Abstract
Proteinuria is a major cause of tubulointerstitial kidney damage, and free fatty acids bound to albumin are thought to play an important role in its pathogenesis. However, the mechanism whereby proteinuria causes tubulointerstitial damage to the kidney is unclear. Using primary human renal proximal tubular cells, we observed that albumin replete with fatty acids (rBSA) and defatted albumin (dBSA) complexed with linoleic acid (LA) induced significantly more apoptosis than did defatted albumin alone. Oxidative stress was partially involved in apoptotic induction by LA/dBSA but not by rBSA. Administration of fatty acid-bound BSA increased the number of lipid droplets (LDs) and the LD-associated proteins, adipocyte differentiation-related protein and TIP47. LDs are organelles that store esterified fatty acids, and the LD-associated proteins are presumed to facilitate LD formation. Knockdown of adipocyte differentiation-related protein or TIP47 by RNA interference enhanced induction of apoptosis by both rBSA and LA/dBSA. Apoptotic induction was observed similarly when either rBSA or LA/dBSA was applied to only the apical surfaces of polarized LLC-PK1 cells. The present results suggest that LDs and LD-associated proteins have protective effects against apoptosis induced by fatty acid-bound albumin by sequestering free fatty acids. Therapeutic manipulation of these LD-associated proteins could aid in the amelioration of nephritic diseases.
Functional impairment of the kidney during glomerulonephritis correlates better with the degree of tubulointerstitial atrophy than glomerular alteration.1 Many studies have shown that proteinuria is a major cause of damage in the renal tubules and interstitium.2,3,4 Albumin is the predominant protein in the urine of nephritic patients. In vitro studies have shown that albumin affects intracellular signaling pathways in proximal tubular epithelial cells,5,6 induces them to generate various chemoattractants and extracellular matrices,7,8 and changes the balance between cell proliferation and cell death.9,10
The mechanism whereby proteinuria causes tubulointerstitial damage is unclear. Although many studies have concluded that albumin itself is important for the development of the pathological changes, other studies have inferred that free fatty acids (FFAs) bound to albumin play critical roles.9,10,11 In mice, FFA-bound albumin caused more severe tubulointerstitial damage, including cortical apoptosis, than albumin depleted of FFA.12,13 Also, in cultured proximal tubular cells (PTCs), FFA-bound albumin induced apoptosis by activating peroxisome proliferator activated receptor (PPAR)-γ.11 These results suggest that FFAs are involved in the pathogenesis of tubulointerstitial damage.
FFAs are potentially harmful to cellular functions, but cells readily esterify them to form triacylglycerol and cholesterol esters. The esters are then stored in lipid droplets (LDs), consisting of a globular mass of lipid esters surrounded by a phospholipid monolayer.14,15 In fact, administration of FFAs increases the number and size of LDs in many kinds of cells in culture, including PTCs.16 Recent studies revealed that a number of proteins are associated with LDs, and that their expression is increased on FFA loading. Furthermore, engagement of LDs in intracellular lipid trafficking, lipid metabolism, signal transduction, and other cellular functions has been suggested.15,17,18,19
In view of the novel functions attributed to LDs and LD-associated proteins, we aimed to study whether their manipulation could modify the effect of FFAs on PTCs. For this purpose, we used primary human PTCs and two PTC lines and confirmed that albumin replete with FFAs as well as defatted albumin complexed with long-chain FFAs induced a higher degree of apoptosis than defatted albumin alone. Using this experimental system, we found that reduction of LD-associated proteins, ie, adipose differentiation-related protein (ADRP) and TIP47, by RNA interference increased apoptosis induced by FFA-bound albumins. The result suggests that manipulation of LD-associated proteins could be a potential target of therapeutic intervention for nephrotic diseases.
Materials and Methods
Reagents
Rabbit anti-human TIP47 antibody was raised as described.20 Mouse anti-ADRP antibody (Progen; Richlands BC, Queensland, Australia), and secondary antibodies (Jackson ImmunoResearch, West Grove, PA; Invitrogen, Carlsbad, CA; Pierce Chemical, Rockford, IL) were obtained commercially. Bovine serum albumin replete with FFAs (rBSA, catalog no. A9306; Sigma Chemical, St. Louis, MO; and endotoxin <0.1 ng/mg, catalog no. 013-15104; Wako Pure Chemical, Osaka, Japan), and essentially FFA-free BSA (dBSA, catalog no. 017-15141; Wako) were used. Oleic acids (OA, Sigma), linoleic acid (LA, Sigma), and docosahexaenoic acid (DHA, Sigma) were vigorously mixed with dBSA in phosphate-buffered saline at a molar ratio of 6:1,21 filter-sterilized, and added to culture media at the final fatty acid concentration of 400 μmol/L (OA, LA) or 100 μmol/L (DHA). This concentration of FFA was used because the molar ratio of FFA to albumin could reach up to 8.59,22 and the albumin concentration in the proximal tubular lumen could be as high as 2.9 mg/ml, or 43 μmol/L.23 In the current experimental condition, the BSA concentration was 4.4 mg/ml when the fatty acid was used at 400 μmol/L. Vitamin E and desferrioxamine were purchased from Sigma. The FFA content of the rBSA preparation was analyzed by gas chromatography by Mitsubishi Kagaku BCL Inc. (Tokyo, Japan).
Cell Culture
Human primary PTCs (RPTECs; Cambrex, Walkersville, MD), showing characteristics of PTCs in vivo, were cultivated in renal epithelial cell basal medium (REBM, Cambrex) supplemented with REGM SingleQuots (0.5 μl/ml hydrocortisone, 10 pg/ml hEGF, 0.5 μg/ml epinephrine, 6.5 pg/ml triiodothyronine, 10 μg/ml transferrin, 5 μg/ml insulin, 50 μg/ml gentamicin, 50 pg/ml amphotericin B, and 0.5% fetal bovine serum). HK-2 cells, an immortalized human PTC line, were obtained from American Type Culture Collection (Rockville, MD) and were cultured in K1 medium. The K1 medium contained Ham’s F-12/Dulbecco’s modified Eagle’s medium (1:1, Sigma), 12.5 mmol/L HEPES, 10% heat-inactivated fetal bovine serum (JRH Biosciences Inc., Lenexa, KS), and select hormones as described.24 LLC-PK1 cells, a pig PTC line, were obtained from the Japanese Collection of Research Bioresources (Tokyo, Japan), and grown in 199 medium (AppiChem, Darmstadt, Germany) supplemented with 10% fetal bovine serum. LLC-PK1 cells were grown in Transwell chambers (Corning, Corning, NY), and used for experiments 3 days after reaching confluence. All of the cells were kept at 37°C in 5% CO2/95% air.
Immunofluorescence Microscopy and Data Analysis
Cells were fixed with 3% formaldehyde and 0.05% glutaraldehyde for 30 minutes, permeabilized with 0.01% digitonin for 30 minutes, and treated with 3% BSA before immunolabeling for ADRP and TIP47. LDs were stained with BODIPY493/503 (Invitrogen). Images were obtained with an Axiovert fluorescence microscope equipped with Apotome (Carl Zeiss, Oberkochen, Germany) and analyzed with Image J software (National Institutes of Health, Bethesda, MD) as described.18 Contrast and brightness of micrographs were adjusted by Adobe Photoshop 7.0 for data presentation.
RNA Interference and cDNA Transfection
For RNA interference (RNAi), siGENOME duplexes (Dharmacon Inc., Lafayette, CO) were used to knock down the expression of TIP47 and ADRP. A control RNA duplex, siControl nontargeting siRNA, was also obtained from Dharmacon. RNAi was conducted by electroporation using the Gene Pulser 2 system (Bio-Rad, Hercules, CA).25 RPTECs (1.5 × 106) were electroporated (600 V, exponential decay 300 ms, 1 pulse) with 10 μg of siRNA in 400 μl of siPORT siRNA electroporation buffer (Ambion, Austin, TX) using 2-mm electroporation cuvettes. Cells were analyzed 3 days after RNAi.
Human ADRP and TIP47 cDNAs were cloned by polymerase chain reaction and inserted into the pcDNA3.1 vector (Invitrogen). The plasmid vectors were introduced into the cells by Lipofectamine2000 (Invitrogen), and the cells were analyzed 3 days later. The transfection protocol was optimized by using pEGFP-C1 vector (Clontech, Mountain View, CA), and the efficiency was estimated as ∼60%.
Western Blotting
Total cell lysates were prepared in a sodium dodecyl sulfate-sample buffer, an equal amount of protein (30 μg) was electrophoresed in 15% acrylamide gels, and the protein was transferred to nitrocellulose membranes. The blots were incubated with primary antibodies followed by horseradish peroxidase-conjugated secondary antibodies. The reaction was detected with the SuperSignal West dura extended duration substrate (Pierce).
Apoptosis and Cell Viability Assays and Thiobarbituric Acid Reactive Substance (TBARS) Assay
DNA fragmentation was detected by terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) using an ApoAlert DNA fragmentation kit (Clontech). Nuclei were counterstained with 4,6-diamidino-2-phenylindole. More than five areas, each containing 30 to 170 cells, were randomly selected for each sample under identical microscopic settings. The ratio of positive cells was counted and given in percentages.
Activated caspases were detected by the CaspACE FITC-VAD-FMK in situ marker (Promega, Madison, WI) that was added directly to living cells and incubated for 20 minutes. The cells were then fixed and observed with a microscope. For flow cytometric analysis, cells were detached from the substrate by a trypsin-ethylenediaminetetraacetic acid solution after fixation and all of the cells were subjected to analysis in a FACSCalibur cytometer (Becton Dickinson, Franklin Lakes, NJ). Fluorescence was excited at 488 nm and measured at 530 nm. For each experiment, control samples obtained from cells cultured in the complete medium containing 10% FCS were analyzed along with the experimental groups, and a threshold was set so that 1% of the control cells were classified as apoptotic. Cell viability was determined with the Cell Counting Kit 8 (Wako). The amount of TBARS including lipid hydroperoxides, which increase as a result of oxidative stress, was measured by the TBARS assay kit (Cayman Chemical, Ann Arbor, MI).
Statistical Analysis
Where appropriate, experimental data were analyzed by unpaired t-tests, assuming unequal variance. A P value of <0.05 was considered statistically significant.
Results
FFA-Bound Albumin Induces Apoptosis in Cultured PTCs
We first tested whether FFAs induce apoptosis of cultured human PTCs (RPTECs). Because albumin itself could induce apoptosis, we compared the effect of FFA-free BSA versus the same concentration of FFA-bound BSA using two different combinations: first, dBSA that was depleted of FFAs was compared with the same concentration (4.4 mg/ml) of dBSA prebound with linoleic acid (18:2) or oleic acid (18:1) (LA/dBSA, OA/BSA; FFA concentration, 400 μmol/L); second, dBSA and rBSA at 30 mg/ml were compared. Gas chromatographic analysis revealed that the medium with 30 mg/ml of rBSA contained ∼380 μmol/L FFAs, among which 56.6% (w/w) were saturated, ie, stearate, palmitate, and myristate, and the rest were unsaturated, ie, linoleate and oleate. An albumin concentration of 30 mg/ml is higher than that is found in the normal proximal tubule lumen in vivo, but was necessary to reproduce the FFA concentration seen in the nephrotic renal tubule.11 The ratio of apoptotic cells was examined by TUNEL staining (Figure 1, A and B) and fluorescence microscopic analysis of the caspase marker (FITC-VAD-FMK) (Figure 1, C and D). Both assays showed that LA/dBSA and rBSA induced a significant increase in apoptosis compared to dBSA at the same concentration. OA/BSA caused a slight increase in apoptosis, but the difference was not statistically significant. The result indicates that, irrespective of whether BSA alone can induce apoptosis, FFAs bound with BSA can exert additional effects to induce apoptosis of human PTCs, and that the amount of FFAs included in the normal rBSA preparation was sufficient for the effect. This may explain the origin of oval fat bodies in the urine, which are likely to be cell debris after fatty degeneration.26 The observed apoptosis did not occur because of lipopolysaccharide contaminating BSAs,27 because rBSA with little endotoxin contamination (<0.1 ng/mg, A9306; Sigma) caused apoptosis in the present experiment.
Figure 1.
Fatty acids bound to BSA increase apoptosis in human PTCs (RPTECs). Human PTCs, cultured in medium containing 0.5% FCS, were treated with five different combinations of BSA and FFAs for 24 hours: i) dBSA (4.4 mg/ml); ii) 400 μmol/L LA/dBSA (BSA concentration, 4.4 mg/ml); iii) 400 μmol/L OA/BSA (BSA concentration, 4.4 mg/ml); iv) dBSA (30 mg/ml); and v) rBSA (30 mg/ml; FFA concentration, 380 μmol/L). A and B: TUNEL assay; C and D: FITC-VAD-FMK assay. A and C: Photographs show representative areas. B and D: The number of positive cells were counted in eight random areas in three independent experiments and averaged (mean ± SD, **P < 0.01). In controls, no protein was added to the culture medium. Both assays showed that LA/dBSA and rBSA significantly increased apoptosis compared to dBSA at the same concentration. Scale bars = 10 μm.
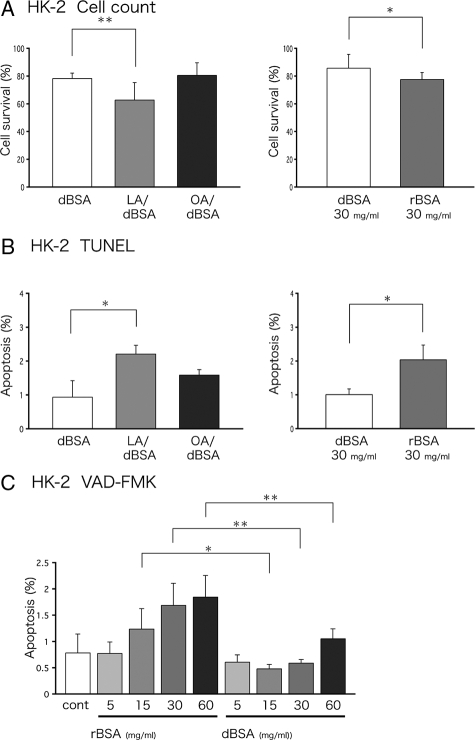
The effect of FFAs was also examined using human HK-2 cells, which have been used as a model of PTCs in many studies.28,29,30 An increase in the apoptotic ratio (Figure 2B) and a decrease in the surviving cell ratio (Figure 2A) were observed by FFA treatments. The relatively low apoptotic ratio in HK-2 cells was probably because they are transformed cells. Nonetheless, the result was essentially the same as that obtained in human PTCs. We further examined the dose effect of rBSA on apoptotic induction. As shown in Figure 2C, the apoptotic ratio increased in a dose-dependent manner, and at any concentration the effect was larger than dBSA at the same concentration.
Figure 2.
Apoptosis in HK-2 cells. A: HK-2 cells, preincubated for 24 hours in serum-free medium, were treated with dBSA (4.4 mg/ml), LA/dBSA, OA/dBSA (FFAs, 400 μmol/L; BSA, 4.4 mg/ml), dBSA (30 mg/ml), or rBSA (30 mg/ml; FFA concentration, 185 μmol/L) for another 24 hours. Cell survival was analyzed with the Cell Counting Kit 8. The number of surviving cells are shown as a ratio to that of control cells kept in 10% FCS. The results of nine samples obtained in three independent experiments were averaged (mean ± SD, *P < 0.05, **P < 0.01). B: HK-2 cells treated in the same manner as described in A were analyzed by TUNEL assay. The number of positive cells was counted in eight random areas in three independent experiments and averaged (mean ± SD, *P < 0.05). C: HK-2 cells were preincubated for 24 hours with 0.5% lipoprotein-deficient serum (LPDS), treated with dBSA or rBSA (5, 15, 30, 60 mg/ml) for another 24 hours, stained by FITC-VAD-FMK, and analyzed by flow cytometry. Control cells were kept in 0.5% LPDS without any further additions. Results obtained in three independent experiments were averaged (mean ± SD, *P < 0.05, **P < 0.01).
We also examined the effect of FFAs that was applied only to the apical surface of the polarized PTC using LLC-PK1 cultured on a Transwell filter support. This experiment was done because PTC in vivo is the simple epithelium with a tight intercellular barrier and FFA-bound albumin bathes the apical surface alone in nephrosis. Formation of an impermeable monolayer was confirmed both by the trans-epithelial resistance measurement and by immunofluorescence microscopy of ZO-1 (data not shown). When various BSA preparations were added to the apical chamber, rBSA and LA/dBSA, but not OA/dBSA, increased the apoptotic ratio than dBSA in both TUNEL and FITC-VAD-FMK assays (Supplementary Figure S1, see http://ajp.amjpathol.org). This result showed that exposure of the apical surface of polarized epithelial cells to FFAs is sufficient to induce apoptosis.
FFA-Bound Albumin Increases LDs, ADRP, and TIP47 in Human PTCs
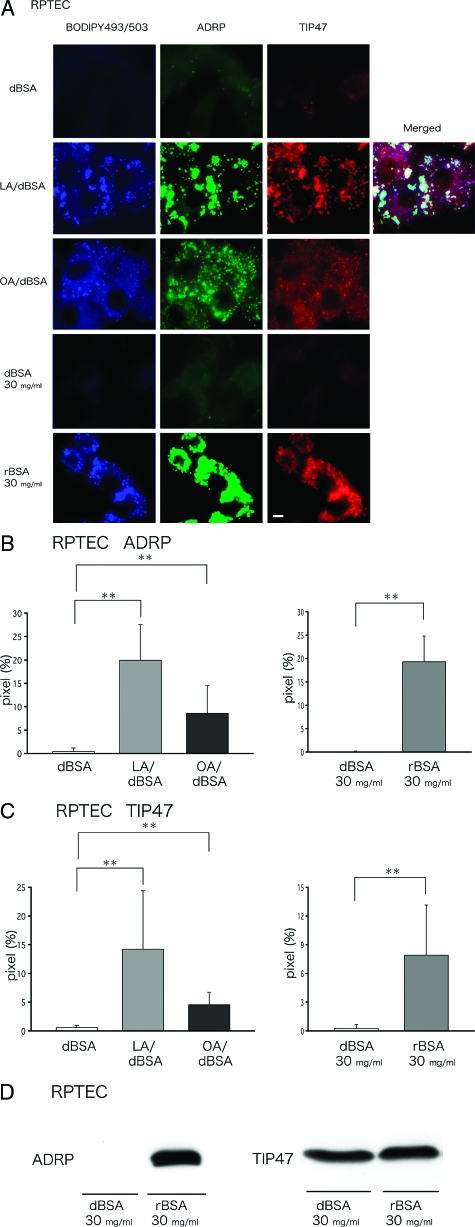
In many cell types including PTCs,16 FFAs were shown to increase the total volume of LDs and expression of LD-associated proteins. We examined whether similar increases occur in human PTCs using the above treatments that induced apoptosis. In human PTCs treated with dBSA alone, either at 4.4 mg/ml or 30 mg/ml, LDs were hardly visible by BODIPY493/503 staining, and labeling for ADRP or TIP47 was negligible (Figure 3A). After treatment with LA/dBSA, OA/BSA, or rBSA, LDs were clearly observed and were positively labeled for ADRP and TIP47. Quantification of the labeling intensity in randomly taken fluorescence micrographs showed that both ADRP and TIP47 labeling in LDs increased after FFA-bound albumin treatment (Figure 3, B and C). A very similar result was obtained in HK-2 cells and after the DHA/dBSA treatment (data not shown). TIP47 can exist stably both as soluble and LD-bound forms, and is rapidly recruited from the cytosol to LDs when FFAs are administered.31,32 On the other hand, ADRP exists in LDs constitutively and is a more reliable marker for estimating the amount of LDs. Consistent with this, Western blotting showed that the total expression of ADRP was greater in cells treated with rBSA than in those treated with dBSA, whereas the expression of TIP47 was not significantly different in the two samples (Figure 3D). An increase of ADRP in LA/dBSA- and OA/dBSA-treated cells was also confirmed by Western blotting (data not shown).
Figure 3.
LDs and LD-associated proteins in human PTCs (RPTECs) increase significantly on treatment with FFAs bound to BSA. A: Human PTCs were treated with dBSA (4.4 mg/ml), LA/dBSA, OA/dBSA (FFAs, 400 μmol/L; BSA, 4.4 mg/ml), dBSA (30 mg/ml), or rBSA (30 mg/ml) for 24 hours as described in Figure 1. LDs were stained by BODIPY493/503 (green), whereas ADRP (blue) and TIP47 (red) were labeled with antibodies. A merged picture showed clearly that ADRP and TIP47 co-localize in the same LDs. Treatment with LA/dBSA, OA/dBSA, or rBSA significantly increased the labeling intensity of LDs, ADRP, and TIP47. B and C: The labeling intensity of ADRP (B) and TIP47 (C) by immunofluorescence microscopy was quantified (mean ± SD). Results obtained in three independent experiments were averaged. Eight areas were randomly chosen and photographed at the same setting. The ordinate is the percentage of pixels that shows the labeling more intense than a threshold (mean ± SD, **P < 0.01) D: Western blotting of ADRP and TIP47 using the total cell lysate. Treatment with rBSA (30 mg/ml) drastically increased the expression of ADRP, whereas the influence on TIP47 expression was minimal. Equal amounts (30 μg) of protein were loaded in each lane. Scale bar = 5 μm.
Antioxidants Suppress FFA-Induced Apoptosis to Various Extents
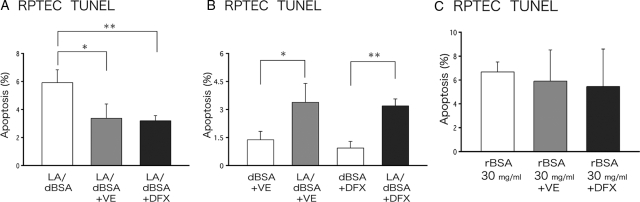
Polyunsaturated FFAs (PUFAs) are substrates of iron-dependent peroxidation reactions that give rise to lipid peroxides, or reactive lipid oxygen species, and increase oxidant stress.33,34 To explore whether lipid peroxidation is involved in FFA-induced apoptosis, we examined the effect of antioxidants: vitamin E (120 μmol/L) and desferrioxamine (100 μmol/L), a specific chelator for iron that inhibits iron-dependent lipid peroxidation,35 were used. After preincubation with vitamin E or desferrioxamine for 1 hour, human PTCs were treated with LA/dBSA or rBSA for another 24 hours in the continued presence of antioxidant. Apoptosis induced by LA/dBSA was suppressed by incubation with either vitamin E or desferrioxamine (Figure 4A), but was still significantly more than that caused by dBSA alone (Figure 4B). In contrast, apoptosis induced by rBSA was not affected by the presence of antioxidants (Figure 4C). Similar results were obtained in HK-2 cells by flow cytometry after FITC-VAD-FMK staining (data not shown). Interestingly, apoptosis caused by DHA/dBSA in HK-2 cells was completely suppressed by antioxidants (Supplementary Figure S2, see http://ajp. amjpathol.org). Consistent with these results, the TBARS assay showed that DHA/dBSA is the strongest oxidative stress, whereas LA/dBSA is significantly weaker than DHA/dBSA, and rBSA was comparable to dBSA (Supplementary Figure S2C, see http://ajp.amjpathol.org). The results corroborate that lipid peroxidation is the major cause of apoptotic induction by polyunsaturated (24:6) DHA/dBSA, whereas it is only partially involved in the induction by LA/dBSA containing two unsaturated bonds (18:2) and is not related to the induction by rBSA primarily consisting of saturated fatty acids.
Figure 4.
Increased FFA-induced apoptosis persists in the presence of antioxidants. Human PTCs were preincubated with 120 μmol/L vitamin E (VE) or 100 μmol/L desferrioxamine (DFX) for 1 hour, and were then incubated with LA/dBSA (LA, 400 μmol/L; BSA, 4.4 mg/ml) or rBSA (30 mg/ml) for 24 hours. Apoptosis was measured by the TUNEL assay. A: Vitamin E and desferrioxamine significantly decreased apoptosis induced by LA/dBSA. B: Even in the presence of vitamin E or desferrioxamine, LA/dBSA caused a higher ratio of apoptotic cells than dBSA alone. C: Apoptosis induced by rBSA was not significantly reduced by vitamin E or desferrioxamine. Results obtained in three independent experiments were averaged (mean ± SD; *P < 0.05, **P < 0.01).
Knockdown of ADRP and TIP47 Augments FFA-Induced Apoptosis
The experiments described above show that treatment with FFA-bound albumin increases LD formation and apoptosis in PTCs. By storing excess FFAs as lipid esters, LDs are thought to protect cells against the toxicity of FFAs.36 This led to speculation that manipulation of LD formation may influence the outcome of FFA loading. To test this hypothesis in PTCs, we knocked down expression of ADRP and TIP47 by RNAi and examined the effect on FFA-induced apoptosis.
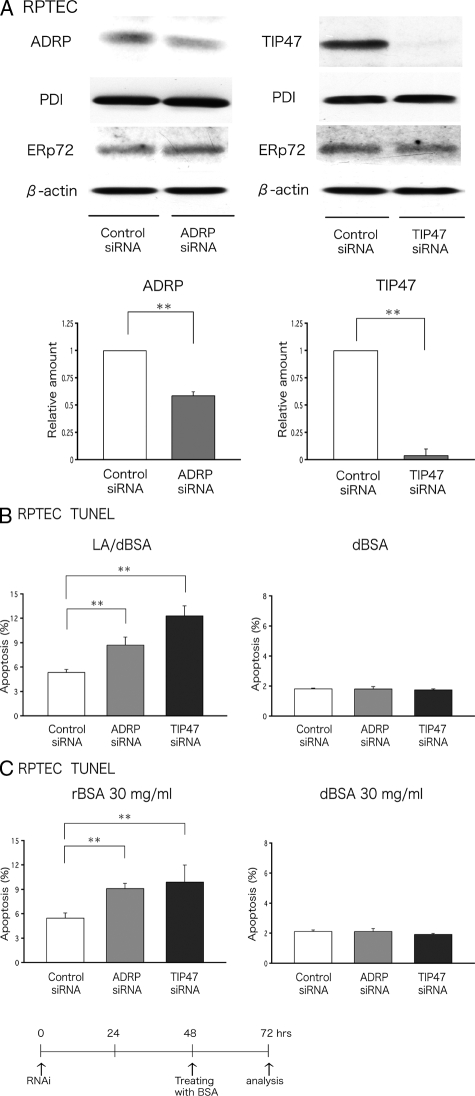
The RNAi procedure using electroporation significantly decreased the expression of both ADRP and TIP47 in human primary PTCs, although the effect was consistently more pronounced for TIP47 than for ADRP (Figure 5A). Two days after RNAi, the cells were treated with LA/dBSA or rBSA, and the apoptotic ratio was examined 24 hours later by the TUNEL assay. The apoptotic ratio was compared between cells transfected with random control siRNA and those treated with siRNAs specific for ADRP or TIP47. Knockdown of either ADRP or TIP47 significantly increased the ratio of apoptotic cells that were induced by both LA/dBSA (Figure 5B) and rBSA (Figure 5C). A similar result was obtained by quantifying the nuclear condensation after the 4,6-diamidino-2-phenylindole staining (Supplementary Figure S3, see http://ajp.amjpathol.org). A basal level of apoptosis observed in the presence of dBSA was not changed by the RNAi procedure. A similar result was obtained in HK-2 cells that were subjected to RNAi and examined by flow cytometry after FITC-VAD-FMK staining (data not shown). These results suggest that endogenous ADRP and TIP47 may protect cells from apoptosis by augmenting storage of FFAs as lipid esters in LDs.
Figure 5.
Knockdown of ADRP or TIP47 augments FFA-induced apoptosis in human PTCs (RPTECs). A: Western blotting showed that expression of ADRP and TIP47 in human PTCs was reduced significantly by RNAi. Equal amounts (30 μg) of total cell lysate were electrophoresed and probed with antibodies to ADRP, TIP47, PDI, ERp72, and β-actin. The RNAi of ADRP and TIP47 reduced expression of respective protein significantly, but did not affect other proteins. Results obtained from three independent experiments were averaged and shown in the bar graph (mean ± SD). B and C: Human PTCs were transfected with either random, ADRP, or TIP47 siRNA, and then challenged with LA/dBSA, rBSA, or dBSA. Apoptosis was measured by the TUNEL assay. The result is shown as the average of four independent experiments (mean ± SD, **P < 0.01). B: Knockdown of either ADRP or TIP47 increases apoptosis induced by LA/dBSA (LA, 400 μmol/L; BSA, 4.4 mg/ml), whereas it does not influence the basal level of apoptosis observed in the presence of dBSA (4.4 mg/ml). C: The knockdown also increased apoptosis by 30 mg/ml of rBSA, but it did not affect the apoptotic ratio in cells treated by the same concentration of dBSA.
Discussion
In the present study, we first showed that dBSA bound with LA or rBSA induced more apoptosis than dBSA at the same concentration using three different cell preparations: human primary PTCs and two immortalized cell lines derived from PTCs, HK-2 and LLC-PK1. Second, we demonstrated that knockdown of either ADRP or TIP47 aggravated FFA-induced apoptosis, suggesting a protective role of those LD-associated proteins in PTCs.
Concerning the first point, the result of LLC-PK1 cells is important because they retain the apico-basolateral polarization when cultured on semipermeable supports and form tight junctions to demarcate the apical and basal fluid compartments. During nephrosis, a drastic increase in protein concentration in the renal tubular fluid causes various changes in the epithelial cells, whereas the basolateral environment should remain primarily unaffected at least during the initial stage. This condition could be produced in vitro by adding BSA to only the apical compartment of polarized LLC-PK1 cells. In the present experiment, apoptosis was similarly induced in nonpolarized human PTCs and HK-2 cells that were exposed to FFA-bound BSAs on all surfaces. But in other experimental conditions, loss of polarization could cause changes in cellular reactions. Conflicting results have been reported concerning the effect of BSA on cultured renal tubular cells,7,9,10,11 and we speculate that some of these discrepancies could be related to lack of cellular polarization in the experimental systems.
FFAs can induce apoptosis through different pathways, and the pro-apoptotic potency of each FFA may vary depending on the cell types.36 In the present study, rBSA and LA/dBSA caused more apoptosis than OA/dBSA in PTCs. Because the induction of apoptosis by DHA/dBSA in HK-2 cells was completely suppressed by either the antioxidant vitamin E or the iron-chelator desferrioxamine, oxidative stress appears to be the major cause of apoptosis in this case, as previously suggested.37 However, apoptosis caused by LA/dBSA was only partially suppressed by these reagents, and apoptosis caused by rBSA, primarily bound with saturated FFAs, was not affected at all. This result suggests that oxidative stress is a major effect that PUFAs exert on cells, but that FFAs with less unsaturation induce apoptosis by other mechanisms. That is, if human serum albumin is primarily bound with saturated FFAs as rBSA, antioxidants would not be effective to block apoptosis in PTCs of the nephrotic kidney.
In many cases including the present study, OA has been shown to be less toxic than saturated FFAs or PUFAs.38 It may be partly because monounsaturated OA may not generate strong oxidative stress and is not a precursor for ceramide synthesis.38 Additionally, OA may counterbalance its toxicity by promoting LD formation, thereby reducing the FFA concentration.36 This supposition can be extended to other FFAs, and we speculated that the proapoptotic potency of each FFA can be determined by the balance between its toxicity and ability to induce LD formation. From this viewpoint, we hypothesized that reduction of the LD-associated proteins, ADRP and TIP47, would compromise the capacity of cells to store lipid esters and increase apoptosis on administration of FFAs. As expected, when either ADRP or TIP47 was down-regulated by RNAi, apoptosis induced by LA/dBSA or rBSA was significantly enhanced. The effect on apoptosis was more consistently observed with TIP47 RNAi than with ADRP RNAi. This difference could result from potential critical roles played by TIP47 in sequestering FFAs to LDs, but it may simply be explained by the fact that knockdown of TIP47 was more efficient than ADRP.
ADRP has been presumed to be involved in the generation of LDs.21,39,40 Consistent with this supposition, ADRP-null mice show a drastic reduction of LDs and lipid esters in the liver.41 Perilipin, a LD-associated protein expressed in adipocytes and steroidogenic cells, prevents cytosolic hormone-sensitive lipases from acting on lipid esters in LDs.42,43 ADRP may also have a similar protective role against lipases,44 and may also reduce the cytotoxic effect of FFAs by functioning in LDs in an undefined manner. On the other hand, the function of TIP47 with regards to LDs is unclear,18,31,45 whereas its involvement in recycling of the mannose-6-phosphate receptor from endosomes to the trans-Golgi network has been reported.46 TIP47 shows significant similarity to ADRP both in amino acid sequence and three-dimensional structure45,47 and is likely to have a related function in some aspect of lipid metabolism. Even though the molecular mechanism is not clear, we speculate that reduction of ADRP and TIP47 compromised the ability of cells to sequester FFAs as lipid esters and thus led to an increase in FFA-induced apoptosis in PTCs (Figure 6).
Figure 6.
Based on the results of the present study, we hypothesize that LD and LD-associated proteins function in PTCs of the nephrotic kidney as described in the figure. Albumin that reached the tubular lumen presents FFAs to PTCs in an unregulated manner. FFAs may induce apoptosis in PTCs, but LDs can sequester FFAs as lipid esters and thereby protect cells from their toxicity. When the LD-associated proteins were reduced, FFA-sequestering ability of LDs may be compromised, and the resultant increase of FFAs in the cytosol may induce an increase in apoptosis.
We expected that cDNA transfection of ADRP or TIP47 would provide a protective effect against FFA-induced apoptosis, but it did not cause a significant change in HK-2 cells (data not shown). We speculate that this negative result was primarily attributable to the low level of protein overexpression after cDNA transfection (data not shown). Expression of ADRP is posttranslationally regulated through polyubiquitylation and proteasomal degradation.48,49 Without binding to LDs, they are likely to be short-lived, and thus an introduction of cDNA did not lead to a significant increase of protein. The regulatory mechanism of TIP47 protein expression is not known, but the lack of a significant increase in expression after cDNA transfection suggests a similar mechanism. Additionally, different LD-related proteins may cooperate with each other, such that they may need to be simultaneously overexpressed to be truly functional. That is, if expression of ADRP, TIP47, and some other proteins can be physiologically increased, they may give rise to an increase in protective function. In this context, it is noteworthy that PPAR ligands inhibited progression of diabetic nephropathy50,51,52 and experimental glomerulonephritis.9,53 This phenotype may be the result of an increase of not only ADRP but also other LD-related proteins.15,54,55
The present study shows that FFAs bound to BSA induce apoptosis in PTCs, and that reduction of ADRP or TIP47 further increases the ratio of apoptotic cells. This result does not exclude the possibility that high concentrations of urinary proteins alone can affect renal tubular cells in the nephrotic kidney. Most importantly, this study demonstrates for the first time that LD-associated proteins play some protective role against FFA-induced cytotoxicity in PTCs. Furthermore, it reveals that the cellular toxicity of FFAs is heterogeneous and not linearly correlated with the number of double bonds. The result suggests that the prognosis for nephrosis may vary depending on the expression level of LD-associated proteins and composition of FFAs bound to serum proteins. It also suggests that LD-associated proteins and their regulatory mechanisms may be used as targets of therapeutic intervention for nephrotic diseases. We hope that the role of LD-associated proteins in PTCs will be confirmed in animal experiments and translated to clinical applications in the near future.
Acknowledgments
We thank Dr. Mikio Furuse (Kobe University, Kobe, Japan) for the transepithelial resistance measurement, and Ms. Kumi Tauchi-Sato and Mr. Tetsuo Okumura for technical assistance.
Footnotes
Address reprint requests to Yoshimichi Urahama, M.D., The Division of Nephrology, Department of Internal Medicine, Nagoya University Graduate School of Medicine, 65 Tsurumai, Showa, Nagoya 466-8550, Japan. E-mail: urahama@med.nagoya-u.ac.jp.
Supported by the Ministry of Education, Culture, Sports, Science, and Technology of Japan (grants-in-aid for scientific research and the 21st century COE program “Integrated Molecular Medicine for Neuronal and Neoplastic Disorders”).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Risdon RA, Sloper JC, De Wardener HE. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet. 1968;2:363–366. doi: 10.1016/s0140-6736(68)90589-8. [DOI] [PubMed] [Google Scholar]
- Eddy AA. Experimental insights into the tubulointerstitial disease accompanying primary glomerular lesions. J Am Soc Nephrol. 1994;5:1273–1287. doi: 10.1681/ASN.V561273. [DOI] [PubMed] [Google Scholar]
- Peterson JC, Adler S, Burkart JM, Greene T, Hebert LA, Hunsicker LG, King AJ, Klahr S, Massry SG, Seifter JL. Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease Study. Ann Intern Med. 1995;123:754–762. doi: 10.7326/0003-4819-123-10-199511150-00003. [DOI] [PubMed] [Google Scholar]
- Remuzzi G, Bertani T. Pathophysiology of progressive nephropathies. N Engl J Med. 1998;339:1448–1456. doi: 10.1056/NEJM199811123392007. [DOI] [PubMed] [Google Scholar]
- Brunskill NJ, Stuart J, Tobin AB, Walls J, Nahorski S. Receptor-mediated endocytosis of albumin by kidney proximal tubule cells is regulated by phosphatidylinositide 3-kinase. J Clin Invest. 1998;101:2140–2150. doi: 10.1172/JCI1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R, Brunskill NJ. Activation of mitogenic pathways by albumin in kidney proximal tubule epithelial cells: implications for the pathophysiology of proteinuric states. J Am Soc Nephrol. 1999;10:1487–1497. doi: 10.1681/ASN.V1071487. [DOI] [PubMed] [Google Scholar]
- Iglesias J, Abernethy VE, Wang Z, Lieberthal W, Koh JS, Levine JS. Albumin is a major serum survival factor for renal tubular cells and macrophages through scavenging of ROS. Am J Physiol. 1999;277:F711–F722. doi: 10.1152/ajprenal.1999.277.5.F711. [DOI] [PubMed] [Google Scholar]
- Zoja C, Donadelli R, Colleoni S, Figliuzzi M, Bonazzola S, Morigi M, Remuzzi G. Protein overload stimulates RANTES production by proximal tubular cells depending on NF-kappa B activation. Kidney Int. 1998;53:1608–1615. doi: 10.1046/j.1523-1755.1998.00905.x. [DOI] [PubMed] [Google Scholar]
- Erkan E, De Leon M, Devarajan P. Albumin overload induces apoptosis in LLC-PK(1) cells. Am J Physiol. 2001;280:F1107–F1114. doi: 10.1152/ajprenal.2001.280.6.F1107. [DOI] [PubMed] [Google Scholar]
- Erkan E, Garcia CD, Patterson LT, Mishra J, Mitsnefes MM, Kaskel FJ, Devarajan P. Induction of renal tubular cell apoptosis in focal segmental glomerulosclerosis: roles of proteinuria and Fas-dependent pathways. J Am Soc Nephrol. 2005;16:398–407. doi: 10.1681/ASN.2003100861. [DOI] [PubMed] [Google Scholar]
- Arici M, Chana R, Lewington A, Brown J, Brunskill NJ. Stimulation of proximal tubular cell apoptosis by albumin-bound fatty acids mediated by peroxisome proliferator activated receptor-gamma. J Am Soc Nephrol. 2003;14:17–27. doi: 10.1097/01.asn.0000042167.66685.ea. [DOI] [PubMed] [Google Scholar]
- Kamijo A, Kimura K, Sugaya T, Yamanouchi M, Hase H, Kaneko T, Hirata Y, Goto A, Fujita T, Omata M. Urinary free fatty acids bound to albumin aggravate tubulointerstitial damage. Kidney Int. 2002;62:1628–1637. doi: 10.1046/j.1523-1755.2002.00618.x. [DOI] [PubMed] [Google Scholar]
- Thomas ME, Harris KP, Walls J, Furness PN, Brunskill NJ. Fatty acids exacerbate tubulointerstitial injury in protein-overload proteinuria. Am J Physiol. 2002;283:F640–F647. doi: 10.1152/ajprenal.00001.2002. [DOI] [PubMed] [Google Scholar]
- Tauchi-Sato K, Ozeki S, Houjou T, Taguchi R, Fujimoto T. The surface of lipid droplets is a phospholipid monolayer with a unique fatty acid composition. J Biol Chem. 2002;277:44507–44512. doi: 10.1074/jbc.M207712200. [DOI] [PubMed] [Google Scholar]
- Murphy DJ. The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Prog Lipid Res. 2001;40:325–438. doi: 10.1016/s0163-7827(01)00013-3. [DOI] [PubMed] [Google Scholar]
- Thomas ME, Morrison AR, Schreiner GF. Metabolic effects of fatty acid-bearing albumin on a proximal tubule cell line. Am J Physiol. 1995;268:F1177–F1184. doi: 10.1152/ajprenal.1995.268.6.F1177. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Kogo H, Ishiguro K, Tauchi K, Nomura R. Caveolin-2 is targeted to lipid droplets, a new “membrane domain” in the cell. J Cell Biol. 2001;152:1079–1085. doi: 10.1083/jcb.152.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsaki Y, Cheng J, Fujita A, Tokumoto T, Fujimoto T. Cytoplasmic lipid droplets are sites of convergence of proteasomal and autophagic degradation of apolipoprotein B. Mol Biol Cell. 2006;17:2674–2683. doi: 10.1091/mbc.E05-07-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Parton RG. Lipid droplets: a unified view of a dynamic organelle. Nat Rev Mol Cell Biol. 2006;7:373–378. doi: 10.1038/nrm1912. [DOI] [PubMed] [Google Scholar]
- Ohsaki Y, Maeda T, Fujimoto T. Fixation and permeabilization protocol is critical for the immunolabeling of lipid droplet proteins. Histochem Cell Biol. 2005;124:445–452. doi: 10.1007/s00418-005-0061-5. [DOI] [PubMed] [Google Scholar]
- Brasaemle DL, Barber T, Kimmel AR, Londos C. Post-translational regulation of perilipin expression. Stabilization by stored intracellular neutral lipids. J Biol Chem. 1997;272:9378–9387. doi: 10.1074/jbc.272.14.9378. [DOI] [PubMed] [Google Scholar]
- Ghiggeri GM, Ginevri F, Candiano G, Oleggini R, Perfumo F, Queirolo C, Gusmano R. Characterization of cationic albumin in minimal change nephropathy. Kidney Int. 1987;32:547–553. doi: 10.1038/ki.1987.243. [DOI] [PubMed] [Google Scholar]
- Lewy JE, Pesce A. Micropuncture study of albumin transfer in aminonucleoside nephrosis in the rat. Pediatr Res. 1973;7:553–559. doi: 10.1203/00006450-197306000-00002. [DOI] [PubMed] [Google Scholar]
- Sato W, Kadomatsu K, Yuzawa Y, Muramatsu H, Hotta N, Matsuo S, Muramatsu T. Midkine is involved in neutrophil infiltration into the tubulointerstitium in ischemic renal injury. J Immunol. 2001;167:3463–3469. doi: 10.4049/jimmunol.167.6.3463. [DOI] [PubMed] [Google Scholar]
- Ovcharenko D, Jarvis R, Hunicke-Smith S, Kelnar K, Brown D. High-throughput RNAi screening in vitro: from cell lines to primary cells. RNA. 2005;11:985–993. doi: 10.1261/rna.7288405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternheimer R. A supravital cytodiagnostic stain for urinary sediments. JAMA. 1975;231:826–832. [PubMed] [Google Scholar]
- Jo SK, Cha DR, Cho WY, Kim HK, Chang KH, Yun SY, Won NH. Inflammatory cytokines and lipopolysaccharide induce Fas-mediated apoptosis in renal tubular cells. Nephron. 2002;91:406–415. doi: 10.1159/000064280. [DOI] [PubMed] [Google Scholar]
- Morigi M, Macconi D, Zoja C, Donadelli R, Buelli S, Zanchi C, Ghilardi M, Remuzzi G. Protein overload-induced NF-kappaB activation in proximal tubular cells requires H(2)O(2) through a PKC-dependent pathway. J Am Soc Nephrol. 2002;13:1179–1189. [PubMed] [Google Scholar]
- Zhang XL, Selbi W, de la Motte C, Hascall V, Phillips A. Renal proximal tubular epithelial cell transforming growth factor-beta1 generation and monocyte binding. Am J Pathol. 2004;165:763–773. doi: 10.1016/s0002-9440(10)63339-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy DA, Daly PJ, Docherty NG, Murphy M, Fitzpatrick JM, Watson RW. Heat shock-induced protection of renal proximal tubular epithelial cells from cold storage and rewarming injury. J Am Soc Nephrol. 2006;17:805–812. doi: 10.1681/ASN.2005090980. [DOI] [PubMed] [Google Scholar]
- Wolins NE, Rubin B, Brasaemle DL. TIP47 associates with lipid droplets. J Biol Chem. 2001;276:5101–5108. doi: 10.1074/jbc.M006775200. [DOI] [PubMed] [Google Scholar]
- Ohsaki Y, Maeda T, Maeda M, Tauchi-Sato K, Fujimoto T. Recruitment of TIP47 to lipid droplets is controlled by the putative hydrophobic cleft. Biochem Biophys Res Commun. 2006;347:279–287. doi: 10.1016/j.bbrc.2006.06.074. [DOI] [PubMed] [Google Scholar]
- Garrido A, Garrido F, Guerra R, Valenzuela A. Ingestion of high doses of fish oil increases the susceptibility of cellular membranes to the induction of oxidative stress. Lipids. 1989;24:833–835. doi: 10.1007/BF02544593. [DOI] [PubMed] [Google Scholar]
- D'Aquino M, Benedetti PC, Di Felice M, Gentili V, Tomassi G, Maiorino M, Ursini F. Effect of fish oil and coconut oil on antioxidant defence system and lipid peroxidation in rat liver. Free Radic Res Commun. 1991;12–13:147–152. doi: 10.3109/10715769109145779. [DOI] [PubMed] [Google Scholar]
- Mishra R, Emancipator SN, Miller C, Kern T, Simonson MS. Adipose differentiation-related protein and regulators of lipid homeostasis identified by gene expression profiling in the murine db/db diabetic kidney. Am J Physiol. 2004;286:F913–F921. doi: 10.1152/ajprenal.00323.2003. [DOI] [PubMed] [Google Scholar]
- Listenberger LL, Han X, Lewis SE, Cases S, Farese RV, Jr, Ory DS, Schaffer JE. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci USA. 2003;100:3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishola DA, Post JA, van Timmeren MM, Bakker SJ, Goldschmeding R, Koomans HA, Braam B, Joles JA. Albumin-bound fatty acids induce mitochondrial oxidant stress and impair antioxidant responses in proximal tubular cells. Kidney Int. 2006;70:724–731. doi: 10.1038/sj.ki.5001629. [DOI] [PubMed] [Google Scholar]
- Artwohl M, Roden M, Waldhausl W, Freudenthaler A, Baumgartner-Parzer SM. Free fatty acids trigger apoptosis and inhibit cell cycle progression in human vascular endothelial cells. FASEB J. 2004;18:146–148. doi: 10.1096/fj.03-0301fje. [DOI] [PubMed] [Google Scholar]
- Gao J, Serrero G. Adipose differentiation related protein (ADRP) expressed in transfected COS-7 cells selectively stimulates long chain fatty acid uptake. J Biol Chem. 1999;274:16825–16830. doi: 10.1074/jbc.274.24.16825. [DOI] [PubMed] [Google Scholar]
- Targett-Adams P, Chambers D, Gledhill S, Hope RG, Coy JF, Girod A, McLauchlan J. Live cell analysis and targeting of the lipid droplet-binding adipocyte differentiation-related protein. J Biol Chem. 2003;278:15998–16007. doi: 10.1074/jbc.M211289200. [DOI] [PubMed] [Google Scholar]
- Chang BH, Li L, Paul A, Taniguchi S, Nannegari V, Heird WC, Chan L. Protection against fatty liver but normal adipogenesis in mice lacking adipose differentiation-related protein. Mol Cell Biol. 2006;26:1063–1076. doi: 10.1128/MCB.26.3.1063-1076.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasaemle DL, Rubin B, Harten IA, Gruia-Gray J, Kimmel AR, Londos C. Perilipin A increases triacylglycerol storage by decreasing the rate of triacylglycerol hydrolysis. J Biol Chem. 2000;275:38486–38493. doi: 10.1074/jbc.M007322200. [DOI] [PubMed] [Google Scholar]
- Sztalryd C, Xu G, Dorward H, Tansey JT, Contreras JA, Kimmel AR, Londos C. Perilipin A is essential for the translocation of hormone-sensitive lipase during lipolytic activation. J Cell Biol. 2003;161:1093–1103. doi: 10.1083/jcb.200210169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasaemle DL. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res. 2007;48:2547–2559. doi: 10.1194/jlr.R700014-JLR200. [DOI] [PubMed] [Google Scholar]
- Miura S, Gan JW, Brzostowski J, Parisi MJ, Schultz CJ, Londos C, Oliver B, Kimmel AR. Functional conservation for lipid storage droplet association among perilipin, ADRP, and TIP47 (PAT)-related proteins in mammals, Drosophila, and Dictyostelium. J Biol Chem. 2002;277:32253–32257. doi: 10.1074/jbc.M204410200. [DOI] [PubMed] [Google Scholar]
- Díaz E, Pfeffer SR. TIP47: a cargo selection device for mannose 6-phosphate receptor trafficking. Cell. 1998;93:433–443. doi: 10.1016/s0092-8674(00)81171-x. [DOI] [PubMed] [Google Scholar]
- Hickenbottom SJ, Kimmel AR, Londos C, Hurley JH. Structure of a lipid droplet protein; the PAT family member TIP47. Structure. 2004;12:1199–1207. doi: 10.1016/j.str.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Xu G, Sztalryd C, Lu X, Tansey JT, Gan J, Dorward H, Kimmel AR, Londos C. Post-translational regulation of adipose differentiation-related protein by the ubiquitin/proteasome pathway. J Biol Chem. 2005;280:42841–42847. doi: 10.1074/jbc.M506569200. [DOI] [PubMed] [Google Scholar]
- Masuda Y, Itabe H, Odaki M, Hama K, Fujimoto Y, Mori M, Sasabe N, Aoki J, Arai H, Takano T. ADRP/adipophilin is degraded through the proteasome-dependent pathway during regression of lipid-storing cells. J Lipid Res. 2006;47:87–98. doi: 10.1194/jlr.M500170-JLR200. [DOI] [PubMed] [Google Scholar]
- Guan Y. Peroxisome proliferator-activated receptor family and its relationship to renal complications of the metabolic syndrome. J Am Soc Nephrol. 2004;15:2801–2815. doi: 10.1097/01.ASN.0000139067.83419.46. [DOI] [PubMed] [Google Scholar]
- Agarwal R, Saha C, Battiwala M, Vasavada N, Curley T, Chase SD, Sachs N, Semret MH. A pilot randomized controlled trial of renal protection with pioglitazone in diabetic nephropathy. Kidney Int. 2005;68:285–292. doi: 10.1111/j.1523-1755.2005.00416.x. [DOI] [PubMed] [Google Scholar]
- Park CW, Zhang Y, Zhang X, Wu J, Chen L, Cha DR, Su D, Hwang MT, Fan X, Davis L, Striker G, Zheng F, Breyer M, Guan Y. PPARalpha agonist fenofibrate improves diabetic nephropathy in db/db mice. Kidney Int. 2006;69:1511–1517. doi: 10.1038/sj.ki.5000209. [DOI] [PubMed] [Google Scholar]
- Saga D, Sakatsume M, Ogawa A, Tsubata Y, Kaneko Y, Kuroda T, Sato F, Ajiro J, Kondo D, Miida T, Narita I, Gejyo F. Bezafibrate suppresses rat antiglomerular basement membrane crescentic glomerulonephritis. Kidney Int. 2005;67:1821–1829. doi: 10.1111/j.1523-1755.2005.00280.x. [DOI] [PubMed] [Google Scholar]
- Schadinger SE, Bucher NL, Schreiber BM, Farmer SR. PPARgamma2 regulates lipogenesis and lipid accumulation in steatotic hepatocytes. Am J Physiol. 2005;288:E1195–E1205. doi: 10.1152/ajpendo.00513.2004. [DOI] [PubMed] [Google Scholar]
- Edvardsson U, Ljungberg A, Linden D, William-Olsson L, Peilot-Sjogren H, Ahnmark A, Oscarsson J. PPARalpha activation increases triglyceride mass and adipose differentiation-related protein in hepatocytes. J Lipid Res. 2006;47:329–340. doi: 10.1194/jlr.M500203-JLR200. [DOI] [PubMed] [Google Scholar]