Abstract
Phosphorylation of types III and IV intermediate filaments (IFs) is known to regulate their organization and function. Phosphorylation of the amino-terminal head domain sites on types III and IV IF proteins plays a key role in the assembly/disassembly of IF subunits into 10 nm filaments, and influences the phosphorylation of sites on the carboxyl-terminal tail domain. These phosphorylation events are largely under the control of second messenger-dependent protein kinases and provide the cells a mechanism to reorganize the IFs in response to the changes in second messenger levels. In mitotic cells, Cdk1, Rho kinase, PAK1 and Aurora-B kinase are believed to regulate vimentin and glial fibrillary acidic protein phosphorylation in a spatio-temporal manner. In neurons, the carboxyl-terminal tail domains of the NF-M and NF-H subunits of heteropolymeric neurofilaments (NFs) are highly phosphorylated by proline-directed protein kinases. The phosphorylation of carboxyl-terminal tail domains of NFs has been suspected to play roles in forming cross-bridges between NFs and microtubules, slowing axonal transport and promoting their integration into cytoskeleton lattice and, in doing so, to control axonal caliber and stabilize the axon. The role of IF phosphorylation in disease pathobiology is discussed.
Keywords: Intermediate filaments, neurofilaments, vimentin, phosphorylation, protein kinases and protein assembly
Introduction
The major constituents of the cell cytoskeleton are microtubules, microfilaments and the intermediate filaments. Intermediate filaments (IFs) are 10 nm thick cytoskeletal structures formed by members of a family of related proteins, which vary in size (40–280 kDa) and their primary structure [1–4]. In mammals, there are about 70 genes coding for IF proteins and these genes are expressed in a tissue- and cell type- and sometimes in a cell differentiation-specific manner [3–6]. IF proteins, in most instances, can self assemble into flexible, non-polar filaments. Based on the gene structure and amino acid sequence, IFs have been classified into five types [2–4. 7–9]. Acidic and basic keratins (types I and II) are typically expressed in epithelial cells. Vimentin (mesenchymal cells), desmin (muscle cells), glial fibrillary acidic protein (GFAP) (glia and astrocytes), and peripherin (neurons of the peripheral nervous system) — are classified as type III IF proteins. Type IV IF proteins are expressed in neurons of the central nervous system and include the neurofilament triplet proteins, NF-L, NF-M, and NF-H and a-internexin. In contrast to the cytoplasmic location of the above described four classes of IF proteins, the type V IF proteins, called lamins, are found exclusively in the nucleus. Proteins belonging to IF superfamily but not included in the five types, such as, nestin and synemins are in some classifications referred to as type VI IFs [5].
The IF proteins exhibit a common tripartite domain structure, with non-helical amino (head)- and carboxyl -terminal (tail) domains flanking a central α-helical core region of ~ 310 amino acid-long in cytoplasmic IF sequences, and ~ 352 amino acid-long in the type V nuclear lamins [3,6,7,9]. The size and amino acid sequence of the amino-terminal head and carboxyl-terminal tail domains vary extensively between individual IF sequences. Several excellent reviews have been published on the domain structure and assembly of IFs [2–4, 9].
IF proteins are known to be phosphorylated on their head and tail domains and the dynamics of their phosphorylation/dephosphorylation plays a major role in regulating the structural organization and function of IFs in a cell- and tissue-specific manner [6, 11–15]. In this review, we discuss the progress made in understanding the role of phosphorylation on the cellular dynamics of IFs, the signaling pathways involved in phosphorylating the specific sites and how phosphorylation of specific sites might regulate the functions of types III and IV IFs.
Amino-terminal phosphorylation regulates intermediate filament assembly/disassembly
Type III filaments
Class III filaments are homopolymers and their assembly is initiated by the formation of dimers and tetramers. The tetramers assemble into protofilaments and ultimately form 10 nm thick filaments [2,4,9]. It has been suggested that head domain promotes lateral association of protofibrils into protofilaments and the tail domain promotes the termination of filaments upon reaching 10 nm [16]. Phosphorylation plays a major role in regulating the assembly/disassembly of these filaments. The phosphorylation of head domain sites by protein kinase A or protein kinase C can block vimentin and desmin subunit polymerization in vitro and disassemble preexisting filaments supported the idea that the site-specific phosphorylation in the amino-terminal head domain of IF proteins induces the disassembly of IFs [17, 18]. This was confirmed in vivo by the studies demonstrating that during mitosis, disassembly of the vimentin filaments is accompanied by hyperphosphorylation of the subunits at Ser-55 on the amino-terminal domain [19, 20]. In these studies the kinase responsible for disassembly of vimentin filaments was identified to be p34cdc2 kinase, but in subsequent studies a number of interphase-specific high turnover sites were identified [21]. Ser-38 and Ser-72 were important phosphorylation targets in vivo and these sites were phosphorylated by PKA in vitro. Interestingly, head domain sites have also been reported as targets for RhoA-binding kinase [22, 23]. Li et al [24] demonstrated that PAK-mediated vimentin phosphorylation at Ser-56 leads to increases in vimentin disassembly on stimulation of cultured smooth muscle cells with 5-hydroxytryptamine (5-HT) induced p21-activated kinase (PAK1). It is, therefore, reasonable to conclude that phosphorylation by various protein kinases on distinct specific sites on a subdomain (amino acid residues 35–75) of amino-terminal head domain of vimentin is involved in assembly/disassembly of vimentin filaments. This is consistent with the findings that part of the head domain on IFs (between residues 31 and 87) is required for early steps in filament assembly [16, 25]. Interestingly, the phosphorylation-induced disassembly of vimentin filaments results in the release of tetrameric subunits [21] suggesting that assembly of tetramers into protofilaments might be the key phosphorylation regulated step in IF assembly.
Peripherin, a neuronal type III IF, assembly into filaments also is most likely regulated by the phosphorylation/dephosphorylation of the head domain sites. The phosphorylation sites on peripherin are located on the amino-terminal region and a strong correlation was reported between phosphorylation of the head domain of peripherin and filament fragmentation [26, 27].
Neurofilaments
The three subunits of NFs are highly phosphorylated, where NF-L, NF-M and NF-H subunit carry between 3–5, 15–26 and 30–60 phosphate groups/molecule each, respectively [28–31]. Pulse-labeling studies using retinal ganglion cells in mice as a model system demonstrated that the phosphate groups on the amino-terminal head domain on NF subunits are added by second messenger-dependent kinases [32–34], where as the carboxyl-terminal phosphate groups are added by second messenger-independent kinases [11, 33–35]. These studies also showed that the amino-terminal head domain contains at least 2 distinct phosphorylation sites on NF-L and 6 distinct phosphorylation sites on NF-M and the phosphate groups on these sites are turned over rapidly as the NFs enter the proximal axon [33, 34, 36]. The major sites of phosphorylation on NF-L and NF-M subunits were identified to be Ser-55 (protein kinase A) and Ser-23 (protein kinase A as well as protein kinase C), respectively [36, 37]. Interestingly, in vitro, calcium/calmodulin-dependent kinase phosphorylated NF-L subunit on Ser-57 [38], and protein kinase C phosphorylated Ser-12, Ser-27, Ser-33 and Ser-51 [39]. The studies carried out to affect directly the activities of protein kinase A and protein kinase C by microinjection of the catalytic subunit of protein kinase A or inhibitors and activators of protein kinase A and Protein kinase C into lamprey neurons further strengthened the findings that NFs are in vivo substrates of these kinases [40].
Filament assembly from purified NF-L subunits showed certain parallels with the results on vimentin and other class III IFs. The phosphorylation of NF-L subunit by protein kinase A or protein kinase C prevented their assembly and disassembled filaments formed from NF-L [41], suggesting that Ser-55 phosphorylation on NF-L subunit plays a key role in NF assembly/disassembly. This is further supported by the findings that the phosphate groups on the head domain of NF-L are turned over rapidly once the NF-L enters the axons [36]. It is of significance to note that the protein kinase A (Ser-55), protein kinase C (Ser-51) and calcium/calmodulin-dependent kinase (Ser-57) phosphorylation sites are localized in the middle of the amino-terminal head domain which is known to be important in filament assembly [16, 25]. However, the reversible polymerization, as observed in homopolymeric filaments like vimentin and desmin, makes perfect sense for IF networks of the dividing cell, which is continually being reorganized; but neurofilaments are present in the postmitotic neurons, and once formed, do not undergo depolymerization. The reason may be that neurofilaments are obligate heteropolymers formed by the assembly of the NF-L, NF-M and NF-H subunits with NF-L subunit forming the ‘core’ of the filaments. NF-M subunit in the absence of NF-L subunit can assemble to form polymers but not 10 nm filaments, and NF-H subunit in the absence of NF-L does not polymerize [3, 4, 9]. Analysis of the roles of head domains of type IV neuronal IFs in filament assembly using domain-swapped chimeric proteins showed that the head domains of rat NF-L and NF-M are required for the obligate heteropolymeric NF filament formation [42]. Also, these studies showed that the head domain is essential for a-internexin’s ability to self-assemble in transfected cells [42]. Phosphorylation of the head domain of a-internexin by protein kinase A was found to regulate its assembly/disassembly into 10 nm filaments in vitro [43].
Spatio-temporal phosphorylation of type III intermediate filament proteins during mitosis
There is increasing evidence that mitotic IF phosphorylation is spatio-temporally regulated by distinct protein kinase(s) [12, 44–47]. By using site- and phosphorylation state-specific antibodies, Inagaki and coworkers demonstrated the spatial and temporal distributions of four distinct phosphorylation sites (Ser-8, Thr-7, Ser-13, and Ser-38) on the amino-terminal head domain of GFAP [48, 49]. Their studies identified that Ser-8 phosphorylation of GFAP in glial cells began in the prometaphase, remained until metaphase, and declined gradually thereafter. In contrast, the phosphorylation of residues Thr-7, Ser-13, and Ser-38 was spatially and temporally distinct, increasing in anaphase, maintained until telophase, and decreasing at the exit of mitosis, and this phosphorylation was localized specifically at the cleavage furrow (CF). Since this kinase seemed to be activated specifically at the CF during cytokinesis, it was named CF kinase [48, 49] and later identified to be a Rho-kinase [50–52].
Similarly, the use of site- and phosphorylation state-specific antibodies has led to the findings that Cdk1 phosphorylates vimentin from prometaphase to metaphase [53], and Aurora-B [54] and Rho-kinase [55] phosphorylates vimentin specifically at the CF from anaphase to the end of mitosis. The additional evidence for the spatio-temporal regulation of vimentin came from mutational studies. Inagaki and coworkers produced several vimentin mutants in which known vimentin kinase sites and an additional serine residue were altered. Among theses mutations, the vimentin mutation at Ser-72 in addition to mutations at sites phosphorylated by known vimentin kinases induced a remarkable phenotype in terms of filament separation. As shown in Fig. 2A, about 60% of cells that were transfected with this mutant formed IF bridge between daughter cells [56]. Mutational studies also revealed that efficient IF separation largely depends on Aurora-B and Rho-kinase activity [54, 57].
Fig. 2.
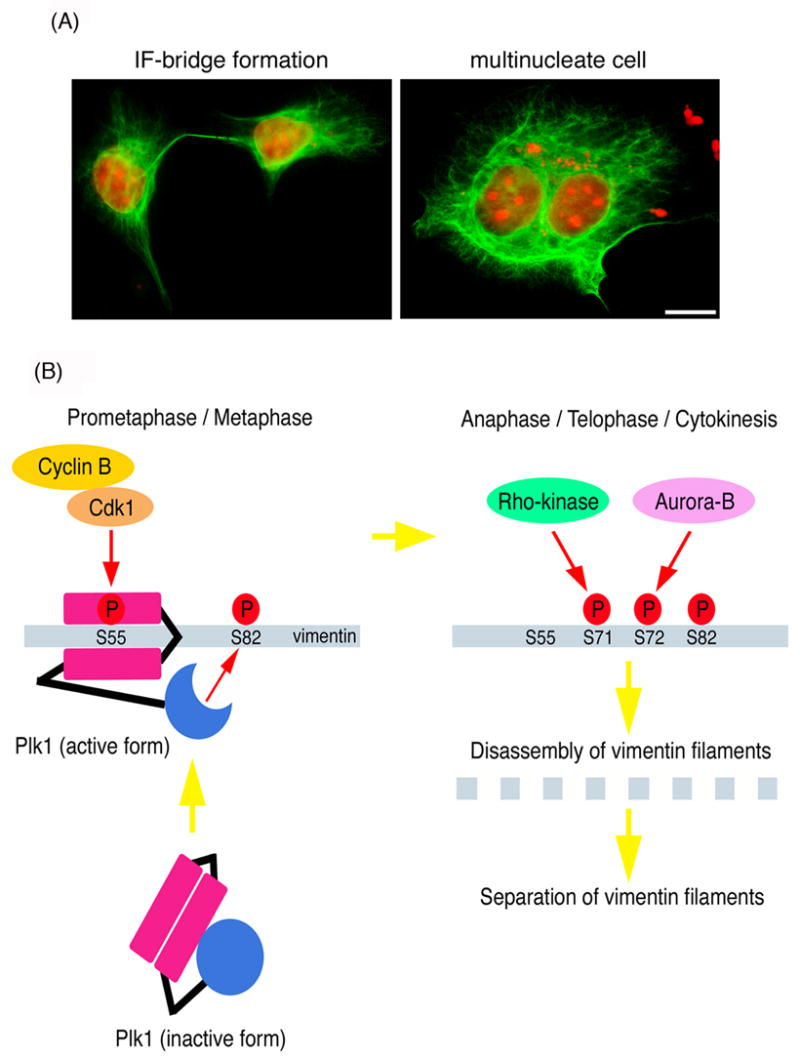
(A). Vimentin IF-bridge formation and multinucleate cell in T24 cells expressing mutant vimentin with mutations of phosphorylation sites by Rho-kinase, Aurora-B, Cdk1, and Plk1. Cells were stained with anti-vimentin antibody (green) and propidium iodide (red). Scale bar is 10 μm. (B). Cdk1 phosphorylation induces Plk1-mediated reorganization of vimentin filaments. From prometaphase to metaphase, Cdk1 phosphorylates Ser-55 on vimentin. Plk1 is recruited to Ser-55-phosphorylated vimentin and then activated. The activated Plk1 then phosphorylates Ser-82 on vimentin. At metaphase-anaphase transition, phospho-Ser-55 is dephosphorylated, but Ser-82 phosphorylation maintains at the high level. After anaphase, Rho-kinase and Aurora-B coordinately phosphorylates vimentin specifically at the cleavage furrow. The phosphorylated vimentin causes filament disassembly, which allows vimentin to efficiently segregate into 2 daughter cells.
More recently, Yamaguchi et al [58] have demonstrated a direct interaction between Plk1, one of mitotic kinase, and vimentin-Ser-55 phosphorylated by Cdk1, an event that led to Plk1 activation. Activated Plk1 induced the phosphorylation of vimentin-Ser-82, which was elevated from metaphase and maintained until the end of mitosis. Mutational analyses revealed that Plk1-induced vimentin-Ser-82 phosphorylation plays an important role in vimentin filaments segregation, coordinately with Rho-kinase and Aurora-B. These studies led to the suggestion of a novel mechanism in which Cdk1 regulated mitotic vimentin phosphorylation via not only a direct enzyme reaction but also Plk1 recruitment to vimentin [58] (Fig. 2B).
Mitotic checkpoint defects promote aneuploidy and tumorigenesis [59]. Polyploid cells are formed during development in otherwise diploid organisms, and also arise during a variety of pathological conditions [60]. It was suggested that genetic instability in tetraploid cells might provide a route to aneuploidy and thereby contribute to the development of cancer [60]. It has been suggested that impairment of IF phosphorylation by the abnormal regulation of these mitotic kinases would contribute to the generation of tetraploid cells that have two nuclei, leading to subsequent apoptotic cell death in normal cells. The loss of tetraploidy checkpoint and a subsequent catastrophic mitosis in these cells might result in the generation of aneuploid cells, giving rise to cancer development.
Carboxyl-terminal tail domain phosphorylation and regulation of neurofilament function in axon
Although NF subunits become phosphorylated in the cell body soon after they are synthesized, most extensive phosphorylation of NFs takes place after their polymerization [31, 61, 62]. Most of the phosphate groups added after NFs enter the axon are incorporated on the carboxyl-terminal tail domain [33–35]. Spatio-temporal phosphorylation of NFs in optic nerve occurs in a proximal-distal gradient fashion along the axon, as the NFs enter the axon hillock and continue down the axon [31, 61, 62]. Similarly, non-phosphorylated or poorly phosphorylated NF-M/H is found in cell bodies of DRG, ventral motor and sympathetic neurons while phosphorylated NF-M/H is found along the axons [63, 64]. Thus, the initial appearance and progressive phosphorylation of NF-M and NF-H in the axons are region-specific and appear to be correlated to synaptogenesis and myelination, as the mature axonal cytoskeleton begins to be established [65, 66]. Interestingly, extensive NF phosphorylation is not seen in embryonic or adult neuronal dendrites, even though NFs are present [63, 64]. This may be either due to lack of specific cellular signals needed to activate the kinases responsible for NF phosphorylation or the NFs are organized in a manner different than in axons and the phosphorylation sites are not accessible to the kinases.
Most of the NF phosphorylation sites on the carboxyl-terminal tail domain of NF-M and NF-H subunits are located on the multiple “KSP” repeat motifs [67–69], although other Ser/Thr residues get phosphorylated. It is now evident that proline-directed kinases Cdk5 and MAP kinases are the principal kinases that phosphorylate the Ser residues on the KSP repeats [70–72], whereas, the Ser/Thr residues on the Glu-rich regions on the tail domain of mammalian NF subunits are phosphorylated by casein kinases [33, 73, 74]. Co-transfection studies of NF-H with p35/Cdk5 showed that KSPXK repeats were phosphorylated by Cdk5 but not the KSPXXXK repeats [71]. By comparison, the KSPXXXK repeats between residues508 and 763 of rodent NF-H, of which there are 41, were preferentially phosphorylated by MAP kinase1/2 (ERK1/2) [71]. Also, there is evidence that phosphorylation of some sites on the KSP repeat motifs by proline-directed kinases might be influenced by the presence or absence of phosphate groups on the head domain of the NF subunit. Pant and colleagues [75] demonstrated that, in rat cortical neurons, the head domain phosphorylation of endogenous NF-M by forskolin-activated protein kinase A inhibited NF-M tail domain phosphorylation, suggesting that assembly and KSP phosphorylation of NF-M in axons depends on prior dephosphorylation of head domain sites. These findings support the idea that phosphorylation of head domain sites of NFs not only regulate the NF assembly/disassembly, they might play a role in determining the phosphorylation state of specific carboxyl-terminal tail domain phosphorylation sites.
There is some evidence that phosphorylation of KSP motif rich tail domains on NF-M and NF-H might be regulated by the activation of proline-directed kinases (ERK1/2, Cdk5, p38 MAP kinase or SAPK) by signal transduction cascades triggered by growth factors [76], Ca+2 influx [77], extracellular matrix constituents through integrins [78], and glial factors like myelin-associated glycoprotein [79] (Fig. 1B). For example, EGF, which is known to specifically activate ERK1/2 [80], was shown to stimulate NF-H phosphorylation [76]. Also, the membrane depolarization induced Ca+2 influx through L type calcium channels resulted in neurite outgrowth, activation of Erk1/2, and increased phosphorylation of NF-M tail domain [77]. By comparison, under stress conditions (osmotic, UV), stress-activated protein kinase (SAPK) becomes active and the Ser residues on KSPXE motifs become phosphorylated in the perikarya [81]. Under these conditions, MAP kinases were not activated. In addition, Li et al [78,82] provided evidence that intracellular signaling by the a1b1 integrin-induced activation of Cdk5 is involved in neurite outgrowth and human NF-H ‘KSP’ tail domain phosphorylation in differentiated human SH-SY5Y cells. The phosphorylation of these ‘KSP’ tail domain sites has been suspected to play roles in forming cross-bridges between neurofilaments and microtubules, slowing axonal transport, immobilizing neurofilaments and promoting their integration into cytoskeleton lattice and, in doing so, to control axonal caliber and stabilize the axon [11–13.83–87].
Fig. 1.
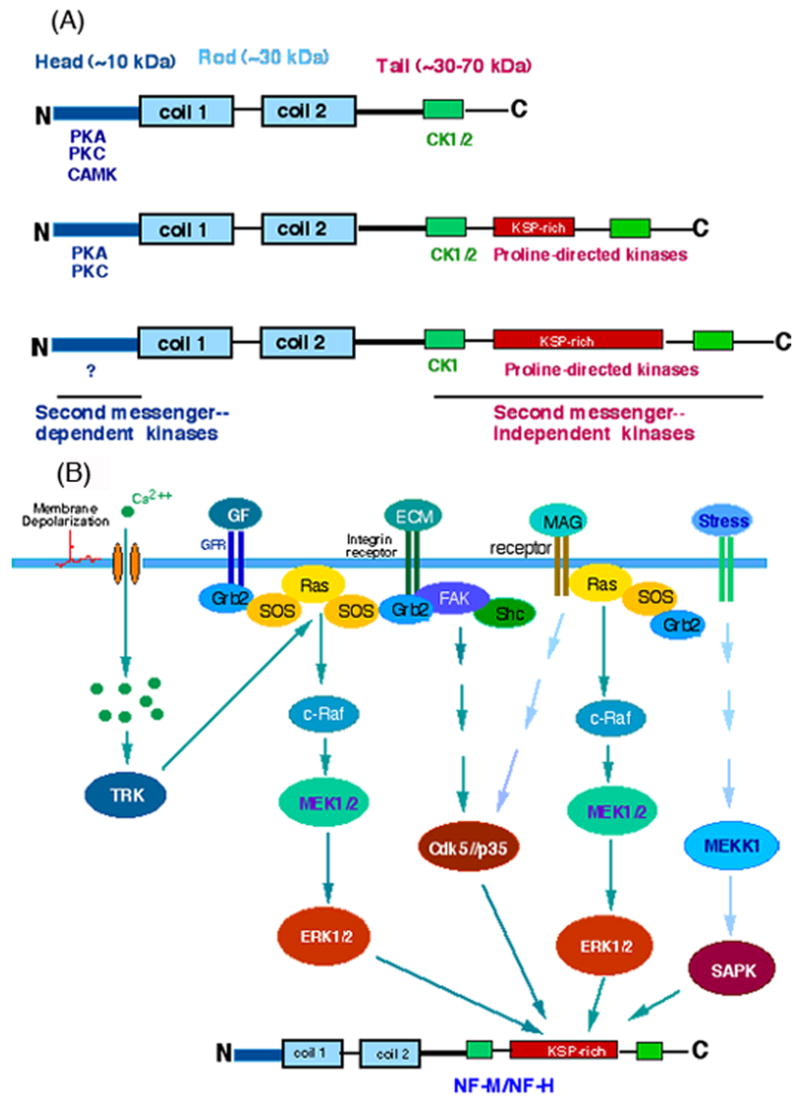
(A). Schematics shows the current information on phosphorylation of neurofilament subunits (NF-L, NF-M and NF-H) by various protein kinases on the amino-terminal head and carboxyl-terminal tail domains. (B). A signal transduction cascade hypothesized to be involved in the phosphorylation of ‘KSP motifs’ on the NF-M/NF-H subunits. According to this hypothesis Ca+2 influx, growth factors (GF), extracellular matrix (ECM), myelin-associated glycoprotein (MAG) and stress (osmotic, UV) are proposed to activate MAP kinases (ERK1/2, SAPK) or Cdk5, which are known to phosphorylate NF-M/NF-H in vivo.
Axonal transport
Neurofilament phosphorylation has long been considered to regulate their axonal transport rate and in doing so to provide stability to mature axons. A considerable body of evidence, from a number of laboratories using diverse systems and approaches, supports the notion that phosphorylation of carboxyl-terminal tail region, in particular those of NF-H, regulates NF axonal transport (88–104). The carboxyl-terminal tail regions of NF-H and NF-M protrude laterally from the filament backbone to form ‘side-arms’ when phosphorylated [11, 13, 83, 105–107]. Phosphorylation of these carboxyl-terminal ‘side-arms’ regulates the interactions of NFs with each other and with other cytoskeletal structures, and in doing so, mediate the formation of a cytoskeletal lattice that supports the mature axon [11, 13, 83, 105–107].
Studies from several laboratories support the idea that the microtubule motors kinesin and dynein mediate anterograde and retrograde NF axonal transport, respectively [108–117]. In this regard, carboxyl-terminal phosphorylation of NF-H progressively restricts association of NFs with kinesin, to the point where monoclonal antibody RT97-reactive phosphorylated epitope containing NFs do not associate with kinesin at all [108] but instead demonstrate selective affinity for dynein [110]; restriction of binding to an anterograde motor provides one mechanism by which carboxyl-terminal phosphorylation can slow NF axonal transport, especially when coupled with promotion of binding to a retrograde motor. The ability of kinesin and dynein – motors which undergo so-called “fast” axonal transport, to translocate cargo such as NFs, the majority of which undergo so-called “slow” transport, has been validated by the demonstration that NFs undergo a series of rapid bursts at a fast rate, interspersed with prolonged pauses, which averages out slow transport [117, 118]. The dynamics of moving and pausing NFs in terms of their association and dissociation with motor systems have been mathematically modeled, and this model agrees with published data from several in vivo systems [119, 120]. This model describes a pool of NFs associated with motors, and a pool that is dissociated but can readily re-associate. While this model did not present any definitive regulatory factors, the above studies would suggest that NF carboxyl-terminal phosphorylation is one factor regulating the shift of NFs between these two pools.
Studies by Rao and colleagues [121–123], who examined the role of NF-M and NF-H carboxyl-terminal tail domain on axon radial growth, NF spacing, and NF transport rate in mutated mice, have questioned the correlation of NF-H/NF-M tail domain phosphorylation to axonal transport of NFs. When NF-H gene in mice was replaced by one deleted in the NF-H tail, at least in optic nerve axons, the loss of NF-H tail did not affect the rate of transport of NF subunits [121–122]. In a similar study, NF-M tail-deleted mutant mice exhibited severely inhibited radial growth of both motor and sensory axons, and reduced axon caliber; but interestingly, the average rate of axonal transport of NFs was unaltered despite these substantial effects on axon morphology [122]. However, the advent of fluorescently conjugated proteins, and the ability to manipulate the activity of various protein kinases in vivo, has provided addition new tools to better understand the molecular basis of NF axonal transport. For example, Miller and colleagues [88] used transfection of cultured cortical neurons with constructs expressing site-directed mutated forms of NF-H along with wild-type NF-H, each of which was tagged with green fluorescent protein to allow real-time monitoring of axonal transport within living neurons. In this study, 7 serines, known to be phosphorylated, on the carboxyl-terminal region of NF-H were mutated to alanine or aspartate to generate “constitutively-nonphosphorylated” and “constitutively-phosphorylated,” NF-H, respectively. Analyses of transport rates demonstrated that NFs containing constitutively-nonphosphorylated NF-H underwent transport 26% faster than those containing wild-type NF-H, while NFs containing constitutively-phosphorylated NF-H underwent transport 35% slower than those containing wild-type NF-H [88]. These findings strongly support the earlier views on correlation between NF-H tail phosphorylation and axonal transport rates of NFs that were largely based on NF transport studies carried out by using NF-radiolabeling methods. In future, studies utilizing new tools and technologies that are becoming available are expected to provide new insights into the mechanism of NF transport.
Axon caliber
Axon caliber is a key determinant of conduction velocity in nerve cells. It has been suggested that regulation of phosphorylation of KSP repeats on the tail domain of NF-H and NF-M may alter charge density on ‘side-arms’ and thereby play a role in NF spacing, and thus the axon caliber, by repulsive interactions of negatively charged sites [10, 12, 64]. Although this view is supported by the fact that addition of most phosphate groups to the tail takes place after the NFs enter the axon and it continues throughout their journey to the distal end [31, 61, 124], the specific dynamics of NF tail extensions, however, have not been easy to demonstrate. The relationship between phosphorylation and carboxyl-terminal tail extension was called into question when Hisanaga and Hirokawa [125] showed that they were able to remove 90% of phosphates on NF-H tail without affecting the extended configuration of the ‘side-arm’, as visualized on grids by low angle rotary shadowing. The production of various NF ‘knockout’ and transgenic mice suggests that ‘side-arm’ phosphorylation of NF-H and NF-M contributes only in part to the radial growth of large axons [126]. Radial growth of axons may correlate with neurofilament number which relies more on NF-L and NF-M expression, since both are more important to filament assembly than NF-H.
Since ‘side-arms’ appear without phosphorylation, what role, if any, does NF-H/NF-M tail phosphorylation play in axon radial growth? NF cross-bridge frequencies estimated in vitro by gelation and viscosity measurements suggest that phosphorylation of KSP motifs on NF-H modulate the rate and extent of cross-bridging [127]. Incubating phosphorylated and unphosphorylated peptides containing KSP motifs with NF proteins in vitro showed a significant competition with cross-bridge interactions between NFs, suggesting that small side arms may extend from unphosphorylated NF-H but these extended much further as phosphorylated KSP repeats uncoil, presumably by charge-charge interactions [127]. This could explain the continued presence of short cross-bridges in transfected NF-H deletion mutants lacking KSP-rich subdomain of the tail and the correlation of ‘side-arms’ length with the number of KSP repeats [128]. Also, available evidence supports a regulatory role of NF tail domain phosphorylation on neurofilament interactions with microtubules and other cytoskeletal proteins. Hisanaga and Hirokawa [125] showed that dephosphorylated NF-H has a high binding affinity to microtubules and phosphorylation dissociates NFs from microtubules. Phosphorylation of NF-H by Cdk5, which phosphorylates KSP motifs, also reduced NF affinity to microtubules [129].
Role of IF phosphorylation in disease pathobiology
The importance of types III and IV IFs in cellular function is evident from the fact that perturbation of their function accounts for several genetically determined protein misfolding/aggregation diseases [130–137]. As an example, studies showing increased axonal accumulation of NFs in transgenic mice or in mice expressing mutant NF subunit have shown that aberrant organization or assembly of NFs is sufficient to cause disease arising from selective dysfunction and degeneration of neurons [138–140]. In fact, perikaryal accumulations/aggregations of aberrantly phosphorylated neurofilaments are a pathological feature of several human neurodegenerative diseases, such as in Alzheimer’s disease, motor neuron diseases and Parkinson’s disease [11–13, 105, 132, 140]. Since the assembly, transport and disposition of NFs is modulated by phosphorylation/dephosphorylation events in a topographic manner, it is easily seen how abnormalities in metabolic events affecting any of these functions could have direct or indirect pathologic consequences. In particular, the differential timing of phosphate additions and turnover at specific sites on three subunits as NFs move from the cell body toward the distal end of the axon, may explain why neurofibrillary lesions predominate in the perikaryon in some neurodegenerative diseases and appear at proximal or distal sites along the axon in other diseases [11, 132, 136, 137, 140]. In this regard, Shea and coworkers [141] demonstrated that increased Cdk5 activity in cultured chicken dorsal-root-ganglion neurons, obtained either by transfection of the Cdk5 and p35 genes or by microinjection of active Cdk5, increased phosphorylation of NFs, caused abnormal thickening in the perikaryon and reduced their axonal transport, supporting the idea that premature phosphorylation of carboxyl-terminal tails in the cell body promotes inappropriate interactions of NFs with cytoskeletal elements, thereby preventing their entry into axons and cause neurofibrillary lesions within perikarya [ 11, 141]. The known inhibition of proteolytic breakdown of NFs due to abnormal phosphorylation and aggregation by Ca+2-activated protease could further contribute to the neurofibrillary lesions [142,143]. It is evident that dysregulation of topographic phosphorylation of IF proteins can result in their misfolding/aggregation but whether or not these events are responsible for causing the diseases such as motor neuron diseases remains to be resolved.
Conclusion and future prospects
There is increasing evidence that site-specific phosphorylation of IF proteins can affect their assembly, structural organization and disposition. Recent analyses on site-specific IF phosphorylation in defined subcellular locations at various mitotic stages has demonstrated that several protein kinases, including Cdk1, Plk1, Rho-kinase, and Aurora-B, phosphorylate IF proteins in a spatiotemporally regulated manner, leading to the efficient separation of IF to daughter cells. It is possible that the impairment of IF phosphorylation by mitotic kinases might result in the formation of multinucleated cells. Similarly, while we have made progress in understanding the role of kinases in the control of NF-phosphorylation in neurons, there are still many questions to be answered. How and why extensive NF-phosphorylation and stabilization is restricted to axonal compartment remains unknown. Is the post-phosphorylation process Pin1-induced cis/trans isomerization of Pro at phospho-Ser/Thr Pro residues in NF-M/H involved in NF function in the axon? What is the role of protein phosphatases in topographic phosphorylation of IFs? How does deregulation of NF phosphorylation lead to abnormal accumulation of phosphorylated NFs in neuronal cell bodies, a pathology characteristic of several neurodegenerative disorders?
In addition, future studies on the identification of novel protein kinases and IF interacting protein might provide us new insights into the molecular basis of IF organization and functions. The elucidation of the mechanism of crosstalk between IFs and intercellular signaling pathways will improve our understanding of tumor development, invasion, and metastasis. Finally, the development of site- and phosphorylation state-specific antibodies of IF proteins should further facilitate our understanding of the cytoskeletal organization, signal transduction, and transcriptional mechanisms as well as pathobiology of diseases, and could be further utilized for developing new drug therapies for protein misfolding/aggregation diseases involving IF proteins.
Abbreviations
- IF
Intermediate filaments
- NFs
Neurofilaments
- NF-L
NF-M, NF-H, molecular weights, neurofilament triplet subunits of (L)ow, (M)iddle and (H)igh
- GFAP
glial fibrillary acidic protein
- Cdk1
cyclin dependent kinase 1
- Cdk5
cyclin dependent kinase 5
- PAK1
p21-activated activated kinase
- CF
cleavage furrow
- Pin1
peptidylprolyl isomerase
- ERK1/2
extracellular signal-regulated kinase 1/2
- MAPK1/2
mitogen-activated kinase1/2
- TRK
tyrosine kinase
- CK1/2
casein kinase1/2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parry DA, Steinert PM. Intermediate filament structure. Curr Opin Cell Biol. 1992;4:94–98. doi: 10.1016/0955-0674(92)90064-j. [DOI] [PubMed] [Google Scholar]
- 2.Fuchs E, Weber K. Intermediate filaments: structure, dynamics, function, and disease. Annu Rev Biochem. 1994;63:345–382. doi: 10.1146/annurev.bi.63.070194.002021. [DOI] [PubMed] [Google Scholar]
- 3.Herrmann H, Hesse M, Reichenzeller M, Aebi U. Functional complexity of intermediate filament cytoskeletons: from structure to assembly to gene ablation. Int Rev Cytol. 2003;223:83–175. doi: 10.1016/s0074-7696(05)23003-6. [DOI] [PubMed] [Google Scholar]
- 4.Herrmann H, Aebi U. Intermediate filaments: molecular structure, assembly mechanism, and integration into functionally distinct intracellular scaffolds. Annu Rev Biochem. 2004;73:749–789. doi: 10.1146/annurev.biochem.73.011303.073823. [DOI] [PubMed] [Google Scholar]
- 5.Coulombe PA, Ma L, Yamada S, Wawersik M. Intermediate filaments at a glance. J Cell Sci. 2001;114:4345–4347. doi: 10.1242/jcs.114.24.4345. [DOI] [PubMed] [Google Scholar]
- 6.Omary MB, Ku NO, Tao GZ, Toivola DM, Liao J. Heads and tails” of intermediate filament phosphorylation: multiple sites and functional insights. Trends Biochem Sci. 2006;31:383–394. doi: 10.1016/j.tibs.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Steinert PM, Parry DA. Intermediate filaments: conformity and diversity of expression and structure. Annu Rev Cell Biol. 1985;1:41–65. doi: 10.1146/annurev.cb.01.110185.000353. [DOI] [PubMed] [Google Scholar]
- 8.Blumenberg M. Evolution of homologous domains of cytoplasmic intermediate filament proteins and lamins. Mol Biol Evol. 1989;6:53–65. doi: 10.1093/oxfordjournals.molbev.a040533. [DOI] [PubMed] [Google Scholar]
- 9.Herrmann H, Aebi U. Intermediate filaments and their associates: multi-talented structural elements specifying cytoarchitecture and cytodynamics. Curr Opin Cell Biol. 2000;12:79–90. doi: 10.1016/s0955-0674(99)00060-5. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs E, Cleveland DW. A structural scaffolding of intermediate filaments in health and disease. Science. 1998;279:514–519. doi: 10.1126/science.279.5350.514. [DOI] [PubMed] [Google Scholar]
- 11.Nixon RA, Sihag RK. Neurofilament phosphorylation: A new look at regulation and function. Trends Neurosci. 1991;14:501–506. doi: 10.1016/0166-2236(91)90062-y. [DOI] [PubMed] [Google Scholar]
- 12.Inagaki M, Matsuoka Y, Tsujimura K, Ando S, Tokui T, Takahashi T, Inagaki N. Dynamic property of intermediate filaments: Regulation by phosphorylation. BioEssays. 1996;18:481–487. [Google Scholar]
- 13.Grant P, Pant HC. Neurofilament protein synthesis and phosphorylation. J Neurocytol. 2000;29:843–872. doi: 10.1023/a:1010999509251. [DOI] [PubMed] [Google Scholar]
- 14.Kesavpanny S, Quarles RH, Pant HC. Intermediate filaments: phosphorylation and signal transduction. In: Paramio J, editor. Intermediate Filaments 2006, Landes Bioscience and Springer Science. 2006. pp. 52–73. [Google Scholar]
- 15.Helfand BT, Chang L, Goldman RD. Intermediate filaments are dynamic and motile elements of cellular architecture. J Cell Sci. 2004;117:133–141. doi: 10.1242/jcs.00936. [DOI] [PubMed] [Google Scholar]
- 16.Heins S, Wong PC, Muller S, Goldie K, Cleveland DW, Aebi U. The rod domain of NF-L determines neurofilament architecture, whereas the end domains specify filament assembly and network formation. J Cell Biol. 1993;123:1517–1533. doi: 10.1083/jcb.123.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inagaki M, Nishi Y, Nishizawa K, Matsuyama M, Sato C. Site-specific phosphorylation induces disassembly of vimentin filaments in vitro. Nature. 1987;328:649–652. doi: 10.1038/328649a0. [DOI] [PubMed] [Google Scholar]
- 18.Geisler N, Weber K. Phosphorylation of desmin in vitro inhibits formation of intermediate filaments; identification of three kinase A sites in the aminoterminal head domain. EMBO J. 1988;7:15–20. doi: 10.1002/j.1460-2075.1988.tb02778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chou YH, Bischoff JR, Beach D, Goldman RD. Intermediate filament reorganization during mitosis is mediated by p34cdc2 phosphorylation of vimentin. Cell. 1990;62:1063–1071. doi: 10.1016/0092-8674(90)90384-q. [DOI] [PubMed] [Google Scholar]
- 20.Chou YH, Ngai KL, Goldman RD. The regulation of intermediate filament reorganization in mitosis. p34cdc2 phosphorylates vimentin at a unique N-terminal site. J Biol Chem. 1991;26:7325–7328. [PubMed] [Google Scholar]
- 21.Eriksson JE, He T, Trejo-Skalli AV, Harmala-Brasken AS, Hellman J, Chou YH, Goldman RD. Specific in vivo phosphorylation sites determine the assembly dynamics of vimentin intermediate filaments. J Cell Sci. 2004;117:919–32. doi: 10.1242/jcs.00906. [DOI] [PubMed] [Google Scholar]
- 22.Goto H, Kosako H, Tanabe K, Yanagida M, Sakusai M, Amano M, Kaibuchi K, Inagaki M. Phosphorylation of vimentin by Rho-associated kinase at a unique amino-terminal site that is specifically phosphorylated during cytokinesis. J Biol Chem. 1998;273:11728–11736. doi: 10.1074/jbc.273.19.11728. [DOI] [PubMed] [Google Scholar]
- 23.Sin WC, Chen XQ, Leung T, Lim L. RhoA-binding kinase alpha translocation is facilitated by the collapse of the vimentin intermediate filament network. Mol Cell Biol. 1998;18:6325–6339. doi: 10.1128/mcb.18.11.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Q-F, Spinelli AM, Wang R, Anfinogenova Y, Singer HA, Tang DD. Critical role of vimentin phosphorylation at Ser-56 by p21-activated kinase (PAK) in vimentin cytoskeleton signaling. J Biol Chem. 2006;281:34716–34724. doi: 10.1074/jbc.M607715200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gill SR, Wong PC, Monteiro MJ, Cleveland DW. Assembly properties of dominant and recessive mutations in the small mouse neurofilament (NF-L) subunit. J Cell Biol. 1990;111:2005–2019. doi: 10.1083/jcb.111.5.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huc C, Escurat M, Djabali K, Derer M, Landon F, Gros F, Portier MM. Phosphorylation of peripherin, an intermediate filament protein, in mouse neuroblastoma NIE 115 cell line and in sympathetic neurons. Biochem Biophys Res Commun. 1989;160:772–779. doi: 10.1016/0006-291x(89)92500-x. [DOI] [PubMed] [Google Scholar]
- 27.Giasson BI, Mushynski WE. Intermediate filament disassembly in cultured dorsal root ganglion neurons is associated with amino-terminal head domain phosphorylation of specific subunits. J Neurochem. 1998;70:1869–1875. doi: 10.1046/j.1471-4159.1998.70051869.x. [DOI] [PubMed] [Google Scholar]
- 28.Julien JP, Mushynski WE. Multiple phosphorylation sites in mammalian neurofilament polypeptides. J Biol Chem. 1982;257:10467–10470. [PubMed] [Google Scholar]
- 29.Jones SM, Williams RC. Phosphate content of mammalian neurofilaments. J Biol Chem. 1982;257:9902–9905. [PubMed] [Google Scholar]
- 30.Ksiezak-Reding H, Yen SH. Phosphatase and carbocyanine dye binding define different types of phosphate groups in mammalian neurofilaments. J Neurosci. 1987;7:3554–3360. doi: 10.1523/JNEUROSCI.07-11-03554.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nixon RA, Lewis SE. Differential turnover of phosphate groups on neurofilament subunits in mammalian neurons in vivo. J Biol Chem. 1986;261:16298–16301. [PubMed] [Google Scholar]
- 32.Sihag RK, Jeng AY, Nixon RA. Phosphorylation of neurofilament proteins by protein kinase C. FEBS Lett. 1988;233:181–185. doi: 10.1016/0014-5793(88)81380-2. [DOI] [PubMed] [Google Scholar]
- 33.Sihag RK, Nixon RA. In vivo phosphorylation of distinct domains of the 70-kilodalton neurofilament subunit involves different protein kinases. J Biol Chem. 1989;264:457–464. [PubMed] [Google Scholar]
- 34.Sihag RK, Nixon RA. Phosphorylation of the amino-terminal head domain of the middle molecular mass 145-kDa subunit of neurofilaments: Evidence for regulation by second messenger-dependent protein kinases. J Biol Chem. 1990;265:4166–4171. [PubMed] [Google Scholar]
- 35.Julien JP, Mushynski WE. The distribution of phosphorylation sites among identified proteolytic fragments of mammalian neurofilaments. J Biol Chem. 1983;258:4019–4025. [PubMed] [Google Scholar]
- 36.Sihag RK, Nixon RA. Identification of Ser-55 as a major protein kinase A phosphorylation site on the 70-kDa subunit of neurofilaments: Early turnover during axonal transport. J Biol Chem. 1991;266:18861–1887. [PubMed] [Google Scholar]
- 37.Sihag RK, Jaffe H, Nixon RA, Rong X. Serine-23 is a major protein kinase A phosphorylation site on the amino-terminal head domain of the middle molecular mass subunit of neurofilament proteins. J Neurochem. 1999;72:491–499. doi: 10.1046/j.1471-4159.1999.0720491.x. [DOI] [PubMed] [Google Scholar]
- 38.Hashimoto R, Nakamura Y, Komai S, Kashiwagi Y, Tamura K, Goto T, Aimoto S, Kaibuchi K, Shiosaka S, Takeda M. Site-specific phosphorylation of neurofilament-L is mediated by calcium/calmodulin-dependent protein kinase II in the apical dendrites during long-term potentiation. J Neurochem. 2000;75:373–382. doi: 10.1046/j.1471-4159.2000.0750373.x. [DOI] [PubMed] [Google Scholar]
- 39.Gonda Y, Nishizawa K, Ando S, Kitamura S, Minoura Y, Nishi Y, Inagaki M. Involvement of protein kinase C in the regulation of assembly-disassembly of neurofilaments in vitro. Biochem Biophys Res Commun. 1990;167:1316–1325. doi: 10.1016/0006-291x(90)90667-c. [DOI] [PubMed] [Google Scholar]
- 40.Hall GF, Kosik KS. Axotomy-induced neurofilament phosphorylation is inhibited in situ by microinjection of PKA and PKC inhibitors into identified lamprey neurons. Neuron. 1993;10:613–625. doi: 10.1016/0896-6273(93)90164-m. [DOI] [PubMed] [Google Scholar]
- 41.Hisanaga S, Gonda Y, Inagaki M, Ikai A, Hirokawa N. Effects of phosphorylation of the neurofilament L protein on filamentous structures. Cell Regul. 1990;1:237–248. doi: 10.1091/mbc.1.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ching GY, Liem RK. Analysis of the roles of the head domains of type IV rat neuronal intermediate filament proteins in filament assembly using domain-swapped chimeric proteins. J Cell Sci. 1999;112:2233–2240. doi: 10.1242/jcs.112.13.2233. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka J, Ogawara M, Ando S, Shibata M, Yatani R, Kusagawa M, Inagaki M. Phosphorylation of a 62 kd porcine alpha-internexin, a newly identified intermediate filament protein. Biochem Biophys Res Commun. 1993;196:115–123. doi: 10.1006/bbrc.1993.2223. [DOI] [PubMed] [Google Scholar]
- 44.Inagaki M, Inagaki N, Takahashi T, Takai Y. Phosphorylation-dependent control of structures of intermediate filaments: A novel approach using site- and phosphorylation state-specific antibodies. J Biochem. 1997;121:407–414. doi: 10.1093/oxfordjournals.jbchem.a021603. [DOI] [PubMed] [Google Scholar]
- 45.Foisner R. Dynamic organization of intermediate filaments and associated proteins during the cell cycle. BioEssays. 1997;19:297–305. doi: 10.1002/bies.950190407. [DOI] [PubMed] [Google Scholar]
- 46.Inagaki N, Ito M, Nakano T, Inagaki M. Spatiotemporal distribution of protein kinase and phosphatase activities. Trends Biochem Sci. 1994;19:448–452. doi: 10.1016/0968-0004(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 47.Ku NO, Liao J, Chou CF, Omary MB. Implications of intermediate filament protein phosphorylation. Cancer Metast Rev. 1998;15:429–444. doi: 10.1007/BF00054011. [DOI] [PubMed] [Google Scholar]
- 48.Nishizawa K, Yano T, Shibata M, Ando S, Saga S, Takahashi T, Inagaki M. Specific localization of phospho-intermediate filament protein in the constricted area of dividing cells. J Biol Chem. 1991;266:3074–3079. [PubMed] [Google Scholar]
- 49.Matsuoka Y, Nishizawa K, Yano T, Shibata M, Ando S, Takahashi T, Inagaki M. Two different protein kinases act on a different time schedule as glial filament kinases during mitosis. EMBO J. 1992;11:2895–2902. doi: 10.1002/j.1460-2075.1992.tb05358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaibuchi K, Kuroda S, Amano M. Regulation of the cytoskeleton and cell adhesion by Rho family GTPases in mammalian cells. Annu Rev Biochem. 1999;68:459–486. doi: 10.1146/annurev.biochem.68.1.459. [DOI] [PubMed] [Google Scholar]
- 51.Kosako H, Amano M, Yanagida M, Tanabe K, Nishi Y, Kaibuchi K, Inagaki M. Phosphorylation of glial fibrillary acidic protein at the same sites by cleavage furrow kinase and Rho-associated kinase. J Biol Chem. 1997;272:10333–10336. doi: 10.1074/jbc.272.16.10333. [DOI] [PubMed] [Google Scholar]
- 52.Yasui Y, Amano M, Nagata K, Inagaki N, Nakamura H, Saya H, Kaibuchi K, Inagaki M. Roles of Rho-associated kinase in cytokinesis: Mutations in Rho-associated kinase phosphorylation sites impair cytokinetic segregation of glial filaments. J Cell Biol. 1998;143:1249–1258. doi: 10.1083/jcb.143.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsujimura K, Ogawara M, Takeuchi Y, Imajoh-Ohmi S, Ha MH, Inagaki M. Visualization and function of vimentin phosphorylation by cdc2 kinase during mitosis. J Biol Chem. 1994;269:31097–31106. [PubMed] [Google Scholar]
- 54.Goto H, Yasui Y, Kawajiri A, Nigg EA, Terada Y, Tatsuka M, Nagata K, Inagaki M. Aurora-B regulates the cleavage furrow-specific vimentin phosphorylation in the cytokinetic process. J Biol Chem. 2003;278:8526–8530. doi: 10.1074/jbc.M210892200. [DOI] [PubMed] [Google Scholar]
- 55.Goto H, Kosako H, Tanabe K, Yanagida M, Sakusai M, Amano M, Kaibuchi K, Inagaki M. Phosphorylation of vimentin by Rho-associated kinase at a unique amino-terminal site that is specifically phosphorylated during cytokinesis. J Biol Chem. 1998;273:11728–11736. doi: 10.1074/jbc.273.19.11728. [DOI] [PubMed] [Google Scholar]
- 56.Yasui Y, Goto H, Matsui S, Manser E, Lim L, Nagata K, Inagaki M. Protein kinases required for segregation of vimentin filaments in mitotic process. Oncogene. 2001;20:2868–2876. doi: 10.1038/sj.onc.1204407. [DOI] [PubMed] [Google Scholar]
- 57.Yasui Y, Urano T, Kawajiri A, Nagata K, Tatsuka M, Saya H, Fukukawa K, Takahashi T, Izawa I, Inagaki M. Autophosphorylation of a newly identified site of Aurora-B is indispensable for cytokinesis. J Biol Chem. 2004;279:12997–13003. doi: 10.1074/jbc.M311128200. [DOI] [PubMed] [Google Scholar]
- 58.Yamaguchi T, Goto H, Yokoyama T, Silljé H, Hanisch A, Uldschmid A, Takai Y, Oguri T, Nigg EA, Inagaki M. Phosphorylation by Cdk1 induces Plk1-mediated vimentin phosphorylation during mitosis. J Cell Biol. 2005;171:431–436. doi: 10.1083/jcb.200504091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kops GJP, Weaver BA, Cleveland DW. On the road to cancer: aneuploidy and the mitotic checkpoint. Nat Rev Cancer. 2005;5:773–785. doi: 10.1038/nrc1714. [DOI] [PubMed] [Google Scholar]
- 60.Storchova Z, Pellman D. From polyploidy to aneuploidy, genome instability and cancer. Nat Rev Mol Cell Biol. 2004;5:45–54. doi: 10.1038/nrm1276. [DOI] [PubMed] [Google Scholar]
- 61.Nixon RA, Lewis SE, Dahl D, Marotta CA, Drager UC. Early posttranslational modifications of the three neurofilament subunits in mouse retinal ganglion cells: neuronal sites and time course in relation to subunit polymerization and axonal transport. Brain Res Mol Brain Res. 1989;5:93–108. doi: 10.1016/0169-328x(89)90001-6. [DOI] [PubMed] [Google Scholar]
- 62.Nixon RA, Lewis SE, Marotta CA. Posttranslational modification of neurofilament proteins by phosphate during axoplasmic transport in retinal ganglion cell neurons. J Neurosci. 1987;7:1145–1158. doi: 10.1523/JNEUROSCI.07-04-01145.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sternberger LA, Sternberger NH. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci USA. 1983;80:6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee VM, Carden MJ, Schlaepfer WW, Trojanowski JQ. Monoclonal antibodies distinguish several differentially phosphorylated states of the two largest rat neurofilament subunits (NF-H and NF-M) and demonstrate their existence in the normal nervous system of adult rats. J Neurosci. 1987;7:3474–3488. doi: 10.1523/JNEUROSCI.07-11-03474.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carden MJ, Trojanowski JQ, Schlaepfer WW, Lee VM. Two-stage expression of neurofilament polypeptides during rat neurogenesis with early establishment of adult phosphorylation patterns. J Neurosci. 1987;7:3489–3504. doi: 10.1523/JNEUROSCI.07-11-03489.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sanchez I, Hassinger L, Sihag RK, Cleveland DW, Mohan P, Nixon RA. Local control of neurofilament accumulation during radial growth of myelinating axons in vivo: Selective role of site-specific phosphorylation. J Cell Biol. 2000;151:1013–1024. doi: 10.1083/jcb.151.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Geisler N, Vandekerckhove J, Weber K. Location and sequence characterization of the major phosphorylation sites of the high molecular mass neurofilament proteins M and H. FEBS Lett. 1987;221:403–407. doi: 10.1016/0014-5793(87)80964-x. [DOI] [PubMed] [Google Scholar]
- 68.Lee VM, Otvos L, Jr, Carden MJ, Hollosi M, Dietzschold B, Lazzarini RA. Identification of the major multiphosphorylation site in mammalian neurofilaments. Proc Natl Acad Sci USA. 1988;85:1998–2002. doi: 10.1073/pnas.85.6.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu ZS, Liu WS, Willard MB. Identification of six phosphorylation sites in the COOH-terminal tail region of the rat neurofilament protein M. J Biol Chem. 1992;267:4467–4471. [PubMed] [Google Scholar]
- 70.Sun D, Leung CL, Liem RK. Phosphorylation of the High Molecular Weight Neurofilament Protein (NF-H) by Cdk5 and p35. J Biol Chem. 1996;271:14245–14251. doi: 10.1074/jbc.271.24.14245. [DOI] [PubMed] [Google Scholar]
- 71.Veeranna V, Amin ND, Ahn NG, Jaffe H, Winters CA, Grant P, Pant HC. Mitogen-activated protein kinases (Erk1, 2) phosphorylate Lys-Ser-Pro (KSP) repeats in neurofilament proteins NF-H and NF-M. J Neurosci. 1998;18:4008–4021. doi: 10.1523/JNEUROSCI.18-11-04008.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jaffe H, Veeranna, Shetty KT, Pant HC. Characterization of the phosphorylation sites of human high molecular weight neurofilament protein by electrospray ionization tandem mass spectrometry and database searching. Biochemistry. 1998;37:3931–3940. doi: 10.1021/bi972518u. [DOI] [PubMed] [Google Scholar]
- 73.Hollander BA, Bennett GS, Shaw G. Localization of sites in the tail domain of the middle molecular mass neurofilament subunit phosphorylated by a neurofilament-associated kinase and by casein kinase I. J Neurochem. 1996;66:412–420. doi: 10.1046/j.1471-4159.1996.66010412.x. [DOI] [PubMed] [Google Scholar]
- 74.Nakamura Y, Hashimoto R, Kashiwagi Y, Wada Y, Sakoda S, Miyamae Y, Kudo T, Takeda M. Casein kinase II is responsible for phosphorylation of NF-L at Ser-473. FEBS Lett. 1999;455:83–86. doi: 10.1016/s0014-5793(99)00832-7. [DOI] [PubMed] [Google Scholar]
- 75.Zheng YL, Li BS, Veeranna, Pant HC. Phosphorylation of the head domain of neurofilament protein (NF-M): a factor regulating topographic phosphorylation of NF-M tail domain KSP sites in neurons. J Biol Chem. 2003;278:24026–24032. doi: 10.1074/jbc.M303079200. [DOI] [PubMed] [Google Scholar]
- 76.Li BS, Veeranna, Gu J, Grant P, Pant HC. Activation of mitogen-activated protein kinases (Erk1 and Erk2) cascade results in phosphorylation of NF-M tail domains in transfected NIH 3T3 cells. Eur J Biochem. 1999;262:211–217. doi: 10.1046/j.1432-1327.1999.00372.x. [DOI] [PubMed] [Google Scholar]
- 77.Li BS, Veeranna, Gu J, Grant P, Pant HC. Calcium influx and membrane depolarization induce phosphorylation of neurofilament (NF-M) KSP repeats in PC12 cells. Brain Res Mol Brain Res. 1999;70:84–91. doi: 10.1016/s0169-328x(99)00142-4. [DOI] [PubMed] [Google Scholar]
- 78.Li BS, Zhang L, Gu J, Amin ND, Pant HC. Integrin alpha(1) beta(1)-mediated activation of cyclin-dependent kinase 5 activity is involved in neurite outgrowth and human neurofilament protein H Lys-Ser-Pro tail domain phosphorylation. J Neurosci. 2000;20:6055–6062. doi: 10.1523/JNEUROSCI.20-16-06055.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dashiell SM, Tanner SL, Pant HC, Quarles RH. Myelin-associated glycoprotein modulates expression and phosphorylation of neuronal cytoskeletal elements and their associated kinases. J Neurochem. 2002;81:1263–1272. doi: 10.1046/j.1471-4159.2002.00927.x. [DOI] [PubMed] [Google Scholar]
- 80.Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 81.Giasson BI, Mushynski WE. Aberrant stress-induced phosphorylation of perikaryal neurofilaments. J Biol Chem. 1996;271:30404–30409. doi: 10.1074/jbc.271.48.30404. [DOI] [PubMed] [Google Scholar]
- 82.Li BS, Daniels MP, Pant HC. Integrins stimulate phosphorylation of neurofilament NF-M subunit KSP repeats through activation of extracellular regulated-kinases (Erk1/Erk2) in cultured motoneurons and transfected NIH 3T3 cells. J Neurochem. 2001;76:703–710. doi: 10.1046/j.1471-4159.2001.00064.x. [DOI] [PubMed] [Google Scholar]
- 83.Hirokawa N, Glicksman MA, Willard MB. Organization of mammalian neurofilament polypeptides within the neuronal cytoskeleton. J Cell Biol. 1984;98:1523–1536. doi: 10.1083/jcb.98.4.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hoffman PN, Griffin JW, Gold BG, Price DL. Slowing of neurofilament transport and the radial growth of developing nerve fibers. J Neurosci. 1985;5:2920–2929. doi: 10.1523/JNEUROSCI.05-11-02920.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hoffman PN, Cleveland DW, Griffin JW, Landes PW, Cowan NJ, Price DL. Neurofilament gene expression: a major determinant of axonal caliber. Proc Natl Acad Sci USA. 1987;84:3472–3476. doi: 10.1073/pnas.84.10.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Watson DF, Hoffman PN, Fittro KP, Griffin JW. Neurofilament and tubulin transport slows along the course of mature motor axons. Brain Res. 1989;477:225–32. doi: 10.1016/0006-8993(89)91410-8. [DOI] [PubMed] [Google Scholar]
- 87.Nixon RA, Paskevich PA, Sihag RK, Thayer CY. Phosphorylation on COOH- terminus domains of neurofilament proteins in retinal ganglion cell neurons in vivo: influences on regional neurofilament accumulation, interneurofilament spacing, and axon caliber. J Cell Biol. 1984;126:1031–1046. doi: 10.1083/jcb.126.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ackerley S, Thornhill P, Grierson AJ, Brownlees J, Anderton BH, Leigh PN, Shaw CE, Miller CJ. Neurofilament heavy chain side-arm phosphorylation regulates axonal transport of neurofilaments. J Cell Biol. 2003;161:489–495. doi: 10.1083/jcb.200303138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Collard JF, Cote F, Julien JP. Defective axonal transport in a transgenic mouse model of amyotrophic lateral sclerosis. Nature. 1995;375:61–64. doi: 10.1038/375061a0. [DOI] [PubMed] [Google Scholar]
- 90.DeWaegh SM, Lee VM, Brady ST. Local modulation of neurofilament phosphorylation, axonal caliber, and slow axonal transport by myelinating Schwann cells. Cell. 1992;68:451–463. doi: 10.1016/0092-8674(92)90183-d. [DOI] [PubMed] [Google Scholar]
- 91.Jung C, Shea TB. Regulation of neurofilament axonal transport by phosphorylation in optic axons in situ. Cell Motil Cytoskel. 1999;43:230–240. doi: 10.1002/(SICI)1097-0169(1999)42:3<230::AID-CM6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 92.Hoffman PN, Lasek RJ, Griffin JW, Price DL. Slowing of the axonal transport of neurofilament protein during development. J Neurosci. 1983;3:1694–1700. doi: 10.1523/JNEUROSCI.03-08-01694.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jung C, Yabe JT, Lee S, Shea TB. Hypophosphorylated neurofilament subunits undergo axonal transport more rapidly than more extenisvely phosphorylated subunits in situ. Cell Motil Cytoskel. 2000;47:120–129. doi: 10.1002/1097-0169(200010)47:2<120::AID-CM3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 94.Jung C, Yabe JT, Shea TB. C-terminal phosphorylation of the heavy molecular weight neurofilament subunit is inversely correlated with neurofilament axonal transport velocity. Brain Res. 2000;856:12–19. doi: 10.1016/s0006-8993(99)02314-8. [DOI] [PubMed] [Google Scholar]
- 95.Lewis SE, Nixon RA. Multiple phosphorylated variants of the high molecular mass subunit of neurofilaments in axons of retinal cell neurons: Characterization and evidence for their differential association with stationary and moving neurofilaments. J Cell Biol. 1988;107:2689–2701. doi: 10.1083/jcb.107.6.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Komiya Y, Cooper NA, Kidman AD. The recovery of slow axonal transport after a single intraperitoneal injection of beta. beta’-iminodipropionitrile in the rat. J Biochem (Tokyo) 1987;102:869–873. doi: 10.1093/oxfordjournals.jbchem.a122127. [DOI] [PubMed] [Google Scholar]
- 97.Marszalek JR, Williamson TL, Lee MK, Xu Z, Hoffman PN, Becher MW, Crawford TO, Cleveland DW. Neurofilament subunit NF-H modulates axonal diameter by selectively slowing neurofilament transport. J Cell Biol. 1996;135:711–724. doi: 10.1083/jcb.135.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shea TB, Fischer I, Paskevich PA, Beermann ML. The protein phosphatase inhibitor okadaic acid increases axonal NFs and neurite caliber, and decreases axonal MTs in NB2a/d1 cells. J Neurosci Res. 1993;35:507–521. doi: 10.1002/jnr.490350507. [DOI] [PubMed] [Google Scholar]
- 99.Tu PH, Elder G, Lazzarini RA, Nelson D, Trojanowski JQ, Lee VMY. Overexpression of the human NFM subunit in transgenic mice modifies the level of endogenous NFL and the phosphorylation state of NFH subunits. J Cell Biol. 1995;129:1629–1640. doi: 10.1083/jcb.129.6.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Watson DF, Glass JD, Griffin JW. Redistribution of cytoskeletal proteins in mammalian axons disconnected from their cell bodies. J Neurosci. 1993;13:4354–4360. doi: 10.1523/JNEUROSCI.13-10-04354.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yabe JT, Chylinski T, Wang FS, Pimenta A, Kattar SD, Linsley MD, Chan WK, Shea TB. Neurofilaments consist of distinct populations that can be distinguished by C-terminal phosphorylation, bundling and axonal transport rate in growing axonal neuritis. J Neurosci. 2001;21:2195–2205. doi: 10.1523/JNEUROSCI.21-07-02195.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yabe JT, Wang F-S, Chylinski T, Katchmar T, Shea TB. Selective accumulation of the high molecular weight neurofilament subunit within the distal region of growing axonal neurites. Cell Motil Cytoskel. 2001;50:1–12. doi: 10.1002/cm.1037. [DOI] [PubMed] [Google Scholar]
- 103.Zhang B, Tu P, Abtahian F, Trojanowski JQ, Lee VM. Neurofilaments and orthograde transport are reduced in ventral root axons of transgenic mice that express human SOD1 with a G93A mutation. J Cell Biol. 1997;139:1307–1315. doi: 10.1083/jcb.139.5.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhu Q, et al. Disruption of the NF-H gene increases axonal microtubule content and velocity of neurofilament transport: Relief of axonopathy resulting from the toxin b′b′-iminodiproprionitrile. J Cell Biol. 1998;143:183–193. doi: 10.1083/jcb.143.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nixon RA. The regulation of neurofilament protein dynamics by phosphorylation: clues to neurofibrillary pathology. Brain Pathol. 1993;3:29–38. doi: 10.1111/j.1750-3639.1993.tb00723.x. [DOI] [PubMed] [Google Scholar]
- 106.Julien JP, Mushynski WE. Neurofilaments in health and disease. Prog Nuc Acid Res and Mol Biol. 1998;61:1–20. doi: 10.1016/s0079-6603(08)60823-5. [DOI] [PubMed] [Google Scholar]
- 107.Nixon RA. The slow transport of cytoskeletal proteins. Curr Opin Cell Biol. 1998;10:87–92. doi: 10.1016/s0955-0674(98)80090-2. [DOI] [PubMed] [Google Scholar]
- 108.Yabe JT, Jung C, Chan W, Shea TB. Phospho-dependent association of neurofilament proteins with kinesin in situ. Cell Motil Cytoskel. 2000;45:249–262. doi: 10.1002/(SICI)1097-0169(200004)45:4<249::AID-CM1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 109.He YF, Francis F, Myers KA, Yu W, Black MM, Baas PW. Role of cytoplasmic dynein in the axonal transport of microtubules and neurofilaments. J Cell Biol. 2005;168:697–703. doi: 10.1083/jcb.200407191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Motil J, Chan WK, Dubey M, Chaudhury P, Pimenta A, Chylinski TM, Ortiz DT, Shea TB. Dynein mediates retrograde neurofilament transport within axonal neurites and anterograde delivery of NFs from perikarya into axons: Regulation by multiple phosphorylation events. Cell Motil Cytoskel. 2006;63:266–286. doi: 10.1002/cm.20122. [DOI] [PubMed] [Google Scholar]
- 111.Prahlad V, Helfand BT, Langford GM, Vale RD, Goldman RD. Fast transport of neurofilament protein along microtubules in squid axoplasm. J Cell Sci. 2000;113:3939–46. doi: 10.1242/jcs.113.22.3939. [DOI] [PubMed] [Google Scholar]
- 112.Shah JV, Flanagan LA, Janmey PA, Leterrier J-F. Bidirectional translocation of neurofilaments along microtubules mediated in part by dynein/dynactin. Mol Biol Cell. 2000;11:3495–3508. doi: 10.1091/mbc.11.10.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Theiss C, Napirei M, Meller K. Impairment of anterograde and retrograde neuro•lament transport after anti-kinesin and anti-dynein antibody microinjection in chicken dorsal root ganglia. Eur J Cell Biol. 2005;84:29–43. doi: 10.1016/j.ejcb.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 114.Xia CH, Roberts EA, Her LS, Liu X, Williams DS, Cleveland DW, Goldstein LS. Abnormal neurofilament transport caused by targeted disruption of neuronal kinesin heavy chain KIF5A. J Cell Biol. 2003;2003;161:55–66. doi: 10.1083/jcb.200301026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yabe JT, Pimenta A, Shea TB. Kinesin-mediated transport of neurofilament protein oligomers in growing axons. J Cell Sci. 1999;112:3799–3814. doi: 10.1242/jcs.112.21.3799. [DOI] [PubMed] [Google Scholar]
- 116.Shea TB, Flanagan L. Kinesin, dynein and neurofilament transport. Trend Neurosci. 2001;2001;24:644–648. doi: 10.1016/s0166-2236(00)01919-6. [DOI] [PubMed] [Google Scholar]
- 117.Wang L, Ho CI, Sun D, Liem RKH, Brown A. Rapid movements of axonal neurofilaments interrupted by prolonged pauses. Nature Cell Biol. 2000;2:137–141. doi: 10.1038/35004008. [DOI] [PubMed] [Google Scholar]
- 118.Roy S, Coffee P, Smith G, Liem RKH, Brady ST, Black MM. Neurofilaments are transported rapidly but intermittently in axons: Implications for slow axonal transport. J Neurosci. 2000;20:6849–6861. doi: 10.1523/JNEUROSCI.20-18-06849.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Craciun G, Brown A, Friedman A. A dynamical system model of neurofilament transport in axons. J Theor Biol. 2005;237:316–322. doi: 10.1016/j.jtbi.2005.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brown A, Wang L, Jung P. Stochastic simulation of neurofilament transport in axons: the “stop-and-go” hypothesis. Mol Biol Cell. 2005;16:4243–4255. doi: 10.1091/mbc.E05-02-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rao MV, Garcia ML, Miyazaki Y, Gotow T, Yuan A, Mattina S, Ward CM, Calcutt NA, Uchiyama Y, Nixon RA, Cleveland DW. Gene replacement in mice reveals that the heavily phosphorylated tail of neurofilament heavy subunit does not affect axonal caliber or the transit of cargoes in slow axonal transport. J Cell Biol. 2002;2002;158:681–693. doi: 10.1083/jcb.200202037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rao MV, Campbell J, Yuan A, Kumar A, Gotow T, Uchiyama Y, Nixon RA. The neurofilament middle molecular mass subunit carboxyl-terminal tail domains is essential for the radial growth and cytoskeletal architecture of axons but not for regulating neurofilament transport rate. J Cell Biol. 2003;163:1021–1031. doi: 10.1083/jcb.200308076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yuan A, Nixon RA, Rao MV. Deleting the phosphorylated tail domain of the neurofilament heavy subunit does not alter neurofilament transport rate in vivo. Neurosci Lett. 2006;393:264–268. doi: 10.1016/j.neulet.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 124.Oblinger M. Characterization of posttranslational processing of the mammalian high-molecular-weight neurofilament protein in vivo. J Neurosci. 1987;7:2510–2521. [PMC free article] [PubMed] [Google Scholar]
- 125.Hisanaga S, Hirokawa N. Dephosphorylation-induced interactions of neurofilaments with microtubules. J Biol Chem. 1990;265:21852–2188. [PubMed] [Google Scholar]
- 126.Hirokawa N, Takeda S. Gene targeting studies begin to reveal the function of neurofilament proteins. J Cell Biol. 1998;143:1–4. doi: 10.1083/jcb.143.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gou JP, Gotow T, Janmey PA, Leterrier JF. Regulation of neurofilament interactions in vitro by natural and synthetic polypeptides sharing Lys-Ser-Pro sequences with the heavy neurofilament subunit NF-H: neurofilament crossbridging by antiparallel sidearm overlapping. Med Biol Eng Comput. 1998;36:371–87. doi: 10.1007/BF02522486. [DOI] [PubMed] [Google Scholar]
- 128.Chen J, Nakata T, Zhang Z, Hirokawa N. The C-terminal tail domain of neurofilament protein-H (NF-H) forms the cross-bridges and regulates neurofilament bundle formation. J Cell Sci. 2000;113:3861–3869. doi: 10.1242/jcs.113.21.3861. [DOI] [PubMed] [Google Scholar]
- 129.Miyasaka H, Okabe S, Ishiguro K, Uchida T, Hirokawa N. Interaction of the tail domain of high molecular weight subunits of neurofilaments with the COOH-terminal region of tubulin and its regulation by tau protein kinase II. J Biol Chem. 1993;268:22695–22702. [PubMed] [Google Scholar]
- 130.Figlewicz DA, Krizus A, Martinoli MG, Meininger V, Dib M, Rouleau GA, Julien JP. Variants of the heavy neurofilament subunit are associated with the development of amyotrophic lateral sclerosis. Hum Mol Genet. 1994;3:1757–1761. doi: 10.1093/hmg/3.10.1757. [DOI] [PubMed] [Google Scholar]
- 131.Al-Chalabi A, Andersen PM, Nilsson P, Chioza B, Andersson JL, Russ C, Shaw CE, Powell JF, Leigh PN. Deletions of the heavy neurofilament subunit tail in amyotrophic lateral sclerosis. Hum Mol Genet. 1999;8:157–164. doi: 10.1093/hmg/8.2.157. [DOI] [PubMed] [Google Scholar]
- 132.Sasaki T, Gotow T, Shiozaki M, Sakaue F, Saito T, Julien JP, Uchiyama Y, Hisanaga S. Aggregate formation and phosphorylation of neurofilament-L pro22 Charcot–Marie–Tooth diaease mutants. Hum Mol Genet. 2006;15:943–952. doi: 10.1093/hmg/ddl011. [DOI] [PubMed] [Google Scholar]
- 133.Arbustini E, Pasotti M, Pilotto A, Pellegrini C, Grasso M, Previtali S, Repetto A, Bellini O, Azan G, Scaffino M, Campana C, Piccolo G, Viganò M, Tavazzi L. Desmin accumulation restrictive cardiomyopathy and atrioventricular block associated with desmin gene defects. Eur J Heart Fail. 2006;8:477–483. doi: 10.1016/j.ejheart.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 134.Momeni P, Cairns NJ, Perry RH, Bigio EH, Gearing M, Singleton AB, Hardy J. Mutation analysis of patients with neuronal intermediate filament inclusion disease (NIFID) Neurobiol Aging 2006. 2006;27:778.e1–778.e6. doi: 10.1016/j.neurobiolaging.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 135.Leung CL, Nagan N, Graham TH, Liem RK. A novel duplication/insertion mutation of NEFL in a patient with Charcot-Marie-Tooth disease. Am J Med Genet A. 2006;140:1021–1025. doi: 10.1002/ajmg.a.31242. [DOI] [PubMed] [Google Scholar]
- 136.Fabrizi GM, Cavallaro T, Angiari C, Bertolasi L, Cabrini I, Ferrarini M, Rizzuto N. Giant axon and neurofilament accumulation in Charcot-Marie-Tooth disease type 2E. Neurology. 2004;62:1429–1431. doi: 10.1212/01.wnl.0000120664.07186.3c. [DOI] [PubMed] [Google Scholar]
- 137.Green SL, Westendorf JM, Jaffe H, Pant HC, Cork LC, Ostrander EA, Vignaux F, Ferrell JE., Jr Allelic variants of the canine heavy neurofilament (NFH) subunit and extensive phosphorylation in dogs with motor neuron disease. J Comp Pathol. 2005;132:33–50. doi: 10.1016/j.jcpa.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 138.Beaulieu JM, Nguyen MD, Julien JP. Late onset of motor neurons in mice overexpressing wild-type peripherin. J Cell Biol. 1999;147:531–544. doi: 10.1083/jcb.147.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Julien JP, Cote F, Collard JF. Mice overexpressing the human neurofilament heavy gene as a model of ALS. Neurobiol Aging. 1995;16:487–490. doi: 10.1016/0197-4580(94)00169-2. [DOI] [PubMed] [Google Scholar]
- 140.Lariviere RC, Julien JP. Functions of intermediate filaments in neuronal development and disease. J Neurobiol. 2004;58:131–148. doi: 10.1002/neu.10270. [DOI] [PubMed] [Google Scholar]
- 141.Shea TB, Yabe JT, Ortiz D, Pimenta A, Loomis P, Goldman RD, Amin N, Pant HC. CDK5 regulates axonal transport and phosphorylation of neurofilaments in cultured neurons. J Cell Sci. 2004;117:933–941. doi: 10.1242/jcs.00785. [DOI] [PubMed] [Google Scholar]
- 142.Goldstein ME, Sternberger NH, Sternberger LA. Phosphorylation protects neurofilaments against proteolysis. J Neuroimmunol. 1987;14:149–60. doi: 10.1016/0165-5728(87)90049-x. [DOI] [PubMed] [Google Scholar]
- 143.Pant HC. Dephosphorylation of neurofilament proteins enhances their susceptibility to degradation by calpain. Biochem J. 1988;256:665–668. doi: 10.1042/bj2560665. [DOI] [PMC free article] [PubMed] [Google Scholar]


