Abstract
Patients with Chagas’ disease remain asymptomatic for many years, presumably by keeping the etiological agent Trypanosoma cruzi in check through protective immunity against. Recently, we found that T. cruzi uses TrkA, a receptor tyrosine kinase responsive to neurotrophin nerve growth factor in vertebrate nervous systems, to invade cells. We also found that TrkA, TrkB, and TrkC, but not T. cruzi, are targets of specific autoantibodies present in the sera of patients with chronic Chagas’ disease. Here we show that TrkA-, TrkB-, and TrkC-specific autoantibodies isolated from the sera of four individuals with chronic indeterminate (asymptomatic) Chagas’ disease potently blocked invasion of Trk-bearing neuronal PC12 cells, neuroglial astrocytes, enteroglial cells, and Schwann cells and Trk-expressing non-neural smooth muscle and dendritic cells. However, these autoantibodies did not inhibit T. cruzi invasion of mutant PC12 cells lacking TrkA or of normal cells lacking Trk receptors, suggesting that autoantibodies interfered with parasite/Trk cross talk to access the intracellular milieu. Passive immunization of susceptible and resistant mouse strains with very small doses of these autoantibodies reduced parasitemia and transferred resistance to an otherwise lethal trypanosome infection. Hence, this exquisitely sensitive and unique regulatory immunity against the host (instead of parasite) could benefit infected individuals by blocking cellular invasion of the obligatory intracellular pathogen, resulting in attenuation of tissue infection and clinical manifestations. Such action is contrary to the horror autotoxicus frequently associated with microbe-related autoimmune responses.
The protozoan Trypanosoma cruzi causes debilitating Chagas’ disease, which afflicts millions of people in the Americas. Lesions in chronic symptomatic disease predominate in the heart and gastrointestinal tract, where strong inflammatory reactions and destruction of cardiac and enteric neurons are believed to cause arrhythmias, congestive heart failure, cardiomegaly, megaviscera (megacolon and/or megaesophagus), and death.1,2,3 This dreadful scenario, however, is a feature of only a relatively small proportion (15% to 30%) of patients, for the majority of them remain for years or decades in the indeterminate form, defined as “asymptomatic individuals with parasitological and/or serological evidence of T. cruzi infection, who exhibit normal electrocardiogram and a normal digestive tract seen by contrasted radiography.”4 It remains a mystery why patients with Chagas’ disease stay asymptomatic for so long, but one logical explanation would be protective immunity against T. cruzi and/or host structures that the parasite exploits to properly colonize tissues. Favoring this hypothesis is the reactivation of infection and emergence of pathology in otherwise asymptomatic chagasic patients subjected to immunosuppressive insults such as drugs5 or co-infection with HIV.6
T. cruzi invades a broad variety of cells in neural and non-neural tissues, including astrocytes, Schwann cells, enteric glial cells, neurons, dendritic cells, and smooth muscle cells.7,8,9 These cells express receptor tyrosine kinases TrkA, TrkB, and/or TrkC,10,11,12 which are activated primarily by the neurotrophins nerve growth factor, brain-derived neurotrophic factor, and neurotrophin-3, respectively, in development and repair of vertebrate nervous systems.13,14 Trks have a conserved amino acid sequence and a common structure, which consists of a multidomain extracellular domain, including a membrane-proximal immunoglobulin-like unit that bears the neurotrophin-binding site, a single trans-membrane domain, and an evolutionary conserved intracellular tyrosine kinase.
Earlier studies showed that the trans-sialidase of T. cruzi, traditionally thought of as a protein that catalyzes the hydrolysis (neuraminidase) and transfer of sialic acid from glycol-conjugates to β-galactosyl substrates (trans-sialidase),15 moonlights as a ligand for TrkA to trigger survival and differentiation of neuronal cells independently of intrinsic enzymatic activities.16,17,18,19 Furthermore, T. cruzi binding to TrkA drives invasion of neuronal and dendritic cells in vitro and in the murine model of Chagas’ disease.20 The survival-promoting action of this parasite-derived neurotrophic factor is not restricted to neuronal cells, for it also promotes survival of endothelial cells through yet-to-be determined receptors.21 Thus, parasite-derived neurotrophic factor resembles also sugar-binding enzyme phosphoglucose isomerase that moonlights as a growth factor for spinal and sensory neurons.22
Recently, autoantibodies specific for TrkA, TrkB, and TrkC (ATA) were discovered in the sera of patients with chronic Chagas’ disease.23 Thus, because TrkA is both an invasion receptor for T. cruzi and a target for autoimmune response in Chagas’ disease patients, the exciting and novel possibility exists for the Trk receptor autoantibodies to directly mediate a protective (ie, inhibitory) or deleterious (ie, enhancing) action in T. cruzi infection. We show here that ATA are extremely effective in blocking invasion in vitro and in a mouse model of Chagas’ disease. The results raise the intriguing and unexpected possibility of autoimmunity against TrkA to play a unique regulatory role in infection and tissue injury in patients with Chagas’ disease.
Materials and Methods
Parasites
The Silvio X-10/4 and Tulahuen strains of T. cruzi were maintained in Vero cell in Dulbecco’s modified Eagle medium containing 1% fetal calf serum (Gibco Laboratories, Grand Island, NY) at 37°C in a 5% CO2 atmosphere as described previously.16 Trypomastigotes were collected 5 or 6 days after Vero cells were infected with T. cruzi. Tulahuen strain was also maintained in mice, as described previously.20
Sera from T. cruzi-Infected and Non-Infected Individuals
We used six sera samples from non-chagasic individual and four sera samples from chagasic patients in the indeterminate phase of Chagas’ disease. These sera were studied in the previous work23 and the chagasic patient sera were chosen because of their availability to perform the study and they had high titers against the extracellular domain (ECD) of TrkA (TrkAECD), representing >50% of the available chagasic sera from indeterminate patients. Their identity in regard to sex and age is unknown. Ethical approval was obtained from Human Investigation Review Committee of Tufts Medical Center and Federal University of Goiàs, Brazil.
Purification of ATA
As previously described,23 10 μg of Fc chimera of the ECD of TrkA, TrkB, or TrkC (R&D Systems) were run by SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane. Receptor location was determined by reacting anti-human IgG Fc antibody followed by alkaline phosphatase-labeled secondary antibody (Promega, Madison, WI) with thin strips cut from each side of the membrane. After identification of the Trk receptor, the strips were aligned with the membrane to guide the location of un-reacted Trk-Fc, which was cut horizontally and incubated with protein G-purified IgG from chagasic sera. The membranes, after gentle shaking overnight at 4°C, were washed twice with 50 mmol/L Tris-buffered saline (pH 7.2), eluted (1 minute) with 50 mmol/L glycine-HCl buffer pH 2.5, and the eluate (ie, ATA) immediately neutralized in 0.1M Tris-buffered saline pH 8.0, concentrated by ultrafiltration (Millipore Corporation), diluted in glycerol (final concentration, 30%), filtered in 0.22 μm membranes, and stored at 4°C until use. As determined by enzyme-linked immunosorbent assay (ELISA) this protocol adsorbed >90% of anti-Trk antibodies from IgG (data not shown). Mock-purification of ATA was performed as above except chagasic IgG was applied to a strip of nitrocellulose containing ovalbumin (Sigma-Aldrich, Saint Louis, MO) or distant from the Trk-, or ovalbumin-bound nitrocellulose. Antibody concentration was determined by absorption at 280 nm in a spectrophotometer or by ELISA (CisBio, Bedford, MA). Contamination of ATA with possible spurious antibodies from chagasic sera was checked by absorption to TrkAECD-Fc or TrkCECD-Fc immobilized on protein G-Sepharose: ATA (10 μg in 200 μl) was passed through protein G-Sepharose (0.1 ml) (GE Health care, Piscataway, NJ) and the effluent re-adsorbed twice over the same column, and reconstituted to twice dilution of the original sample (400 μl) or equivalent to 25 μg/ml assuming no adsorption of the antibodies to TrkA-Fc or TrkC-Fc/protein G-Sepharose. Adsorption was also performed on p75ECD-Fc/protein G-Sepharose, as above. The stock solution of the unabsorbed antibodies (50 μg/ml) was likewise diluted to 20 μg/ml. Effluent and unabsorbed antibodies were tested for binding to TrkA, TrkB, and TrkC by ELISA and for infection, as described below.
ELISA
ELISA was performed as described before.23 In brief, microtiter wells were coated overnight at 4°C with growth factor receptorECD (R&D Systems), incubated with sera (1:200), non-chagasic IgG, or ATA (adsorbed or not to Trk or p75 protein G-Sepharose, as above) IgG in 5% bovine serum albumin/PBS, pH7.2, blocked with 5% goat serum (2 hours, 37°C), followed by antibody to human κ light chain and alkaline phosphatase-labeled secondary relevant antibody. ATA isotyping was performed by ELISA with commercially available kits (Sigma-Aldrich, Saint Louis, MO) based on mouse mAb to human IgG isotypes and goat antibodies specific for IgA and IgM.
Infection in Vitro and in Vivo
PC12wt and PC12nnr5 cells were gifts from Dr. Lloyd Greene, College of Physicians and Surgeons, Columbia University, NY, and grown as described earlier.17 The mouse astrocyte cell line C8-D1A and the rat enteroglial cells were from ATCC. The permanent human Schwann cells cell line were used in previous study.23 Primary cultures of human vascular smooth muscle cells were a gift from Dr. Herbert Tanowitz, Albert Einstein College of Medicine, NY, and mouse bone marrow-derived dendritic cells were a gift from Kristin Stephan, Tufts Medical School, Boston. Cells were infected with T. cruzi and the infection quantified by fluorescence microscopy and Diff-Quik as previously described.23 For inhibition of infection, cells were pre-incubated with ATA, control antibodies, or a rabbit IgG antibody specific for the extracellular domain of TrkA (Abcam, Cambridge, MA) in 1% bovine serum albumin/ Dulbecco’s modified Eagle’s medium for 60 minutes followed by parasites (Silvio or Tulahuen strain) in the same medium for 2 hours, and parasites that did not attach or invade cells were removed by washing the monolayers. Monolayers, which contain infected and non-infected cells, were maintained at 37°C in complete medium (2.5% fetal calf serum in Dulbecco’s modified Eagle’s medium) 2 or 3 days before fixation and staining. PC12 cells were also fixed with 4% paraformaldehyde 3 days after infection, permeabilized with 0.2% Triton X-100, blocked with 5% goat serum, and reacted with chagasic serum (1:200) and Alexa-594-labeled secondary anti-human antibody (Molecular Probe), and counterstained with 4,6-diamidino-2-phenylindole (0.25 μg/ml, 1 minute) to visualize host cell nuclei. For in vivo experiments, BALB/c and C57BL/6 mice (female, 7 to 8 weeks old) were primed and inoculated in the footpad with the Tulahuen strain of T. cruzi and parasitemia determined as described before.24 Experiments with mice were approved by the Department of Laboratory Animal Medicine of Tufts University-New England Medical Center. Statistical analysis and graphs through the manuscript made with GraphPad software (Instat 3.0 and Prism 4.0).
Results
Characterization of Antibodies Specific for the ECD of Trk Receptors in the Sera of Four Patients with Indeterminate (Asymptomatic) Chagas’ Disease
To determine whether ATA interfere with T. cruzi infection, we studied six non-chagasic sera as negative control and four sera of chagasic patients in the indeterminate phase.23 These four sera, chosen because of their high titers against TrkA, are named sera #1, #2, #3, and #4. Consistent with previous results,23 the four sera reacted robustly by ELISA with the ECD of TrkA, TrkB, and TrkC, with highest titers against TrkCECD; the sera of the chagasic individuals did not react with other growth factor receptors, including the pan-neurotrophin receptor p75 (Figure 1A). The four sera also reacted robustly with lysates of T. cruzi (Figure 1A), confirming they were obtained from chagasic individuals.
Figure 1.
Characterization of autoantibodies to Trk receptors in the sera of four patients with indeterminate Chagas’ disease. A: Reaction of four chagasic sera (#1, #2, #3, and #4) and six non-chagasic sera (1:200 dilution) with microtiter plates coated with the ECD of TrkA, TrkB, or TrkC, p75, and freeze/thaw lysates of T. cruzi, followed by alkaline phosphatase-labeled secondary antibody. Reaction read 2 hours after addition of alkaline phosphatase substrate. Average of triplicate wells/antigen. B: Similar to (A) except the coated microtiter wells were reacted with affinity-purified ATA or protein G-purified non-chagasic IgG, all at 0. 3 μg/ml.
Autoantibodies affinity-purified on a TrkECD matrix (see Materials and Methods), hereafter referred to as ATA, reacted with TrkAECD, TrkBECD, TrkCECD, but not with p75ECD (Figure 1B); furthermore, ATA reacted to about the same extent with the ECD of the three Trk receptors (Figure 1B). ATA did not bind to T. cruzi, in agreement with previous results.23 Also in agreement with previous results,23 affinity-purified ATA were of the IgG2 isotype and all had κ light chain (data not shown). ATA mock purified on a strip of nitrocellulose containing ovalbumin or devoid of Trk receptors and other proteins did not yield Trk-reacting antibody, as determined by ELISA (data not shown).
ATA Disrupt the Cellular Invasion Cycle of T. cruzi
To complete its life cycle in mammals, T. cruzi must differentiate and replicate in the cell cytoplasm.7,25,26 Because recent studies showed that TrkA is a cellular receptor for T. cruzi invasion,20 the possibility exists that ATA interferes with T. cruzi invasion. Fluorescence microscopy revealed that ATA (patient #1) at 0.3 μg/ml inhibit T. cruzi (Silvio strain) infection of paradigm TrkA-expressing sympathetic-like PC12 cells (Figure 2A, panel b), compared to the infection in the presence of non-chagasic IgG (Figure 2A, panel a). Similar experiments showed that ATA also inhibited PC12 cell infection by the Tulahuen strain of T. cruzi (data not shown). Dose-response experiments showed ATA (#1) to be extraordinarily potent in blocking T. cruzi infection of PC12 cells, with observable effects in the low (∼1.5 ng/ml) range (Figure 2B); non-chagasic IgG isolated from six sera did not inhibit infection even at relatively high concentrations (Figure 2B). Adsorption of ATA on TrkAECD-Fc/protein G-Sepharose completely removed the inhibitory power of the autoantibodies, whereas adsorption of the autoantibodies on control p75ECD-Fc/protein G-Sepharose did not (Figure 2B). The inhibitory power of ATA was also abrogated by adsorption of the autoantibodies to TrkCECD-Fc/protein G-Sepharose (data not shown). These results suggest that ATA inhibit T. cruzi infection by binding to host cell Trk receptors.
Figure 2.
ATA Inhibit T. cruzi Infection of PC12 Cells A: Visualization of T. cruzi (Silvio strain) infection of PC12wt cells by fluorescence microscopy. PC12wt cells were infected with T. cruzi (∼30 parasites/cell) for 3 days in the presence of 0.3 μg/ml non-chagasic IgG (panel a) or ATA (#1) (panel b), fixed in paraformaldehyde, stained with 4,6-diamidino-2-phenylindole (to visualize host cell nuclei) and chagasic IgG/secondary Alexa-labeled anti-human IgG (to identify intracellular parasites). Arrows in panel a indicate host cells fully loaded with amastigotes. Non-Ch, non-chagasic IgG. B: Dose-response of ATA-induced inhibition of T. cruzi infection of PC12 cells. Protocol similar to that in (A) except infection was performed in the presence of various concentrations of ATA (#1, unadsorbed) and visualized by phase contrast microscopy after staining with Diff-Quik. Also shown is the effect of ATA adsorbed on TrkAECD-Fc/protein G-Sepharose (ATA ads on protein G) and on p75ECD-Fc/protein G-Sepharose (p75 ads on p75/proteinG). Results plotted as inhibition of infection relative to infection in the presence of non-chagasic IgG (open squares). The lack of inhibition by non-chagasic IgG (average of the protein G-Sepharose-purified IgG from three sera) is indicated by open triangles. Experiment repeated twice with similar results.
To further determine whether ATA interfere with infection of other cells targeted by T. cruzi in invasion in the nervous system [astrocytes, Schwann cells, and enteric glial cells (EGC)],8,27,28 relevant cell lines preincubated with ATA (to allow surface receptor binding), infected with the parasites for 3 days, and fixed and stained with Diff-Quick to identify infected and non-infected cells by phase contrast microscopy. The results showed that ATA (#1, #2, #3, and #4; 0.3 μg/ml) significantly inhibited T. cruzi infection of the astrocyte cell line C8-D1A compared to non-chagasic IgG (Figure 3A).
Figure 3.
ATA Inhibit Infection of Trk-Expressing Neural and Non-Neural cells A: Infection of EGC pre-incubated for one hour in medium without (No Ab), with control antibody (Non-Ch IgG), or ATA (#1, #2, #3, and #4), all at 0.30 μg/ml. Inhibition of infection and P values indicated under the bar-graph: ns, not significant; *, P < 0.05; and **, P < 0.01. The result of non-chagasic IgG is the average of the six non-chagasic sera of Figure 1. Experiment repeated twice with similar results. B: Inhibition of infection (relative to medium without added IgG) by ATA (#1, #2, and #3) (0.30 μg/ml). Schwann, immortalized human Schwann cell line; EGC, enteric glial cells; SMC, smooth muscle cells; and dendritic, bone marrow-derived mouse dendritic cells. Experiment repeated three times with similar results. Expression of TrkA, TrkB, and TrkC by the cell lines is indicated under the bar graph. The non-chagasic IgG is from one serum sample.
Another set of experiments showed that ATA (#1, #2, and #3, 0.3 μg/ml) blocked infection of neural (astrocytes, EGC, Schwann cell, and PC12 cells) and non-neural (bone marrow dendritic cells and vascular smooth muscle cells)10,11cells (Figure 3B), all of which express TrkA, or TrkB and TrkC (Figure 3B). However, ATA (#1, #2, and #3) did not significantly inhibit T. cruzi invasion of the PC12nnr5 cells (Figure 4A), a PC12 cell mutant that lacks the TrkA gene.29 In addition, ATA (#1, #2, and #3) were ineffective in blocking infection of kidney epithelial Vero cells (Figure 4B), which do not express Trk receptors. Furthermore, a rabbit antibody specific for the ECD of TrkA inhibited infection of wild-type PC12 cells, which express TrkA, but not of PC12 mutant nnr5, which does not express TrkA (Figure 4A). And the rabbit anti-TrkA antibody was inactive in blocking T. cruzi infection of enteroglial and Vero cells, which, not coincidentally, do not express Trk receptors (Figure 4B). Preliminary experiments had shown that ATA adsorbed on TrkA or TrkC/protein G-Sepharose was unable to bind TrkA, TrkB, and TrkC by ELISA (data not shown). Furthermore, preincubation of ATA (0.3 μg/ml) with TrkAECD (1.0 μg/ml) reversed the inhibitory power of the autoantibodies, as did adsorption of ATA through TrkA-Fc/protein G-Sepharose column (Figure 4A). Thus, these results further suggest that ATA block T. cruzi invasion of cells by interfering the parasite use of TrkA and perhaps other Trk receptors to enter host cells.
Figure 4.
ATA Failed to Block Infection of Cells that Do Not Express Trk Receptors A: ATA and rabbit IgG against TrkA do not inhibit T. cruzi infection of TrkA-deficient PC12nnr5 cells. Duplicate wells of TrkA-expressing PC12wt and TrkA-deficient PC12nnr5 cells were pre-incubated with ATA (#1, white bar; #2, stippled bar; and #3, black bar), ATA #1 (0.3 μg/ml) pre-incubated with TrkAECD (1.0 μg/ml) or after pass through TrkA-Fc/protein G-Sepharose, and rabbit α-TrkA IgG (0.30 μg/ml) for 60 minutes, infected with T. cruzi for 2 days and infection visualized by phase-contrast microscopy after Diff-Quick staining. The results plotted as inhibition of infection relative to non-chagasic IgG used as the same concentration as ATA. Experiments repeated three times with similar results. B: ATA do not block infection of kidney epithelial Vero cells. Protocol and reagents were similar to (A) except for the use of Vero cells (experimental) and of positive control EGC.
Passive Immunization of Mice with Very Low Doses of ATA Before or After Inoculation with T. cruzi Reduces Parasitemia, Inflammation, and Mortality in Otherwise Lethal Infection
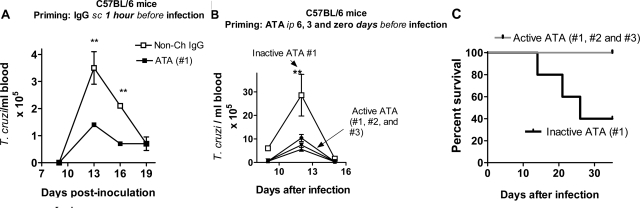
To study whether ATA alter the course of infection in mice relatively resistant to T. cruzi infection,30 groups of five female C57BL/6 mice were sensitized subcutaneously in the footpad with ATA (#1) or non-chagasic IgG (6 ng IgG/mouse) followed, 1 hour later, by a non-lethal inoculum (2 × 103 trypomastigotes, Tulahuen strain) in the sensitization site. The choice of the IgG dose was based on the results in Figures 1–4 and in exploratory experiments. The rational for the immunization protocol is as follows: if ATA bind to Trk invasion receptors and, consequently, block cellular invasion in the inoculation site, as they did in vitro (Figures 1–4), then the reduced infection in the inoculation site should trickle down to other parts of the body and be reflected in blood parasite counts. Indeed, immunization with a very small, single dose, of ATA significantly brought down parasitemia compared to the effect of control non-chagasic IgG (Figure 5A).
Figure 5.
ATA Inhibit Parasitemia and Mortality of C58BL/6 Mice Infected with T. cruzi. A: C57Bl/6 mice (5 mice per group) were sensitized with 6 ng IgG/mouse (footpad) followed 1 hour later by inoculation of 2 × 103 T. cruzi trypomastigotes/mouse in the same priming site. **, P < 0.01. B and C: Groups of five C57Bl/6 mice were sensitized i.p. with 50 ng of inactive, heat-inactivated (100°C, 5 minutes) ATA, or active ATA (#1, #2, and #3), followed by sensitizations 3 and 6 days later with the same amount of inactive or active ATA, for a total of 150 ng IgG. Mice were infected with 6 × 103 parasites in the footpad 6 days after the first sensitizing dose and assessed for parasitemia (B) and survival (C). *, P < 0.05; **, P < 0.01. Experiment performed three times with similar results.
Next, we sought to determine whether systemically administered ATA would still inhibit disease progression even if the parasites were inoculated at a site distinct from the sensitization site. In this case the autoantibodies will, presumably, redistribute throughout the body, including the inoculation site. C57BL/6 mice were primed intraperitoneally with ATA (#1, #2, and #3) at 6, 3, and 0 days with equal dose of the antibody (total of 150 ng/mouse) before a T. cruzi inoculum (6 × 103 parasites/mouse footpad). Preliminary experiments and previous work in our lab showed that this inoculum can kill even resistant C57BL/6 mice. In this experiments, ATA, here named “active ATA” because they bound Trk receptors by ELISA (see Figure 1) and to distinguish from control heat-treated (100°C, 5 minutes) “inactive ATA,” which were unable to bind Trk receptors as determined by ELISA (data not shown). Active ATA significantly reduced parasitemia compared to the effect of inactive ATA (Figure 5B). Furthermore, all C57BL/6 mice immunized with active ATA survived the infection produced by the high T. cruzi inoculum, while mice that received inactive ATA did not (Figure 5C).
To determine whether ATA reduce disease progression in a paradigm susceptible strain of mice,30 footpads of BALB/c mice were sensitized with 6 ng of ATA (#1) or control non-chagasic human IgG, followed, 1 hour later, by a lethal dose (5 × 103/mouse) of Tulahuen strain of T. cruzi in the sensitizing footpad. ATA-sensitized mice displayed a small but statistically significant (P < 0.05) decline in parasitemia at 9 and 11 days postinoculation (PI) relative to control mice (Figure 6A). Most dramatically, all five ATA-immunized mice survived the infection for the 63 days of observation, quite contrary to the death of all control mice at day 34 PI (Figure 6B).
Figure 6.
ATA Inhibit Parasitemia and Mortality of BALB/c Mice Infected with T. cruzi. A and B: Groups of five BALB/c mice were primed in the footpads with 6 ng of ATA (#1) or control non-chagasic IgG and, after 60 minutes, inoculated in the sensitizing footpad with 5 × 103 trypomastigotes (Tulahuen strain). Mice were assessed for parasitemia (A) at the indicated times and observed daily for survival (B) and clinical symptoms. Experiment performed twice; results are plotted as average of the two experiments. C and D: Groups of five BALB/c mice were inoculated each in the footpad with T. cruzi (5 × 103 trypomastigotes) and, 2 days later, injected intraperitoneally with 2.6 ng of control non-chagasic IgG or ATA (#1), and every second or third day up to 12 days, for a total of 250 ng autoantibody. Parasitemia (C) and survival (D) were assessed at the indicated times. *, P < 0.05; **, P < 0.001.
In addition, ATA transferred resistance to BALB/c mice when administered days after parasite inoculation and at a site distinct from parasite inoculation site. Thus, passive administration of ATA 2, 4, 6, 9, and 12 days (five times, total dose: 250 ng/mouse) after T. cruzi infection into a site (i.p.) far from the parasite inoculation site (footpad), significantly diminished parasitemia (Figure 6C) and mortality (Figure 6D) compared to mice similarly immunized with non-chagasic IgG. Mice in the ATA-immunized group were alive and without clinical alterations at the maximum time (65 days) of observation, except for one, which died 2 days before the control group began to succumb (Figure 6D). Because this is the only ATA-sensitized mouse that died after T. cruzi infection, and because we were unable to perform histo- and anatomo-pathological analysis, we are not sure whether death was caused by the infection per se or by tissue damage consequent to the multiple i.p. injections. This experiment was repeated one more time and produced similar result, except now all ATA-immunized mice survived the otherwise lethal infection.
Identification of parasites by immunofluorescence in blood smears of C57BL/6 mice underscored the difference in parasitemia between mice primed with inactive (Figure 7A) and active ATA (Figure 7D), corroborating the parasitemia results of Figure 6.
Figure 7.
Histology of Control and ATA-Primed T. cruzi-Infected Mice. A and D: Blood smears of C57Bl/6 mice primed with inactive ATA (A) and active ATA at the peek of parasitemia (Figure 5B). Blood smears were fixed with 4% paraformaldehyde, stained with chagasic IgG and secondary Alexa594-labeled antibody to reveal trypomastigotes (in red). Magnification = original ×200. B and C: Photomicrograph of H&E-stained left ventricular myocardium (B) and auricle (C) of one of the T. cruzi-infected mice primed with non-chagasic IgG (Figure 6A) (day 34 PI). Note small foci of infiltrating lymphocytes in the myocardium (arrows), and infiltrating lymphocytes and proliferating epicardial cells in the auricle (arrows). E and F: Photomicrograph of left ventricular myocardium (E) and auricle (F) of one ATA-primed T. cruzi-infected mouse (Figure 6A) (day 34 PI). Abnormalities are not seen in the myocardium and auricle. Magnification = original ×200 (B and E); ×100 (C) and (F).
H&E-stained histological sections of the heart in control mice at the time of death (two mice, 34 days PI) revealed strong inflammatory infiltrates, mostly mononuclear cells, in the ventricles (Figure 7B) and auricles, particularly epicardium (Figure 7C), indistinguishable from the inflammatory response of T. cruzi-infected mice untreated with IgG (not shown). This response was in sharp distinction to the absence of detectable inflammation and other abnormalities in the heart of ATA-immunized mice sacrificed at the same PI day (Figure 7, E and F, respectively), akin to the heart histology of uninfected mice (data not shown).
Discussion
It is well known that autoimmunity or horror autotoxicus31,32 can alter function of the body’s own constituents and initiate inflammatory cascades, leading to tissue injury and pathology in the nervous system and other organs.33,34,35 Infectious agents such as Campylobacter jejuni, group A streptococcus, and hepatitis C virus have long been considered a trigger of horror autotoxicus.35,36,37 T. cruzi elicits immune responses to many autoantigens, like ribosomal P protein,38 M2 muscarinic and β1 adrenergic receptors,39 myosin,40 and undefined antigens on neurons and nerves.41,42
Recently, we identified autoantibodies to Trk tyrosine kinases in the sera of patients with chronic Chagas’ disease.23 Because microbial infections can trigger pathology-generating autoimmunity, we first thought that ATA should be harmless like many autoantibodies, or harmful as the ones against the receptor tyrosine kinase MuSK that trigger tissue injury in myasthenia gravis.43 Instead, we found ATA to be extremely potent in blocking T. cruzi invasion of neuronal and glial cells and of Trk-expressing non-neural dendritic cells and smooth muscle cells in vitro (Figures 2–4). Most significantly, ATA were protective in vivo, for mice passively immunized with very low doses of the autoantibodies derived from asymptomatic patients exhibited lower parasitemia and mortality than control mice injected with either non-chagasic IgG or heat-treated ATA that could no longer bind Trk receptor (by ELISA). The therapeutic doses of ATA (∼10 μg antibody i.p.-injected antibody per kg body weight) were orders of magnitude lower that those for several other therapeutic antibodies such as α4β1 integrin antibodies, which prevented experimental autoimmune encephalomyelitis at ∼1.0 mg/rat or ∼300 μg/kg.44
How could ATA-immunized mice show dramatic reduction in mortality and parasitemia compared to control mice? A logical explanation relate to the ATA-dependent inhibition of cellular invasion in the T. cruzi inoculation site, which will result in reduced local infection and consequently lower parasite load elsewhere. But another non-exclusive explanation relates to tissue invasion by bloodstream parasites. To infect cells in tissue parenchyma from the bloodstream, T. cruzi must cross the vascular endothelium and vessel wall. It is believed that T. cruzi infects myocardial cells by first invading endothelial cells and smooth muscle cells in the vasculature.45 And vascular endothelial cells and smooth muscle cells selectively express TrkB and TrkC.46,47 Thus, it is conceivable that ATA block T. cruzi invasion of the heart by inhibiting infection in the vascular endothelium, which, if the case, should reduce access of the parasites to heart parenchyma. Reduced extravasation of T. cruzi into the heart parenchyma should recruit less inflammatory cells, as observed experimentally (Figure 7). Similar explanation could hold for T. cruzi invasion of other organs.
Resistance to T. cruzi infection in mice passively immunized with ATA could relate to the natural course of infection in humans. Most chronic chagasic patients do not exhibit symptoms or infection-related abnormalities for years or decades (indeterminate form), as best illustrated by celebrated patient Berenice, who was diagnosed with acute Chagas’ disease by Carlos Chagas in 1909 when she was less than 2 years old. Berenice died in 1981, aged 73 years, not of complications of megaviscera or cardiomyopathy but of presumed natural causes.48 It remains unknown why chagasic patients remain asymptomatic for so many years, but it may be that ATA are beneficial to Chagas’ disease patients by keeping T. cruzi infection in check and helping patients stay the course of chronic infection without pathological alterations. Like other parameters of host-parasite interactions, the regulatory action of ATA suggested by our results cannot apply to all patients because ATA were found in most, but not all, sera of all patients with chronic indeterminate Chagas’ disease.23 Furthermore, it remains to be determined whether ATA isolated from patients with symptomatic disease (cardiomegaly, megaesophagus, and megacolon), which is characterized by destruction of peripheral neurons,1 have beneficial or detrimental effects to the host.
The hypothesis that ATA benefit chagasic patients is coherent with an emerging immunological concept from a number of laboratories, which remarks that inflammation of the nervous system is a double-edged sword that can destroy or protect tissues.49,50,51 Pioneering experiments showed that transfer of activated myelin basic protein-specific T cells into mice after optic nerve injury prevented neurons from degeneration52 and enhanced survival of retinal ganglion cells.53 It will be of interest to identify the Trk epitope(s) recognized by ATA, for they could in theory lead to a therapeutic vaccine to boost protection of Chagas’ disease patients. Mapping of ATA binding site(s) has yet to be determined but, given that ATA cross-react nearly equally well with the three Trks, it would not be unreasonable to think the epitopes to be located in the second Ig-like and second cysteine-rich domains of the receptors, for they are most similar between different human Trks.54
In conclusion, this study shows for the first time an association between autoimmunity to neurotrophic receptors – Trks, and a chronic disease produced by a parasite invader of the nervous system – Chagas’ disease. Considering that ATA are present in chagasic sera at concentrations (58.8 μg/ml) orders of magnitude above the threshold to neutralize infection in vitro (>0.8 pg/ml) and in vivo (250 ng/mouse), and that the autoantibodies are highly prevalent in chagasic individuals (>80%), the pan-Trk receptor autoantibodies may very well represent a regulatory host response that evolved to inhibit T. cruzi cellular invasion. Such response could be of mutual benefit to the parasite (persistently low levels of parasitemia and tissue parasitism) and host (maintenance of long-term memory and resistance to reinfection).
Acknowledgments
We thank Eugene M. Johnson, Jr. and Angela Vincent for reading the manuscript and for providing helpful and insightful suggestions. We also thank Milena de Melo-Jorge for bacterially expressed TrkA.
Footnotes
Address reprint requests to Mercio PereiraPerrin, Parasitology Research Center, Department of Pathology, Tufts University School of Medicine, 150 Harrison Avenue, Boston, Massachusetts 02111. E-mail: Mercio.perrin@tufts.edu.
Supported by NIH Grants NS40574 and NS42960.
The authors have no conflicting financial interests.
References
- Koberle F. Chagas’ disease and Chagas’ syndromes: the pathology of American trypanosomiasis. Adv Parasitol. 1968;6:63–116. doi: 10.1016/s0065-308x(08)60472-8. [DOI] [PubMed] [Google Scholar]
- Oliveira JS. A natural human model of intrinsic heart nervous system denervation: Chagas’ cardiopathy, Am Heart J. 1985;110:1092–1098. doi: 10.1016/0002-8703(85)90222-4. [DOI] [PubMed] [Google Scholar]
- Rezende JM, Luquetti AO. Chagasic megaviscera. Pan American Health Organization Scientific, editor. Chagas Disease and the Nervous System. 1994:64–82. Publication No. 547. [Google Scholar]
- Andrade ZA. Chagas’ disease: pathology of chronic lesions. Rev Patol Trop. 2002;31:151–160. [Google Scholar]
- Kohl S, Pickering LK, Frankel LS, Yaeger RG. Reactivation of Chagas’ disease during therapy of acute lymphocytic leukemia. Cancer. 1982;50:827–828. doi: 10.1002/1097-0142(19820901)50:5<827::aid-cncr2820500503>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Rosemberg S, Chaves CJ, Higuchi ML, Lopes MB, Castro LH, Machado LR. Fatal meningoencephalitis caused by reactivation of Trypanosoma cruzi infection in a patient with AIDS. Neurology. 1992;42:640–642. doi: 10.1212/wnl.42.3.640. [DOI] [PubMed] [Google Scholar]
- Brener Z. Biology of Trypanosoma cruzi. Annu Rev Microbiol. 1973;27:347–382. doi: 10.1146/annurev.mi.27.100173.002023. [DOI] [PubMed] [Google Scholar]
- Tafuri WL. Pathogenesis of lesions of the autonomic nervous system of the mouse in experimental acute Chagas’ disease. Light and electron microscope studies, Am J Trop Med Hyg. 1970;19:405–417. doi: 10.4269/ajtmh.1970.19.405. [DOI] [PubMed] [Google Scholar]
- Tanowitz HB, Kirchhoff LV, Simon D, Morris SA, Weiss LM, Wittner M. Chagas’ disease. Clin Microbiol Rev. 1992;5:400–419. doi: 10.1128/cmr.5.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan MJ, Miranda RC, Kraemer R, McCaffrey TA, Tessarollo L, Mahadeo D, Sharif S, Kaplan DR, Tsoulfas P, Parada L, Toran-Allerand CD, Hajjar DP, Hempstead BL. Neurotrophin and neurotrophin receptors in vascular smooth muscle cells. Regulation of expression in response to injury. Am J Pathol. 1995;147:309–324. [PMC free article] [PubMed] [Google Scholar]
- Vega JA, Garcia-Suarez O, Hannestad J, Perez-Perez M, Germana A. Neurotrophins and the immune system. J Anat. 2003;203:1–19. doi: 10.1046/j.1469-7580.2003.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci. 2001;24:1217–1281. doi: 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- Bibel M, Barde YA. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000;14:2919–2937. doi: 10.1101/gad.841400. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Schenkman S, Eichinger D, Pereira ME, Nussenzweig V. Structural and functional properties of Trypanosoma trans-sialidase. Annu Rev Microbiol. 1994;48:499–523. doi: 10.1146/annurev.mi.48.100194.002435. [DOI] [PubMed] [Google Scholar]
- Chuenkova MV, Pereira MA. A trypanosomal protein synergizes with the cytokines ciliary neurotrophic factor and leukemia inhibitory factor to prevent apoptosis of neuronal cells. Mol Biol Cell. 2000;11:1487–1498. doi: 10.1091/mbc.11.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuenkova MV, PereiraPerrin M. Chagas’ disease parasite promotes neuron survival and differentiation through TrkA nerve growth factor receptor. J Neurochem. 2004;91:385–394. doi: 10.1111/j.1471-4159.2004.02724.x. [DOI] [PubMed] [Google Scholar]
- Chuenkova MV, PereiraPerrin M. A synthetic peptide modeled on PDNF. Chagas’ disease parasite neurotrophic factor, promotes survival and differentiation of neuronal cells through TrkA receptor, Biochemistry. 2005;44:15685–15694. doi: 10.1021/bi0512039. [DOI] [PubMed] [Google Scholar]
- Chuenkova MV, PereiraPerrin M. Enhancement of tyrosine hydroxylase expression and activity by Trypanosoma cruzi parasite-derived neurotrophic factor. Brain Res. 2006;1099:167–175. doi: 10.1016/j.brainres.2006.04.128. [DOI] [PubMed] [Google Scholar]
- de Melo-Jorge M, PereiraPerrin M. The Chagas’ disease parasite Trypanosoma cruzi exploits nerve growth factor receptor TrkA to infect mammalian hosts. Cell Host Microbe. 2007;1:251–261. doi: 10.1016/j.chom.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Dias WB, Fajardo FD, Graca-Souza AV, Freire-de-Lima L, Vieira F, Girard MF, Bouteille B, Previato JO, Mendonca-Previato L, Todeschini AR. Endothelial cell signalling induced by trans-sialidase from Trypanosoma cruzi. Cell Microbiol. 2008;10:88–99. doi: 10.1111/j.1462-5822.2007.01017.x. [DOI] [PubMed] [Google Scholar]
- Jeffery CJ. Moonlighting proteins. Trends Biochem Sci. 1999;24:8–11. doi: 10.1016/s0968-0004(98)01335-8. [DOI] [PubMed] [Google Scholar]
- Lu B, Petrola Z, Luquetti AO, PereiraPerrin M. Auto-antibodies to receptor tyrosine kinases TrkA, TrkB, and TrkC in patients with chronic Chagas’ disease, Scand J Immunol. 2008;67:603–609. doi: 10.1111/j.1365-3083.2008.02102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuenkova M, Pereira ME. Trypanosoma cruzi trans-sialidase: enhancement of virulence in a murine model of Chagas’ disease. J Exp Med. 1995;181:1693–1703. doi: 10.1084/jem.181.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade LO, Andrews NW. The Trypanosoma cruzi-host-cell interplay: location, invasion, retention. Nat Rev Microbiol. 2005;3:819–823. doi: 10.1038/nrmicro1249. [DOI] [PubMed] [Google Scholar]
- Burleigh BA. Host cell signaling and Trypanosoma cruzi invasion: do all roads lead to lysosomes? Sci STKE. 2005;2005:pe36. doi: 10.1126/stke.2932005pe36. [DOI] [PubMed] [Google Scholar]
- Ruhl A, Trotter J, Stremmel W. Isolation of enteric glia and establishment of transformed enteroglial cell lines from the myenteric plexus of adult rat. Neurogastroenterol Motil. 2001;13:95–106. doi: 10.1046/j.1365-2982.2001.00246.x. [DOI] [PubMed] [Google Scholar]
- Da Mata JR, Camargos MR, Chiari E, Machado CR. Trypanosoma cruzi infection and the rat central nervous system: proliferation of parasites in astrocytes and the brain reaction to parasitism. Brain Res Bull. 2000;53:153–162. doi: 10.1016/s0361-9230(00)00326-9. [DOI] [PubMed] [Google Scholar]
- Green SH, Rydel RE, Connolly JL, Greene LA. PC12 cell mutants that possess low- but not high-affinity nerve growth factor receptors neither respond to nor internalize nerve growth factor. J Cell Biol. 1986;102:830–843. doi: 10.1083/jcb.102.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trischmann T, Tanowitz H, Wittner M, Bloom B. Trypanosoma cruzi: role of the immune response in the natural resistance of inbred strains of mice. Exp Parasitol. 1978;45:160–168. doi: 10.1016/0014-4894(78)90055-3. [DOI] [PubMed] [Google Scholar]
- Himmelweit F: Collected papers of Paul Ehrlich. Edited by London, Pergamon, 1956, 1960: p253 [Google Scholar]
- Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance, Proc Natl Acad Sci USA. 2002;99:351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archelos JJ, Hartung HP. Pathogenetic role of autoantibodies in neurological diseases. Trends Neurosci. 2000;23:317–327. doi: 10.1016/s0166-2236(00)01575-7. [DOI] [PubMed] [Google Scholar]
- Martin F, Chan AC. Pathogenic roles of B cells in human autoimmunity; insights from the clinic. Immunity. 2004;20:517–527. doi: 10.1016/s1074-7613(04)00112-8. [DOI] [PubMed] [Google Scholar]
- Martin F, Chan AC. B cell immunobiology in disease: evolving concepts from the clinic. Annu Rev Immunol. 2006;24:467–496. doi: 10.1146/annurev.immunol.24.021605.090517. [DOI] [PubMed] [Google Scholar]
- Wucherpfennig KW. Mechanisms for the induction of autoimmunity by infectious agents. J Clin Invest. 2001;108:1097–1104. doi: 10.1172/JCI14235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar SM, Porcelli SA. Mechanisms of autoimmune disease induction. The role of the immune response to microbial pathogens, Arthritis Rheum. 1995;38:458–476. doi: 10.1002/art.1780380403. [DOI] [PubMed] [Google Scholar]
- Mesri EA, Levitus G, Hontebeyrie-Joskowicz M, Dighiero G, Van Regenmortel MH, Levin MJ. Major Trypanosoma cruzi antigenic determinant in Chagas’ heart disease shares homology with the systemic lupus erythematosus ribosomal P protein epitope. J Clin Microbiol. 1990;28:1219–1224. doi: 10.1128/jcm.28.6.1219-1224.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borda ES, Sterin-Borda L. Antiadrenergic and muscarinic receptor antibodies in Chagas’ cardiomyopathy. Int J Cardiol. 1996;54:149–156. doi: 10.1016/0167-5273(96)02592-2. [DOI] [PubMed] [Google Scholar]
- Cunha-Neto E, Bilate AM, Hyland KV, Fonseca SG, Kalil J, Engman DM. Induction of cardiac autoimmunity in Chagas heart disease: a case for molecular mimicry. Autoimmunity. 2006;39:41–54. doi: 10.1080/08916930500485002. [DOI] [PubMed] [Google Scholar]
- Khoury EL, Ritacco V, Cossio PM, Laguens RP, Szarfman A, Diez C, Arana RM. Circulating antibodies to peripheral nerve in American trypanosomiasis (Chagas’ disease). Clin Exp Immunol. 1979;36:8–15. [PMC free article] [PubMed] [Google Scholar]
- Ribeiro dos Santos R, Marquez JO, Von Gal Furtado CC, Ramos de Oliveira JC, Martins AR, Koberle F. Antibodies against neurons in chronic Chagas’ disease. Tropenmed Parasitol. 1979;30:19–23. [PubMed] [Google Scholar]
- Hoch W, McConville J, Helms S, Newsom-Davis J, Melms A, Vincent A. Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med. 2001;7:365–368. doi: 10.1038/85520. [DOI] [PubMed] [Google Scholar]
- Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992;356:63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- Tanowitz HB, Burns ER, Sinha AK, Kahn NN, Morris SA, Factor SM, Hatcher VB, Bilezikian JP, Baum SG, Wittner M. Enhanced platelet adherence and aggregation in Chagas’ disease: a potential pathogenic mechanism for cardiomyopathy. Am J Trop Med Hyg. 1990;43:274–281. doi: 10.4269/ajtmh.1990.43.274. [DOI] [PubMed] [Google Scholar]
- Donovan MJ, Lin MI, Wiegn P, Ringstedt T, Kraemer R, Hahn R, Wang S, Ibanez CF, Rafii S, Hempstead BL. Brain derived neurotrophic factor is an endothelial cell survival factor required for intramyocardial vessel stabilization. Development. 2000;127:4531–4540. doi: 10.1242/dev.127.21.4531. [DOI] [PubMed] [Google Scholar]
- Lin MI, Das I, Schwartz GM, Tsoulfas P, Mikawa T, Hempstead BL. Trk C receptor signaling regulates cardiac myocyte proliferation during early heart development in vivo. Dev Biol. 2000;226:180–191. doi: 10.1006/dbio.2000.9850. [DOI] [PubMed] [Google Scholar]
- Lewinsohn R. Prophet in his own country: Carlos Chagas and the Nobel Prize. Perspect Biol Med. 2003;46:532–549. doi: 10.1353/pbm.2003.0078. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Cohen I, Lazarov-Spiegler O, Moalem G, Yoles E. The remedy may lie in ourselves: prospects for immune cell therapy in central nervous system protection and repair. J Mol Med. 1999;77:713–717. doi: 10.1007/s001099900047. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease–a double-edged sword. Neuron. 2002;35:419–432. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner M, Stadelmann C, Dechant G, Wekerle H, Hohlfeld R. Neurotrophic cross-talk between the nervous and immune systems: implications for neurological diseases. Ann Neurol. 2003;53:292–304. doi: 10.1002/ana.10446. [DOI] [PubMed] [Google Scholar]
- Moalem G, Leibowitz-Amit R, Yoles E, Mor F, Cohen IR, Schwartz M. Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nat Med. 1999;5:49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]
- Kipnis J, Yoles E, Schori H, Hauben E, Shaked I, Schwartz M. Neuronal survival after CNS insult is determined by a genetically encoded autoimmune response. J Neurosci. 2001;21:4564–4571. doi: 10.1523/JNEUROSCI.21-13-04564.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton DL, Sutherland J, Gripp J, Camerato T, Armanini MP, Phillips HS, Carroll K, Spencer SD, Levinson AD. Human TRKs: molecular cloning, tissue distribution, and expression of extracellular domain immunoadhesins. J Neurosci. 1995;15:477–491. doi: 10.1523/JNEUROSCI.15-01-00477.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]