Abstract
The anatomical and functional arrangement of the gastrointestinal tract suggests that this organ, beside its digestive and absorptive functions, regulates the trafficking of macromolecules between the environment and the host through a barrier mechanism. Under physiological circumstances, this trafficking is safeguarded by the competency of intercellular tight junctions, structures whose physiological modulation is mediated by, among others, the recently described protein zonulin. To prevent harm and minimize inflammation, the same paracellular pathway, in concert with the gut-associated lymphoid tissue and the neuroendocrine network, controls the equilibrium between tolerance and immunity to nonself antigens. The zonulin pathway has been exploited to deliver drugs, macromolecules, or vaccines that normally would not be absorbed through the gastrointestinal mucosal barrier. However, if the tightly regulated trafficking of macromolecules is jeopardized secondary to prolonged zonulin up-regulation, the excessive flow of nonself antigens in the intestinal submucosa can cause both intestinal and extraintestinal autoimmune disorders in genetically susceptible individuals. This new paradigm subverts traditional theories underlying the development of autoimmunity, which are based on molecular mimicry and/or the bystander effect, and suggests that the autoimmune process can be arrested if the interplay between genes and environmental triggers is prevented by re-establishing intestinal barrier competency. Understanding the role of zonulin-dependent intestinal barrier dysfunction in the pathogenesis of autoimmune diseases is an area of translational research that encompasses many fields.
The intestinal epithelium is the largest mucosal surface, providing an interface between the external environment and the mammalian host. Its exquisite anatomical and functional arrangements and the finely-tuned coordination of digestive, absorptive, motility, neuroendocrine, and immunological functions are testimonial of the complexity of the gastrointestinal (GI) system. Also pivotal is the regulation of molecular trafficking between the intestinal lumen and the submucosa via the paracellular space. The dimensions of the paracellular space are estimated to be between 10 and 15 Å, suggesting that under physiological circumstances, solutes with a molecular radius exceeding 15 Å (∼3.5 kDa) will be excluded from this uptake route. Macromolecule trafficking is dictated mainly by intestinal paracellular permeability, the regulation of which depends on the modulation of intercellular tight junctions (TJs). A century ago, TJs were conceptualized as a secreted extracellular cement forming an absolute and unregulated barrier within the paracellular space. The contribution of the paracellular pathway of the GI tract to the general economy of molecule trafficking between environment and host, therefore, was judged to be negligible. It is now apparent that TJs are extremely dynamic structures involved in several key functions of the intestinal epithelium both under physiological and pathological circumstances. TJ modulation has also been exploited for the delivery of drugs and macromolecules normally not absorbed through the GI tract. Given the structural and functional characteristics of intercellular TJs and the protean nature of the intestinal content, the gut mucosa represent the battlefield where friends (ie, nutrients and enteric microflora) and foes (ie, pathogenic microorganisms and their toxins) must be selectively recognized to reach an ideal balance between tolerance and immune response to nonself antigens. Antigen trafficking through TJs and their sampling by the gut-associated lymphoid tissue (GALT) are among the key strategies applied to reach this balance.
This review is mainly focused on the role of zonulin, a modulator of intercellular TJs, on the pathophysiology of intestinal permeability and its use for therapeutic applications. For a more complete overview on the topic, the reader is referred to an excellent review article recently published in The American Journal of Pathology.1
Structural Composition of Intercellular TJs
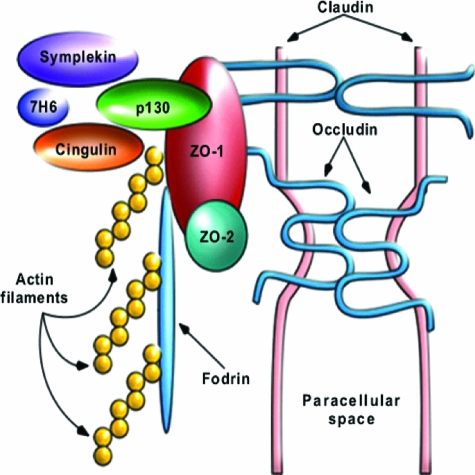
The intercellular TJs are complex structures composed by a multiprotein complex anatomically and functionally connected with the underlying apical actomyosin ring (Figure 1). Among the TJ proteins identified so far are the transmembrane proteins occludin, the claudins, and junctional adhesion molecules (JAMs), and cytoplasmic plaque proteins ZO-1, ZO-2, ZO-3, cingulin, and 7H6.2 In vitro, the electrical resistance is a measure of charge flow across the membrane and thus reflects the permeability of the paracellular shunt pathway and the tightness of the intercellular TJ. TJ assembly is the result of a complex cascade of biochemical events that ultimately lead to the formation of an organized network of TJ elements, the composition of which has been only partially characterized. An in depth discussion of the complexity of TJ composition, the protein-protein interactions that govern their assembly and competency, and the multiple signaling pathways involved in the orchestration of all these events goes beyond the scope of this review. Therefore, only a general overview of the TJ structural composition will be covered herein to provide proper background and rationale to the main focus of the review.
Figure 1.
Composition of intercellular TJs. TJs are composed by integral membrane proteins, including occludin, the claudin family, and JAM; the scaffold proteins, including ZO-1, ZO-2, ZO-3, symplekin, cingulin, and 7H6, and the cell cytoskeleton. This figure has been reused with permission from Advanced Drug Delivery Reviews (2004, 56:795–807).
Transmembrane TJ Proteins
To date, multiple proteins that make up the TJ strands have been identified and include occludin3 and members of the claudin family,4 a group of at least 20 tissue-specific proteins. The JAMs, proteins that belong to the Ig superfamily, have been described as additional components of the TJ fibrils.5 JAMs reportedly bind to ZO-1, aiding its localization to the junctional complex.6
Occludin is a transmembrane TJ phosphoprotein of ∼65 kDa.7 It is expressed in TJs of both epithelial and endothelial cells. The proposed folding topology places both NH2 and COOH terminals within the cytoplasm, allowing the polypeptide to pass out and back inside twice within the NH2 terminal half.7 Occludin has recently been shown to function as a cell-cell adhesion molecule and appears to participate in maintaining the intramembrane diffusion barrier.8 Occludin was previously considered to be involved in establishing the seal at the sites of junctional strands.9 However, discrepancies between the presence of fibrils and occludin expression in several tissues, including human testis and endothelial cells, led to the discovery of the claudins as integral membrane TJ proteins.4
The claudins are 20- to 25-kDa tetraspan membrane proteins that each contain two extracellular loops with variably charged amino acid residues and short intracellular tails.9 The claudins determine the barrier properties of the paracellular pathway because they can completely tighten the intercellular space to solutes, and they can form paracellular ion pores. It is assumed that the extracellular loops specify these claudin functions.
JAMs are characterized by the molecular structure composed of two extracellular immunoglobulin loops, a single transmembrane domain, and a cytoplasmic domain with a PDZ-binding motif.5 JAMs interact with several ligands in both homophilic and heterophilic manners by the extracellular domain and also bind to various PDZ domain-containing proteins at the C terminus. Through these protein-protein interactions, JAMs are implicated in diverse biological functions at the TJ, including cell-cell adhesion, junctional assembly, regulation of paracellular permeability, and leukocyte transmigration.10,11
Cytoplasmic TJ Proteins
The cytoplasmic plaque of the TJ includes multiple proteins that have been defined at the molecular level as well as several others that await further characterization. Through interactions with each other and with cytoskeletal proteins, these scaffold elements (ZO-1, ZO-2, ZO3, and ZO-1-associated protein kinase or ZAK) functionally couple integral membrane TJ proteins to actin microfilaments.12 TJ plaque proteins also appear to be direct targets and effectors of different signaling pathways. Additional cytoplasmic plaque proteins have been localized to the TJ, including 7H6,9 Rab 13 and 3b,13 Gαi-2,14 PKC,15 symplekin (126.5 kDa),16 ZA-1TJ, and cingulin (140-kDa phosphoprotein).17
GI Mucosal Immune System
The GALT
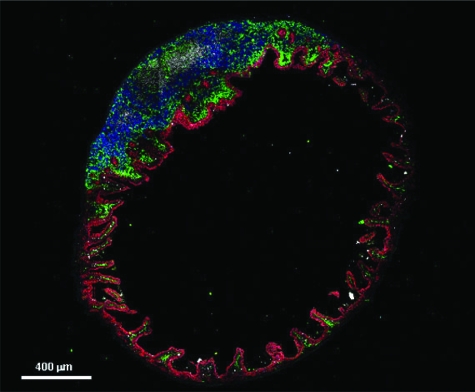
Paracellular passage of macromolecules, under either physiological or pathological circumstances, is safeguarded by the GALT (Figure 2). The GALT serves as a containment system preventing potentially harmful intestinal antigens from reaching the systemic circulation and induces systemic tolerance against luminal antigens by a process that involves polymeric IgA secretion and the induction of regulatory T cells. Another important factor for the intestinal immunological responsiveness is the major histocompatibility complex. Human leukocyte antigen (HLA) class I and class II genes are located in the major histocompatibility complex on chromosome 6. These genes code for glycoproteins, which bind peptides, and this HLA-peptide complex is recognized by certain T-cell receptors in the intestinal mucosa.18,19 Susceptibility to at least 50 diseases is associated with specific HLA class I or class II alleles.
Figure 2.
Immunofluorescence microscopy of mouse small intestine (ileum) cross section. The confocal image shows the anatomical arrangement of epithelial cells (stained in red) and immune cells (dendritic cells stained in green, B cells stained in blue, and T cells stained in white). This arrangement suggests functional interactions between epithelial and immune cells for handling nonself antigens and microbiota present in the intestinal lumen. (Image provided by Marcello Chieppa, Lymphocyte Biology Section, Laboratory of Immunology, National Institute of Allergy and Infectious Diseases, National Institutes of Health).
The balance between immunity and tolerance is essential for a healthy intestine, and abnormal or inappropriate immune responses may result in inflammatory pathologies. There is evidence that antigen-presenting dendritic cells, key players in antigen sampling, are educated by memory T cells and subsequently induce naïve T cells,20 thereby supporting the role for dendritic cells in coupling innate and adaptive immune responses that affect intestinal permeability.
Innate and Adaptive Immunity and Their Interactions
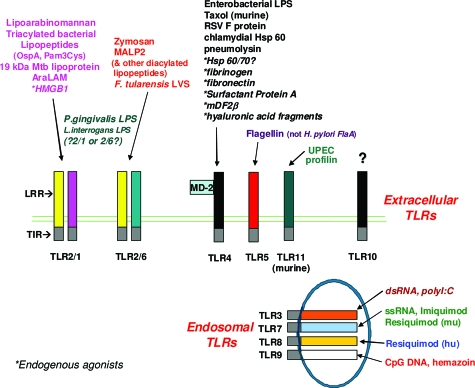
Recognition of antigens by dendritic cells triggers a family of pattern recognition receptors, Toll-like receptors (TLRs), which change dendritic cell phenotype and function. TLRs are the major receptors involved in the discrimination between self and nonself based on the recognition of conserved bacterial molecular patterns (Figure 3). In intestinal epithelial cells, TLRs play a role in normal mucosal homeostasis and are particularly important in the interaction between the mucosa and the luminal flora.21 There are a number of TLRs, all of which are present in the gut and respond to different stimuli resulting in different adaptive immune responses.22,23,24 There is now evidence for a differential response to stimuli arising from TLRs located at the basolateral versus the apical surface.25
Figure 3.
TLR. Currently, at least 10 different TLRs have been described, recognizing different ligands that activate the innate immune system. TLRs 3, 7, 8, and 9 are endosomal TLRs, whereas the remaining TLRs are surface, extracellular receptors.
TLRs direct immune responses by activating signaling events leading to elevated expression of factors, such as cytokines and chemokines that recruit and regulate the immune and inflammatory cells, which then either initiate or enhance host immune responses.26 The peripheral memory T-cell response is a critical outcome of adaptive immunity, and TLRs likely are required for the generation and maintenance of memory T cells.27 TLRs are implicated in chronic diseases such as enteric inflammation and may have both proinflammatory and protective roles. Of interest, commensal flora acting through TLR4 positively influence the susceptibility to food antigens28 and implicate TLRs in the regulation of intestinal permeability. This concept is supported by recent in vitro studies using intestinal epithelial cell cultures, which show that TLR-2 enhances epithelial integrity by a rearrangement of the TJ protein, ZO-1.22
The Zonulin System
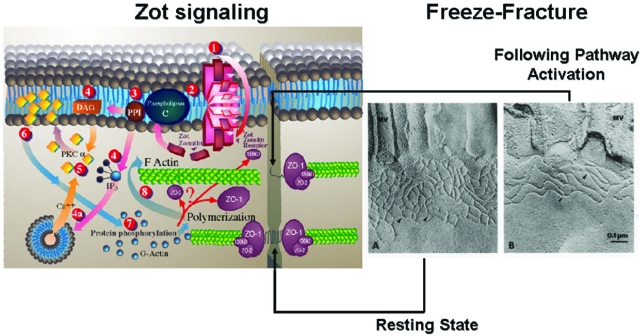
To meet the diverse physiological challenges to which the intestinal epithelial barrier is subjected, TJs must be capable of rapid and coordinated responses. This requires the presence of a complex regulatory system that orchestrates the state of assembly of the TJ multiprotein network. Although knowledge about TJ ultrastructure and intracellular signaling events has progressed significantly during the past decade, relatively little is known about their pathophysiological regulation. The discovery of zonula occludens toxin (Zot), an enterotoxin expressed by Vibrio cholerae that reversibly opens TJs,29 has increased our understanding of the intricate mechanisms that regulate the intestinal epithelial paracellular pathway. Zot is a single polypeptide chain of 44.8 kDa, encoded by the cholera toxin bacteriophage (CTXφ) present in toxigenic strains of V. cholerae.29 Zot action is mediated through a cascade of intracellular events that lead to PKC-α-dependent polymerization of actin microfilaments and subsequent TJ disassembly.30 Immunofluorescence binding studies have shown that Zot binding varies within the intestine, being detectable in the jejunum and distal ileum, but not in the colon, and decreasing along the villous-crypt axis.31 This binding distribution coincides with the differential intestinal epithelial barrier responsiveness and actin reorganization that occurs along the villous axis32,33 as well as with the regional effect of Zot on intestinal permeability.30,31 These combined data demonstrate that Zot regulates TJs in a rapid, reversible, and reproducible manner. Based on these observations, it has been postulated that Zot mimics an immunologically related, endogenous modulator of epithelial TJs. The combination of affinity-purified anti-Zot antibodies and the Ussing chamber assay allowed the identification of a human intestinal Zot homolog that was designated as zonulin (Figure 4).34 Affinity-purified zonulin reduced transepithelial electrical resistance compared to the media control in both monkey jejunum and ileum, but not in the colon.34 V. cholerae-derived Zot and human zonulin both act on intestinal TJs29,35,36 and display the same regional barrier responsiveness31 coincident with Zot receptor distribution within the intestine,30,33 These findings suggest that these two molecules could potentially interact with the same receptor(s). Comparison of the primary amino acid structures of Zot and zonulin provided insights into the structural requirements for ligand engagement of the receptor coupled to intestinal TJ regulation. The NH2-termini of zonulin and the Zot active fragment share a common octapeptide motif (GGVLVQPG) that is critical for intestinal receptor binding.34,36 The synthetic octapeptide motif, named AT1001, is an efficient inhibitor of both Zot and zonulin because it binds to the target receptor, but it does not activate the intracellular signaling leading to TJ disassembly and antigen trafficking.34,36 Zonulin cloning and characterization have revealed that it belongs to a family of serine proteases with structure similarities with a series of growth hormones, including epidermal growth factor (A. Fasano, unpublished).
Figure 4.
Proposed zonulin intracellular signaling leading to the opening of intestinal TJ. Zonulin interacts with a specific surface receptor (1) whose distribution within the intestine varies. The protein then activates phospholipase C (2) that hydrolyzes phosphatidyl inositol (3) to release inositol 1,4,5-Tris phosphate (PPI-3) and diacylglycerol (DAG) (4). PKC-α is then activated (5), either directly (via DAG) (4) or through the release of intracellular Ca2+ (via PPI-3) (4a). Membrane-associated, activated PKC-α (6) catalyzes the phosphorylation of target protein(s), with subsequent polymerization of soluble G-actin in F-actin (7). This polymerization causes the rearrangement of the filaments of actin and the subsequent displacement of proteins (including ZO-1) from the junctional complex (8). As a result, intestinal TJs become looser (see freeze fracture electron microscopy). Once zonulin signaling is over, the TJs resume their baseline steady state. Portions of this figure have been reused with permission from Proceedings of the National Academy of Sciences U.S.A. (1991, 88:5242–5246) and Molecular Genetics and Metabolism (1998, 64:12–18).
Physiology of the Zonulin System
The physiological role of the zonulin system remains to be established; however, it is likely that this system is involved in several functions, including TJ regulation responsible for the movement of fluid, macromolecules, and leukocytes between body compartments. Another possible physiological role of zonulin is the protection against microorganism colonization.37 In the absence of enteric infections, the mammalian small intestine is virtually sterile. The colonization of the proximal gut by enteric microorganisms (even without apparent mucosal damage or elaboration of specific toxins) may lead to a leaky intestine.38 However, the mechanism(s) by which this disturbed physiological regulation of the intestinal TJ permeability secondary to proximal bacterial contamination occurs remains unclear. It has been reported that both normal enteric bacterial flora isolates (well characterized for not harboring any known pathogenic traits) and pathogenic bacteria induce alteration of the TJ competency as suggested by changes in epithelial resistance and increased passage of inulin.37 These changes were mirrored by the concomitant expression of zonulin in organ culture systems and occurred even when bacteria were killed by gentamicin treatment.37 These results suggest that the presence of enteric microorganisms in the small intestine, but not in the colon, where the zonulin system is not operative,29,30,31 induces a host-dependent mucosal response that leads to the luminal secretion of zonulin. The fact that the interaction of bacteria with the intestinal mucosa induces zonulin release, irrespective of their pathogenic traits or viability, can be interpreted as a bacteria-independent mechanism of defense of the host that reacts to the abnormal presence of microorganisms on the surface of the small intestine. After the zonulin-induced opening of TJs, water is secreted into the intestinal lumen after hydrostatic pressure gradients31 and bacteria are flushed out of the small intestine.
Pathology of the Zonulin System
Inappropriate activation of the zonulin pathway, both in terms of timing and duration, can lead to an excessive and unregulated paracellular passage of nonself antigens in the lamina propria. Intestinal microorganisms have been long claimed to be possibly involved in autoimmune disease pathogenesis.39 Therefore, it is possible to hypothesize that the same zonulin innate immunity response that protect us against proximal microorganism contamination can cause a loss of intestinal barrier function when this pathway is chronically or exaggeratedly stimulated by enteric bacteria and/or viruses. Nevertheless, the environmental triggers of autoimmunity remain elusive for all autoimmune diseases except celiac disease (CD), for which gluten, the staple protein of wheat, has been identified as the undisputable trigger.40 We have demonstrated that, like bacterial colonization,37 gluten and its toxic component gliadin activate the zonulin pathway by “mistake of evolution.”41 Recently we have shown that gliadin binds to CXCR3 on epithelial cells to initiate an increase in intestinal permeability through a MyD88-dependent release of zonulin that enables the paracellular passage of gliadin (and possibly other nonself antigens) from the intestinal lumen to the gut mucosa.42 In genetically predisposed individuals, gliadin may attract and stimulate other CXCR3-expressing cells, including γδ T cells, CD3+CD8+αβ T cells, and NK cells, leading to the early activation of the innate immune arm of the CD inflammatory response.42
Role of Zonulin in Autoimmune Diseases
Role of Intestinal Permeability in Autoimmune Disease Pathogenesis
Intestinal TJ dysfunction occurs in a variety of clinical conditions, including food allergies, infections of the GI tract, autoimmune diseases, and inflammatory bowel disease (IBD).43 Healthy, mature gut mucosa with its intact TJ serves as the main barrier to the passage of macromolecules. During the healthy state, small quantities of immunologically active antigens cross the gut host barrier. These antigens are absorbed across the mucosa through at least two pathways. The vast majority of absorbed proteins (up to 90%) crosses the intestinal barrier via the transcellular pathway, followed by lysosomal degradation that converts proteins into smaller, nonimmunogenic peptides. These residual peptides are transported as intact proteins, through the paracellular pathway; it involves a subtle but sophisticated regulation of intercellular TJ that leads to antigen tolerance. When the integrity of the TJ system is compromised, a deleterious immune response to environmental antigens may be elicited. Several autoimmune diseases are characterized by loss of intestinal barrier function,43 suggesting a role of altered intestinal permeability in the pathogenesis of autoimmune diseases.
Specific Autoimmune Diseases in Which Zonulin Involvement Has Been Proved or Hypothesized
CD: CD represents the best testimonial of this theory. CD is a unique model of autoimmunity in which, in contrast to most other autoimmune diseases, a close genetic association with HLA genes, a highly specific humoral autoimmune response against tissue transglutaminase, and, most importantly, the triggering environmental factor (gliadin) are all known. Early in the disease, TJs are opened44,45 and severe intestinal damage ensues45 (Figure 5). The up-regulation of the zonulin innate immunity pathway is directly induced by the exposure to the disease’s antigenic trigger gliadin.46 To determine whether zonulin production is perturbed during the acute phase of CD, intestinal tissues from patients with active CD and non-CD controls were probed for zonulin expression.47 Quantitative immunoblotting of intestinal tissue lysates from active CD patients confirmed the increase in zonulin protein compared to control tissues.47 Zonulin up-regulation during the acute phase of CD was confirmed by measuring zonulin concentration in sera of 189 CD patients using a sandwich enzyme-linked immunosorbent assay. Compared to healthy controls, CD patients in the acute phase of the disease had higher zonulin serum concentrations that decreased after adherence to a gluten-free diet.47
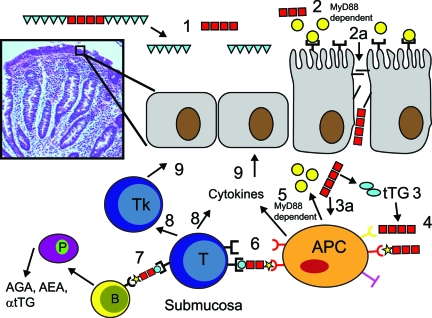
Figure 5.
Proposed role of abnormal intestinal permeability in the pathogenesis of CD. Gliadin and its immunomodulatory/inflammatory fragments are present in the intestinal lumen (1), inducing MyD88-dependent zonulin release (2) that causes opening of TJs (2a) and gliadin passage across the TJ barriers in patients with dysregulation of the zonulin system (2a). After TTG deamidation (3), gliadin peptides bind to HLA receptors present on the surface of antigen-presenting cells (APCs) (4). Alternatively, gliadin can act directly on APC (3a) causing MyD88-dependent release of both zonulin and cytokines (5). Gliadin peptides are also presented to T lymphocytes (6), followed by an aberrant immune response, both humoral (7) and cell-mediated (8) in genetically susceptible individuals. This interplay between innate and adaptive immunity is ultimately responsible for the autoimmune process targeting intestinal epithelial cells, leading to the intestinal damage typical of celiac disease (9). This figure has been redrawn with permission from Nature Clinical Practice Gastroenterology & Hepatology (2005, 2:416–422).
Gliadin has been shown to be also a potent stimulus for macrophage proinflammatory gene expression and cytokine release.48 Our recent data suggest that signaling of both functions is independent of TLR4 and TLR2 but is dependent on MyD88, a key adapter molecule in TLR/interleukin-1 receptor signaling.49 These data indicate that gliadin initiates intestinal permeability through the CXCR3-mediated, MyD88-dependent release of zonulin,42,49 which enables paracellular translocation of gliadin and its subsequent interaction with macrophages within the intestinal submucosa (Figure 5). The key role of gliadin-induced zonulin release and subsequent loss of intestinal barrier function in CD pathogenesis has been supported by clinical trials with the zonulin inhibitor AT1001.50
Gliadin interaction with macrophages initiates signaling through a TLR-like pathway, resulting in the establishment of a pro-inflammatory (Th1-type) cytokine milieu that results in mononuclear cell infiltration into the submucosa. This, in turn, may permit the interaction of T cells with antigen-presenting cells, including macrophages, leading ultimately to the antigen-specific adaptive immune response seen in patients with CD. Once gluten is removed from the diet, serum zonulin levels decrease, the intestine resumes its baseline barrier function, the autoantibody titers are normalized, the autoimmune process shuts off, and consequently, the intestinal damage (that represents the biological outcome of the autoimmune process) heals completely.
IBDs: The pathogenesis of IBD remains unknown, although in recent years there is convincing evidence to implicate genetic, immunological, and environmental factors in initiating the autoimmune process. Several lines of evidence, however, suggest that an increased intestinal permeability plays a central role in the pathogenesis of IBD.39,51 Like CD, IBD may be related to an innate immune deficiency, leading to the inappropriate access of nonself antigens to the GALT. In clinically asymptomatic Crohn’s disease patients, increased intestinal epithelial permeability precedes clinical relapse by as much as 1 year, suggesting that a permeability defect is an early event in disease exacerbation.51 The hypothesis that abnormal intestinal barrier function is a genetic trait involved in the pathogenesis of IBD is further supported by the observation that clinically asymptomatic first-degree relatives of Crohn’s disease patients may have increased intestinal permeability.39,51 We have recently generated evidence suggesting that zonulin up-regulation is detectable in the acute phase of IBD and that its serum levels decrease (but are still higher than normal) once the inflammatory process subsides after specific treatment (Bai J. and Fasano A., unpublished data). Although a primary defect of the intestinal barrier function (possibly secondary to activation of the zonulin pathway) may be involved in the early steps of the pathogenesis of IBD, the production of cytokines, including interferon-γ and tumor necrosis factor-α, secondary to the inflammatory process serve to perpetuate the increased intestinal permeability by reorganizing TJ proteins ZO-1, JAM 1, occludin, claudin-1, and claudin-4.52,53,54,55 In this manner, a vicious cycle is created in which barrier dysfunction allows further leakage of luminal contents, thereby triggering an immune response that in turn promotes further leakiness.
Type 1 Diabetes (T1D): The trigger of the autoimmune destruction of pancreatic β cells in T1D is unknown. T1D has the same pathogenic challenges as other autoimmune diseases: defining the environmental triggers and how these triggers cross the intestinal barrier to interact with the immune system.43 It is the interplay between environmental factors and specific susceptibility genes that dictates the aberrant immune response responsible for the onset of disease. Less than 10% of those with increased genetic susceptibility progress to clinical disease, suggesting a strong environmental trigger in the prediabetic process. Environmental factors are also likely affecting the outcome of the process and the rate of progression to disease in those who develop T1D. One theory is that antigens absorbed through the gut may be involved. The specific cells that are key for this immune response lie in close proximity to the intestinal epithelial barrier (Figure 2).56,57
Another critical factor for intestinal immunological responsiveness is the major histocompatibility complex. Certain HLA class II alleles account for 40% of the genetic susceptibility to T1D in Caucasians; however, the majority of genetically predisposed individuals do not develop T1D. This supports the concept that reaction to some environmental products triggers autoimmune destruction of β cells and leads to T1D. Recent studies have shown that altered intestinal permeability occurs in T1D before the onset of complications,58,59 which is not the case in type 2 diabetes.60 This has led to the suggestion that increased intestinal permeability attributable to alterations in intestinal TJs is responsible for the onset of T1D.59,61,62 This hypothesis is supported by a recent study performed in an animal model that develops T1D spontaneously.63 The authors of this study demonstrated increased permeability of the small intestine (but not of the colon) in BioBreeding diabetic-prone (BBDP) rats that preceded the onset of diabetes by at least a month. Further, histological evidence of pancreatic islet destruction was absent at the time of increased permeability but was clearly present at a later time.63 Therefore, the authors presented evidence that increased permeability occurred before either histological or overt manifestation of diabetes in this animal model. Unaffected family members of probands with autoimmune diseases have also been found to have increased gut permeability.43 The BBDP rat animal model of T1D resembles this human scenario since it has been shown that “leaky gut” is part of the pathogenic equation.63 We have confirmed these data by reporting in this animal model of T1D that zonulin-dependent increases in intestinal permeability precedes the onset of T1D by 2 to 3 weeks.64 Oral administration of the zonulin inhibitor, AT1001, to BBDP rats blocked autoantibody formation and zonulin-induced loss in intestinal barrier function, reducing the incidence of diabetes.64 The involvement of zonulin in T1D pathogenesis was corroborated by our studies in humans showing that a large subgroup of T1D patients has elevated serum zonulin levels that correlated with increased intestinal permeability.65 We also provided preliminary evidence suggesting that, as in the BBDP rat model of the disease, zonulin up-regulation precedes the intestinal permeability in T1D patients.65
Therapeutic Use of the Zonulin System
Although the chronic and unregulated paracellular passage of nonself antigens can lead to allergic, inflammatory, and/or autoimmune reactions, the controlled and self-limiting modulation of intercellular TJ could represent an innovative approach for the delivery of drugs and/or molecules normally not absorbed through the GI tract.43 Considering the limitations of the TJ modulators currently tested for drug delivery,66 it was reasonable to explore whether findings from basic research on the zonulin system could be applied to developing new approaches to the enhancement of drug absorption via the regulation of intercellular TJs. Until recently, the main source of zonulin remains its biochemical purification from human cadavers. Therefore, Zot was used as a valid alternative to use the zonulin system for drug delivery, because both proteins seem to activate the same intracellular signaling leading to the modulation of intercellular TJs.
In vitro experiments in the rabbit ileum demonstrated that Zot reversibly increased intestinal absorption of insulin (MW 5733 Da) by 72% and IgG (140 to 160 kDa) by 52% in a time-dependent manner.67 In vivo31,67 and in vitro29,30 studies showed that the effect of Zot on tissue permeability occurs within 20 minutes of the addition of the protein to the intestinal mucosa and reaches a peak effect in 80 minutes. Zot was also tested in an in vivo primate model of diabetes mellitus. Insulin was intragastrically administered to diabetic monkeys either alone or in combination with increasing amounts of Zot. Measurements of blood insulin levels revealed that insulin bioavailability increased from 5.4% in controls to 10.7% and 18% when Zot 2 μg/kg and 4 μg/kg were administered.68
In the BB Wor rat model of T1D, the bioavailability of oral insulin co-administered with Zot was sufficient to lower serum glucose concentrations to levels comparable to those obtained after parenteral injection of the hormone.67 The survival time of diabetic animals chronically treated with oral insulin + Zot was comparable to that observed in parenterally treated rats. None of the animals treated with insulin and Zot experienced diarrhea, fever, or other systemic symptoms,67 providing the first in vivo evidence that the zonulin system can be exploited as an innovative strategy for the oral delivery of drugs and proteins normally not absorbed through the intestine.
Karyekar and colleagues69 have recently demonstrated that the zonulin pathway is also operative at the endothelial levels, demonstrating that Zot increases the permeability of molecular weight markers (sucrose, inulin) and chemotherapeutic agents (paclitaxel and doxorubicin) across the bovine brain microvessel endothelial cells in a reversible and concentration-dependant manner. A good correlation was established between the transport enhancement and the molecular size with r2 of 0.875. Because many therapeutic agents including anticancer drugs have molecular weights ranging from 300 to 1000 Da, these studies introduced Zot as a potential absorption enhancer for the effective delivery of therapeutic agents to the central nervous system.69
Moreover, studies have shown that Zot enhances the transport of drug candidates of varying molecular weight (mannitol, PEG4000, inulin) or low bioavailability (doxorubicin, paclitaxel, acyclovir, cyclosporin A, anticonvulsant enaminones) up to 30-fold as seen with paclitaxel across Caco-2 cell monolayers without modulating the transcellular transport.70,71 In addition, the transport-enhancing effect of Zot was reversible and nontoxic.71 Recent studies have identified a smaller 12-kDa fragment of Zot, referred to as ΔG that retains Zot’s biological activity on TJs.36 In vitro studies showed that ΔG is capable of significantly increasing the apparent permeability coefficients for a wide variety of therapeutic agents and markers across the Caco-2 cell model.72,73,74 In addition, ΔG improved the bioavailability of paracellular markers, mannitol, inulin, and PEG4000 after intraduodenal administration to rats.72,73 The transport/absorption of different therapeutic agents exhibiting different physicochemical properties (lipophilicity/hydrophilicity, molecular weights, efflux properties, structural differences) and belonging to a wide range of drug classes was tested with ΔG. For example, when the anti-HIV protease inhibitors were administered orally to Sprague Dawley rats with ΔG, the rate and/or extent of drug absorption were significantly ameliorated, with the exception of drugs such as saquinavir, whose low oral bioavailability is mainly attributable to extensive first pass metabolism.74 The in vivo studies with ΔG displayed up to 32-fold increases in drug bioavailability as seen with cyclosporin A after metabolic protection was provided.74
Finally, Zot is a good mucosal adjuvant, considering its ability to interfere with the suppression of specific cell-mediated immunity, probably as a result of the increased dose and/or altered processing of antigen at the mucosal level.75 To characterize further the role of Zot as an adjuvant, its ability to abrogate nasal tolerance to an unrelated protein as gliadin was examined.75 When mice were intranasally administered Zot with gliadin, the cytokine pattern showed reduced down-regulation of interleukin-2 and interferon-γ secretions, together with significantly less suppression in T-cell proliferation.75 This suggested that the mechanism of adjuvanticity mediated by Zot preferentially leads to a functional activation of Th1-cell differentiation.
Additional studies have shown that Zot could be exploited to deliver soluble antigens (Ag) through the nasal mucosa for the induction of Ag-specific systemic and mucosal immune responses.76,77 The co-administration of Zot and ovalbumin (Ova) was found to induce anti-Ova serum IgG (IgG) titers that were ∼40-fold higher than those induced by immunization with Ag alone.76 Zot also stimulated Ag-specific IgA titers in vaginal and intestinal mucosa.77
Conclusions
The GI tract has been extensively studied for its digestive and absorptive functions. A more attentive analysis of its anatomo-functional characteristics, however, clearly indicates that its functions go well beyond the handling of nutrients and electrolytes. The exquisite regional-specific anatomical arrangements of cell subtypes and the finely-regulated cross talk between epithelial, neuroendocrine, and immune cells highlights other less-studied, yet extremely important functions of the GI tract. Of particular interest is the regulation of antigen trafficking and intestinal mucosa-microbiota interactions. These functions dictate the switch from tolerance to immunity and are likely integral mechanisms involved in the pathogenesis of GI inflammatory processes.
The classical paradigm of autoimmune pathogenesis involving specific genetic makeup and exposure to environmental triggers has been challenged recently by the addition of a third element, the loss of intestinal barrier function. Genetic predisposition, miscommunication between innate and adaptive immunity, exposure to environmental triggers, and zonulin-dependent loss of intestinal barrier function secondary to a dysfunction of the intercellular TJs, all seem to be key ingredients involved in the pathogenesis of several autoimmune diseases. This new theory implies that once the autoimmune process is activated, it is not autoperpetuating. Rather it can be modulated or even reversed by preventing the continuous interplay between genes and the environment. Because TJ dysfunction allows such interactions, new therapeutic strategies aimed at re-establishing the intestinal barrier function offer innovative and hitherto unexplored approaches for the management of these devastating chronic diseases. Finally, the zonulin pathway has been exploited to facilitate delivery of drugs and antigens through several epithelial and endothelial barriers, thus allowing the passage of therapeutic agents in the GI tract, the airways, and the blood-brain-barrier.
Acknowledgments
Special thanks to Benjamin E. Weston for expertly redrawing Figure 5.
Footnotes
Address reprint requests to Alessio Fasano, M.D., University of Maryland School of Medicine, Mucosal Biology Research Center, Health Science Facility II, Room S345, 20 Penn St., Baltimore, MD 21201. E-mail: afasano@mbrc.umaryland.edu.
A.F. has financial interest as stock holder in Alba Therapeutics, which is currently investigating a zonulin peptide antagonist. He also serves as chair of the scientific advisory board of the company.
References
- Turner JR. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am J Pathol. 2006;169:1901–1909. doi: 10.2353/ajpath.2006.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusrat A, Turner JR, Madara JL. Molecular physiology and pathophysiology of tight junctions. IV. Regulation of tight junctions by extracellular stimuli: nutrients, cytokines, and immune cells. Am J Physiol. 2000;279:G851–G857. doi: 10.1152/ajpgi.2000.279.5.G851. [DOI] [PubMed] [Google Scholar]
- Furuse M, Hirase M, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Padura I, Lostgalio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Vill A, Simmons D, Dejana E. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142:117–127. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebnet K, Suzuki A, Ohno S, Vestweber D. Junctional adhesion molecules (JAMs): more molecules with dual functions? J Cell Sci. 2004;117:19–29. doi: 10.1242/jcs.00930. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Van Itallie CM. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol. 1995;269:G467–G475. doi: 10.1152/ajpgi.1995.269.4.G467. [DOI] [PubMed] [Google Scholar]
- Mitic LL, Anderson JM. Molecular architecture of tight junctions. Annu Rev Physiol. 1998;60:121–142. doi: 10.1146/annurev.physiol.60.1.121. [DOI] [PubMed] [Google Scholar]
- Mitic LL, Van Itallie Cereijido M, Anderson JM, editors. Boca Raton: CRC Press, Inc.; Occludin and claudins: transmembrane proteins of the tight junction. Tight Junctions. 2001:pp 213–230. [Google Scholar]
- Hirabayashi S, Hata Yutaka. Gonzales-Mariscal L, editor. New York: CRC Press; JAM family proteins: tight junction proteins that belong to the immunoglobulin superfamily. Tight Junctions. 2006:pp 43–63. [Google Scholar]
- Laukoetter MG, Nava P, Lee WY, Severson EA, Capaldo CT, Babbin BA, Williams IR, Koval M, Peatman E, Campbell JA, Dermody TS, Nusrat A, Parkos CA. JAM-A regulates permeability and inflammation in the intestine in vivo. J Exp Med. 2007;204:3067–3076. doi: 10.1084/jem.20071416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citi S. The cytoplasmic plaque proteins of the tight junction. Cereijido M, Anderson JM, editors. Boca Raton: CRC Press, Inc.,; Tight Junctions. 2001:pp 231–264. [Google Scholar]
- Zahraoui A, Joberty G, Arpin M, Fontaine JJ, Hellio R, Tavitian A, Louvard D. A small rab GTPase is distributed in cytoplasmic vesicles in non polarized cells but colocalized with the tight junction marker Zo-1 in polarized epithelial cells. J Cell Biol. 1994;124:101–115. doi: 10.1083/jcb.124.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denker BM, Saha C, Khawaja S, Nigam SK. Involvement of a heterotrimeric G protein alpha subunit in tight junction biogenesis. J Biol Chem. 1996;271:25750–25753. doi: 10.1074/jbc.271.42.25750. [DOI] [PubMed] [Google Scholar]
- Dodane V, Kachar B. Identification of isoforms of G proteins and PKC that colocalize with tight junctions. J Membr Biol. 1996;149:199–209. doi: 10.1007/s002329900020. [DOI] [PubMed] [Google Scholar]
- Keon BH, Schafer S, Kuhn C, Grund C, Franke WW. Symplekin, a novel type of tight junction plaque protein. J Cell Biol. 1996;134:1003–1018. doi: 10.1083/jcb.134.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citi S, Sabannay H, Jakes R, Geiger B, Kendrich-Jones J. Cingulin, a new peripheral component of tight junctions. Nature. 1988;333:272–275. doi: 10.1038/333272a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman PJ, Saper MA, Samraoui B, Bennett WS, Strominger JL, Wiley DC. Structure of the human class-I histocompatibility antigen, Hla-A2. Nature. 1987;329:506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Cuvelier C, Barbatis C, Mielants H, Devos M, Roels H, Veys E. Histopathology of intestinal inflammation related to reactive arthritis. Gut. 1987;28:394–401. doi: 10.1136/gut.28.4.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpan O, Bachelder E, Isil E, Arnheiter H, Matzinger P. ‘Educated’ dendritic cells act as messengers from memory to naive T helper cells. Nat Immunol. 2004;5:615–622. doi: 10.1038/ni1077. [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology. 2004;127:224–238. doi: 10.1053/j.gastro.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Rumio C, Besusso D, Palazzo M, Selleri S, Sfondrini L, Dubini F, Menard S, Balsari A. Degranulation of Paneth cells via Toll-like receptor 9. Am J Pathol. 2004;165:373–381. doi: 10.1016/S0002-9440(10)63304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte JM, Cario E, Podolsky DK. Mechanisms of cross hyporesponsiveness to toll-like receptor bacterial ligands in intestinal epithelial cells. Gastroenterology. 2004;126:1054–1070. doi: 10.1053/j.gastro.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Lee J, Mo JH, Katakura K, Alkalay I, Rucker AN, Liu YT, Lee HK, Shen C, Cojocaru G, Shenouda S, Kagnoff M, Eckmann L, Ben-Neriah Y, Raz E. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol. 2006;8:1327–1336. doi: 10.1038/ncb1500. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll-dependent control mechanisms of CD4 T cell activation. Immunity. 2004;21:733–741. doi: 10.1016/j.immuni.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Bashir ME, Louie S, Shi HN, Nagler-Anderson C. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J Immunol. 2004;172:6978–6987. doi: 10.4049/jimmunol.172.11.6978. [DOI] [PubMed] [Google Scholar]
- Fasano A, Baudry B, Pumplin DW, Wasserman SS, Tall BD, Ketley J, Kaper JB. Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proc Natl Acad Sci USA. 1991;88:5242–5246. doi: 10.1073/pnas.88.12.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A, Fiorentini C, Donelli G, Uzzau S, Kaper JB, Margaretten K, Ding X, Guandalini S, Comstock L, Goldblum SE. Zonula occludens toxin modulates tight junctions through protein kinase C-dependent actin reorganization, in vitro. J Clin Invest. 1995;96:710–720. doi: 10.1172/JCI118114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A, Uzzau S, Fiore C, Margaretten K. The enterotoxic effect of zonula occludens toxin (Zot) on rabbit small intestine involves the paracellular pathway. Gastroenterology. 1997;112:839–846. doi: 10.1053/gast.1997.v112.pm9041245. [DOI] [PubMed] [Google Scholar]
- Marcial MA, Carlson SL, Madara JL. Partitioning of paracellular conductance along the ileal crypt-villus axis: a hypothesis based on structural analysis with detailed consideration of tight junction structure-function relationships. J Membr Biol. 1984;80:59–70. doi: 10.1007/BF01868690. [DOI] [PubMed] [Google Scholar]
- Uzzau S, Lu R, Wang W, Fiore C, Fasano A. Purification and preliminary characterization of the zonula occludens toxin receptor from human (CaCo2) and murine (IEC6) intestinal cell lines. FEMS Microbiol Lett. 2001;194:1–5. doi: 10.1111/j.1574-6968.2001.tb09437.x. [DOI] [PubMed] [Google Scholar]
- Wang W, Uzzau S, Goldblum SE, Fasano A. Human zonulin, a potential modulator of intestinal tight junctions. J Cell Sci. 2000;113:4435–4440. doi: 10.1242/jcs.113.24.4435. [DOI] [PubMed] [Google Scholar]
- Baudry B, Fasano A, Ketley JM, Kaper JB. Cloning of a gene (zot) encoding a new toxin produced by Vibrio cholerae. Infect Immun. 1992;60:428–434. doi: 10.1128/iai.60.2.428-434.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pierro M, Lu R, Uzzau S, Wang W, Margaretten K, Pazzani C, Maimone F, Fasano A. Zonula occludens toxin structure-function analysis. Identification of the fragment biologically active on tight junctions and of the zonulin receptor binding domain. J Biol Chem. 2001;276:19160–19165. doi: 10.1074/jbc.M009674200. [DOI] [PubMed] [Google Scholar]
- El Asmar R, Panigrahi P, Bamford P, Berti I, Not T, Coppa GV, Catassi C, Fasano A. Host-dependent activation of the zonulin system is involved in the impairment of the gut barrier function following bacterial colonization. Gastroenterology. 2002;123:1607–1615. doi: 10.1053/gast.2002.36578. [DOI] [PubMed] [Google Scholar]
- von Herrath MG, Fujinami RS, Whitton JL. Microorganisms and autoimmunity: making the barren field fertile? Nat Rev Microbiol. 2003;1:151–157. doi: 10.1038/nrmicro754. [DOI] [PubMed] [Google Scholar]
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- Fasano A, Shea-Donohue T. Mechanisms of disease: the role of intestinal barrier function in the pathogenesis of gastrointestinal autoimmune diseases. Nat Clin Pract Gastroenterol Hepatol. 2005;2:416–422. doi: 10.1038/ncpgasthep0259. [DOI] [PubMed] [Google Scholar]
- Fasano A. Celiac disease—how to handle a clinical chameleon. N Engl J Med. 2003;348:2568–2570. doi: 10.1056/NEJMe030050. [DOI] [PubMed] [Google Scholar]
- Lammers KM, Lu R, Brownley J, Lu B, Gerard C, Thomas K, Rallabhandi P, Shea-Donohue T, Tamiz A, Alkan S, Netzel-Arnett S, Antalis T, Vogel SN, Fasano A. Gliadin induces an increase in intestinal permeability and intestinal zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology. 2008;135:194–204. doi: 10.1053/j.gastro.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A. Pathological and therapeutic implications of macromolecule passage through the tight junction. Cereijido M, Anderson JM, editors. Boca Raton: CRC Press, Inc.,; Tight Junctions. 2001:pp 697–722. [Google Scholar]
- Madara JL, Trier JS. Structural abnormalities of jejunal epithelial cell membranes in celiac sprue. Lab Invest. 1980;43:254–261. [PubMed] [Google Scholar]
- Schulzke JD, Bentzel CJ, Schulzke I, Riecken EO, Fromm M. Epithelial tight junction structure in the jejunum of children with acute and treated celiac sprue. Pediatr Res. 1998;43:435–441. doi: 10.1203/00006450-199804000-00001. [DOI] [PubMed] [Google Scholar]
- Fasano A, Not T, Wang W, Uzzau S, Berti I, Tommasini A, Goldblum SE. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet. 2000;358:1518–1519. doi: 10.1016/S0140-6736(00)02169-3. [DOI] [PubMed] [Google Scholar]
- Clemente MG, De Virgiliis S, Kang JS, Macatagney R, Musu MP, Di Pierro MR, Drago S, Congia M, Fasano A. Early effects of gliadin on enterocyte intracellular signaling involved in intestinal barrier function. Gut. 2003;52:218–223. doi: 10.1136/gut.52.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikulina M, Habich C, Flohé SB, Scott FW, Kolb H. Wheat gluten causes dendritic cell maturation and chemokine secretion. J Immunol. 2004;173:1925–1933. doi: 10.4049/jimmunol.173.3.1925. [DOI] [PubMed] [Google Scholar]
- Thomas KE, Sapone A, Fasano A, Vogel SN. Gliadin stimulation of murine macrophage inflammatory gene expression and intestinal permeability are Myd88-dependent: role of the innate immune response in celiac disease. J Immunol. 2006;176:2512–2521. doi: 10.4049/jimmunol.176.4.2512. [DOI] [PubMed] [Google Scholar]
- Paterson BM, Lammers KM, Arrieta MC, Fasano A, Meddings JB. The safety, tolerance, pharmacokinetic and pharmacodynamic effects of single doses of AT-1001 in coeliac disease subjects: a proof of concept study. Aliment Pharmacol Ther. 2007;26:757–766. doi: 10.1111/j.1365-2036.2007.03413.x. [DOI] [PubMed] [Google Scholar]
- Weber CR, Turner JR. Inflammatory bowel disease: is it really just another break in the wall? Gut. 2007;56:6–8. doi: 10.1136/gut.2006.104182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Schwarz BT, Graham WV, Wang Y, Su L, Clayburgh DR, Abraham C, Turner JR. IFN-gamma-induced TNFR2 expression is required for TNF-dependent intestinal epithelial barrier dysfunction. Gastroenterology. 2006;131:1153–1163. doi: 10.1053/j.gastro.2006.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166:409–419. doi: 10.1016/s0002-9440(10)62264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye D, Ma I, Ma TY. Molecular mechanism of tumor necrosis factor-alpha modulation of intestinal epithelial tight junction barrier. Am J Physiol. 2006;290:G496–G504. doi: 10.1152/ajpgi.00318.2005. [DOI] [PubMed] [Google Scholar]
- Al-Sadi RM, Ma TY. IL-1beta causes an increase in intestinal epithelial tight junction permeability. J Immunol. 2007;178:4641–4649. doi: 10.4049/jimmunol.178.7.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P, Halstensen TS, Kett K, Krajci P, Kvale D, Rognum TO, Scott H, Sollid LM. Immunobiology and immunopathology of human gut mucosa: humoral immunity and intraepithelial lymphocytes. Gastroenterology. 1989;97:1562–1584. doi: 10.1016/0016-5085(89)90406-x. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Overview of the mucosal immune system. Curr Top Microbiol Immunol. 1989;146:13–25. doi: 10.1007/978-3-642-74529-4_2. [DOI] [PubMed] [Google Scholar]
- De Magistris L, Secondulfo M, Iafusco D, Carbone AG, Urio A, Pontoni G, Carratu R. Altered mannitol absorption in diabetic children. Ital J Gastroenterol. 1996;28:367. [PubMed] [Google Scholar]
- Carratu R, Secondulfo M, de Magistris L, Iafusco D, Urio A, Carbone MG, Pontoni G, Carteni M, Prisco F. Altered intestinal permeability to mannitol in diabetes mellitus type I. J Pediatr Gastroenterol Nutr. 1999;28:264–269. doi: 10.1097/00005176-199903000-00010. [DOI] [PubMed] [Google Scholar]
- Secondulfo M, De Magistris L, Sapone A, Di Monda G, Esposito P, Carratu R. Intestinal permeability and diabetes mellitus type 2. Minerva Gastroenterol Dietol. 1999;45:187–192. [PubMed] [Google Scholar]
- Mooradian AD, Morley JE, Levine AS, Prigge WF, Gebhard RL. Abnormal intestinal permeability to sugars in diabetes mellitus. Diabetologia. 1986;29:221–224. doi: 10.1007/BF00454879. [DOI] [PubMed] [Google Scholar]
- Cooper BT, Ukabam SO, O'Brien IA, Hare JP, Corrall RJ. Intestinal permeability in diabetic diarrhoea. Diabet Med. 1987;4:49–52. doi: 10.1111/j.1464-5491.1987.tb00828.x. [DOI] [PubMed] [Google Scholar]
- Meddings JB, Jarand J, Urbanski SJ, Hardin J, Gall DG. Increased gastrointestinal permeability is an early lesion in the spontaneously diabetic BB rat. Am J Physiol. 1999;276:G951–G957. doi: 10.1152/ajpgi.1999.276.4.G951. [DOI] [PubMed] [Google Scholar]
- Watts T, Berti I, Sapone A, Gerarduzzi T, Not T, Zielke R, Fasano A. Role of the intestinal tight junction modulator zonulin in the pathogenesis of type I diabetes in BB diabetic-prone rats. Proc Natl Acad Sci USA. 2005;102:2916–2921. doi: 10.1073/pnas.0500178102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapone A, de Magistris L, Pietzak M, Clemente MG, Tripathi A, Cucca F, Lampis R, Kryszak D, Cartenì M, Generoso M, Iafusco D, Prisco F, Laghi F, Riegler G, Carratu R, Counts D, Fasano A. Zonulin up-regulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes. 2006;55:1443–1449. doi: 10.2337/db05-1593. [DOI] [PubMed] [Google Scholar]
- Kondoh M, Yagi K. Tight junction modulators: promising candidates for drug delivery. Curr Med Chem. 2007;14:2482–2488. doi: 10.2174/092986707782023640. [DOI] [PubMed] [Google Scholar]
- Fasano A, Uzzau S. Modulation of intestinal tight junctions by zonula occludens toxin permits enteral administration of insulin and other macromolecules in an animal model. J Clin Invest. 1997;99:1158–1164. doi: 10.1172/JCI119271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts TL, Alexander T, Hansen B, Fasano A. Utilizing the paracellular pathway: a novel approach for the delivery of oral insulin in diabetic rhesus macaques. J Invest Med. 2000;48:185. [Google Scholar]
- Karyekar CS, Fasano A, Raje S, Lu R, Dowling TC, Eddington ND. Zonula occludens toxin increases the permeability of molecular weight markers and chemotherapeutic agents across the bovine brain microvessel endothelial cells. J Pharm Sci. 2003;92:414–423. doi: 10.1002/jps.10310. [DOI] [PubMed] [Google Scholar]
- Cox D, Gao H, Raje S, Scott KR, Eddington ND. Enhancing the permeation of marker compounds and enaminone anticonvulsants across Caco-2 monolayers by modulating tight junctions using zonula occludens toxin. Eur J Pharm Biopharm. 2001;52:145–150. doi: 10.1016/s0939-6411(01)00172-2. [DOI] [PubMed] [Google Scholar]
- Cox D, Raje S, Gao H, Salama NN, Eddington ND. Enhanced permeability of molecular weight markers and poorly bioavailable compounds across Caco-2 cell monolayers using the absorption enhancer, zonula occludens toxin. Pharm Res. 2002;19:1680–1688. doi: 10.1023/a:1020709513562. [DOI] [PubMed] [Google Scholar]
- Salama NN, Fasano A, Lu R, Eddington ND. Effect of the biologically active fragment of zonula occludens toxin, ΔG, on the intestinal paracellular transport and oral absorption of mannitol. Int J Pharm. 2003;251:113–121. doi: 10.1016/s0378-5173(02)00589-6. [DOI] [PubMed] [Google Scholar]
- Salama NN, Fasano A, Thakar M, Eddington ND. The effect of ΔG on the transport and oral absorption of macromolecules. J Pharm Sci. 2004;93:1310–1319. doi: 10.1002/jps.20052. [DOI] [PubMed] [Google Scholar]
- Salama NN, Fasano A, Thakar M, Eddington ND. The effect of ΔG on the oral bioavailability of low bioavailable therapeutic agents. J Pharmacol Exp Ther. 2005;312:199–205. doi: 10.1124/jpet.104.073205. [DOI] [PubMed] [Google Scholar]
- Rossi M, Maurano F, Luongo D, Fasano A, Uzzau S, Auricchio S, Troncone R. Zonula occludens toxin (Zot) interferes with the induction of nasal tolerance to gliadin. Immunol Lett. 2002;81:217–221. doi: 10.1016/s0165-2478(02)00038-x. [DOI] [PubMed] [Google Scholar]
- Marinaro M, Di Tommaso A, Uzzau S, Fasano A, De Magistris MT. Zonula occludens toxin is a powerful mucosal adjuvant for intranasally delivered antigens. Infect Immun. 1999;67:1287–1291. doi: 10.1128/iai.67.3.1287-1291.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinaro M, Fasano A, De Magistris MT. Zonula occludens toxin acts as an adjuvant through different mucosal routes and induces protective immune responses. Infect Immun. 2003;71:1897–1902. doi: 10.1128/IAI.71.4.1897-1902.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]