reprinted with permission from Ingber, 1993).
Another unique property of tensegrities and living materials is that both are constructed as structural hierarchies (i.e., systems within systems within systems) (Fuller, 1961; Ingber, 2003b; Ingber, 2006). For example, we know that cells are hierarchical structures because it is possible to remove the nucleus from one cell and transplant into another enucleated cell, as is done during embryonic cloning. This means that the nucleus is able to retain its own structural and functional integrity in isolation, yet when inserted into the cytoplasm of another cell, it can reform its natural structural connections, such that the whole cell and nucleus once again behave as one hierarchically-integrated structure.
To explore this from a structural perspective, a large spherical, stick-and-string, tensegrity ‘cell’ model was constructed that contained another smaller spherical tensegrity built in a similar manner at its center; the ‘nucleus’ was connected to the ‘cell surface’ by additional elastic cables that mimicked cytoskeletal filaments in order to provide tensional integrity (Fig. 3). When this round structure was attached to anchoring points distributed across a solid foundation, both the cell and nucleus spread in a coordinated manner, and the nucleus polarized (moved closer to the cell base). Later studies confirmed that the same behaviors are exhibited by living cells when they attach and spread on ECM substrates( Ingber et al., 1986; Ingber et al., 1987; Ingber, 1990).
Tensegrity also has been used to describe how whole organisms, including mammals, insects and plants stabilize themselves at larger size scales in the hierarchy of life (Ingber, 1993; Ingber, 2003b; Ingber, 2006). For instance, the bones that constitute our skeleton are pulled up against the force of gravity and stabilized by the pull of tensed muscles, tendons, ligaments and fascia, and the shape stability (stiffness) of our bodies depends on the tone (tensile prestress) in our muscles. Insect muscles act in a similar manner to stabilize the form of their bodies; however, they pull on their insertions on a stiffened exoskeleton, rather than internal compression-resistant bones. Plants similarly must tensionally prestress their cell walls to maintain their stiffness and shape stability (and not ‘wilt’). But they do this by using turgor forces to swell their compression-resistant cell bodies against surrounding non-extensible cell walls. In other words, their compression elements push outward and tense the surrounding network, rather than having the tensed network actively pull in against rigid compression elements, as in mammalian systems.
Work from my laboratory and others has confirmed that many different types of cells use tensegrity to stabilize their shape and cytoskeletal structure (Ingber, 1993; Ingber, 2003b; Ingber, 2006). The cytoskeleton of mammalian cells is composed of three major filament systems – microfilaments, intermediate filaments and microtubules. Microfilaments are actin polymers that can associate with myosin filaments to form ‘contractile microfilaments’ that generate tension. When free of myosin, they also can form flexible networks or self-assemble into cross-linked bundles that become relatively rigid (e.g., as in filopodia). Intermediate filaments are tough polymers composed of vimentin, keratin, desmin or neurofibrillar proteins depending on the cell types; they form flexible cables that extend from the cell surface to the cell center where they form a cage that envelops the nucleus. Microtubules are larger hollow polymers composed of tubulin that polymerize outward from a microtubule organizing center positioned near the nucleus, and extend across long distances of cytoplasm to the cell periphery; they also form bundles that move chromosomes in the mitotic spindle.
Cells use tensegrity to mechanically integrate and stabilize these three interconnecting biopolymer networks. Mammalian cells adhere to substrates by binding their cell surface integrin receptors to immobilized ECM molecules. When bound to ECM, these receptors become activated (undergo a change in molecular shape), such that they promote binding of proteins, such as talin, vinculin, α-actinin, paxillin and zyxin, to their cytoplasmic tails. These proteins form a specialized cytoskeletal complex, known as the ‘focal adhesion’, that physically links the integrins to the ends of contractile microfilament bundles (‘stress fibers’), thereby forming a molecular bridge between ECM and the cytoskeleton.
When the cell exerts traction on its relative rigid substrate adhesions, the level of tension applied to these receptors increases. This shift in the balance of forces across integrins triggers a series of biophysical and biochemical signaling events that lead to activation of the small GTPase Rho that further activates myosin-dependent tension generation. This positive feedback loop results in increased traction on these adhesions, causing increased integrin binding and clustering, as well as additional recruitment of more focal adhesion proteins. This is why focal adhesions appear as large, streak-like anchoring structures in adherent cells, and why focal adhesion size scales with the level of tension applied across transmembrane integrin receptors (Riveline et al., 2001).
If a cell is constructed like a tent or prestressed tensegrity structure, then the integrins and focal adhesions correspond to tent pegs anchored in the ground. Given that contractile microfilaments generate tension, and span from one focal adhesion to another when bundled within stress fibers, it seemed likely that they would form the tension cables in the cellular tensegrity structure. In fact, we were able to demonstrate directly that stress fibers are tensionally prestressed by using a laser nanoscissor to probe these nanometer-scale cytoskeletal bundles in living cells expressing YGFP-labelled actin (Fig. 4A) (Kumar et al., 2006).
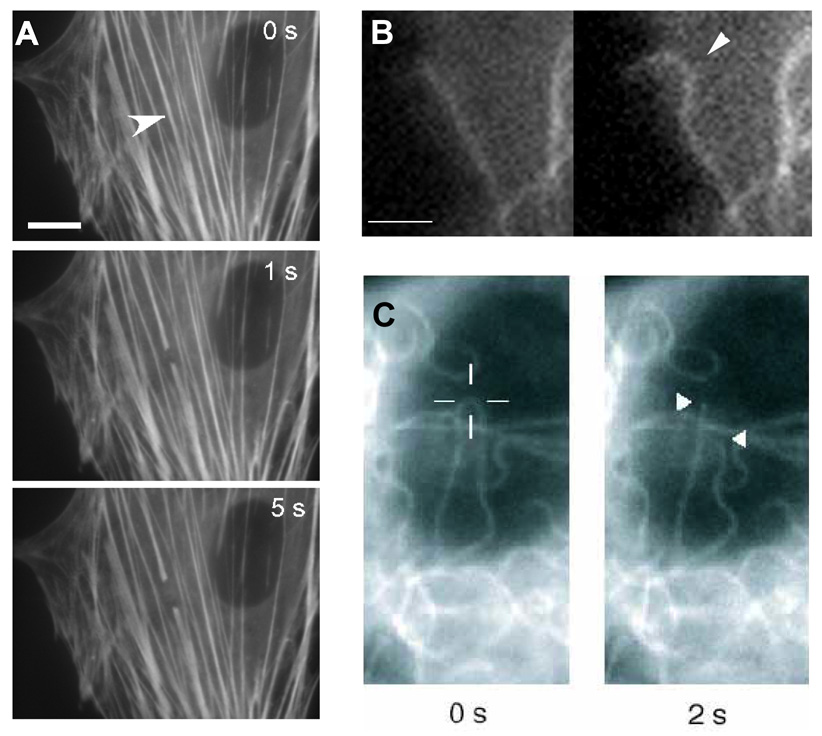
Fig. 4. Mechanical Behavior of Cytoskeletal Filaments in Living Cells.
A) Severing and spontaneous retraction of a single stress fiber bundle in an endothelial cell expressing EYFP-actin using a femtosecond-based nanoscissor reveals prestress in these cytoskeletal bundles (arrowhead indicates position of the laser spot; bar = 10 µm; from Kumar et al., 2006). B) Fluorescence video microscopy images of a cell expressing GFP-tubulin showing buckling of a microtubule (arrowhead) as it polymerizes and impinges end-on on the cell cortex (right vs. left; bar = 2 µm; from Wang et al., PNAS 2001). C) Fluorescence micrographs of a GFP-labeled microtubule in an endothelial cell before (left) and 2 sec after (right) it was incised with the laser nanoscissor. Note that the previously bent microtubule rapidly snaps back to a straight shape immediately after it is cut; the cross hair shows the position targeted by the laser (from Heisterkamp et al., 2005).
When a femtosecond laser focused through a microscope objective was used to punch a 300 nm hole in the center of a single living stress fiber, the hole immediately stretched lengthwise along the main tension axis of the stress fiber and took on an elliptical form (Kumar et al., 2006). This demonstration of residual strain in this nanometer-sized structure, confirmed that this structure was in a state of isometric tension prior to the material ablation. Moreover, when we sliced a single stress fiber, both ends spontaneously retracted much like when a tensed muscle is cut during whole body surgery (Fig. 4A).
When nanosurgery was carried out in cells adherent to rigid dishes, only a local response within the single cut stress fiber was observed. However, when the same experiment was done in a cell attached to a flexible ECM substrate with a compliance more similar to that of living tissues, cutting one stress fiber resulted in coordinated structural rearrangements throughout the entire cytoskeleton, as well as a global change in cell shape. This occurred because the force balance between the cell and the prestressed ECM was disrupted when the internal stress fiber was cut, and the resulting elastic recoil of the tensed ECM physically pulled the remaining cell and cytoskeleton outward until a new force balance was obtained. Using pharmacological modifiers of myosin-based tension generation, we also confirmed that actin-containing stress fibers experience both active and passive prestress in living cells (Kumar et al., 2006). Thus, if cells are tensegrities, then actin-containing contractile microfilaments are the major tension elements in these structures that winch in the cytoskeleton and membrane against the cell’s tent peg-like adhesions.
For many years, the major skepticism relating to the cellular tensegrity theory focused on whether cells could possibly contain internal compression struts. Microtubules seemed to be perfectly suited to provide this function because they are larger than the other cytoskeletal fibers, hollow, and therefore much stiffer. In fact, in vitro analysis of microtubules revealed that they have a persistence length of 2 millimeters, which means that they appear straight over this length scale after isolation. Yet, microtubules almost always appear curved in living cells, which could indicate that they are buckling under compressive loads.
Various studies have indeed confirmed that some microtubules bear compression in living cells. For example, dynamic real-time analysis of cells expressing GFP-tubulin revealed that individual growing microtubules buckle and display the classic small wavelength curvature observed in fixed cells when they polymerize and impinge end-on against other stiff cellular components or the cell periphery (Fig. 4B) (Brangwynne et al., 2006; Kaech et al., 1996; Wang et al., 2001). Individual bent microtubules also snap back to a straight position (i.e., display elastic recoil) when cut with a laser (Fig. 4C) (Heisterkamp et al., 2005). Moreover, physical analysis and modeling of microtubule curvature has confirmed that the characteristic 2–3 um wavelength of buckling observed in virtually all cells is caused by these biopolymers being compressed when they are connected to, or surrounded by, other viscoelastic cytoskeletal elements (Brangwynne et al., 2006). Furthermore, microtubule buckling and breakage increase when the level of tension in the surrounding actin cytoskeleton is raised, whereas microtubules straighten when cytoskeletal tension is dissipated (Waterman-Storer and Salmon, 1997; Wang et al., 2001; Brangwynne et al., 2006). Hence, microtubules appear to function as compression struts that balance tensile forces in the surrounding cytoskeleton in living cells.
Studies with nucleated tensegrity cell models predict that living cells are ‘hard-wired’ such that structural components in the cytoplasm mechanically couple anchoring sites on the cell surface to the nucleus. Again, this has been confirmed experimentally by applying tensional forces directly to bound cell surface integrin receptors (using ECM-coated micropipettes with a micromanipulator or magnetic microbeads in conjunction with applied magnetic fields). These studies demonstrate that pulling on integrins results rearrangements of cytoskeletal filaments, movement of organelles (e.g., mitochondria), and nuclear shape changes, as well as molecular rearrangements in nucleoli in the center of the nucleus (Maniotis et al., 1997b; Wang et al., 2001; Hu et al., 2003). Importantly, only local effects at the cell membrane result when similar forces are applied to other transmembrane receptors (e.g., metabolic receptors) that do not form focal adhesion linkages to the deep cytoskeleton (Maniotis et al., 1997b; Wang et al., 2001). Also, long-distance force transfer through the cytoplasm can be inhibited by disrupting intermediate filaments, either using drugs or gene knock out approaches (Maniotis et al., 1997b; Eckes et al., 1998). Intermediate filaments therefore structurally integrate the cell, cytoplasm and nucleus, in addition to tensionally stiffening microtubules and other cytoskeletal elements by connecting along their length and functioning like ‘guy wires’ that stabilize these elongated structures (Brodland and Gordon, 1990).
In summary, these and other studies provide strong evidence in support of the theory that cells are organized and stabilized as tensegrity structures. The fundamental concept that arises from this work is that prestress is the unifying principle behind cell shape stability. Cell form is stabilized through a mechanical force balance in which cytoskeletal struts and adhesive tethers resist and balance the pull of the cell’s contractile cytoskeleton, thereby placing the entire network in a tensionally prestressed state of isometric tension.
Tensegrity and Cellular Mechanotransduction
Recognition that cells use tensegrity led to new insights into the molecular basis of cellular mechanotransduction (Ingber and Jamieson, 1985; Ingber, 1991; Ingber, 1997; Ingber, 2006). Rather than sensing mechanical signals through generalized membrane deformation, tensegrity predicts that cell surface adhesion receptors that act like tent pegs and mechanically couple the cytoskeleton to the ECM should be among the first molecules on the membrane to sense physical forces. Hence, ECM receptors such as integrins may act as ‘mechanoreceptors’ (Ingber and Jamieson, 1985; Ingber, 1991; Wang et al., 1993).
To test this hypothesis, we developed a magnetic cytometry technique in which controlled mechanical stresses (shear or tension) are applied to integrins bound to magnetic microbeads (1–10 um diameter) coated with integrin ligands using applied magnetic fields (Wang et al., 1993; Wang and Ingber, 1994; Alenghat et al., 2004; Overby et al., 2005; Matthews et al., 2006). These studies revealed that cells stiffen when stresses are applied to integrins, whereas only a minimal response is observed when the same stresses are applied to transmembrane scavenger receptors, growth factor receptors and histocompatibility antigens that do not form focal adhesions (Wang et al., 1993; Yoshida et al., 1996). Furthermore, this stiffening response was mediated by mechanical interplay between all three cytoskeletal filaments systems (microfilaments, microtubules, and intermediate filaments), and the level of cell stiffness could be immediately increased or decreased by enhancing cytosketal tension (prestress) or dissipating it, respectively (Pourati et al., 1998; Wang et al., 1993; Wang and Ingber, 1994). Even changes in cell stiffness due to osmotic swelling are governed by the level of prestress in the cytoskeleton (Cai et al., 1998).
In our early studies, we showed that stick-and-elastic string tensegrity sculptures can mimic many of the cell mechanical behaviors described above, including linear increases in structural stiffness as the level of applied stress is raised (Wang et al., 1993). Importantly, working with Dimitrije Stamenovic (Boston U.), we developed mathematical models of the cell based on tensegrity starting from first mechanistic principles that provide even more powerful a priori predictions relating to both cell static and dynamic mechanical behavior, which have now been confirmed experimentally in various cell types (Stamenovic et al., 1996; Coughlin and Stamenovic, 1998; Stamenovic and Coughlin, 1999; Stamenovic and Coughlin, 2000; Wang and Stamenovic, 2000; Stamenovic, 2005). Behaviors exhibited by living cells that can be predicted by the tensegrity model include: 1) linear relation between stiffness and applied stress (Wang et al., 1993; Wang and Ingber, 1994), 2) cell mechanics depends on prestress (Lee et al., 1998; Wang and Ingber, 1994), 3) linear relation between stiffness and prestress (Wang et al., 2001; Wang et al., 2002), 4) hysteresivity is independent of prestress (Maksym et al., 2000; Wang et al., 2001); 5) quantitative predictions of cellular elasticity (Stamenovic and Coughlin, 2000), 6) predictions of dynamic mechanical behavior (Sultan et al., 2004), and 7) mechanical contribution of intermediate filaments to cell mechanics.
Recently, other groups have found that the red blood cell membrane, which has a structure similar to that of the submembranous ‘cortical’ cytoskeleton of nucleated cells, can be effectively modeled as a tensegrity as well (Vera et al., 2005). On a smaller size scale, the geodesic nuclear lamina and mitotic spindle composed of microtubule struts that push out against a tensed mechanically continuous network of chromosomes and linked nuclear matrix scaffolds (Maniotis et al., 1997a) also have been described as prestressed tensegrity structures (Ingber et al., 1994; Pickett-Heaps et al., 1997). Because all viruses exhibit geodesic forms, they have been long recognized to be tensegrities, and tensegrity models were used in early studies delineating their structure (Caspar, 1980).
Geodesic forms also are observed in transport vesicles and enzyme complexes (Ingber, 1998), and individual molecular biopolymers, such as actin filaments, have been described as tensegrity masts (Schutt et al., 1997). At this size scale, ‘things don’t touch’; instead, each molecular subunit attracts (pulls or tenses) its neighboring subunits due to intermolecular bonding forces, and these inward directed forces are balanced by each subunit’s ability to resist being deformed when compressed. Even individual molecules, such as single proteins, may be viewed as tensegrities because their peptide backbone has stiffened subregions (e.g., α-helix, β-strand) separated by flexible hinge areas that pull on each other due to intramolecular bonding forces, and thereby prestress the entire molecule when they resist inward-direct deforming forces (Ingber, 1998; Ingber, 2000; Ingber, 2003b; Zanottiand Guerra, 2003). This prestress explains why proteins lose shape stability when they are cleaved, or why certain proteins (e.g., hemagglutinin) can undergo dramatic unfolding or other shape transformations when a small region of the molecule is destabilized.
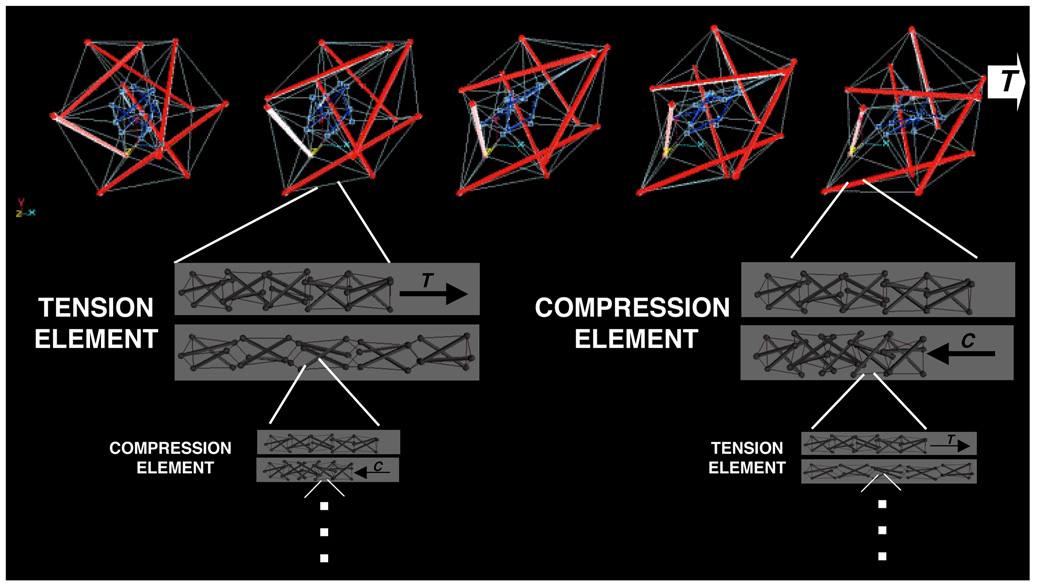
Thus, our bodies are constructed as systems within systems, such that an individual tension or compression element of a tensegrity at one size scale may itself be a tensegrity composed of multiple smaller tension and compression elements at a reduced size scale (Fig. 5). If our bodies are structural hierarchies that use tensegrity to mechanically integrate across different size scales, then this should have significant impact on how mechanical signals are transferred from the macro- to the micro-scale. Specifically, forces exerted at the level of the whole organ (e.g., due to rhythmic heart contraction) will be channeled over ECMs and linked integrins, and thereby focused on focal adhesions and the cytoskeleton where mechanochemical transduction may then proceed.
Fig. 5. Computer models depicting multiscale structural rearrangements with a prestressed tensegrity hierarchy.
Top) A two-tier hierarchical tensegrity composed of concentric large (red) and small (blue) spherical (polyhedral) strut and cable structures connected by tension elements. Note that the structure exhibits coordinated structural rearrangements of its internal elements as it extends to the right in response to tension (T) application (movies showing dynamic movements in tensegrities can be seen at: www.childrenshospital.org/research/Site2029/mainpageS2029P23sublevel24.html). Lower panels show how individual struts and cables of the structure may themselves be organized as compressed (C) and tensed (T) tensegrity mast structures at smaller and smaller size scales ad infinitum. A stress applied at the macroscale will result in global rearrangements at multiple size scales, rather than local bending or breakage, as long as tensional integrity and stabilizing prestress are maintained throughout the hierarchical network (reprinted with permission from Ingber, 2006).
Mechanochemical Transduction via Solid-State Biochemistry
The concept that tensegrity may facilitate force focusing on the cytoskeleton is important because this molecular framework is more than a structural support scaffold for the cell. It also orients most of the cell’s metabolic machinery. For example, many of the enzymes and substrates that mediate DNA synthesis, transcription, RNA processing, protein synthesis, glycolysis and signal transduction are not floating free in the cytoplasm or in the lipid bilayer; instead, they function when immobilized on these insoluble cytoskeletal scaffolds in the cytoplasm and nucleus (Ingber, 1993). Thus, forces focused on these cytoskeletal filaments may result in physical deformation of these associated molecules.
At the molecular biophysical level, altering molecular shape or deformation rates (kinetics) may directly influence biochemical activities (Ingber, 1997; Ingber, 2006). Examples include the effects of membrane shear on stress-activated ion channels; tension applied to molecular motors or enzymes using optical tweezers; exposure of new binding sites on molecules when they are unfolded using pulling forces applied using AFM; and the effects of tension and compression on polymerization dynamics of linear polymers, such as microtubules.
These concepts of force channeling and solid-state biochemistry led to the hypothesis that focal adhesions that both mediate transmembrane force transfer and orient much of the signal transduction machinery of the cell, might be key sites of mechanochemical signal transduction(Ingber, 1991; Geiger and Bershadsky, 2002). In addition to integrins, the cytoskeletal backbone of the focal adhesion physically associates with multiple protein kinases (FAK, Src, ERKs), inositol lipid signaling molecules, small and large G proteins, ion channels, and some growth factor receptors, among other signaling molecules (Plopper et al., 1995; Miyamoto et al., 1995; Geiger and Bershadsky, 2002).
Importantly, we and others have been able to confirm that forces applied directly to bound integrins activate many of these signaling molecules and stimulate gene transcription in a specific manner (Alenghat and Ingber, 2002). For instance, we have found that applying tension to integrins through bound magnetic microbeads activates calcium influx through stress-sensitive ion channels within milliseconds after force application (Matthews, Thodeti, Tytell and Ingber, unpublished results). With sustained pulling at nN levels of force, enough calcium enters the cell to trigger release of intracellular stores and induce a global wave of calcium within seconds after force application (Matthews et al., 2006). In separate studies, applying shear to bound cell surface integrins was shown to stimulate the entire cAMP signaling cascade from activation of large G proteins, to increasd cAMP production by adenylyl cyclase, to release of the catalytic portion of protein kinase A, which translocates into the nucleus and activates transcription of genes containing multiple cAMP response elements (Meyer et al., 2000). In contrast, applying the same level of shear stress to transmembrane metabolic receptors failed to produce these signaling effects. Hence, generalized membrane deformation is not sufficient to activate mechanotransduction, and instead integrins specifically mediate mechano-chemical signal conversion by producing deformation-dependent changes in the activities of distinct signaling molecules inside the cell.
The focal adhesion is now thought of as a ‘mechanosensory organelle’ (Ingber, 1991; Geiger and Bershadsky, 2002). However, this signaling complex exhibits an even more novel property: it changes its size and shape to optimally meet the needs of its mechanical environment. High stresses stimulate focal adhesion assembly, whereas well formed focal adhesions disassemble and shrink when tension is released (Riveline et al., 2001; Chen et al., 2003; Lele et al., 2006). This can be demonstrated directly at the level of changes in molecular binding kinetics in cells expressing GFP-labelled focal adhesion proteins, such as vinculin and zyxin (Lele et al., 2006). When these cells were analyzed using fluorescence recovery after photobleaching (FRAP) techniques, zyxin was found to almost double its unbinding constant (koff) when tension was dissipated by adding myosin inhibitors or severing a single stress fiber using laser nanosurgery. In contrast, the koff of vinculin remained essentially unchanged under similar conditions. Thus, forces transmitted over integrins and focused on focal adhesions result in specific changes in molecular binding activities of a subset of these molecules on the millisecond time scale that translate into large-scale changes in focal adhesion size and structure over seconds to minutes.
Taken together, these findings confirm that the focal adhesion is one of the major sites where mechano-chemical signal conversion is carried out in the cell. However, the nucleated tensegrity model suggests that forces applied locally to integrin adhesion sites also will be simultaneously transmitted to multiple other locations in the cell because they are channeled across discrete load-bearing elements in the cytoskeleton and nucleus. This type of force channeling has been clearly visualized in living cells using an ‘intracellular’ traction force microscopy technique developed by Ning Wang (U. Illinois Champaign-Urbana), which demonstrates that forces applied to apical integrins travels to the nucleus and to basal focal adhesions along discrete paths (Hu et al., 2003; Hu et al., 2005; Hu and Wang, 2006). Moreover, these stresses can be concentrated at distant sites in the cell due to geometric constraints (e.g., many elements converging on a common site), and the efficiency of force transfer is again totally dependent on the level of prestress in the cytoskeleton, as predicted by the tensegrity paradigm (Hu et al., 2003; Hu et al., 2005; Hu and Wang, 2006). It also provides a structural basis for why direct distortion of apical primary cilia activates mechanical signaling (calcium influx through stress-sensitive polycystin channels) under normal conditions, but not when basal integrin binding or the internal actin cytoskeleton is disrupted (Nauli et al., 2003; Alenghat et al., 2004). Thus, this cell-wide scheme for integrating force transmission and deforming multiple structures at different sites simultaneously may explain why so many different molecules and components have been found to contribute to cellular mechanotransduction, including integrins, stress-sensitive ion channels, cadherins, caveolae, focal adhesions, primary cilia, cytoskeletal filaments, nuclear structures and ECM proteins, among others (Ingber, 2006).
Cytoskeletal Tension, Cell Shape and Developmental Control
Integrins and focal adhesions are now recognized as ubiquitous mediators of mechanosensation. However, we have found that while applying forces directly to integrins activates early signaling events to a similar degree in flat and round cells (e.g., cAMP production; Meyer et al., 2000), spread cells integrate this early cue with other physical stimuli associated with its stretched form and proliferate, whereas round cells devoid of these additional cues switch on the apoptosis program (Chen et al., 1997). Hence, additional mechanical signals are conveyed by the overall degree to which cells experience shape distortion, and these latter signals govern cell fate switching.
Analysis of the mechanism by which cell shape controls cell cycle progression in capillary cells has revealed that the small GTPase, Rho, plays a central role in this mechanoregulatory mechanism. As mentioned earlier, tension application to integrins activates Rho, which stimulates actin polymerization and additional myosin-based tension generation by activation of its downstream effectors, mDia and Rho-associated kinase (ROCK), respectively. This plays a major role in focal adhesion formation and stress fiber assembly (Riveline et al., 2001). However, the balance between mDia and ROCK activities also regulates the F-box protein Skp2, which controls degradation of the critical cyclin-dependent kinase (cdk) inhibitor, p27, which regulates the G1/S transition (Mammoto et al., 2004).
Although Rho signaling and cell cycle progression can be regulated by growth factors and integrin binding directly, we found that physical distortion of cell shape and the cytoskeleton can regulate this pathway independently through the Skp2-p27 pathway, and thereby govern whether or not cells will proliferate (Huang et al., 1998; Huangand Ingber, 2002; Numaguchi et al., 2003; Mammoto et al., 2004). Interestingly, changes in the structure of the cytoskeleton impact this pathway by promoting calpain-dependent cleavage of the cytoskeletal protein filamin, which controls entry of the upstream inhibitor of Rho, p190RhoGAP, into lipid rafts where it modulates Rho activity (Mammoto et al., 2007). Thus, Rho is both upstream and downstream of mechanical forces and the cytoskeleton in these cells.
The identification of Rho and ROCK as a critical regulators of cytoskeletal tension in cells allowed us to return to our initial question of whether local variations in the mechanical force balance between the cytoskeleton and the ECM might contribute to control of tissue branching during embryogenesis (Fig. 1). To explore this directly, we treated embryonic lung rudiments isolated on day 12 of development with stimulators and inhibitors of Rho-dependent tension generation (Moore et al., 2005). These studies revealed that both epithelial budding morphogenesis and capillary branching (angiogenesis) can be stimulated by increasing cytoskeletal tension in whole developing organs, whereas dissipating tension with specific inhibitors suppresses development of both tissues. In addition, increasing Rho activity and tension to extremely high levels causes physical compaction of the whole organ and inhibits its growth and development. Interestingly, dissipating tension in the developing gland also prevented thinning of the basement membrane that is normally observed beneath the tips of growing lung buds. Using the run in the stocking analogy: it was as if there were a cut in the fabric, but no tension to stretch and thin the compromised material.
Thus, the ECM and cytoskeleton appear to be critical mediators of developmental control in part because they mediate mechanical signaling. Although cell binding to soluble cytokines and insoluble ECM molecules convey signals that are important for development, control of signal integration and cellular decision-making lies in the balance of forces across transmembrane integrin receptors. Pulling on ECM tugs on integrins and associated focal adhesion proteins, thereby deforming the shape of molecules that elicit biochemical signals, which leads to changes in intracellular metabolism and gene expressions. However, the reverse is also true: cell traction on ECM adhesions promotes ECM fibril assembly and modulates expression and activities of matrix-modulating enzymes (e.g., matrix metalloproteinases) (Pankov et al., 2000; Yan et al., 2000; Sarasa-Renedo and Chiquet, 2005; Gee et al., 2008). In this manner, cells and tissues remodel their internal and external structure in a coordinated manner so that one rarely sees a tissue growing free of its surrounding ECM scaffolds.
All organs also use structural hierarchies to mediate the mechanotransduction response. Contraction and dilation of the heart, for example, result in deformation of its component ECMs, which distort cells and their integrin adhesions as well as internal focal adhesions and linked cytoskeletal and nuclear components. At every size scale and level of organization, the level of tone or prestress in these discrete structural networks governs their overall response to stress, both mechanically and biochemically (Ingber, 2006). This is also true in sound sensation in the ear, responsiveness to air movements in the lung, hemodynamic stresses in blood vessels, and compression in bone and cartilage. Thus, tensegrity helps to guide force transmission and orchestrate multimolecular responses to stress at all size scales and in all organ systems.
Conclusion
Work over the past few decades has revealed that mechanical forces are as potent regulators of cell and tissue development as soluble hormones and insoluble ECM proteins. Analysis of how cells and tissues structure themselves at the nanometer scale has led to the discovery that living systems are organized as structural hierarchies that use tensegrity architecture to mechanically stabilize themselves. Use of this structural system for shape stability may explain how cells and tissues can immediately sense and respond to external mechanical signals, as well as how they channel and focus forces on cell surface integrin mechanoreceptor molecules.
The finding that cells are not bits of viscous cytoplasm surrounded by an elastic membrane, but instead structured as tensegrities with internal load bearing struts and tensed cables also has led to novel insights into developmental control and pathobiology. For example, the finding that microtubules bear compression in living cells is extremely relevant for heart physiology because an increased density of the microtubule component of the extramyofilament portion of the cardiocyte cytoskeleton caused by pressure overload can physically interfere with inward-directed shortening of the myofibrillar bundle, and hence lead to contractile dysfunction associated with cardiac hypertrophy (Tagawa et al., 1997). The integrated nature of biolological architecture also helps to explain why cardiac diseases and developmental abnormalities can be caused by mutations in various ostensibly unrelated molecules, including integrins, cytoskeletal filaments, ion channels, or nuclear components (Ingber, 2003a).
Forces channeled over ECMs and to integrins are converted into biochemical changes by producing changes in deformation of other load-bearing mechantransducer molecules, such as stress-sensitive ion channels, protein kinases, G proteins, and other signaling molecules, inside the cell. Disruption of normal paths or structures that mediate this fine coordination between living tissue deformation and activation of signal transducers (e.g., stress-sensitive ion channels) that can be caused by hypoxic events (e.g., myocardial infarct) or abnormal physical distension of organs (e.g., volume overload) may therefore lead to aberrant mechano-electrical coupling that are observed during development of heart arrhythmias. Recent advances in mathematics, engineering, and statistical mechanical models of tensegrity structures, and in the use of nanotechnology to create artificial cell-material control interfaces (Mannix et al., 2008), may provide new ways to investigate, model, manipulate, probe, and control these fundamental mechanotransduction mechanisms in living cells, tissues and organs, including heart, in the future.
Acknowledgements
I would like to thank all of my past and present students, fellows, staff and collaborators for their hard work and creativity over the past 25 years that stand behind all of the findings I summarized in this lecture. This work was supported by grants from NIH, NASA, NSF, and DARPA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alenghat FJ, Ingber DE. Mechanotransduction: all signals point to cytoskeleton, matrix, and integrins. Sci STKE. 2002. 2002:PE6. doi: 10.1126/stke.2002.119.pe6. [DOI] [PubMed] [Google Scholar]
- Alenghat FJ, Nauli SM, Kolb R, Zhou J, Ingber DE. Global cytoskeletal control of mechanotransduction in kidney epithelial cells. Exp Cell Res. 2004;301:23–30. doi: 10.1016/j.yexcr.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Ausprunk DH, Folkman J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res. 1977;14:53–65. doi: 10.1016/0026-2862(77)90141-8. [DOI] [PubMed] [Google Scholar]
- Bernfield MR, Banerjee SD. The basal lamina in epithelial-mesenchymal interactions. In: Kefalides N, editor. Biology and Chemistry of Basement Membranes. New York: Academic Press; 1978. pp. 137–148. [Google Scholar]
- Brangwynne CMF, Kumar S, Geisse NA, Mahadevan L, Parker KK, Ingber DE, Weitz D. Microtubules can bear enhanced compressive loads in living cells due to lateral reinforcement. J. Cell Biol. 2006 doi: 10.1083/jcb.200601060. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock A, Chang E, Ho CC, LeDuc P, Jiang X, Whitesides GM, Ingber DE. Geometric determinants of directional cell motility revealed using microcontact printing. Langmuir. 2003;19:1611–1617. doi: 10.1021/la026394k. [DOI] [PubMed] [Google Scholar]
- Brodland GW, Gordon R. Intermediate filaments may prevent buckling of compressively loaded microtubules. J Biomech Eng. 1990;112:319–321. doi: 10.1115/1.2891190. [DOI] [PubMed] [Google Scholar]
- Bursac N, Parker KK, Iravanian S, Tung L. Cardiomyocyte cultures with controlled macroscopic anisotropy: a model for functional electrophysiological studies of cardiac muscle. Circ Res. 2002;91:e45–e54. doi: 10.1161/01.res.0000047530.88338.eb. [DOI] [PubMed] [Google Scholar]
- Cai S, Pestic-Dragovich L, O'Donnell ME, Wang N, Ingber D, Elson E, De Lanerolle P. Regulation of cytoskeletal mechanics and cell growth by myosin light chain phosphorylation. Am J Physiol. 1998;275:C1349–C1356. doi: 10.1152/ajpcell.1998.275.5.C1349. [DOI] [PubMed] [Google Scholar]
- Caspar DL. Movement and self-control in protein assemblies. Quasi-equivalence revisited. Biophys J. 1980;32:103–138. doi: 10.1016/S0006-3495(80)84929-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CS, Alonso JL, Ostuni E, Whitesides GM, Ingber DE. Cell shape provides global control of focal adhesion assembly. Biochem Biophys Res Commun. 2003;307:355–361. doi: 10.1016/s0006-291x(03)01165-3. [DOI] [PubMed] [Google Scholar]
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- Chen CS, Ostuni E, Whitesides GM, Ingber DE. Using self-assembled monolayers to pattern ECM proteins and cells on substrates. Methods Mol Biol. 2000;139:209–219. doi: 10.1385/1-59259-063-2:209. [DOI] [PubMed] [Google Scholar]
- Clark ER, Clark EL. Microscopic observations on the growth of blood capillaries in the living mammal. Am. J. Anat. 1938;64:251–301. [Google Scholar]
- Coughlin MF, Stamenovic D. A tensegrity model of the cytoskeleton in spread and round cells. J Biomech Eng. 1998;120:770–777. doi: 10.1115/1.2834892. [DOI] [PubMed] [Google Scholar]
- Dike LE, Chen CS, Mrksich M, Tien J, Whitesides GM, Ingber DE. Geometric control of switching between growth, apoptosis, and differentiation during angiogenesis using micropatterned substrates. In Vitro Cell Dev Biol Anim. 1999;35:441–448. doi: 10.1007/s11626-999-0050-4. [DOI] [PubMed] [Google Scholar]
- Eckes B, Dogic D, Colucci-Guyon E, Wang N, Maniotis A, Ingber D, Merckling A, Langa F, Aumailley M, Delouvee A, Koteliansky V, Babinet C, Krieg T. Impaired mechanical stability, migration and contractile capacity in vimentin-deficient fibroblasts. J Cell Sci. 1998;111(Pt 13):1897–1907. doi: 10.1242/jcs.111.13.1897. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Folkman J, Moscona A. Role of cell shape in growth control. Nature. 1978;273:345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Fuller B. Tensegrity. Portfolio Artnews Annual. 1961;4:112–127. [Google Scholar]
- Gee EPS, Ingber DE, Stultz CM. Fibronectin unfolding revisited: modeling cell traction-mediated unfolding of the tenth type-III repeat. Proc Natl Acad Sci USA. 2008 doi: 10.1371/journal.pone.0002373. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A. Exploring the neighborhood: adhesion-coupled cell mechanosensors. Cell. 2002;110:139–142. doi: 10.1016/s0092-8674(02)00831-0. [DOI] [PubMed] [Google Scholar]
- Heisterkamp A, Maxwell IZ, Mazur E, Underwood JM, Nickerson JA, Kumar S, Ingber DE. Pulse energy dependence of subcellular dissection by femtosecond laser pulses. Opt Express. 2005;13:3690–3696. doi: 10.1364/opex.13.003690. [DOI] [PubMed] [Google Scholar]
- Heuser JE, Kirschner MW. Filament organization revealed in platinum replicas of freeze-dried cytoskeletons. J Cell Biol. 1980;86:212–234. doi: 10.1083/jcb.86.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Chen J, Butler JP, Wang N. Prestress mediates force propagation into the nucleus. Biochem Biophys Res Commun. 2005;329:423–428. doi: 10.1016/j.bbrc.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Hu S, Chen J, Fabry B, Numaguchi Y, Gouldstone A, Ingber DE, Fredberg JJ, Butler JP, Wang N. Intracellular stress tomography reveals stress focusing and structural anisotropy in cytoskeleton of living cells. Am J Physiol Cell Physiol. 2003;285:C1082–C1090. doi: 10.1152/ajpcell.00159.2003. [DOI] [PubMed] [Google Scholar]
- Hu S, Wang N. Control of stress propagation in the cytoplasm by prestress and loading frequency. Mol Cell Biomech. 2006;3:49–60. [PubMed] [Google Scholar]
- Huang S, Chen CS, Ingber DE. Control of cyclin D1, p27(Kip1), and cell cycle progression in human capillary endothelial cells by cell shape and cytoskeletal tension. Mol Biol Cell. 1998;9:3179–3193. doi: 10.1091/mbc.9.11.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Ingber DE. The structural and mechanical complexity of cell-growth control. Nat Cell Biol. 1999;1:E131–E138. doi: 10.1038/13043. [DOI] [PubMed] [Google Scholar]
- Huang S, Ingber DE. A discrete cell cycle checkpoint in late G(1) that is cytoskeleton-dependent and MAP kinase (Erk)-independent. Exp Cell Res. 2002;275:255–264. doi: 10.1006/excr.2002.5504. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Fibronectin controls capillary endothelial cell growth by modulating cell shape. Proc Natl Acad Sci USA. 1990;87:3579–3583. doi: 10.1073/pnas.87.9.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber DE. Integrins as mechanochemical transducers. Curr Opin Cell Biol. 1991;3:841–848. doi: 10.1016/0955-0674(91)90058-7. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Cellular tensegrity: defining new rules of biological design that govern the cytoskeleton. J Cell Sci. 1993;104(Pt 3):613–627. doi: 10.1242/jcs.104.3.613. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol. 1997;59:575–599. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- Ingber DE. The architecture of life. Sci Am. 1998;278:48–57. doi: 10.1038/scientificamerican0198-48. [DOI] [PubMed] [Google Scholar]
- Ingber DE. The origin of cellular life. Bioessays. 2000;22:1160–1170. doi: 10.1002/1521-1878(200012)22:12<1160::AID-BIES14>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Mechanobiology and diseases of mechanotransduction. Ann Med. 2003a;35:564–577. doi: 10.1080/07853890310016333. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Tensegrity I. Cell structure and hierarchical systems biology. J Cell Sci. 2003b;116:1157–1173. doi: 10.1242/jcs.00359. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Cellular mechanotransduction: putting all the pieces together again. Faseb J. 2006;20:811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- Ingber DE, Dike L, Hansen L, Karp S, Liley H, Maniotis A, McNamee H, Mooney D, Plopper G, Sims J, et al. Cellular tensegrity: exploring how mechanical changes in the cytoskeleton regulate cell growth, migration, and tissue pattern during morphogenesis. Int Rev Cytol. 1994;150:173–224. doi: 10.1016/s0074-7696(08)61542-9. [DOI] [PubMed] [Google Scholar]
- Ingber D, Jamieson JD. Cells as tensegrity structures: architectural regulation of histodifferentiation by physical forces tranduced over basement membrane. In: Andersson LC, Gahmberg CG, Ekblom P, editors. Gene Expression During Normal and Malignant Differentiation. Orlando: Academic Press; 1985. pp. 13–32. [Google Scholar]
- Ingber DE, Madri JA, Folkman J. Endothelial growth factors and extracellular matrix regulate DNA synthesis through modulation of cell and nuclear expansion. In Vitro Cell Dev Biol. 1987;23:387–394. doi: 10.1007/BF02620997. [DOI] [PubMed] [Google Scholar]
- Ingber DE, Madri JA, Jamieson JD. Role of basal lamina in neoplastic disorganization of tissue architecture. Proc Natl Acad Sci U S A. 1981;78:3901–3905. doi: 10.1073/pnas.78.6.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber DE, Madri JA, Jamieson JD. Basement membrane as a spatial organizer of polarized epithelia. Exogenous basement membrane reorients pancreatic epithelial tumor cells in vitro. Am J Pathol. 1986;122:129–139. [PMC free article] [PubMed] [Google Scholar]
- Kaech S, Ludin B, Matus A. Cytoskeletal plasticity in cells expressing neuronal microtubule-associated proteins. Neuron. 1996;17:1189–1199. doi: 10.1016/s0896-6273(00)80249-4. [DOI] [PubMed] [Google Scholar]
- Kumar S, Maxwell IZ, Heisterkamp A, Polte TR, Lele TP, Salanga M, Mazur E, Ingber DE. Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization, and extracellular matrix mechanics. Biophys J. 2006;90:3762–3773. doi: 10.1529/biophysj.105.071506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E. Actin, alpha-actinin, and tropomyosin interaction in the structural organization of actin filaments in nonmuscle cells. J Cell Biol. 1976;68:202–219. doi: 10.1083/jcb.68.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Tsai KY, Wang N, Ingber DE. Extracellular matrix and pulmonary hypertension: control of vascular smooth muscle cell contractility. Am J Physiol. 1998;274:H76–H82. doi: 10.1152/ajpheart.1998.274.1.H76. [DOI] [PubMed] [Google Scholar]
- Lele TP, Pendse J, Kumar S, Salanga M, Karavitis J, Ingber DE. Mechanical forces alter zyxin unbinding kinetics within focal adhesions of living cells. J Cell Physiol. 2006;207:187–194. doi: 10.1002/jcp.20550. [DOI] [PubMed] [Google Scholar]
- Maksym GN, Fabry B, Butler JP, Navajas D, Tschumperlin DJ, Laporte JD, Fredberg JJ. Mechanical properties of cultured human airway smooth muscle cells from 0.05 to 0.4 Hz. J Appl Physiol. 2000;89:1619–1632. doi: 10.1152/jappl.2000.89.4.1619. [DOI] [PubMed] [Google Scholar]
- Mammoto A, Huang S, Ingber DE. Cell spreading controls Rho activity by promoting calpain-dependent cleavage of filamin and translocation of p190RhoGAP to lipid rafts. 2007 [Google Scholar]
- Mammoto A, Huang S, Moore K, Oh P, Ingber DE. Role of RhoA, mDia, and ROCK in cell shape-dependent control of the Skp2-p27kip1 pathway and the G1/S transition. J Biol Chem. 2004;279:26323–26330. doi: 10.1074/jbc.M402725200. [DOI] [PubMed] [Google Scholar]
- Maniotis AJ, Bojanowski K, Ingber DE. Mechanical continuity and reversible chromosome disassembly within intact genomes removed from living cells. J Cell Biochem. 1997a;65:114–130. [PubMed] [Google Scholar]
- Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci U S A. 1997b;94:849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannix RJ, Kumar S, Cassiola F, Montoya-Zavala M, Ingber DE. Magnetic actuation of receptor-mediated signal transduction. Nature Nanotech. 2008 doi: 10.1038/nnano.2007.418. in press. [DOI] [PubMed] [Google Scholar]
- Matthews BD, Overby DR, Mannix R, Ingber DE. Cellular adaptation to mechanical stress: role of integrins, Rho, cytoskeletal tension, and mechanosensitive ion channels. J Cell Sci. 2006;119:508–518. doi: 10.1242/jcs.02760. [DOI] [PubMed] [Google Scholar]
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- Meyer CJ, Alenghat FJ, Rim P, Fong JH, Fabry B, Ingber DE. Mechanical control of cyclic AMP signalling and gene transcription through integrins. Nat Cell Biol. 2000;2:666–668. doi: 10.1038/35023621. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Teramoto H, Coso OA, Gutkind JS, Burbelo PD, Akiyama SK, Yamada KM. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KA, Polte T, Huang S, Shi B, Alsberg E, Sunday ME, Ingber DE. Control of basement membrane remodeling and epithelial branching morphogenesis in embryonic lung by Rho and cytoskeletal tension. Developmental Dynamics. 2005;232:268–281. doi: 10.1002/dvdy.20237. [DOI] [PubMed] [Google Scholar]
- Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- Numaguchi Y, Huang S, Polte TR, Eichler GS, Wang N, Ingber DE. Caldesmon-dependent switching between capillary endothelial cell growth and apoptosis through modulation of cell shape and contractility. Angiogenesis. 2003;6:55–64. doi: 10.1023/a:1025821517679. [DOI] [PubMed] [Google Scholar]
- Overby DR, Matthews BD, Alsberg E, Ingber DE. Novel dynamic rheological behavior of focal adhesions measured within single cells using electromagnetic pulling cytometry. Acta Biomateriala. 2005;3:295–303. doi: 10.1016/j.actbio.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Pankov R, Cukierman E, Katz BZ, Matsumoto K, Lin DC, Lin S, Hahn C, Yamada KM. Integrin dynamics and matrix assembly: tensin-dependent translocation of alpha(5)beta(1) integrins promotes early fibronectin fibrillogenesis. J Cell Biol. 2000;148:1075–1090. doi: 10.1083/jcb.148.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KK, Brock AL, Brangwynne C, Mannix RJ, Wang N, Ostuni E, Geisse NA, Adams JC, Whitesides GM, Ingber DE. Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces. Faseb J. 2002;16:1195–1204. doi: 10.1096/fj.02-0038com. [DOI] [PubMed] [Google Scholar]
- Parker KK, Ingber DE. Extracellular matrix, mechanotransduction and structural hierarchies in heart tissue engineering. Philos Trans R Soc Lond B Biol Sci. 2007;362:1267–1279. doi: 10.1098/rstb.2007.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett-Heaps JD, Forer A, Spurck T. Traction fibre: toward a "tensegral" model of the spindle. Cell Motil Cytoskeleton. 1997;37:1–6. doi: 10.1002/(SICI)1097-0169(1997)37:1<1::AID-CM1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Plopper GE, McNamee HP, Dike LE, Bojanowski K, Ingber DE. Convergence of integrin and growth factor receptor signaling pathways within the focal adhesion complex. Mol Biol Cell. 1995;6:1349–1365. doi: 10.1091/mbc.6.10.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polte TR, Eichler GS, Wang N, Ingber DE. Extracellular matrix controls myosin light chain phosphorylation and cell contractility through modulation of cell shape and cytoskeletal prestress. Am J Physiol Cell Physiol. 2004;286:C518–C528. doi: 10.1152/ajpcell.00280.2003. [DOI] [PubMed] [Google Scholar]
- Pourati J, Maniotis A, Spiegel D, Schaffer JL, Butler JP, Fredberg JJ, Ingber DE, Stamenovic D, Wang N. Is cytoskeletal tension a major determinant of cell deformability in adherent endothelial cells? Am J Physiol. 1998;274:C1283–C1289. doi: 10.1152/ajpcell.1998.274.5.C1283. [DOI] [PubMed] [Google Scholar]
- Prime KL, Whitesides GM. Science. Vol. 252. Washington, DC, United States: 1991. Self-assembled organic monolayers: model systems for studying adsorption of proteins at surfaces; pp. 1164–1167. [DOI] [PubMed] [Google Scholar]
- Rafferty NS, Scholz DL. Actin in polygonal arrays of microfilaments and sequestered actin bundles (SABs) in lens epithelial cells of rabbits and mice. Curr Eye Res. 1985;4:713–718. doi: 10.3109/02713688509017667. [DOI] [PubMed] [Google Scholar]
- Rathke PC, Osborn M, Weber K. Immunological and ultrastructural characterization of microfilament bundles: polygonal nets and stress fibers in an established cell line. Eur J Cell Biol. 1979;19:40–48. [Google Scholar]
- Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, Kam Z, Geiger B, Bershadsky AD. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol. 2001;153:1175–1186. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarasa-Renedo A, Chiquet M. Mechanical signals regulating extracellular matrix gene expression in fibroblasts. Scand J Med Sci Sports. 2005;15:223–230. doi: 10.1111/j.1600-0838.2005.00461.x. [DOI] [PubMed] [Google Scholar]
- Schutt CE, Kreatsoulas C, Page R, Lindberg U. Plugging into actin's architectonic socket. Nat Struct Biol. 1997;4:169–172. doi: 10.1038/nsb0397-169. [DOI] [PubMed] [Google Scholar]
- Singhvi R, Kumar A, Lopez GP, Stephanopoulos GN, Wang DI, Whitesides GM, Ingber DE. Engineering cell shape and function. Science. 1994;264:696–698. doi: 10.1126/science.8171320. [DOI] [PubMed] [Google Scholar]
- Stamenovic D. Effects of cytoskeletal prestress on cell rheological behavior. Acta Biomater. 2005;1:255–262. doi: 10.1016/j.actbio.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Stamenovic D, Coughlin MF. The role of prestress and architecture of the cytoskeleton and deformability of cytoskeletal filaments in mechanics of adherent cells: a quantitative analysis. J Theor Biol. 1999;201:63–74. doi: 10.1006/jtbi.1999.1014. [DOI] [PubMed] [Google Scholar]
- Stamenovic D, Coughlin MF. A quantitative model of cellular elasticity based on tensegrity. J Biomech Eng. 2000;122:39–43. doi: 10.1115/1.429631. [DOI] [PubMed] [Google Scholar]
- Stamenovic D, Fredberg JJ, Wang N, Butler JP, Ingber DE. A microstructural approach to cytoskeletal mechanics based on tensegrity. J Theor Biol. 1996;181:125–136. doi: 10.1006/jtbi.1996.0120. [DOI] [PubMed] [Google Scholar]
- Sultan C, Stamenovic D, Ingber DE. A computational tensegrity model predicts dynamic rheological behaviors in living cells. Ann Biomed Eng. 2004;32:520–530. doi: 10.1023/b:abme.0000019171.26711.37. [DOI] [PubMed] [Google Scholar]
- Tagawa H, Wang N, Narishige T, Ingber DE, Zile MR, Cooper Gt. Cytoskeletal mechanics in pressure-overload cardiac hypertrophy. Circ Res. 1997;80:281–289. doi: 10.1161/01.res.80.2.281. [DOI] [PubMed] [Google Scholar]
- Vera C, Skelton R, Bossens F, Sung LA. 3-d nanomechanics of an erythrocyte junctional complex in equibiaxial and anisotropic deformations. Ann Biomed Eng. 2005;33:1387–1404. doi: 10.1007/s10439-005-4698-y. [DOI] [PubMed] [Google Scholar]
- Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- Wang N, Ingber DE. Control of cytoskeletal mechanics by extracellular matrix, cell shape, and mechanical tension. Biophys J. 1994;66:2181–2189. doi: 10.1016/S0006-3495(94)81014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Naruse K, Stamenovic D, Fredberg JJ, Mijailovich SM, Tolic-Norrelykke IM, Polte T, Mannix R, Ingber DE. Mechanical behavior in living cells consistent with the tensegrity model. Proc Natl Acad Sci U S A. 2001;98:7765–7770. doi: 10.1073/pnas.141199598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Stamenovic D. Contribution of intermediate filaments to cell stiffness, stiffening, and growth. Am J Physiol Cell Physiol. 2000;279:C188–C194. doi: 10.1152/ajpcell.2000.279.1.C188. [DOI] [PubMed] [Google Scholar]
- Wang N, Tolic-Norrelykke IM, Chen J, Mijailovich SM, Butler JP, Fredberg JJ, Stamenovic D. Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. Am J Physiol Cell Physiol. 2002;282:C606–C616. doi: 10.1152/ajpcell.00269.2001. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer CM, Salmon ED. Actomyosin-based retrograde flow of microtubules in the lamella of migrating epithelial cells influences microtubule dynamic instability and turnover and is associated with microtubule breakage and treadmilling. J Cell Biol. 1997;139:417–434. doi: 10.1083/jcb.139.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Moses MA, Huang S, Ingber DE. Adhesion-dependent control of matrix metalloproteinase-2 activation in human capillary endothelial cells. J Cell Sci. 2000;113(Pt 22):3979–3987. doi: 10.1242/jcs.113.22.3979. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Westlin WF, Wang N, Ingber DE, Rosenzweig A, Resnick N, Gimbrone MA., Jr Leukocyte adhesion to vascular endothelium induces E-selectin linkage to the actin cytoskeleton. J Cell Biol. 1996;133:445–455. doi: 10.1083/jcb.133.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanotti G, Guerra C. Is tensegrity a unifying concept of protein folds? FEBS Lett. 2003;534:7–10. doi: 10.1016/s0014-5793(02)03853-x. [DOI] [PubMed] [Google Scholar]