Abstract
Epithelial appendages, such as mammary glands and hair, arise as a result of epithelial-mesenchymal interactions. Bone morphogenetic proteins (BMPs) are important for hair follicle morphogenesis and cycling and are known to regulate a wide variety of developmental processes. For example, overexpression of BMPs inhibits hair follicle formation. We hypothesized that the down-regulation of the BMP signaling pathway in the basal epidermis expands regions that are competent to form hair follicles and could alter the fate of the epithelium in the mouse nipple to a hair-covered epidermal phenotype. To test our hypothesis, we used a transgenic mouse model in which keratin 14 (KRT14) promoter-mediated overexpression of Noggin, a BMP antagonist, modulates BMP activity. We observed the conversion of nipple epithelium into pilosebaceous units. During normal mammary gland organogenesis, BMPs are likely used by the nipple epithelium to suppress keratinocyte differentiation, thus preventing the formation of pilosebaceous units. In this report, we characterize the morphology and processes that influence the development of hairs within the nipple of the KRT14-Noggin mouse. We demonstrate that Noggin acts, in part, by reducing the BMP signal in the epithelium. Reduction of the BMP signal in turn leads to a reduction in the levels of parathyroid hormone-related protein. We propose that during evolution of the nipple, the BMP pathway was co-opted to suppress hair follicle formation and create a more functional milk delivery apparatus.
The development of diverse ectodermal organs such as mammary glands, feathers, and hair has much in common and shares developmental stages such as induction, morphogenesis, and differentiation.1 Many ectodermal organs have even become defining characteristics of specific vertebrate classes; ie, feathers and hair are cardinal characteristics of the Aves and Mammalia classes, respectively.2 These different ectodermal organs show regional specificity whereby their developmental potential varies in different regions of the skin. For instance nails grow from the tips of fingers and toes, teeth form within the mouth, hair of different coarseness, length, and pigmentation form from the scalp, beard, eyebrows, chest, arms, and so forth.
Although ectodermal organs arise from different developmental mechanisms, several aspects of their development and morphogenesis run in parallel. Early stages of development include the formation of a dense mesenchyme underlying an epithelial bud followed by invagination or evagination coupled to differential growth. Then the epithelial appendage undergoes a process of differentiation to unveil the unique ectodermal organ structure and function. Each of these ectodermal organs expand the skin surface area in different ways to provide methods for display, camouflage, feeding of the young, warmth or cooling, flight, and so forth. We presume that each region of the skin has the initial potential to generate each type of ectodermal organ, but the fate is chosen through molecular signals in the local environment.3 Small changes in molecular expression may tip the balance toward a different developmental program. To further explore this concept, we focused on the ventral skin that can form interfollicular skin, pilosebaceous units, or nipples. Specifically, we analyzed ectodermal organs that lie along the mammary line.
In the mouse, the differentiation of stem cells into a mammary gland is first observed by the appearance of five symmetric (left-right) pairs of placodes visible by scanning electron microscopy at embryonic day 11.5 (E11.5). These are seen as elevations above the surrounding surface ectoderm.4 At this stage, the epidermis of male mice is under the influence of androgens and the epithelial bud becomes separated from the epidermis as a result of apoptosis in the underlying mammary mesenchyme at approximately E14.5 In their female counterparts at E15.5, the epithelial bud forms a mammary sprout and invaginates through the underlying mesenchyme. This is followed at E16.5 by the formation of a rudimentary ductal tree derived from the branching of the epithelial sprout into the mammary fat pad and the induction of the nipple sheath in the overlying epithelium.6 Thus male mice fail to form both mammary glands and nipples. The nipple sheath results from epidermal thickening that invaginates down into the underlying dermis, forming a halo surrounding the location of the mammary sprout that is formed as a result of parathyroid hormone-related protein (PTHrP)/PTH-R1 signaling.4 The nipple is considered to be a type of specialized epidermis that shows distinct patterns of differentiation and keratin expression to withstand the mechanical strain of nursing.7
In normal mice, hair follicles do not develop in the nipple epidermis, but they are found covering the rest of the ventral trunk with the exception of the genital regions. In the skin of the trunk, proliferating keratinocytes can systematically differentiate, giving rise to the basic structure of the hair follicle. This leads to the formation of various structures in the hair follicle including the inner and outer root sheath, medulla, cortex, and central cuticle of the hair shaft. The epithelium can further differentiate into a sebaceous gland that is comprised of lipid-filled sebocytes that release their contents (sebum) onto the surface of the epidermis. Stem cells have been proposed to lie in a specialized region of the hair follicle known as the bulge and appear to be responsible for replenishing the sebaceous gland in addition to generating the hair lineages.8,9
Multiple signaling pathways have been implicated in various stages of the hair follicle development and its regenerative cycle in the adult, including Wnt, fibroblast growth factor, sonic hedgehog (Shh), and the transforming growth factor-β superfamily signaling pathways. The bone morphogenetic protein (BMP) pathway consists of more than 20 secreted growth factors and their receptors that are involved in many aspects of development. Various BMPs and secreted antagonists of the BMPs such as Noggin and Follistatin are of particular importance for hair follicle morphogenesis and cycling.10,11,12 BMPs have been described as being classical morphogens because they have the ability to alter ectodermal organ specificity.13,14 BMPs such as BMP2, -4, and -7 signal through a heteromeric complex of type I and II receptor serine/threonine kinases.15 Binding of the BMPs to the receptors induces the phosphorylation of the receptor regulated SMAD family (rSMADs) members (SMAD1, -5, and -8) that then associate with co-SMAD (SMAD4). This phosphorylated complex can then translocate into the nucleus and regulate the transcription of various genes.16,17 Hair follicle morphogenesis is dependent on the inhibition of BMP signaling as demonstrated by the observation that mice that overexpress Noggin at the dermal-epidermal interface have an increased number of hair follicles.18
In this study, we set forth to examine the role of the BMP pathway in fate determination within the ventral skin by using Noggin as a tool to inhibit BMP activity. We demonstrate that BMP signaling is central to a series of cell fate decisions altering the competence status in the nipple epithelium. This leads to the formation of hairs, sebaceous glands, and normal nipple epithelium within the same nipple.
Materials and Methods
Animals
KRT14-Noggin mice were identified as described previously.18 Noggin-lacZ mice were obtained as a gift from Dr. Richard Harland (University of California, Berkeley) and Msx2−/− mice were obtained as a gift from Dr. Robert Maxson (University of Southern California). Msx2−/− mice were backcrossed onto a C57BLl/6 background and then identified as previously described.19 All animals were anesthetized and their nipples removed and processed for staining or scanning electron microscopy as described below.
Cell Culture and Transient Transfection
Immortalized primary human keratinocytes (a kind gift from Dr. David Woodley, University of Southern California) were used for all cell culture experiments and described previously.20 Keratinocytes were plated at 5 × 104 cells/well in 24-well plates and grown in keratinocyte serum-free media (Invitrogen, Carlsbad, CA) containing pituitary extract, epidermal growth factor supplement, 2% fetal bovine serum, and penicillin/streptomycin. For reporter gene assays, keratinocytes were transiently transfected using TargeFect F1 reagent (Targeting Systems, El Cajon, CA). A total of 1.0 μg of plasmid DNA was incubated with F1 reagent in OptiMEM at 37°C for 20 minutes and then added to the corresponding wells for 3 hours, followed by the addition of serum containing media to each well in the presence or absence of 10 nmol/L BMP4 protein (R&D Systems, Minneapolis, MN) or 10 nmol/L Noggin protein (R&D). Each well was washed with phosphate-buffered saline (PBS) before preparing cell extracts. Cell extracts were prepared and assayed for luciferase activity 48 hours after transfection, according to the manufacturer’s protocol (Promega, Madison, WI). The results shown are the means and SD of triplicate points. Intact P3-Luciferase construct described previously21 and mutated SMAD binding site (P3M3) also described previously22 were used to assess activation of the PTHrP promoter by BMP4 and Noggin. A Student’s t-test was performed to determine statistical significance (P < 0.05).
Histological, Histochemical, and Immunohistological Staining
Hematoxylin and Eosin (H&E)
After excision of the mouse nipples, the specimens were fixed in 4% paraformaldehyde in PBS overnight at 4°C followed by a dehydration series and embedding in paraffin wax. Five μm sections of the fourth inguinal nipple were cut longitudinally. Standard H&E staining was performed for basic histological analysis.
Immunostaining Sections
Specimens were fixed, dehydrated, and embedded as described above. Sections of the fourth inguinal nipple were stained with antibodies against keratin 14 (KRT14) (Lab Vision, Fremont, CA) and β-catenin (Sigma, St. Louis, MO) to visualize signaling. Staining for pSMAD1,5,8 (Cell Signaling Beverly, MA) was performed to visualize BMP signaling. Antibodies against NCAM23 were used to examine alterations in dermal signaling. Ki-67 antibodies (DAKO, Carpinteria, CA) were used to localize proliferating cells. TUNEL (Roche, Indianapolis, IN) staining was used to visualize apoptosis. Binding of the secondary antibody and visualization using AEC (Vector Laboratories, Burlingame, CA) were done according to the manufacturer’s protocol and as described previously.24 Visualization and photos were taken using the MedMicroscopy (Trestle, Irvine, CA) slide scanner fitted on a BX51 microscope (Olympus, Melville, NY).
Staining of Sebocytes with Oil Red O
Oil Red O staining was modified and performed based on the protocol described by Guha.25 Briefly, 5-μm frozen sections of the adult mouse nipple were postfixed in 4% paraformaldehyde, washed with distilled water, followed by two changes of 100% propylene glycol for 5 minutes each. The sections were stained for 7 minutes with Oil Red O, followed by rinsing in 85% propylene glycol and then in distilled water for 3 minutes each, and then counterstained with hematoxylin and mounted.
In Situ Hybridization
Section in situ hybridization was performed as previously described but with some modifications.24 Proteinase K treatment was performed for 10 minutes and all washing steps were performed for 10 minutes each. PTHrP probe was a kind gift from Dr. Marcel Karperien (Leidin University Medical Center) and described previously.26 Shh and Noggin probes were a kind gift from Dr. Saverio Bellusci (University of Southern California).
Scanning Electron Microscopy
The specimens were excised and fixed overnight at 4°C in Karnovsky’s fixative (2% paraformaldehyde, 2% glutaraldehyde, 5 mmol/L CaCl2 in 0.1 mol/L cacodylate buffer, pH 7.4). After fixation, the specimens were rinsed several times with 0.1 mol/L cacodylate buffer for a minimum of 15 minutes, followed by postfixation with 2% osmium tetroxide in 0.1 mol/L cacodylate buffer for 2 hours in the hood. This is followed by rinsing with 0.1 mol/L cacodylate buffer for a minimum of 15 minutes followed by dehydration of the specimens using a graded alcohol series (50% for 15 minutes, 70% for 15 minutes, 85% for 15 minutes, 95% for 15 minutes, and two changes of 100% for 5 minutes each). The samples are then chemically dried using a series of hexamethyldisilazane (HMDS) (50% HMDS in ethyl alcohol for 15 minutes followed by 75%, 85%, 95%, and two changes of 100%). After the final change of 100% HMDS, the excess is removed and the samples are allowed to air-dry in a hood overnight. The samples are then mounted on aluminum stubs with adhesive tabs and sputter-coated for 3 minutes.
Semiquantitative Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
A total of five nipples were excised from WT, KRT14-Noggin+/−, KRT14-Noggin+/+, and Msx2−/− mice. Tissues were then ground using a mortar and pestle in liquid nitrogen and homogenized using the QIAShredder (Qiagen, Valencia, CA). RNA was extracted using the RNeasy kit (Qiagen). Finally RNA was transcribed into cDNA using a first-strand reverse transcriptase kit for RT-PCR (Invitrogen). We analyzed the tissues for levels of ectopic KRT14-Noggin,18 endogenous Noggin sense 5′-GAGCAAGAAGCTGAGGAGGA-3′ and antisense 5′-GTGAGGTGCACAGACTTGGA-3′, Bmpr1a sense 5′-TCTCAAGCAGGGGTCGTTAC-3′ and antisense 5′-CGCCATTTACCCATCCATAC-3′, and L32 sense 5′-AGAAGGTTCAAGGGCCAGAT-3′ and antisense 5′-CAGCTCCTTGACATTGTGGA-3′ as a control. PCR was performed using the MJ mini (Bio-Rad, Hercules, CA) as follows: an initial dissociation at 95°C for 3 minutes followed by 30 cycles of dissociation at 95°C for 30 seconds, annealing at 55 to 58°C for 30 seconds, and extension at 72°C for 30 seconds. A final extension time of 10 minutes was then performed and PCR products were held at 4°C until run on a 1% agarose gel for visualization of the bands.
Results
Overexpression of Noggin Leads to the Conversion of Nipple Epithelium into Competent Hair Follicle-Forming Epithelium
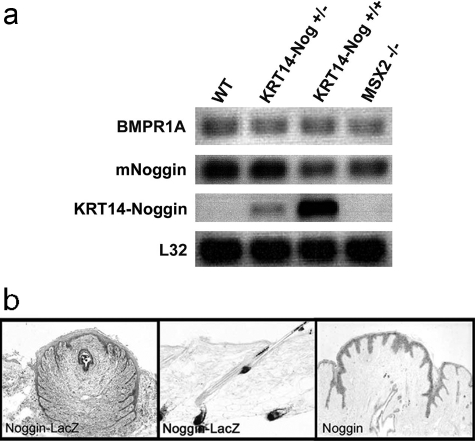
To characterize the expression of endogenous Noggin in Noggin transgenic and Msx2−/− mice compared to controls we performed RT-PCR (Figure 1a). Endogenous Noggin is expressed to the same level in each mouse line. On the other hand, ectopic Noggin is expressed in a dose-dependent manner in our Noggin transgenic mice (Figure 1a). We also compared the levels of Bmpr1a to test whether the expression level of this receptor is changed because of the decreased BMP signaling in the epithelium. Bmpr1a expression was not altered in the nipples as a result of decreasing BMP activity in both Noggin-overexpressing and Msx2−/− mice (Figure 1a). Control gene (L32) expression was consistent throughout the samples. Next we examined the location of endogenous Noggin expression. Using a transgenic mouse in which the Noggin promoter drives expression of LacZ27 and expresses β-galactosidase, we identified the endogenous pattern of Noggin expression in the adult nipple. Interestingly, in the nipple area Noggin is mainly expressed in the epithelium of the adult mouse nipple but is also present to a low level in the mesenchyme (Figure 1b). In contrast, LacZ staining shows that Noggin is expressed in the dermal papilla of dorsal skin in agreement with published observations.11 We further tested the location of Noggin expression in the adult nipple by performing in situ hybridization on a control mouse. These data confirm that Noggin is expressed primarily by the epithelium in the adult mouse nipple (Figure 1b) and suggest that the BMP signaling pathway likely exerts complex regulation on the nipple epithelium.
Figure 1.
Expression of Noggin and BMP signaling in nipples of 6- to 8-month-old WT, Noggin-overexpressing, and Msx2−/− mice. a: Nipples were harvested from WT and transgenic mice and analyzed for levels of endogenous Noggin, ectopic levels of KRT14-Noggin, Bmpr1a, and control L32 genes by RT-PCR. b: A Noggin-lacZ mouse was used to locate Noggin expression and further confirmed by in situ hybridization for Noggin on a control nipple.
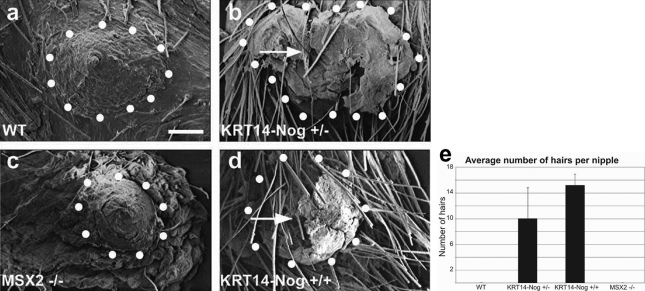
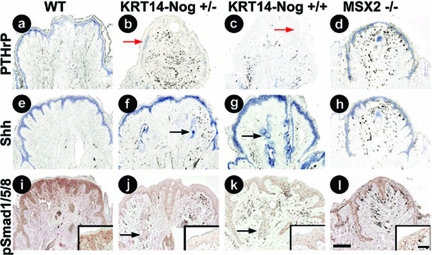
To test the function of BMP in establishing the specialized nipple structures, we used transgenic mice in which Noggin expression was regulated by the keratin 14 (KRT14) promoter. These KRT14-Noggin mice express Noggin in the basal layer of the epithelium at the junction of the epithelium and dermis. This expression pattern mostly overlaps with the endogenous Noggin expression pattern (compare Figure 1b with Figure 3i). Scanning electron microscopy was performed to visualize the presence of hair follicles formed within the nipples of two types of Noggin-overexpressing mice and wild-type (WT) littermates. We also examined the nipples of Msx2−/− mice (Figure 2, a–d) because Msx2 is known to have a SMAD binding site in its promoter region and this homeodomain transcription factor mediates BMP signaling in several tissues. Furthermore, it was shown that BMP and PTHrP signaling up-regulates Msx2 expression in the mammary gland and suppresses hair growth in the nipple. The disruption of Msx2 induces new hair follicle growth on the ventral skin of PTHrP-overexpressing mice.28 The images obtained from scanning electron microscopy allowed us to count the number of follicles protruding from the nipples. The effects of KRT14-Noggin suggested a dose-dependent impact of the BMP repressor on hair follicle formation. Heterozygous Noggin mice had an average of 10.00 ± 4.83 hairs per nipple, whereas homozygous mice averaged 15.25 ± 1.71 hairs per nipple. No hair follicles were observed in the nipples of control or Msx2−/− mice (Figure 2e).
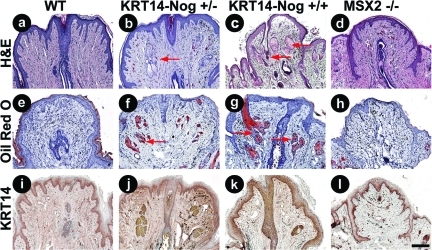
Figure 3.
Histological and immunohistochemical analysis of adult, parous 6- to 8-month-old WT and transgenic mouse nipples with decreased BMP activity. H&E-stained sections of control (a), KRT14-Noggin+/− (b), KRT14-Noggin+/+ (c), and Msx2−/− mice (d). e–h: Oil Red O staining to indicate the presence of sebaceous glands in the Noggin-overexpressing mice compared to Msx2−/− and control mice. KRT14 immunostaining showing no change in nipple epithelium from control, Noggin-overexpressing transgenic, or Msx2−/− mice (i–l) but increased epithelial expression surrounding ectopic hair follicles in Noggin-overexpressing mice (j, k). Red arrows point to pilosebaceous units. Scale bar = 200 μm.
Figure 2.
Scanning electron microscopy and quantitation of hair follicles from adult, 6 to 8 months old, parous mice. a and c: Control and Msx2−/− mice show no hair follicles within the nipple. b and d: Noggin-overexpressing mice show ectopic hair follicles extruding from the nipples. e: A graph indicates the trend that increased hair follicle numbers correlates with increasing Noggin expression. Arrows point to an example of a hair follicle growing within the nipple. Scale bar = 200 μm.
Because the nipple is part of a specialized ectodermal organ (mammary gland) derived from epithelial-mesenchymal interactions, altering BMP pathway activity at the epithelial-dermal junction may change the competence of the epithelium, allowing for the formation of hair follicles. After histological staining was performed we examined the nipples for any morphological changes that had occurred. Noggin-expressing mice showed hair follicles and sebaceous glands within the nipple (Figure 3, b and c) that were absent from WT nipples (Figure 3a) and those of the Msx2−/− (Figure 3d) mice. These data suggest that suppression of BMP activity by Noggin leads to a conversion of nipple epithelial cells into different ectodermal organs including hair follicles and sebaceous glands. This activity does not appear to be mediated via Msx2 because neither hairs nor sebaceous glands are present in the nipples of Msx2−/− mice.
Oil Red O staining was performed on frozen sections of the mouse nipples to confirm the presence of sebaceous glands within the nipples of the transgenic Noggin mice. Results show that Noggin acts in a dose-dependent manner that increases the number and size of ectopic sebaceous glands within the nipple (Figure 3, f and g). WT and Msx2−/− mice lacked sebaceous glands within the nipple (Figure 3, e and h, respectively). KRT14 staining indicated the location of the transgene expression in KRT14-Noggin mice (Figure 3, j and k) and also the antibody labeled the newly formed hair follicles in the noggin mice. The pattern of KRT14 staining was not altered in the nipple of the transgenic mice compared to controls (Figure 3, i–l).
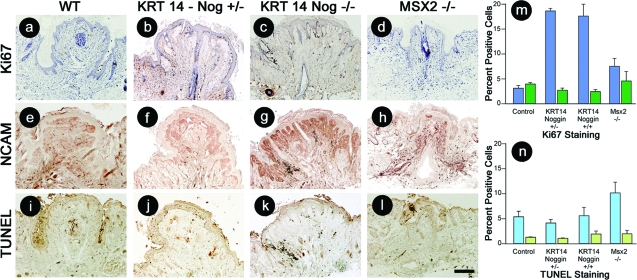
We next characterized the proliferation, differentiation, and apoptosis status of the control, Msx2−/−, and KRT14-Noggin mouse nipples. To identify cells within the proliferative cell pool, sections were immunostained for Ki-67 (Figure 4, a–d). The percentage of proliferating cells within the nipple epithelium (blue bars) and dermis (green bars) was determined (n = 3). Proliferation was increased 5.2-fold in the homozygous and heterozygous KRT14-Noggin mice epithelium compared to the WT mice. Proliferating epithelial cells were increased 2.1-fold in Msx2−/− mice compared to WT mice (Figure 4, a–d and m). Noggin has been observed previously in the hair-producing mesenchyme.11 Therefore we stained WT, Msx2−/−, and KRT14-Noggin mouse nipples with neural cell adhesion molecule (NCAM), another mesenchymal marker to detect changes that may have taken place in the nipple mesenchyme (Figure 4, e–h). NCAM expression levels were increased in the dermis immediately underlying the epithelium in the nipple of the homozygous KRT14-Noggin mice. There was less increase in the heterozygous KRT14-Noggin mice and the response appears to reflect dose dependence. Apoptosis was detected in the epithelium (blue bars) and dermis (green bars) using TUNEL staining (Figure 4, i–l). These data were quantified and demonstrated that there did not appear to be any changes in the levels of apoptotic cells in the KRT14-Noggin mice (Figure 4n). TUNEL staining was elevated in the epithelium of the Msx2−/− mice. We also stained for keratin to detect differentiation using AE13 monoclonal antibodies, but the staining in the pilosebaceous units within the nipple was weak (data not shown). We suspect that the pilosebaceous units in the nipples are not completely differentiated because the majority of the structures observed are sebaceous glands with occasional hair follicles.
Figure 4.
Immunohistochemical analysis of proliferation, differentiation, and apoptosis of adult, parous, 6- to 8-month-old WT and transgenic mouse nipples with decreased BMP activity. Control (a), KRT14-Noggin+/− (b), KRT14-Noggin+/+ (c), and Msx2−/− (d) mouse nipples stained for Ki-67 to detect proliferating cells. e–h: NCAM staining for mesenchymal cells. i–l: TUNEL staining to locate apoptotic cells. The percent Ki-67 (m)- and TUNEL (n)-positive epithelial (blue bars) and dermal (green bars) cells were quantified and the average and SD determined (n = 3). Scale bar = 200 μm.
Overexpression of Noggin Leads to Suppression of PTHrP and Ectopic Expression of Shh
We were interested in determining what molecular pathways were affected as a result of suppressing BMP pathway activity in the nipple. In situ hybridization results revealed that Noggin overexpression decreased the expression levels of PTHrP transcripts compared to controls (Figure 5, a–c). PTHrP expression does not appear to be affected in the Msx2−/− mice (Figure 5d). Shh transcripts were expressed in the epithelium of the ectopically formed hair follicles in the nipple (Figure 5, e–h). We also demonstrate that antibody labeling of phospho-smad 1/5/8, an indicator of BMP signaling, was reduced in the epithelium of the Noggin mice (Figure 5, i–l). Subsequently, the expression levels of PTHrP and Shh were altered in the nipples of these mice. The nipples of Msx2−/− mice; however, do not have hair follicles present suggesting that Msx2-mediated BMP signaling is not required to repress hair follicle formation. We demonstrate that inhibiting BMP signaling within the basal keratinocytes is permissive for an epithelial fate change that allows for the formation of hair follicles in the mouse nipple where they normally would fail to develop.
Figure 5.
Expression patterns for PTHrP, Shh, and pSmad1/5/8 in the nipples of adult, parous, 6- to 8-month-old WT, Noggin-overexpressing, and Msx2−/− mice. a–d: PTHrP expression appears to decrease in a dose-dependent manner in Noggin-overexpressing mice compared to WT and Msx2−/− mice. e–h: Noggin-overexpressing mice show an increase in epithelial Shh expression surrounding the ectopic hair follicles but no change in nipple epithelial expression compared to control and Msx2−/− mice. i–l: p-Smad1/5/8 immunostaining is reduced in the nipple epithelium and increased in the epithelium surrounding the ectopic hair follicles in Noggin-overexpressing mice compared to WT and Msx2−/− mice. Red arrows in b and c indicate areas with reduced PTHrP expression whereas black arrows in f, g, j, and k point to increased Shh surrounding ectopic hair follicles. Scale bars: 200 μm; 60 μm (inset).
PTHrP Promoter Activity Is Enhanced by BMP4
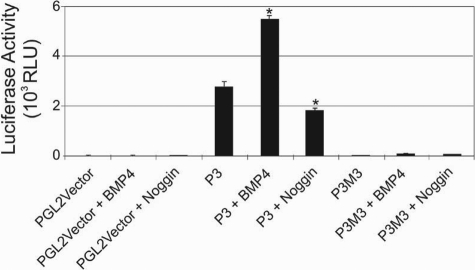
Based on in situ hybridization results our data indicated that PTHrP was down-regulated in the epidermis of the nipple of the KRT14-Noggin mice. To test whether BMP4 is directly involved in transcriptional activation of PTHrP via binding to a SMAD binding element in the promoter, we used a transient transfection assay using two human PTHrP reporter genes and immortalized human keratinocytes. The human PTHrP gene has three distinct promoters (P1 to P3) that regulate PTHrP transcription.21 The human P3 promoter is more than 90% identical to the single rodent promoter in the region of the TATA box and core activator domain containing transcription factor binding sites GAS, Ets, SP1, and SMAD binding sites.21 A WT human P3 promoter-driven luciferase construct containing 200 bp of sequence upstream of the transcription start site (P3) and a second construct that had a site directed mutation of the SMAD binding site (P3M3) were used in this assay.21,22 Reporter gene activity of the WT P3-luciferase construct was enhanced on addition of 10 nmol/L BMP4 to the culture media and was found to be statistically significant (P < 0.05). Conversely, on addition of Noggin protein to the culture media, the baseline luciferase activity of the WT P3 construct was decreased and also found to be statistically significant (P < 0.05). Results also show that mutation of the SMAD binding site in the P3M3 construct severely reduced basal construct activity, and this could not be activated by the addition of exogenous BMP4 (Figure 6).
Figure 6.
PTHrP promoter activation by the BMP pathway. Immortalized primary human keratinocytes were transfected in 24-well plates with PGL2Vector-LUC (1 μg), P3-Luciferase (1 μg), and P3M3 (1 μg) as indicated and grown in the presence or absence of BMP4 and Noggin proteins. Cell extracts were assayed for luciferase activity. Results shown are representative of three independent experiments, each performed in triplicate. A two-tailed t-test was performed on the data and was found to be statistically significant (*P < 0.05).
Discussion
Signaling Pathways Involved in Hair Follicle and Nipple Formation
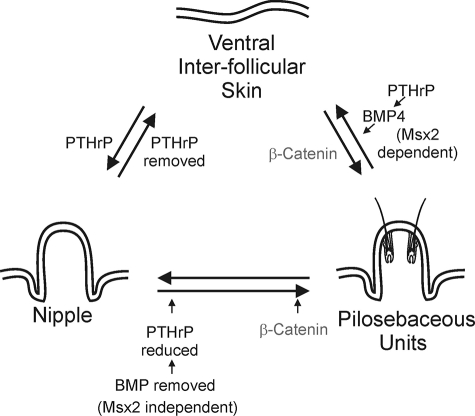
Because of regional specificity, the nipple only develops on the ventral side of a female mouse. In fact three possible fate choices are available to cells residing within the ventral skin. Ventral skin can become interfollicular skin, pilosebaceous units (hairs plus sebaceous glands), or nipples (Figure 7). This choice is primarily determined through epithelial-mesenchymal interactions.1 Each of the choices produces dramatically different morphologies and molecular expression. Here we sought to characterize some of these differences, focusing on the role of BMP signaling.
Figure 7.
Molecules regulating the fate of ventral skin. Ventral skin can form interfollicular skin, pilosebaceous units, or nipples. The choice of cell fates lies in the balance of molecular pathway activities. The BMP pathways shown at the bottom (Msx2-independent) are from this current study. Those in the top right (Msx2-dependent) are from Hens and colleagues.28 Those in the top left are from Foley and colleagues.36 Gat and colleagues42 clarified the involvement of β-catenin along the ventral interfollicular skin to pilosebaceous unit pathway (top right) whereas Miyoshi and colleagues43 identified β-catenin along the nipple to pilosebaceous unit pathway (bottom right).
We initially examined the distribution and function of Noggin in the murine nipple and surrounding hair follicle containing skin (interfollicular plus pilosebaceous containing) to test the involvement of the BMP pathway in distinguishing between these different regions of the ventral skin. Noggin is normally expressed in the hair follicle dermal papilla in dorsal skin.11,18 To our surprise its expression shifted to the epithelium within the nipple (Figure 1).
To further explore BMP’s role in nipple development, we misexpressed Noggin from a keratin 14 promoter. The KRT-14 promoter clearly misexpressed the Noggin transgene in the entire basal layer of the epithelium in these animals as previously reported18 in a region that mostly coincided with the endogenous expression pattern. Ectopic pilosebaceous unit formation including hair and sebaceous glands has been reported on the ventral side of the paw and eyelids of these KRT14-Noggin transgenic mice.18 Here we show that the transgene induces hairs to form in the nipple suggesting that inhibition of BMPs in the nipple microenvironment via KRT14-noggin expression surmounts a threshold level and drives keratinocytes toward hair follicle fate determination in the epithelium. Lineage selection by the common progenitor cells in the skin is therefore dependent on the BMP signaling pathway. Increased Noggin expression in the transgenic mice led to increased epithelial cell proliferation within the nipple consistent with the finding that BMP signaling suppresses proliferation in hair-producing epidermal cells.29 The cyclic suppression and up-regulation of BMP regulates the hair cycle producing hair waves30 by acting through BMP receptor 1a.31 In hairs, BMP apparently functions through the dermal papilla because BMPR1a-null dermal papilla cells failed to form hair follicles when combined with epithelial stem cells.32
Recent studies have shown that removal of Shh signaling in the epithelium secondarily causes increased levels of Shh expression in the mesenchyme, contributing to induction of hair follicles.33 Our results indicate that Noggin-overexpressing mice exhibit epithelial Shh expression in the abnormal hair follicles that form within the nipple. Shh expression is not altered in the overlying nipple epithelium, suggesting that the increase in Shh expression is secondary to the formation of the hair follicles in the nipple.
PTHrP signaling is required for the proper formation of the nipple. In the absence of PTHrP signaling, mammary mesenchyme fails to form and in turn does not direct the formation of nipple sheath in the overlying epidermis.5,6,34 Conversely, in the KRT14-PTHrP mice that overexpress PTHrP in basal keratinocytes, peptide signaling causes the conversion of subepidermal mesenchyme into condensed mammary mesenchyme.36 This leads to the suppression of hair follicle development and causes the entire ventral epidermis between the mammary lines to acquire characteristics of the nipple sheath.35 We found that PTHrP expression was decreased in our Noggin-overexpressing transgenic mice and that the pilosebaceous units formed within the nipple.
One way that BMPs can exert their effects is through Msx1 and Msx2, which are BMP-responsive homeodomain-containing transcription factors that participate in epithelial-mesenchymal signaling during development.19,36,37 Both Msx1 and Msx2 are important to the normal development of epithelial appendages including hair and mammary glands. In mice that are deficient for Msx1 and Msx2, mammary development fails at the placode stage.19 Msx2 alone has been detected within the dense mammary mesenchyme.19,36 Satokata and colleagues19 reported that mammary buds form in Msx2−/− mice but the development subsequently arrests at E16.5, and no ductal outgrowth is formed. Although Msx2 was previously thought to be required for the outgrowth of the mammary bud in mice,19 in our hands these mice can form normal and functional mammary ducts and nipples. Recently, Hens and colleagues28 also found that these mice are capable of forming normal mammary buds. They reported that in ventral skin PTHrP can induce BMP signaling by increasing the amount of Bmpr1a mRNA as assessed by RT-PCR and sensitizes the mammary mesenchyme to BMP4, which in turn induces Msx2 expression, potentially leading to the lateral inhibition of hair follicle formation within the developing nipple sheath.28 However, using semiquantitative RT-PCR we saw no changes in BMPR1a expression levels in the nipples of adult KRT14-Noggin or Msx2−/− mice (Figure 5). Therefore the BMP receptor is not up-regulated as a result of increasing Noggin levels or decreased because of the lack of Msx2 levels in the nipple. Furthermore, using a reporter construct in vitro we showed that BMP is capable of inducing transcription of a PTHrP reporter gene via a SMAD binding element in cultured keratinocytes (Figure 6). Mutating the SMAD binding site within the promoter region, prevents the activation of the construct, suggesting that the BMP pathway is upstream of PTHrP. Thus, the regulatory interaction between BMP and PTHrP may differ between specific regions of the ventral skin and/or between developing and adult tissue. The nipples may have additional signaling pathways in place that repress hair follicle formation. It would be interesting to see whether hair growth was induced in the nipples of the Msx2−/− mice from Hens and colleagues.28 This raises the intriguing possibility that there is a positive feedback loop whereby BMP activates PTHrP gene expression in epithelia that stimulates the PTH receptor leading to an up-regulation of the BMP receptors in the mesenchyme.
From a morphological standpoint, both nipple and pilosebaceous unit epithelium invaginate into the surrounding mesenchyme. Because they are configured differently this causes them to have a very distinct appearance. The mesenchyme of both of these organs is highly enriched in NCAM. Furthermore, we found that suppression of BMP signaling increased the expression of NCAM within the nipple. Also, both the nipple and pilosebaceous units are not at the same level as the surrounding epithelium; nipples protrude out from the skin surface and hair follicles sink into the skin.
The molecular characterization of the nipple is still at an early stage. We surmise that as more information becomes available it will help to explain these alterations in morphology. We presume that all regions of the ventral skin initially have an equal chance of becoming interfollicular skin, pilosebaceous units, and nipples given the proper microenvironments. This can be accomplished by the differential regulation of molecular networks leading to a change in equilibrium. To this end, we have summarized the existing results describing how molecular pathways affect the ventral skin (Figure 7). In this study we demonstrated that whereas hair follicles do not normally form in the nipple, expression of exogenous Noggin in the nipple epithelium suppresses BMP signaling and allows progression of pilosebaceous units. Decreases in BMP pathway activity as well as PTHrP expression may play a key role in this process. Levels of Shh in the nipple epithelium of Noggin-overexpressing mice were unchanged from controls while Shh is expressed within the ectopically formed hair follicles, preventing reversion back to nipple epithelium. This conversion of nipple epithelium to hair-forming epithelium occurs in an Msx2-independent manner.
Ectopic PTHrP expression converted ventral epithelium to nipple skin while PTHrP knockout mice form interfollicular skin.36 Increased PTHrP expression was found to increase BMP signaling in the ventral skin.28 PTHrP in conjunction with BMP increases Msx2 expression that suppresses hair follicle growth.28 The conditional perturbation of Shh signaling in the skin led to increased Shh signaling in the mesenchyme and the conversion of hair follicles into mammary gland-like structures.33
Previous studies show that the epidermis contains a population of stem cells that can proliferate and give rise to differentiating daughter cells that signal to the mesenchyme to form the various ectodermal organs including mammary glands, hair follicles, and sebaceous glands.38 Our study brings out the possibility that the epithelial stem cells in the mammary gland and nipple region can be altered to produce the formation of hair instead of hairless nipple epithelium through exposure to regulatory molecules. Our results combined with those from the literature have led us to speculate that high PTHrP activity favors the formation of nipples, whereas high levels of BMP activity promote interfollicular skin formation and high β-catenin pathway activity may lead to pilosebaceous unit formation. We are aware that PTHrP levels can vary within hair follicle producing skin from low to high depending on the hair cycle.39 PTHrP is very low in interfollicular epidermis at all times. In the hair follicle PTHrP is localized around the neck of the hair follicle but becomes very highly expressed in the outer root sheath during late anagen. Despite these discrepancies, we find it helpful to speculate that shifts in the expression of these molecules regulate ectodermal organ specificity within different skin regions.
Developments in stem cell biology and regenerative medicine are focused toward converting nonneural stem cells into specific desired ectodermal organs. Therefore, it is even more important to understand how different gene networks are wired differently in different body regions. Our work studying these transgenic mice begins to unravel some of the clues about this fundamental process that would contribute to stem cell biology.
Evolution of the Nipple and Mammary Gland
The evolution of the nipple and mammary gland is interesting and of particular importance because the development of these structures allows for improved milk delivery to the young and thus increased survival. Many species have evolved ways to feed their young with varying success. Interestingly, eggs have been proposed as an important element in mammary gland evolution.40,41 Early Synapsids, are believed to have used secretions from their glandular skin to replenish fluids to the egg shells surrounding their young, as do current amphibians such as salamanders.2 The secretions of Monotremes, on the other hand, trickled from mammary patches consisting of nippleless areolae-like skin, to a hairy coat to feed their young. Today’s platypus uses specialized mammary hairs located along two presumptive mammary lines that run along their ventral surface.2 Because altricial newborns rely more on early feedings from their mothers than their precocial counterparts, a hairless areola with a nipple was developed as an efficient means to feed the young. Mice do not exhibit an areolar complex as humans do, but merely contain nipple epithelium adjacent to normal skin. Therefore, we speculate that the evolution of mammary glands and nipples may be associated not only with the live birth of mammals but also with the molecular gain of BMP pathway activity. This would allow for specialized mammary glands and nipples to form while preventing hair formation in this region.
Acknowledgments
We thank Maksim Plikus and Jacqueline Veltmaat for helpful discussions and Dr. Robert Maxson (University of Southern California) for providing the Msx2−/− mice.
Footnotes
Address reprint requests to Randall Widelitz, Ph.D., Department of Pathology, University of Southern California, 2011 Zonal Ave., Los Angeles, CA 90033. E-mail: widelitz@usc.edu.
Supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grants AR052397 to R.B.W., AR2177 and AR47364 to C.M.C., and AR45585 to J.F.), the Department of Defense (congressionally mandated breast cancer program grant BC 044808 to J.M.), and the University of Southern California/Norris Breast Cancer Research Training Program (predoctoral fellowship to J.M.).
References
- Chuong CM. A model of the formation of epithelial appendages. Chuong CM, editor. Austin: Landes Bioscience,; Molecular Basis of Epithelial Appendage Morphogenesis. 1998:pp 3–14. [Google Scholar]
- Widelitz RB, Veltmaat JM, Mayer JA, Foley J, Chuong CM. Mammary glands and feathers: comparing two skin appendages which help define novel classes during vertebrate evolution. Semin Cell Dev Biol. 2007;18:255–266. doi: 10.1016/j.semcdb.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Hou L, Plikus M, Hughes M, Scehnet J, Suksaweang S, Widelitz R, Jiang TX, Chuong CM. Evo-Devo of amniote integuments and appendages. Int J Dev Biol. 2004;48:249–270. doi: 10.1387/ijdb.041825pw. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailleux AA, Spencer-Dene B, Dillon C, Ndiaye D, Savona-Baron C, Itoh N, Kato S, Dickson C, Thiery JP, Bellusci S. Role of FGF10/FGFR2b signaling during mammary gland development in the mouse embryo. Development. 2002;129:53–60. doi: 10.1242/dev.129.1.53. [DOI] [PubMed] [Google Scholar]
- Wysolmerski JJ, Philbrick WM, Dunbar ME, Lanske B, Kronenberg H, Broadus AE. Rescue of the parathyroid hormone-related protein knockout mouse demonstrates that parathyroid hormone-related protein is essential for mammary gland development. Development. 1998;125:1285–1294. doi: 10.1242/dev.125.7.1285. [DOI] [PubMed] [Google Scholar]
- Foley J, Dann P, Hong J, Cosgrove J, Dreyer B, Rimm D, Dunbar M, Philbrick W, Wysolmerski J. Parathyroid hormone-related protein maintains mammary epithelial fate and triggers nipple skin differentiation during embryonic breast development. Development. 2001;128:513–525. doi: 10.1242/dev.128.4.513. [DOI] [PubMed] [Google Scholar]
- Eastwood J, Offutt C, Menon K, Keel M, Hrncirova P, Novotny MV, Arnold R, Foley J. Identification of markers for nipple epidermis: changes in expression during pregnancy and lactation. Differentiation. 2007;75:75–83. doi: 10.1111/j.1432-0436.2006.00112.x. [DOI] [PubMed] [Google Scholar]
- Oshima H, Rochat A, Kedzia C, Kobayashi K, Barrandon Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell. 2001;104:233–245. doi: 10.1016/s0092-8674(01)00208-2. [DOI] [PubMed] [Google Scholar]
- Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102:451–461. doi: 10.1016/s0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Botchkareva NV, Roth W, Nakamura M, Chen LH, Herzog W, Lindner G, McMahon JA, Peters C, Lauster R, McMahon AP, Paus R. Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nat Cell Biol. 1999;1:158–164. doi: 10.1038/11078. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Botchkareva NV, Sharov AA, Funa K, Huber O, Gilchrest BA. Modulation of BMP signaling by noggin is required for induction of the secondary (nontylotrich) hair follicles. J Invest Dermatol. 2002;118:3–10. doi: 10.1046/j.1523-1747.2002.01645.x. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Matzuk MM, Gerstmayer B, Bosio A, Lauster R, Miyachi Y, Werner S, Paus R. Control of pelage hair follicle development and cycling by complex interactions between follistatin and activin. FASEB J. 2003;17:497–499. doi: 10.1096/fj.02-0247fje. [DOI] [PubMed] [Google Scholar]
- Gurdon JB, Bourillot PY. Morphogen gradient interpretation. Nature. 2001;413:797–803. doi: 10.1038/35101500. [DOI] [PubMed] [Google Scholar]
- Rosen V. BMP and BMP inhibitors in bone. Ann NY Acad Sci. 2006;1068:19–25. doi: 10.1196/annals.1346.005. [DOI] [PubMed] [Google Scholar]
- Massagué J. TGFbeta signaling: receptors, transducers, and Mad proteins. Cell. 1996;85:947–950. doi: 10.1016/s0092-8674(00)81296-9. [DOI] [PubMed] [Google Scholar]
- Massagué J, Chen YG. Controlling TGF-beta signaling. Genes Dev. 2000;14:627–644. [PubMed] [Google Scholar]
- von Bubnoff A, Cho KW. Intracellular BMP signaling regulation in vertebrates: pathway or network? Dev Biol. 2001;239:1–14. doi: 10.1006/dbio.2001.0388. [DOI] [PubMed] [Google Scholar]
- Plikus M, Wang WP, Liu J, Wang X, Jiang TX, Chuong CM. Morpho-regulation of ectodermal organs: integument pathology and phenotypic variations in K14-Noggin engineered mice through modulation of bone morphogenic protein pathway. Am J Pathol. 2004;164:1099–1114. doi: 10.1016/S0002-9440(10)63197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satokata I, Ma L, Ohshima H, Bei M, Woo I, Nishizawa K, Maeda T, Takano Y, Uchiyama M, Heaney S, Peters H, Tang Z, Maxson R, Maas R. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat Genet. 2000;24:391–395. doi: 10.1038/74231. [DOI] [PubMed] [Google Scholar]
- Sung CC, O'Toole EA, Lannutti BJ, Hunt J, O'Gorman M, Woodley DT, Paller AS. Integrin alpha 5 beta 1 expression is required for inhibition of keratinocyte migration by ganglioside GT1b. Exp Cell Res. 1998;239:311–319. doi: 10.1006/excr.1997.3897. [DOI] [PubMed] [Google Scholar]
- Cataisson C, Gordon J, Roussiere M, Abdalkhani A, Lindemann R, Dittmer J, Foley J, Bouizar Z. Ets-1 activates parathyroid hormone-related protein gene expression in tumorigenic breast epithelial cells. Mol Cell Endocrinol. 2003;204:155–168. doi: 10.1016/s0303-7207(02)00298-8. [DOI] [PubMed] [Google Scholar]
- Richard V, Nadella MV, Green PL, Lairmore MD, Feuer G, Foley JG, Rosol TJ. Transcriptional regulation of parathyroid hormone-related protein promoter P3 by ETS-1 in adult T-cell leukemia/lymphoma. Leukemia. 2005;19:1175–1183. doi: 10.1038/sj.leu.2403787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong CM, McClain DA, Streit P, Edelman GM. Neural cell adhesion molecules in rodent brains isolated by monoclonal antibodies with cross-species reactivity. Proc Natl Acad Sci USA. 1982;79:4234–4238. doi: 10.1073/pnas.79.13.4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang TX, Stott S, Widelitz RB, Chuong CM. Current methods in the study of avian skin appendages. Chuong CM, editor. Austin: Landes Bioscience,; Molecular Basis of Epithelial Appendage Morphogenesis. 1998:p 395. [Google Scholar]
- Guha U, Mecklenburg L, Cowin P, Kan L, O'Guin WM, D'Vizio D, Pestell RG, Paus R, Kessler JA. Bone morphogenetic protein signaling regulates postnatal hair follicle differentiation and cycling. Am J Pathol. 2004;165:729–740. doi: 10.1016/S0002-9440(10)63336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karperien M, Lanser P, de Laat SW, Boonstra J, Defize LH. Parathyroid hormone related peptide mRNA expression during murine postimplantation development: evidence for involvement in multiple differentiation processes. Int J Dev Biol. 1996;40:599–608. [PubMed] [Google Scholar]
- McMahon JA, Takada S, Zimmerman LB, Fan CM, Harland RM, McMahon AP. Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev. 1998;12:1438–1452. doi: 10.1101/gad.12.10.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hens JR, Dann P, Zhang JP, Harris S, Robinson GW, Wysolmerski J. BMP4 and PTHrP interact to stimulate ductal outgrowth during embryonic mammary development and to inhibit hair follicle induction. Development. 2007;134:1221–1230. doi: 10.1242/dev.000182. [DOI] [PubMed] [Google Scholar]
- Blessing M, Schirmacher P, Kaiser S. Overexpression of bone morphogenetic protein-6 (BMP-6) in the epidermis of transgenic mice: inhibition or stimulation of proliferation depending on the pattern of transgene expression and formation of psoriatic lesions. J Cell Biol. 1996;135:227–239. doi: 10.1083/jcb.135.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, Mayer JA, de la Cruz D, Baker RE, Maini PK, Maxson R, Chuong CM. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451:340–344. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobielak K, Pasolli HA, Alonso L, Polak L, Fuchs E. Defining BMP functions in the hair follicle by conditional ablation of BMP receptor IA. J Cell Biol. 2003;163:609–623. doi: 10.1083/jcb.200309042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendl M, Polak L, Fuchs E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 2008;22:543–557. doi: 10.1101/gad.1614408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritli-Linde A, Hallberg K, Harfe BD, Reyahi A, Kannius-Janson M, Nilsson J, Cobourne MT, Sharpe PT, McMahon AP, Linde A. Abnormal hair development and apparent follicular transformation to mammary gland in the absence of hedgehog signaling. Dev Cell. 2007;12:99–112. doi: 10.1016/j.devcel.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar ME, Dann PR, Robinson GW, Hennighausen L, Zhang JP, Wysolmerski JJ. Parathyroid hormone-related protein signaling is necessary for sexual dimorphism during embryonic mammary development. Development. 1999;126:3485–3493. doi: 10.1242/dev.126.16.3485. [DOI] [PubMed] [Google Scholar]
- Abdalkhani A, Sellers R, Gent J, Wulitich H, Childress S, Stein B, Boissy RE, Wysolmerski JJ, Foley J. Nipple connective tissue and its development: insights from the K14-PTHrP mouse. Mech Dev. 2002;115:63–77. doi: 10.1016/s0925-4773(02)00092-8. [DOI] [PubMed] [Google Scholar]
- Phippard DJ, Weber-Hall SJ, Sharpe PT, Naylor MS, Jayatalake H, Maas R, Woo I, Roberts-Clark D, Francis-West PH, Liu YH, Maxson R, Hill RE, Dale TC. Regulation of Msx-1, Msx-2, Bmp-2 and Bmp-4 during foetal and postnatal mammary gland development. Development. 1996;122:2729–2737. doi: 10.1242/dev.122.9.2729. [DOI] [PubMed] [Google Scholar]
- Satoh K, Ginsburg E, Vonderhaar BK. Msx-1 and Msx-2 in mammary gland development. J Mammary Gland Biol Neoplasia. 2004;9:195–205. doi: 10.1023/B:JOMG.0000037162.84758.b5. [DOI] [PubMed] [Google Scholar]
- Niemann C, Watt FM. Designer skin: lineage commitment in postnatal epidermis. Trends Cell Biol. 2002;12:185–192. doi: 10.1016/s0962-8924(02)02263-8. [DOI] [PubMed] [Google Scholar]
- Cho YM, Woodard GL, Dunbar M, Gocken T, Jimenez JA, Foley J. Hair-cycle-dependent expression of parathyroid hormone-related protein and its type I receptor: evidence for regulation at the anagen to catagen transition. J Invest Dermatol. 2003;120:715–727. doi: 10.1046/j.1523-1747.2003.12147.x. [DOI] [PubMed] [Google Scholar]
- Oftedal OT. The origin of lactation as a water source for parchment-shelled eggs. J Mammary Gland Biol Neoplasia. 2002;7:253–266. doi: 10.1023/a:1022848632125. [DOI] [PubMed] [Google Scholar]
- Oftedal OT. The mammary gland and its origin during synapsid evolution. J Mammary Gland Biol Neoplasia. 2002;7:225–252. doi: 10.1023/a:1022896515287. [DOI] [PubMed] [Google Scholar]
- Gat U, DasGupta R, Degenstein L, Fuchs E. De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- Miyoshi K, Rosner A, Nozawa M, Byrd C, Morgan F, Landesman-Bollag E, Xu X, Seldin DC, Schmidt EV, Taketo MM, Robinson GW, Cardiff RD, Hennighausen L. Activation of different Wnt/beta-catenin signaling components in mammary epithelium induces transdifferentiation and the formation of pilar tumors. Oncogene. 2002;21:5548–5556. doi: 10.1038/sj.onc.1205686. [DOI] [PubMed] [Google Scholar]