Abstract
Sjögren’s syndrome (SS) is a chronic autoimmune exocrinopathy associated with variable lymphocytic infiltration of the affected organs (primarily salivary and lacrimal glands) and broad clinical manifestations, including lymphoma development. To investigate the potential implication of Foxp3+ T-regulatory cells in the regulation of SS inflammatory responses, we studied their incidence in the minor salivary glands (MSGs) and their relationship with histopathological and clinical disease parameters. Similar percentages of infiltrating Foxp3+ cells were observed in the MSG lesions of all SS patients (n = 30) and non-SS sialadenitis controls (n = 7). Foxp3+ cells were not detected in sicca-complaining controls with negative biopsy (n = 6). In SS patients, Foxp3+ cell frequency varied according to lesion severity, with the highest and lowest frequencies obtained in intermediate and mild MSG lesions, respectively. In the peripheral blood of these patients, reverse distribution of Foxp3+ cells was observed. Furthermore, the frequency of Foxp3+ cells in the MSG lesions and peripheral blood was negatively associated (r = −0.6679, P = 0.0065). MSG-infiltrating Foxp3+ cells were found to positively correlate with biopsy focus score (P = 0.05), infiltrating mononuclear cells, dendritic cells, and macrophages (P ≤ 0.024 each), and serum C4 levels (P = 0.0328), whereas lower Foxp3+ cell incidence correlated with adverse predictors for lymphoma development, such as the presence of C4 hypocomplementemia (P = 0.012) and SG enlargement (tendency, P = 0.067). Our findings suggest that the Foxp3+ T-regulatory cell frequency in the MSG lesions of SS patients correlates with inflammation grade and certain risk factors for lymphoma development.
The establishment and maintenance of self-tolerance is regulated by complex mechanisms that include the central deletion of self-reactive T cells and the active regulation of those that escape deletion. T-regulatory cells (Tregs) play a pivotal role in immune homeostasis by suppressing the proliferation and function of effector T lymphocytes, as well as of other immunocytes.1,2,3,4 Several subsets of T cells with regulatory properties have been described.5 Among them, CD4+CD25+ T cells represent one of the most extensively studied subpopulation of Tregs. They are characterized by the expression of the forkhead/winged-helix transcription factor (Foxp3), which is a key regulator of Treg development and suppressive activity.6,7,8,9,10,11
The significance of Tregs to the immune equilibrium is revealed by emerging evidence that implicate them in almost every situation in which suppression of immune responses might be relevant, such as allergies, infections, tumor immunity, and autoimmune diseases.10,12,13,14 Although depletion and/or dysfunction of Tregs has been shown to result in severe or fatal systemic autoimmunity,7,10 their implication and/or effectiveness on the control of human autoimmune disorders is not fully understood. Reduced or elevated numbers of Tregs with enhanced, decreased, or unaffected suppressive capacity have been reported at the affected tissues of patients with rheumatoid arthritis, inflammatory bowel disease, psoriasis, and primary biliary cirrhosis.13,15,16,17,18,19 The factors that mediate the differentiation and/or accumulation of Tregs at the site of inflammation continue to be dissected and are thought to include a conducive cytokine milieu and favorable interactions with dendritic cells (DCs).20,21,22
Sjögren’s syndrome (SS) is a rather common, chronic autoimmune exocrinopathy (predominantly of the salivary and lacrimal glands) with features extending from organ-specific to systemic autoimmunity.23 The destruction of the glandular tissue is associated with lymphocytic infiltrates that tend to develop around ducts and extend from mild to advanced lesions with concomitant loss of tissue architecture. The lymphocytic infiltrates mainly consist of activated T and B cells, whereas classical antigen-presenting cells (macrophages and DCs) are primarily observed in heavy infiltrates and their frequency is associated with the severity of the autoimmune lesions.24,25,26 In addition, intense salivary gland inflammation has been associated with the presence of extraglandular systemic manifestations in SS, suggesting that these patients constitute a distinct subgroup with more severe disease and autoimmune responses.27 Despite extensive studies, the etiopathogenic factors that lead to the loss of the immune balance and the massive infiltration of the exocrine glands in SS are unknown. Incessant activation, defective regulation, and/or inherent defects of the immune system might participate. In this context, Tregs could be implicated in SS pathogenesis, and their role is of great interest.
Herein, we sought to explore the implication of Tregs in the regulation of the inflammatory infiltrates of the minor salivary glands (MSGs) of SS patients. The low number of infiltrating cells hampered the study of the infiltrating Treg function. Thus, our study was focused on the evaluation of the incidence of Foxp3+ Tregs in the MSG inflammatory lesions and their relationship with the grade of infiltration and certain clinical disease parameters, which are considered as predictors for lymphoma development.
Materials and Methods
Patients and MSG Biopsies
MSG biopsies were obtained with informed consent from 43 individuals undergoing diagnostic evaluation for sicca symptoms indicative of SS, as approved by the Ethics Committee of the School of Medicine, National University of Athens, Athens, Greece. Thirty primary SS patients (all women), diagnosed according to the revised American-European classification criteria,28 were included in the study. Based on the Tarpley and colleagues29 MSG biopsy score, SS samples were categorized in three groups. The first group (SS-I, n = 10) included specimens with 1+ biopsy score, the second (SS-II, n = 10) samples with 2+ score, and the third (SS-III, n = 10) biopsies of 3+ or 4+ score. In all SS patients, the biopsy focus score (lymphocytic foci/4 mm2 of tissue) was ≥1. None of the SS patients had evidence of lymphoma, sarcoidosis, or infection by hepatitis B, hepatitis C, or human immunodeficiency virus. The control group (n = 13) consisted of six individuals (four women and two men) complaining of sicca symptoms who did not fulfill the aforementioned SS criteria and had negative biopsy focus scores (less than one foci/4 mm2), as well as of seven patients with non-SS sialadenitis (five women and two men), including three that suffered from sarcoidosis and four with viral-related sialadenitis (three HCV+ and one HIV+ patients). The inflammatory MSG lesions were mild (Tarpley score, 1+) in five of the sialadenitis controls, whereas intermediate (Tarpley score, 2+) and advanced (Tarpley score, 3+) infiltrates were noticed in one each. The characteristics of the individuals included in the study are summarized in Table 1. None of the patients studied had received any glucocorticoid and/or immunosuppressive drug treatment until biopsy performance. The files of SS patients were retrospectively evaluated for various clinical and serological parameters, including persistent SG enlargement, palpable purpura, C3- and/or C4-hypocomplementemia and cryoglobulinemia that are considered as adverse prognostic factors for lymphoma development and increased mortality (Table 1).30,31,32,33 Certain manifestations indicative of systemic disease, such as palpable purpura, peripheral neuropathy, pulmonary involvement, and cryoglobulinemia were exclusively observed in patients of the SS-III subgroup (advanced MSG lesions). Furthermore, compared to the SS-I and SS-II subgroups, SS-III patients displayed higher incidence of persistent SG enlargement and lower disease duration (Table 1).
Table 1.
Characteristics of the Individuals Included in the Study
| Features | SS patients
|
Control patients
|
||||
|---|---|---|---|---|---|---|
| Total (n = 30) | SS-I (n = 10) | SS-II (n = 10) | SS-III (n = 10) | Sialadenitis (n = 7) | Sicca-complaining (n = 6) | |
| Age (years), median (range) | 53 (32 to 68) | 32 (27 to 66) | 58 (51 to 65) | 55 (32 to 68) | 68 (38 to 72) | 60 (44 to 75) |
| No of SS criteria fulfilled, median (range) | 4 (4 to 6) | 4 (4 to 4) | 5 (4 to 5) | 5 (4 to 6) | 1.5 (1 to 3) | 0.5 (0 to 3) |
| Mean duration (years) of sicca symptoms, median (range) | 3 (0 to 13) | 4 (1.5 to 8) | 3 (2 to 11) | 1.75 (0 to 13) | 7.5 (0 to 15) | 0.5 (0.5 to 0.5) |
| MSG biopsy focus score (number of lymphocytic foci per 4 mm2), median (range) | 3.38 (1.00 to 10.00) | 1.25 (1.00 to 1.44) | 3.21 (2.20 to 3.82) | 6.17 (4.21 to 10.00) | 1.07 (0.80 to 4.40) | 0.09 (0.00 to 0.42) |
| Tarpley MSG biopsy score, median (range) | 2 (1 to 4) | 1 (1 to 1) | 2 (2 to 2) | 3 (3 to 4) | 1 (1 to 3) | 0 (0 to 0) |
| Anti-Ro(SSA)-positive (%) | 63.3 | 40.0 | 80.0 | 70.0 | 0.0 | 0.0 |
| Anti-La(SSA)-positive (%) | 33.3 | 20.0 | 50.0 | 30.0 | 0.0 | 0.0 |
| C3-hypocomplementemia (%) | 6.7 | 10.0 | 0.0 | 10.0 | 0.0 | 0.0 |
| C4-hypocomplementemia (%) | 43.3 | 40.0 | 40.0 | 50.0 | 50.0 | 0.0 |
| Leukopenia (%) | 10.0 | 10.0 | 20.0 | 0.0 | 57.1 | 0.0 |
| Cryoglobulinemia (%) | 20.0 | 0.0 | 0.0 | 60.0 | 0.0 | 0.0 |
| SG enlargement (SGE) (%) | 40.0 | 10.0 | 30.0 | 80.0 | 0.0 | 0.0 |
| Palpable purpura (%) | 13.3 | 0.0 | 0.0 | 40.0 | 0.0 | 0.0 |
| Raynaud’s phenomenon (%) | 40.0 | 40.0 | 10.0 | 30.0 | 0.0 | 0.0 |
| Peripheral neuropathy (%) | 0.0 | 0.0 | 0.0 | 20.0 | 0.0 | 0.0 |
| Pulmonary involvement (%) | 0.0 | 0.0 | 0.0 | 30.0 | 14.3 | 0.0 |
| Renal involvement (%) | 0.0 | 0.0 | 0.0 | 10.0 | 0.0 | 0.0 |
SS- I, -II, and -III: SS patients with mild, intermediate, and advanced MSG lesions, respectively.
Primary Antibodies
Mouse monoclonal antibodies (mAbs) to human Foxp3 (236A/E7) and fascin (SPM133) were purchased from Abcam (Cambridge, UK). mAbs against CD68 (PG-M1), CD20 (L26), and CD4 (OPD4), as well as rabbit polyclonal antibodies to S100 and CD3 were from DAKO (Glostrup, Denmark). APC- and PE-conjugated mAbs to human CD4 (SK3), Foxp3 (259D/C7), and CD127 (hIL-7R-M21) were from Becton-Dickinson (San Jose, CA). Fluorescein isothiocyanate-conjugated mAb against human CD25 (IL-2Ra, 7G7B6) was from Ancell (Bayport, MN).
Immunohistochemical Analysis of the Incidence of Various Types of Infiltrating Cells at MSG Lesions
Serial sections of paraffin-embedded MSG tissues were immunohistochemically analyzed for the presence of Tregs, T lymphocytes, B cells, macrophages, follicular- and interdigitating DCs by antibodies to the Foxp3-, CD3-, CD20-, CD68-, fascin-, and S100-specific markers, respectively. The identification of macrophages and DCs was based in both positive staining and typical morphology. A standard immunoperoxidase technique using the EnVision system (DAKO) was used.34 Where appropriate, double staining by the EnVision double-stain system (DAKO) was applied, according to the manufacturer’s instructions. Tris-buffered saline buffer supplemented with 10% normal nonimmune fetal bovine serum and 0.1% Triton X, as well as 0.5% H2O2 in methanol were used to block nonspecific antibody binding and endogenous peroxidase activity, respectively. Negative-control staining was performed by replacing primary with irrelevant isotype-matched antibodies. Positively-stained cells were counted field-by-field in each section (consisted of at least four MSG lobules) by two independent observers (M.I.C., E.K.K.). The positively-stained populations of infiltrating mononuclear cells that display high density, such as CD3+ T cells and CD20+ B cells, were manually counted using the Cell Counter plugin of the ImageJ software (http://rsb.info.nih.gov/ij, National Institutes of Health, Bethesda, MD) on serial images of the entire section, acquired by a computerized image acquisition system (ProgRes-CapturePro software; Jenoptik-Laser, Optik-Systeme, Jena, Germany) connected to an Axioskop-40 microscope (Carl Zeiss, Thornwood, NY) at ×10 objective magnification. The number of total infiltrating mononuclear cells in each section was automatically estimated by the ITCN plugin (which evaluates nuclei) of the ImageJ software on serial images of the entire section.
Analysis of Treg Frequency in the Peripheral Blood
Peripheral blood mononuclear cells from 15 patients of the SS cohort with mild, intermediate, and advanced MSG lesions (six, five, and four, respectively), and 7 healthy donors were isolated by density gradient centrifugation on Ficoll/Paque (Amersham-Pharmacia Biotech, Uppsala, Sweden). In SS patients, peripheral blood was obtained at the time of MSG biopsy performance. Treg incidence was estimated by flow cytometric analysis of the CD4+Foxp3+ and CD4+CD25+CD127low-Treg population in a FACSCalibur flow cytometer using fluorescence-conjugated antibodies and standard techniques, as described previously.35,36
Statistical Analyses
Statistical analyses were performed by Mann-Whitney or Spearman’s rank correlation tests, using the GraphPad Prism 4.0 software (GraphPad Software, San Diego, CA). Correlations of histopathological parameters with the presence or absence of clinical and serological manifestations were investigated by Mann-Whitney test, whereas the small population size did not permit the application of multivariate analyses of clinical correlations. Only the statistically significant differences are reported.
Results
The Infiltration by Foxp3+ Tregs Is Associated with the Grade of the MSG Inflammatory Lesions
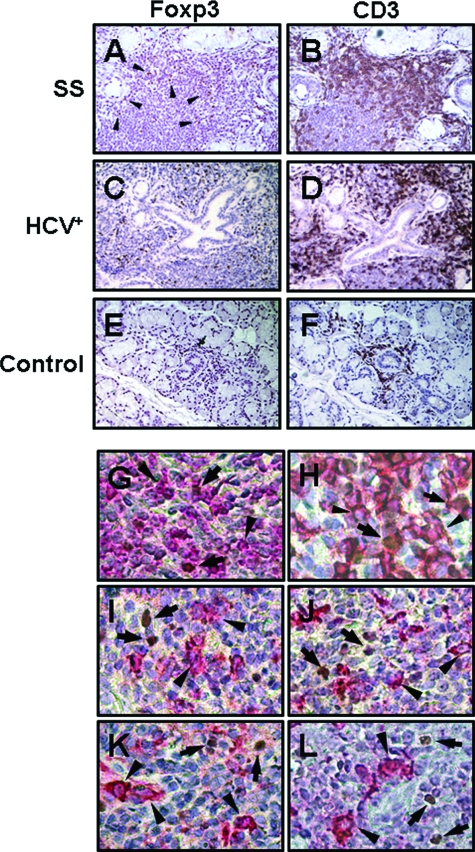
Foxp3+ cells displaying the typical nuclear staining were readily identified among infiltrating mononuclear cells in MSG tissues obtained from SS patients and non-SS sialadenitis controls (sarcoidosis- and virus-related), but not in sicca-complaining controls that did not fulfill the SS classification criteria (possibly because of the low number of infiltrating mononuclear cells) (Figure 1, A–F). Staining of serial sections revealed that Foxp3+ Tregs are solely detected in the areas of CD3+ T cells (Figure 1, A–F). Double staining confirmed that Foxp3+ cells bear the T-cell-specific CD3 and the CD4 phenotype, whereas they were negative for molecules characterizing B cells, macrophages, or DCs (such as CD20, CD68, or fascin and S100, respectively) (Figure 1, G–L). In preliminary experiments, the numbers of infiltrating of Foxp3+ cells were not found to be affected by the tissue section level, since similar counts were observed in distant sections (differing at least 150 μm) (data not shown).
Figure 1.
Immunohistochemical stainings of paraffin-embedded MSG biopsies that were obtained from one patient with SS (A, B), non-SS sialadenitis (related to infection by HCV, designated as HCV+) (C, D), and a sicca-complaining control (control) with negative biopsy (E, F). Foxp3+ cells displaying the typical nuclear staining (A, C, E) were solely detected among the infiltrating mononuclear cells and were located at the areas of CD3+ T cells (B, D, F). Double stainings revealed that Foxp3+ cells (brown, indicated by arrows) were also positive for the T-cell-specific CD3 (G) and the CD4 (H) antigens (red, arrowheads), but not the B-cell marker CD20 (I), the macrophage-specific CD68 molecule (J), or fascin (K) and S100 (L) proteins that characterize follicular and interdigitating DCs, respectively (red-stained cells, designated by arrowheads). Representative examples are shown. Original magnifications: ×100 (A–F); ×400 (G–L).
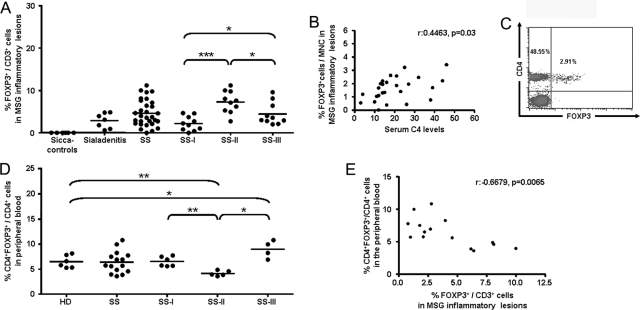
Similar levels of infiltrating Foxp3+ cells were observed at the MSG lesions of SS patients and sialadenitis controls (Table 2). Mann-Whitney test showed that the number of Foxp3+ cells per mm2 of tissue was significantly increased in intermediate and severe lesions (SS-II and SS-III groups, respectively), compared to mild infiltrates (SS-I, Table 2), whereas Spearman’s analysis revealed a strong correlation with the MSG biopsy focus score (number of lymphocytic foci/4 mm2; r = 0.7191, P < 0.0001). However, this finding was anticipated and of rather low informative value for the understanding of Treg distribution at the MSG lesions, because the increased Treg number can be attributed to the higher levels of mononuclear cells (MNCs) that infiltrate tissues with intermediate or advanced lesions (compared to mild ones). Furthermore, the microscopic evaluation of the samples indicated that the frequency of Foxp3+ cells varied among SS specimens with distinct degree of lymphocytic infiltration (Figure 2). Thus, the infiltrating Foxp3+ cells were expressed as percentage of total infiltrating MNCs, which also showed that the higher incidence of Foxp3+ cells is observed in intermediate (SS-II) MSG lesions (Table 2). Between-group Mann-Whitney analysis revealed that the differences obtained between the SS-I (mild) and SS-II (intermediate), as well as the SS-II and SS-III (severe) subgroups are significant (Table 2). T-cell incidence in the MSG infiltrates of SS patients varies according to lesion severity. Indeed, the mean percentage ± SE of CD3+ T cells/MNCs was found 64.73 ± 4.26, 32.91 ± 2.31, and 28.15 ± 3.91 in the SS-I, SS-II, and SS-III subgroups, respectively. Hence, to exclude that the distinct Foxp3+ cell distribution in the SS subgroups owes to T-cell variation, Foxp3+ cells were expressed as a percentage of CD3+ cells. In line with the Foxp3+/MNC percentage, the distribution pattern of Foxp3+/CD3+ cells significantly varied among SS subgroups. The highest percentage of Foxp3+/CD3+ cells was detected in MSG tissues with intermediate infiltrates, whereas the lowest in mild lesions (Table 2 and Figure 3A). Notably, the frequency of Foxp3+ cells was significantly decreased in advanced lesions, compared to intermediate ones, suggesting that the reduced Treg incidence might be implicated in advanced immune deregulation. The differences of the Foxp3+/CD3+ cell percentages among the three SS subgroups were statistically significant, as indicated by Mann-Whitney analysis (Figure 3A).
Table 2.
Infiltration by Foxp3+ Cells (Mean Values ± SE) in the MSG Tissues Obtained from SS Patients or Sialadenitis Controls
| Infiltration by Foxp3+ cells (expressed as) | Sjögren’s syndrome
|
Sialadenitis
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 30) | SS-I (n = 10) | SS-II (n = 10) | SS-III (n = 10) | Statistical significance (P)
|
Total (n = 7) | I (n = 5) | II (n = 1) | III (n = 1) | |||
| SS-I versus SS-II | SS-II versus SS-III | SS-I versus SS-III | |||||||||
| Cell number/mm2-tissue | 21.05 ± 3.59 | 2.52 ± 1.02 | 22.59 ± 3.50 | 31.17 ± 4.70 | < 0.0001 | NS | < 0.0001 | 19.08 ± 10.59 | 6.31 ± 2.67 | NA | NA |
| Percentage of MNC | 1.79 ± 0.25 | 1.34 ± 0.37 | 2.35 ± 0.28 | 1.26 ± 0.19 | 0.044 | 0.01 | NS | 1.72 ± 0.66 | 1.16 ± 0.28 | NA | NA |
| Percentage of CD3+-T cells | 4.64 ± 0.57 | 2.19 ± 0.53 | 7.28 ± 0.83 | 4.45 ± 0.84 | 0.0001 | 0.04 | 0.05 | 2.85 ± 0.67 | 2.06 ± 0.64 | NA | NA |
Statistical analyses were performed by Mann-Whitney test.
NA, not applicable; NS, not significant.
The subgroups I, II, and III of patients with SS and sialadenitis correspond to patients with mild, intermediate, and advanced MSG lesions, respectively.
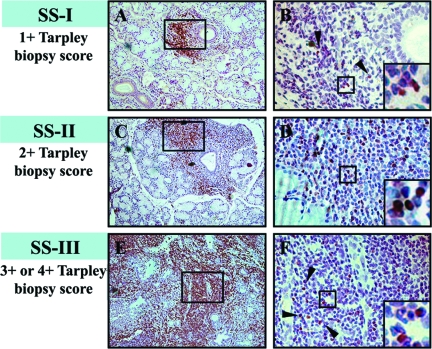
Figure 2.
Representative examples of MSG tissues that belong to the three SS subgroups, as these classified by the grade of inflammatory lesion (SS-I, mild; SS-II, intermediate; and SS-III, advanced MSG lesions). A, C, and E: Tissues stained by anti-CD3 antibody, which reveal the areas of T cells. B, D, and F: Detection of Foxp3+ cells (shown by arrowheads) in a serial section corresponding to the tissue area marked by square in A, C, and E, respectively. The figures that are incorporated in B, D, and F represent detail of the squared area, showing the typical nuclear staining of Foxp3+ cells. Differential incidence of Foxp3+-stained cells among the three SS subgroups is readily detected and cannot be attributed to the lack or decreased numbers of T cells, as shown in the respective panels presenting CD3+ T-cell distribution (A, C, E). Original magnifications: ×100 (A, C, E); ×200 (B, D, F); ×400 (insets B, D, F).
Figure 3.
A: Plot indicating the distribution of infiltrating Foxp3+/CD3+ cells at the MSG inflammatory lesions of sicca-complaining controls with negative biopsy (designated as sicca-controls), non-SS sialadenitis controls (sialadenitis), SS patients (SS), and SS subgroups, as these classified by the grade of MSG autoimmune lesions (SS-I, SS-II, and SS-III: mild, intermediate, and advanced MSG lesions, respectively). Foxp3+/CD3+ cell percentage was not found to statistically differ between the SS cohort and sialadenitis-controls, whereas Foxp3+ cells were not detected in sicca-controls. Mann-Whitney test revealed that Foxp3+/CD3+ cell distribution varied significantly among the three SS subgroups, with the highest observed at the group with the intermediate grade of infiltration (SS-II) and the lowest at the group with mild lesions (SS-I). P values are designated by asterisks (*P < 0.05, ***P < 0.0001). Horizontal bars represent the mean value of the group. B: Plot showing the Spearman’s rank correlation analysis between the percentages of Foxp3+ cells to total infiltrating MNCs in the MSG inflammatory lesions and the serum C4 levels of SS patients (n = 26). C: Representative density plot presenting the flow cytometric analysis of the CD4+Foxp3+ cells in the peripheral blood of an SS patient, using a fluorescein isothiocyanate-conjugated anti-CD4 and a PE-conjugated anti-Foxp3 antibody. For analyses, CD4+Foxp3+ cells were expressed as a percentage of total CD4+ cells. D: Plot indicating the percentage of CD4+Foxp3+ cells to CD4+ T cells in the peripheral blood of healthy donors (HDs) and SS patients (SS), as well as SS subgroups (SS-I, SS-II, and SS-III), analyzed by flow cytometry. Mann-Whitney analyses revealed that similar distribution of CD4+Foxp3+ cells is observed in healthy donors and SS patients. The percentages of CD4+Foxp3+ cells were found to significantly vary among the three SS subgroups. The lowest percentages are observed in the subgroup with intermediate MSG lesions (SS-II), whereas the highest in patients with advanced MSG lesions (SS-III). P values are designated by asterisks (*P < 0.05, **P < 0.01). Horizontal bars represent the group mean. E: Plot displaying the significant negative correlation (r = −0.6679, P = 0.0065) between the incidence of Foxp3+ cells in the peripheral blood (expressed as percentage of CD4+ cells) and the autoimmune MSG lesions (expressed as a percentage of CD3+ cells). Analysis was performed by Spearman’s rank correlation test.
Correlation of Foxp3+ Tregs with Histopathological and Clinical Parameters
Spearman’s rank correlation test revealed that the levels of infiltrating Foxp3+ cells positively correlated with the MSG biopsy focus score (Foxp3+/mm2: r = 0.7191, P < 0.0001 and Foxp3+/CD3+ percentage: r = 0.3599, P = 0.05). Moreover, strong correlations were obtained between the number of Foxp3+ cells and total infiltrating MNCs, CD3+ T cells, CD20+ B lymphocytes, fascin+-follicular DCs, S100+-interdigitating DCs, or CD68+ macrophages (Table 3). Analysis of each SS subgroup separately revealed a rather intriguing finding. The correlation of Foxp3+ cells to total infiltrating MNCs in the mild and intermediate lesions were strongly significant and comparable, whereas in advanced lesions became insignificant (Table 3). However, this inconsistency resulted in the slight reduction of the regression in the entire SS group and it was not evident, possibly due to of regularization by the SS-I and SS-II subgroups’ values (Table 3). Similar correlation patterns between Foxp3+ cells and the other types of infiltrating MNCs were found to operate in non-SS sialadenitis controls; nevertheless, the significance rates were lower, possibly because of the low number of specimens (Table 3).
Table 3.
Correlation of Foxp3+ Tregs with Various Histopathological Parameters as Analyzed by Spearman’s Rank Regression Test
| Histopathological parameters | Foxp3+ Tregs
|
Mononuclear cells
|
||||||
|---|---|---|---|---|---|---|---|---|
| Sjögren’s syndrome
|
Sialadenitis
|
Sjögren’s syndrome
|
Sialadenitis
|
|||||
| Regression | P value | Regression | P value | Regression | P value | Regression | P value | |
| Mononuclear cells | 0.90* | <0.0001 | 0.96 | 0.0028 | ||||
| CD3+ T cells | 0.80 | <0.0001 | 0.96 | 0.0028 | 0.90 | <0.0001 | 0.93 | 0.0067 |
| CD20+ B cells | 0.85 | <0.0001 | 0.96 | 0.0028 | 0.98 | <0.0001 | 0.93 | 0.0067 |
| Fascin+ follicular DCs | 0.90 | <0.0001 | 0.93 | 0.0067 | 0.84 | <0.0001 | 0.96 | 0.0028 |
| S100+ interdigitating DCs | 0.53 | 0.0237 | 0.75 | 0.0663 | 0.75 | <0.0001 | 0.79 | 0.0480 |
| CD68+ MΦ | 0.86 | <0.0001 | 0.86 | 0.0238 | 0.87 | <0.0001 | 0.82 | 0.0341 |
SS-I and SS-II, 0.94, P < 0.0001; SS-III, not significant.
SS-I and SS-II, SS patients with mild and intermediate MSG lesions; and SS-III, SS patients with advanced MSG lesions.
The incidence of Foxp3+ cells (expressed as percentage of total infiltrating MNCs) correlated with serum C4 levels (r = 0.4463, P = 0.03; Spearman’s rank correlation test) (Figure 3B). In addition, as revealed by Mann- Whitney analyses, the occurrence of C4-hypocomplementemia correlated with lower Foxp3+/MNC percentages at the MSG infiltrates (1.23 ± 0.17 in patients with versus 2.47 ± 0.41 patients without C4-hypocomplementemia, P = 0.012), whereas the presence of persistent SG enlargement tended to associate with reduced Foxp3+ cell incidence (1.34 ± 0.49 in patients with versus 1.91 ± 0.28 patients without, P = 0.067). Foxp3+ cells were not found to correlate with the formation of structures resembling germinal centers, as well as with other clinical and serological parameters tested.
Tregs Incidence in the Peripheral Blood of SS Patients and Controls
The incidence of CD4+Foxp3+ cells (expressed as a percentage of CD4+ cells) in the peripheral blood was analyzed by flow cytometry (Figure 3C). Mann-Whitney analysis showed that the levels of CD4+Foxp3+ cells in the peripheral blood of SS patients and healthy individuals were comparable (mean percentage of CD4+Foxp3+ cells to CD4+ cells ± SE: 6.36 ± 0.57 and 6.48 ± 0.51 in SS patients and healthy donors, respectively). Within-group Mann-Whitney analysis of the SS patients revealed significantly different distribution of the peripheral blood Foxp3+ cells in three SS subgroups (6.51 ± 0.40, 4.09 ± 0.20, and 8.97 ± 0.87 in patients classified in the SS-I, SS-II, and SS-III subgroups, respectively) (Figure 3D). Similar findings were obtained from the analysis of CD4+CD25+CD127low Treg population in the peripheral blood of the SS patients and healthy controls. The mean percentage ± SE was 6.21 ± 0.63 and 5.80 ± 0.32 in SS patients and controls, respectively, and 5.72 ± 0.26, 3.98 ± 0.25, and 9.73 ± 0.67 in SS-I, SS-II, and SS-III subgroups, respectively. The comparison of the distribution of Foxp3+ cells at the MSG lesions (Figure 3A) and the peripheral blood (Figure 3D) indicate that reverse distribution of Foxp3+ cells occurs in the affected tissue and the periphery, which is further supported by the significant negative correlation between the Foxp3+ cell incidence at the MSG lesions and the periphery (r = −0.6679, P = 0.0065), as analyzed by Spearman’s test (Figure 3E).
Discussion
Foxp3+ Tregs are considered to play an important role in the control of autoimmunity. In this study, we showed that Foxp3+ Tregs are present at the MSG lesions of SS patients and sialadenitis controls, but not in sicca-complaining controls with negative MSG biopsy score. In contrast with a previous study, reporting the detection of Foxp3+ cells at the MSG inflammatory lesions of 5 of 30 SS patients examined,37 our findings indicate that Foxp3+ cells are present in the SS autoimmune lesions. Moreover, the similar patterns of Foxp3+ Treg distribution in the MSG inflammatory lesions of SS patients and sialadenitis controls most likely suggest that the number of Foxp3+ cells is not defective in SS. The levels of infiltrating Foxp3+ cells was found to significantly differ among MSG tissues with mild, intermediate, or advanced SS inflammatory lesions. Furthermore, the number of infiltrating Foxp3+ cells was positively correlated to the MSG biopsy focus score (number of lymphocytic foci/4 mm2), the number of total, as well as of certain subpopulations of infiltrating mononuclear cells. These findings strongly suggest that the occurrence of Foxp3+ Tregs at MSG tissues is associated with the grade of the autoimmune lesion. This is in accordance with studies in other autoimmune disorders, which support that, compared to peripheral blood, the frequency of Tregs at the site of inflammation is elevated.17,18,19
Interestingly, Foxp3+ cells were not found to correlate to the infiltrating mononuclear cells in the SS subgroup with advanced MSG infiltrates. In these patients, the majority of the glandular tissue is destroyed, the infiltrating cells dominate, the proportion of B cells is increased, and aberrant immune activation is thought to occur.27 Furthermore, these patients probably constitute a distinct SS subgroup, characterized by severe disease with systemic features and expression of adverse prognostic factors for lymphoma development and increased mortality, such as persistent SG enlargement, cryoglobulinemia, and palpable purpura (Table 1).27,30,31,32,33 It is rather appealing to speculate that the lower percentages of Foxp3+ Tregs in advanced SS inflammatory lesions are translated to inability of efficient control of local immune responses, thus resulting in the ultimate loss of immune balance and systemic disease. Intriguingly, the percentage of infiltrating Foxp3+ cells was found to correlate with serum C4 levels, whereas lower Foxp3+ cell incidence at the MSG inflammatory lesions was found to correlate to C4-hypocomplementemia and possibly to persistent SG enlargement. These correlations may imply a cause-and-effect relationship; however, irrelevant phenomena driven by common disease processes might also participate.
Comparative analyses revealed that the lower incidence of Tregs is observed in the peripheral blood of SS-II patients (intermediate MSG infiltrates), which present the higher percentages of infiltrating Foxp3+ cells at the MSG lesions. In addition, the percentage of Foxp3+/CD3+ cells at the MSG lesions was negatively correlated to the Foxp3+ cell incidence (percentage of CD4+ cells) in the peripheral blood. These findings suggest a reverse regulation of Tregs in the periphery and the affected tissue and possibly indicate that Tregs outflow from the circulation and accumulate at the inflamed tissue. In patients with advanced MSG lesions (SS-III), high percentages of Foxp3+ cells are detected in the peripheral blood, possibly signifying the systemic features of the disease in these patients.
The mechanisms that mediate the recruitment, differentiation, and/or expansion of Tregs at the site of inflammation continue to be dissected. The conducive cytokine milieu and favorable interactions with DCs are thought to participate.21,22 In addition, the ability of macrophages to modulate the suppressive capacity of Tregs has been reported.22 At the SS autoimmune lesions, Foxp3+ Tregs were found to highly correlate with the infiltration by DCs and macrophages. It would be tempting to presume that the strong correlation observed is indicative of the implication of infiltrating DCs and macrophages in the accumulation and/or differentiation of Tregs at the site of SS inflammatory responses. However, since the number of infiltrating DCs and macrophages strongly correlates to the degree of SS inflammation, we should not omit the possibility this association to be coincidental and attributed to the lesion severity. Furthermore, the factors implicated in the reduced incidence of Tregs in advanced SS autoimmune lesions need to be clarified. Unfavorable conditions for Treg recruitment, differentiation, expansion, and/or survival might operate.
Unfortunately, the low number of Tregs that infiltrate MSG tissues hampers the study of their functional properties. As reported previously, the functional properties of CD4+CD25high in the peripheral blood of SS patients are not impaired.38 Studies in other autoimmune or inflammatory diseases tend to support that effective Tregs accumulate at the site of inflammation.17,18,19,20,39,40 Nevertheless, recent studies suggest reduced suppressive activity of Foxp3+ Tregs at the inflamed tissue, a feature regulated by the cytokine milieu.41
This study shows the occurrence of Tregs at the SS inflammatory lesions and presents evidence for their distinct distribution according to lesion severity. Although further studies are needed to elucidate the role of Tregs in the modulation of SS autoimmune responses, the mechanisms that operate their recruitment, differentiation, and/or expansion at the site of inflammation, as well as their interplay with other types of infiltrating cells, their presence at the MSG inflammatory lesions suggests an immunoregulatory role.
Acknowledgments
We thank Spyros Paikos, D.D.S., for obtaining MSG biopsies; Stamatis Pagakis, Ph.D., for his help and training on ImageJ software; and Efstathia Bourazopoulou, B.Sc., and Ioannis A. Tsonis, M.D., for their assistance.
Footnotes
Address reprint requests to H.M. Moutsopoulos, M.D., F.A.C.P., F.R.C.P., Department of Pathophysiology, School of Medicine, National University of Athens, 75 Mikras Asias St., Athens 11527, Greece. E-mail: hmoutsop@med.uoa.gr.
Supported by the Hellenic Secretariat for Research and Technology and the Lilian Voudouri Foundation.
M.I.C. and E.K.K. contributed equally to the study.
References
- Schwartz RH. Natural regulatory T cells and self-tolerance. Nat Immunol. 2005;6:327–330. doi: 10.1038/ni1184. [DOI] [PubMed] [Google Scholar]
- Taams LS, van Amelsfort JM, Tiemessen MM, Jacobs KM, de Jong EC, Akbar AN, Bijlsma JW, Lafeber FP. Modulation of monocyte/macrophage function by human CD4+CD25+ regulatory T cells. Hum Immunol. 2005;66:222–230. doi: 10.1016/j.humimm.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338–344. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- Wahl SM, Wen J, Moutsopoulos NM. The kiss of death: interrupted by NK-cell close encounters of another kind. Trends Immunol. 2006;27:161–164. doi: 10.1016/j.it.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6:353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- Lan RY, Ansari AA, Lian ZX, Gershwin ME. Regulatory T cells: development, function and role in autoimmunity. Autoimmun Rev. 2005;4:351–363. doi: 10.1016/j.autrev.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Wraith DC, Nicolson KS, Whitley NT. Regulatory CD4+ T cells and the control of autoimmune disease. Curr Opin Immunol. 2004;16:695–701. doi: 10.1016/j.coi.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Lan RY, Cheng C, Lian ZX, Tsuneyama K, Yang GX, Moritoki Y, Chuang YH, Nakamura T, Saito S, Shimoda S, Tanaka A, Bowlus CL, Takano Y, Ansari AA, Coppel RL, Gershwin ME. Liver-targeted and peripheral blood alterations of regulatory T cells in primary biliary cirrhosis. Hepatology. 2006;43:729–737. doi: 10.1002/hep.21123. [DOI] [PubMed] [Google Scholar]
- Sugiyama H, Gyulai R, Toichi E, Garaczi E, Shimada S, Stevens SR, McCormick TS, Cooper KD. Dysfunctional blood and target tissue CD4+CD25high regulatory T cells in psoriasis: mechanism underlying unrestrained pathogenic effector T cell proliferation. J Immunol. 2005;174:164–173. doi: 10.4049/jimmunol.174.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004;50:2775–2785. doi: 10.1002/art.20499. [DOI] [PubMed] [Google Scholar]
- Maul J, Loddenkemper C, Mundt P, Berg E, Giese T, Stallmach A, Zeitz M, Duchmann R. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868–1878. doi: 10.1053/j.gastro.2005.03.043. [DOI] [PubMed] [Google Scholar]
- Möttönen M, Heikkinen J, Mustonen L, Isomaki P, Luukkainen R, Lassila O. CD4+ CD25+ T cells with the phenotypic and functional characteristics of regulatory T cells are enriched in the synovial fluid of patients with rheumatoid arthritis. Clin Exp Immunol. 2005;140:360–367. doi: 10.1111/j.1365-2249.2005.02754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Backstrom BT, Sobel RA, Wucherpfennig KW, Strom TB, Oukka M, Kuchroo VK. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluestone JA, Tang Q. How do CD4+CD25+ regulatory T cells control autoimmunity? Curr Opin Immunol. 2005;17:638–642. doi: 10.1016/j.coi.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Fox DA. Regulatory T cell defects in rheumatoid arthritis. Arthritis Rheum. 2007;56:710–713. doi: 10.1002/art.22415. [DOI] [PubMed] [Google Scholar]
- Moutsopoulos HM. Fauci AS, Isselbacher KJ, Wilson JD, Martin JB, Kasper DL, Hauser SL, Longo DL, editors. New York: McGraw-Hill,; Sjogren’s Syndrome. 1998:pp 1901–1904. [Google Scholar]
- Manoussakis MN, Boiu S, Korkolopoulou P, Kapsogeorgou EK, Kavantzas N, Ziakas P, Patsouris E, Moutsopoulos HM. Rates of infiltration by macrophages and dendritic cells and expression of interleukin-18 and interleukin-12 in the chronic inflammatory lesions of Sjogren’s syndrome: correlation with certain features of immune hyperactivity and factors associated with high risk of lymphoma development. Arthritis Rheum. 2007;56:3977–3988. doi: 10.1002/art.23073. [DOI] [PubMed] [Google Scholar]
- Moutsopoulos HM, Hooks JJ, Chan CC, Dalavanga YA, Skopouli FN, Detrick B. HLA-DR expression by labial minor salivary gland tissues in Sjogren’s syndrome. Ann Rheum Dis. 1986;45:677–683. doi: 10.1136/ard.45.8.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xanthou G, Tapinos NI, Polihronis M, Nezis IP, Margaritis LH, Moutsopoulos HM. CD4 cytotoxic and dendritic cells in the immunopathologic lesion of Sjogren’s syndrome. Clin Exp Immunol. 1999;118:154–163. doi: 10.1046/j.1365-2249.1999.01037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerli R, Muscat C, Giansanti M, Danieli MG, Sciuto M, Gabrielli A, Fiandra E, Vitali C. Quantitative assessment of salivary gland inflammatory infiltration in primary Sjogren’s syndrome: its relationship to different demographic, clinical and serological features of the disorder. Br J Rheumatol. 1997;36:969–975. doi: 10.1093/rheumatology/36.9.969. [DOI] [PubMed] [Google Scholar]
- Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, Daniels TE, Fox PC, Fox RI, Kassan SS, Pillemer SR, Talal N, Weisman MH. Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarpley TM, Jr, Anderson LG, White CL. Minor salivary gland involvement in Sjogren’s syndrome. Oral Surg Oral Med Oral Pathol. 1974;37:64–74. doi: 10.1016/0030-4220(74)90160-1. [DOI] [PubMed] [Google Scholar]
- Ioannidis JP, Vassiliou VA, Moutsopoulos HM. Long-term risk of mortality and lymphoproliferative disease and predictive classification of primary Sjogren’s syndrome. Arthritis Rheum. 2002;46:741–747. doi: 10.1002/art.10221. [DOI] [PubMed] [Google Scholar]
- Skopouli FN, Dafni U, Ioannidis JP, Moutsopoulos HM. Clinical evolution, and morbidity and mortality of primary Sjogren’s syndrome. Semin Arthritis Rheum. 2000;29:296–304. doi: 10.1016/s0049-0172(00)80016-5. [DOI] [PubMed] [Google Scholar]
- Sutcliffe N, Inanc M, Speight P, Isenberg D. Predictors of lymphoma development in primary Sjogren’s syndrome. Semin Arthritis Rheum. 1998;28:80–87. doi: 10.1016/s0049-0172(98)80040-1. [DOI] [PubMed] [Google Scholar]
- Voulgarelis M, Dafni UG, Isenberg DA, Moutsopoulos HM. Malignant lymphoma in primary Sjogren’s syndrome: a multicenter, retrospective, clinical study by the European Concerted Action on Sjogren’s Syndrome. Arthritis Rheum. 1999;42:1765–1772. doi: 10.1002/1529-0131(199908)42:8<1765::AID-ANR28>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Katsiougiannis S, Kapsogeorgou EK, Manoussakis MN, Skopouli FN. Salivary gland epithelial cells: a new source of the immunoregulatory hormone adiponectin. Arthritis Rheum. 2006;54:2295–2299. doi: 10.1002/art.21944. [DOI] [PubMed] [Google Scholar]
- Kapsogeorgou EK, Moutsopoulos HM, Manoussakis MN. Functional expression of a costimulatory B7.2 (CD86) protein on human salivary gland epithelial cells that interacts with the CD28 receptor, but has reduced binding to CTLA4. J Immunol. 2001;166:3107–3113. doi: 10.4049/jimmunol.166.5.3107. [DOI] [PubMed] [Google Scholar]
- Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Li X, Qian L, Wang G, Zhang H, Wang X, Chen K, Zhai Z, Li Q, Wang Y, Harris DC. T regulatory cells are markedly diminished in diseased salivary glands of patients with primary Sjogren’s syndrome. J Rheumatol. 2007;34:2438–2445. [PubMed] [Google Scholar]
- Gottenberg JE, Lavie F, Abbed K, Gasnault J, Le Nevot E, Delfraissy JF, Taoufik Y, Mariette X. CD4 CD25high regulatory T cells are not impaired in patients with primary Sjogren’s syndrome. J Autoimmun. 2005;24:235–242. doi: 10.1016/j.jaut.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Marshall NA, Christie LE, Munro LR, Culligan DJ, Johnston PW, Barker RN, Vickers MA. Immunosuppressive regulatory T cells are abundant in the reactive lymphocytes of Hodgkin lymphoma. Blood. 2004;103:1755–1762. doi: 10.1182/blood-2003-07-2594. [DOI] [PubMed] [Google Scholar]
- Rad R, Brenner L, Bauer S, Schwendy S, Layland L, da Costa CP, Reindl W, Dossumbekova A, Friedrich M, Saur D, Wagner H, Schmid RM, Prinz C. CD25+/Foxp3+ T cells regulate gastric inflammation and Helicobacter pylori colonization in vivo. Gastroenterology. 2006;131:525–537. doi: 10.1053/j.gastro.2006.05.001. [DOI] [PubMed] [Google Scholar]
- van Amelsfort JM, van Roon JA, Noordegraaf M, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. Proinflammatory mediator-induced reversal of CD4+,CD25+ regulatory T cell-mediated suppression in rheumatoid arthritis. Arthritis Rheum. 2007;56:732–742. doi: 10.1002/art.22414. [DOI] [PubMed] [Google Scholar]