Abstract
The benzothiophene SERMs raloxifene and arzoxifene, in the clinic or clinical trials for treatment of breast cancer and postmenopausal symptoms, are highly susceptible to oxidative metabolism and formation of electrophilic metabolites. 4′F-DMA, fluoro-substituted desmethyl arzoxifene (DMA), showed attenuated oxidation to quinoids in incubation with rat hepatocytes as well as in rat and human liver microsomes. Incubations of 4′F-DMA with hepatocytes yielded only one glucuronide conjugate and no GSH conjugates; whereas DMA underwent greater metabolism giving two glucuronide conjugates, one sulfate conjugate, and two GSH conjugates. Phase I and phase II metabolism was further evaluated in human small intestine microsomes and in human intestinal Caco-2 cells. In comparison to DMA, 4′F-DMA formed significantly less glucuronide and sulfate conjugates. The formation of quinoids was futher explored in hepatocytes in which DMA was observed to give concentration and time dependent depletion of GSH accompanied by damage to DNA which showed inverse dependence on GSH; in contrast, GSH depletion and DNA damage were almost completely abrogated in incubations with 4′F-DMA. 4′F-DMA shows ligand binding affinity to ERα and ERβ with similarity to both raloxifene and to DMA. ER-mediated biological activity was measured with the ERE-luciferase reporter system in transfected MCF-7 cells and Ishikawa cells, and in MCF-7 cells proliferation was measured. In all systems, 4′F-DMA exhibited anitestrogenic acitivty of comparable potency to raloxifene, but did not manifest estrogenic properties, mirroring previous results on inhibition of estradiol-mediated induction of alkaline phosphatase activity in Ishikawa cells. These results suggest that 4′F-DMA might be an improved benzothiophene SERM with similar antiestrogenic activity to raloxifene, but improved metabolic stability and attenuated toxicity; showing that simple chemical modification can abrogate oxidative bioactivation to potentially toxic metabolites without loss of activity.
Introduction
Hormone replacement therapy (HRT) remains a cornerstone of contemporary women’s healthcare, despite recent outcomes from the Women’s Health Initiative clinical trials questioning the benefits of HRT in light of the demonstrated risks, including increased breast cancer and stroke.(1,2) However, benefits of HRT were confirmed including reductions in the incidence of colon cancer, osteoporotic fractures, and menopausal symptoms. Estrogens play a central role in female reproduction and exert beneficial effects on the skeletal, cardiovascular, and central nervous systems. In clinical observations, postmenopausal women have a higher incidence of osteoporotic fractures, coronary heart disease (CHD), hot flashes, night sweats, and lower reported quality of life. There remains a role in women’s health for HRT, however, the place of the current agents, based upon conjugated equine estrogens, may be filled by selective estrogen receptor modulators (SERMs), particularly for women at high risk of breast cancer.
SERMs as a class of therapeutic agents and drug candidates evoke their actions through selective agonist and antagonist action at the estrogen receptor (ER). An ideal SERM would be tissue selective having pro-estrogenic or agonist activity in liver, bone, the cardiovascular, and central nervous systems whilst exerting antagonist or anti-estrogenic activity in the breast and uterus (3-6). The therapeutic objectives for a SERM of use in HRT include eliciting the desired estrogenic effects (e.g. alleviation of menopausal symptoms such as hot flashes, vaginal dryness, and affective or emotional symptoms), whilst not eliciting adverse effects such as uterine and breast carcinogenesis and venous thromboembolic events.
Tamoxifen is the prototype SERM (Scheme 1), displaying a mixed tissue-selective estrogen agonist/estrogen antagonist character, and is in use for the treatment and prevention of hormone-dependent breast cancer. Raloxifene (Evista®) is a benzothiophene SERM with improved tissue selectivity, approved for the treatment and prevention of postmenopausal osteoporosis (Scheme 1) (7). The National Cancer Institute supported “Study of Tamoxifen and Raloxifene” (STAR Trial) is currently being conducted comparing the safety and effectiveness of these two agents for the prevention of breast cancer in postmenopausal women (8). Subsequent generations of SERMs, such as arzoxifene and acolbifene, are in development (Scheme 1) (9). Recently, lasofoxifene, a new SERM structurally related to tamoxifen, received a non-approvable letter from the FDA for treatment of osteoporosis (10).
Scheme 1.
It has long been recognized that the carcinogenic actions of endogenous estrogens can result from chemical mechanisms in addition to hormonal actions (11). These chemical mechanisms involve oxidative metabolism to catechol and subsequently quinoid metabolites (12). The seminal work of Liehr on fluoroestradiols, in which catechol formation was impeded by chemical modification using ring substitution with fluorine, demonstrated decreased carcinogenicity relative to the endogenous estrogen (13,14). On the basis of ablation of the chemical carcinogenesis pathways, fluoroestradiols were even proposed as drug candidates for HRT. In simile with human estrogens, the equine estrogen equilenin is oxidatively metabolized to catechols and quinones, however, equilenin as a polyaromatic phenol is more susceptible to oxidation and able to form more stable electrophilic quinones that elicit cellular damage (15) . Clinically, the most widely used form of HRT consists of conjugated equine estrogens.
The active, oxidative metabolite, 4-hydroxytamoxifen, is a polyaromatic phenol that can be oxidized further to reactive intermediates including quinoids that have been observed to form adducts with glutathione (GSH) and nucleic acids (16). The detection of DNA adducts in women on tamoxifen therapy supports a genotoxic pathway resulting from oxidative bioactivation (17). Clinical data suggest that prolonged use of tamoxifen is associated with development of uterine cancer and an increase in the incidence of endometrial cancer (18-22). Furthermore, long term treatment of male and female rats with tamoxifen resulted in a dose related increase in the incidence of liver tumors (23). Later generation SERMs, including raloxifene, arzoxifene and acolbifene, are also polyaromatic phenols, susceptible to oxidative metabolism to quinoids (24-29). Oxidative bioactivation to eletrophilic, quinoid metabolites that can modify proteins and nucleic acids in addition to promoting oxidative stress has been reported in a number of studies (26,27,29). Arzoxifene has been reported to have a more favorable therapeutic and safety profile as compared to raloxifene, although the active metabolite of arzoxifene, desmethylated arzoxifene (DMA), can be oxidized to quinoids, in the presence of rat or human liver microsomes, which also have the potential to cause toxicity in vivo (27).
Given continuing safety concerns and therapeutic benefit, there remains a need to develop new SERMs with improved metabolic stability and attenuated toxicity, but retaining ER modulating activity. Studies of the benzothiophene SERMs, raloxifene and DMA, indicated that replacement of the 4′-hydroxyl group would attenuate quinoid formation by preventing formation of the di-quinone methide form (27,30). Thus, the new SERM, 4′FDMA, was designed to improve metabolic stability, attenuate toxicity via quinoid formation, whilst minimizing impact upon ER-mediated biological activity(27). Our previous study showed that quinoid formation from 4′F-DMA is attenuated in simple liver microsomal incubations (27).
In the present study, phase I and phase II metabolism of 4′F-DMA was compared with DMA using rat hepatocytes, human small intestinal microsomes, and human intestinal Caco-2 cells. The antiestrogenic activity of 4′F-DMA was studied in a number of cell-based assays in comparison with other SERMs. The results suggest that 4′F-DMA might be a promising SERM with similar antiestrogenic activity to DMA and raloxifene, but with improved metabolic stability and attenuated toxicity. The use of ring substituents to attenuate oxidative bioactivation to potentially toxic metabolites can be readily incorporated into the design and discovery of novel SERMs.
Materials and Methods
Caution: DMA di-quinone methide was handled in accordance with the NIH Guidelines for the Laboratory Use of Chemical Carcinogens
All solvents and reagents were purchased from either Aldrich Chemical (Milwaukee, WI), Fisher Scientific (Itasca, IL), or Sigma (St. Louis, MO) unless stated otherwise. Hepatocyte thawing medium (HTM) and rat cryopreserved hepatocytes were purchased from In Vitro Technology, Inc. (Baltimore, MD). DMA and 4′F-DMA were synthesized as described previously (27). The comet assay kit for the detection of DNA damage was purchased form Trevigen Inc. (Gaithersburg, MD).
HPLC
HPLC analysis was performed using a Beckman 4.6 × 250 mm Ultrasphere C18 column on an Agilent (PaloAlto, CA) 1100 UV/Vis photodiode array detector with UV absorbance at 316 nm and an Agilent ion trap mass spectrometer equipped with a model 1100 HPLC system and electrospray ionization. Method A: The mobile phase consisted of a linear gradient from 10 - 30% acetonitrile in water which contains 10% methanol and 0.1% formic acid at a flow rate of 1 mL/min over 40 min, 10 min gradient from 30 - 70% acetonitrile, and then 70 - 90% acetonitrile over 5 min. Method B: The mobile phase consisted of a 20 min linear gradient from 10 - 30% acetonitrile with a cosolvent of water containing 10% methanol and 0.1% formic acid at a flow rate of 1 mL/min, 10 min gradient from 30 - 70% acetonitrile, and then 70 - 90% acetonitrile over 5 min.
Cell culture conditions
MCF-7 cells were maintained in MEME supplemented with 1% penicillin, streptomycin, fungizome, 10 mg/L insulin, 1% nonessential amino acids, 1 mM glutamax, and 10% fetal bovine serum (FBS) (Atlanta Biologicals, Atlanta, GA). Ishikawa cells were maintained in Dulbecco’s modified eagle essential medium (DMEM)/F12 media with 10% heat-inactivated FBS, sodium pyruvate (1%), nonessential amino acid (1%), and glutamax (1 mM). The cryopreserved rat hepatocytes were thawed according to the protocol described by In Vitro Technologies. On the day of assay, the vials containing the rat cryopreserved hepatocytes were removed from liquid nitrogen storage. The cells were thawed by gently shaking the vials in a 37 °C water bath. The hepatocyte suspension was transferred into the prewarmed 50 mL HTM, the cells were centrifuged at 50 × g for 3 min, and the resulting pellet was resuspended in Krebs Hensleit buffer (KHB) at appropriate cell density for further experiments. Viability was determined using the trypan blue exclusion assay. Caco-2 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM)/F12 supplemented with 1% penicillin, streptomycin, fungizome, 1% nonessential amino acids, 1% sodium pyruvate, 0.15% sodium bicarbonate, and 20% fetal bovine serum (FBS) (Atlanta Biologicals, Atlanta, GA).
Metabolism of DMA and 4′F-DMA in Rat Hepatocytes
After thawing cryopreserved rat hepatocytes as described above, the hepatocytes were resuspended in KHB buffer at a density of 2 × 106 cells/mL in a 6-well plate and cultured in KHB buffer for 30 min prior to metabolic studies. DMA and 4′F-DMA dissolved in DMSO were added to the hepatocyte cultures to give a final concentration of 20 μM. DMSO concentration was 0.1% (v/v). After incubation at 37 °C, 5% CO2 for 2 h, the culture medium was acidified with 10% aqueous TFA. The reaction mixtures were centrifuged at 13,000 rpm for 5 min, and the solutions were extracted using PrepSep™ C18 solid-phase extraction cartridges, eluted with methanol, and concentrated to a final volume of 200 μL. Aliquots (100 μL) were analyzed directly using HPLC method A.
Metabolism of 4′F-DMA and DMA in Microsomes Prepared from Human Small Intestine in the presence of UDP-glucuronic acid
A solution containing the substrate (20 μM), human small intestine microsomes (1 mg/mL), UDP-glucuronic acid (1 mM), MgCl2 (8 mM), alamethicin (50 mg/μL) in 25 mM Tris-HCl (pH 7.1, 1 mL total volume) (31) was incubated for 2 h at 37 °C. For control incubations, UDP-glucuronic acid was omitted. The reactions were terminated by chilling in an ice bath followed by the addition of 2 mL of acetonitrile. The reaction mixtures were centrifuged at 13,000 rpm for 5 min and the protein was removed using ultrafiltration through a 30,000 molecular weight cutoff regenerated cellulose filter (Baltimore, MD). Samples (100 μL) were analyzed using HPLC method B.
Metabolism of DMA and 4′F-DMA in Caco-2 cells
Human intestinal Caco-2 cells were plated (1 × 105 cells/well) in duplicate onto a 12-well plate. Caco-2 cells were cultured in DMEM/F12 medium for 10 days before beginning the metabolism study. The medium was changed every other day. SERMs dissolved in DMSO were added to the cells to give a final concentration of 20 μM. DMSO concentration was 0.1% (v/v). After treatment, medium was collected and cells were lysed by the addition of 1 mL MeOH. The medium and MeOH solution were combined and were centrifuged at 13,000 rpm for 10 min, and the protein was removed using ultrafiltration through a 30,000 molecular weight cutoff regenerated cellulose filter (Baltimore, MD). Samples (100 μL) were analyzed using HPLC method B.
DNA Damage in Rat Hepatocytes using the Alkaline Single Cell Gel Electrophoresis Assay (Comet assay)
The comet assay was carried out as recommended by the manufacturer (Trevigen Inc.). Briefly, the cells (2 × 105 cells/mL) were incubated with the test compounds for 60 min, the cells were collected, centrifuged at 50 × g for 5 min, and the resulting pellet was resuspended in PBS at a concentration of 2 × 105 cells/mL. Cells (50 μL) were combined with LMAgarose (500 μL, 42 °C) and 50 μL of the mixture was immediately placed onto a CometSlide. The slides were incubated at 4 °C in the dark for 10 min followed by immersion in prechilled lysis solution at 4 °C for 30 min and then incubated in freshly prepared alkali electrophoresis solution at room temperature in the dark for 45 min. Following electrophoresis in alkali solution at 320 mA for 30 min, the slides were immersed in ethanol for 5 min and air-dried. The slides were then stained with SYBR green and viewed under a fluorescence microscope. The DNA was scored from 0 (intact DNA) to 4 (completely damaged DNA with tail only). Scores were calculated using the following formula in which NA (intact DNA), NB, NC, ND, and NE (completely damaged DNA) were the number of different kinds of comets: score (S) = (NA×0 +NB ×1 + NC × 2 + ND × 3 + NE × 4) / (NA + NB + NC + ND + NE) × 100. Duplicated samples were prepared for each treatment and at least 100 cells were scored per sample.
Measurement of GSH levels in Rat Hepatocytes
The cellular GSH levels were measured by an enzymatic recycling procedure (32). Briefly, the cells on 12-well plates were incubated with test compounds or DMSO. After treatment, the cells were collected and centrifuged at 50 × g for 5 min. The resulting pellet was resuspended in 0.5 mL 125 mM sodium phosphate buffer containing 6.3 mM EDTA (pH 7.5, sodium phosphate buffer). After sonication, the reaction mixture for determination of GSH content consisted of 20 μL lysates, 0.3 mM NADPH, 0.6 mM 5,5′-dithiobis-(2 -nitrobenzoic acid), and 0.5 unit/mL of GSH reductase. The absorbance at 412 nm was measured at 25 °C using a UV-vis spectrophotometer (Hewlett-Packard, Palo Alto, CA) 1 min after addition of the reaction mixture. The content of GSH was calculated from the change in the rate of absorbance on the basis of a standard curve.
Determination of Inhibition of 17β-estradiol-induced Luciferase Activity in MCF-7 cells
Breast cancer cell lines MCF-7 cells were cultured in MEME estrogen-free media for 3 days, and plated (4 × 105 cells/well) in duplicate onto a 12-well plate. By using eletroporation (950 μF/250V), cells were transiently transfected with a plasmid containing a luciferase reporter gene driven by ERE (2 μg) and with an internal control plasmid pRLTK (1 μg). AT 24 h post-transfection, cells were treated with test compounds with and without 1 nM 17β-estradiol for 24 h at which time the cells were harvested with 200 μL of passive lysis buffer (Promega, Madison, WI). Luciferase activity was determined determined using the dual-luciferase assay kit (Promega, Madison, WI) as per manufacturer’s instructions and read on a Lumistar galaxy luminameter (BNG Labtechnologies, Durham, NC) in a 96 well plate format. The luciferase activity was normalized by the expression of the internal control plasmid. Percent estrogen induction was calculated as follows: [(Luciferasesample - LuciferaseDMSO) / (Luciferaseestradiol - LuciferaseDMSO)] × 100. The results are expressed as the average ± SD of three determination.
Determination of Inhibition of Estrogen-induced Luciferase Activity in Ishikawa cells
Endometrial cancer cell lines Ishikawa cells were cultured in DMEM-F estrogen-free media for 3 days, and plated (2 × 105 cells/well) in duplicate onto a 12-well plate. Cells were transiently transfected with a plasmid containing a luciferase reporter gene driven by ERE (4.8 μg) and with an internal control plasmid pRLTK (2.4 μg) by using Effectene Transfection Reagent (Qiagen, Valencia, CA). The remainder of the assay follows that of MCF-7 cells described above.
Cell Proliferation Assay
MCF-7 cells were cultured in estrogen-free medium for 3 days before beginning the proliferation assay (day 0) by plating 0.5 × 103 cells in 1 mL of estrogen-free medium per well in 24-well plates. Medium containing the appropriate test compounds with and without 1 nM 17β-estradiol was added on day 1. All compounds were dissolved in 100% DMSO and added to the medium at a 1:1000 dilution. Compound containing medium was changed on days 3 and 5, and the experiment was stopped on day 7. The DNA content of cells was measured using fluorescent DMA quantitation kit (BIO-RAD, Hercules, CA) as described previously (33) with a Lumistar galaxy luminameter (BNG Labtechnologies, Durham, NC) in a 96 well plate format.
Results
Metabolism of DMA and 4′F-DMA in Rat Hepatocytes
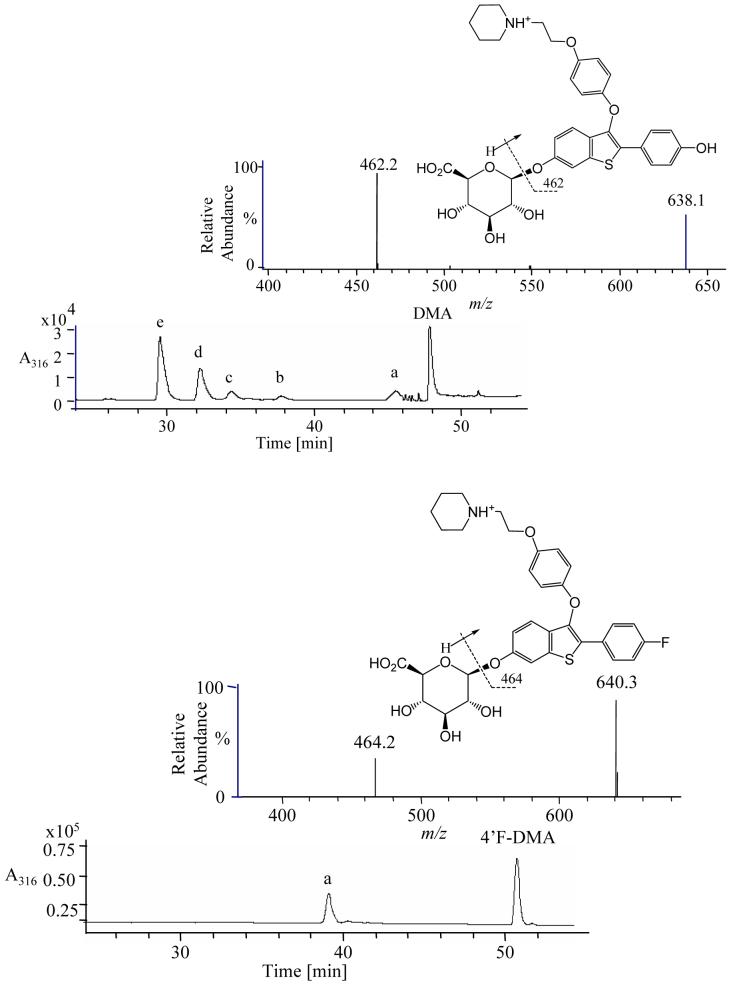
Incubations of DMA in cryopreserved rat hepatocytes resulted in the formation of two glucuronide conjugates, two GSH conjugates, and one sulfate conjugate (Figures 1, 2). The two GSH conjugates (DMA-SG1, DMA-SG2) were identified by co-injection of the three fully characterized GSH conjugates generated by the reaction of GSH with DMA di-quinone methide generated by chemical oxidation (34). The two DMA glucuronide conjugates were arbitrarily assigned as DMA-Glu1 and DMA-Glu2. MS-MS with CID of the protonated molecules of m/z 638, DMA-Glu1 and DMA-Glu2 showed similar fragmentation patterns, fo example, both produced a fragment ion of m/z 462, which corresponded to the protonated aglycon formed from cleavage of the glycosidic bond (MH+-176) (Figure 2). In incubations of 4′F-DMA with rat hepatocytes, only one glucuronide conjugate (4′F-DMA-Glu) was detected; no GSH conjugates were detected using LC-MS-MS (Figure 2). MS analysis of 4′F-DMA-Glu produced a protonated molecule of m/z 640. MS-MS with CID of this protonated molecule produced fragment ions of m/z 464, corresponding to the cleavage of the glycosidic bond (MH+-176).
Figure 1.
A) Formation of glutathione conjugates from DMA di-quinone methide; B) formation of glucoronide conjugate from 4′F-DMA, which cannot form a di-quinone methide.
Figure 2.
LC-UV-MS-MS analysis of DMA or 4′F-DMA (LOWER) incubation with cryopreserved rat hepatocytes: DMA UPPER (a) DMA-SO4, (b) DMA-SG1, (c) DMA-SG2, (d) DMA-Glu1, (e) DMA-Glu2; 4′F-DMA LOWER, (a) 4′F-DMA-Glu.
Phase II metabolism of DMA and 4′F-DMA with Microsomes Prepared from Human Small Intestine
Absolute bioavailability of raloxifene is only 2%. This poor bioavailability is thought to be the result of extensive phase II glucuronidation (31,35). We presume that 4′F-DMA may undergo less phase II glucuronidation as compared to DMA since the 4′F-DMA does not contain a 4′-hydroxyl group. In the incubation of DMA (20 μM) and 4′F-DMA (20 μM) with microsomes prepared from human small intestine in the presence of UDP-glucuronic acid, 4′F-DMA formed 4-fold less glucuronide conjuates as compared to DMA (Figure 3).
Figure 3.
HPLC chromatograms of human small intestine microsomal incubations of 4′F-DMA and DMA in the presence of UDP-glucuronic acid. (d) DMA-Glu1, (e) DMA-Glu2; (a) 4′F-DMA-Glu.
Metabolism of SERMs with Human Intestinal Caco-2 cells
In incubations of 4′F-DMA, DMA, and raloxifene with Caco-2 cells (Figure 4), no GSH conjugates were detected using LC-MS-MS. 4′F-DMA formed one glucuronide conjugate and one sulfate conjugate. DMA formed two glucuronide conjugates and two sulfate conjugate. Raloxifene formed two glucuronide conjugates and one sulfate conjugate. DMA, raloxifene and 4′F-DMA all caused a time-dependent increase in the formation of glucuronide conjugates and sulfate conjugates. At 24 h, 4′F-DMA formed 30-fold and 5-fold less glucuronide and sulfate conjugates, respectively, as compared to DMA, and 10-fold and 3-fold less glucuronide and sulfate conjugates, respectively, as compared to raloxifene.
Figure 4.
A) Formation of glucuronide conjugates of SERMs in human intestinal Caco-2 cells. B) Formation of sulfate conjugates of SERMs in human intestinal Caco-2 cells: closed circles, DMA; open circles, raloxifene; closed squares, 4′F-DMA. Data represent the mean ± SD of three determinations.
DNA Damage in Rat Hepatocytes
The comet assay was used to investigate the relative ability of the antiestrogens to induce DNA damage (Figure 5). A dose-dependent increase in the extent of DNA damage caused by DMA was observed in rat hepatocytes. Treating cells with diethyl maleate (DEM), which depleted cellular GSH prior to DMA treatment, led to enhanced DNA damage in rat hepatocytes as shown in Figure 5. 4′F-DMA caused much less DNA damage as compared with DMA with and without the addition of DEM, which further confirmed that 4′F-DMA does not form a quinoid electrophile in rat hepatocytes and that a di-quinone methide might play a role in the DNA damage caused by DMA .
Figure 5.
Induction of DNA damage by SERMs in cryopreserved rat hepatocytes. Cells (5 × 105 cells/mL) were treated with compounds or vehicle for 60 min: closed circles, DMA; open circles, DMA + DEM; closed squares, 4′F-DMA; open squares, 4′F-DMA + DEM. Data represent the mean ± SD of three determinations.
Effect of SERMs on GSH levels in Rat Hepatocytes
We have shown that DMA can be metabolized by rat liver microsomes and rat hepatocytes to a DMA di-quinone methide which reacted with GSH to form DMA GSH conjugates (34). However, in the incubation of 4′F-DMA with either rat liver microsomes or rat hepatocytes, no GSH conjugates were detected. Since GSH represents the major intracellular nonprotein thiol whose role is to protect cells from oxidative injury, we examined the effect of DMA and 4′F-DMA on GSH content in rat hepatocytes. We found that DMA (25 μM) decreased GSH levels to about 70% within 30 minutes in rat hepatocytes (Figure 6). In contrast, 4′F-DMA did not cause any change in GSH levels in rat hepatocytes.
Figure 6.
Effect of DMA and 4′F-DMA on cellular GSH levels in cryopreserved rat hepatocytes: A) DMA (25 μM, circles) or 4′F-DMA (25 μM, squares); B) 30 min treatment with varied concentrations of DMA (circles) or 4′F-DMA (squares). GSH levels after each treatment were measured as described in Materials and Mmethods. Data represent the mean ± SD of three determinations.
Measurement of Estrogenic and Antiestrogenic Activity of SERMs using the ERE-luciferase reporter assay cell culture
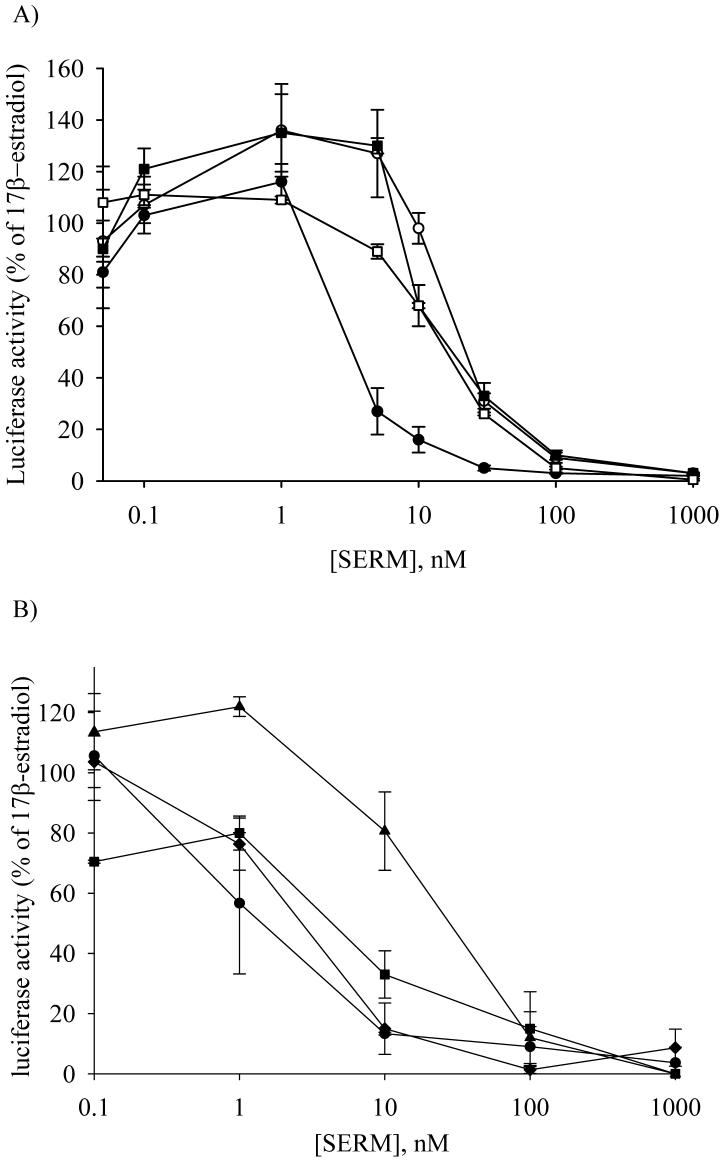
MCF-7 cells have been used for many years as a model cell line to investigate hormonally responsive breast cancer (36). Ishikawa cells are a ERα positive cell line, which is an established human endometrial cancer cell line that responds to estrogens and antiestrogens at concentrations approximating physiological levels (37). To evaluate the estrogenic activity of 4′F-DMA, both cell lines were treated with increasing concentrations of 4′F-DMA in the absence of 17β-estradiol. For comparison, raloxifene and DMA were included. However, none of the compounds generated estrogenic activity in the range of 10-10 -10-6 M (data not shown). We next evaluated the antiestrogenic activity of 4′F-DMA in the presence of 1 nM 17β-estradiol. For comparison, raloxifene, DMA, and ICI were included. All of the compounds exhibited significant antiestrogenic activities on the cells at the concentration of 10-9 -10-6 M (Figure 7). 4′F-DMA has similar antiestrogenic activity as compared with raloxifene and ICI; however, 10-fold lower antiestrogenic activity as compared to DMA.
Figure 7.
Antiestrogenic activity of SERMs using ERE-luciferase reporter assay in (A) MCF-7 cells and (B) Ishikawa cells. Cells (4 × 105 cells/well, MCF-7; 2 × 105 cells/well, Ishikawa cells) were treated with test compounds with 17β-estradiol (1 nM) for 24 h: closed circles, DMA; closed squares, 4′F-DMA; open circles, raloxifene; open squares, ICI. Data represent the mean ± SD of three independent experiments. Details are described in Materials and Methods.
Proliferative and antiproliferative acitivity of 4′F-DMA using proliferation assay in MCF-7 cells
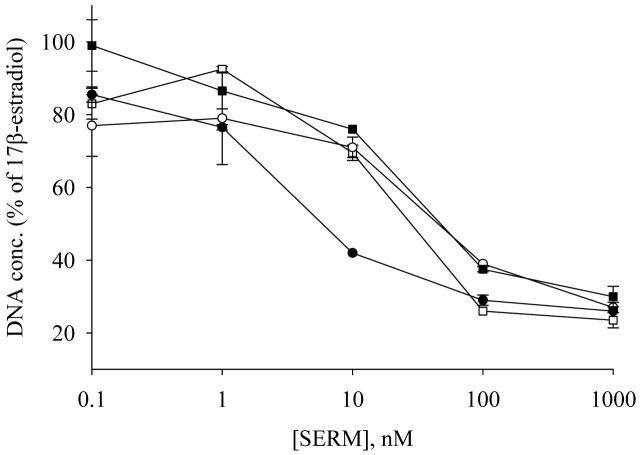
To evaluate the proliferative activity of 4′F-DMA, MCF-7 cells were treated with increasing concentrations of 4′F-DMA in the absence of 17β-estradiol. 4′F-DMA did not show any growth stimulatory activity at the concentration of 10-10 -10-6 M (data not shown). In contrast, 17β-estradiol at 1 nM stimulated MCF-7 proliferation ∼5-fold. Antiproliferative activity of 4′F-DMA in the presence of 1 nM of 17β-estradiol was then examined. All of the SERMs exhibited significant antiproliferative activities on the cells in the range of 10-9 -10-6 M (Figure 8). 4′F-DMA has similar antiproliferative activity as compared to raloxifene and ICI; however, 10-fold lower antiproliferative activity compared to DMA.
Figure 8.
MCF-7 in vitro proliferation study with SERMs. Cells (1 × 104 cells/well) treated with test compounds and 17β-estradiol (1 nM) for 7 days closed circles, DMA; closed squares, 4′F-DMA; open circles, raloxifene; open squares, ICI. Medium containing compounds was changed on days 3 and 5, and the experiment was stopped on day 7. Data represent the mean ± SD of three independent experiments; details are described in Materials and Methods..
Discussion
Raloxifene undergoes rapid absorption, extensive first-pass glucuronidation, and enterohepatic cycling after oral administration, resulting in an absolute bioavailability of just 2% and apparent oral clearance of 44 L/kg/h (31,35). Clinical studies have shown that glucuronidation occurs primarily at the 4′-position of raloxifene, with lower levels of the 6-glucuronide and 4′,6-diglucuronide conjugate found in vivo. Intestinal glucuronidation may be a significant contributor to the presystemic clearance of raloxifene in vivo (38). In addition, previous research has shown that raloxifene can be bioactivated by rat and human liver microsomes to an electrophilic di-quinone methide and in a very minor pathway to o-quinones (26,29), which might contribute to cytotoxicity and/or genotoxicity through mechanisms including depletion of GSH, alkylation of proteins, and induction of DNA damage in vivo.
Arzoxifene is a structural congener of raloxifene in which the carbonyl hinge of raloxifene has been replaced by an ether linkage. In addition, a methoxy group has been substituted for the 4′-hydroxyl group to both reduce first-pass glucuronidation and increase ClogP (by approximately 0.6 log units relative to raloxifene). The intended effect of these modifications is to increase the bioavailability of the active drug relative to raloxifene (39), however, arzoxifene is metabolically desmethylated in vivo to yield DMA, an active metabolite that is a more potent ligand for both ERα and ERβ than both arzoxifene and raloxifene (IC50-DMA = 17nM, 20 nM, respectively; IC50-raloxifene = 24, 400 nM, respectively (30)). Moreover, there is some ambiguity as to whether DMA is the active form of arzoxifene, since reported DMA plasma concentrations are highly variable. For example, the overall mean steady-state plasma concentrations of arzoxifene were 2.88 ng/mL and 8.36 ng/mL for the 20-mg and 50-mg dose respectively, in patients who had quantifiable DMA; even in these pateints the individual observed steady-state concentrations ranged from 0.051 to 1.132 ng/mL for the 20-mg dose and 0.051 to 2.798 ng/mL for the 50-mg dose (40). Our previous studies showed that DMA can be oxidized to a quinoid, in the presence of rat or human liver microsomes (30), which has the potential to cause toxicity in vivo. Thus, it is postulated that 4′F-DMA represents a new benzothiophene SERM that will manifest reduced first and second pass metabolism, attenuated toxicity, and increased bioavailability.
Chemical modification has been used to change both the route and the rate of metabolism of estradiol whilst retaining estrogenic activity (41). Exposure to estrogens is associated with an increase in the incidence of breast cancer and of various uterine lesions, including tumors (42,43). Endogenous estrogens undergo extensive oxidative metabolism to the 2-, and 4-hydroxylated catechol metabolites, which has been proposed to contribute to the carcinogenic action of estrogens (44). Catechols can be oxidized to electrophilic o-quinones which bind covalently to proteins, peptides and to DNA and hence promote estrogen carcinogenesis (14,44). In an animal model, 4-hydroxy-estradiol was considerably more carcinogenic than the 2-hydroxy-isomer probably because of the rapid metabolism of the latter (45).
Strong evidence for a chemical carcinogenesis mechanism derives from studies on estrogens chemically modified at the 2- or 4-positions to block catechol and subsequent quinone formation. In the Syrian hamster model, the carcinogenicity observed in treatment with estradiol or hydroxyl-estradiol was completely abrogated in treatment with 2-fluoroestradiol, and significantly attenuated with 4-fluoroestradiol. However, both 2- and 4-fluoroestradiol were highly estrogenic in vivo and in vitro, comparable with estradiol itself (14). The separation of estrogenic from carcinogenic action shows that these properties are not intrinsically linked with each other but dependent on the influence of structure on chemical reactivity towards oxidation. This structural influence can be seen in variable rates and extent of: (i) the oxidation, and the oxidative dehalogenation of the fluoroestradiols; (ii) the methylation of the resulting catechols by catechol-O-methyl transferase (COMT); and, (iii) glucuronidation and sulfation by Phase II enzymes (46-49).
In addition to estradiol, 4-fluoro analogs of the equine estrogens equilenin and 17β-equilenin have been reported and shown to have a much lower propensity for oxidation to quinones in vitro (50). These fluoro-derivatives were shown to have identical ligand binding ability to their parent estrogens at both ERα and ERβ and to show comparable estrogenic effects in vitro; however, in vivo, the fluoro derivatives showed more modest activity relative to the parent estrogens. These results again emphasize the importance of metabolism in determining both toxicity and biological activity.
Oxidation of DMA to a di-quinone methide represents the major bioactivation pathway for DMA from data obtained in vitro (30). Thus, a fluoro-derivative, 4′F-DMA, was synthesized to disallow formation of this quinoid. This new SERM is designed to allow differentiation of the biological activity mediated by ER binding, from toxicity and activity associated with oxidative metabolism to quinoids. In preliminary studies, it was shown that 4′F-DMA was not metabolized to the di-quinone methide by rat and human liver microsomes, whereas 4′F-DMA showed similar ER ligand binding affinity to DMA itself, and similar antiestrogenic activity to raloxifene (27). However, in preparation for in vivo studies, a number of questions about the reactivity, metabolism, and activity of 4′F-DMA need to be answered.
In the current study, cryopreserved rat hepatocytes were selected as an experimental model to compare the phase I and phase II metabolism and toxicological behavior of DMA and 4′F-DMA. Two DMA GSH conjugates, two DMA glucuronide conjugates, and one DMA sulfate conjugate were detected in incubations of DMA with rat hepatocytes and identified by LC-MS-MS. In contrast, 4′F-DMA did not yield any GSH conjugates in incubation with rat hepatocytes, which demonstrated that 4′-fluoro substitution of DMA successfully blocked quinoid formation at the cellular level. Moreover, 4′F-DMA was observed to form twofold less glucuronide conjugates relative to DMA in hepatocytes, which might be expected by the absence of the 4′OH group, since in raloxifene this is the major site of glucuronidation. No evidence was seen for oxidative dehalogenation of 4′F-DMA in LC-MS-MS chromatograms.
Intestinal glucuronidation of raloxifene is the major contributor to the presystemic clearance of raloxifene in vivo (38). Therefore, microsomes prepared from human small intestine were used to further evaluate the glucuronidation of DMA and 4′F-DMA. 4′F-DMA formed fourfold less glucuronide conjugates as compared to DMA in the incubation with human small intestinal microsomes in the presence of UDP-glucuronic acid. In order to extend the data to obtain time dependence, human intestinal Caco-2 cells were used. After 24 h, incubation of 4′F-DMA with human intestinal Caco-2 cells thirtyfold less glucuronide conjugates and fivefold less sulfate conjugates were observed as compared to DMA. The collected results predict that 4′F-DMA will demonstrate improved bioavailability relative to DMA and raloxifene.
SERM-induced DNA damage was assessed in rat hepatocytes using the comet assay to measure single strand breaks. 4′F-DMA caused almost no DNA damage as compared to DMA and the GSH-depleting agent, DEM, did not increase the very low level of DNA damage resulting from 4′F-DMA. However, DMA caused concentration dependent DNA damage which could be potentiated by DEM. Since DMA was observed to form GSH conjugates in rat hepatocytes, it was of interest to ascertain if DMA itself could cause GSH depletion in rat hepatocytes. Therefore, the effects of DMA and 4′F-DMA on GSH content in rat hepatocytes were measured. GSH levels were unaffected by 4′F-DMA in rat hepatocytes. In contrast, DMA caused a dose and time dependent depletion of GSH levels in rat hepatocytes. This assay measured GSH depletion due to alkylation by the electrophilic quinoid metabolite, however, depletion due to GSH oxidation by quinoids is a further pathway that may contribute to loss of cellular antioxidant defenses.
The ER-dependent activity of 4′F-DMA was examined by measuring ERE-luciferase activity in transiently transfected MCF-7 cells and Ishikawa cells. None of the SERMs studied showed estrogenic activity in either cell line, however, estradiol-induced luciferase activity was significantly antagonized by raloxifene, DMA, 4′F-DMA, and the pure antiestrogen, ICI 182780, in both cell lines. 4′F-DMA showed less antiestrogenic activity as compared to DMA, but comparable activity compared to raloxifene and ICI 182780 in both cell lines. Furthermore, 4′F-DMA antagonized the actions of estradiol in the MCF-7 cell proliferation assay without itself showing estrogenic activity; again, the antiestrogenicity of 4′F-DMA was comparable to raloxifene and ICI 182780.
In conclusion, the new SERM, 4′F-DMA, showed binding potency towards ERα and ERβ with similarities to both raloxifene and DMA. Antiestrogenic activity was seen in a variety of cell-based assays to be comparable to raloxifene. No estrogenic activity was observed and 4′F-DMA was seen to be antiproliferative in MCF-7 cells in response to estradiol treatment. More importantly, in contrast to DMA and raloxifene, 4′F-DMA was resistant to oxidation to quinoids in rat hepatocytes and in liver microsomes. Furthermore, DMA caused both significant depletion of GSH and DNA damage in hepatocytes, whereas 4′F-DMA did not deplete GSH nor cause DNA strangle strand breaks. In human microsomes and in cell culture, 4′F-DMA was observed to form significantly less Phase II enzyme metabolites than DMA. Taken together, these data suggest that 4′F-DMA represents a promising SERM with comparable antiestrogenic activity to raloxifene, but improved metabolic stability and a greatly attenuated potential for toxicity compared to current benzothiophene SERMs. The present data support the strategy of structural modification, to provide control of oxidative metabolism and toxicity, as a general approach towards an improved SERM; these data are currently being extended to provide in vivo observations.
Acknowledgements
This work is supported by NIH Grants CA102590 and CA79870. We thank Dr. Minsun Chang and Dr. Yan Li for technical assistance.
Abbreviations
- DEM
diethyl maleate
- DMA
desmethylated arzoxifene
- DMA-SG
glutathionyl-desmethylated arzoxifene
- DMEM
dulbecco’s modified eagle essential medium
- DMSO
dimethyl sulfoxide
- ER
estrogen receptor
- FBS
fetal bovine serum
- GSH
glutathione
- HTM
hepatocyte thawing medium
- ICI
ICI182780
- KHB
Krebs Hensleit buffer
- LC-MS-MS
liquid chromatography-tandem mass spectrometry
- LMAgarose
low melting agarose
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- SERM
selective estrogen receptor modulator.
References
- (1).Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. Jama. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- (2).Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419–427. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- (3).Cosman F, Lindsay R. Selective estrogen receptor modulators: clinical spectrum. Endocr. Rev. 1999;20:418–434. doi: 10.1210/edrv.20.3.0371. [DOI] [PubMed] [Google Scholar]
- (4).Conzen SD. Current status of selective estrogen receptor medulators (SERMs) Cancer J. 2003;9:4–11. doi: 10.1097/00130404-200301000-00003. [DOI] [PubMed] [Google Scholar]
- (5).Shang Y, Brown M. Molecular determinants for the tissue specificity of SERMs. Science. 2002;295:2465–2468. doi: 10.1126/science.1068537. [DOI] [PubMed] [Google Scholar]
- (6).Osborne CK, Zhao H, Fuqua SA. Selective estrogen receptor modulators: structure, function, and clinical use. J. Clin. Oncol. 2000;18:3172–3186. doi: 10.1200/JCO.2000.18.17.3172. [DOI] [PubMed] [Google Scholar]
- (7).Delmas PD, Bjarnason NH, Mitlak BH, Ravoux AC, Shah AS, Huster WJ, Draper M, Christiansen C. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med. 1997;337:1641–1647. doi: 10.1056/NEJM199712043372301. [DOI] [PubMed] [Google Scholar]
- (8).Kelminski A. The Study of Tamoxifen and Raloxifene (STAR trial) for the prevention of breast cancer. Hawaii Med. J. 2002;61:209–210. [PubMed] [Google Scholar]
- (9).Riggs BL, Hartmann LC. Selective estrogen-receptor modulators -- mechanisms of action and application to clinical practice. N Engl J Med. 2003;348:618–629. doi: 10.1056/NEJMra022219. [DOI] [PubMed] [Google Scholar]
- (10).Gennari L. Lasofoxifene (Pfizer) Curr Opin Investig Drugs. 2005;6:1067–1078. [PubMed] [Google Scholar]
- (11).Yager JD, Liehr JG. Molecular mechanisms of estrogen carcinogenesis. Annu Rev Pharmacol Toxicol. 1996;36:203–232. doi: 10.1146/annurev.pa.36.040196.001223. [DOI] [PubMed] [Google Scholar]
- (12).Bolton JL, Trush MA, Penning TM, Dryhurst G, Monks TJ. Role of quinones in toxicology. Chem Res Toxicol. 2000;13:135–160. doi: 10.1021/tx9902082. [DOI] [PubMed] [Google Scholar]
- (13).Liehr JG. Modulation of estrogen-induced carcinogenesis by chemical modifications. Arch Toxicol. 1984;55:119–122. doi: 10.1007/BF00346049. [DOI] [PubMed] [Google Scholar]
- (14).Liehr JG. 2-Fluoroestradiol. Separation of estrogenicity from carcinogenicity. Mol Pharmacol. 1983;23:278–281. [PubMed] [Google Scholar]
- (15).Yu L, Liu H, Li W, Zhang F, Luckie C, Van Breemen RB, Thatcher GRJ, Bolton JL. Oxidation of Raloxifene to Quinoids: Potential Toxic Pathways via a Diquinone Methide and o-Quinones. Chem Res Toxicol. 2004;17:879–888. doi: 10.1021/tx0342722. [DOI] [PubMed] [Google Scholar]
- (16).Zhang F, Fan PW, Liu X, Shen L, van Breeman RB, Bolton JL. Synthesis and reactivity of a potential carcinogenic metabolite of tamoxifen: 3,4-dihydroxytamoxifen-o-quinone. Chem Res Toxicol. 2000;13:53–62. doi: 10.1021/tx990145n. [DOI] [PubMed] [Google Scholar]
- (17).Shibutani S, Ravindernath A, Suzuki N, Terashima I, Sugarman SM, Grollman AP, Pearl ML. Identification of tamoxifen-DNA adducts in the endometrium of women treated with tamoxifen. Carcinogenesis. 2000;21:1461–1467. [PubMed] [Google Scholar]
- (18).King CM. Tamoxifen and the induction of cancer. Carcinogenesis. 1995;16:1449–1454. doi: 10.1093/carcin/16.7.1449. [DOI] [PubMed] [Google Scholar]
- (19).van Leeuwen FE, Benraadt J, Coebergh JW, Kiemeney LA, Gimbrere CH, Otter R, Schouten LJ, Damhuis RA, Bontenbal M, Diepenhorst FW, et al. Risk of endometrial cancer after tamoxifen treatment of breast cancer. Lancet. 1994;343:448–452. doi: 10.1016/s0140-6736(94)92692-1. [DOI] [PubMed] [Google Scholar]
- (20).Fornander T, Rutqvist LE, Cedermark B, Glas U, Mattsson A, Silfversward C, Skoog L, Somell A, Theve T, Wilking N, et al. Adjuvant tamoxifen in early breast cancer: occurrence of new primary cancers. Lancet. 1989;1:117–120. doi: 10.1016/s0140-6736(89)91141-0. [DOI] [PubMed] [Google Scholar]
- (21).Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- (22).Fisher B, Costantino JP, Redmond CK, Fisher ER, Wickerham DL, Cronin WM. Endometrial cancer in tamoxifen-treated breast cancer patients: findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J. Natl. Cancer Inst. 1994;86:527–537. doi: 10.1093/jnci/86.7.527. [DOI] [PubMed] [Google Scholar]
- (23).Williams GM, Iatropoulos MJ, Djordjevic MV, Kaltenberg OP. The triphenylethylene drug tamoxifen is a strong liver carcinogen in the rat. Carcinogenesis. 1993;14:315–317. doi: 10.1093/carcin/14.2.315. [DOI] [PubMed] [Google Scholar]
- (24).Shen L, Pisha E, Huang Z, Pezzuto JM, Krol E, Alam Z, van Breemen RB, Bolton JL. Bioreductive activation of catechol estrogen-ortho-quinones: aromatization of the B ring in 4-hydroxyequilenin markedly alters quinoid formation and reactivity. Carcinogenesis. 1997;18:1093–1101. doi: 10.1093/carcin/18.5.1093. [DOI] [PubMed] [Google Scholar]
- (25).Han X, Liehr JG, Bosland MC. Induction of a DNA adduct detectable by 32P-postlabeling in the dorsolateral prostate of NBL/Cr rats treated with estradiol-17 beta and testosterone. Carcinogenesis. 1995;16:951–954. doi: 10.1093/carcin/16.4.951. [DOI] [PubMed] [Google Scholar]
- (26).Yu L, Liu H, Li W, Zhang F, Luckie C, van Breemen RB, Thatcher GR, Bolton JL. Oxidation of raloxifene to quinoids: potential toxic pathways via a diquinone methide and o-quinones. Chem. Res. Toxicol. 2004;17:879–888. doi: 10.1021/tx0342722. [DOI] [PubMed] [Google Scholar]
- (27).Liu H, Liu J, van Breemen RB, Thatcher GR, Bolton JL. Bioactivation of the selective estrogen receptor modulator desmethylated arzoxifene to quinoids: 4′-fluoro substitution prevents quinoid formation. Chem Res Toxicol. 2005;18:162–173. doi: 10.1021/tx049776u. [DOI] [PubMed] [Google Scholar]
- (28).Liu J, Liu H, van Breemen RB, Thatcher GR, Bolton JL. Bioactivation of the selective estrogen receptor modulator acolbifene to quinone methides. Chem. Res. Toxicol. 2005;18:174–182. doi: 10.1021/tx0497752. [DOI] [PubMed] [Google Scholar]
- (29).Chen Q, Ngui JS, Doss GA, Wang RW, Cai X, DiNinno FP, Blizzard TA, Hammond ML, Stearns RA, Evans DC, Baillie TA, Tang W. Cytochrome P450 3A4-Mediated Bioactivation of Raloxifene: Irreversible Enzyme Inhibition and Thiol Adduct Formation. Chem Res Toxicol. 2002;15:907–914. doi: 10.1021/tx0200109. [DOI] [PubMed] [Google Scholar]
- (30).Liu H, Liu J, van Breemen RB, Thatcher GRJ, Bolton JL. Bioactivation of the selective estrogen receptor modulator desmethylated arzoxifene to quinoids: 4′-fluoro substitution prevents quinoid formation. Chem Res Toxicol. 2005;18:162–173. doi: 10.1021/tx049776u. [DOI] [PubMed] [Google Scholar]
- (31).Snyder KR, Sparano N, Malinowski JM. Raloxifene hydrochloride. Am. J. Health Syst. Pharm. 2000;57:1669–1675. quiz 1676-1668. [PubMed] [Google Scholar]
- (32).Takemura H, Urasaki Y, Yoshida A, Fukushima T, Ueda T. Simultaneous treatment with 1-beta-D-arabinofuranosylcytosine and daunorubicin induces cross-resistance to both drugs due to a combination-specific mechanism in HL60 cells. Cancer Res. 2001;61:172–177. [PubMed] [Google Scholar]
- (33).Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal. Biochem. 1980;102:344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- (34).Liu J, Liu H, van Breemen RB, Thatcher GRJ, Bolton JL. Bioactivation of the selective estrogen receptor modulator acolbifene to quinone methides. Chem Res Toxicol. 2005;18:174–182. doi: 10.1021/tx0497752. [DOI] [PubMed] [Google Scholar]
- (35).Hochner-Celnikier D. Pharmacokinetics of raloxifene and its clinical application. Eur. J. Obstet. Gynecol. Reprod. Biol. 1999;85:23–29. doi: 10.1016/s0301-2115(98)00278-4. [DOI] [PubMed] [Google Scholar]
- (36).Horwitz KB, Costlow ME, McGuire WL. MCF-7; a human breast cancer cell line with estrogen, androgen, progesterone, and glucocorticoid receptors. Steroids. 1975;26:785–795. doi: 10.1016/0039-128x(75)90110-5. [DOI] [PubMed] [Google Scholar]
- (37).Albert JL, Sundstrom SA, Lyttle CR. Estrogen regulation of placental alkaline phosphatase gene expression in a human endometrial adenocarcinoma cell line. Cancer Res. 1990;50:3306–3310. [PubMed] [Google Scholar]
- (38).Kemp DC, Fan PW, Stevens JC. Characterization of raloxifene glucuronidation in vitro: contribution of intestinal metabolism to presystemic clearance. Drug. Metab. Dispos. 2002;30:694–700. doi: 10.1124/dmd.30.6.694. [DOI] [PubMed] [Google Scholar]
- (39).Sato M, Turner CH, Wang T, Adrian MD, Rowley E, Bryant HU. LY353381.HCl: a novel raloxifene analog with improved SERM potency and efficacy in vivo. J Pharmacol Exp Ther. 1998;287:1–7. [PubMed] [Google Scholar]
- (40).Buzdar A, O’Shaughnessy JA, Booser DJ, Pippen JE, Jr., Jones SE, Munster PN, Peterson P, Melemed AS, Winer E, Hudis C. Phase II, randomized, double-blind study of two dose levels of arzoxifene in patients with locally advanced or metastatic breast cancer. J Clin Oncol. 2003;21:1007–1014. doi: 10.1200/JCO.2003.06.108. [DOI] [PubMed] [Google Scholar]
- (41).Raynaud JP, Bouton MM, Gallet-Bourquin D, Philibert D, Tournemine C, Azadian-Baulanger G. Comparative study of estrogen action. Mol. Pharmacol. 1973;9:520–533. [PubMed] [Google Scholar]
- (42).Dutertre M, Smith CL. Molecular mechanisms of selective estrogen receptor modulator (SERM) action. J Pharmacol Exp Ther. 2000;295:431–437. [PubMed] [Google Scholar]
- (43).Lonard DM, Smith CL. Molecular perspectives on selective estrogen receptor modulators (SERMs): progress in understanding their tissue-specific agonist and antagonist actions. Steroids. 2002;67:15–24. doi: 10.1016/s0039-128x(01)00133-7. [DOI] [PubMed] [Google Scholar]
- (44).Morgan P, Maggs JL, Bulman-Page PC, Hussain F, Park BK. The metabolism of 2- and 4-fluoro-17 beta-oestradiol in the rat and its implications for oestrogen carcinogenesis. Biochem. Pharmacol. 1992;43:985–993. doi: 10.1016/0006-2952(92)90603-g. [DOI] [PubMed] [Google Scholar]
- (45).Liehr JG, Fang WF, Sirbasku DA, Ari-Ulubelen A. Carcinogenicity of catechol estrogens in Syrian hamsters. J. Steroid. Biochem. 1986;24:353–356. doi: 10.1016/0022-4731(86)90080-4. [DOI] [PubMed] [Google Scholar]
- (46).Li JJ, Purdy RH, Appelman EH, Klicka JK, Li SA. Catechol formation of fluoro- and bromo-substituted estradiols by hamster liver microsomes. Evidence for dehalogenation. Mol. Pharmacol. 1985;27:559–565. [PubMed] [Google Scholar]
- (47).Ashburn SP, Han X, Liehr JG. Microsomal hydroxylation of 2- and 4-fluoroestradiol to catechol metabolites and their conversion to methyl ethers: catechol estrogens as possible mediators of hormonal carcinogenesis. Mol Pharmacol. 1993;43:534–541. [PubMed] [Google Scholar]
- (48).Stalford AC, Maggs JL, Gilchrist TL, Park BK. Catecholestrogens as mediators of carcinogenesis: correlation of aromatic hydroxylation of estradiol and its fluorinated analogs with tumor induction in Syrian hamsters. Mol. Pharmacol. 1994;45:1268–1272. [PubMed] [Google Scholar]
- (49).Morgan P, Maggs JL, Page PCB, Hussain F, Park BK. The metabolism of 2- and 4-fluoro-17b-estradiol in the rat and its implications for estrogen carcinogenesis. Biochem. Pharmacol. 1992;43:985–994. doi: 10.1016/0006-2952(92)90603-g. [DOI] [PubMed] [Google Scholar]
- (50).Liu X, Zhang F, Liu H, Burdette JE, Li Y, Overk CR, Pisha E, Yao J, van Breemen RB, Swanson SM, Bolton JL. Effect of halogenated substituents on the metabolism and estrogenic effects of the equine estrogen, equilenin. Chem Res Toxicol. 2003;16:741–749. doi: 10.1021/tx030001f. [DOI] [PubMed] [Google Scholar]