Abstract
Spinal cord injury (SCI) causes an increase of inhibitory factors that may restrict axonal outgrowth after trauma. During the past decade, the Eph receptors and ephrin ligands have emerged as key repulsive cues known to be involved in neurite outgrowth, synapse formation, and axonal pathfinding during development. Given the non-permissive environment for axonal regeneration after SCI, we questioned whether enhanced-expression of the EphA4 receptor with repulsive activity for axonal outgrowth is potentially responsible for the regenerative failure. To address this possibility we have examined the expression of EphA4 after SCI in adult rats following a contusion SCI. EphA4 expression studies demonstrated a time-dependent change for EphA4 protein without alterations in β-actin. EphA4 was downregulated initially and upregulated 7 days after injury. Blockade of EphA4 upregulation with antisense oligonucleotides did not produced an anatomical or physiological response monitored with anterograde tracing studies or transcranial magnetic motor evoked potentials (tcMMEP), respectively. These results demonstrated that upregulation of EphA4 receptors after trauma is not related to axonal regeneration or return of nerve conduction across the injury site.
Keywords: EphA, trauma, regeneration, ephrin, sprouting, tracing
The adult central nervous system (CNS) has the plasticity to promote axonal elongation after trauma, if a supportive substrate and a permissive environment are provided. However, many inhibitory factors within the CNS restrict axonal regeneration. Molecular cues of myelin origin, as NOGO, MAG, and OMgp, are the best-known and intensively studied neurite outgrowth inhibitors [12]. Neutralization of NOGO-A with antibodies against this repulsive molecule enhanced nerve regeneration over long distances [1], but not complete suggesting the presence of additional blockers to neurite outgrowth.
Other molecular signals with repellent properties that may influence axonal regeneration after CNS injury include semaphorins/collapsins, tenascin, Slit proteins, sulfated proteoglycans [24], and the Eph receptor protein tyrosine kinase (RPTK) [3,11,22,23] and its ligands, the ephrins [5]. Although these molecules are predominantly recognized as developmental signals, many are constitutively expressed in the adult CNS and show altered expression after trauma [8]. Recent publications [5,6,10,13,30,31] showed that Eph and ephrin expression are markedly altered after SCI.
The Eph receptor tyrosine kinase family is the largest RPTK family known, and is divided in two groups: EphA and EphB receptors [9]. They are classified according to the type of ligands that activates them. The ephrin-A ligands are anchored to the plasma membrane by a glycosylphosphatidylinositol group and the ephrin-B ligands are anchored by a transmembrane domain. Therefore, those receptors activated by ephrin-A ligands are called EphA receptors and those receptors activated by ephrin-B ligands are called EphB receptors [9]. An interesting exception to this rule is EphA4 RTK, which can be activated by ephrin-B2 and ephrin-B3 [16].
The main characteristic of the Eph receptors is their ability to mediate cell-cell repulsion through the binding of their ligand on an adjacent cell surface [16]. Many members of the Eph RPTK family are expressed exclusively in the CNS, and their temporal expression patterns as well as functional activity suggest that they are involved in development, maturation, and maintenance of the CNS [16]. While the expression of Eph RPTK has been shown to be critical during early stages of neural development, it is unknown if re-expression of these molecules after SCI affect axonal regeneration.
Recently, Cruz-Orengo and colleagues (2006) reported changes in EphA4 mRNA expression after SCI. Moreover, they observed that blockade of EphA4 expression with antisense oligonucleotides after injury did not improved locomotor behavior in treated rats. However, more sensitive assays like anterograde tracing or transcranial magnetic motor evoke potentials (tcMMEPs) may uncover whether EphA4 plays a subtle but relevant role in axonal outgrowth or the return of nerve conduction after injury. We hypothesize that EphA4 plays a role in the injury response of the adult CNS generating a repulsive environment and blockade of its expression could promote axonal outgrowth and tcMMEP responses.
Adult female Sprague Dawley rats (225 g) were anesthetized with a cocktail of Ketamine/Xylazine/Acepromazine, 40/4/0.9 mg/kg, respectively (Fort Dodge Animal Health, Fort Dodge, IO) and the T10 spinal cord segment exposed, as described previously [6,10,22]. Briefly, the animals received a moderate contusion (10 g, 12.5 mm) SCI, using the NYU impactor device and sham control rats had only a laminectomy. Rats (n=5) were allowed to recover for 2, 4, 7, 14 and 28 days post-injury (DPI) to analyze the protein expression profile of EphA4.
EphA4 protein levels after SCI was determined using standardized Western blot analysis. Tissue from the lesion epicenter (5 mm) was dissected out and homogenized in ice-cold lysis buffer consisting of 20 mM Tris (pH 7.5), 150 mM NaCl, 5 mM NaF, 1 mM EDTA and 1 mM EGTA, and a cocktail of proteinase inhibitors: 2 μg/ml antipain, 10 μg/ml aprotinin, 5 mM benzamidine, 1 mM DTT, 10 μg/ml leupeptin, 1mM Na3VO4, 1 mM PMSF, and 10 μg/ml trypsin inhibitors [18]. Lysates were centrifuged at 20,000 × g for 90 minutes at 4°C. Pellets were resuspended in the same lysis buffer with 1% NP-40 and incubated at 4°C for 45 minutes while shaking. Following centrifugation at 20,000 × g for 10 minutes at 4°C, the protein concentration of the resulting supernatant was determined using DCprot with bovine serum albumin as a standard following the manufacturer's instructions (Bio-Rad Laboratories, Hercules, CA).
Protein lysates (25 μg) were analyzed on 6% and 10% SDS-PAGE gels, for EphA4 and actin immunoblots, respectively. Proteins were electroblotted to a nitrocellulose membrane and the membranes were stained with 0.1% Ponceus S to confirm equal loading and appropriate transfer. The membranes were blocked for 3 hours in Blotto and incubated overnight with the primary antibody (1:4000) sc-921 rabbit anti-EphA4 (Santa Cruz Biotechnologies, CA), or 1:1000 A4700 monoclonal anti-actin (Sigma-Aldrich, MO). Blots were incubated one hour with HRP-conjugated secondary goat anti-rabbit IgG (Sigma-Aldrich, MO) or rabbit anti-mouse IgG. Chemiluminescence was activated using SuperSignal West Dura (Pierce Chemical Company, IL) following the manufacturer's directions. Membranes were exposed and developed using Kodak (Rochester, NY) films.
Antibody specificity against EphA4 receptor was determined by preabsorbing the primary antibody with 0.5 μg sc-921P blocking peptide, which was used to generate the polyclonal antibody. A single band of 120 kDa was detected but no immunoreactivity was obtained after preabsorption with the blocking peptide. Western Blot autoradiograms were quantified by densitometry using the Bio-Rad Gel Doc 2000 (Bio-Rad Laboratories, CA) and QuantiScan software (Biosoft, Ferguson, MO). Immunoblots with a series of membrane protein concentrations were used to determine the linear range for analysis. Statistical analysis to compare sham versus injured groups were performed with ANOVA, followed by Bonferroni post-hoc test using InStat version 3.0 software (GraphPad Software Inc., San Diego, CA). Statistical significance was set at p < 0.05.
Intrathecal infusion of EphA4 antisense oligonucleotide (AS) to contused spinal cord with a miniosmotic-pump blocked the expression of EphA4 by approximately 50%, but did not produce any locomotor recovery [6]. However, studies to analyze the effect of EphA4 gene expression blockade at more sensitive anatomical and physiological level were not previously investigated. Therefore, rats were treated with AS oligonucleotides immediately after contusion for a period of 28 days to block EphA4 expression after SCI as described by Cruz-Orengo and colleagues (2006) and axonal regeneration monitored with anterograde tracing studies or tcMMEP responses to determine any return of nerve conduction.
After 28 DPI, antisense and control treated rats were assessed by tcMMEP as previously described [10]. Briefly, two sterile platinum subdermal needle electrodes were introduced in both gastrocnemius muscles and the reference electrode in the distal tendon of the gastrocnemius. Once the magnetic coil was placed over the skull, magnetic pulses were generated and electromyograms recorded from the gastrocnemius muscles. tcMMEPs were recorded at 70%, 85% and 100% intensity of the magnetic field to validate the obtained responses. Latency onset, defined as the time between stimulation and muscle evoked potential EMG, was monitored and analyzed for both hindlimbs. Analyses of the converted recordings were done using Axoscope 8.2 software (Molecular Devices Corporation).
The extent of axonal outgrowth across the lesion epicenter was monitored with anterograde tracing studies after 42 DPI, giving enough time to observe some neuronal regrowth in a permissive environment. Antisense- and control-treated rats were anesthetized as described above, for sterotaxic surgery using the Stoelting Stereotaxic Apparatus model 51650 (Stoelting Co., IL). The skull was exposed through a linear skin incision allowing visualizing of both bregma and lambda sutures. The anteroposterior (AP), mediolateral (ML), and dorsoventral (DV) stereotaxic coordinates for bregma were calculated. For tracing the right motor cortex, layer V (MCV) at three points, the coordinates selected were: (1) AP, −0.8; ML, −1.0; DV, −2.0 (2) AP, −1.8; ML, −1.2; DV, −2.0 and (3) AP, −2.8; ML, −1.7; DV, −2.0. The bilateral red nuclei (RN) tracing coordinates selected were AP, −5.8; ML, ±0.7; DV, −6.2. Both targets were selected according to the landmarks described in Paxinos and Watson (1998).
Once the coordinates were marked with a pencil on the skull, small holes were done with a Dremel 750-02 Mini-drill (Robert Bosch Tool Corp, Racine, WI). Five microliters of 10% biotinylated dextran amine (BDA, Molecular Probes Invitrogen Detection Technologies, OR) were injected at each MCV and RN point at a 0.5 μl/min flow rate, using a 5 μl, 24-g Hamilton syringe model 7105 (Hamilton Company, NV) set on a Stoelting Motorized Nanopump model 53310 (Stoelting Co., IL). To avoid reflux of the injected BDA, the syringe remained in place for 5 additional minutes before retraction of the needle for each particular injection site. A thin layer of dental cement (Shofu Dental Corp., San Marcos, CA) was used to cover the bone openings prior to a subdermal suture closure of the skin. Two weeks after injection, the animals were sacrificed and spinal cords containing regions rostral and caudal (1 cm/each) to the lesion epicenter were sectioned as described previously [6].
Sections were washed twice for 5 minutes with Tris-PBS and then incubated for 15 minutes in 0.3% H2O2/MEOH. After rinsing 3 times in 0.3% Triton X-100/Tris-PBS for 10 minutes, the sections were incubated in the ABC reagent, according to Vector PK-6101 kit directions (Vector Laboratories, CA). Following that incubation, the sections were washes 3 times in TBS for 10 minutes and incubated in Vector SK-4100 DAB reagent. After clearing with water, the slides were dehydrated sequentially with graded ethanol and xylene. Finally, the sections were coverslipped in VectaMount (Vector Laboratories) and dried at 60°C for 30 minutes. The sections were visualized with a bright field microscope and the pictures analyzed with Motic® software (Motic Images 2000, version 1.2; Micro-Optic Industrial Group, CO, LTD). Finally, the pictures were assembled with Adobe Photoshop® 5 and Adobe Illustrator® 7 software.
Western Blots detected a single 120 kDa polypeptide in adult spinal cords extracts (Fig. 1), as shown previously for other tissues [18]. Preabsorbing the antibody with the sc-921P peptide used to generate it abolished the signal, demonstrating antibody specificity. Since other EphA receptors had been shown to be upregulated after CNS trauma and other neuropathologies [4,14,23,28], the specificity of the EphA4 antibody was previously demonstrated [6]. Determination of the linear phase for Western blot detection was performed with several amounts of protein extracts and various exposure times to avoid film saturation (data not shown).
Fig. 1.

Expression profile of EphA4 receptor after spinal cord injury. A single band of 120 kDa was detected (top panel) from spinal cord of naïve animals, and the specificity of the antibody determined by preabsorbing it with sc-921P blocking peptide. Representative immunoblots showed EphA4 expression at the lesion epicenter (T10 segment) at 2, 4, 7, 14 or 28 days post-injury from sham and injured animals. β-actin levels was used as a housekeeping protein that remained constant. Densitometric analysis of EphA4 immunoreactivity from sham and injured rats, expressed as percentage values relative to control levels.
Following SCI, the levels of EphA4 at the lesion epicenter initially decreased to 75% (p<0.0011) and 50% (p<0.007) of baseline levels at 2 and 4 DPI, respectively (Figure 1B). At 7 DPI, EphA4 protein levels were close to baseline and there was a tendency to increase at 14 (130%) and 28 (150%) days (F = 2.5844; df = 5, 24; p < 0.0525). The levels of β-actin, were not altered in the injured rats confirming the specificity of the effects generated by the trauma to the spinal cord.
As demonstrated before by Cruz-Orengo, et al. (2006), EphA4-antisense ODN treatment blocked EphA4 expression and affected spontaneous exploratory behavior, but did not exerted any direct effect on locomotion. In addition, suppression of EphA4 expression after SCI increased pain evoked behavior when direct measurements of somatic allodynia responses to non-noxious stimuli were performed with the Von Frey filaments [6]. Therefore, to determine if the absence of locomotor behavior recovery observed with the BBB, grid walking and beam crossing assays after EphA4 AS treatment [6] was also related to the absence of axonal elongation across the lesion site we employed tcMMEPs recordings. This technique allowed us to monitor whether some axonal regeneration or sprouting occurred, that could have been missed by the less sensitive behavioral experiments.
TcMMEP potentials are carried in the ventrolateral funicullus (VLF), a major pathway in the control of hindlimb locomotion [20,26]. TcMMEP responses provide an unbiased and quantitative electrophysiological assessment of return of spinal cord function: descending monosynaptic conduction or reorganized polysynaptic connections in the VLF. Blockade of EphA4 with AS oligonucleotides did not restore tcMMEP responses from either the right or left limbs (Fig. 2). Responses were seen only from sham animals within the first 4-7 msec. These data eliminate the possibility of axonal outgrowth in tracts traveling through the VLF or re-organization of propiospinal connections with descending information controlling motor function. However, some outgrowth may take place across the injury site without the formation of a synapse with its appropriate target and no tcMMEP responses would be observed. Therefore, anterograde tracing was used as another sensitive assay to monitor fiber outgrowth after blockade of EphA4 repulsive protein with AS oligonucleotides in lesioned rats.
Fig. 2.
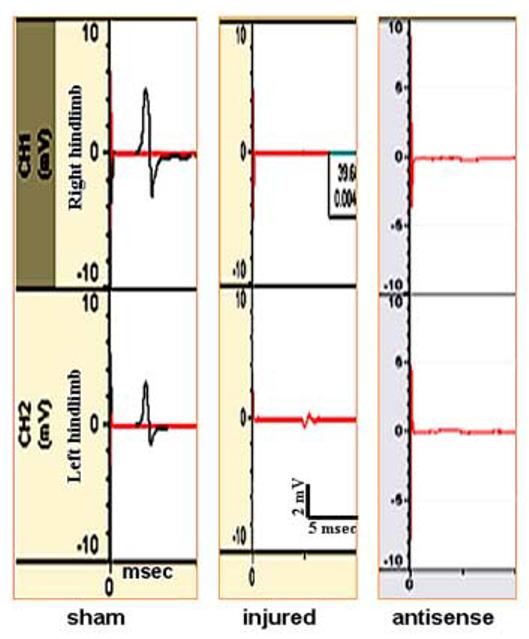
Effect of EphA4 antisense oligonucleotide treatment on the return of nerve conduction. TcMMEPs were recorded from the gastrocnemius muscles (right and left hindlimbs) and these are representative traces from a sham, injured and injured rats treated with antisense oligonucleotides. No responses were observed in the tcMMEPs recorded from antisense and control (data not shown) treated rats.
BDA injections in layer V of the motor cortex (right side) and both red nuclei were performed one week after the tcMMEP recording and animals were sacrificed 14 days after stereotaxic surgery. No changes in DAB staining were observed in antisense-versus control-treated rats in corticospinal- or rubrospinal-tracts anterograde tracing (Fig. 3). This sensitive assay did not show any staining caudal to the lesion epicenter, confirming that EphA4 receptors are not involved in axonal regeneration after SCI. These descending spinal tracts are known to be involved in fine movement and locomotor activity, respectively [19,26,29]. Therefore, anterograde tracing studies supported the idea that supraspinal circuitry was not involved in the observed pain related behavioral changes [6] as expected by the lack of locomotive recovery.
Fig. 3.
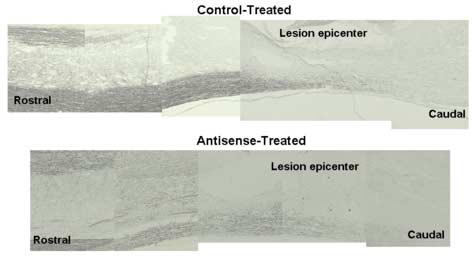
Anterograde tracing studies after EphA4 antisense treatment in contused rats. BDA was injected at the Motor Cortex (layer V), and Red Nuclei of injured rats, that were infused intrathecally with oligonucleotides. Anatomical tracing was assessed by DAB/HRP and no staining was observed in antisense- or control-treated rats in either the corticospinal or rubrospinal tracts caudal to the lesion epicenter.
Upregulation of Eph receptors has been reported in some models of CNS injury, suggesting a possible role in the non-permissive environment generated after trauma. Moreno-Flores and Wandosell (1999) reported that kainate neurotoxicity elicited a widespread hippocampal expression of EphA4, EphA5, and EphB2. Also, Biervert et al. (2001) reported changes in EphRTK after traumatic brain injury. Similar results were demonstrated by Knöll et al. (2001) with the A-subfamily of Eph receptors following optic nerve transection. Additional data [5,11,22,30,31] show an increase in the expression of some members of the Eph RTK family after SCI. During development, their roles are predominantly to mediate axonal retraction, target recognition, and axonal fasciculation mediated by repulsive mechanisms after ligand binding. However, after trauma, their overexpression could retard axonal outgrowth, contributing to the CNS non-permissive environment for regeneration.
The expression of the EphA4 receptor is important for the development of the corticospinal tract [17], the interconnections of locomotor pacemaker cells [15], the formation of the neuromuscular junction [18], and motorneuron pathfinding [7]. All these studies support a role for this protein in the organization of tracts related to locomotor activity and in the maintenance of the circuitry already formed in the CNS. Consistent with previous reports [21,30], the present data confirm that the adult spinal cord has a basal expression of EphA4. The basal or induced expression of the EphA4 receptor, as a repellent-mediated protein, may be an important factor to block sprouting or the formation of incorrect synapses that may generate pain as reported by Cruz-Orengo et al., 2006.
The initial period of low EphA4 protein expression after SCI could be associated to cell death in the gray matter [2]. The tendency for increased EphA4 protein expression 14 DPI may result from upregulated expression in reactive astrocytes and/or increased migration of reactive astrocytes to glia scar. Typically, white matter has been associated with neurite growth inhibition during development and after trauma [12], due mainly to increased production of proteins, which restrict the regeneration of adult CNS axons by forming a chemical and physical barrier [24,27]. The observed expression of EphA4 [6] after SCI in reactive astrocytes suggests it may function as one of the inhibitory cues that play a part in the pathophysiology mediated by gliosis at the lesion site.
The delay in protein levels relative to the EphA4 mRNA upregulation at later time points [6] likely reflects differences in their synthesis and/or turnover. It is noteworthy that the protein lysates for Western Blot analysis were performed with tissue from whole epicenter. Therefore, changes in EphA4 protein levels could be missed due to dilution of the protein content in our samples. To address this issue, densitometric analysis of EphA4 immunoreactivity in the ventrolateral funiculus and ventral columns was performed [30]. These results showed an increased protein expression similar to the changes observed with the mRNA studies which implies that molecular changes were restricted to a specific region of the white matter, and with less contribution by other structures of the cord.
Spinal cord injury generates a complex set of events. Among them is the increase of inhibitory factors that may restrict or block neurite outgrowth after trauma, although the adult CNS has the plasticity for axonal regeneration. Several key reports from our group and of others, show that some Eph receptors are upregulated after injury to the CNS [5,6,10,11,13,22,30,31], and many revert to similar levels when compared with early stages of development. The unexpected failure of axonal outgrowth across the lesion epicenter or the return of nerve conduction after antisense oligonucleotide treatment, support the idea that EphA4 after SCI is involved in other events, not related to locomotion [6]. Unlike its putative role in the formation of locomotor pathways during development, EphA4 may contribute to synapse stabilization during the adulthood or after traumatic spinal cord lesions.
Acknowledgement
Supported by NS39405, S06-GM008224, GM-68138, EPS-9874782, and G12RR03051.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bareyre FM, Haudenschild B, Schwab ME. Long-lasting sprouting and gene expression changes induced by the monoclonal antibody IN-1 in the adult spinal cord. J. Neurosci. 2002;22:7097–110. doi: 10.1523/JNEUROSCI.22-16-07097.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beattie MS, Hermann GE, Rogers RC, Bresnahan JC. Cell death in models of spinal cord injuryc. Prog Brain Res. 2002;137:37–47. doi: 10.1016/s0079-6123(02)37006-7. [DOI] [PubMed] [Google Scholar]
- 3.Benson MD, Romero MI, Lush ME, Lu QR, Henkemeyer M, Parada LF. Ephrin-B3 is a myelin-based inhibitor of neurite outgrowth. Proc Natl Acad Sci U S A. 2005;102:10694–10699. doi: 10.1073/pnas.0504021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biervert C, Horvath E, Fahrig T. Semiquantitative expression analysis of ephrine-receptor tyrosine kinase mRNA's in a rat model of traumatic brain injury. Neurosci Let. 2001;315:25–28. doi: 10.1016/s0304-3940(01)02312-6. [DOI] [PubMed] [Google Scholar]
- 5.Bundesen LQ, Scheel TA, Bregman BS, Kromer LF. Ephrin-B2 and EphB2 regulation of astrocyte-meningeal fibroblast interactions in response to spinal cord lesions in adult rats. J Neurosci. 2003;23:7789–7800. doi: 10.1523/JNEUROSCI.23-21-07789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruz-Orengo L, Figueroa JD, Velazquez I, Torrado A, Ortiz C, Hernandez C, Puig A, Segarra AC, Whittemore SR, Miranda JD. Blocking EphA4 upregulation after spinal cord injury results in enhanced chronic pain. Exp. Neurol. 2006;202:421–433. doi: 10.1016/j.expneurol.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Eberhart J, Swartz M, Koblar SA, Pasquale EB, Tanaka H. Expression of EphA4, ephrin-A2 and ephrin-A5 during axon outgrowth to the hindlimb indicates potential roles in the pathfinding. Dev Neurosci. 2000;22:237–250. doi: 10.1159/000017446. [DOI] [PubMed] [Google Scholar]
- 8.Emery DL, Royo NC, Fischer I, Saatman KE, McIntosh TK. Plasticity following injury to the adult central nervous system: is recapitulation of a developmental state worth promoting? J Neurotrauma. 2003;12:1271–1292. doi: 10.1089/089771503322686085. [DOI] [PubMed] [Google Scholar]
- 9.Eph Nomenclature Committee Unified nomenclature for Eph family receptors and their ligands, the ephrins. Cell. 1997;90:403–404. doi: 10.1016/s0092-8674(00)80500-0. [DOI] [PubMed] [Google Scholar]
- 10.Figueroa JD, Benton RL, Velazquez I, Torrado AI, Ortiz C, Hernandez C, Diaz JJ, Magnuson DS, Whittemore SR, Miranda JD. Inhibition of EphA7 upregulation after spinal cord injury reduces apoptosis and promotes locomotor recovery. J.Neurosci.Res. 2006;84:1438–51. doi: 10.1002/jnr.21048. [DOI] [PubMed] [Google Scholar]
- 11.Goldshmit Y, Galea MP, Graham W, Barlett PF, Turnley AM. Axonal regeneration and lack of astrocytic gliosis in EphA4-deficient mice. J Neurosci. 2004;24:10064–10075. doi: 10.1523/JNEUROSCI.2981-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grados-Munro EM, Fournier AE. Myelin-associated inhibitors of axon regeneration. J Neurosci Res. 2003;74:479–85. doi: 10.1002/jnr.10803. [DOI] [PubMed] [Google Scholar]
- 13.Irizarry-Ramírez M, Willson CA, Cruz-Orengo L, Figueroa JD, Velázquez I, Jones H, Foster RD, Whittemore SR, Miranda JD. Upregulation of EphA3 receptor after spinal cord injury. J Neurotrauma. 2005;22:929–935. doi: 10.1089/neu.2005.22.929. [DOI] [PubMed] [Google Scholar]
- 14.Knöll B, Isenmann S, Kilic E, Walkenhorst J, Engel S, Wehinger J, Bähr M, Drescher U. Graded expression patterns of ephrin-As in the superior colliculus after lesion of the adult mouse optic nerve. Mech. Dev. 2001;106:119–127. doi: 10.1016/s0925-4773(01)00431-2. [DOI] [PubMed] [Google Scholar]
- 15.Kullander K, Butt SJ, Lebret JM, Lunfald L, Restrepo CE, Rydström A, Klein R, Kiehn O. Role of EphA4 and ephrinB3 in local neuronal circuits that control walking. Science. 2003;299:1889–1892. doi: 10.1126/science.1079641. [DOI] [PubMed] [Google Scholar]
- 16.Kullander K, Klein R. Mechanisms and functions of Eph and ephrins signaling. Nature Rev. Mol. Cell Biol. 2002;3:475–486. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- 17.Kullander K, Mather NK, Diella F, Dottori M, Boyd AW, Klein R. Kinase-dependent and kinase-independent functions of EphA4 receptors in major axon tract formation in vivo. Neuron. 2001;29:73–84. doi: 10.1016/s0896-6273(01)00181-7. [DOI] [PubMed] [Google Scholar]
- 18.Lai KO, Ip FC, Cheung J, Fu AK, Ip NY. Expression of Eph receptors in the skeletal muscle and their localization at the neuromuscular junction. Mol. Cell Neurosci. 2001;17:1034–1047. doi: 10.1006/mcne.2001.0997. [DOI] [PubMed] [Google Scholar]
- 19.Lavoie S, Drew T. Discharge characteristics of neurons in the red nucleus during voluntary gait modifications: a comparison with the motor cortex. J Neurophysiol. 2002;88:1791–1814. doi: 10.1152/jn.2002.88.4.1791. [DOI] [PubMed] [Google Scholar]
- 20.Loy DN, Magnuson DS, Zhang YP, Onifer SM, Mills MD, Cao QL, Darnall JB, Fajardo LC, Burke DA, Whittemore SR. Functional redundancy of ventral spinal locomotor pathways. J. Neurosci. 2002;22:315–323. doi: 10.1523/JNEUROSCI.22-01-00315.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martone ME, Holash JA, Bayardo A, Pasquale EB, Ellisman MH. Immunolocalization of the receptor tyrosine kinase EphA4 in the adult rat central nervous system. Brain Research. 1997;771:238–250. doi: 10.1016/s0006-8993(97)00792-0. [DOI] [PubMed] [Google Scholar]
- 22.Miranda JD, White LA, Marcillo AE, Willson CA, Jagid J, Whittemore SR. Induction of Eph B3 after spinal cord injury. Exp. Neurol. 1999;156:218–222. doi: 10.1006/exnr.1998.7012. [DOI] [PubMed] [Google Scholar]
- 23.Moreno-Flores MT, Wandosell F. Upregulation of Eph receptors after excitotoxic injury in adult hippocampus. Neuroscience. 1999;91:193–201. doi: 10.1016/s0306-4522(98)00568-5. [DOI] [PubMed] [Google Scholar]
- 24.Niclou SP, Ehlert EM, Verhaagen J. Chemorepellent axon guidance molecules in spinal cord injury. J. Neurotrauma. 2006;23:409–421. doi: 10.1089/neu.2006.23.409. [DOI] [PubMed] [Google Scholar]
- 25.Paxinos G, Watson C. The Rat Brain in stereotaxic coordinates. 4th edition Academic Press Limited; San Diego: 1998. [Google Scholar]
- 26.Schucht P, Raineteau O, Schwab ME, Fouad K. Anatomical correlates of locomotor recovery following dorsal and ventral lesions of the rat spinal cord. Exp. Neurol. 2002;176:143–153. doi: 10.1006/exnr.2002.7909. [DOI] [PubMed] [Google Scholar]
- 27.Silver J, Miller JH. Regeneration beyond the glial scar. Nature Rev. Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 28.Sobel R. Ephrin A receptors and ligands in lesions and normal-appearing white matter in multiple sclerosis. Brain Pathol. 2005;15:35–45. doi: 10.1111/j.1750-3639.2005.tb00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Webb AA, Muir GD. Unilateral dorsal column and rubrospinal tract injuries affect overground locomotion in the unrestrained rat. Eur. J Neurosci. 2003;18:412–422. doi: 10.1046/j.1460-9568.2003.02768.x. [DOI] [PubMed] [Google Scholar]
- 30.Willson CA, Irizarry-Ramírez M, Gaskins HE, Cruz-Orengo L, Figueroa JD, Whittemore SR, Miranda JD. Up-regulation of EphA receptor expression in the injured adult spinal cord. Cell Transplantation. 2002;11:229–239. [PubMed] [Google Scholar]
- 31.Willson CA, Miranda JD, Foster RD, Onifer SM, Whittemore SR. Transection of the adult rat spinal cord upregulates EphB3 receptor and ligand expression. Cell Transplantation. 2003;12:279–290. doi: 10.3727/000000003108746830. [DOI] [PubMed] [Google Scholar]


