Abstract
It is well established that motor neurons depend for their survival on many trophic factors. In this study, we show that the precursor form of NGF (proNGF) can induce the death of motor neurons via engagement of the p75 neurotrophin receptor. The pro-apoptotic activity was dependent upon the presence of sortilin, a p75 co-receptor expressed on motor neurons. One potential source of proNGF is reactive astrocytes, which upregulate the levels of proNGF in response to peroxynitrite, an oxidant and producer of free radicals. Indeed, motor neuron viability was sensitive to conditioned media from cultured astrocytes treated with peroxynitrite and this effect could be reversed using a specific antibody against the pro-domain of proNGF. These results are consistent with a role for activated astrocytes and proNGF in the induction of motor neuron death and suggest a possible therapeutic target for the treatment of motor neuron disease.
Introduction
The selective death of spinal motor neurons is the central pathology of neurodegenerative diseases such as amyotrophic lateral sclerosis and spinal muscular atrophy. A great deal of effort has been made to identify trophic factors that support the survival of motor neurons (Henderson et al. 1994; Oppenheim et al. 1995). In addition to ciliary neurotrophic factor (CNTF) and glial cell derived neurotrophic factor (GDNF), the neurotrophins brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT-3) and neurotrophin 4/5 (NT-4/5) are potent trophic factors for spinal motor neuron survival in different experimental paradigms (Sendtner et al. 1992; Yan et al. 1992; Henderson et al. 1993; Koliatsos et al. 1994).
The family of neurotrophins exert their activity by interacting with two types of receptors - the tyrosine kinase (Trk) family and p75 (Chao et al. 1998; Chao 2003; Teng and Hempstead 2004). The p75 receptor has been shown to act as a co-receptor during Trk-mediated signaling (Hempstead et al. 1991; Bothwell 1995). During injury or inflammatory conditions and specific developmental time windows, p75 can mediate cell death in several cell types (Casaccia-Bonnefil et al. 1996; Roux et al. 1999; Terrado et al. 2000; Beattie et al. 2002; Kraemer 2002). It has been known for many years that p75 is expressed in embryonic spinal motor neurons (Ernfors et al. 1989; Chiu et al. 1993). Indeed, many efforts to isolate motor neurons rely upon using antibodies against p75 for immuno-purification (Camu and Henderson 1992; Arce et al. 1999).
Several studies have demonstrated that the level of expression and signaling activity of p75 can influence motor neuron survival. For example, following facial nerve axotomy, motor neuron loss was increased by NGF treatment in newborn wild-type but not in p75-null mice (Wiese et al. 1999). The expression of p75 normally continues until the second post-natal week, at which time it is down-regulated and it can no longer be detected in spinal motor neurons (Yan and Johnson 1988; Urschel and Hulsebosch 1992; Bothwell 1995). Re-expression of p75 occurs in the spinal cord motor neurons of rats during aging (Xie et al. 2003) and in response to injury (Koliatsos et al. 1991). This re-expression of p75 increases their susceptibility to apoptosis (Ferri et al. 1998).
There is also evidence indicating that p75 may have a role in motor neuron dysfunction. In amyotrophic lateral sclerosis, p75 immuno-reactivity is increased in spinal motor neurons (Seeburger et al. 1993; Copray et al. 2003) while both TrkA and TkB are absent (Seeburger et al. 1993). In a mouse model of the disease, the onset of degeneration was delayed in p75-null female mice (Kust et al. 2003) and antisense-mediated knockdown of p75 in adult animals delayed disease progression (Turner et al. 2003).
Recent studies have shown that proneurotrophins binding to p75 mediates cell death after injury or neurodegenerative diseases such as Alzheimer’s dementia (Harrington et al. 2004; Peng et al. 2004; Pedraza et al. 2005; Volosin et al. 2006). Pro-neurotrophins are produced and then cleaved by furin and other proteases to produce the mature neurotrophin (Lee et al. 2001). The precursor form of NGF, proNGF is responsible for inducing apoptotic death in particular cellular contexts (Lee et al. 2001; Beattie et al. 2002; Harrington et al. 2004; Volosin et al. 2006). Proneurotrophins, such as proNGF and proBDNF, preferentially interact with sortilin together with p75 to form a complex capable of activating an apoptotic cascade (Lee et al. 2001; Nykjaer et al. 2004; Teng et al. 2005).
Sortilin is a Vps10p domain containing transmembrane protein, which is involved in trafficking and endocytosis of proteins such as neurotensin (Petersen et al. 1997; Mazella et al. 1998; Nielsen et al. 1999; Nielsen et al. 2001; Nykjaer et al. 2004). Moreover, binding of pro-neurotrophins and the subsequent engagement of sortilin with p75 have defined a new mechanism for pro-neurotrophin action during neuronal death (Lee et al. 2001; Nykjaer et al. 2004; Teng et al. 2005).
Although the elements that lead to motor neuron degeneration are not well understood, mounting evidence indicate that non-neuronal cells contribute to motor neuron dysfunction. Astrocytes play a crucial role in maintaining central nervous system (CNS) physiology during development and in adulthood (Heales et al. 2004; Benarroch 2005). In contrast to the release of neurotrophic factors for neuronal survival, astrocytes can be detrimental in an activated state or during injury (Heales et al. 2004; Pehar et al. 2004). One potential trophic factor, NGF, had been proposed to induce motor neuron degeneration (Pehar et al. 2004). In this study, we demonstrate that the precursor form of NGF, and not the mature form, can induce the death of embryonic spinal motor neurons. In addition, we have found that stimulated astrocytes represent a cellular source for proNGF.
Results
Sortilin expression in spinal motor neurons
It has been proposed that p75 and sortilin can form a complex that results in neuronal cell death induced by proneurotrophins (Nykjaer et al. 2004; Teng et al. 2005). Spinal motor neurons express p75 (Camu and Henderson 1992; Pitts and Miller 1995), however, it is not known whether sortilin is specifically expressed in motor neurons. Although sortilin immunoreactivity was previously reported in spinal cord lysates (Petersen et al. 1997), the cellular localization of sortilin and its potential co-expression with p75 has not been investigated.
To explore this question, cryosections were obtained from the spinal cord of adult mice and subjected to immuno-fluorescence using a specific antibody raised against sortilin. The antibody shows no immuno-reactivity when used on sortilin−/− tissue and its specificity has been previously documented (Martin et al. 2002; Chen et al. 2005). A representative image showing sortilin expression in large cell bodies within the ventral aspect of the spinal cord is presented in Figure 1a. Immunofluorescence microscopy of cultured embryonic motor neurons, which were identified by expression of the specific marker Isl-1, demonstrated that sortilin (Figure 1b–c) and p75 (Figure 1d–e) are localized to both somata and neurites. Hence in spinal motor neurons, there is expression of sortilin protein along with p75.
Figure 1. Expression of sortilin by motor neurons.
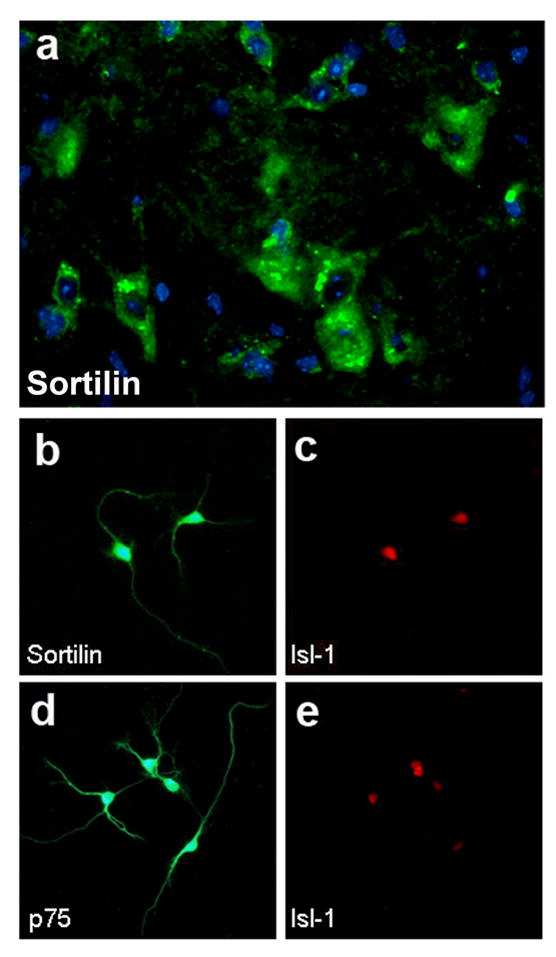
a) Transversal section of spinal cord from 5 month-old wild-type immunostained with antibody against sortilin (green) and Hoechst 33342 (blue). High power view within the lumbar ventral horn. b–c) E14.5 motor neurons 1 d.i.v. immunostained for sortilin (b) and the motor neuron marker Isl-1 (c). d–e) E14.5 motor neurons 1 d.i.v. immunostained for p75 (d) and the motor neuron marker Isl-1 (e).
Pro-neurotrophins induce cell death in cultured spinal motor neurons
The observation that motor neurons express sortilin and p75 raises the possibility that they may complex with pro-neurotrophins to mediate neuronal death. Therefore we assessed the effects of recombinant pro-neurotrophins on rat embryonic E14.5 spinal motor neurons cultures (Camu and Henderson 1992). We first sought to determine the optimal time course for our experiments, thus the motor neurons were cultured for 7 days under control conditions and their viability was assessed daily by counting Calcein AM positive cells extending neurites (Figure 2a). Cultures were maintained at all times in the presence of appropriate concentrations of neurotrophic factors (Henderson et al. 1993; Henderson et al. 1994; Sendtner et al. 1996; Raoul et al. 1999). The same neurotrophic factor cocktail was also included in the media for all subsequent experiments. Approximately 56% of the plated neurons survived the first day in vitro and their numbers remained constant for the following six days (Figure 2c). Based on these results, we adopted an experimental paradigm where motor neurons were plated and cultured for 24 hours before being exposed to test conditions for an additional 48 hours.
Figure 2. Viability of embryonic spinal motor neurons in culture.
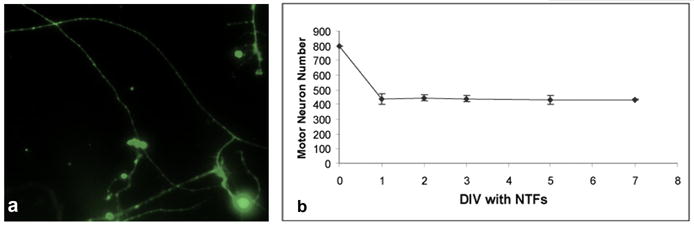
a) E14.5 motor neurons were visualized with Calcein AM after 48 hours incubation under control conditions. b) Viability of E14.5 SMNs in culture was monitored for 7 days by Calcein AM staining. The graph show the averages of four independent experiments.
His-tagged proNGF was produced using a baculovirus expression system and purified using Ni-bead chromatography as described in Teng et al. 2005. A point mutation at the furin-cleavage site was introduced to avoid intracellular processing and to promote the secretion of the precursor species (Lee et al. 2001). Because it was previously demonstrated that 24 hours are sufficient for the induction of apoptosis in superior cervical ganglia neurons by proNGF (Nykjaer et al. 2004), we stained motor neuron cultures with a nucleic acid dye, Hoechst 33334, to visualize condensed chromatin after 24 hours treatment. Addition to the culture media of recombinant proNGF resulted in a 2.4-fold increase in the number of apoptotic nuclei as compared to control conditions (Figure 3a). The pro-domain of NGF (amino acids E19 to R121) expressed as a glutathione S-transferase fusion protein (GST-pro), but not GST alone, selectively prevents binding to sortilin and it abolished the effects of proNGF (Figure 3a). Meanwhile, neither GST nor GST-pro had any effect under control conditions.
Figure 3. ProNGF treatment induces spinal motor neurons apoptosis in culture via a mechanism mediated by p75 and sortilin.
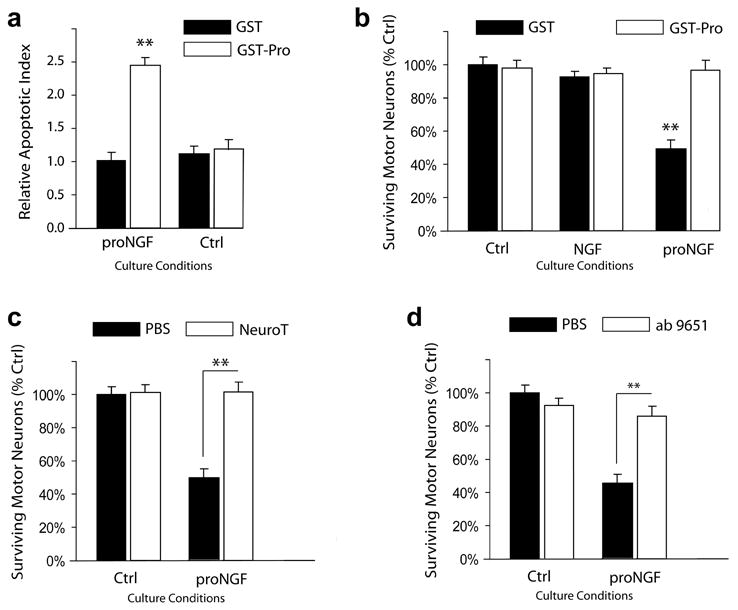
a) Induction of motor neuron apoptosis after 24 hours treatment was measured by Hoechst staining and analysis of fragmented nuclei and condensed chromatin. Neither pro-GST nor GST alone induced neuronal apoptosis. b) Motor neuron viability after 48 hours treatment was measured by Calcein AM staining. ProNGF (2 ng/ml), but not mature NGF, reduced motor neuron survival. The simultaneous addition of GST-pro, but not GST alone, eliminated motor neuron death. c) Co-incubation with neurotensin (10 μM) abrogated the proNGF apoptotic activity. d) Co-incubation with a function blocking anti-p75 antibody abrogated the proNGF apoptotic activity. Motor neurons were cultured at all times in complete media supplemented with neurotrophic factors. Experiments were conducted in quadruplicates. Shown are averages of 3 independent experiments. Statistical significance was determined by one-way ANOVA and Tukey’s test. ** = p<0.001.
After 48 hours of treatment, we measured neuronal survival by calcein AM uptake. The results, shown in Figure 3b, indicated that the addition of proNGF resulted in a 49% decrease in motor neuron survival as compared to control conditions. As in the previous experiment, GST-pro, but not GST alone, abolished the effects of proNGF while having no effect on survival under control conditions. Moreover, addition of mature NGF did not have an effect on motor neuron survival (Fig. 3b). Thus, proNGF can induce motor neuron cell death in culture and neither of its domains, pro or mature, is sufficient by itself for this activity.
ProNGF-induced motor neuron apoptosis requires sortilin and p75
To investigate the mechanism by which proNGF induces motor neuron death, we tested for the involvement of sortilin and p75. Neurotensin competes with proNGF for binding to sortilin and also prevents proNGF-induced neuronal apoptosis (Nykjaer et al. 2004; Teng et al. 2005). In fact, treatment with neurotensin reduced the number of apoptotic nuclei (data not shown) and improved cell survival (Figures 3c) in response to proNGF. Importantly, neurotensin alone did not have an effect on neuronal survival (Fig. 3c).
To asses the role of p75, we treated motor neurons with an antibody against p75 which blocks NGF binding (Huber and Chao 1995). Addition of the antibody prevented the proNGF-induced decline in neuronal survival (Fig. 3d). Taken together, these results indicate that proNGF effects on motor neuron survival require the expression of both sortilin and p75.
Pro-neurotrophins production by astrocytes
Reactive glia has been shown to secrete neurotrophic factors in response to various stimuli (Ridet et al. 1997; Yoshida and Toya 1997, Toyomoto, 2004 #49, Aschner, 1998 #98) In particular, astrocytes were shown to up-regulate synthesis of NGF following treatment with LPS (Galve-Roperh et al. 1997; Xiong et al. 1999), glutamate (Wu et al. 2004) and nitric oxide donors, such as SIN1 and peroxynitrite (Tanaka et al. 1999; Cassina et al. 2001), and also after injury (Yu et al. 1996; Goss et al. 1998; Micera et al. 1998; Krenz and Weaver 2000). Recently, induction of proNGF in vivo was shown in GFAP-positive cells within the basal forebrain following kainic acid seizures {Volosin, 2006 #127}.
To determine whether astrocytes are a source of proNGF, we isolated astrocytes from spinal cords of newborn rats and stimulated them with nitric oxide donors. Since both peroxynitrite and SIN1 have an extremely short half-life at neutral pH, stimulations were performed by rapid addition of the stock solutions to the dish while swirling to mix the solution. Each treatment was repeated three times to ensure the exposure of the cells to the active reagents. The same protocol was followed in the control experiments where cells were either treated with PBS or a degraded peroxynitrite solution.
Cultured astrocytes, with or without stimulation, were immunostained with an antibody against the pro-domain of proNGF (Harrington et al. 2004). The results show a strong induction of proNGF synthesis following peroxynitrite treatment (Fig. 4b) while immunoreactivity was absent under control conditions (Fig. 4a).
Figure 4. Stimulated astrocytes production of proNGF.
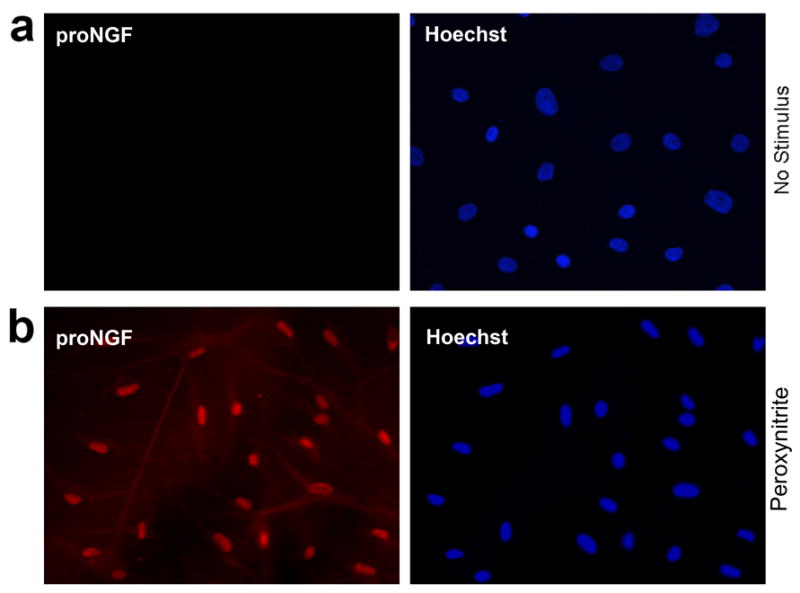
Cultured astrocytes, untreated (a) or treated with 1 mM peroxynitrite (b) were immunostained for the pro-domain of proNGF (red) and counterstained with Hoechst 33342.
Because proNGF immunoreactivity could simply be an indication of NGF production, we analyzed the growth media following stimulation. Western blot analysis of astrocyte-conditioned media indicated that spinal astrocytes in culture up-regulated secretion of proNGF in response to peroxynitrite (1 mM, Fig. 5 – Ln1) and the nitric oxide donor SIN-1 (100 μM, Fig. 5 – Ln2). The stimulation by peroxynitrite and SIN-1 resulted in the production of a 30kD NGF species (arrowhead) which co-migrates with the purified proNGF loaded as control (Fig. 5 – Ln 5 and 6). No induction was observed in response to degraded peroxynitrite or vehicle. To verify that similar cell numbers were stimulated in each treatment, the cultures were lysed after collection of the conditioned media. Equal amounts of lysates were separated by SDS/PAGE and probed with an antibody against tubulin (Fig. 5). We conclude that activated astrocytes can secrete unprocessed proNGF to the media.
Figure 5. Stimulated astrocytes secretion of proNGF.
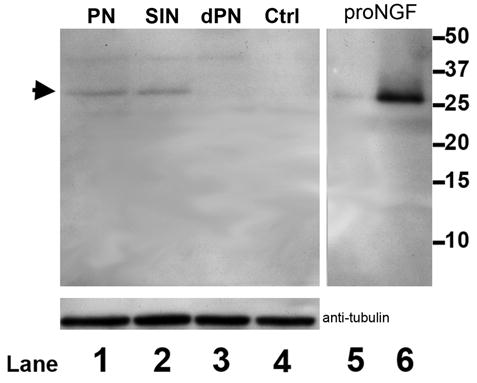
Media from astrocytes cultures treated with peroxynitrite (1 mM, Ln 1), SIN1 (100 μM, Ln 2), degraded peroxynitrite (Ln 3) or vehicle control were subjected to SDS-PAGE. Membranes were probed with an antibody against the pro-domain of proNGF. Recombinant proNGF was loaded as control in lanes 5 and 6, 0.2 and 2 μg respectively. Arrowhead points to the high molecular weight (approx. 30 kD) proNGF species. Lysates from the astrocyte cultures were immunoblotted for tubulin to verify comparable cell numbers.
Pro-neurotrophins secreted by astrocytes induce motor neuron death in culture
We proceeded to test whether the proNGF secreted by stimulated astrocytes was biologically active. Astrocyte-conditioned Neurobasal media was used to prepare motor neuron media by adding B27 and neurotrophic factors as required. The media was then used to treat spinal motor neuron cultures and to determine whether proNGF secreted by the astrocytes can induce motor neuron death in culture. The media conditioned by astrocytes, which had been stimulated with either peroxynitrite (PN) or SIN1, promoted a decrease in motor neuron survival, 54% and 51% respectively (Fig. 6a). The selective removal of proNGF from the media by immuno-depletion with an antibody specific for the pro-domain of proNGF (Harrington et al. 2004) significantly reduced the apoptotic effects of the conditioned media (Fig. 6b). Consistent with previous findings, addition of neurotensin reversed the detrimental effects of proNGF on motor neuron survival (Fig. 6c). These data supports the hypothesis that, following stimulation with strong oxidizing agents, astrocytes can secrete proNGF in sufficient amounts to bind sortilin and induce motor neuron death.
Figure 6. Astrocyte conditioned media induces motor neuron death in culture.
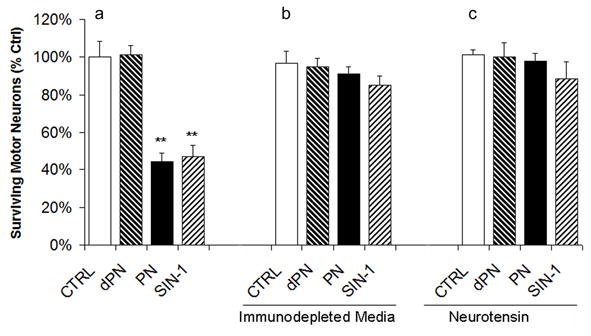
a) Conditioned media from stimulated astrocyte cultures (PN: peroxynitrite; SIN: SIN1; dPN: degraded PN) were used to prepare motor neuron media. The media were supplemented with neurotrophic factors immediately prior to use. Survival of motor neurons was quantified 48 hours after application of the astrocyte-conditioned MN media. b) Prior to survival experiments, astrocyte-conditioned MN media were immuno-depleted using an antibody against the pro-domain of proNGF. c) To confirm that the reduction in MN survival was due to the activation of sortilin, neurotensin was added to the neuronal cultures.
Discussion
The neurotrophins, nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3), regulate the development, maintenance and survival of cells in the nervous system (Chao 2003; Teng and Hempstead 2004). They are synthesized as precursor forms (proNTs: proNGF, proBDNF and proNT-3), which are either secreted from cells or cleaved intracellularly into mature neurotrophin dimers (Lee et al. 2001; Chao and Bothwell 2002). Neurotrophins are induced in response to injury and play a role in the degeneration and regeneration of the CNS (Isackson 1995), but, until recently, only the mature forms were considered biologically active. Evidence has now emerged to indicate new roles for unprocessed neurotrophins in neural cell death {Beattie, 2002 #80; Harrington, 2004 #69; Lee, 2001 #15; Nykjaer, 2004 #46; Teng, 2005 #55; Volosin, 2006 #127}. These pro-apoptotic activities were shown to depend upon binding a dual receptor complex consisting of sortilin and p75 that facilitated the binding of proneurotrophins (Nykjaer et al. 2004).
Interestingly, both p75 (Turner et al. 2004) and neurotrophins (Pehar et al. 2004) have also been implicated in motor neuron dysfunction. Pehar et. al. demonstrated that reactive astrocytes can induce p75-dependent motor neuron death by secreting NGF. Since the reagents used in these experiments were specific for the mature domain of NGF, an obvious question raised by the findings is whether the pro-apoptotic activity was mediated by mature NGF or proNGF. In this study, we demonstrate that proNGF effectively promotes motor neuron death at a concentration 50-fold lower than the one previously reported for NGF (100 ng/ml). We also observed that in the presence of proNGF, although viable, the Calcein AM positive motor neurons exhibited fewer and shorter processes, possibly due to the concomitant activation of a p75-mediated pathway which inhibits neurite outgrowth (Wang et al. 2002). This observation will be the subject of future analysis.
In contrast to previous reports (Sedel et al. 1999; Pehar et al. 2004), recombinant mature NGF did not affect motor neuron survival. A possible explanation for the previous findings may lay in trace amounts of proNGF still present in natural NGF preparations following its purification from mouse submaxillary glands (Supplementary Fig. S1b).
Consistent with previous studies, stimulation of cultured astrocytes with peroxynitrite resulted in the upregulation of neurotrophin synthesis. Although NGF immunoreactivity has been detected in astrocyte-conditioned media (Pehar et al. 2004), the molecular weight of the species had not been documented. We found evidence that proNGF is secreted by reactive astrocytes (Fig. 5). Our results indicate that motor neuron survival is sensitive to astrocyte-conditioned media. This response could be prevented by depletion of proNGF or by blocking sortilin (Fig. 6), thereby confirming that motor neuron survival is affected by proNGF and not by the mature NGF form.
Proteolytic processing of proNGF determines the molecular form and activity of secreted NGF (Lee et al. 2001; Chao and Bothwell 2002). Since proteolytic processing is altered in many neurological disorders (Rosenberg 2002) neurotrophic factor functions may be altered to influence disease progression. For example, increased levels of proNGF have been detected in Alzheimer’s disease patients (Peng et al. 2004; Pedraza et al. 2005), but the cellular sources were not fully identified. Whether the abnormal proNGF secretion by astrocytes is the result of decreased protease activity or a general increase in neurotrophin synthesis requires further investigation. Also unclear is whether a mechanism for the control of neurotrophic and neurotoxic events by astrocytes is deregulated during pathological conditions in vivo. However, our data suggest that, under abnormal conditions and following re-expression of p75, spinal motor neuron survival may be affected by proNGF secreted by reactive astrocytes. Further understanding of this mechanism may contribute to the development of therapeutic tools aimed at delaying the progression of motor neuron diseases.
Material and Methods
Reagents
Mouse monoclonal antibodies against glial fibrillary acidic protein (GFAP, clone GA5), CD11b (clone OX-42), beta III tubulin (clone Tu-20), choline acetyltransferase (ChAT, clone 1E6) and p75 (clone IgG-192) were purchased from Chemicon International. Anti-sortilin rabbit antibody was a gift from Dr. Nykjaer (Aarhus University, Denmark). Monoclonal antibody against Islet-1 (clone 39.4D5) was purchased from Developmental Studies Hybridoma Bank (University of Iowa). Antibodies against Trk (C14), Akt (B1) and NGF (M20) were purchased from Santa Cruz Biotechnologies. Antibodies against phospho-TrkA (Y490), phospho-Akt (Ser473), phospho-p42/44 MAPK (Thr202/Tyr204), p42/44 MAPK and cleaved caspase-3 (Asp175) were purchased from Cell Signaling. Alexa Fluor-conjugated secondary antibodies, Hoechst 33342 and Calcein AM were purchased from Molecular Probes.
Recombinant human NGF was purchased from Calbiochem. The product was tested for activity (Supplementary Fig. S1a) as well as for trace amounts of proNGF (Supplementary Fig. S1b).
Peroxynitrite (active and degraded) was purchased from Upstate. Prior to experimentation, peroxynitrite was quantitated spectrophotometrically (extinction coefficient at 302 nm = 1670 M−1 cm−1) (Radi et al. 1991).
All other reagents were purchased from Sigma unless otherwise stated.
Purification and culture of motor neurons
Spinal motor neuron cultures prepared from E14.5 Sprague Dawley rats (Charles River Labs) as previously described (Camu and Henderson 1992; Arce et al. 1999). Briefly, dissected spinal cords were digested in 0.025% trypsin (Gibco) for 8 minutes at 37°C. The tissue was transferred to a solution containing L-15 medium (Gibco) supplemented with 2% horse serum (Gibco), insulin (5 μg/ml); putrescine (1 × 10−4 M), conalbumin (100 μg/ml), sodium selenite (3 × 10−8 M), progesterone (2 × 10−8 M), glucose (3.6 mg/ml), penicillin (100 IU/ml), streptomycin (100 μg/ml), DNAse (100 μg/ml) and bovine serum albumin (BSA; 0.4%). The tissue was then triturated using a 1-ml pipetman and the suspension was layered over a cushion of 10.4% Optiprep (Nycomed Pharma) in L-15 in a 15-ml Falcon tube. The layered suspension was centrifuged at 830 × g for 15 min. The cells at the interface were suspended in PBS containing 0.5% BSA and separated by immunoaffinity using the IgG-192 p75-specific antibody followed by cell sorting using magnetic microbeads (Miltenyi Biotec) to purify the motor neurons. The motor neurons medium consists of neurobasal medium (Gibco) supplemented with B27 supplement (Gibco), glutamate (25 μM), 2-mercaptoethanol (25μM) and 2% horse serum. The motor neurons were plated onto laminin-coated coverslips at a density of 3000 cells/coverslip unless otherwise stated. A cocktail of neurotrophic factors (NTFs: 1 ng/ml BDNF, 100 pg/ml GDNF, 10 ng/ml CNTF) was added at the time of cell seeding. After 16 hr in culture, motor neurons were treated by addition of the different reagents diluted in motor neurons medium supplemented with NTFs.
Purification and culture of spinal astrocytes
Spinal astrocytes were isolated from P0–P2 Sprague Dawley rats as previously described (Saneto and De Vellis 1987). Spinal cords were dissociated in 0.25% trypsin (Gibco) for 30 min at 37°C and triturated with a 5-ml pipette. Cells were collected by centrifugation, filtered through a 70 μm mesh cell dissociation sieve, and plated in 75 cm2 culture flasks (Falcon) that were pre-coated with PDL at a density of 2 × 107 cells/flask. Astrocyte medium contains Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, penicillin (100 IU/ml), and streptomycin (100 g/ml). After 5 days, the astroglial layer was confluent and the flasks were rinsed with phosphate-buffered saline (PBS) and shaken for 24 hours at 180 rpm at 37°C to remove contaminating non-astroglial cells. The medium was replaced with fresh astrocyte medium and cytosine arabinoside (20 μM) was added to inhibit fibroblast contamination. After 48 hour the media was replaced with astrocyte media and the cells were allowed to recover for 24 hours before repeating the procedure one more time. The resulting cultures were >98% pure as determined by glial fibrillary acidic protein (GFAP) immunoreactivity and devoid of CD11b-positive microglia (data not shown).
Astrocyte conditioned media
Astrocytes were washed with phosphate-buffered saline (PBS) and then equilibrated in PBS for 5 min. PBS, rather than Neurobasal media, was used as the medium for treatments to avoid interfering reactions of media constituents. Peroxynitrite (1 mM), SIN-1 (100 μM), degraded peroxynitrite or vehicle were delivered as a single bolus against one side of the dish while rapidly swirling the medium to guarantee optimal exposure of the cells before decomposition (van der Vliet et al. 1998). The wash/stimulation cycle was repeated three times. The final wash was replaced with unsupplemented Neurobasal medium and the cells were cultured at 37°C for 48 hours prior to collection of the media. Conditioned media was used immediately in PAGE/Western blotting experiments or to prepare motor neuron media. The astrocyte-conditioned motor neuron media were stored at −80°C and supplemented with NTFs prior to use with motor neuron cultures.
Survival Assay
Spinal motor neurons were prepared from E14.5 rat embryos as described. Cells were cultured in the presence of NTFs for 24 hr and viability (V1) was estimated by counting the number of Calcein AM positive cells. For culture viability studies, motor neurons were cultured 7DIV in the presence of NTFs with one medium change at day 3. Viability was estimated at DIV 2,3,5 and 7 (V2–7). The percentage of viable motor neurons was calculated as follows: [(V2–7)/(V1)] × 100. For toxicity experiments, the indicated reagents were added to cultures at DIV 1 and motor neurons were cultured for additional 48 hours before measurement of viability. In all cases, NTFs were maintained for the duration of the experiments.
Statistical Analysis
The number of surviving motor neurons at DIV 3 for each treatment was expressed as a percentage of the number of motor neurons surviving at DIV 1 in the presence of NTFs alone. Quadruplicate dishes were used for each condition. Differences between treatments were analyzed for their statistical significance by one-way ANOVA with post-hoc Tukey’s testing.
Cryosection preparation and immunolabeling
Adult wild-type mice (5 months) were perfused with PBS and then 4% (w/v) paraformaldehyde in PBS. Lumbar spinal cords were dissected, post-fixed 2 hours in 4% (w/v) paraformaldehyde and cryoprotected with 30% (w/v) sucrose in PBS overnight before embedding in optimal cutting temperature medium by freezing in isopentane cooled on dry ice. Cryosections (12 μm) were cut using a Leica cryostat and mounted onto glass slides (Superfrost Plus; Fisher Scientific). The sections were rinsed in PBS and blocked with 3% donkey serum, 3% BSA and 0.3% TritonX-100 in PBS for 30 min at RT. Sections were immunostained with rabbit anti-sortilin antibody (1:200) and counterstained with Hoechst 33342 followed by washing three times in PBS and mounting with Vectashield. Immunostaining was viewed on a Nikon E800 fluorescence microscope and digital images were obtained using CCD camera and Axioplot software.
Immunostaining of astrocytes and spinal motor neuron cultures
Coverslips were rinsed PBS and then fixed in 4% paraformaldehyde for 10 min at room temperature. The coverslips were rinsed in PBS three times, incubated for 30 min with 3% donkey serum, 3% BSA and 0.3% TritonX-100 for blocking of nonspecific binding and permeabilization, rinsed three times in PBS, and incubated overnight at 4°C with primary antibodies diluted at 1:500 in blocking buffer. They were rinsed in PBS and incubated with secondary antibodies, donkey anti-mouse Alexa 594 and donkey anti-rabbit Alexa 488 diluted at 1:300, for 1 hour at room temperature. Finally, the coverslips were rinsed and mounted with Vectashield onto a glass slide. Immunostaining was viewed on a Nikon E800 UV Fluorescence microscope and digital images were obtained using CCD camera and Axioplot software.
Western blotting
Aliquots of astrocyte-conditioned Neurobasal media were collected on ice and supplemented with sodium orthovanadate (100 μM) and protease inhibitors aprotinin (10 μg/ml), PMSF (1 mM) and leupeptin (20 μg/ml). Samples were centrifuged at 12,000 × g for 10 minutes to remove cellular debris and concentrated approximately 10-fold using Centriprep YM-3 spin columns (Millipore). Protein concentration in the retentate was determined by DC-Protein Assay (Bio-Rad). Proteins were separated in 12% SDS-PAGE, transferred to Immobilon-P membranes (Millipore) and blocked for 1 hour at room temperature in TBS with 0.1% Tween 20 and 5% BSA. Membranes were incubated with anti-proNGF antibody (1:1000 in TBS-T) at 4°C overnight. After washing in TBS-T, membranes were incubated with HRP-conjugated anti-rabbit antibody (1:7500 in TBS-T) at room temperature for 1 hour. For detection, an ECL chemiluminescence system (Amersham-Pharmacia) was used in accordance with the manufacturer’s instructions. After collection of the conditioned media, protein extracts were obtained from the astrocyte cultures, separated on 12% SDS-PAGE and transferred to membranes which were probed with anti-tubulin antibody (1:5000 in TBS-T) to confirm that cell numbers were comparable among all treatment conditions.
Immunodepletion of proNGF
Aliquots (5 ml) of astrocyte-conditioned Neurobasal media were collected on ice and centrifuged at 12,000 × g for 10 minutes to remove cellular debris. Supernatants were incubated with 25 μg each of anti-proNGF antibody or 50 μg of control antibody (rabbit anti-mouse Ig-G) in the presence of 100 μl 50% protein A agarose beads slurry for 4 hours at 4°C with gentle mixing. The beads were collected by centrifugation at 1000 × g at 4°C, and the supernatant was saved for immunoblotting and preparation of motor neuron media. After removal of the supernatant, the resin was washed three times with wash buffer (20 mM Tris-HCl pH 8, 200 mM NaCl, 0.2 mM EDTA, 10% glycerol, and 0.1% IGEPAL) and boiled in 1× SDS gel loading buffer. Control and proNGF-depleted extracts, as well as the immunoprecipitated material, were analyzed by Western blotting. As an additional control of depletion efficiency, 5 ml astrocyte-conditioned Neurobasal media containing 10 μg proNGF was subjected to the same depletion protocol and immunoblotting.
Supplementary Material
Analysis of NGF purity and activity.
a) PC12-615 cells were plated in poly-D-lysine coated wells until 75% confluency. Cells were then serum-starved for 24 hours, stimulated for 10 minutes with NGF from different sources and lysed in SDS sample buffer. Equal amounts of each lysate were separated by PAGE and western blotted for TrkA, Akt, MAPK, phospho-TrkA, phospho-Akt and phospho-MAPK. We tested recombinant human NGF (rhNGF) from Calbiochem and two lots of purified mouse NGF from Harlan. All lots tested successfully activated TrkA. b–c) Aliquots of each sample of NGF were separated by PAGE and western blotted with an antibody against mature NGF (b) or against the pro-domain of proNGF (c). High molecular weight bands immunoreactive for proNGF were observed in both the mouse lots, but were absent from the rhNGF samples.
ProNGF induces the cleavage of caspase-3 in cultured motor neurons.
To investigate the participation of activated caspase-3 in the events induced by proNGF in cultured spinal motor neurons, we used immunofluorescence with antibodies that recognize only the large fragment (17/19 kDa) of active caspase-3. Cultured motor neurons treated with proNGF for 24 hours were immunostained with antibodies to both ChAT (b, red) and active caspase-3 (c, green), and chromatin was viewed with Hoechst 33334 (a, blue). After proNGF treatment, caspase-3 was cleaved in motor neurons that had fragmented nuclei (arrowheads). Healthy motor neurons did not show caspase-3 activity.
Acknowledgments
This work was supported by National Institutes of Health Grants NS21072 and HD023315, and The Robert Packard Center for ALS Research at Johns Hopkins University. We thank Michael Sendtner and Chris Henderson for graciously sharing their time and protocols with us. We acknowledge the advice and help of Juan Carlos Arevalo and Mercedes Beyna. We thank Risa Torkin and Ramiro Almeida for assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Marco Domeniconi, Molecular Neurobiology Program, Skirball Institute of Biomolecular Medicine, Departments of Cell Biology, Physiology and Neuroscience, New York University School of Medicine, 540 First Avenue, New York, New York 10016, USA.
Barbara L. Hempstead, Division of Hematology Oncology, Weill Medical College of Cornell University, 1300 York Avenue, New York, New York 10021, USA
Moses V. Chao, Molecular Neurobiology Program, Skirball Institute of Biomolecular Medicine, Departments of Cell Biology, Physiology and Neuroscience, New York University School of Medicine, 540 First Avenue, New York, New York 10016, USA
References
- Arce V, et al. Cardiotrophin-1 requires LIFRbeta to promote survival of mouse motoneurons purified by a novel technique. J Neurosci Res. 1999;55(1):119–26. doi: 10.1002/(SICI)1097-4547(19990101)55:1<119::AID-JNR13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Beattie MS, et al. ProNGF induces p75-mediated death of oligodendrocytes following spinal cord injury. Neuron. 2002;36(3):375–86. doi: 10.1016/s0896-6273(02)01005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE. Neuron-astrocyte interactions: partnership for normal function and disease in the central nervous system. Mayo Clin Proc. 2005;80(10):1326–38. doi: 10.4065/80.10.1326. [DOI] [PubMed] [Google Scholar]
- Bothwell M. Functional interactions of neurotrophins and neurotrophin receptors. Annu Rev Neurosci. 1995;18:223–53. doi: 10.1146/annurev.ne.18.030195.001255. [DOI] [PubMed] [Google Scholar]
- Camu W, Henderson CE. Purification of embryonic rat motoneurons by panning on a monoclonal antibody to the low-affinity NGF receptor. J Neurosci Methods. 1992;44(1):59–70. doi: 10.1016/0165-0270(92)90114-s. [DOI] [PubMed] [Google Scholar]
- Casaccia-Bonnefil P, et al. Death of oligodendrocytes mediated by the interaction of nerve growth factor with its receptor p75. Nature. 1996;383(6602):716–9. doi: 10.1038/383716a0. [DOI] [PubMed] [Google Scholar]
- Cassina P, et al. Adaptative responses of spinal astrocytes to oxidative stress. Prog Brain Res. 2001;132:413–25. doi: 10.1016/S0079-6123(01)32092-7. [DOI] [PubMed] [Google Scholar]
- Chao M, et al. Neurotrophin receptors: mediators of life and death. Brain Res Brain Res Rev. 1998;26(2–3):295–301. doi: 10.1016/s0165-0173(97)00036-2. [DOI] [PubMed] [Google Scholar]
- Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4(4):299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- Chao MV, Bothwell M. Neurotrophins: to cleave or not to cleave. Neuron. 2002;33(1):9–12. doi: 10.1016/s0896-6273(01)00573-6. [DOI] [PubMed] [Google Scholar]
- Chen ZY, et al. Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. J Neurosci. 2005;25(26):6156–66. doi: 10.1523/JNEUROSCI.1017-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu AY, et al. A motor neuron-specific epitope and the low-affinity nerve growth factor receptor display reciprocal patterns of expression during development, axotomy, and regeneration. J Comp Neurol. 1993;328(3):351–63. doi: 10.1002/cne.903280303. [DOI] [PubMed] [Google Scholar]
- Copray JC, et al. Expression of the low affinity neurotrophin receptor p75 in spinal motoneurons in a transgenic mouse model for amyotrophic lateral sclerosis. Neuroscience. 2003;116(3):685–94. doi: 10.1016/s0306-4522(02)00755-8. [DOI] [PubMed] [Google Scholar]
- Ernfors P, et al. Expression of nerve growth factor receptor mRNA is developmentally regulated and increased after axotomy in rat spinal cord motoneurons. Neuron. 1989;2(6):1605–13. doi: 10.1016/0896-6273(89)90049-4. [DOI] [PubMed] [Google Scholar]
- Ferri CC, et al. Effects of facial nerve injury on mouse motoneurons lacking the p75 low-affinity neurotrophin receptor. J Neurobiol. 1998;34(1):1–9. [PubMed] [Google Scholar]
- Galve-Roperh I, et al. Regulation of nerve growth factor secretion and mRNA expression by bacterial lipopolysaccharide in primary cultures of rat astrocytes. J Neurosci Res. 1997;49(5):569–75. doi: 10.1002/(SICI)1097-4547(19970901)49:5<569::AID-JNR7>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Goss JR, et al. Astrocytes are the major source of nerve growth factor upregulation following traumatic brain injury in the rat. Exp Neurol. 1998;149(2):301–9. doi: 10.1006/exnr.1997.6712. [DOI] [PubMed] [Google Scholar]
- Harrington AW, et al. Secreted proNGF is a pathophysiological death-inducing ligand after adult CNS injury. Proc Natl Acad Sci U S A. 2004;101(16):6226–30. doi: 10.1073/pnas.0305755101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heales SJ, et al. Neurodegeneration or neuroprotection: the pivotal role of astrocytes. Neurochem Res. 2004;29(3):513–9. doi: 10.1023/b:nere.0000014822.69384.0f. [DOI] [PubMed] [Google Scholar]
- Hempstead BL, et al. High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature. 1991;350(6320):678–83. doi: 10.1038/350678a0. [DOI] [PubMed] [Google Scholar]
- Henderson CE, et al. Neurotrophins promote motor neuron survival and are present in embryonic limb bud. Nature. 1993;363(6426):266–70. doi: 10.1038/363266a0. [DOI] [PubMed] [Google Scholar]
- Henderson CE, et al. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994;266(5187):1062–4. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- Huber LJ, Chao MV. A potential interaction of p75 and trkA NGF receptors revealed by affinity crosslinking and immunoprecipitation. J Neurosci Res. 1995;40(4):557–63. doi: 10.1002/jnr.490400415. [DOI] [PubMed] [Google Scholar]
- Isackson PJ. Trophic factor response to neuronal stimuli or injury. Curr Opin Neurobiol. 1995;5(3):350–7. doi: 10.1016/0959-4388(95)80048-4. [DOI] [PubMed] [Google Scholar]
- Koliatsos VE, et al. Neurotrophin 4/5 is a trophic factor for mammalian facial motor neurons. Proc Natl Acad Sci U S A. 1994;91(8):3304–8. doi: 10.1073/pnas.91.8.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koliatsos VE, et al. Axotomy induces nerve growth factor receptor immunoreactivity in spinal motor neurons. Brain Res. 1991;549(2):297–304. doi: 10.1016/0006-8993(91)90471-7. [DOI] [PubMed] [Google Scholar]
- Kraemer R. Reduced apoptosis and increased lesion development in the flow-restricted carotid artery of p75(NTR)-null mutant mice. Circ Res. 2002;91(6):494–500. doi: 10.1161/01.res.0000035245.83233.2a. [DOI] [PubMed] [Google Scholar]
- Krenz NR, Weaver LC. Nerve growth factor in glia and inflammatory cells of the injured rat spinal cord. J Neurochem. 2000;74(2):730–9. doi: 10.1046/j.1471-4159.2000.740730.x. [DOI] [PubMed] [Google Scholar]
- Kust BM, et al. Reduced p75NTR expression delays disease onset only in female mice of a transgenic model of familial amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2003;4(2):100–5. [PubMed] [Google Scholar]
- Lee R, et al. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294(5548):1945–8. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Martin S, et al. Neurotensin receptor-1 and -3 complex modulates the cellular signaling of neurotensin in the HT29 cell line. Gastroenterology. 2002;123(4):1135–43. doi: 10.1053/gast.2002.36000. [DOI] [PubMed] [Google Scholar]
- Mazella J, et al. The 100-kDa neurotensin receptor is gp95/sortilin, a non-G-protein-coupled receptor. J Biol Chem. 1998;273(41):26273–6. doi: 10.1074/jbc.273.41.26273. [DOI] [PubMed] [Google Scholar]
- Micera A, et al. Changes of NGF presence in nonneuronal cells in response to experimental allergic encephalomyelitis in Lewis rats. Exp Neurol. 1998;154(1):41–6. doi: 10.1006/exnr.1998.6864. [DOI] [PubMed] [Google Scholar]
- Nielsen MS, et al. Sortilin/neurotensin receptor-3 binds and mediates degradation of lipoprotein lipase. J Biol Chem. 1999;274(13):8832–6. doi: 10.1074/jbc.274.13.8832. [DOI] [PubMed] [Google Scholar]
- Nielsen MS, et al. The sortilin cytoplasmic tail conveys Golgi-endosome transport and binds the VHS domain of the GGA2 sorting protein. Embo J. 2001;20(9):2180–90. doi: 10.1093/emboj/20.9.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykjaer A, et al. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427(6977):843–8. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW, et al. Developing motor neurons rescued from programmed and axotomy-induced cell death by GDNF. Nature. 1995;373(6512):344–6. doi: 10.1038/373344a0. [DOI] [PubMed] [Google Scholar]
- Pedraza CE, et al. Pro-NGF isolated from the human brain affected by Alzheimer’s disease induces neuronal apoptosis mediated by p75NTR. Am J Pathol. 2005;166(2):533–43. doi: 10.1016/S0002-9440(10)62275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehar M, et al. Astrocytic production of nerve growth factor in motor neuron apoptosis: implications for amyotrophic lateral sclerosis. J Neurochem. 2004;89(2):464–73. doi: 10.1111/j.1471-4159.2004.02357.x. [DOI] [PubMed] [Google Scholar]
- Peng S, et al. Increased proNGF levels in subjects with mild cognitive impairment and mild Alzheimer disease. J Neuropathol Exp Neurol. 2004;63(6):641–9. doi: 10.1093/jnen/63.6.641. [DOI] [PubMed] [Google Scholar]
- Petersen CM, et al. Molecular identification of a novel candidate sorting receptor purified from human brain by receptor-associated protein affinity chromatography. J Biol Chem. 1997;272(6):3599–605. doi: 10.1074/jbc.272.6.3599. [DOI] [PubMed] [Google Scholar]
- Pitts AF, Miller MW. Expression of nerve growth factor, p75, and trk in the somatosensory and motor cortices of mature rats: evidence for local trophic support circuits. Somatosens Mot Res. 1995;12(3–4):329–42. doi: 10.3109/08990229509093666. [DOI] [PubMed] [Google Scholar]
- Radi R, et al. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991;266(7):4244–50. [PubMed] [Google Scholar]
- Raoul C, et al. Programmed cell death of embryonic motoneurons triggered through the Fas death receptor. J Cell Biol. 1999;147(5):1049–62. doi: 10.1083/jcb.147.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridet JL, et al. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997;20(12):570–7. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA. Matrix metalloproteinases in neuroinflammation. Glia. 2002;39(3):279–91. doi: 10.1002/glia.10108. [DOI] [PubMed] [Google Scholar]
- Roux PP, et al. p75 neurotrophin receptor expression is induced in apoptotic neurons after seizure. J Neurosci. 1999;19(16):6887–96. doi: 10.1523/JNEUROSCI.19-16-06887.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saneto RP, De Vellis J. Neuronal and glial cells: cell culture of the central nervous system. In: Turner AJ, Brachelard HS, editors. Neurochemistry: a Practical Approach. Washington, DC: IRL Press; 1987. pp. 27–63. [Google Scholar]
- Sedel F, et al. Nerve growth factor (NGF) induces motoneuron apoptosis in rat embryonic spinal cord in vitro. Eur J Neurosci. 1999;11(11):3904–12. doi: 10.1046/j.1460-9568.1999.00814.x. [DOI] [PubMed] [Google Scholar]
- Seeburger JL, et al. Spinal cord motoneurons express p75NGFR and p145trkB mRNA in amyotrophic lateral sclerosis. Brain Res. 1993;621(1):111–5. doi: 10.1016/0006-8993(93)90304-6. [DOI] [PubMed] [Google Scholar]
- Sendtner M, et al. The response of motoneurons to neurotrophins. Neurochem Res. 1996;21(7):831–41. doi: 10.1007/BF02532307. [DOI] [PubMed] [Google Scholar]
- Sendtner M, et al. Brain-derived neurotrophic factor prevents the death of motoneurons in newborn rats after nerve section. Nature. 1992;360(6406):757–9. doi: 10.1038/360757a0. [DOI] [PubMed] [Google Scholar]
- Tanaka J, et al. Astrocytes prevent neuronal death induced by reactive oxygen and nitrogen species. Glia. 1999;28(2):85–96. doi: 10.1002/(sici)1098-1136(199911)28:2<85::aid-glia1>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Teng HK, et al. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci. 2005;25(22):5455–63. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng KK, Hempstead BL. Neurotrophins and their receptors: signaling trios in complex biological systems. Cell Mol Life Sci. 2004;61(1):35–48. doi: 10.1007/s00018-003-3099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrado J, et al. NGF-induced motoneuron cell death depends on the genetic background and motoneuron sub-type. Neuroreport. 2000;11(7):1473–7. [PubMed] [Google Scholar]
- Turner BJ, et al. Antisense peptide nucleic acid-mediated knockdown of the p75 neurotrophin receptor delays motor neuron disease in mutant SOD1 transgenic mice. J Neurochem. 2003;87(3):752–63. doi: 10.1046/j.1471-4159.2003.02053.x. [DOI] [PubMed] [Google Scholar]
- Turner BJ, et al. Effect of p75 neurotrophin receptor antagonist on disease progression in transgenic amyotrophic lateral sclerosis mice. J Neurosci Res. 2004;78(2):193–9. doi: 10.1002/jnr.20256. [DOI] [PubMed] [Google Scholar]
- Urschel BA, Hulsebosch CE. Distribution and relative density of p75 nerve growth factor receptors in the rat brain as a function of age and treatment with antibodies to nerve growth factor. Brain Res. 1992;591(2):223–38. doi: 10.1016/0006-8993(92)91702-g. [DOI] [PubMed] [Google Scholar]
- van der Vliet A, et al. Peroxynitrite induces covalent dimerization of epidermal growth factor receptors in A431 epidermoid carcinoma cells. J Biol Chem. 1998;273(48):31860–6. doi: 10.1074/jbc.273.48.31860. [DOI] [PubMed] [Google Scholar]
- Volosin M, et al. Interaction of survival and death signaling in basal forebrain neurons: roles of neurotrophins and proneurotrophins. J Neurosci. 2006;26(29):7756–66. doi: 10.1523/JNEUROSCI.1560-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KC, et al. P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature. 2002;420(6911):74–8. doi: 10.1038/nature01176. [DOI] [PubMed] [Google Scholar]
- Wiese S, et al. The role of p75NTR in modulating neurotrophin survival effects in developing motoneurons. Eur J Neurosci. 1999;11(5):1668–76. doi: 10.1046/j.1460-9568.1999.00585.x. [DOI] [PubMed] [Google Scholar]
- Wu H, et al. Differential regulation of neurotrophin expression in basal forebrain astrocytes by neuronal signals. J Neurosci Res. 2004;76(1):76–85. doi: 10.1002/jnr.20060. [DOI] [PubMed] [Google Scholar]
- Xie Y, et al. Expression and role of low-affinity nerve growth factor receptor (p75) in spinal motor neurons of aged rats following axonal injury. Dev Neurosci. 2003;25(1):65–71. doi: 10.1159/000071469. [DOI] [PubMed] [Google Scholar]
- Xiong H, et al. Regulation of nerve growth factor release by nitric oxide through cyclic GMP pathway in cortical glial cells. Mol Pharmacol. 1999;56(2):339–47. doi: 10.1124/mol.56.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, et al. Brain-derived neurotrophic factor rescues spinal motor neurons from axotomy-induced cell death. Nature. 1992;360(6406):753–5. doi: 10.1038/360753a0. [DOI] [PubMed] [Google Scholar]
- Yan Q, Johnson EM., Jr An immunohistochemical study of the nerve growth factor receptor in developing rats. J Neurosci. 1988;8(9):3481–98. doi: 10.1523/JNEUROSCI.08-09-03481.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Toya S. Neurotrophic activity in cytokine-activated astrocytes. Keio J Med. 1997;46(2):55–60. doi: 10.2302/kjm.46.55. [DOI] [PubMed] [Google Scholar]
- Yu J, et al. Altered NGF protein levels in different brain areas after immunolesion. J Neurosci Res. 1996;43(2):213–23. doi: 10.1002/(SICI)1097-4547(19960115)43:2<213::AID-JNR9>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Analysis of NGF purity and activity.
a) PC12-615 cells were plated in poly-D-lysine coated wells until 75% confluency. Cells were then serum-starved for 24 hours, stimulated for 10 minutes with NGF from different sources and lysed in SDS sample buffer. Equal amounts of each lysate were separated by PAGE and western blotted for TrkA, Akt, MAPK, phospho-TrkA, phospho-Akt and phospho-MAPK. We tested recombinant human NGF (rhNGF) from Calbiochem and two lots of purified mouse NGF from Harlan. All lots tested successfully activated TrkA. b–c) Aliquots of each sample of NGF were separated by PAGE and western blotted with an antibody against mature NGF (b) or against the pro-domain of proNGF (c). High molecular weight bands immunoreactive for proNGF were observed in both the mouse lots, but were absent from the rhNGF samples.
ProNGF induces the cleavage of caspase-3 in cultured motor neurons.
To investigate the participation of activated caspase-3 in the events induced by proNGF in cultured spinal motor neurons, we used immunofluorescence with antibodies that recognize only the large fragment (17/19 kDa) of active caspase-3. Cultured motor neurons treated with proNGF for 24 hours were immunostained with antibodies to both ChAT (b, red) and active caspase-3 (c, green), and chromatin was viewed with Hoechst 33334 (a, blue). After proNGF treatment, caspase-3 was cleaved in motor neurons that had fragmented nuclei (arrowheads). Healthy motor neurons did not show caspase-3 activity.


