Abstract
Keloids are tumor-like skin scars that grow as a result of the aberrant healing of skin injuries, with no effective treatment. We provide new evidence that both overexpression of plasminogen activator inhibitor-1 (PAI-1) and elevated collagen accumulation are intrinsic features of keloid fibroblasts and that these characteristics are causally linked. Using seven strains each of early passage normal and keloid fibroblasts, the keloid strains exhibited inherently elevated collagen accumulation and PAI-1 expression in serum-free, 0.1% ITS+ culture; larger increases in these parameters occurred when cells were cultured in 3% serum. To demonstrate a causal relationship between PAI-1 overexpression and collagen accumulation, normal fibroblasts were infected with PAI-1-expressing adenovirus. Such cells exhibited a two- to fourfold increase in the accumulation of newly synthesized collagen in a viral dose-dependent fashion in both monolayers and fibrin gel, provisional matrix-like cultures. Three different PAI-1-targeted small interfering RNAs, alone or in combination, produced greater than an 80% PAI-1 knockdown and reduced collagen accumulation in PAI-1-overexpressing normal or keloid fibroblasts. A vitronectin-binding mutant of PAI-1 was equipotent with wild-type PAI-1 in inducing collagen accumulation, whereas a complete protease inhibitor mutant retained approximately 50% activity. Thus, PAI-1 may use more than its protease inhibitory activity to control keloid collagen accumulation. PAI-1-targeted interventions, such as small interfering RNA and lentiviral short hairpin RNA-containing microRNA sequence suppression reported here, may have therapeutic utility in the prevention of keloid scarring.
Keloids are exuberant scars that grow beyond the margins of the original wound as a result of aberrant healing of skin injuries. They are characterized by thick and abundant collagen fibers that lack the nodular structures present in hypertrophic scars and an amorphous pericellular fibroblast matrix.1,2,3 As part of their clinical phenotype, keloid lesions are preceded by excessive inflammation, pruritis, and pain, and they progress with a proliferative edge and eventually a collapsing center to form tissue that is less cellular and vascular than that in hypertrophic scars. Keloid formation is significantly more common in dark skinned races, ie, people of African, Hispanic, and Asian descents, pointing to a possible genetic predisposition.2,4 Altered expression and regulation of various extracellular matrix (ECM) components by keloid fibroblasts, such a collagen, fibronectin (Fn), elastin, proteoglycans, and matrix-directed protease and protease inhibitors, have been implicated in keloid fibrosis.5,6,7,8,9,10,11,12 Modifications in growth factor-regulated cell growth and apoptosis, intracellular signal transduction, and epidermis-dermis interactions have been reported.13,14,15,16,17,18,19,20,21 Changes in the level of genes involved in the above phenomena were also detected in gene profiling studies.22,23 Despite these findings, knowledge-based specific approaches for prevention and efficacious treatment of keloids are still absent, and the lack of proper in vitro and animal models that recapitulate the pathophysiology of keloids further hampers progress. Consequentially, intralesional injection of steroid, which might soften the scar tissue and cause limited scar regression, remains the standard treatment.3 Surgical excision of keloids is usually reserved for large debilitating lesions because growth and expansion of keloids reoccur due to skin injury caused by the surgical procedure.3
By establishing and using a three-dimensional in vitro fibrin matrix-cell culture model that recapitulates conditions of in vivo fibroplasia, we discovered that keloid fibroblasts not only produce excessive collagen but are also defective in fibrin degradation because of an overexpression of plasminogen activator inhibitor (PAI-1).7 The increased expression of PAI-1 protein is also present in fibroblasts of keloid lesions.24 Importantly, PAI-1 may cause excessive accumulation of newly synthesized collagen by keloid fibroblasts because reducing PAI-1 activity with PAI-1 neutralizing antibody normalizes collagen accumulation by keloid fibroblasts.24 This link between PAI-1 and collagen accumulation represents a novel mechanism participating in keloid pathogenesis.
PAI-1 is the major physiological inhibitor of the plasminogen activator/plasmin protease system.25 Plasmin not only is the primary effective enzyme in fibrinolysis and clot resolution but also participates in the breakdown of other ECM proteins and activates matrix metalloproteinases and growth factors.26 Although a balanced action between plasminogen activator and PAI-1 maintains the normal function of the protease system, altered plasminogen activator/PAI-1 has been implicated in the development of thrombotic diseases and metabolic disorders that are linked with the development of arteriosclerosis,27 cancers,28 and chronic wounds.29 Increased PAI-1 activity has been a hallmark of fibrosis evident by a direct correlation between genetically determined level of PAI-1 and the extent of collagen accumulation that follows inflammation during injury repair in various organs such as liver,30 lung,31 kidney,32 blood vessels,33 and skin.26 In addition to keloids, PAI-1 overexpression has been found in skin fibroblasts of Werner’s fibrosis and scleroderma.34
PAI-1 is secreted as an active serine protease inhibitor and has a short half-life. Its activity, however, is stabilized by vitronectin (Vn) present both in plasma and ECM.35,36 Recently, PAI-1 has been implicated in modulating urokinase plasminogen activator (uPA) and uPA receptor (uPAR)-mediated cell adhesion and migration independent of its inhibitor function.37 These additional functions of PAI-1 are based on the evidence that both PAI-1 and uPAR bind to Vn through its somatomedin domain adjacent to the RGD site where integrins bind Vn.38,39 Thus PAI-1 has the capacity to influence ECM metabolism and integrin/ligand binding (both Vn-integrin and uPA/uPAR-Vn-integrin) that modulate cell phenotype and affect cell differentiation and/or ECM production.37,39,40,41 Because ECM synthesis, degradation, and remodeling are essential features of injury repair, we hypothesize that increased PAI-1 is a precursor to elevated collagen accumulation in keloid fibroblasts and that it functions through inhibiting the uPA/plasmin protease cascade and/or modulating Vn-dependent cell adhesion. We have addressed the precursor role and mechanism of PAI-1 by demonstrating i) increased collagen accumulation in normal fibroblasts through viral overexpression of wild-type hPAI-1 (Wt PAI-1) in monolayer culture and fibrin gels, ii) suppression of collagen accumulation by treatment with PAI-1-directed small interfering RNA (siRNA) and short hairpin RNA-containing microRNA sequences (shRNAmir) in PAI-1-overexpressing normal cells, iii) suppression of collagen accumulation in keloid fibroblasts with PAI-1 siRNA, and iv) differences in protease inhibitor (PAI-1INH−) and Vn-binding (PAI-1VN−) mutants of PAI-1 in their capacity to drive increased accumulation of newly synthesized collagen.
Materials and Methods
Materials
Collagenase type 1 (filtered; 190 U/mg, 13,659 U/vial) was purchased from Worthington Biochemical Corporation (Lakewood, NJ). Dulbecco’s PBS (without calcium and magnesium) and Dulbecco’s modified Eagle’s medium (DMEM; 1× with 4.5 g/L glucose, l-glutamine, and sodium pyruvate) were purchased from Mediatech Inc. (Herndon, VA). Fetal bovine serum (FBS) was from Atlas Biologicals (Fort Collins, CO). HEPES-buffered DMEM (HDMEM) was prepared by adding HEPES free-acid (Sigma-Aldrich Co., St. Louis, MO) to DMEM powder (Sigma-Aldrich Co., St. Louis, MO) per the manufacture’s instructions. Primocin was from InvivoGen (San Diego, CA). ITS+ is a premixed universal culture supplement (BD Biosciences, San Jose, CA), containing 12.5 mg of human recombinant insulin, 12.5 mg of human transferrin, 12.5 μg of selenous acid, 2.5 g of bovine serum albumin, and 10.7 mg of linoleic acid in 20 ml of water. Nylon cell strainers (100 μm) were purchased from BD Biosciences Discovery Labware (Bedford, MA). PicoGreen DNA assay was purchased from Molecular Probes (Eugene, OR). Proteinase K (recombinant PCR grade) was from Roche Diagnostics (Indianapolis, IN). Sources of antibodies were as follows: mouse monoclonal to α-tubulin 1 mg/ml (Abcam, Cambridge, MA); mouse monoclonal to human PAI-1 (catalog no. 612025; BD Biosciences); mouse monoclonal to Lamin A/C (catalog no. 612163; BD Biosciences); anti-mouse IgG, horseradish peroxidase-conjugated antibody (goat antiserum; 1 mg/ml; catalog no.; Sigma-Aldrich Co.); affinity-purified rabbit antibody to collagen type 1 (catalog no. 600-401-103-0.5; Rockland, Gilbertsville, PA); anti-rabbit IgG, horseradish peroxidase-conjugated antibody (Cell Signaling Technology, Danvers, MA). Precision Plus Protein Dual Color molecular weight standards (catalog no. 161-0374) were obtained from Bio-Rad (Hercules, CA).
Human PAI-1 siRNAs, siCONTROL nontargeting siRNA 1, siGLO Green Transfection Indicator, and transfection reagents were purchased from Dharmacon Inc. (Chicago, IL). The sequences of the three siRNAs for human PAI-1 (SERPINE1) gene are J-019376-05 (siRNA5) sense sequence, 5′-CGACAUGUUCAGACAGUUUUU-3′, and antisense sequence, 5′-P.AAACUGUCUGAACAUGUCGUU-3′; J-019376-06 (siRNA6) sense sequence, 5′-GCUAUGGGAUUCAAGAUUGUU-3′, and antisense sequence, 5′-P.CAAUCUUGAAUCCCAUAGCUU-3′; and J-019476-07 (siRNA7) sense sequence, 5′-CUAGAGAACCUGGGAAUGAUU-3′, and antisense sequence, 5′-P.UCAUUCCCAGGUUCUCUAGUU-3′. PAI-1 protease inhibitory activity was determined using Spectrolyse (pL) PAI, which was purchased from American Diagnostica (Stamford, CT). Lentiviral shRNAmir hairpin sequence for PAI-1 645 (V2LHS 111645) is 5′-TGCTGTTGACAGTGAGCGAGGACACCCTCAGCATGTTCATTAGTGAAGCCACAGATGTAATGAACATGCTGAGGGTGTCCCTGCCTACTGCCTCGGA-3′ (Open BioSystems, Huntsville, AL).
Fibroblast Isolation
The protocol for obtaining clinical discards of human normal skin, scar, and keloid specimens was approved by the Institutional Review Board of both the Childrens Hospital Los Angeles (T.-L.T.) and University Mississippi Medical Center (A.W.). Samples were kept on ice during shipping and handling. Samples were rinsed with ice-cold Dulbecco’s PBS (without calcium and magnesium) to remove blood clots and trimmed by sharp dissection to remove adipose tissue and 2 to 5 mm of tissue from the epidermal surface. Fibroblasts from each keloid specimen were isolated from this core without distinction between central and peripheral progressive regions using collagenase digestion (Worthington Collagenase Type 1-Filtered 190 U/mg and 13,659 U/vial/30 ml HDMEM). Samples were weighed for estimation of the amount of collagenase/HDMEM to be used (2000 U collagenase/g tissue). Samples were subsequently cut and minced into fine pieces with surgical blades and collected in a 50-ml conical tube filled with 20 ml of ice-cold Dulbecco’s PBS (without calcium and magnesium). The tube was set on ice undisturbed for 10 minutes to collect tissue fragments, which were subsequently resuspended in an appropriate volume of collagenase/HDMEM solution and incubated at 37°C in a 5% CO2 environment for 2 hours with gentle mixing (collagenase pretreatment step). Tissue fragments were then transferred into fresh collagenase solution (320 U/ml in 10% FBS/HDMEM) and incubated as above for 18 hours. The digested dermal tissues were filtered through a 100-μm cell strainer to separate the released cells from undigested tissue. Cells were collected by centrifugation, resuspended in 10% FBS/DMEM, and cultured in monolayer at 37°C. These freshly isolated and early passage cells (≤2 passages) were used in subsequent experiments. For these experiments, cell passage is defined as lifting and transferring confluent cells from one plate into three new culture plates of the equal size every 7 days.
Recombinant Adenovirus and Cell Transduction
The Ad5-based E1-deleted recombinant vector expressing human PAI-1, AdCMVPAI-1 (Wt PAI-1), mutant PAI-1 protein defective in Vn binding (pACCMVPAI-1VN− or PAI-1VN−), mutant PAI-1 protein defective in plasminogen activator inhibition (pACCMVPAI-1INH− or PAI-1INH−), and the empty vector AdRR5 have been described before42 and were kindly provided by Dr. Robert Gerard (University of Texas Southwestern Medical Center, Dallas, TX). pACCMVPAI-1INH− contains the double mutation R346M and M347S, which renders PAI-1 defective in serpin function. The PAI-1 inhibitory activity was confirmed by inhibition of a known quantity of uPA using an indirect chromogenic substrate assay that measures plasminogen activation42 and by the Spectrolyse (pL) PAI assay (American Diagnostica). pACCMVPAI-1VN− encoding PAI-1 with the Q123K mutation is deficient in Vn binding, which was confirmed by Vn-binding assay using human Vn-coated microtiter plates and the monoclonal antibody to human PAI-1 MA-12A4-horseradish peroxidase.42 On receiving the viral stocks, adenoviruses were repurified by plaque purification and two rounds of dilution to 0.5 plaque-forming unit/well and propagated in 293 cells. Viral stocks were stored as lysates in aliquots at −80°C in 10 mmol/L Tris, pH 8.0, 2 mmol/L MgCl2, and 5% sucrose.43 Titers were determined in 293 cells using the Clontech Adeno-X Rapid Titer procedure (BD Biosciences) and corrected to plaque-forming units per milliliter.
Cells were plated in 10% FBS/DMEM in 48-well plates at confluent density (6 × 104 cells/well) by layering beneath 5% FBS/DMEM. Cells were dispensed slowly with an electronic multichannel pipette after placing the tips at the bottom of the wells beneath 5% FBS; this eliminated the uneven plating density within the wells that was caused by the meniscus when cells were plated without the overlay of 5% FBS. Cells were allowed to attach for 24 hours, washed with DMEM, and cultured or infected with viral vectors at the indicated concentrations for 24 hours in 0.125 ml of 0.3% ITS+/DMEM. Subsequently, cells were fed with the testing medium (0.1% ITS+, 1% FBS, 3% FBS, or 10% FBS) for additional time periods as indicated in the legend of each figure with a change of medium every other day.
Preparation of Fibrin Gels
All fibrinogen used was >95% clottable as determined with a previously described procedure.24 Human skin fibroblasts suspended in DMEM were added to a fibrinogen solution at 24°C. Final concentrations of fibrinogen and fibroblasts were 2.5 mg/ml and 0.5 × 106 cells/ml, respectively. Aliquots (80 μl) of the fibroblast/fibrinogen mixtures were placed in wells of 48-well tissue culture plates (Costar, Cambridge, MA) with 1 U of thrombin per sample. Each aliquot occupied an area outlined by an 8-mm-diameter circular score within the well. The preparations were incubated at 37°C for 1 hour in a humidified incubator containing 5% CO2 to ensure polymerization of fibrin. At the end of the incubation period, 0.5 ml of 10% FBS was added to each well to cover the gel and allowed 24 hours for incubation before being infected with adenoviral vectors at the indicated concentrations for 24 hours in 0.125 ml of 0.3% ITS+. Subsequently, cells were fed with the 3% FBS for an additional 6 days with a change of medium every other day.
Collagen Analysis
Accumulation of newly synthesized collagen was determined by monitoring [3H]proline incorporation into pepsin-resistant collagen chains separated by SDS-PAGE. Cells were treated with labeling medium [testing medium plus 62.5 μg/ml β-aminoproprionitrile, 25 μg/ml ascorbate, and 40 μCi/ml [5-3H]proline (Amersham Biosciences, Piscataway, NJ)] over a 24-hour period before the termination of the experiment. Cultures (medium and cell layer, together) were frozen (terminated), thawed, adjusted to 0.5 N acetic acid and 0.5 mg/ml pepsin, and rocked overnight at 4°C. After two cycles of lyophilization in the culture plate, samples were dissolved in 0.25 ml of SDS-PAGE sample buffer without reducing agent at 55°C for 30 minutes before SDS-PAGE, fluorography,43 and digital image analysis of α1(I) collagen bands (identified by prior comparison with authentic collagen standards). Triplicate samples were analyzed separately for statistical analysis or pooled to provide an averaged sample for visualization/analysis. Pooled samples routinely produced values equivalent to the mean of individually analyzed samples, but without data for the SD. DNA content was used to normalize data obtained from cultures of different duration conducted in the presence of different serum concentrations. Pepsin digestion of noncollagenous proteins and acid depurination of DNA occur in the first step of collagen analysis, respectively preventing the use of cellular proteins as loading controls or accurate DNA analysis in aliquots of these samples for data normalization. Consequently, the cell layers of parallel cultures were digested with Proteinase K and analyzed for DNA using Pico Green (Molecular Probes).
Western Blotting
Medium and cell layer samples from cell cultures were separated at the end of each experiment for PAI-1 Western analysis. Cell layers were rinsed once with Dulbecco’s PBS (without calcium and magnesium) and then treated in the well with 1× hot SDS-PAGE sample buffer containing 5% β-mercaptoenthanol. Medium samples were also treated with hot SDS-PAGE sample buffer (4× and final concentration, 1×). Proteins were boiled before SDS-PAGE and transferred to nitrocellulose in buffer containing 25 mmol/L Tris base, 0.2 mol/L glycine, 20% methanol (pH 8.5), and 0.02% SDS. Membrane blots were subsequently blocked for 2 hours at 23°C with 5% nonfat dry milk in 50 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, and 0.1% Tween 20 (Tris-buffered saline-Tween 20) and incubated with primary antibodies (i.e., 1:1000 dilution of mouse monoclonal to human PAI-1) overnight at 4°C in 5% bovine serum albumin in Tris-buffered saline-Tween 20. After brief washing in Tris-buffered saline-Tween 20, blots were incubated at 23°C for 40 minutes with horseradish peroxidase-coupled second antibody (i.e., 1:3000 dilution of anti-mouse IgG, horseradish peroxidase-conjugated antibody) in 5% nonfat dry milk in Tris-buffered saline-Tween 20. The protein bands in washed blots were visualized on film with chemiluminescence using SuperSignal West Dura substrate (Pierce Biotechnology, Rockford, IL). Membrane blots were reprobed with mouse monoclonal antibody to Lamin A/C (1:1000 dilution) or α-tubulin (1:2000) as interchangeable loading controls. Molecular weight standards were run with each SDS-PAGE gel. Detected antigens exhibited molecular weights of 47 kDa (PAI-1), 74/65 kDa (Lamin A/C), and 52 kDa (α-tubulin). For collagen Western blots, samples were processed as described for collagen analysis (above) until completion of electrophoresis and then processed for Western blotting with an affinity-purified anti-collagen type I antibody (1:1000 dilution). Triplicate samples were analyzed separately or as a physical pool (see collagen analysis, above) by digital image analysis of the protein bands.
Digital Image Analysis
Data recorded on preflashed film, ie, collagen fluorographs and chemiluminescent Western blots, were analyzed by digital image analysis (Kodak 1D Image Analysis Software; Eastman Kodak, Rochester, NY) using multiple exposures of increasing duration such that integrated intensities for bands of interest were in the linear response range of the film. Band intensities from different exposures were indexed using a band at the low end of the linear range in short exposures and the same band at the high end of the linear range in longer exposures. Control and experimental samples were routinely run on the same gel/blot to ensure accurate comparison, and samples common to each pair of gels were included to enable comparison between gels in the same experiment and between gels of all cell strains.
PAI-1 Activity Assay
A two-stage, indirect enzymatic assay, Spectrolyse (pL) PAI (American Diagnostica), was used for the quantitative determination of PAI-1 activity. The PAI-1 activity (IU) is defined as the amount of PAI that inhibits 1 IU of a human single-chain tissue type plasminogen activator as calibrated against the International Standard for tissue type plasminogen activator (National Institute for Biological Standards and Control, Holly Hill, London, UK).
PAI-1 Knockdown by PAI-1 siRNA
Cells were plated using the underlayering technique described above and transfected the next day with siRNA (final concentration 100 nmol/L) in 0.15 ml of 10% FBS using DharmaFECT 1 transfection reagent (Dharmacon Inc.) for 24 hours. After transfection, cells were washed with DMEM and cultured or infected with adenoviral vectors for 24 hours in 0.25 ml of 0.1% ITS+/DMEM. Subsequently, cells were fed with the testing media (1% FBS, 3% FBS, or 10% FBS) for additional time periods indicated in the legend of each figure with a change of medium every other day before termination.
PAI-1 Lentiviral shRNAmir
A lentiviral stock was created by cotransfecting optimized packaging plasmid mix and transfer vector into the TLA-HEK293T cell line (Open Biosystems), to produce replication-incompetent lentivirus. HEK-293T cells were plated at a density of 5.5 × 106 cells per 100-mm plate and transfected with PAI-1-targeted pGIPZ shRNAmir transfer vector (PAI-1 645) or a nonsilencing vector according to the manufacturer’s protocol (Open Biosystems). Virus was concentrated by PEG precipitation (System Biosciences, Mountain View, CA). For transduction, normal fibroblasts (strain N144) were plated at a density of 8 × 105 cells per 35-mm dish, infected with lentivirus in 2% FBS/DMEM for 24 hours, and expanded by culture in 10%FBS/DMEM. Transduced fibroblasts were selected with puromycin over a 10-day period, resulting in 95 to 100% green fluorescent protein (GFP)-positive cells. These cells were then infected with Wt PAI-1 adenoviral vector [100 multiplicity of infection (moi)] and monitored for PAI-1 and collagen accumulation as described above.
Data Analysis and Statistics
All pairwise mean ± SD comparisons were performed using Student’s t-test function (two-tailed distribution) with either equal or unequal variance determined by F-test. Differences were considered statistically significant at P ≤ 0.05. Statistical analysis for the difference between pooled normal fibroblast strain data and pooled keloid strain data were based on the general linear model of analysis of variance in Minitab. Differences were considered statistically significant at P ≤ 0.05.
Results
Increased PAI-1 and Collagen Are Reproducible Features of Keloid Fibroblasts in Cell Monolayers
Cell monolayers were used in the current study to bypass the influence of fibrin matrix44 and test directly the link between PAI-1 expression and collagen accumulation. In addition, cell monolayers can be readily varied in size, permit rapid verification of experimental manipulations and retention of cells, and avoid the loss of cells from fibrin gels cultured under serum-free conditions.45 Seven strains each of freshly isolated primary, first, or second passage fibroblasts from normal skin or keloids (Table 1) were cultured for 2 or 6 days in DMEM containing 0.1% ITS+, 3% FBS, or 10% FBS. The serum-free condition, ie, 0.1% ITS+, was used to reveal the inherent differences in PAI-1 expression and accumulation of newly synthesized collagen between normal and keloid fibroblasts; 3 and 10% FBS were used to determine the effects of serum-derived growth factors. Short-term 2-day cultures were used to investigate the levels of PAI-1 and collagen while fibroblasts established a new matrix environment, and 6-day cultures were used to examine function within this maturing matrix.
Table 1.
List of Freshly Isolated Strains of Normal and Keloid Fibroblasts Used in the Study
| Type | Cell strain | Ethnic background | Gender | Age | Location |
|---|---|---|---|---|---|
| Normal skin | N-A* | Unknown | Male | NB | Foreskin |
| Normal skin | N144 | African American | Male | NB | Foreskin |
| Normal skin | N-J | Caucasian | Female | 28 | Chest/breast |
| Normal skin | N-M | African American | Female | 29 | Ear |
| Normal skin | N-N | African American | Female | 29 | Chest/breast |
| Normal skin | N-R | Caucasian | Female | 53 | Facial/eyelid |
| Normal skin | N-Y | Caucasian | Female | 25 | Post ear |
| Normal skin | N-(K-F) | African American | Female | 50 | Ear |
| Keloid | K-A | African American | Male | 59 | Neck |
| Keloid | K-C3 | African American | Male | 17 | Ear |
| Keloid | K-F | African American | Female | 50 | Ear Lobe |
| Keloid | K-G | African-American | Male | 38 | Post ear |
| Keloid | K-I | Middle Eastern | Female | 15 | Post ear |
| Keloid | K-Ea | Middle Eastern | Female | 15 | Face |
| Keloid | K-J | African American | Female | 70 | Back |
Strain not included in Figure 2. NB = New Born.
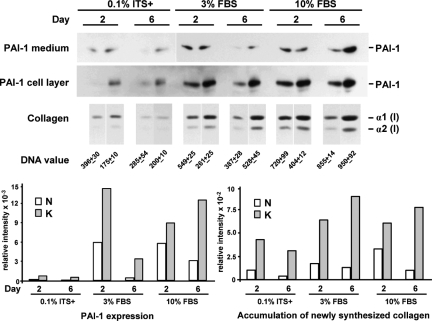
PAI-1 expression and accumulation of newly synthesized collagen were elevated two- to fourfold in keloid fibroblasts (strain K-C3) when compared with that in normal fibroblasts (strain N-M) (Figure 1). This comparison is presented because it represented the closest donor match with regard to age, ethnicity, and anatomical location. As summarized graphically after correction for DNA content (Figure 1, bottom panel), differences between normal and keloid fibroblasts were apparent under serum-free (0.1% ITS+) conditions and were maintained with 3 and 10% FBS, even though serum produced a substantial increase in expression of both markers regardless of cell strain. Thus, serum is neither required for the expression of these differences between normal and keloid fibroblasts nor does the presence of serum mask or saturate these differences.
Figure 1.
Comparison of PAI-1 protein and accumulation of newly synthesized collagen between a pair of keloid (strain K-C3) and normal (strain N-M) fibroblasts best matched in donor age, ethnic background, and anatomical location (Table 1). Each protein band represents the physical pool of triplicate samples. Medium and cell layer fractions were analyzed separately for PAI-1 protein expression and combined for collagen accumulation. The images from 1-hour exposure of PAI-1 Western blots are presented for cell layer samples, and 10-minute images are presented for medium samples, except the 0.1% ITS samples (also 1 hour). Tritiated proline fluorographic images of pepsin-treated collagen after SDS-PAGE are presented for all collagen samples. DNA values (mean of triplicate samples ± SD) from parallel cultures were used for normalization of the relative intensities of PAI-1 and collagen [α1(I)] bands obtained by digital image analysis. PAI-1 values from the medium and cell layer were combined for the normalized bar graphs that summarize these results (bottom panel). PAI-1 = 47 kDa.
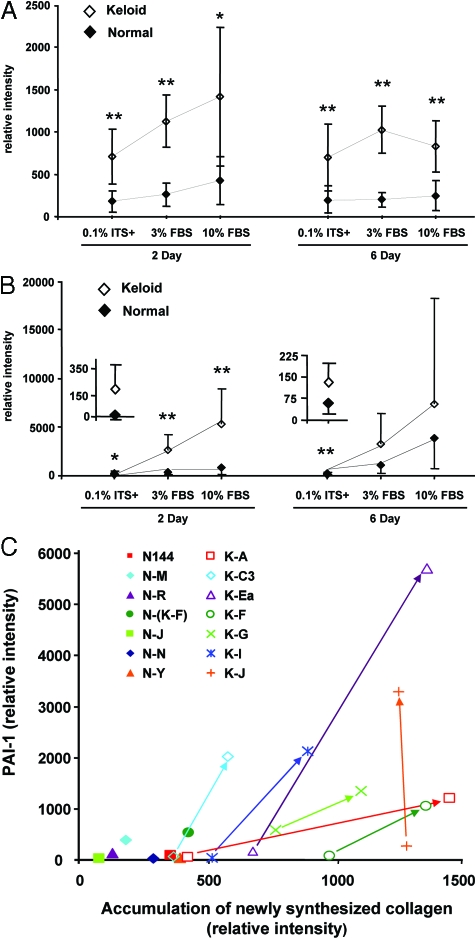
Twelve additional strains of normal and keloid fibroblasts (six strains of each) were analyzed with the same methods to determine whether elevated PAI-1 expression and accumulation of newly synthesized collagen were consistently observed and represent an element of the keloid phenotype. Mean values for the seven keloid and seven normal cell strains (Table 1) are presented in Figure 2A for collagen and Figure 2B for PAI-1, whereas strain-specific data are presented in supplementary material (Supplemental Figure S1, A and B, respectively, see http://ajp.amjpathol.org). The strain-specific DNA analyses used for normalization of these data are also presented (Supplemental Figure S2, see http://ajp.amjpathol.org) and demonstrated a slight lag in growth or less-efficient cell attachment for keloid cells during the first 2 days of culture, followed by similar growth of both cell types in 3 and 10% FBS. Neither keloid nor normal fibroblasts grew in 0.1% ITS+, but the cultures were stable, without significant loss of cells, through day 6.
Figure 2.
Comparison of accumulation of newly synthesized collagen (A) and PAI-1 expression (B) between seven strains each of freshly isolated and low passage (≤2) keloid and normal fibroblast cultures. Fibroblasts were cultured in 0.1% ITS+, 3% FBS, or 10% FBS for 2 or 6 days. Triplicate samples were analyzed as a physical pool for each cell strain as described in the legend to Figure 1. The relative intensities of PAI-1 and collagen [α1(I)] bands from both the medium and cell layer were quantified by digital image analysis and normalized with cellular DNA content. Digital image analysis was based on pairwise determination of band intensity from multiple film exposures. Samples common to each pair of gels were included to enable comparison between gels in the same experiment and between gels of all cell strains. Each point represents the mean ± SD from seven strains each of normal or keloid fibroblasts and includes the strains analyzed in Figure 1. ⋄, Keloid fibroblasts; ♦, normal fibroblasts. Insets contain a magnified scale for data points from normal and keloid fibroblasts cultured in 0.1% ITS+. *P ≤ 0.05; **P ≤ 0.01. Plots of strain-specific data are presented as a supplement (Supplemental Figure S1, A and B). C: A combined plot of PAI-1 and collagen strain-specific data for normal and keloid fibroblasts analyzed on day 2 after culture in ITS+ or 3% FBS. For each keloid strain, the data point for culture in ITS+ is connected to that in 3% FBS by an arrow originating at the ITS+ point. The angled arrows indicate simultaneous increases in both the PAI-1 and collagen parameters. Because all data from normal strains in ITS+ fell closer to the origin than the corresponding data from normal strains in 3% FBS, only the data from the more stimulatory 3% FBS culture of normal cells are presented. Normal fibroblast strains, filled symbols; keloid fibroblast strains, open symbols, +, ×, and *. Strain identifiers are presented in the legend and in Table 1.
Similar to the results from cell strains K-C3 and N-M in Figure 1, the larger collection of cell strains demonstrated that PAI-1 expression and accumulation of newly synthesized collagen were two- to fivefold higher in keloid fibroblasts when compared pairwise with normal fibroblasts at each serum level and time period (Figure 2, A and B). These results were always statistically significant, with the exception of PAI-1 expression in 3 and 10% FBS on day 6. This exception was primarily caused by elevated PAI-1 expression in two normal strains, N-(K-F) and N-R (Supplemental Figure S1B). Even when all data from normal cell strains were compared with all data from keloid cell strains by analysis of variance, the differences were significant for both collagen accumulation (normal, mean 256 ± 30 SEM; keloid, mean 974 ± 77 SEM; P = 0.001) and PAI-1 production (normal, mean 996 ± 313 SEM; keloid, mean 3191 ± 313 SEM; P = 0.014).
The combination of both PAI-1 and collagen accumulation as phenotypic markers allows clear distinction between individual strains of normal and keloid fibroblasts (Figure 2C; Supplemental Figure S3, see http://ajp.amjpathol.org). In Figure 2C, keloid fibroblast strains exhibited increases in both markers during the transition from culture in basal, serum-free, ITS+ supplemented medium (open symbols at arrow origin) to culture in 3% FBS (open symbols at the arrowhead), leading to angled arrows. Under both conditions, keloid strains exceeded normal fibroblasts strains cultured in 3% FBS (Figure 2C, filled symbols). Only keloid strains K-A and K-C3 in 0.1% ITS+ did not exceed the highest normal strains in 3% FBS. Another exception was K-J, which only increased in PAI-1 expression in response to 3% FBS. When evaluated for response to the transition from 3 to 10% FBS, keloid strains in the less stimulatory condition, 3% FBS, again exceeded production of both PAI-1 and collagen accumulation in normal strains in 10% FBS (Supplemental Figure S3). In contrast to the results in Figure 2C, however, arrows linking keloid strains in 3 and 10% were vertical, indicating only an increase in PAI-1 expression and apparent saturation of the mechanism regulating collagen accumulation by serum. Informative exceptions were the keloid strain K-F and normal strain N-(K-F), which was isolated from the “normal” skin adjacent to K-F. N-(K-F) produced levels of both markers intermediate between other normal and keloid strains and exhibited a similar transition from 3 to 10% FBS when compared with its corresponding keloid strain, K-F. This suggests that such adjacent skin may have begun a transition toward a keloid phenotype, even though apparently normal by visual inspection. Collectively, the results demonstrate that freshly isolated, early passage keloid fibroblasts from a wide range of donors and different sites persistently express higher levels of PAI-1 and collagen than normal fibroblasts, regardless of the presence or concentration of serum during culture and the status of matrix deposition/maturation (day 2 versus 6). The question of whether PAI-1 plays a role in increased collagen accumulation was addressed directly by the following gain- and loss-of-function experiments.
Overexpression of Wild-Type PAI-1 Protein in Normal Fibroblasts Results in Increased Accumulation of Newly Synthesized Collagen
Elevated PAI-1 and collagen in keloid fibroblasts may occur together because both are downstream targets of the pro-fibrotic growth factor TGF-β1 that is present in the serum or because PAI-1 directly stimulates accumulation of newly synthesized collagen. To test the latter hypothesis, we transduced normal fibroblasts with an adenoviral vector expressing the wild-type hPAI-1 gene (Wt PAI-1; gift of Dr. Robert Gerard)42 to increase PAI-1 protein expression in these cells. This approach provided 85% transduction efficiency, and rapid manipulation of PAI-1 expression without consumption of cellular life span and the risk of stable viral integration. In addition, we were able to vary expression by increasing the moi so that PAI-1 expression approached or surpassed that of keloid fibroblasts. Normal fibroblasts infected with adenoviral Wt PAI-1 expressed PAI-1 protein in a dose-dependent manner (5 to 100 moi) in serum-free, 0.1% ITS+-supplemented medium and produced PAI-1 levels at 100 moi that surpassed that of fibroblasts cultured in 10% FBS without virus (Supplemental Figure S4A, see http://ajp.amjpathol.org). Fibroblasts infected with virus at 100 moi and subsequently cultured in 0.1% ITS+, 1% FBS, 3% FBS, or 10% FBS for 48 hours exhibited a serum dose-response superimposed on a 10-fold virus-dependent increase in PAI-1 expression (Supplemental Figure S4, B and C). When this 10-fold increase in PAI-1 is applied to any of the normal strain data in Figure 2B, the PAI-1 levels approach or exceed those of the keloid strains. A direct comparison within an independent experiment conducted with infection at 100 moi and culture in 3% FBS for a longer 6-day period produced greater than twofold higher PAI-1 levels in virally transduced normal cells than in a keloid strain (Supplemental Figure S4D). Thus, the combined effects of serum, its effects on the CMV viral promoter for PAI-1, and the viral dose were capable of producing PAI-1 levels above that exhibited by keloid fibroblasts.
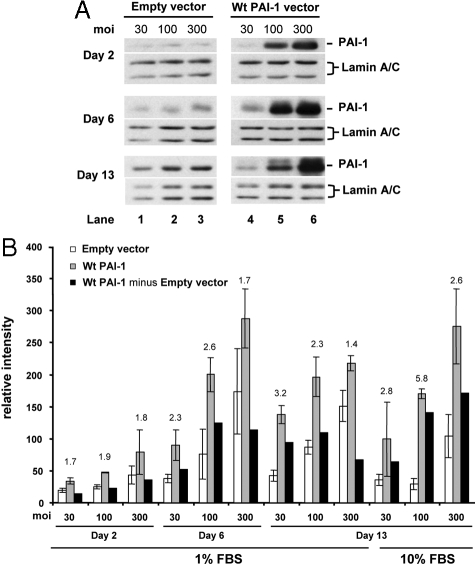
The effects of PAI-1 overexpression on accumulation of newly synthesized collagen were tested over a broad culture period of 2, 6, and 13 days to mimic the time frame of early wound repair and remodeling and in 1% FBS to minimize effects of serum stimulation, if these exist. Normal fibroblasts were infected with 30, 100, and 300 moi of adenoviral Wt PAI-1 (Ad-Wt PAI-1) or empty vector to vary the PAI-1 dose delivered to the cultured normal fibroblasts (strain N144). A viral dose-response was observed from days 2 to 13, with PAI expression reaching a maximum at day 6 and declining only slightly by day 13, consistent with the expected efficacy of adenoviral transduction (Figure 3A). The empty vector showed little effect on PAI-1 protein expression after correction with Lamin A/C for protein loading. Under these culture conditions, PAI-1 overexpression did not increase cell proliferation, as indicated by constant Lamin A/C expression. Importantly, the increase of PAI-1 protein expression in Wt PAI-1 but not empty vector-infected fibroblasts was accompanied by a corresponding increase in PAI-1-specific protease inhibitory activity (see below).
Figure 3.
PAI-1 overexpression stimulates accumulation of newly synthesized collagen in normal fibroblasts infected with adenoviral vector carrying the wild-type PAI-1 gene. Normal fibroblasts from the N144 strain were infected with empty vector (control) and Wt PAI-1 adenovirus at 30, 100, or 300 moi for 24 hours in 0.1% ITS+ before culturing in 1% FBS for 2, 6, or 13 days or in 10% FBS for 13 days. A: Western blots of PAI-1 protein expression (cell layer) under 1% FBS use the nuclear envelope proteins Lamin A/C as loading controls and represent the physical pool of triplicate samples. B: Analyses of accumulation of newly synthesized collagen use DNA content for normalization and represent the separate analysis of triplicate samples (□, empty vector; ░⃞, Wt PAI-1 vector; ▪, Wt PAI-1 minus Empty vector). The results represent the means ± SD. PAI-1 = 47 kDa; Lamin A/C = 74/65 kDa.
PAI-1 overexpression in 1% FBS increased accumulation of newly synthesized collagen over the entire time course of the experiment (Figure 3B). Compared with empty vector, the PAI-1-specific stimulation averaged twofold with a range of 1.4- to 3.2-fold. The total increase in collagen under each experimental condition was influenced by a dose-dependent increase in collagen due to adenovirus-dependent mechanisms, as demonstrated by the effects of empty vector. When this contribution was removed (Figure 3B, black bars), the lower doses of 30 and 100 moi generated the highest PAI-1-specific collagen responses, regardless of the length of culture. The empty vector response was suppressed on day 13 when cells were cultured in 10% FBS. When empty vector and Wt PAI-1 vector were used at 100 moi, PAI-1-dependent accumulation of newly synthesized collagen reached 5.8-fold compared with empty vector. This observation that PAI-1 overexpression is capable of inducing increased collagen accumulation has been replicated in eight independent experiments, including Figures 3 and 4, and five additional experiments not presented. Five of these experiments used 3% FBS (strains N-A, N-N, and N144), two used 1% FBS (strain N144), and two used 10% FBS (strain N144). Thus, under a variety of conditions of serum concentration and culture duration, the single manipulation of vector-driven PAI-1 production in normal fibroblasts resulted in accumulation of newly synthesized collagen that approached the levels detected in keloid fibroblasts (compare PAI-1-dependent fold stimulations in Figure 3B with differences between normal and keloid fibroblasts) (Supplemental Figure S1A; Figure 2A). In addition, a direct comparison within the same experiment (see below) demonstrated that PAI-1-dependent increased collagen accumulation in a normal cell strain (N144) was equal to the level in the keloid strain, K-C3.
Figure 4.

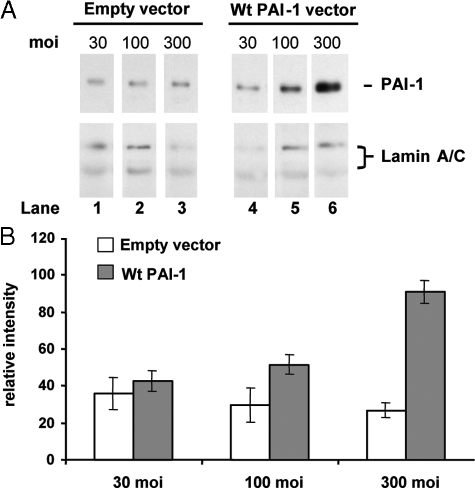
The effect of increased levels of PAI-1 was also studied in normal fibroblasts incorporated in fibrin gels, a situation that mimics the wound environment and where multiple phenotypic differences between keloid and normal fibroblasts have been observed.24,45 Fibroblasts in fibrin gels were infected at 30, 100, and 300 moi with Ad-Wt PAI-1 or empty vector and subsequently cultured for 6 days in 3% FBS. These conditions avoid gel lysis by normal cells under lower serum conditions45,46 and minimize possible effects of growth factors in higher serum concentrations. Ad-Wt PAI-1, but not empty vector, led to increased levels of PAI-1 protein in a viral dose-dependent manner (Figure 4A). Similar to the results in monolayer culture, increased PAI-1 production in fibrin gels stimulated the accumulation of newly synthesized collagen 3.3-fold (Figure 4B; 300 moi). Similar results were obtained with a different normal cell strain, N-A (data not shown). In this fibrin gel culture model, empty vector had no effect on collagen. Thus, in a model of provisional matrix, normal fibroblasts still exhibited PAI-1-dependent collagen regulation.
PAI-1 siRNAs Suppress the Level of PAI-1 Protein and Reduce Collagen Accumulation in Either Normal Fibroblasts Transduced with Ad-Wt PAI-1 or Keloid Fibroblasts
To remove any ambiguity regarding PAI-1-dependent regulation of collagen caused by the stimulatory effect of empty vector and to demonstrate this PAI-1-dependent function in keloid fibroblasts in the absence of adenoviral vectors, we used a siRNA approach. Normal fibroblasts were transduced with Ad-Wt PAI-1 vector to increase both PAI-1 and collagen as described in Figure 3. In this situation, the effects of adenoviral infection (either Wt PAI-1 or empty vector) on the accumulation of newly synthesized collagen were expected to remain after exposure to siRNAs targeted to PAI-1. Thus, if the only effect on collagen accumulation was due infection, siRNAs would be without effect; similarly, any reduction of collagen accumulation caused by siRNA would only be due to PAI-1-dependent regulation. Three different siRNA sequences targeting the human PAI-1 gene were used to decrease the level of PAI-1, and a mammalian nontargeting sequence siCONTROL siRNA was used as a negative control. Fluorescent siGLO was cotransfected with each targeting siRNA to follow transfection efficiency (∼90%). This stable, fluorescent, non-targeting control siRNA has been modified so that it does not engage the RNA-induced silencing complex (RISC) system or interfere with target gene silencing. Transfection of normal fibroblasts with siRNA was performed in 10% FBS immediately before infection with Ad-Wt PAI-1. Cells were then cultured in 3% FBS for 6 days before analysis.
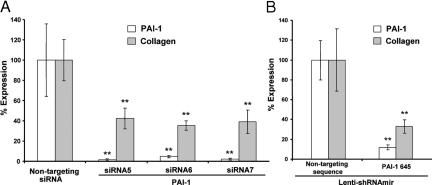
PAI-1 protein expression was reduced by more than 90% with each PAI-1-targeting siRNA sequence (siRNA5, siRNA6, and siRNA7) when compared with the nontargeting sequence siCONTROL siRNA (nontargeting siRNA) (Figure 5A, open bars). This caused greater than 60% reduction in accumulation of newly synthesized collagen (Figure 5A, filled bars). Given that the level of accumulation in the presence of the nontargeting sequence siRNA represents the sum of adenoviral (alone) and PAI-1-dependent increases in accumulation, the observed reductions caused by targeting siRNAs are consistent with elimination of the PAI-1-dependent effects (compare with Figure 3B).
Figure 5.

To eliminate effects of siRNA transfection and incomplete transduction, we used an alternative method of establishing RNA interference to PAI-1 expression. Normal fibroblasts (N144) were transduced with a lentiviral vector expressing a short hairpin RNA-containing microRNA sequence (shRNAmir; Open Biosystems) and expanded for selection of transduced cells. Such cells were selected with puromycin and verified by their expression of GFP before transduction with Ad-Wt PAI-1 to increase collagen accumulation over 6 days of culture in 3% FBS. The results with shRNAmir targeted to a different PAI-1 sequence (and compared with a different nontargeting sequence) demonstrated slightly better reduction in collagen accumulation (Figure 5B) than with siRNA in Figure 5A, probably due to continued expression of shRNAmir in the full population of evaluated cells for 6 days. Thus, two different RNA interference delivery vehicles and different targeting and control sequences produced the same loss-of-function result; PAI-1 expression supported collagen accumulation, and decreased PAI-1 led to decreased collagen accumulation.
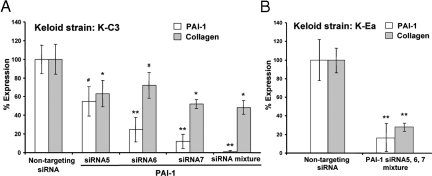
Next, we studied the effect of PAI-1 siRNA on PAI-1 expression and collagen accumulation by keloid fibroblasts. In this case, no adenoviral exposure was involved, but an unknown level of non-PAI-1-mediated support for keloid-specific collagen accumulation was expected. In addition to the individual PAI-1 siRNAs, we also used a mixture of the three siRNAs, because the effectiveness of the individual PAI-1 siRNAs varied in keloid fibroblasts. The mixture of PAI-1 siRNAs had the strongest effect (> 90%) on decreasing the level of PAI-1 protein in keloid fibroblasts (strain K-C3) compared with individual siRNAs (Figure 6A, open bars). Treatment with siRNAs for PAI-1 also resulted in decreased accumulation of newly synthesized collagen; the siRNA mix provided the largest decrease, to ∼50%, when compared with the nontargeting sequence (Figure 6A, filled bars). Using the siRNA mix and a different keloid cell strain, K-Ea, collagen accumulation was reduced to 30% of the nontargeting control (Figure 6B). Collectively, these siRNA experiments demonstrate that PAI-1 protein is capable of enhancing the accumulation of newly synthesized collagen and makes a significant contribution to the pathological levels of collagen accumulation produced by keloid fibroblasts.
Figure 6.

Evaluation of PAI-1 Loss-of-Function Mutants for Their Effects on Collagen Accumulation
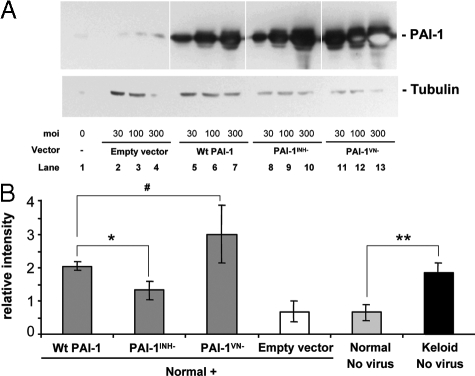
To determine whether the effect of PAI-1 on the accumulation of newly synthesized collagen was mediated by its inhibition of protease activity or by its interference with Vn-dependent cell function, adenoviral vectors carrying PAI-1 mutant genes were used to infect normal fibroblasts and compared with Wt PAI-1 for their effect on collagen accumulation. Two mutants were studied, PAI-1INH− and PAI-1VN−; the former has been demonstrated to lack the capacity for protease inhibition, whereas the latter lacks the capacity to bind to Vn and alter its normal ligand interactions.42 Normal fibroblasts transduced with each of the three PAI-1 adenoviral vectors expressed a similar dose-dependent increase in PAI-1 protein expression (Figure 7A). However, at each dose, cells treated with PAI-1VN− expressed higher levels of mutant PAI-1 than occurred with Wt PAI-1 or the PAI-1INH− mutant. Importantly, the level of PAI-1-dependent protease inhibitory activity in PAI-1INH− mutant treated cells was <6% of that detected in Wt or VN− mutant treated cells (Figure 8A), in agreement with the original literature.42 Consequently, if protease inhibition is responsible for the effects of PAI-1 on collagen, this loss-of-function mutant should be >94% less effective than Wt PAI-1 (Figure 8A). The PAI-1VN− mutant caused a small fraction of the treated fibroblasts to round-up, consistent with its lack of association with the Vn.42 Fibroblasts from all other treatment groups exhibited morphology similar to uninfected cells (Figure 8B).
Figure 7.
PAI-1 protein expression and collagen accumulation in N144 normal fibroblasts infected with adenoviral vectors carrying wild-type PAI-1 or mutant genes. The protease inhibitor mutant (PAI-1INH−), vitronectin-binding mutant (PAI-1VN−), or Wt PAI-1 adenoviral vectors at 30,100, and 300 moi were used to infect cells for 24 hours in 0.1% ITS+ before 6 days of culture in 3% FBS. A: PAI-1 protein expression was analyzed by Western blotting with α-tubulin (52 kDa) as the loading control. Empty vector was used as the control. Each protein band represents a physical pool of triplicates samples. Light spots inside the PAI-1 protein bands in lanes 12 and 13 indicate local exhaustion of chemiluminescent substrate. B: Accumulation of newly synthesized collagen as analyzed in Figure 5. Keloid strain K-C3 was used for comparison with normal strain N144 and N144 transduced with empty vector or viruses expressing wild-type and mutant PAI-1 at 100 moi during infection and culture as in A. n = 3, *P ≤ 0.05; **P ≤ 0.01; #P = 0.13.
Figure 8.
PAI-1-specific protease inhibitory activity of PAI-1 protein from normal fibroblasts (N144) infected with adenoviral vectors expressing Wt PAI-1, PAI-1INH−, or PAI-1VN−. A: Fibroblasts were infected with three concentrations of adenoviruses, 30, 100, or 300 moi for 24 hours in 0.1% ITS+ before culturing for 6 days in 3% FBS. The activity of PAI-1 protein from culture media was analyzed using Spectrolyze (pL) PAI-1 assay (American Diagnostica). Tubulin levels in the cell layer were determined by Western blotting and image analysis and used to normalize the data for protease inhibition. n = 3; mean ± SD. B: Phase contrast images from cultures described above. Note morphological similarity, except for cells infected with Ad-PAI-1VN−, which display some cell rounding. Scale bar = 15 μm.
In parallel samples, Wt PAI-1 and PAI-1VN− increased the accumulation of newly synthesized collagen two- to fourfold, which was comparable with the level of keloid fibroblasts and well above the empty vector control or untreated normal fibroblasts (Figure 7B). The PAI-1INH− vector also increased collagen accumulation above the empty vector control, reaching 50% of the level produced by the Wt PAI-1 vector (Figure 7B). The significant stimulation produced by this mutant that exhibits essentially no protease inhibitory activity calls into question, but does not eliminate, the possibility that PAI-1 exerts its effects on collagen through protease inhibition. In addition, the results with the PAI-1VN− mutant argue strongly against any involvement of the Vn binding capacity of PAI-1 in its control of collagen accumulation.
Discussion
Keloids result from an aberrant wound-healing response, and the high diversity and complex synchrony of wound repair provide multiple opportunities for initiation of pathology. Knowledge-based directed therapy requires detailed understanding of molecular mechanisms and identification of cause and effect relationships, yet little is known regarding keloid pathogenesis. We have previously extended the molecular phenotype of keloid fibroblasts to include overexpression of PAI-1. In culture, this was demonstrated at the protein and mRNA level, and in vivo by immunohistochemistry.24,45 In normal skin, PAI-1 was present at low levels and associated with the vasculature, whereas in keloid tissue, PAI was dramatically increased and shifted to a fibroblast/territorial matrix localization.24 In addition, we demonstrated that treatment of keloid fibroblasts with a PAI-1-specific polyclonal antibody reduced collagen synthesis to levels exhibited by normal fibroblasts.24 The intent of the present studies was to clarify the correlation between PAI-1 expression and collagen accumulation in keloid fibroblasts, to provide new and different evidence of a causal relationship between PAI-1 expression and collagen accumulation, and to test the capacity of PAI-1-directed RNA interference to reduce collagen accumulation as a possible therapeutic strategy.
Using seven strains of normal and keloid fibroblasts and different lengths and conditions of culture, we have exposed two clear differences that serve to characterize keloid fibroblasts; these are most easily recognized in Figure 2C. First, under basal conditions (0.1% ITS+), keloid fibroblasts, but not normal fibroblasts, demonstrated a high but variable level of accumulation of newly synthesized collagen, inherent accumulation, associated with very low PAI-1 (values along the x axis). Second, only keloid fibroblasts responded to 3% FBS with large changes in collagen accumulation and PAI-1 expression, providing angular plots in Figure 2C. Inherent collagen accumulation did not require exogenous serum-derived growth factors and was maintained at least 6 days. Such stability suggests the operation of autocrine or paracrine growth factor signaling. Similarly, elevated type I collagen gene expression after 24 hours of serum-free culture has recently been reported for keloid fibroblasts derived from three spontaneous keloids using genome-wide arrays.23 COL1A1 expression in fibroblasts from three different lesional locations, the expanding periphery, center bottom (origin), and center top (collapsing), was ∼8-fold higher than in normal controls. Although 24 hours of serum-free culture may not be adequate for complete decay of the effects of serum exposure, these results support the concept of cell-centric inherent collagen accumulation based on regulation of transcription/translation rather than collagen turnover, although both likely participate.
Considerable variation of inherent collagen accumulation is apparent in different strains of keloid fibroblasts in Figure 2C. This may be due to genetic differences that mark susceptible individuals (rev. in Ref. 22) or to differences in donor or lesion location. Because multiple aspects of the keloid phenotype may be differentially and independently retained/lost during subculture, we have sought to minimize this by using strains at less than three passages and by using direct enzymatic release from lesional tissue rather than a cell outgrowth technique. Similar culture protocols were used in the spontaneous keloid gene array study above23 and may increase the detection of keloid markers, including collagen expression. The importance of rapid isolation and characterization has also been demonstrated by the loss of integrin subpopulations in freshly isolated keloid fibroblasts within 4 days of culture.47 Such choices for analysis will also minimize cell selection but may allow inclusion of a small proportion vascular cells or epithelial cells that inform epithelial-mesenchymal communication to support the fibroblast keloid phenotype.18
The molecular mechanisms that drive inherent collagen accumulation and dictate other aspects of the keloid phenotype remain elusive, but several possibilities have been suggested by both gene arrays and directed studies. Among these are two that interact with the PAI-1-uPA system. Insulin-like growth factor binding proteins (IGFBPs) have been consistently identified in gene array studies as being markedly up-regulated in keloid fibroblasts.22,23,48 It is not clear whether the role of IGFBPs in fibrosis depends on insulin-like growth factor binding and the modulation of this by binding to collagen or Vn or on insulin-like growth factor-independent activity. However, the single manipulation of IGFBP5 overexpression in cultured normal lung fibroblasts by adenoviral transduction leads to marked increases in collagen and Fn production.49 Application of adenoviral expression of IGFBP5 to the in vivo environment has led to compelling models of skin50 and lung50 fibrosis.
Expression, processing, and signaling of the pro-fibrotic growth factor, TGF-β, provides multiple opportunities for initiation of pathology in a serum-free fibroblast-dependent autocrine environment. It influences PAI-1 by stimulating PAI-1 expression51 and by driving matrix synthesis that may be under PAI-1 regulatory control. In addition, TGF-β induction of PAI-1 prevents plasmin-dependent fibroblast apoptosis through its inhibition of plasminogen activation, providing a survival advantage for fibroblasts in an active fibrotic environment.40 TGF-β is up-regulated in keloid gene arrays,23and protein expression of the β1- and β2-isoforms that increase incisional scaring52 has been reported in keloid fibroblasts.53 These cells also exhibit differential expression of TGF-β receptors I and II, increased Smad phosphorylation compared with normal fibroblasts,54 and suppression of TGF-β-induced collagen synthesis on exposure to a truncated type II TGF-β receptor.55 Pharmacological treatment with quercetin suppresses collagen and Fn expression in keloid fibroblasts due to TGF-β receptor and Smad down-regulation and decreased Smad2 and 3 activation.56 That a single alteration in TGF-signaling can lead to pathological fibrosis has been shown by the targeted expression of a kinase-negative TGF-β type II receptor mutant in transgenic mice. Low-level expression of this usually dominant-negative receptor distorts signaling from the type I receptor and leads to increased production and processing of TGF-β, increased expression of the wild-type type II receptor, and development of both skin and lung fibrosis mediated by the type I receptor.57,58 Regulation of collagen synthesis can also occur through the noncanonical TGF-β-activated kinase 1 (TAK1) pathway as we have shown by mimicking TGF-β-stimulated type II collagen synthesis using adenoviral overexpression of TGF-β-activated kinase 1 with or without its activator, TGF-β-activated kinase 1 binding protein 1.43
Regardless of whether the TGF-β and IGFBP pathways are responsible for inherent collagen accumulation under serum-free conditions, they both will be engaged during the transition from 0.1% ITS+ to 3% FBS because of the increase in exogenous TGF-β and insulin-like growth factor. Six of seven keloid strains exhibited simultaneous increases in PAI-1 expression and collagen accumulation as demonstrated in Figure 2C. In contrast, during the transition from 3 to 10% FBS, six of seven keloid strains showed vertical plots, indicating that saturation of collagen accumulation but not PAI-1 occurred at ≥3% FBS. The uniformity of these transitions in keloid fibroblasts was unexpected. The only exception to saturation in 3% FBS was strain K-F (Supplemental Figure S3, angled plot), which exhibited continued increases in collagen and PAI-1 from 0.1% ITS+ to 10% FBS and produced the highest level of collagen accumulation. It is unclear why this strain diverges from the above pattern. However, strain K-F is unique in another way; it is the only strain that has a matched normal strain, N-(K-F), obtained from apparently normal perilesional tissue. The level of collagen accumulation and serum-response profile of N-(K-F) were midway between clustered normal and keloid strains (Figure 2C; Supplemental Figure S3). This is consistent with an early stage of development of the keloid phenotype or a low percentage of mature keloid cells. For comparison, perilesional elevation of IGFBP expression has also been reported in patients with systemic sclerosis,49,59 and both observations suggest staging of pathological changes.
Serum induced, simultaneous increases in collagen accumulation and PAI-1 expression were expected because serum, and more specifically TGF-β, cause transactivation of both promoters.51 However, our previous work demonstrating inhibition of keloid collagen synthesis by PAI-1 neutralizing antibodies suggested a more direct link. We therefore asked whether direct specific manipulation of PAI-1 expression would alter collagen accumulation. Adenoviral expression of PAI-1 in normal fibroblasts provided a range of PAI-1 concentrations that mimicked or exceeded that produced by keloid fibroblasts and increased the accumulation of newly synthesized collagen. This effect of PAI-1 on collagen accumulation required considerable time to develop, because PAI-1 expression on day 2 with 300 moi was similar to that on day 6 with 100 moi (Figure 3A), and yet collagen accumulation was ∼3.5-fold higher on day 6 (Figure 3B, compare black bars for these conditions). This is consistent with a cascade of events downstream of PAI-1 expression rather than an immediate result of its increased abundance. PAI-1 expression over 6 days caused a similar effect on normal cells cast in fibrin gels (Figure 4) suggesting that this mechanism of enhancing collagen accumulation should be operational in the three-dimensional fibrin-dominated matrix of early wound repair.
Three different siRNA sequences were selected from the wild-type human PAI-1 coding sequence for our loss-of-function experiments. Each produced PAI-1 knockdown at the protein level and reduced collagen accumulation in the 6-day experimental protocol when PAI-1 and collagen accumulation were initially elevated by adenoviral overexpression of PAI-1 in normal cells. This result was replicated when a fourth PAI-1 sequence was targeted by expression of an shRNAmir using a lentiviral vector, demonstrating that the result was independent of the delivery mechanism and further demonstrating specificity to PAI-1. These complex RNA interference protocols take time to develop both the up-regulation of PAI-1 and the down-regulation of PAI-1 and collagen, and they may not be optimal. They clearly demonstrate, however, that both the increase and decrease of collagen accumulation are PAI-1 dependent and not the result of adenoviral infection (empty vector). As a test of the potential clinical relevance of PAI-1 siRNA in reducing collagen accumulation, this strategy was applied to two different keloid fibroblast strains, K-C3 and K-Ea. The most effective knockdown of both PAI-1 and collagen accumulation was provided by the mixture of all three siRNAs. This produced statistically significant 50 and 70% reductions, respectively, in collagen accumulation and brought accumulation near (K-C3) or within (K-Ea) the range of normal fibroblasts under the identical conditions used for these siRNA experiments (namely, 3% FBS and 6 days of culture) (Supplemental Figure S1A).
Collectively, these results demonstrate that PAI-1 drives at least a major part of collagen accumulation in keloid fibroblasts. PAI-1 is necessary but may not be sufficient for this function and may require cooperation with TGF-β and insulin-like growth factor-1, factors that enhance matrix synthesis. Much of the research on keloids and other fibrotic diseases has been focused on synthetic mechanisms rather than on those that alter matrix degradation/turnover. The principal role of PAI-1 is to balance the uPA/tissue type plasminogen activator-dependent generation of plasmin from plasminogen.25 Excess PAI-1 inhibits plasminogen activators and prevents plasmin generation and its activation of matrix metalloproteinases, including collagenases, as well as its proteolysis of matrix proteins, including fibrin44 and Fn.40 Thus, in the presence of PAI-1, even normal matrix synthesis would yield higher levels of matrix because of the lack of plasmin activity and its attendant matrix turnover. This is the predominant in vivo fibrotic mechanism in a model of kidney fibrosis in which a protease inhibitor-deficient mutant of PAI-1 that maintained its wild-type Vn binding capacity competitively displaced wild-type PAI-1 on tissue Vn. This led to increased plasmin activity and decreased collagen accumulation in a plasmin-dependent manner.60,61 Such a protease-dependent mechanism is consistent with direct effects of PAI-1 on collagen accumulation in keloid and normal fibroblasts but may be modulated by the excess of keloid IGFBP-5, which binds PAI-1 and reduces its inhibitory capacity.62
PAI-1 also exhibits effects on cells not related to its protease inhibitory capacity, some of which are mediated by binding to Vn (rev. in Ref. 25). We have tested a protease inhibitory mutant and a Vn-binding mutant for their capacity to induce collagen accumulation. The latter was found to be equipotent with wild-type PAI-1. Thus Vn binding is dispensable for the effects of PAI-1 on collagen accumulation. The complete protease inhibitor mutant still retained ∼50% of the capacity of wild-type PAI-1 for inducing collagen accumulation. Because this is a cleavage-resistant mutant, it is expected to maintain its interactions with uPA without inhibiting the catalytic activity of uPA (R. Gerard, personal communication). The partial reduction in accumulation by the mutant may reflect the fact that PAI-1 uses protease inhibition and another of its functions to control accumulation or that protease inhibition is not significantly involved but a conformational change in the inhibitor mutant makes it less capable of enhancing collagen accumulation. It is important that the mutant is not limited in its activity by issues of access, because the mutant was simultaneously synthesized and secreted by infected cells and would efficiently compete with wild-type PAI-1 in all time- and location-dependent protein-protein interactions.
PAI-1 is capable of interacting with a range of binding partners that can directly or indirectly influence the biosynthetic and regulatory machinery of the cell. Such mechanisms may represent alternatives to protease inhibition/suppression of plasmin generation for PAI-1-mediated stimulation of the keloid phenotype. For example, PAI-1 binds to preformed uPA:uPAR complexes and then to very low density lipoprotein receptor to stabilize the phosphorylation of extracellular signal-regulated kinase 1/2 induced by uPA:uPAR. This extended activation of extracellular signal-regulated kinase renders the uPA signal mitogenic63 and may also enhance the effects of other growth factors that signal through or collaborate with extracellular signal-regulated kinase. Potentially more important is the role of PAI-1 in mediating integrin ligation. uPAR interacts with β1, β2, β3 and β5 integrins64 and one of these, α1β1-integrin, is a principle collagen I receptor in mesenchymal cells and is elevated in keloid fibroblasts.47 This integrin has been proposed as a key mediator of matrix collagen feedback on collagen synthesis, based on the dermal phenotype of α1-integrin-null mice65 The observations are important because PAI-1 has been shown to cause disengagement of αvβ3 from its matrix ligands, including collagen and Fn in a variety of cell types by binding to uPA:uPAR complexes and subsequently causing endocytosis and recycling of the integrin.37 This process is mediated by PAI-1 binding to uPA:uPAR-integrin complexes, reducing integrin affinity for its ligand. Disengagement of the integrin would suppress its outside-in signaling as described for integrin-specific antibodies66and reduce its feedback inhibition of collagen synthesis, leading to collagen accumulation. We have shown that keloid fibroblasts are responsive to matrix feedback by demonstrating that their collagen synthesis was dramatically reduced when cultured in three-dimensional collagen gels,24 and similar cultures have shown that such inhibition is less profound in α1-integrin-null fibroblasts than in normal cells.65 Thus, a speculative PAI-uPA-uPAR-α1 or β5-integrin-mediated feedback regulatory pathway may provide a substantial part of the increased collagen accumulation characteristic of the keloid phenotype. Such a mechanism may cooperate with the recently described function of uPAR-associated protein/Endo180 in fibroblast-mediated collagen degradation,67 where a role for PAI-1 has not yet been described. Validation of these mechanisms will require focused experiments to demonstrate the function of each element and the whole as well as its operation in keloid fibroblasts.
The decrease in collagen accumulation by keloid fibroblasts treated either with PAI-1 siRNAs, as shown in this study, or by PAI-1 neutralizing antibody, as in our previous study,24 establishes a novel PAI-1-mediated regulatory intervention for keloids that may be useful in the clinical setting. RNA interference has been shown to be effective at decreasing PAI-1 expression and consequently increasing adherence in vascular endothelial cells68 and suppressing replicative senescence.69 It is not known, however, whether siRNA targeted to PAI-1 can be effective in dermal fibroblasts maintained for long periods of time in three-dimensional models of fibroplasia45 and in diverse samples of keloid fibroblasts, using collagen synthesis as the outcome measure. Only when this has been demonstrated can investigations proceed to in vivo delivery techniques for eventual prophylactic treatment of patients at risk for keloid formation.
Acknowledgments
We thank Drs. Larry Nichter, William C. Lineaweaver, and Tanya Oswald for providing the surgical discards of normal skin and keloid specimens. We thank Dr. Robert Gerard for providing PAI-1 adenoviral vectors. Mr. Travis Nichter and Mr. Peter Hwu are acknowledged for their technical assistance. We gratefully acknowledge the assistance of the Biostatistics Core at Childrens Hospital Los Angeles.
Footnotes
Address reprint requests to Tai-Lan Tuan, PhD, The Saban Research Institute of Childrens Hospital Los Angeles, 4650 Sunset Blvd., MS 35, Los Angeles, CA 90027. E-mail: ttuan@chla.usc.edu.
Supported by National Institute of Health grant R01GM055081 (to T.-L.T.) and by the Los Angeles Orthopaedic Hospital Foundation (to P.D.B.).
T.-L.T. and P.B. contributed equally to this work.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Ehrlich HP, Desmouliere A, Diegelmann RF, Cohen IK, Compton CC, Garner WL, Kapanci Y, Gabbiani G. Morphological and immunochemical differences between keloid and hypertrophic scar. Am J Pathol. 1994;145:105–113. [PMC free article] [PubMed] [Google Scholar]
- Tuan TL, Nichter LS. The molecular basis of keloid and hypertrophic scar formation. Mol Med Today. 1998;4:19–24. doi: 10.1016/S1357-4310(97)80541-2. [DOI] [PubMed] [Google Scholar]
- Al-Attar A, Mess S, Thomassen JM, Kauffman CL, Davison SP. Keloid pathogenesis and treatment. Plast Reconstr Surg. 2006;117:286–300. doi: 10.1097/01.prs.0000195073.73580.46. [DOI] [PubMed] [Google Scholar]
- Marneros AG, Norris JE, Watanabe S, Reichenberger E, Olsen BR. Genome scans provide evidence for keloid susceptibility loci on chromosomes 2q23 and 7p11. J Invest Dermatol. 2004;122:1126–1132. doi: 10.1111/j.0022-202X.2004.22327.x. [DOI] [PubMed] [Google Scholar]
- Uitto J, Perejda AJ, Abergel RP, Chu ML, Ramirez F. Altered steady-state ratio of type I/III procollagen mRNAs correlates with selectively increased type I procollagen biosynthesis in cultured keloid fibroblasts. Proc Natl Acad Sci USA. 1985;82:5935–5939. doi: 10.1073/pnas.82.17.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abergel RP, Pizzurro D, Meeker CA, Lask G, Matsuoka LY, Minor RR, Chu ML, Uitto J. Biochemical composition of the connective tissue in keloids and analysis of collagen metabolism in keloid fibroblast cultures. J Invest Dermatol. 1985;84:384–390. doi: 10.1111/1523-1747.ep12265471. [DOI] [PubMed] [Google Scholar]
- Tuan TL, Zhu JY, Sun B, Nichter LS, Nimni ME, Laug WE. Elevated levels of plasminogen activator inhibitor-1 may account for the altered fibrinolysis by keloid fibroblasts. J Invest Dermatol. 1996;106:1007–1011. doi: 10.1111/1523-1747.ep12338552. [DOI] [PubMed] [Google Scholar]
- Diegelmann RF, Cohen IK, McCoy BJ. Growth kinetics and collagen synthesis of normal skin, normal scar and keloid fibroblasts in vitro. J Cell Physiol. 1979;98:341–346. doi: 10.1002/jcp.1040980210. [DOI] [PubMed] [Google Scholar]
- Babu M, Diegelmann R, Oliver N. Fibronectin is overproduced by keloid fibroblasts during abnormal wound healing. Mol Cell Biol. 1989;9:1642–1650. doi: 10.1128/mcb.9.4.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver N, Babu M, Diegelmann R. Fibronectin gene transcription is enhanced in abnormal wound healing. J Invest Dermatol. 1992;99:579–586. doi: 10.1111/1523-1747.ep12667776. [DOI] [PubMed] [Google Scholar]
- Alaish SM, Yager DR, Diegelmann RF, Cohen IK. Hyaluronic acid metabolism in keloid fibroblasts. J Pediatr Surg. 1995;30:949–952. doi: 10.1016/0022-3468(95)90319-4. [DOI] [PubMed] [Google Scholar]
- Meyer LJ, Russell SB, Russell JD, Trupin JS, Egbert BM, Shuster S, Stern R. Reduced hyaluronan in keloid tissue and cultured keloid fibroblasts. J Invest Dermatol. 2000;114:953–959. doi: 10.1046/j.1523-1747.2000.00950.x. [DOI] [PubMed] [Google Scholar]
- Bettinger DA, Yager DR, Diegelmann RF, Cohen IK. The effect of TGF-beta on keloid fibroblast proliferation and collagen synthesis. Plast Reconstr Surg. 1996;98:827–833. doi: 10.1097/00006534-199610000-00012. [DOI] [PubMed] [Google Scholar]
- Myles ME, Russell JD, Trupin JS, Smith JC, Russell SB. Keloid fibroblasts are refractory to inhibition of DNA synthesis by phorbol esters: altered response is accompanied by reduced sensitivity to prostaglandin E2 and altered down-regulation of phorbol ester binding sites. J Biol Chem. 1992;267:9014–9020. [PubMed] [Google Scholar]
- Russell SB, Trupin JS, Myers JC, Broquist AH, Smith JC, Myles ME, Russell JD. Differential glucocorticoid regulation of collagen mRNAs in human dermal fibroblasts: keloid-derived and fetal fibroblasts are refractory to down-regulation. J Biol Chem. 1989;264:13730–13735. [PubMed] [Google Scholar]
- Russell SB, Trupin KM, Rodriguez-Eaton S, Russell JD, Trupin JS. Reduced growth-factor requirement of keloid-derived fibroblasts may account for tumor growth. Proc Natl Acad Sci USA. 1988;85:587–591. doi: 10.1073/pnas.85.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SB, Russell JD, Trupin JS. Alteration of amino acid transport by hydrocortisone: different effects in human fibroblasts derived from normal skin and keloid. J Biol Chem. 1982;257:9525–9531. [PubMed] [Google Scholar]
- Phan TT, Lim IJ, Aalami O, Lorget F, Khoo A, Tan EK, Mukhopadhyay A, Longaker MT. Smad3 signalling plays an important role in keloid pathogenesis via epithelial-mesenchymal interactions. J Pathol. 2005;207:232–242. doi: 10.1002/path.1826. [DOI] [PubMed] [Google Scholar]
- Ong CT, Khoo YT, Tan EK, Mukhopadhyay A, Do DV, Han HC, Lim IJ, Phan TT. Epithelial-mesenchymal interactions in keloid pathogenesis modulate vascular endothelial growth factor expression and secretion. J Pathol. 2007;211:95–108. doi: 10.1002/path.2081. [DOI] [PubMed] [Google Scholar]
- Lim IJ, Phan TT, Bay BH, Qi R, Huynh H, Tan WT, Lee ST, Longaker MT. Fibroblasts cocultured with keloid keratinocytes: normal fibroblasts secrete collagen in a keloidlike manner. Am J Physiol Cell Physiol. 2002;283:C212–C222. doi: 10.1152/ajpcell.00555.2001. [DOI] [PubMed] [Google Scholar]
- Ghazizadeh M, Tosa M, Shimizu H, Hyakusoku H, Kawanami O. Functional implications of the IL-6 signaling pathway in keloid pathogenesis. J Invest Dermatol. 2007;127:98–105. doi: 10.1038/sj.jid.5700564. [DOI] [PubMed] [Google Scholar]
- Smith JC, Boone BE, Opalenik SR, Williams SM, Russell SB. Gene profiling of keloid fibroblasts shows altered expression in multiple fibrosis-associated pathways. J Invest Dermatol. 2007;128:1298–1310. doi: 10.1038/sj.jid.5701149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert O, Bayat A, Geffers R, Dienus K, Buer J, Lofgren S, Matussek A. Identification of unique gene expression patterns within different lesional sites of keloids. Wound Repair Regen. 2008;16:254–265. doi: 10.1111/j.1524-475X.2007.00343.x. [DOI] [PubMed] [Google Scholar]
- Tuan TL, Wu H, Huang EY, Chong SS, Laug W, Messadi D, Kelly P, Le A. Increased plasminogen activator inhibitor-1 in keloid fibroblasts may account for their elevated collagen accumulation in fibrin gel cultures. Am J Pathol. 2003;162:1579–1589. doi: 10.1016/S0002-9440(10)64292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cale JM, Lawrence DA. Structure-function relationships of plasminogen activator inhibitor-1 and its potential as a therapeutic agent. Curr Drug Targets. 2007;8:971–981. doi: 10.2174/138945007781662337. [DOI] [PubMed] [Google Scholar]
- Li WY, Chong SS, Huang EY, Tuan TL. Plasminogen activator/plasmin system: a major player in wound healing? Wound Repair Regen. 2003;11:239–247. doi: 10.1046/j.1524-475x.2003.11402.x. [DOI] [PubMed] [Google Scholar]
- De Taeye B, Smith LH, Vaughan DE. Plasminogen activator inhibitor-1: a common denominator in obesity, diabetes and cardiovascular disease. Curr Opin Pharmacol. 2005;5:149–154. doi: 10.1016/j.coph.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Andreasen PA. PAI-1: a potential therapeutic target in cancer. Curr Drug Targets. 2007;8:1030–1041. doi: 10.2174/138945007781662346. [DOI] [PubMed] [Google Scholar]
- Wysocki AB, Kusakabe AO, Chang S, Tuan TL. Temporal expression of urokinase plasminogen activator, plasminogen activator inhibitor and gelatinase-B in chronic wound fluid switches from a chronic to acute wound profile with progression to healing. Wound Repair Regen. 1999;7:154–165. doi: 10.1046/j.1524-475x.1999.00154.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhang Y, Heuckeroth RO. PAI-1 deficiency reduces liver fibrosis after bile duct ligation in mice through activation of tPA. FEBS Lett. 2007;581:3098–3104. doi: 10.1016/j.febslet.2007.05.049. [DOI] [PubMed] [Google Scholar]
- Sisson TH, Simon RH. The plasminogen activation system in lung disease. Curr Drug Targets. 2007;8:1016–1029. doi: 10.2174/138945007781662319. [DOI] [PubMed] [Google Scholar]
- Huang Y, Noble NA. PAI-1 as a target in kidney disease. Curr Drug Targets. 2007;8:1007–1015. doi: 10.2174/138945007781662373. [DOI] [PubMed] [Google Scholar]
- Vaughan DE, De Taeye BM, Eren M. PAI-1 antagonists: predictable indications and unconventional applications. Curr Drug Targets. 2007;8:962–970. doi: 10.2174/138945007781662364. [DOI] [PubMed] [Google Scholar]
- Higgins PJ, Slack JK, Diegelmann RF, Staiano-Coico L. Differential regulation of PAI-1 gene expression in human fibroblasts predisposed to a fibrotic phenotype. Exp Cell Res. 1999;248:634–642. doi: 10.1006/excr.1999.4466. [DOI] [PubMed] [Google Scholar]
- Declerck PJ, De Mol M, Alessi MC, Baudner S, Paques EP, Preissner KT, Muller-Berghaus G, Collen D. Purification and characterization of a plasminogen activator inhibitor 1 binding protein from human plasma: identification as a multimeric form of S protein (vitronectin). J Biol Chem. 1988;263:15454–15461. [PubMed] [Google Scholar]
- Seiffert D, Mimuro J, Schleef RR, Loskutoff DJ. Interactions between type 1 plasminogen activator inhibitor, extracellular matrix and vitronectin. Cell Differ Dev. 1990;32:287–292. doi: 10.1016/0922-3371(90)90041-t. [DOI] [PubMed] [Google Scholar]
- Czekay RP, Aertgeerts K, Curriden SA, Loskutoff DJ. Plasminogen activator inhibitor-1 detaches cells from extracellular matrices by inactivating integrins. J Cell Biol. 2003;160:781–791. doi: 10.1083/jcb.200208117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson S, Lawrence DA. The serpin PAI-1 inhibits cell migration by blocking integrin alpha V beta 3 binding to vitronectin. Nature. 1996;383:441–443. doi: 10.1038/383441a0. [DOI] [PubMed] [Google Scholar]
- Loskutoff DJ, Curriden SA, Hu G, Deng G. Regulation of cell adhesion by PAI-1. APMIS. 1999;107:54–61. doi: 10.1111/j.1699-0463.1999.tb01526.x. [DOI] [PubMed] [Google Scholar]
- Horowitz JC, Rogers DS, Simon RH, Sisson TH, Thannickal VJ. Plasminogen activation induced pericellular fibronectin proteolysis promotes fibroblast apoptosis. Am J Respir Cell Mol Biol. 2008;38:78–87. doi: 10.1165/rcmb.2007-0174OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danø K, Behrendt N, Hoyer-Hansen G, Johnsen M, Lund LR, Ploug M, Romer J. Plasminogen activation and cancer. Thromb Haemost. 2005;93:676–681. doi: 10.1160/TH05-01-0054. [DOI] [PubMed] [Google Scholar]
- Praus M, Collen D, Gerard RD. Both u-PA inhibition and vitronectin binding by plasminogen activator inhibitor 1 regulate HT1080 fibrosarcoma cell metastasis. Int J Cancer. 2002;102:584–591. doi: 10.1002/ijc.10767. [DOI] [PubMed] [Google Scholar]
- Qiao B, Padilla SR, Benya PD. Transforming growth factor (TGF)-{beta}-activated kinase 1 mimics and mediates TGF-{beta}-induced stimulation of type II collagen synthesis in chondrocytes independent of Col2a1 transcription and Smad3 signaling. J Biol Chem. 2005;280:17562–17571. doi: 10.1074/jbc.M500646200. [DOI] [PubMed] [Google Scholar]
- de Giorgio-Miller A, Bottoms S, Laurent G, Carmeliet P, Herrick S. Fibrin-induced skin fibrosis in mice deficient in tissue plasminogen activator. Am J Pathol. 2005;167:721–732. doi: 10.1016/S0002-9440(10)62046-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuan TL, Song A, Chang S, Younai S, Nimni ME. In vitro fibroplasia: matrix contraction, cell growth, and collagen production of fibroblasts cultured in fibrin gels. Exp Cell Res. 1996;223:127–134. doi: 10.1006/excr.1996.0065. [DOI] [PubMed] [Google Scholar]
- Tuan TL, Grinnell F. Fibronectin and fibrinolysis are not required for fibrin gel contraction by human skin fibroblasts. J Cell Physiol. 1989;140:577–583. doi: 10.1002/jcp.1041400324. [DOI] [PubMed] [Google Scholar]
- Szulgit G, Rudolph R, Wandel A, Tenenhaus M, Panos R, Gardner H. Alterations in fibroblast alpha1beta1 integrin collagen receptor expression in keloids and hypertrophic scars. J Invest Dermatol. 2002;118:409–415. doi: 10.1046/j.0022-202x.2001.01680.x. [DOI] [PubMed] [Google Scholar]
- Satish L, Lyons-Weiler J, Hebda PA, Wells A. Gene expression patterns in isolated keloid fibroblasts. Wound Repair Regen. 2006;14:463–470. doi: 10.1111/j.1743-6109.2006.00135.x. [DOI] [PubMed] [Google Scholar]
- Pilewski JM, Liu L, Henry AC, Knauer AV, Feghali-Bostwick CA. Insulin-like growth factor binding proteins 3 and 5 are overexpressed in idiopathic pulmonary fibrosis and contribute to extracellular matrix deposition. Am J Pathol. 2005;166:399–407. doi: 10.1016/S0002-9440(10)62263-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuoka H, Jukic DM, Zhou Z, Choi AM, Feghali-Bostwick CA. Insulin-like growth factor binding protein 5 induces skin fibrosis: a novel murine model for dermal fibrosis. Arthritis Rheum. 2006;54:3001–3010. doi: 10.1002/art.22084. [DOI] [PubMed] [Google Scholar]
- Keski-Oja J, Raghow R, Sawdey M, Loskutoff DJ, Postlethwaite AE, Kang AH, Moses HL. Regulation of mRNAs for type-1 plasminogen activator inhibitor, fibronectin, and type I procollagen by transforming growth factor-beta: divergent responses in lung fibroblasts and carcinoma cells. J Biol Chem. 1988;263:3111–3115. [PubMed] [Google Scholar]
- Shah M, Foreman DM, Ferguson MW. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci. 1995;108:985–1002. doi: 10.1242/jcs.108.3.985. [DOI] [PubMed] [Google Scholar]
- Lee TY, Chin GS, Kim WJ, Chau D, Gittes GK, Longaker MT. Expression of transforming growth factor beta 1, 2, and 3 proteins in keloids. Ann Plast Surg. 1999;43:179–184. [PubMed] [Google Scholar]
- Chin GS, Liu W, Peled Z, Lee TY, Steinbrech DS, Hsu M, Longaker MT. Differential expression of transforming growth factor-beta receptors I and II and activation of Smad 3 in keloid fibroblasts. Plast Reconstr Surg. 2001;108:423–429. doi: 10.1097/00006534-200108000-00022. [DOI] [PubMed] [Google Scholar]
- Chu Y, Guo F, Li Y, Li X, Zhou T, Guo Y. A novel truncated TGF-beta receptor II downregulates collagen synthesis and TGF-beta I secretion of keloid fibroblasts. Connect Tissue Res. 2008;49:92–98. doi: 10.1080/03008200801913924. [DOI] [PubMed] [Google Scholar]
- Phan TT, Lim IJ, Chan SY, Tan EK, Lee ST, Longaker MT. Suppression of transforming growth factor beta/smad signaling in keloid-derived fibroblasts by quercetin: implications for the treatment of excessive scars. J Trauma. 2004;57:1032–1037. doi: 10.1097/01.ta.0000114087.46566.eb. [DOI] [PubMed] [Google Scholar]
- Denton CP, Black CM. Targeted therapy comes of age in scleroderma. Trends Immunol. 2005;26:596–602. doi: 10.1016/j.it.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Denton CP, Zheng B, Evans LA, Shi-wen X, Ong VH, Fisher I, Lazaridis K, Abraham DJ, Black CM, de Crombrugghe B. Fibroblast-specific expression of a kinase-deficient type II transforming growth factor beta (TGFbeta) receptor leads to paradoxical activation of TGFbeta signaling pathways with fibrosis in transgenic mice. J Biol Chem. 2003;278:25109–25119. doi: 10.1074/jbc.M300636200. [DOI] [PubMed] [Google Scholar]
- Feghali CA, Wright TM. Identification of multiple, differentially expressed messenger RNAs in dermal fibroblasts from patients with systemic sclerosis. Arthritis Rheum. 1999;42:1451–1457. doi: 10.1002/1529-0131(199907)42:7<1451::AID-ANR19>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Huang Y, Border WA, Lawrence DA, Noble NA. Noninhibitory PAI-1 enhances plasmin-mediated matrix degradation both in vitro and in experimental nephritis. Kidney Int. 2006;70:515–522. doi: 10.1038/sj.ki.5000353. [DOI] [PubMed] [Google Scholar]
- Huang Y, Haraguchi M, Lawrence DA, Border WA, Yu L, Noble NA. A mutant, noninhibitory plasminogen activator inhibitor type 1 decreases matrix accumulation in experimental glomerulonephritis. J Clin Invest. 2003;112:379–388. doi: 10.1172/JCI18038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie J, Allan GJ, Lochrie JD, Flint DJ. Insulin-like growth factor-binding protein-5 (IGFBP-5): a critical member of the IGF axis. Biochem J. 2006;395:1–19. doi: 10.1042/BJ20060086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb DJ, Thomas KS, Gonias SL. Plasminogen activator inhibitor 1 functions as a urokinase response modifier at the level of cell signaling and thereby promotes MCF-7 cell growth. J Cell Biol. 2001;152:741–752. doi: 10.1083/jcb.152.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuning U, Magdolen V, Hapke S, Schmitt M. Molecular and functional interdependence of the urokinase-type plasminogen activator system with integrins. Biol Chem. 2003;384:1119–1131. doi: 10.1515/BC.2003.125. [DOI] [PubMed] [Google Scholar]
- Gardner H, Broberg A, Pozzi A, Laato M, Heino J. Absence of integrin alpha1beta1 in the mouse causes loss of feedback regulation of collagen synthesis in normal and wounded dermis. J Cell Sci. 1999;112:263–272. doi: 10.1242/jcs.112.3.263. [DOI] [PubMed] [Google Scholar]
- Ravanti L, Heino J, Lopez-Otin C, Kahari VM. Induction of collagenase-3 (MMP-13) expression in human skin fibroblasts by three-dimensional collagen is mediated by p38 mitogen-activated protein kinase. J Biol Chem. 1999;274:2446–2455. doi: 10.1074/jbc.274.4.2446. [DOI] [PubMed] [Google Scholar]
- Madsen DH, Engelholm LH, Ingvarsen S, Hillig T, Wagenaar-Miller RA, Kjoller L, Gardsvoll H, Hoyer-Hansen G, Holmbeck K, Bugge TH, Behrendt N. Extracellular collagenases and the endocytic receptor, urokinase plasminogen activator receptor-associated protein/Endo180, cooperate in fibroblast-mediated collagen degradation. J Biol Chem. 2007;282:27037–27045. doi: 10.1074/jbc.M701088200. [DOI] [PubMed] [Google Scholar]
- Hecke A, Brooks H, Meryet-Figuiere M, Minne S, Konstantinides S, Hasenfuss G, Lebleu B, Schafer K. Successful silencing of plasminogen activator inhibitor-1 in human vascular endothelial cells using small interfering RNA. Thromb Haemost. 2006;95:857–864. [PubMed] [Google Scholar]
- Kortlever RM, Higgins PJ, Bernards R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat Cell Biol. 2006;8:877–884. doi: 10.1038/ncb1448. [DOI] [PMC free article] [PubMed] [Google Scholar]