Abstract
When ciliogenesis first occurs in sea urchin embryos, the major building block proteins, tubulin and dynein, exist in substantial pools, but most 9+2 architectural proteins must be synthesized de novo. Pulse-chase labeling with [3H]leucine demonstrates that these proteins are coordinately up-regulated in response to deciliation so that regeneration ensues and the tubulin and dynein pools are replenished. Protein labeling and incorporation into already-assembled cilia is high, indicating constitutive ciliary gene expression and steady-state turnover. To determine whether either the synthesis of tubulin or the size of its available pool is coupled to the synthesis or turnover of the other 9+2 proteins in some feedback manner, fully-ciliated mid- or late-gastrula stage Strongylocentrotus droebachiensis embryos were pulse labeled in the presence of colchicine or taxol at concentrations that block ciliary growth. As a consequence of tubulin autoregulation mediated by increased free tubulin, no labeling of ciliary tubulin occurred in colchicine-treated embryos. However, most other proteins were labeled and incorporated into steady-state cilia at near-control levels in the presence of colchicine or taxol. With taxol, tubulin was labeled as well. An axoneme-associated 78 kDa cognate of the molecular chaperone HSP70 correlated with length during regeneration; neither colchicine nor taxol influenced the association of this protein in steady-state cilia. These data indicate that 1) ciliary protein synthesis and turnover is independent of tubulin synthesis or tubulin pool size; 2) steady-state incorporation of labeled proteins cannot be due to formation or elongation of cilia; 3) substantial tubulin exchange takes place in fully-motile cilia; and 4) chaperone presence and association in steady-state cilia is independent of background ciliogenesis, tubulin synthesis, and tubulin assembly state.
INTRODUCTION
The first permanent structure in sea urchin development is the single cilium assembled by each blastomere before hatching. During ciliogenesis, the synthesis of tubulin and dynein, already present in large pools, is up-regulated. Most 9+2 architectural proteins are made in discrete, lesser amounts. Some of these, such as the integral outer doublet microtubule component tektin-A, are synthesized in limited, length-proportionate amounts, a process referred to as quantal synthesis (Stephens, 1977, 1989). The synthesis of all of these building blocks is up-regulated in response to hypertonic deciliation: the larger pools of tubulin and dynein and the smaller pools of most architectural proteins are replenished while another quantal amount of the length-limiting proteins is made. Since the process of regeneration recapitulates both the morphologic and the synthetic events characteristic of ciliogenesis without altering the progress of development — regardless of how often embryos are induced to regenerate cilia —ciliogenesis has been referred to as a subroutine in the program of development by analogy to the computer programming counterpart (Stephens, 1995a). As such, it is a highly convenient system with which to study inducible gene expression and organelle assembly.
Complicating this simple reiterative theme is the fact that ciliary proteins, after assembly, can be labeled to a level approaching that of full regeneration (Stephens, 1991, 1994a). This background synthesis is reflected in steady-state, ciliary length-related levels of tektin mRNAs (Norrander et al., 1995). Little protein labeling can be attributed to ciliary growth (Stephens, 1994a). Furthermore, steady-state turnover is not metabolic since the ciliary protein pools do not lose significant label in the presence of unlabeled amino acid (Stephens, 1989).
Tubulin pool size has long been considered a potential controlling factor in ciliary growth. Since the synthesis of 9+2 proteins appears to be under coordinate control during regeneration and continues at a reduced rate after assembly, it would be useful to document these processes quantitatively and determine whether the pool of available tubulin or its synthesis influences either the stoichiometric synthesis of other ciliary proteins (e.g., feed-back) or the turnover/exchange process after growth (e.g., cotransport). It is well established that colchicine leads to the depolymerization of cytoplasmic microtubules while taxol favors their assembly. Colchicine quite effectively prevents the assembly of sea urchin embryonic cilia but, once formed, the cilia are not structurally responsive to the drug (Tilney and Gibbins, 1968). As a consequence of autoregulation mediated by an increased amount of free tubulin, the synthesis of tubulin can be inhibited selectively by treating sea urchin embryos with colchicine (Gong and Brandhorst, 1988). Furthermore, taxol, which does not inhibit tubulin synthesis, can be used to decrease the free tubulin concentration and stabilize existing microtubules. Thus it is feasible to inhibit specifically both tubulin synthesis and assembly or to vary the effective tubulin pool size while retaining fully-functional cilia.
This study investigates ciliary protein synthesis and turnover with respect to several related questions. What are the baseline quantitative synthetic relationships among the 9+2 proteins in multiple regenerations and during steady-state turnover? Does turnover occur when tubulin synthesis, assembly, or effective pool size is modulated by tubulin-specific inhibitors? If so, are the architectural proteins of the cilium still proportionately synthesized? This approach also provides a rigorous assessment of the degree of protein turnover when ciliary regeneration and elongation are blocked. The recent discovery of a molecular chaperone concentrated at the distal growth tip of Chlamydomonas flagella (Bloch and Johnson, 1995) prompted a search for its equivalent in embryonic cilia. Is a chaperone associated with the 9+2 axoneme at steady state or during regrowth and does blockage of tubulin assembly or disassembly influence the amount or distribution of this potential mediator of transport and assembly? Brief accounts of this work have been presented in meeting proceedings (Stephens, 1994b, 1995b).
MATERIALS AND METHODS
Sea Urchin Embryos and Protein Labeling
Eggs and sperm were obtained from the sea urchin Strongylocentrotus droebachiensis, collected off the coast of Maine (Ocean Resources, Isle au Haut, ME), by intracoelomic injection of 0.5 M KCl. The eggs were washed by decanting and then fertilized, washed again with 0.45-μm Millipore-filtered sea water buffered at pH 8.0 with 10 mM Tris-HCl, and grown as a 1–2% suspension at 7.5–8°C in magnetically stirred 500-ml tissue culture flasks (Corning 26503–500 Slow Speed Stirring Vessel System, Corning, NY) at 30 rpm. The developing embryos were washed thoroughly with Millipore-filtered sea water immediately after hatching to rid the cultures of debris. The embryos were returned to the culture flasks at a concentration of 1–2% and grown to the mid- or late gastrula stage.
The culture was generally split in two: one was incubated with 0.5–1 mM colchicine (or 10 μm taxol) for 8 h, the length of one full ciliary regeneration, while the other served as a control. In some experiments, a single large culture was split into four: one subculture was deciliated before labeling to assess the incorporation rate during full regeneration while the others consisted of colchicine- and taxol-treated experimental conditions and a steady-state turnover control. Parallel cultures, consisting of 3 ml of embryos in 90 ml of sea water contained in stirred 125-ml tissue culture flasks (Corning 26501–125), were pulse labeled for 8 h with 225 μCi (2.5 μCi/ml) of [3H]leucine (ICN 2003205, ICN Radiochemicals, Irvine, CA, 110 Ci/mmol). In pulse-chase experiments, embryos were labeled during the first 4 h of regeneration (linear growth phase), washed free of isotope, and incubated in sea water containing 0.25% leucine for another 4 h before deciliation.
Tritiated leucine uptake was measured by comparing the 3H counts from zero-time and final samples of the incubation media, centrifuged free of embryos and cell debris, and then analyzed by scintillation counting. Incorporation into total protein was estimated by precipitation with cold 10% trichloroacetic acid (TCA), filtration onto a Whatman GF/A glass fiber filter (Whatman, Clifton, NJ), thorough washing with cold 10% TCA, and scintillation counting. Parallel cultures, conditions, sampling, and processing were used, permitting a direct comparison of incorporated counts.
Cilia Isolation and Fractionation
At the end of the labeling period, the embryos were recovered by gentle manual centrifugation, washed twice with cold sea water, and treated for 2 min with double-tonicity sea water (32 g NaCl per liter of Tris-buffered sea water), a process that results in complete deciliation but permits full regeneration (Auclair and Siegel, 1966; Stephens, 1986). The embryos were removed by manual centrifugation. Cilia were recovered from the chilled hypertonic supernatant by centrifugation for 10 min at 10,000 × g and then washed once with cold normal-strength sea water.
The pelleted cilia were demembranated by extraction for 2–5 min at 0°C with >10 volumes of 0.25% peroxide-free NP-40 (Pierce 28324, Pierce Chemical, Rockford, IL) in 3 mM MgCl2, 30 mM Tris-HCl, pH 8.0, containing 1 mM phenylmethylsulfonyl fluoride. The suspension was centrifuged for 5 min at 35,000 × g. The resulting pellet of 9+2 axonemes was either resuspended to the original extraction volume in fresh extraction solution for direct SDS-PAGE comparison with the membrane/matrix fraction or it was further fractionated.
To fractionate the axonemes thermally into ninefold ciliary remnants and solubilized axonemal tubulin, the preparation was resuspended to the initial extraction volume in 10 mM Tris-HCl (pH 8), 1 mM EDTA, and 0.1% 2-mercaptoethanol extraction, heated at 45°C for 10–15 min, and then chilled on ice (Stephens et al., 1989). The sample was centrifuged at 45,000 × g for 15 min, the tubulin-containing supernatant was withdrawn, and the pellet of ciliary remnants was resuspended to the initial extraction volume with this same buffer.
SDS-PAGE, Immunoblot, and Fluorographic Analysis
The membrane/matrix and axonemal fractions were mixed with 5×-concentrated SDS-sample buffer, boiled for 2 min, and analyzed on uniform 8% T/2.5% C polyacrylamide slab gels (1.5 mm thick × 10 cm long × 15 cm wide), using 0.1% SDS (Sigma L-5750, Sigma, St. Louis, MO) and the Laemmli (1970) discontinuous ionic system. Gels were stained by the equilibrium method of Fairbanks et al. (1971), using Coomassie blue.
Immunoblotting was carried out by the method of Dunn (1986). The mouse monoclonal antibody 3a3 and the rat monoclonal antibody 7.10 to the HSP70 family of proteins were obtained from Affinity Bioreagents (Golden, CO) and used at dilutions of 1:1,000. The antibodies were detected with alkaline phosphatase-conjugated secondary antibodies and visualized with Western Blue-stabilized substrate, all obtained from Promega (Madison, WI).
For fluorography, the gels were treated with EN3HANCE or ENTENSIFY (New England Nuclear, Boston, MA), dried, and fluorographed at −80°C against preflashed Kodak X-Omat AR film (Eastman Kodak, Rochester, NY); multiple film exposures were obtained to remain within the linear limits of film response (Laskey and Mills, 1975). Densitometry was performed with the Jandel Video Analysis (JAVA) hardware/software package (Jandel Scientific, San Rafael, CA), using the general principles outlined by Haselgrove et al. (1985). Full-range digital images were obtained by adjusting the background to a pixel intensity near 255 with the camera lens aperture and including an opaque black object in the field to define a pixel intensity of 1. Optical density (OD) values were obtained from the eight-bit video intensity scans of gel or fluorogram lanes by applying the mathematical transform, OD = log(transmitted background intensity/data point intensity). Protein and radioactivity calibrations for gels and fluorograms were obtained by running known amounts of bovine serum albumin and known total protein counts. Using Sigma Gel (Jandel Scientific), digital images of immunoblots were converted to relative reflectance [log(255/pixel intensity)] and quantified in terms of integrated band density above the average blot background with the software’s “flood-fill” feature.
RESULTS
On the Validity of Video Densitometry
Within certain experimental limits (primarily dye saturation or silver depletion), the light transmitted by stained polyacrylamide gels or their corresponding autoradiograms or fluorograms obeys the classic Lambert/Beer Law, namely that the absorbance, or OD, is directly proportional to the optical path length (which is constant) and the concentration (amount of dye or density of silver grains). In the case of immunoblots, the light reflected from a spot (like the light transmitted by gel or film bands) is dependent inversely on the light absorbed, given that too much opaque product doesn’t accumulate. Absorbance (or reflectance) is a log function, log(incident intensity/transmitted or reflected intensity), while the pixel intensity of an eight-bit video or scanner digital image is a linear function with 28 steps. It has become common, although incorrect, to quantify gels, fluorograms, autoradiograms, and immunoblots simply in terms of the pixel intensity (or reverse-image pixel intensity) of bands or spots. This can be valid if one includes standards within each run or expresses the comparative transmitted or reflected intensities on a log scale, but only in digitally captured fluorescent images is pixel intensity directly proportional to concentration.
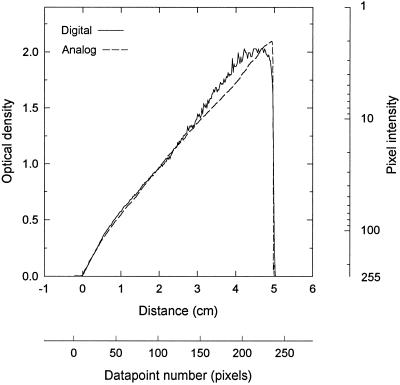
The validity of the approach used here is illustrated in Figure 1 where a standard linear beam splitter was scanned conventionally in analog manner with a photon-counting densitometer and also imaged digitally with a CCD camera and frame-grabber, converting pixel intensity to OD mathematically. The two data sets are in excellent agreement up to an OD of about 1.3, above which the inherent video signal-to-noise limitations become evident. Quantitative densitometric measurements are ordinarily restricted to values below 1 OD since neither dye binding nor film response is linear much beyond this point, rendering this deviation of little practical consequence.
Figure 1.
Comparison of photon-counting analog and video-based digital densitometry. A 0–2.0 OD linear beam splitter (Edmund Scientific 41960, Edmund Scientific, Barrington, NJ) was scanned with an Ortec 4310 densitometer (Ortec, Oak Ridge, TN), giving a direct OD read-out, and also with a COHU CCD camera and JAVA system, producing a digital image. The pixel intensity along the wedge axis was converted to OD by the transform OD = log(255/pixel intensity). The two methods deviated only at high OD values, below a pixel intensity of 10, where the error due to precision of measurement (±1 pixel value) becomes progressively more significant.
Limited Architectural Protein Synthesis Recurs during Successive Regenerations
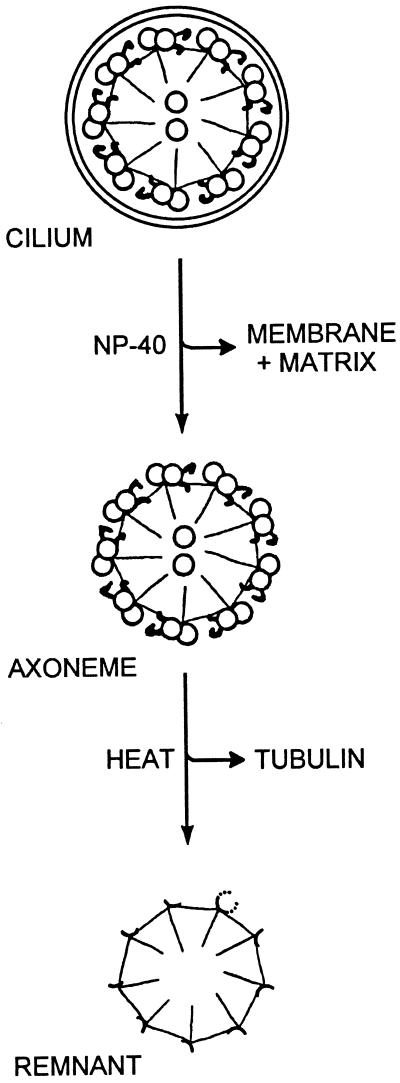
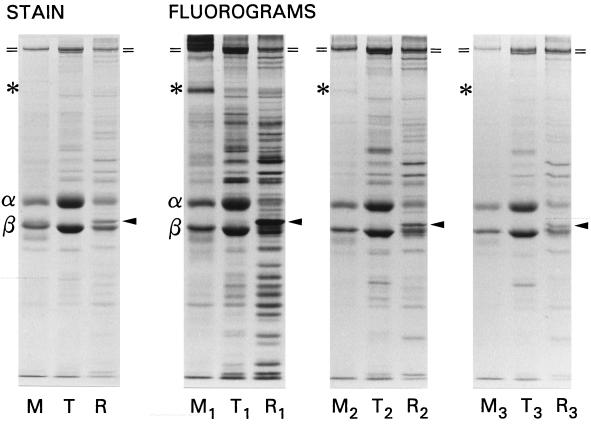
The synthesis and utilization of tubulin and tektin-A have been described (Stephens, 1991, 1994a), but little quantitative information is available for other ciliary proteins, particularly after multiple regenerations. After high-salt deciliation, S. droebachiensis embryos regenerate full-length cilia in 8 h at 7.5°C, and this procedure may be repeated at will (Stephens, 1977). To evaluate consecutive protein synthesis, embryos were first deciliated and pulse labeled during the 4-h near-linear ciliary growth phase. After a chase of unlabeled leucine during completion of growth, the embryos were deciliated and then allowed to regenerate twice more. To detect any differences that might occur in the synthesis or incorporation of proteins from different locations surrounding or within the 9+2 structure, labeled cilia were first fractionated into detergent-solubilized membrane plus soluble matrix components, thermally solubilized axonemal tubulin plus associated proteins, and stable ninefold skeletal remnants, as shown schematically in Figure 2. An SDS-PAGE and fluorographic analysis of these fractions for one labeled regeneration and two cold regenerations is illustrated and quantified in Figures 3 and 4.
Figure 2.
Fractionation of a cilium by detergent (NP-40) solubilization of the membrane plus matrix, followed by the thermal depolymerization of most of the tubulin from the 9+2 axoneme, yields a ninefold remnant that retains most of the architectural proteins and a vane-like segment of the A-tubule. For spatial orientation, a partially depolymerized A-tubule is shown on the remnant. [Based on results of Stephens et al. (1989).]
Figure 3.
SDS-PAGE and 3H-fluorographic analysis of ciliary proteins synthesized during one regeneration and replaced with unlabeled proteins in two successive regenerations. The detergent-solubilized membrane/matrix (M), thermally solubilized tubulin (T), and stable ninefold remnant (R) were loaded stoichiometrically; the subscripts denote the first, second, and third regenerations. A prominently labeled membrane protein is designated with an asterisk; the positions of the barely resolved dynein heavy chains are marked (=); the α and β tubulin chains are so designated; the solid arrowheads mark tektin A.
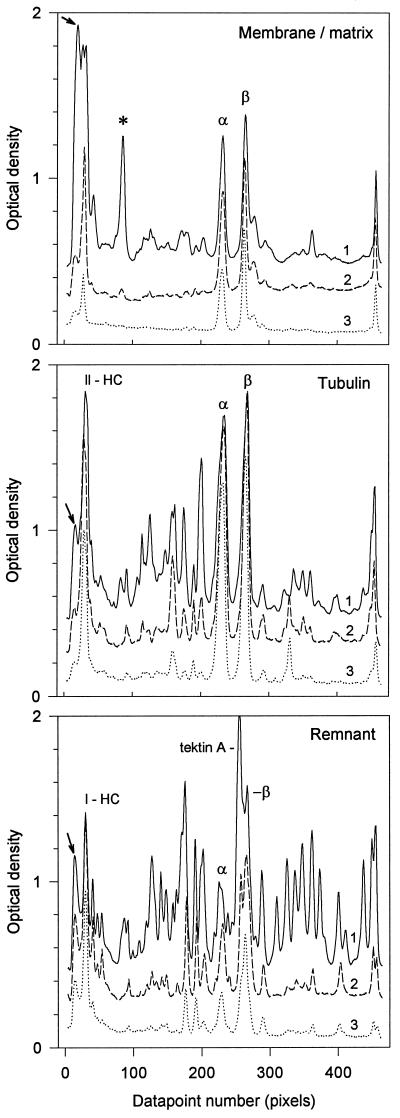
Figure 4.
Comparative densitometry of the 3H-fluorogram of the membrane/matrix, tubulin, and remnant fractions from Figure 3. The traces in each panel compare the designated fractions from the pulse-chase-labeled first (1), second (2), and third (3) regenerations. The data have been normalized for sample-loading differences, and traces 1 and 2 have been displaced vertically by 0.40 and 0.20 OD units, respectively, to avoid intersection. Peak designations correspond to the band designations of Figure 3. The arrows (extreme left) mark material barely penetrating the top of each gel.
Inspection of the fluorograms (Figure 3) reveals three major features. First, the label present in the two major axonemal building blocks, tubulin and dynein, decreased only minimally with successive regenerations. This is indicative of large labeled pools of these proteins, to which new protein, synthesized with unlabeled leucine, contribute relatively little. Second, certain architectural proteins were uniformly replaced in successive regenerations, indicative of more limited pools of these coordinately synthesized constituents. Third, many heavily labeled proteins, particularly in the remnant fraction, were almost fully replaced after only one unlabeled regeneration (the definition of quantal synthesis).
Comparative densitometry of each of the three fractions (Figure 4) makes these points more apparent. In the membrane/matrix fraction, very high molecular weight material, barely penetrating the gel, and a prominent 190 kDa acylated protein (Stephens, 1991) were heavily labeled in the first (pulse-chase) regeneration but were rapidly replaced with proteins containing unlabeled leucine in the two successive regenerations, in both cases decreasing by >80%. Many of the minor membrane/matrix proteins, few of which correspond in size to the prominently labeled architectural proteins discussed below, also decreased by a similar amount. In contrast, tubulin α- and β-chain label decreased significantly only after the second regeneration.
In the thermally solubilized tubulin fraction, the label in the α and β tubulin chains and in the comigrating heavy chains of dynein were also minimally replaced with unlabeled proteins. Tubulin label increased slightly in the second regeneration (since first regeneration cilia must utilize initially unlabeled large pools) and then decreased by about 20% in the third. Also high in the second regeneration, dynein decreased by about one-third in the third. However, the label in many of the architectural proteins, particularly those that migrate between dynein and tubulin, decreased by two-thirds or more in both unlabeled regenerations but remained apparent as peaks on the third regeneration trace.
Although representing less than one-third of the total protein of the cilium and retaining only about one-fifth of the tubulin, the thermally stable remnant, in which most of the 9+2 determining architectural proteins are located, contained >40% of the label. With the exception of a high-molecular weight band identified by an antibody specific for inner arm dynein heavy chain (Stephens and Prior, 1995) and the residual α and β tubulin chains, many labeled proteins decreased by >two-thirds in successive regenerations. The most prominently labeled architectural protein was tektin-A, shown previously to correlate with elongation and length. Its label decreased by >80%, as was also true for a number of other proteins noted earlier as also being potentially limiting or quantal in their synthesis (Stephens, 1989). These proteins were barely detectable on the third regeneration trace.
Colchicine Inhibition of Tubulin Synthesis or Assembly Does Not Inhibit Protein Turnover
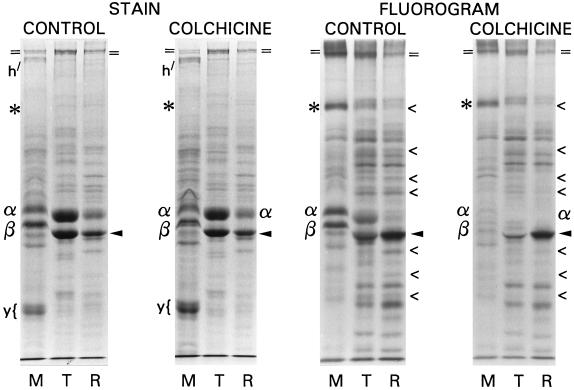
To determine whether turnover is coupled to tubulin synthesis or assembly, subcultures having virgin cilia were treated with and without colchicine during labeling. The membrane/matrix, axonemal tubulin, and remnant fractions from equivalent amounts of cilia were resolved on the same gel and then analyzed by fluorography (Figure 5).
Figure 5.
SDS-PAGE and 3H-fluorographic analysis of ciliary protein fractions from control and 1 mM colchicine-treated midgastrula stage embryos. Left panel: Coomassie blue-stained gel; right panel: fluorogram of the same gel. Tubulin synthesis is inhibited by colchicine, but protein distribution and labeling are similar under both conditions. The retarded migration in the M lanes is due to the presence of hyalin, normally removed when embryos are predeciliated during culture, which these intentionally were not. Designations as in Figure 3; “<” denotes proteins whose labeling, but not amount, is diminished by colchicine. Nonciliary, nonlabeled proteins originating from high-salt solubilization of the hyaline layer during deciliation are noted: h, hyalin; y, a yolk granule component.
Even though inhibiting ciliary regeneration fully, 1 mM colchicine treatment resulted in no redistribution of tubulin into the membrane/matrix fraction (Figure 5, α/β, stain). This observation directly addresses the question of whether membrane-associated tubulin might be derived from a breakdown of assembling or recently assembled axonemal microtubules, circumstances under which colchicine would be expected to increase the relative proportion of tubulin in the detergent-soluble fraction. The relative proportions and labeling of other proteins of the membrane/matrix fraction were qualitatively the same in both cases.
In the presence of colchicine, no significant labeling occurred in the tubulin of the membrane/matrix fraction (Figure 5, α/β, fluorogram), nor was axonemal tubulin itself labeled. However, the labeling of most other major axonemal proteins was similar to the control, indicating that more general ciliary protein synthesis was not markedly inhibited by long-term colchicine treatment. This conclusion was further confirmed for the whole embryo by direct measurement of [3H]leucine incorporation into TCA-precipitated total protein. In the case illustrated, there was no inhibition of synthesis in colchicine-treated versus the untreated embryo culture (16,270 cpm/mg vs. 15,556 cpm/mg, respectively), nor was there a difference in [3H]leucine uptake (88.7% vs. 88.8% of total added counts, respectively).
Incorporation into the major labeled architectural protein, tektin A (Figure 5, arrowhead), was also quite similar, as was the qualitative labeling pattern of the other architectural components. However, there were quantitative differences in labeling, but not in amount, in some of the latter (Figure 5, <). These labeling differences were consistently seen in three separate analyses of both mid- and late-gastrula stage embryos.
In regenerated cilia, protein label directly reflects the characteristic composition of each fraction (Figure 3) but during steady-state turnover, most of the labeled proteins detected in the thermally solubilized tubulin fraction were more characteristic of the remnant fraction, i.e., the overall labeling pattern is dominated by newly synthesized proteins that are destined for incorporation into the architectural elements of the 9+2 structure (Stephens, 1994a). Since neither colchicine nor taxol treatment changed this relative distribution, further comparative experiments are illustrated below with unfractionated (i.e., 9+2) axonemes.
Synthesis and Turnover Continue in the Presence of Oppositely Acting Tubulin Inhibitors
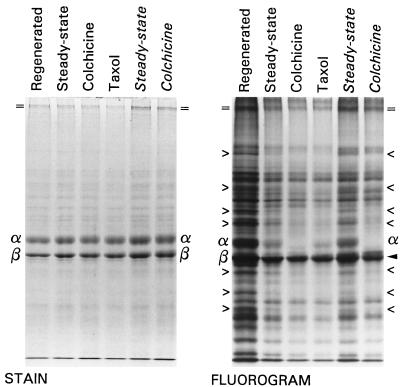
To compare the actions of colchicine and taxol, the labeling of axonemes from 1) regenerated, 2) untreated control, and 3) colchicine- and 4) taxol-treated late-gastrula stage embryos were examined (Figure 6). The uptake and incorporation values for embryos from these four conditions shown were experimentally indistinguishable, averaging 77.1 ± 1.1% and 19,002 ± 328 cpm/mg, respectively (mean ± SD). That protein synthesis was not significantly decreased by colchicine would indicate that new tubulin synthesis must be a very small fraction of total protein synthesis, at least at this late stage. For further comparison, axonemes from steady-state and colchicine-treated midgastrula stage embryos, as in Figure 5, were included.
Figure 6.
SDS-PAGE and 3H fluorographic analysis of ciliary axonemes from parallel cultures of Regenerated, Steady-state control, and 1 mM Colchicine- or 10 μM Taxol-treated late-gastrula stage embryos and from Steady-state control and 1 mM Colchicine-treated midgastrula stage embryos. Left panel: Coomassie blue-stained gel; right panel: fluorogram of the same gel. The labeling patterns are similar between control and either colchicine or taxol-treated conditions, but both drugs cause similar diminution of label in certain proteins. Designations as in Figures 3 and 5; “> and <” mark the same proteins as Figure 5.
Compared with the steady-state control, the labeling of cilia regenerated from late-gastrula stage embryos was relatively high since deciliation up-regulates the stage-suppressed ciliary protein synthetic rate better than twofold (Harlow and Nemer, 1987; Stephens, 1991). In midgastrula stage embryos, the steady-state synthesis rate is characteristically high, a fact that is apparent if one compares the late- and midgastrula stage controls (Figure 6, Steady-state vs. Steady-state). Regardless of these differences, the same overall labeling pattern prevailed.
The relative degree and qualitative pattern of labeling of most nontubulin proteins was similar in untreated steady-state controls versus the colchicine- or taxol-treated cases. This is particularly obvious when one compares tektin-A among lanes 2–4 of Figure 6. The above-noted architectural proteins whose relative labeling was diminished by colchicine were similarly affected by taxol. In comparison with flanking bands, many of these same proteins are more heavily labeled in regenerated than in steady-state cilia, indicating a disproportionate up-regulation in response to experimental deciliation (Figure 6, > vs. <).
In contrast to colchicine, taxol does not inhibit tubulin synthesis (Gong and Brandhorst, 1988), verified by the observation of control-level tubulin labeling in the membrane/matrix fraction. In the example illustrated, the labeling of α-tubulin in axonemes from taxol-treated embryos was 51% of the steady-state control whereas the architecturals averaged about 70%, comparable to the reduction seen with colchicine.
Most Axonemal Proteins Are Proportionately Labeled during Colchicine Treatment
Discounting the inhibition of tubulin synthesis, the degree of labeling (specific activity) of ciliary axonemes in the presence of colchicine was about 20% less at the late gastrula stage and as much as one-third less at the midgastrula stage. The yield of cilia at each stage correlated with the observed decrease in labeling, consistent with the inhibition by colchicine (and taxol) of both cell division and the subsequent regrowth of cilia on the resulting daughter blastomeres. Thus the decrease in labeling most likely reflects that fraction of cilia not assembled during the time period, and, hence, any remaining label that is incorporated must reflect true turnover.
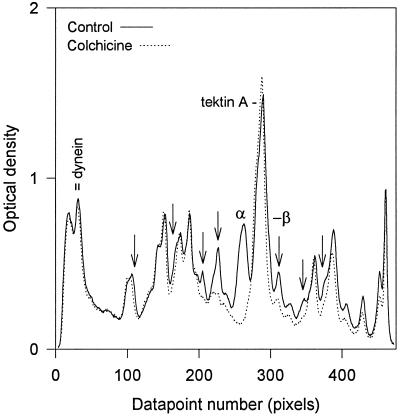
When densitometer traces of fluorograms of axonemes from untreated and colchicine-treated embryos are compared directly, the proportionate synthesis of most axonemal components, including the dynein heavy chains, becomes more apparent. Representative traces, shown in Figure 7, were taken from the midgastrula Steady-state and Colchicine lanes of Figure 6. These data were normalized with respect to the incorporation differences noted above. Only α- and β-tubulin (the latter migrating slightly ahead of tektin-A and hence obscured by its heavy label) were essentially unlabeled while the above noted architectural proteins in cilia from colchicine-treated embryos contained disproportionately less label than the untreated controls.
Figure 7.
Comparative densitometry demonstrates stoichiometric labeling of most ciliary proteins despite colchicine inhibition of tubulin synthesis. The final two lanes of Figure 5 were scanned, normalized by a factor of 1.30 for the difference in total nontubulin counts, and plotted together. The arrows designate the proteins whose labeling was diminished by colchicine treatment, as noted in Figure 5.
Neither Colchicine nor Taxol Influences the Distribution of a Molecular Chaperone Cognate
The finding of a molecular chaperone in Chlamydomonas flagella by Bloch and Johnson (1995) suggested to them that the chaperone might be involved in targeting tubulin and other unassembled 9+2 components to the cilium for assembly. Because of steady-state turnover, it is important to know whether a similar molecule is present in sea urchin embryonic cilia and whether inhibition of regeneration by colchicine or taxol influences its presence or distribution.
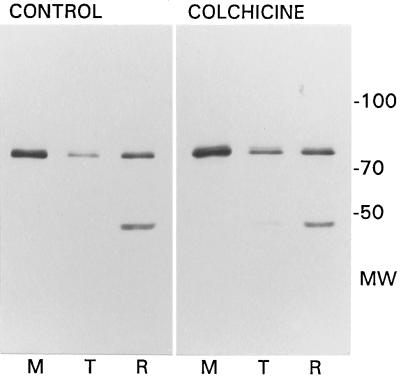
Axonemes from steady-state control, colchicine-, and taxol-arrested late-gastrula stage embryos were thermally fractionated into soluble tubulin and insoluble ninefold remnants and compared stoichiometrically with the initial detergent-soluble membrane/matrix extract by SDS-PAGE and immunoblotting, using mouse (3a3) or rat (7.10) monoclonal antibodies to human or Drosophila HSP70, respectively. One such comparison with monoclonal antibody 7.10 is shown in Figure 8.
Figure 8.
Immunoblot analysis of ciliary protein fractions from control and colchicine-treated late-gastrula stage embryos using the monoclonal antibody 7.10 to molecular chaperones of the HSP70 family shows that the localization of a 78-kDa cognate to HSP70 is minimally affected by colchicine treatment, but its total amount is greater. The antibody also cross-reacted with a 46-kDa axonemal protein. The original unfractionated axonemes that yielded these samples are shown in Figure 6, lanes 2 and 3, respectively.
This antibody detected a single 78-kDa protein in all three fractions, as did monoclonal 3a3, but monoclonal 7.10 also cross-reacted with a 46-kDa protein found principally in the remnant fraction. (Another sea urchin, Tripneustes gratilla, had only the 78-kDa protein, so the presence of a 46-kDa antigen is not characteristic of urchins.) By separate analysis of purified tektin filaments, the monoclonal 7.10 did not cross-react with tektins, typically migrating in the 47–55 kDa region. Although the molecular weight of the major component was coincident with that of certain KDEL proteins (Grp78 or BiP), a broad range antibody to the KDEL epitope (StressGen SPA-827, StressGen Biotech, Victoria, British Columbia, Canada) cross-reacted with neither protein.
Contrary to expectations based on either background ciliogenesis or microtubule-mediated transport, neither colchicine inhibition of tubulin synthesis and assembly nor taxol promotion of tubulin assembly decreased the amount of HSP70 cognate or markedly changed its distribution. Rather, quantification of this and two other analyses indicated that there was typically 40–50% more total HSP70 antigen in cilia from the colchicine-treated embryos than in untreated controls. However, the distributions of this antigen in the membrane/matrix, solubilized tubulin, and remnant fractions were similar: 59%, 11%, and 30% in the control and 55%, 19%, and 26% in the colchicine-treated case illustrated here. There was consistently 8–11% more total HSP70 antigen in the cilia from taxol-treated embryos than in the controls. The distribution was 57%, 19%, and 24% in the membrane/matrix, solubilized tubulin, and remnant fractions, respectively. In spite of their opposite modes of action, both colchicine and taxol nearly doubled the amount of HSP70 cognate that was released from the axoneme by heating but, regardless of any differences in either total or thermally released antigen, the fraction that was initially axoneme-associated was 41–45% under all three incubation conditions.
Bound Chaperone Is Proportional to Length during Regeneration
The mere presence of an HSP70 cognate in steady-state cilia says nothing about its behavior during ciliogenesis. If such a protein is associated along the length of the cilium, the amount in the axoneme fraction should correlate with length as the axoneme elongates during regeneration. In contrast, if the HSP70 cognate is predominately tip-associated (Block and Johnson, 1995), a fixed amount per axoneme would be expected. To assess these points, embryos were deciliated and allowed to regenerate cilia of varying length. The cilia were removed, demembranated to produce membrane/matrix and axoneme fractions, and analyzed by quantitative immunoblotting. The amount of 78-kDa protein in each fraction, expressed relative to the antigen in steady-state cilia before deciliation, is illustrated in Figure 9.
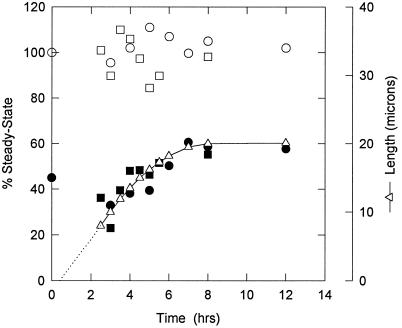
Figure 9.
Time after deciliation vs. the amount of HSP70 cognate found in whole cilia (open circles/squares) and associated with the axoneme (solid circles/squares) with respect to the total amount of antigen found in steady-state cilia before deciliation, taken as 100%. The two sets of symbols represent two independent experiments (monoclonal 7.10). The amount of HSP70 cognate in whole cilia is nearly constant while the axoneme-associated amount increases in parallel with ciliary length (triangles/line).
The total amount of HSP70 cognate from a given number of cilia during regeneration remained relatively constant and did not differ from that found at steady-state before deciliation (99.9 ± 7.7 SD, n = 15). However, the fraction of this total amount that was axoneme-associated increased steadily in parallel with elongation, eventually exceeding that found before deciliation. The membrane-matrix fraction contained better than two-thirds of the 78-kDa protein when the cilia were half grown, but it accounted for less than one-half when they achieved their final length. (Shorter lengths were not analyzed due to decreased yield and increased potential contamination.)
DISCUSSION
Upon successive regenerations, the synthesis of ciliary proteins follows a distinctive pattern, heretofore not presented in comparative, quantitative detail. Large pools of tubulin and dynein are replenished with newly synthesized protein, changing little in specific activity after a chase with unlabeled amino acid. The architectural proteins are synthesized in discrete amounts: one class is present in two- to threefold excess while the other is synthesized in only slight excess, as judged by their respective dilutions with unlabeled protein. Most of these proteins are labeled to a similar extent in steady-state cilia, indicating continued coordinate synthesis and subsequent exchange.
In spite of this coordinate synthesis, the turnover or exchange of most ciliary proteins is not dependent on either the synthesis of tubulin or its ability to assemble since colchicine effectively prevents both, yet labeling continues. Furthermore, the proportionate synthesis of dynein and most of the 9+2 architectural proteins is not coordinated with the available tubulin pool since near-normal labeling occurs in the presence of either colchicine or taxol, both of which influence tubulin assembly but in a converse manner. However, the labeling of a minor subset of architectural proteins is suppressed to the same degree by each of these drugs. These proteins are more prominently labeled in experimentally regenerated cilia, suggesting that they may be specifically up-regulated during background ciliogenesis or regeneration. Enhanced labeling of similar-sized proteins, particularly in the region between tubulin and dynein, may be seen in regenerated versus steady-state cilia from both late-gastrula and zinc-arrested blastula embryos from this and a different sea urchin species in unrelated experiments in which neither colchicine nor taxol was involved (Stephens, 1994a).
These results should lay to rest the persistent quandary of whether axonemal labeling might originate from unrecognized ciliary growth. Unequivocally, the steady-state incorporation of labeled proteins into cilia, still observed in the presence of sufficient colchicine or taxol to prevent ciliary regeneration, cannot be due to regrowth or elongation during the labeling period since these drugs prevent both. Thus the turnover is real, but its mechanism and function remain enigmatic.
Essentially all of the components that show substantial turnover are associated directly with the microtubules of the 9+2 axoneme, namely dynein arms and, by definition, the architectural proteins. Consequently, these proteins are potentially accessible for exchange with their soluble or transported counterparts. An apparent exception is tektin-A, an integral structural determinant of certain doublet microtubule protofilaments (Nojima et al., 1995). However, biochemically there are two subclasses of tektin-A, one readily soluble in chaotropic agents and one not. Only the more soluble of the two exhibits significant labeling (Stephens, 1994a), perhaps reflecting a more accessible location within the tubulin lattice.
The labeling of axonemal tubulin in taxol-treated embryos demonstrates that tubulin within 9+2 microtubules can turn over, although not to the same extent as in the steady-state control. Sequestration of tubulin with taxol may simply make it less available. Previous work demonstrated that the tubulin of steady-state blastula cilia was labeled to 64–74% of the level seen in regenerated cilia, not accounting for any simultaneous ciliary growth (Stephens, 1991). Correcting for background ciliogenesis using the colchicine inhibition results presented above would reduced this to >45% turnover. Furthermore, 42–48% tubulin turnover was estimated for cilia from both late gastrula and zinc-arrested blastula from a different sea urchin in which cell division and up-regulation of synthesis by deciliation were insignificant (Stephens, 1994a). Thus, these two independent estimates are in reasonable agreement with the present observation of tubulin labeling during taxol treatment. Exactly how tubulin in an intact microtubule might exchange remains a mystery.
Turnover is seen in terminally differentiated molluscan gill epithelial cilia, and it likewise cannot be attributed to ciliary growth (Stephens, 1996). As in sea urchin embryos, the process is dominated by synthesis and exchange of the 9+2 architectural proteins, displaying the same distinctions in tektin-A solubility subclass labeling. However, no significant labeling of axonemal dynein or tubulin was seen, although membrane-associated tubulin was labeled. That such similar processes take place in cilia from two very different tissues in organisms from two major branches of the evolutionary tree suggests a highly conserved functional mechanism.
Turnover of protozoan axonemal proteins has been recognized for some time, approaching 50% of the regeneration rate for Ochromonas flagella (Rosenbaum and Child, 1967) or Tetrahymena cilia (Nelsen, 1975). In these studies, elongation or regrowth during labeling was judged to be insignificant. More recently, using dikaryon rescue approaches, workers have shown that epitope-tagged tubulin or radial spokes (Johnson and Rosenbaum, 1992, 1993) and various dynein components (Piperno et al., 1996) can assemble, fill vacant sites, or replace existing structures in fully grown Chlamydomonas flagella, typically starting from the distal tip. The latter study demonstrated that a kinesin-like protein is required for dynein inner arm but not outer arm transport, suggesting the coexistence of several mechanisms for translocation. This particular kinesin-like protein is involved in intraflagellar transport, the bidirectional conveyance of rafts of granule-like particles beneath the flagellar membrane and adjacent to the outer doublets (Kozminski et al., 1995).
The finding of an axoneme-associated sea urchin HSP70 cognate in both regenerating cilia and steady-state cilia undergoing protein turnover is the first report of a chaperone in metazoan cilia and is consistent with the proposal that such a molecule may be involved in the transport and assembly of axonemal precursors (Bloch and Johnson, 1995). The finding that the membrane/matrix versus axonemal distribution of this chaperone is little influenced by colchicine or taxol would suggest that neither association with newly synthesized tubulin nor the assembly state of tubulin is a requisite for chaperone complex formation, transport, or function. In fact, with the notable exception of membrane-associated tubulin, most newly synthesized axonemal proteins are axoneme associated before their incorporation and do not appear in the membrane–matrix fraction (Stephens, 1992, 1994a). Furthermore, the constancy of the total amount of this protein would suggest that its presence is not simply the consequence of hypertonic shock-induced deciliation or of background ciliogenesis. Thus the similar amounts of chaperone in steady-state and regenerating cilia may reflect the substantial turnover seen in the former.
It is not clear why colchicine and, to a lesser extent, taxol should increase the amount of HSP70 cognate in steady-state cilia, in contrast to deciliation and regeneration. These inhibitors should have opposite effects on any cytoplasmic microtubules involved in endoplasmic reticulum to basal body transport, although perhaps both disruption and overstabilization of microtubules somehow lead to an accumulation of chaperone in the steady-state cilium by preventing recycling. Alternatively, this increase could be a true shock response to antimitotic drugs.
It may be relevant that Spec3, an ectoderm-specific protein whose synthesis correlates with ciliogenesis, appears first in the Golgi complex before incorporation into motile cilia, but prevention of its translocation to the axoneme does not impair motility (Eldon et al., 1987, 1990). Although its N-terminal region is exposed at the ciliary membrane surface, the protein remains anchored to the axoneme after detergent extraction, suggesting that it may be part of a transmembrane–axonemal complex that is not part of the motile apparatus per se but may be involved in protein translocation. Spec3 itself does not appear to be related to any known chaperone, nor is its function in cilia yet known, but its translocation after ciliary assembly strongly suggests postciliogenesis protein trafficking.
The association between components of the protein synthetic machinery and sea urchin embryonic cilia has been long recognized. Well-oriented endoplasmic reticulum and Golgi membranes are apically convergent. Elements of the Golgi apparatus surround the striated rootlet, assuming the shape of an inverted funnel whose apex is centered around the centriole and basal body of mature cilia (Gibbins et al., 1969; Anstrom, 1992). Whether this is guilt by association or indicative of a working relationship remains to be determined.
The function of protein turnover in a motile cilium or flagellum may be related to the maintenance of the organelle. Rapid protein turnover/exchange has been reported for a number of other supposedly stable cytoplasmic structures, for example actin thin filaments (Kreis et al., 1982), myosin thick filaments (Saad et al., 1991), and intermediate filaments (Vikstrom et al., 1992). Similar processes for axonemal proteins might not be too surprising were it not for the topology involved. What sets the cilium or flagellum apart is the fact that 9+2 axonemes are uniquely compartmentalized with respect to the somatic plasma membrane, the protein synthetic machinery, and the soluble precursor pools necessary for its construction, length limitations, and maintenance. This provides important experimental advantages for analyzing pathways, transport, and regulatory mechanisms.
ACKNOWLEDGMENTS
I thank Nicole Lemieux for her skilled technical assistance. Taxol was generously provided by the Drug Synthesis and Chemistry Branch of the National Cancer Institute, Division of Cancer Treatment. This work was supported by United States Public Health Service grant GM-20,644 from the National Institute of General Medical Sciences.
REFERENCES
- Anstrom JA. Organization of the ciliary basal apparatus in embryonic cells of the sea urchin, Lytechinus pictus. Tissue Cell Res. 1992;269:305–313. doi: 10.1007/BF00319622. [DOI] [PubMed] [Google Scholar]
- Auclair W, Siegel BW. Cilia regeneration in the sea urchin embryo: evidence for a pool of ciliary proteins. Science. 1966;154:913–915. doi: 10.1126/science.154.3751.913. [DOI] [PubMed] [Google Scholar]
- Bloch MA, Johnson KA. Identification of a molecular chaperone in the eukaryotic flagellum and its localization to the site of microtubule assembly. J Cell Sci. 1995;108:3541–3545. doi: 10.1242/jcs.108.11.3541. [DOI] [PubMed] [Google Scholar]
- Dunn SD. Effects of the modification of transfer buffer composition and the renaturation of proteins in gels on the recognition of proteins on western blots by monoclonal antibodies. Anal Biochem. 1986;157:144–153. doi: 10.1016/0003-2697(86)90207-1. [DOI] [PubMed] [Google Scholar]
- Eldon ED, Angerer LM, Angerer RC, Klein WH. Spec3: embryonic expression of a sea urchin gene whose product is involved in ectodermal ciliogenesis. Genes Dev. 1987;1:1280–1292. doi: 10.1101/gad.1.10.1280. [DOI] [PubMed] [Google Scholar]
- Eldon ED, Montpetit IC, Nguyen T, Decker G, Valdizan MC, Klein WH, Brandhorst BP. Localization of the sea urchin Spec3 protein to cilia and Golgi complexes of embryonic ectoderm cells. Genes Dev. 1990;4:111–122. doi: 10.1101/gad.4.1.111. [DOI] [PubMed] [Google Scholar]
- Fairbanks GT, Steck T, Wallach DFH. Electrophoretic analysis of the major peptides of the human erythrocyte. Biochemistry. 1971;10:2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gibbins JR, Tilney LG, Porter KR. Microtubules in the formation and development of the primary mesenchyme in Arbacia punctulata. I. The distribution of microtubules. J Cell Biol. 1969;41:201–226. doi: 10.1083/jcb.41.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Z, Brandhorst B. Autogenous regulation of tubulin gene transcription via RNA stability during sea urchin embryogenesis. Development. 1988;102:31–43. doi: 10.1242/dev.102.1.31. [DOI] [PubMed] [Google Scholar]
- Harlow P, Nemer M. Developmental and tissue-specific regulation of β-tubulin gene expression in the embryo of the sea urchin Strongylocentrotus purpuratus. Genes Dev. 1987;1:147–160. doi: 10.1101/gad.1.2.147. [DOI] [PubMed] [Google Scholar]
- Haselgrove JC, Lyons G, Rubenstein N, Kelly A. A rapid, inexpensive, quantitative, general purpose densitometer and its application to one-dimensional gel electrophoretograms. Anal Biochem. 1985;150:449–456. doi: 10.1016/0003-2697(85)90534-2. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Rosenbaum JL. Polarity of flagellar assembly in Chlamydomonas. J Cell Biol. 1992;72:67–85. doi: 10.1083/jcb.119.6.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Rosenbaum JL. Flagellar regeneration in Chlamydomonas: a model system for studying organelle assembly. Trends Cell Biol. 1993;3:156–161. doi: 10.1016/0962-8924(93)90136-o. [DOI] [PubMed] [Google Scholar]
- Kozminski KG, Beech PL, Rosenbaum JL. The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. J Cell Biol. 1995;131:1517–1527. doi: 10.1083/jcb.131.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis TE, Geiger B, Schlessinger J. Mobility of microinjected rhodamine actin within living chicken gizzard cells determined by fluorescence photobleaching recovery. Cell. 1982;29:835–845. doi: 10.1016/0092-8674(82)90445-7. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey RA, Mills AD. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975;56:335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Nelsen EM. Regulation of tubulin during ciliary regeneration in non-growing Tetrahymena. Exp Cell Res. 1975;94:152–158. doi: 10.1016/0014-4827(75)90542-x. [DOI] [PubMed] [Google Scholar]
- Nojima D, Linck RW, Egelman EH. At least one of the protofilaments in flagellar microtubules is not composed of tubulin. Curr Biol. 1995;5:158–167. doi: 10.1016/s0960-9822(95)00037-6. [DOI] [PubMed] [Google Scholar]
- Norrander JM, Linck RW, Stephens RE. Transcriptional control of tektin A mRNA correlates with cilia development and length determination during sea urchin embryogenesis. Development. 1995;121:1615–1623. doi: 10.1242/dev.121.6.1615. [DOI] [PubMed] [Google Scholar]
- Piperno G, Mead K, Henderson S. Inner dynein arms but not outer dynein arms require the activity of kinesin homologue protein KHP1FLA10 to reach the distal part of flagella in Chlamydomonas. J Cell Biol. 1996;133:371–379. doi: 10.1083/jcb.133.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum JL, Child FM. Flagellar regeneration in protozoan flagellates. J Cell Biol. 1967;34:345–364. doi: 10.1083/jcb.34.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad AD, Dennis JE, Tan IP, Fischman DA. Visualization of myosin exchange between synthetic thick filaments. J Muscle Res Cell Motil. 1991;12:225–234. doi: 10.1007/BF01745111. [DOI] [PubMed] [Google Scholar]
- Stephens RE. Differential protein synthesis and utilization during cilia formation in sea urchin embryos. Dev Biol. 1977;61:311–329. doi: 10.1016/0012-1606(77)90301-3. [DOI] [PubMed] [Google Scholar]
- Stephens RE. Isolation of embryonic cilia and sperm flagella. Methods Cell Biol. 1986;27:217–227. doi: 10.1016/s0091-679x(08)60350-7. [DOI] [PubMed] [Google Scholar]
- Stephens RE. Quantal tektin synthesis and ciliary length in sea urchin embryos. J Cell Sci. 1989;92:403–413. doi: 10.1242/jcs.92.3.403. [DOI] [PubMed] [Google Scholar]
- Stephens RE. Tubulin in sea urchin embryonic cilia: characterization of the membrane-periaxonemal matrix. J Cell Sci. 1991;100:521–531. doi: 10.1242/jcs.100.3.521. [DOI] [PubMed] [Google Scholar]
- Stephens RE. Tubulin in sea urchin embryonic cilia: post-translational modifications during regeneration. J Cell Sci. 1992;101:837–845. doi: 10.1242/jcs.101.4.837. [DOI] [PubMed] [Google Scholar]
- Stephens RE. Tubulin and tektin in sea urchin embryonic cilia: pathways of protein incorporation during turnover and regeneration. J Cell Sci. 1994a;107:683–692. [PubMed] [Google Scholar]
- Stephens RE. Developmental regulation of ciliogenesis and ciliary length in sea urchin and surf clam embryos. In: Wilson WH Jr, Stricker SA, Shinn GL, editors. Reproduction and Development of Marine Invertebrates. Baltimore, MD: Johns Hopkins University Press; 1994b. pp. 129–139. [Google Scholar]
- Stephens RE. Ciliogenesis in sea urchin embryos — a subroutine in the program of development. BioEssays. 1995a;17:331–340. doi: 10.1002/bies.950170409. [DOI] [PubMed] [Google Scholar]
- Stephens RE. Rapid protein turnover in embryonic sea urchin cilia continues during colchicine-inhibition of tubulin synthesis. Mol Biol Cell. 1995b;6:358a. doi: 10.1091/mbc.8.11.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens RE. Selective incorporation of architectural proteins into terminally-differentiated molluscan gill cilia. J Exp Zool. 1996;274:330–309. doi: 10.1002/(SICI)1097-010X(19960401)274:5<300::AID-JEZ5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Stephens RE, Oleszko S, Linck RW. Retention of ciliary ninefold structure after removal of microtubules. J Cell Sci. 1989;92:391–402. doi: 10.1242/jcs.92.3.391. [DOI] [PubMed] [Google Scholar]
- Stephens RE, Prior G. Dynein inner arm heavy chain identification in cAMP-activated flagella using class-specific polyclonal antibodies. Cell Motil Cytoskeleton. 1995;30:261–271. doi: 10.1002/cm.970300404. [DOI] [PubMed] [Google Scholar]
- Tilney LG, Gibbins JR. Differential effects of antimitotic agents on the stability and behavior of cytoplasmic and ciliary microtubules. Protoplasma. 1968;65:167–179. doi: 10.1007/BF01666377. [DOI] [PubMed] [Google Scholar]
- Vikstrom KL, Lim SS, Goldman RD, Borisy GG. Steady state dynamics of intermediate filament networks. J Cell Biol. 1992;118:121–129. doi: 10.1083/jcb.118.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]