Abstract
Smoking is the preferred method of administration for two of the most frequently abused drugs, marijuana and nicotine. The high temporal and spatial resolution of functional magnetic resonance imaging (fMRI) make it a natural choice for studying the neurobiological effects of smoked drugs if the challenges of smoking in a magnetic resonance (MR) scanner can be overcome. We report on a design for an MR-compatible smoking device that can be used for smoking marijuana (or tobacco) during fMRI examinations. Nine volunteers smoked marijuana cigarettes (3.51% Δ9-THC) on two occasions: with and without the device. The device allowed subjects to smoke while they lay in the scanner, while containing all smoke and odors. Plasma Δ9-THC, subjective reports of intoxication, and heart rate increases are reported, and were all similar in individuals smoking marijuana either with or without the device. The use of this device will help advance research studies on smoked drugs including marijuana, tobacco and crack cocaine.
Introduction
Functional magnetic resonance imaging (fMRI) has become a critical tool for probing the function of the human brain. In addition to providing a window into understanding basic processes associated with language, memory, perception, signal detection and motor function (Gaillard 2004; Latchaw et al 1995), fMRI has also become increasingly important in the study of both acute and chronic brain response to drugs of abuse. In particular, studies of cocaine (Breiter et al 1997; Cowan et al 2000a; Kaufman et al 1998; Maas et al 1998), opiates (Sell et al 1997), alcohol (Streeter et al 1998), amphetamines (Cowan et al 2000b), nicotine (Jacobsen et al 2002; Kumari et al 2003; Stein et al 1998; Tregellas et al 2005), and marijuana (Gruber and Yurgelun-Todd 2005; Pillay et al 2004) have been conducted. However, these studies have been limited to a parental or oral route of administration or assessing the effects of chronic drug administration. While current procedures are adequate for drugs that are typically self-administered by the oral or intravenous routes, the available procedures have been inadequate to evaluate the effects of smoked materials on brain function and mood.
Smoking is the preferred route of administration for nearly all marijuana and tobacco users. To date, it has not been possible to administer smoked drugs in a natural manner during fMRI experiments because of a number of factors relating to safety, discomfort, residual odors and technical aspects of drug delivery. The nature of a smoked product requires that it be burned, and having a lit cigarette near a subject who is supine in the magnet places him/her at risk. Second, it is impossible for a subject to mechanically smoke a cigarette while in the scanner due to the tight space inside the bore of the magnet. Third, both the side stream smoke and second hand smoke will “stick” to the plastic liner of the scanner, making the scanning process uncomfortable for subsequent subjects. Fourth, the smoke particles can enter the coil, rendering problems with the electronics. Finally, the physics of smoke make it difficult to administer remotely. Smoke is composed of hundreds of chemicals, some of which are gases and some that are particulates, and THC is an oil that adheres to most surfaces. Thus, for a smoking device to be used successfully in the MRI suite, it must overcome these obstacles. We have developed and validated a system that addresses these issues and permits real time measures of brain activation during smoking marijuana.
Design
The overall goal of this project was to design and build a system that would allow subjects to smoke marijuana in as natural a manner as possible during fMRI sessions, and contain the expired smoke in such a manner that residual odors would not remain on the scanner or in the scanning suite.
Concretely, this meant that the following criteria had to be satisfied: 1) The system had to be as unobtrusive as possible to the subject, so as not to make subjects physically uncomfortable or distracted during the imaging session, or inhibit their ability to see visual displays and operate response devices; 2) The act of smoking must be natural, i.e. excessive suction should not be necessary to get smoke, and the smoke delivered must be fresh and not depleted of active ingredients such as THC; 3) The system could not interfere with the operation of the MR scanner by generating electrical noise or containing any magnetic materials; 4) All smoke generated by the system (exhaled and sidestream) must be fully contained within the system or vented from the MR environment (outside). No smoke could enter the scan room or come into contact with scanner components. 5) The system must be modular so that system installation and removal, in addition to all scanning, could be performed in a 2-hour scanning time slot (we use 1.5 hour slots currently); and 6) All components that came into contact with bodily fluids must be cleanable/sanitizable or disposable.
The system was divided into two elements, the smoke delivery system and the exhaust system. These are described separately.
Smoke Delivery System
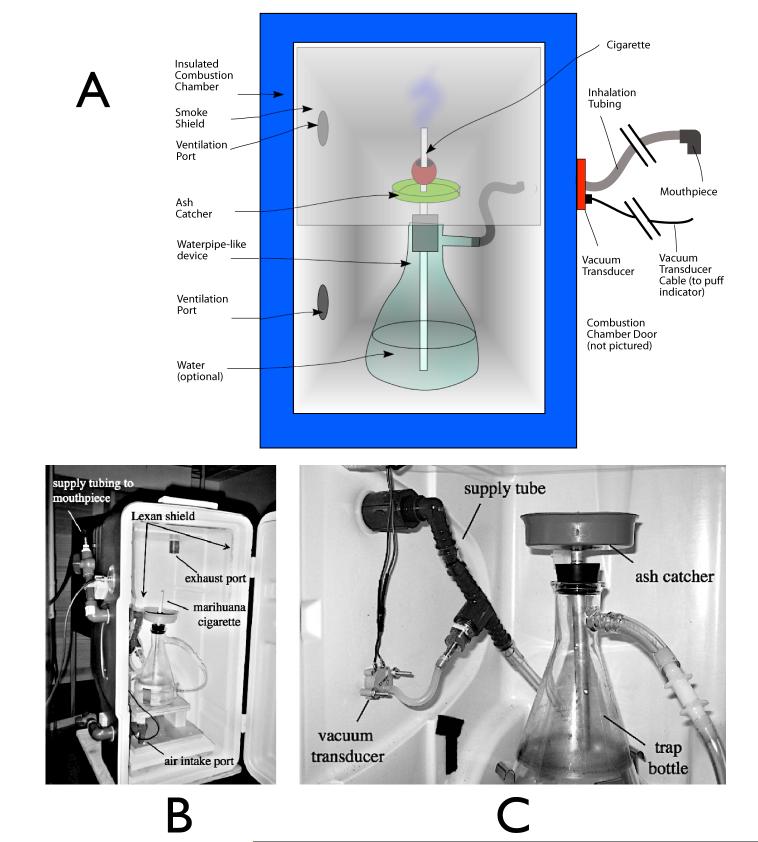
The smoke delivery system is derived from a previous design devised for smoking marijuana in a controlled setting (Lukas et al 1994). The revised model, shown in Figure 1a, depicts a marijuana cigarette that is attached to a vertical brass tube passing through the center of a 4” diameter plastic ash catcher. Smoke from the cigarette is drawn through the tube into the bottom of a cooling chamber made from a 1 L conical filtering flask. Smoke is drawn from the top of the chamber through vinyl tubing attached to the flask’s side tube leading to a nylon mouthpiece in the subject’s mouth. Traditional water pipes have the tube extend into water to trap particulates. However, were the subject to cough or blow into the tube, water could be forced up the tube and saturate the cigarette. On the other hand, a one-way valve would restrict airflow. In fact, we have found that the device works quite well with no water in the chamber. The smoke is adequately cooled by passing through the air chamber and the accompanying tubing, so the subject can smoke comfortably, with no risk of wetting the cigarette. A vacuum transducer is attached to the pipe between the filter flask and the tube leading to the mouthpiece. This transducer closes a switch when the pressure in the tube falls 3.3 torr below the outside pressure (this value was determined empirically from previous studies with a similar device); this allows us to record when and for how long the subject takes a puff from the cigarette.
Figure 1.
Smoking device. (a) shows a schematic representation of the major elements of the combustion chamber. (b) shows a photograph of the actual device; the door (on the right of the photo) remains closed once the cigarette is lit. (c) Shows a detail of the pipe, including the vacuum transducer used to detect when the subject is puffing.
This smoking device is placed at the head end of the magnet to keep the length of the tubing from the cigarette to the subject at a minimum (some slack in the tubing is required to allow the mouthpiece to be positioned prior to moving the subject into the magnet bore; the total tubing length is 2.40 m. In order to keep smoke from the cigarette from entering the magnet room, the entire device is enclosed in a ventilated, airtight container made from a 70 quart picnic cooler placed on its end. The filter flask is secured to the bottom of the chamber, and an acrylic shield covers the top of the chamber, to keep smoke from escaping when the door to the chamber is open. Ports on the top of the chamber are attached to an exhaust system that vents outside the building, and vents on the bottom of the chamber allow air to enter the chamber, maintaining a bottom-to-top airflow through the chamber. The chamber is shown in Figure 1b.
Almost all of the components of the smoke delivery system are nonmagnetic (plastic, glass, brass, and aluminum). There is a very small amount of magnetic material (internal parts of the pressure transducer and some components of the lighter) all of which is securely attached to the interior of the device. As a result, there is no perceptible force exerted on the chamber even in the region of the strongest field gradient of an actively shielded 3T magnet, and there are no loose magnetic components that could be pulled into the magnet and cause injury.
Exhaust system
In order to permit smoking studies to be performed during normal scan hours, the exhaust system must prevent any exhaled or sidestream smoke from entering the scanner room, even in the event of a cough that would make the data unusable. Since scanner time is usually fully booked, it was not practical to wait for the smell of smoke to dissipate after every smoking session. In addition to handling the “normal” airflow due to smoke inhalation and exhalation, the system needs to prevent smoke from escaping even if the subject were to cough (a relatively common occurrence in marijuana smokers, but, as it turned out, extremely infrequent during the fMRI scans performed for this study). Any solution that we developed also had to fit within the radiofrequency (RF) head coil used for imaging, as smoke could not be allowed to come in contact with the coil. This was for two reasons; first, the coil is a complicated structure that would be difficult to fully deodorize between subjects, and second, if smoke particles were to build up over time on the high voltage internal components of the coil, it would likely lead to internal arcing and coil failure.
Our initial design concept for the smoke removal was to use a close fitting mask attached to a suction system to capture all of the exhaled smoke, with a deflated bag attached to absorb large boluses of gas from a cough. This type of design turned out to be impractical. It was important that the system be unobtrusive and not cause the subjects any discomfort or distress; the close fitting mask [with suction] was uncomfortable, difficult to fit in the RF coil, and could not be fully decoupled from the smoking device (the constant suction from the exhaust system tended to “smoke” the cigarette when the subject was not inhaling). We therefore decided to use an open, non-contact design.
We elected to use a device that would create airflow around the head that would sweep all smoke into an exhaust hose and rapidly vent it from the building. This has a number of advantages. First, no component of the exhaust system actually touches the subject, so there is no uncomfortable or distracting contact, and no need to individually fit components to each subject. This also significantly speeds installation during the scan preparation period. Second, since the net airflow all around the subject is into the exhaust pipe, any smoke that initially escapes during a cough is rapidly recaptured by the system. This fact has been tested repeatedly and the exhaust system has been found to handle subject coughs with no smoke escaping.
We replaced the facemask with a half cylinder of acrylic that conformed to the inner diameter of the RF coil (26.7 cm O.D., 64 mm wall thickness). This shield extended from the bridge of the nose, just below the eyes, to halfway down the neck. Along the central axis over the nose, a section of PVC pipe was glued on to give more clearance for the nose. Seven 190 mm diameter nylon barbed tube fittings were mounted on this pipe to provide exhaust ports, and were connected to an exhaust manifold using vinyl tubing. The number, position, and size of the exhaust ports were varied during the design process; this configuration provided high, uniform airflow over the nose and mouth, was compatible with the spacing of the conductors of the radiofrequency coil, and did not create uncomfortable suction for the subject (large exhaust ports right over the nose or mouth tended to make inhalation difficult) or drying of the eyes or lips.
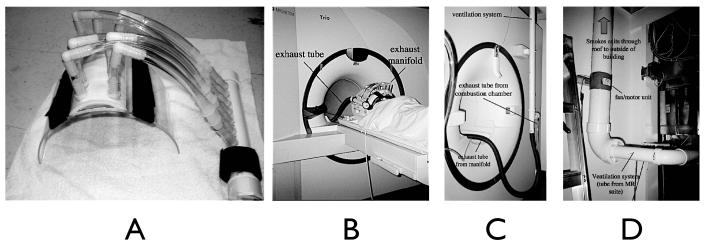
The exhaust manifold was attached to a 3.8 cm diameter flexible hose leading to a coupling at the back of the magnet to a permanently mounted exhaust system. This consisted of a 5.08 cm PVC pipe that exited the magnet room through a waveguide, and was coupled to a 10.2 cm PVC pipe with an inline 12V, 240 CFM duct fan that vented the smoke out of the building. The major components of the exhaust system are shown in Figure 2.
Figure 2.
Exhaust system components. (a) closeup of the exhaust manifold and face shield. (b) the face shield installed. Visual presentation is done via rear-projection, so the tubing does not obstruct the subject’s view of the behavioral rating scales. (c) exhaust tube exiting the “magnet” or “head” end of the magnet bore, and coupling to the exhaust pipe in the ceiling. The combustion chamber is on the floor just to the left of the pictured region. (d) exhaust fan outside the magnet room.
Installation and Usage
At the beginning of an experimental session, the smoking chamber is positioned at the head end of the magnet, the face shield is secured to the RF coil with Velcro straps, the exhaust tubing from the face mask is attached to the fixed tubing at the magnet end, and all vents are opened using plastic valves. The door to the chamber is opened, and the cigarette is lit using a lighter that is tethered to the interior of the chamber. The door to the smoking chamber is closed, and the subject can draw on the cigarette either ad libidum or paced with an audio cue. The experimenter can verify that the cigarette is burning by viewing it through an 8 cm acrylic window in the front of the chamber.
When the subject has finished smoking, the airflow through the chamber continues, and sweeps most of the smoke out of the chamber; the very mild suction clears the smoke from the mouthpiece tubing. When the imaging session is over, all the valves to the chamber are shut, and the sealed smoking device is removed from the MR suite to be opened at another location for cleaning. This prevents any smoke or odor from escaping into the MR suite.
Validation
A number of tests were performed to ensure the proper performance of the system. The criteria for proper performance were simple; the device must allow a subject to smoke marijuana or tobacco while lying in the magnet, the smoke must not enter the magnet room, the smoke delivered must have a sufficient amount of Δ9-THC to match the levels typically experienced while self-administering the drug, and the device must not interfere with fMRI data acquisition, either by increasing patient motion, or by directly affecting the acquired MR signal (through electronic noise or field distortion).
All system tests involving human subjects, and the fMRI study of marijuana smoking, were approved by the McLean Hospital Institutional Review Board.
Exhaust Efficiency
The simplest parameter to verify was that the device fully contained and exhausted all the smoke generated during the experiment. To test this, a subject underwent the MR imaging protocol while smoking both a placebo and real tobacco cigarette (tobacco is easier to obtain than marijuana, and is considered to have a stronger smell). Before, during, and after the imaging procedure, a total of 4 volunteers (age 22-35 and all non-smokers) were instructed to sniff the area around the apparatus, focusing on the combustion chamber and the area around the subjects face. They were asked to rate the air quality in the room and the degree to which they could smell burning tobacco. During the experiment, the smoker was instructed to cough several times after filling his lungs with smoke. In no instance was a tobacco odor detected by any of the subjects either during the normal smoking procedures or after the subject coughed. We have subsequently used this system for 54 scans (44 marijuana and 28 tobacco) and there has not been any detectable smell of smoke in the scan room at any time during or after these experiments.
System Generated Noise Measurement
To verify that the system itself did not introduce any distortion or noise into the functional images, two functional runs were performed on a spherical test phantom with and without the smoking apparatus installed in the magnet, using the same protocol employed for smoking studies. Data were analyzed in Brain Voyager QX. There was no observable image distortion caused by the presence of the device, and a comparison of the timecourses in an ROI in the center of the phantom showed no increase in noise when the smoking device was installed with the exhaust system running.
Delivery of THC via Measurement of Plasma Drug Levels
Having met this minimum standard, it was then necessary to determine if the device significantly decreased the bioavailability of the cigarette by actually measuring plasma drug levels. This experiment was conducted outside of the magnet to facilitate the collection of the blood samples; these data were then matched with parallel studies conducted in the magnet while physiological and behavioral indices of drug effects were monitored.
The subjects for this validation were a subgroup of the earliest subjects who subsequently participated in an fMRI study of smoked marijuana (N=9). Subjects participated in two experimental sessions in the laboratory, counterbalanced for order of exposure that included smoking a marijuana cigarette either with or without the MR compatible device. Sessions were separated by a minimum of 5 days. During both sessions subjects smoked a standard (3.51% THC) marijuana cigarette as they normally would, except that they followed audiotaped instructions for a paced smoking paradigm. They were asked to breathe normally for the first 5 minutes of the experiment; at 5 minutes, the cigarette was lit, and from 5 minutes to 10 minutes into the experiment, the subject performed paced smoking (auditory cues were given to “inhale” (3 sec), “hold” (5 sec), and “exhale”. This was repeated every 20 seconds during the smoking period.). For the remaining 15 minutes of the experiment, the subject breathed normally. Heart rate and subjective behavioral ratings were recorded throughout the sessions using the procedure described below. Blood samples were taken at two minute intervals for the first 14 minutes of the experiment, and at 4 minute intervals for the next 10 minutes. The samples were sent to a commercial laboratory (National Medical Service, Inc., Willow Grove, PA) for analysis of Δ9-THC and other cannabinoid levels using GC/MS.
Tests in the fMRI scanner
The final necessary verification steps were a) to confirm that the system would produce physiological and behavioral responses during an fMRI experiment that were comparable to those achieved in a natural setting, and b) to determine if the use of the system would not cause excessive motion by the subject. To perform these tests, we analyzed physiological, behavioral, and motion obtained during actual marijuana smoking fMRI experiments.
Imaging Protocol
Data were acquired using a Siemens 3T Trio whole body scanner with a transmit-receive quadrature birdcage head coil. After an initial localizer scan, a high-resolution “match-warped” T1-weighted anatomical image set was acquired for later alignment of activation with neuroanatomy. The “match-warped” scan undergoes exactly the same geometric distortion arising from field inhomogeneity as the fMRI data collected later (Frederick et al 2004; Rohan et al 2001), so fMRI activation maps directly onto the anatomy shown in the match-warped image. When the localizer was complete, an arterial spin labeled (ASL) image was obtained to measure baseline blood flow (ASL scans were acquired as part of our standard marijuana smoking protocol; it is not relevant to evaluation of the device function, and will not be discussed further in this manuscript). fMRI data were then collected as follows: single shot gradient echo EPI, TR/TE= 3000/18ms, 64x64 matrix, 220x220 mm FOV, 50 interleaved transverse slices 3mm thick with no gap, 1 average, 500 repetitions (25:00) (N.B. the 18ms echo time was required to fit the 50 slice whole head acquisition into a 3 second TR. While this gives a slightly lower contrast-to-noise ratio relative to a longer echo time, testing in our laboratory with a visual stimulation experiment using a range of echo times has not found that this has a major impact on the quality of detected activation). This scan was followed by another 5 minutes of ASL data collection, followed by whole head field maps acquired for distortion correction (3:28), in addition to a high resolution T1-weighted MPRAGE3D anatomical image (FOV=256x256x170mm, 256x256x128 (8:59)).
Physiological and behavioral measures
The subject’s heart rate and SaO2 levels were continuously monitored with a patient monitoring device (In Vivo MRI Devices, Orlando, FL), timestamped, and recorded on a PC for correlation with the fMRI data.
In order to determine subjective mood states during marijuana smoking, computerized visual analog scales were presented to the subject during the fMRI scans using Presentation® software (Version 0.92, Neurobehavioral Systems, Inc., Albany, CA) with a rear-projection system. While we had previously developed a non ferrous device for recording behavioral responses in magnetic fields (Lukas et al 1993), we have now switched to using a commercial MR compatible 4-button fiberoptic keypad (Fiberoptic Response Pad (fORP), Current Designs, Inc.), to move a cursor on the scale. A set of scales (Crave, High, Like drug, Anxious, Irritable, Feel drug effects, Dislike drug) were presented for 9 seconds apiece on a 150 second cycle, and the subject’s responses were recorded (only Crave and High showed any consistent changes associated with the smoking paradigm, so the other measures will not be discussed further). The presentation program also provided the subject with auditory cues to puff on the smoking device, and provided a periodic checkerboard visual stimulus to control for global blood flow changes (8 Hz black and white reversing logarithmic radial checkerboard, off-on-off-on-off in 12s epochs, presented every 150s). The fORP also provided an interface to synchronize the presentation program to a scanner-generated trigger signal.
Marijuana Smoking
During all fMRI scans subjects smoked a single NIDA cigarette containing 3.51% Δ9-THC using the “paced” smoking paradigm described above with minor modifications described below. The subject breathed normally for the first 5 minutes of the experiment (baseline). At 5 minutes, the subject was cued to begin puffing on the smoking device (the subject was instructed by auditory cues to “inhale” (3 sec), “hold” (5 sec), and “exhale”). This was repeated every 20 seconds during the smoking period. From 5 to 10 minutes, the cigarette was not lit (sham puff). At 10 minutes, the cigarette was lit and smoking continued at the same pace until 15 minutes into the scan (MJ smoking). At 15 minutes the cues ended and the subject breathed normally for the remaining 10 minutes of the session (Post-MJ Measurement Period).
Processing
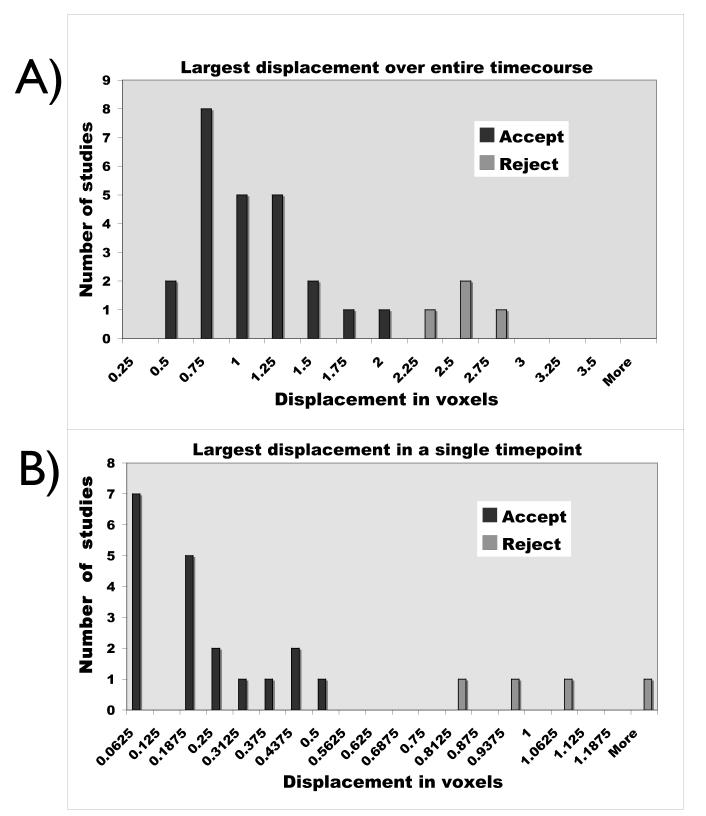
All BOLD data analysis was performed in Brain VoyagerQX (Brain Innovation, Maastricht, The Netherlands). The measured motion time courses are output from BrainVoyager’s 3D motion correction routine A concern when designing this experiment was that the long scan time, coupled with having the subject smoke in the magnet, would lead to excessive motion, which would make analysis of the BOLD time courses difficult. For this reason, we took great care to a) make the smoking device as comfortable as possible, b) minimize the effort required to puff on the cigarette, and c) secure the subject in the head coil using the restraints built into the RF coil and additional foam padding such that their head naturally returned to the baseline position. This approach worked quite well; the subject motion is quite acceptable, even during the smoking period, and is likely that a large component of the apparent motion is actually scanner drift over the long acquisition (a fact which has been verified by a phantom acquisition). Because of the extremely long acquisition, and the drift component to the motion, we used a somewhat relaxed motion standard; scans were discarded if either a) the absolute displacement in any direction over the entire time course was greater than two voxels, or b) there was a 0.5 voxel displacement in any direction over a single time point (voxel size =3.43mm). In an initial study of 29 scans, 6 were eliminated due to motion. Typical motion parameters during the fMRI session measured during the motion correction process for three subjects are shown in Figure 3. Figure 4 shows histograms of the maximum displacement (any direction) over the full timecourse, and the maximum displacement change over a single timepoint for the 29 studies.
Figure 3.
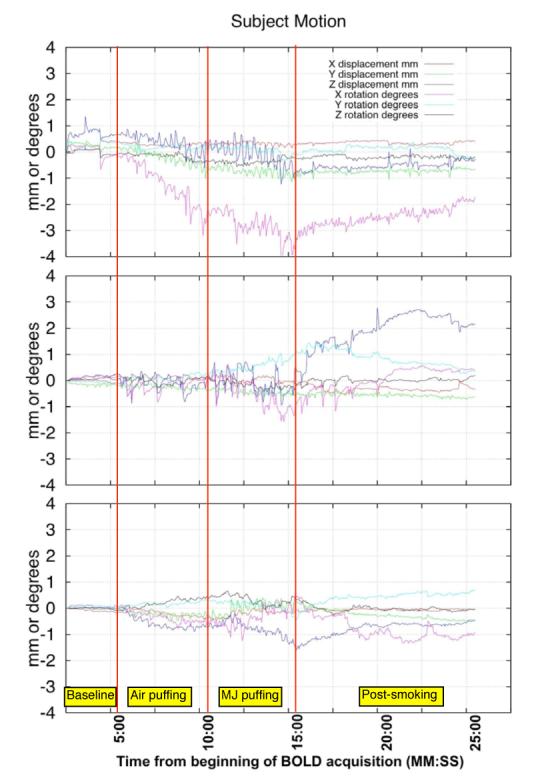
Motion parameters from the first of two smoking time courses for three subjects. Plots show X, Y, and Z motion in mm, and X, Y, and Z rotation in degrees. For reference, the baseline period covers from the start of the experiment to 5:00. Sham smoking goes from 5:00 to 10:00, marijuana smoking is from 10:00 to 15:00. From 15:00 to the end of the scan is the post-smoking measurement period.
Figure 4.
Summary histograms of motion parameters from 29 scans during marijuana or placebo marijuana smoking. A) shows the distribution of the maximum displacement from the initial head position due to any of the six measured motion parameters over the entire timecourse in voxel units (NOTE: rotation values were converted to displacement by calculating displacement at a radius of 75 mm due to the rotation). Subjects with a maximum displacement of >2 voxels are considered to have excessive motion. B) shows the distribution of the maximum change in displacement over a single timepoint in voxel units. Subjects with a single point displacement > 0.5 voxels have excessive motion. In this subject group, six subjects exceeded one or both thresholds.
Visual timecourses were processed by extracting the 20 timepoints (60 seconds) of each checkerboard presentation from the preprocessed timecourse into a separate file, and then performing a multirun general linear model (GLM) on all ten repetitions (there were two repetitions each during the presmoking, air puffing period, and marijuana smoking periods, and 4 during the post-smoking period). Timecourses were percentage normalized and corrected for serial correlations. Individual and overall activations were then calculated.
Results
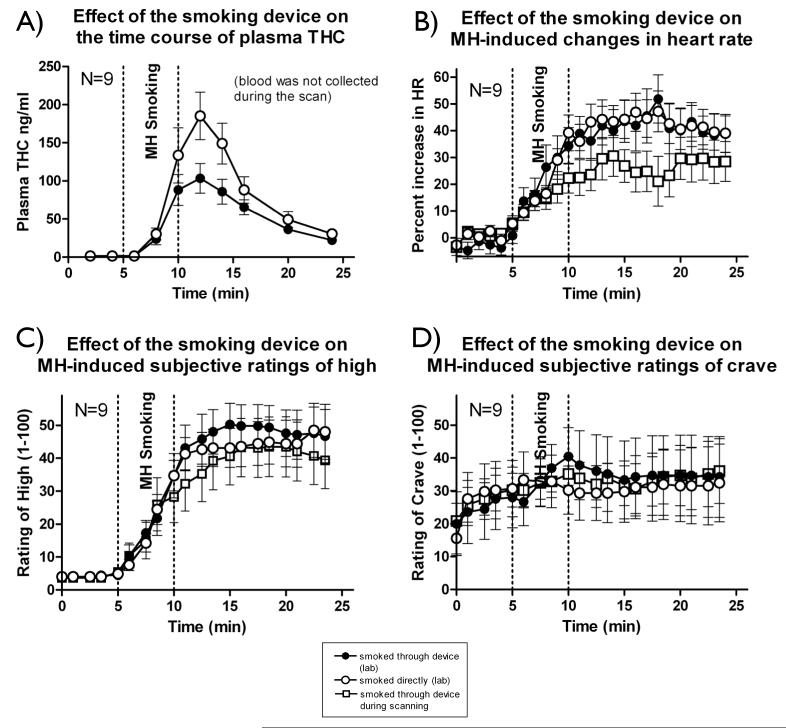
The averaged plasma THC levels from 9 subjects after smoking a single cigarette either with or without the MR compatible smoking device are summarized in Figure 5A. The plasma THC level timecourses in these individuals are quite consistent with our previous results using a similar smoking device (Lukas et al 1994; Lukas et al 1992; Lukas and Orozco 2001), and the peak levels measured (103.4±19.4 (s.e.) ng/ml smoking through the device and 185.3±31.3 ng/ml directly) both fall well within expected laboratory standards (50-270 ng/ml at 6-9 minutes after smoking a 1.75% or 3.51% THC marijuana cigarette (National Medical Services 2005)). The mean peak plasma levels achieved with the device were, as expected, lower than without the device (about 50% of the level achieved without the device), however if higher concentrations of plasma THC are needed, a higher potency marijuana cigarette can be used.
Figure 5.
Comparison of marijuana smoking (3.51% THC marijuana cigarettes) using in three conditions (N=9); 1) smoking the cigarette directly from a holder in a naturalistic setting, 2) smoking the cigarette through the smoking device in the naturalistic setting, and 3) smoking the cigarette through the device while in the MR scanner. The graphs show the following data; A) Average plasma THC timecourses in conditions 1 and 2; B) Heart rate measured with a pulse oximeter (and standard deviation bounds) in all three conditions, and Subjective rating of high (C) and craving (D) over the same time period for all three conditions.
Figure 5 also shows the averaged physiological (Figure 5B), and behavioral (Figures 5C and 5D) responses from the same 9 subjects who smoked marijuana under these two conditions, and in the third condition of smoking marijuana through the device in the scanner. As expected, marijuana caused a pronounced increase in heart rate that had remained stable throughout the sham smoking period. Increases of about 50% from baseline were observed, and this lasted for about 10 minutes after the onset of marijuana smoking. There was no observable difference in heartrate change between using and not using the device outside the scanner. The heartrate increase was lower in the scanner, but this is likely due to the difference in the subject position (prone in the scanner versus sitting in a chair outside). These findings are also consistent with our previous studies.
We should note that it is probably unavoidable that smoking a marijuana cigarette through a length of tubing will decrease the available THC. However, it is important to note that: 1) The subjects show no significant differences in the physiological and behavioral responses with or without the device 2) The decrease in plasma THC is small (less than 50%) and can be compensated by using a cigarette with a higher THC concentration (7% THC cigarettes are available)(or by allowing subjects to smoke a greater quantity of marijuana). In any case, the plasma levels obtained are within laboratory norms for smoking this type of cigarette. 3) The THC delivery is consistent (the standard deviation in the THC levels delivered was not greater when the device was used.), so the effect can be accounted for in analysis 4) Since the subject is smoking and can control the depth of inhalation, they can titrate their dose to compensate.
In order to demonstrate that use of the device does not impair the collection of fMRI data, we examined the data from the visual stimulus delivered during the smoking run. A brief visual stimulus was interleaved with the behavioral data acquisition to control for global bloodflow effects. Figure 6A shows the overall GLM response to all periods of visual stimulus. As expected, there is a strong, focal activation cluster in the visual cortex. Figure 6B shows the timecourses from this cluster, separated by epoch (Baseline, Air puffing, Marijuana Puffing, and Post-smoking period). The timecourses do not show any significant degradation during the periods where the subject is actively smoking.
Figure 6.
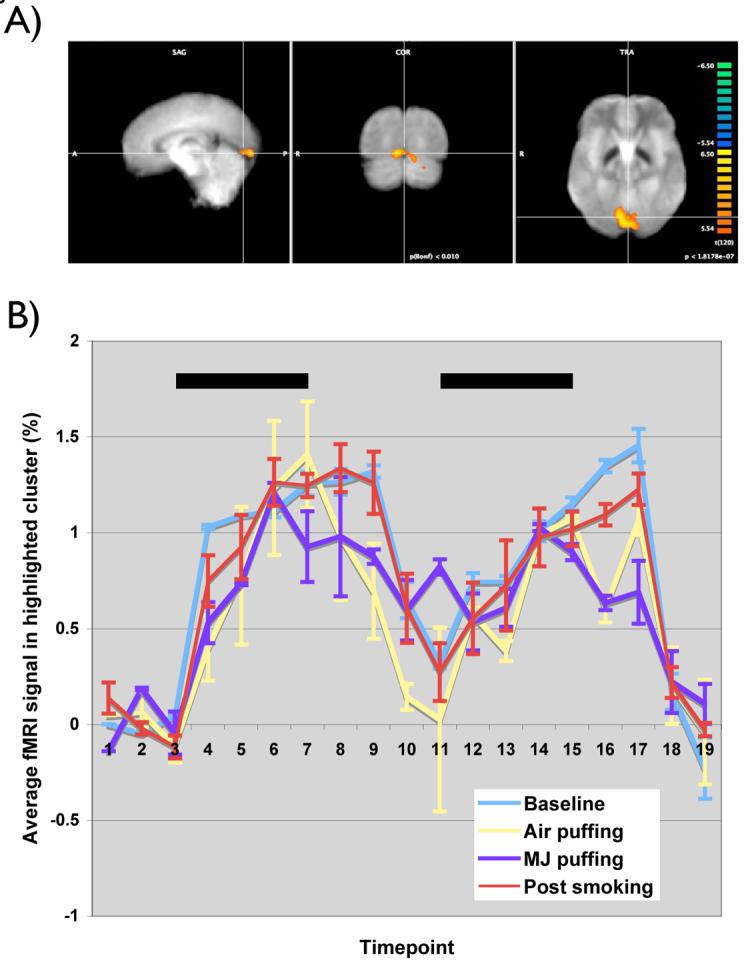
Visual activation in response to a reversing checkerboard stimulus. A) shows the visual cortical activation in a typical subject in response to the 10 visual stimulus presentations that occur during a smoking session (Bonferroni corrected p<0.01) B) shows the timecourses from the cluster indicated, separated by epoch (Baseline, Air puffing, Marijuana puffing, and Postsmoking period. There is no apparent difference between the responses in any of the epochs.
Discussion
We have developed a device that permits subjects to smoke either marijuana or tobacco cigarettes while in an MR scanner, and have verified that the device does not permit any smoke to contaminate the scanner area or components. We have demonstrated that the device delivers physiologically active levels of Δ9-THC to subjects in a manner that closely replicates the physiological and behavioral responses elicited by smoking outside the magnet in a natural setting.
One notable feature is that the heart rate increase for the subjects in the scanner was lower than for either of the smoking conditions outside of the scanner. This is most likely an effect of the subject’s supine position during the scanning sessions (the subject is reclining) in the natural setting experiment). Nevertheless, the heart rate increases resulting from marijuana smoking, coupled with the time courses of both the positive and negative behavioral ratings provides a good deal of confidence that pharmacologically active amounts of Δ9-THC are being delivered to the subject and they are responding in a manner similar to how they react outside of the scanner.
Finally, we have demonstrated that neither the use of the device nor the act of smoking in the magnet impair our ability to record high quality fMRI data. This device will allow us to perform measurements of brain response to marijuana or tobacco in a range of subject populations using the delivery method of choice for these drugs. This device will permit the design of experiments that can explore how the brain reacts to smoked drugs of abuse. Additional experiments can now be designed to explore the role that other non-drug factors (e.g., carbon monoxide) play in the response to smoked drug. We expect that the use of this device will greatly enhance our understanding of how the brain responds acutely to smoked drugs.
Acknowledgements
This project was supported by the following NIDA Grants: DA14013 (BBF), DA019238 (KPL), DA017712 (LDN), DA03994, DA019238 (SEL), and DA00343 (SEL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Breiter H, Gollub R, Weisskoff R, Kennedy D, Makris N, Berke J, et al. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Cowan R, Frederick B, Rainey M, Levin J, Maas L, Bang J, et al. Photic Activation in Cocaine Withdrawn Subjects: An fMRI BOLD Study; 62nd annual meeting of the College of Problems in Drug Dependance; San Juan, Puerto Rico. 2000a.p. 297. [Google Scholar]
- Cowan R, Frederick B, Rohan M, Bang J, Levin J, Lukas S, Renshaw P. Effect of d-amphetamine on response to red and blue light primary visual cortex of humans: a BOLD fMRI study; Society for Neuroscience, 2000 annual meeting; New Orleans. 2000b. [Google Scholar]
- Frederick B, Rohan M, Elman I, Lukas S, Renshaw P. Improved localization of fMRI activation in the basal forebrain at high field using match warped anatomic images; 66th Annual meeting of the College on Problems of Drug Dependance; San Juan, PR. 2004. [Google Scholar]
- Gaillard WD. Functional MR imaging of language, memory, and sensorimotor cortex. Neuroimaging Clin N Am. 2004;14:471–485. doi: 10.1016/j.nic.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Yurgelun-Todd DA. Neuroimaging of marijuana smokers during inhibitory processing: a pilot investigation. Cognitive Brain Research. 2005;23:107–118. doi: 10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Gore JC, Skudlarski P, Lacadie CM, Jatlow P, Krystal JH. Impact of intravenous nicotine on BOLD signal response to photic stimulation. Magn Reson Imaging. 2002;20:141–145. doi: 10.1016/s0730-725x(02)00494-0. [DOI] [PubMed] [Google Scholar]
- Kaufman MJ, Levin JM, Maas LC, Rose SL, Lukas SE, Mendelson JH, et al. Cocaine decreases relative cerebral blood volume in humans: a dynamic susceptibility contrast magnetic resonance imaging study. Psychopharmacology. 1998;138:76–81. doi: 10.1007/s002130050647. [DOI] [PubMed] [Google Scholar]
- Kumari V, Gray JA, ffytche DH, Mitterschiffthaler MT, Das M, Zachariah E, et al. Cognitive effects of nicotine in humans: an fMRI study. Neuroimage. 2003;19:1002–1013. doi: 10.1016/s1053-8119(03)00110-1. [DOI] [PubMed] [Google Scholar]
- Latchaw RE, Ugurbil K, Hu X. Functional MR imaging of perceptual and cognitive functions. Neuroimaging Clin N Am. 1995;5:193–205. [PubMed] [Google Scholar]
- Lukas S, Dobrosielski M, Chiu T, Woods B, Teoh S, Mendelson J. A non-ferrous instrumental joystick device for recording behavioral responses during magnetic resonance imaging and spectroscopy. Pharmacol Biochem Behav. 1993;46:781–785. doi: 10.1016/0091-3057(93)90201-4. [DOI] [PubMed] [Google Scholar]
- Lukas S, Sholar M, Kouri E, Fukozako H, Mendelson J. Marihuana Smoking Increases Plasma Cocaine Levels and Subjective Reports of Euphoria in Male Volunteers. Pharmacology Biochemistry and Behavior. 1994;48:715–721. doi: 10.1016/0091-3057(94)90338-7. [DOI] [PubMed] [Google Scholar]
- Lukas SE, Benedikt R, Mendelson JH, Kouri E, Sholar M, Amass L. Marihuana attenuates the rise in plasma ethanol levels in human subjects. Neuropsychopharmacology. 1992;7:77–81. [PubMed] [Google Scholar]
- Lukas SE, Orozco S. Ethanol increases plasma Δ9-tetrahydrocannabinol (THC) levels and subjective effects after marihuana smoking in human volunteers. Drug Alc Depend. 2001;64:143–149. doi: 10.1016/s0376-8716(01)00118-1. [DOI] [PubMed] [Google Scholar]
- Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, et al. Functional magnetic resonance imaging of cue-induced cocaine craving in the human brain. American Journal of Psychiatry. 1998;155:124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- National Medical Services Inc Delta-9 THC Laboratory Analysis Report. 2005.
- Pillay SS, Rogowska J, Kanayama G, Jon DI, Gruber S, Simpson N, et al. Neurophysiology of motor function following cannabis discontinuation in chronic cannabis smokers: an fMRI study. Drug & Alcohol Dependence. 2004;76:261–271. doi: 10.1016/j.drugalcdep.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Rohan M, Killgore W, Eskesen J, Renshaw P, Yurgelun-Todd D. Match-Warped EPI Anatomic Images and the Amygdala: Imaging in Hard Places; International Society for Magnetic Resonance and Medicine 9th Scientific Meeting and Exhibition and the European Society for Magnetic Resonance in Medicine and Biology 18th Annual Meeting and Exhibition; Glasgow, Scotland. 2001. p. 1237. [Google Scholar]
- Sell LA, Simmons A, Lemmens GM, Williams SC, Brammer M, Strang J. Functional magnetic resonance imaging of the acute effect of intravenous heroin administration on visual activation in long-term heroin addicts: results from a feasibility study. Drug Alcohol Depend. 1997;49:55–60. doi: 10.1016/s0376-8716(97)00140-3. [DOI] [PubMed] [Google Scholar]
- Stein EA, Pankiewicz J, Harsch HH, Cho JK, Fuller SA, Hoffmann RG, et al. Nicotine-induced limbic cortical activation in the human brain: a functional MRI study. Am J Psychiatry. 1998;155:1009–1015. doi: 10.1176/ajp.155.8.1009. [DOI] [PubMed] [Google Scholar]
- Streeter CC, Ciraulo DA, Harris GJ, Kaufman MJ, Lewis RF, Knapp CM, et al. Functional magnetic resonance imaging of alprazolam-induced changes in humans with familial alcoholism. Psychiatry Research. 1998;82:69–82. doi: 10.1016/s0925-4927(98)00009-2. [DOI] [PubMed] [Google Scholar]
- Tregellas JR, Tanabe JL, Martin LF, Freedman R. FMRI of response to nicotine during a smooth pursuit eye movement task in schizophrenia. Am J Psychiatry. 2005;162:391–393. doi: 10.1176/appi.ajp.162.2.391. [DOI] [PubMed] [Google Scholar]