Abstract
Toll-like receptors (TLRs) are key factors of innate immunity that detect pathogen invasion and trigger a host response. TLR4 can mediate a response through adaptor molecules, MyD88 or TRIF. In the present study, streptomycin-treated MyD88−/−, Tlr4−/−, Trif Lps2/Lps2, and C57BL/6 wild-type (WT) mice were infected with either Shiga toxin (Stx)-producing or non-producing Escherichia coli O157:H7. Moderate to severe clinical signs of disease developed in MyD88−/− (n = 21/21), Tlr4−/− (n = 12/16), Trif Lps2/Lps2 (n = 7/15) and WT mice (n = 6/20) infected with Stx-producing E. coli O157:H7 but not in mice inoculated with the Stx non-producing strain (n = 0/54, P < 0.001). MyD88−/− mice infected with Stx-producing E. coli O157:H7 developed the most severe disease and had the highest bacterial burden. Hematological analysis of sick MyD88−/− mice showed reduced red blood cell counts and reticulocytosis, suggesting hemolysis. Thrombocytopenia developed in MyD88−/−, Trif Lps2/Lps2, and WT mice, and creatinine levels were elevated in both MyD88−/− and WT mice infected with the Stx-producing strain. Renal histopathology showed evidence of glomerular capillary congestion, tubular desquamation, and fibrinogen deposition, and intestinal histopathology showed mucosal injury, edema, and inflammation in sick mice. Administration of purified Stx2 to MyD88−/− and WT mice led to severe disease in both groups, suggesting that MyD88−/− mice are not more sensitive to Stx than WT mice. As MyD88−/− mice developed the most severe disease hematological and pathological changes, the results suggest that dysfunctional innate immune responses via MyD88 enhanced Stx-induced disease.
Gastrointestinal infection with enterohemorrhagic Escherichia coli (EHEC) strains causes diarrhea and hemorrhagic colitis, and is the leading cause of hemolytic uremic syndrome (HUS), which is characterized by a triad of hemolytic anemia, thrombocytopenia, and acute renal failure, occurring predominantly in infants and young children.1 Shiga toxin (Stx), which is a major virulence factor, has been implicated as the main causative agent in EHEC-associated HUS since only Stx-producing bacteria have been linked with HUS.2,3 Other virulence factors include intimin, lipopolysaccharide (LPS), and E. coli-secreted proteins.4
Initially, orally ingested EHEC must be able to survive the conditions in the stomach and then to colonize the gut by adhering to intestinal epithelial cells.5,6 Stx produced by EHEC within the intestinal tract is capable of crossing the epithelial barrier in the intestine.6 Following translocation, the toxin remains biologically active6 and enters the bloodstream circulating bound to blood cells,7,8 allowing systemic dissemination and targeting the organs that express the specific toxin receptor Gb3.9 The kidney expresses high levels of the receptor10 and is a main target organ.
Previous animal models using whole EHEC bacteria for infection in mice have reproduced some aspects of human HUS. Mice developed gastrointestinal, neurological and systemic manifestations of disease following infection with the entire microorganism11 providing insight into the interactions of virulence factors required for EHEC pathogenesis. Histopathological changes in kidneys included acute tubular necrosis,12 mesangial cell and matrix expansion, vascular congestion, and interstitial inflammation.11 Hematological analysis showed fragmented erythrocytes11 but other blood parameters including hemoglobin concentration, hematocrit, platelet counts, and creatinine were not consistent with a diagnosis of HUS.13 The role of Stx, alone or in combination with LPS, for disease was also studied in mice (reviewed in14). Only mice that received both purified Stx2 and LPS developed characteristic signs of HUS.15
Early detection and defense against enteric bacterial pathogens requires the activation of the innate immune system, which uses a range of receptors to detect and respond to microorganisms and their components through signaling pathways16 involving Toll-like receptors (TLRs).17 Individual TLRs recognize distinct components of microbial pathogens18 and their respective ligands recruit the common adaptor protein MyD88 with subsequent activation of a signaling cascade that promotes the production of inflammatory cytokines and the recruitment of new effector cells. These events are necessary for clearing mucosal infections.17 In addition, there is MyD88-independent pathway that uses alternative adaptor molecules.18 Toll-like receptor 4 (TLR4), which has been identified as a receptor for LPS,16 can elicit an inflammatory response through the adaptor molecule MyD88 or through an alternative adaptor molecule known as TRIF that has been implicated in the MyD88-independent pathway for TLR4 signaling.19 MyD88 activation leads to early phase NF-κB activation,17 whereas TRIF activation initiates the type 1 interferon response as well as late NF-κB activation.20
This study examined the TLR4, MyD88, and TRIF signaling pathways in host response to EHEC infection. A wild-type C57BL/6 mouse model and knock-out mice (MyD88−/−, Tlr4−/−) as well as mutated mice (Trif Lps2/Lps2) on the same background were compared. The specific importance of Stx was addressed using Stx-producing and non-producing E. coli O157:H7 for inoculation.
Materials and Methods
Mice
MyD88−/−21 and Tlr4−/−22 mice were generated in the C57BL/6 mouse background at the BIKEN animal facilities (Osaka, Japan). Lps2, a non-functional codominant allele of Trif, was induced by N-ethyl-N-nitrosourea mutagenesis on the C57BL/6 mouse background19 at the Scripps Institute animal facilities (La Jolla CA). MyD88−/−, Tlr4−/−, Trif Lps2/Lps2 and C57BL/6 wild-type (WT) mice used in this study were bred in the animal facilities of the Department of Microbiology, Immunology and Glycobiology, Institute of Laboratory Medicine, Lund University. Male mice were used at 6 to 14 weeks of age.
Bacterial Strains and Cultures
The E. coli O157:H7 strains, 86-24 Stx2-producing, and 87-23 Stx2 non-producing were isolated during the Walla Walla Washington State outbreak of hemorrhagic colitis and HUS, in November 1986 and were kindly provided by A. D. O'Brien (Department of Microbiology and Immunology, Uniformed Services University of the Health Sciences, Bethseda, MD).23 These strains were previously fully genotypically and phenotypically characterized11 regarding presence of stx and eae genes, enterohemorrhagic E. coli 60-MDa plasmid, enteroaggregative E. coli localized adherence probe, enteropathogenic E. coli adherence factor, diffuse adherence E. coli probe, plasmid content, enterohemolysin, serotype, and the Vero cell assay. The strains were found to differ only in the production of Stx2 and consequently cytotoxicity for Vero cells. Spontaneous streptomycin (Str)-resistant derivatives of these strains were developed by growth on Luria-Bertani (LB) agar with a 50-μg-streptomycin disk, as previously described,24 and were termed 86-24 stx2+ (Strr) and 87-23 stx2− (Strr), respectively.
To enhance the virulence and to develop a strain capable of causing systemic infection, 86-24 stx2+ (Strr) and 87-23 stx2− (Strr) strains were first intragastrically inoculated into streptomycin-treated C57BL/6 WT mice (5g/L streptomycin sulfate; MP Biomedicals, Ohio, in drinking water 24 hours before challenge and throughout the experiment). Four mice were inoculated with the 86-24 stx2+ (Strr) strain, three displayed clinical signs of disease (described below). Bacteria were recovered on day 10 from colonic content of a sick mouse. Five mice were inoculated with the 87-23 stx2− (Strr) strain, which did not cause clinical signs of disease but colonized the intestine. Bacteria were recovered on day 5 from colonic content of an infected mouse. The resulting recovered strains termed P86-24 stx2+ (Strr) and P87-23 stx2− (Strr), respectively, were used in this study. Stock cultures of these strains were stored in LB/glycerol (85:15%, v/v) at −80°C until used.
Strains P86-24 stx2+ (Strr) and P87-23 stx2− (Strr) were subject to phenotypic analyses. The presence of the O157 serogroup antigen was confirmed by slide agglutination using an E. coli O157 latex test kit according to the manufacturer’s protocol (Oxoid, Basingstoke, UK). The presence or absence of Stx2 in E. coli strains P86-24 stx2+ (Strr) and P87-23 stx2− (Strr), respectively, was tested by the cytotoxic effects on Vero cells as described previously.11,25
Before inoculation strains were grown overnight at 37°C in LB broth supplemented with 50 μg/ml streptomycin and harvested by centrifugation. The pellet was washed in sterile phosphate buffer saline (PBS, pH 7.4; Medicago AB, Uppsala, Sweden) and resuspended in a solution of 20% (w/v) sucrose and 10% (w/v) NaHCO3 in sterile water.
Infection Protocol
Mice inoculated with E. coli O157:H7 strains P86-24 stx2+ (Strr) and P87-23 stx2− (Strr) are listed in Table 1. Mice were fasted 20 to 24 hours before the administration of bacteria and treated with streptomycin sulfate as described above to reduce the normal commensal flora, thereby enabling streptomycin-resistant strains to colonize the gastrointestinal tract.12,26 Under isoflurane anesthesia (Forene; Abbott, Wiesbaden, Germany), 100 μl of bacterial suspensions at 109 colony forming units/ml were administered intragastrically11 through a soft polyethylene catheter (0.61 mm OD; 0.28 mm ID; Clay Adams, Parsippany, NJ). Control mice received 100 μl of sterile PBS. After challenge the catheter was removed, food was reintroduced and provided ad libitum. Over the course of infection, mice were monitored three times per day and a final disease score was given to each mouse according to clinical signs observed as summarized in Table 2. After 8 days or when evident signs of disease (score 2 to 3 as per Table 2) were observed, infected and control mice were sacrificed by cervical dislocation. All experiments were approved by the Animal Ethics Committee of Lund University (protocol no. M231-05).
Table 1.
Numbers of Mice Inoculated with Stx2-Producing and Non-Producing E. coli O157:H7
| Inoculum | Number of mice
|
|||
|---|---|---|---|---|
| MyD88−/− | Tlr4−/− | TrifLps2/Lps2 | WT | |
| P86-24 stx2+ (Strr) | 21 | 16 | 15 | 20 |
| P87-23 stx2− (Strr) | 15 | 14 | 10 | 15 |
| PBS | 12 | 10 | 11 | 11 |
Table 2.
Disease Score
| Score | Characterization | Clinical signs |
|---|---|---|
| 0 | No clinical signs | — |
| 1 | Mild clinical signsa | Ruffled fur |
| 2 | Moderate clinical signs | Ruffled fur plus, lethargy, hunched posture, decreased activity |
| 3 | Severe clinical signs | Paresis, paralysis, tremor, shivers, ataxia, rigidity, coma |
| 4 | Death | — |
Mice that exhibited only mild clinical signs, did not show further signs of disease and recovered until the end of the experiment were not considered ill.
Confirmation of Colonization
To confirm the colonization of bacteria, fecal samples were collected 24 and 72 hours after challenge, plated on LB agar supplemented with 50 μg/ml streptomycin and tested for the presence of the O157 serogroup by latex agglutination.
Weight Measurement
To account for weight change during the experiment, weight was measured daily before and after infection in MyD88−/−, Tlr4−/−, Trif Lps2/Lps2 and WT mice infected with E. coli O157:H7 strains P86-24 stx2+ (Strr) and P87-23 stx2− (Strr) as well as control mice. Body weight changes were calculated as a percentage of initial body weight.
Bacterial Burden
Fresh feces were collected daily from MyD88−/−, Tlr4−/−, Trif Lps2/Lps2and WT infected with E. coli O157:H7 strains P86-24 stx2+ (Strr) and P87-23 stx2− (Strr). Feces were weighed and homogenized in 1 ml sterile PBS. Serial dilutions were plated onto LB agar plates supplemented with 50 μg/ml streptomycin, and colony forming units per gram of feces were calculated.
Hematological Analysis
Blood samples were collected retroorbitally with citrated Pasteur pipettes (0.129M citrate; BD Diagnostics, Plymouth, England) and used for analysis of creatinine levels, platelet counts, red blood cell (RBC) counts, and blood smears. Blood samples for creatinine measurement were centrifuged at 1000 × g for 10 minutes at 4°C, plasma samples were collected, passed through 0.2-μm pore size filters (Whatman, Dessel, Germany) and stored at −80°C until analyzed. Creatinine levels were measured using a creatinine assay kit (Cayman Chemical, Ann Arbor, MI) following the manufacturer’s instructions. RBC and platelet counts were performed using a hematocytometer (Scherf, Meiningen-Dreissigacker, Germany). Blood samples for platelet counts (100 μl) were diluted in 200 μl PBS and centrifuged at 200 × g for 2 minutes, platelet rich plasma samples were collected, and 10 μl of platelets were then diluted in 190 μl of PBS. Blood samples collected for films were smeared onto glass slides, allowed to dry for 30 minutes at room temperature and stained with May-Grünwald’s eosin-methylene blue and Giemsa solution (Merck, Darmstadt, Germany). Percentage of reticulocytes was determined by counting the number of reticulocytes per 1000 RBC in the blood smears, a reticulocyte was defined as an erythrocyte containing two or more stained intraerythrocytic particles.
Histological Analysis
Kidneys, and proximal and distal colon samples were collected for histological analysis. Samples were subject to macroscopic inspection and fixed overnight in 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO) followed by embedding in paraffin. For light microscopy, paraffin-embedded kidneys and intestines were sectioned (3 μm) and stained with H&E (Merck, Darmstadt, Germany) and periodic acid-Schiff, respectively. Kidneys were examined for glomerular, tubular, and vascular changes. Intestines were examined for inflammation. All samples were coded, examined in a blinded fashion and the degree of pathological findings was defined as not observed (−), mild (+), moderate (++), or severe (+++).
Immunofluorescence and Immunohistochemistry
For immunofluorescence, cryosections of tissue samples were obtained by fixation of kidneys in 4% paraformaldehyde at 4°C overnight, followed by 15% sucrose at 4°C for 24 hours, after which tissue was embedded in optimal cutting temperature compound (Tissue-Tek Sakura; Zoeterwoude, Netherlands), frozen over cold 2-methylbutane and kept at −80°C until analyzed. Frozen kidneys were sectioned (3 μm) and placed onto positively charged slides (SuperFrost Plus; Menzel-Gläser, Braunschweig, Germany). Slides were placed into a glass holder, washed in PBS for 3 minutes and rinsed in Milli-Q water. Fibrinogen deposition was examined in cryosections from kidneys with polyclonal rabbit anti-human fibrinogen fluorescein isothiocyanate-conjugated antibody (Dako, Glostrup, Denmark). Human and murine fibrinogen show a high degree of homology.27 The ability of this antibody to recognize murine fibrinogen was first confirmed in mouse plasma by immunoblot. Sections were then incubated with 50 μl of 160 μg/ml polyclonal rabbit anti-human fibrinogen fluorescein isothiocyanate-conjugated antibody, or with 50 μl of 160 μg/ml rabbit IgG fluorescein isothiocyanate as the control antibody (Abcam, Cambridge, UK) in a humidified atmosphere in the dark for 30 minutes at 37°C. Slides were rinsed in Milli-Q water, washed twice in PBS for 10 minutes in the dark, rinsed again in Milli-Q water and mounted with fluorescent mounting medium (Dako). Stained tissue sections were then examined under an Axiostar plus fluorescence microscope and an Axiocam MRc5 camera (Carl Zeiss, Göttingen, Germany). AxioVision AC software version 4.4 (Carl Zeiss, Göttingen, Germany) was used for image processing.
Fibrinogen was also detected by immunohistochemistry in paraffin sections from kidneys using polyclonal rabbit anti-human fibrinogen antibody (Dako) at 1 μg/ml. Rabbit immunoglobulin fraction (Dako) at 1 μg/ml was used as the control antibody. Signal was detected using EnVision+ System anti-rabbit horseradish peroxidase (DakoCytomation, Carpinteria, CA). Specificity of the secondary antibody was tested by omission of the primary antibody. Positive signal stained brown. Slides were examined by light microscopy (Axiostar Zeiss microscope, mounted with an Axiocam MRc5 camera; Carl Zeiss, Göttingen, Germany).
Stx2 Injection
Stx2 (a kind gift from T.G. Obrig, Dept of Nephrology, University of Virginia) was suspended in sterile PBS. The endotoxin content was tested by the limulus amebocyte assay (Coatex AB, Gothenburg, Sweden) and found to be 0.05 ng/μg Stx2. The Stx2 cytotoxicity was confirmed on Vero cells.11,25 WT and MyD88−/− mice received an intraperitoneal injection of Stx2 at 285 ng/kg. PBS injection was used in control mice. Mice were weighed daily and monitored for clinical signs of disease.
Statistical Analysis
Differences between mice infected with strains P86-24 stx2+ (Strr), P87-23 stx2− (Strr), or PBS controls, as well as differences between mouse strains regarding disease score, creatinine levels, and RBC and platelet counts were assessed by the Mann-Whitney U-test. A P value ≤0.05 was considered significant. Statistical analyses were performed using SPSS version 11 (SPSS, Chicago, IL).
Results
Clinical Signs of Disease Developed in Mice Infected with the P86-24 stx2+ (Strr) Strain
MyD88−/−, Tlr4−/−, Trif Lps2/Lps2 and WT streptomycin-treated mice were intragastrically infected with E. coli O157:H7 strains P86-24 stx2+ (Strr) and P87-23 stx2− (Strr) and compared with regard to clinical signs of disease. Mice infected with the P86-24 stx2+ (Strr) strain developed clinical signs in all mouse groups. Of all mice infected with the P86-24 stx2+ (Strr) strain, 46/72 (64%) developed illness (disease score ≥2), of which MyD88−/− 21/21 (100%), Tlr4−/− 12/16 (75%), Trif Lps2/Lps2 7/15 (47%), and WT mice 6/20 (30%). However, no mice (0/54) infected with the P87-23 stx2− (Strr) strain developed signs of disease, P < 0.001 comparing all mice inoculated with P86-24 stx2+ (Strr) to mice inoculated with P87-23 stx2− (Strr).
MyD88−/− Mice Exhibited the Most Severe Clinical Signs of Disease
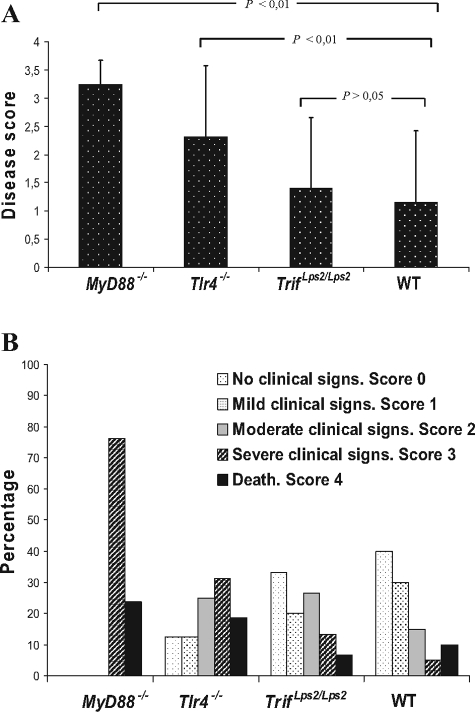
MyD88−/− mice exhibited the highest disease score after infection with the P86-24 stx2+ (Strr) strain, followed by Tlr4−/− mice, Trif Lps2/Lps2, and WT mice (Figure 1A). Clinical signs started with ruffled fur, hunched posture, decreased activity, and progressed to limb paresis or paralysis, tremor, ataxia, rigidity, dehydration, and coma occurring within 2 to 8 days after challenge. All MyD88−/− mice developed terminal disease (Figure 1B). In comparison, severe clinical signs (disease score ≥3) occurred in 8/16 (50%) Tlr4−/− mice within 4 to 8 days after challenge, 3/15 (20%) Trif Lps2/Lps2 mice within 5 to 8 days after challenge and 3/20 (15%) WT mice within 6 to 8 days after challenge. Trif Lps2/Lps2and WT mice had fewer clinical signs and 5/15 (33%) and 8/20 (40%) mice, respectively, remained asymptomatic (disease score 0). Taken together, these data show that MyD88−/− mice are highly susceptible to infection with the P86-24 stx2+ (Strr) strain.
Figure 1.
Clinical signs of disease in mice infected with the P86-24 stx2+ (Strr) strain. A: Disease score of mice infected with the P86-24 stx2+ (Strr) strain. The disease score was calculated as the median of all mice in each group as per Table 1. Bars show medians and error bars show standard deviations. B: Clinical signs of disease in mice infected with the P86-24 stx2+ (Strr) strain are presented as a percentage of the total number of inoculated mice in each group. Columns are lacking where mice did not have clinical signs of disease in that score. Data represent seven experiments with reproducible results.
Body Weight Changes during Infection
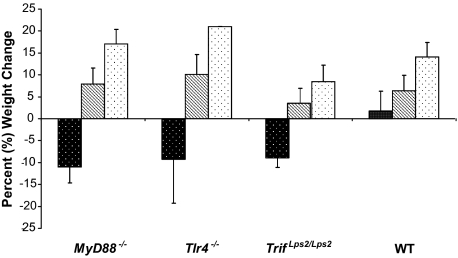
Mice were weighed before infection as well as daily after inoculation until the end of the experiment or when signs of terminal disease developed. Body weight changes were calculated as a percentage of the initial body weight. After eight days, the PBS control groups and the groups of mice inoculated with the P87-23 stx2− (Strr) strain did not show clinical signs of disease and exhibited weight gain, however, mice inoculated with the P87-23 stx2− (Strr) strain did not gain weight to the same extent as PBS control mice. In contrast, mice infected with the P86-24 stx2+ (Strr) strain exhibited weight loss at an average of 11% in the MyD88−/− group, 9,2% in the Tlr4−/− group, 8,8% in the Trif Lps2/Lps2 group, and the mice in the WT group exhibited an average weight gain of 1.7% (Figure 2).
Figure 2.
Body weight changes during the length of the experiment. Weight changes in mice infected with strain P86-24 stx2+ (Strr) (black) after a median of 5.2 days (range, 3 to 8 days) for the MyD88−/− group (n = 5); 7.1 days (range, 4 to 8 days) for the Tlr4−/− group (n = 8); 7 days (range, 6 to 8 days) for the Trif Lps2/Lps2 group (n = 5); and 7.7 days (range, 7 to 8 days) for the WT group (n = 8). Differences regarding the duration of the experiment in the different mouse groups reflect the time at sacrifice. Weight changes in mice infected with strain P87-23 stx2− (Strr) (hatched) (n = 24) and PBS controls (white) (n = 13) at the end of the experiment 8 days after inoculation. Bars show means and error bars show standard deviations.
Bacterial Burden
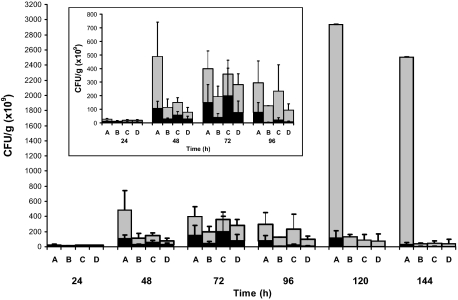
Mice in all groups (at least five mice per group and strain) infected with the P86-24 stx2+ (Strr) strain exhibited higher fecal counts of bacteria compared with mice infected with the P87-23 stx2− (Strr) strain (Figure 3). MyD88−/− mice exhibited elevated bacterial counts compared to the other mouse groups each day and particularly elevated levels on days 5 and 6. The other mouse groups infected with the P86-24 stx2+ (Strr) strain exhibited a peak between day 3 and 4 and a decrease after day 6 (Figure 3 see insert). By day 4, mice infected with the P87-23 stx2− (Strr) strain exhibited a progressive decrease in bacterial loads, except in MyD88−/− mice that did not show this decrease even by day 8 (data not shown).
Figure 3.
Bacterial burden. Bacterial counts in feces from mice infected with strains P86-24 stx2+ (Strr) (gray) and P87-23 stx2− (Strr) (black) were taken daily during eight days, the results from the first six days are presented. A: MyD88−/− mice. B: Tlr4−/− mice. C: Trif Lps2/Lps2 mice. D: WT mice. Standard deviations are presented in MyD88−/− mice up to day 4 as fewer (n = 2) MyD88−/− mice infected with strain P86–24 stx2+ (Strr) were alive from day 5. The insert shows details of days 1 to 4. Bars show means and error bars show standard deviations.
RBC Counts and Blood Smears Indicated Anemia and Hemolysis in Infected Mice
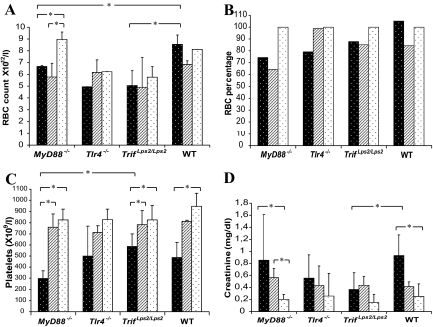
As EHEC infection may lead to HUS manifesting with hemolytic anemia, thrombocytopenia and renal failure the RBC counts, reticulocyte levels, platelet counts and creatinine levels were assayed in infected mice (at least five mice per group and strain). RBC counts were reduced in MyD88−/− mice infected with strains P86-24 stx2+ (Strr) and P87-23 stx2− (Strr) compared with the uninfected MyD88−/− controls (Figure 4A). RBC counts were also reduced in MyD88−/− mice when compared to WT mice infected with the P86-24 stx2+ (Strr) strain (Figure 4A). As a certain degree of variability was detected between PBS controls in the different mouse groups with regard to RBC counts, which may reflect genotypic differences in the various groups, the results are also presented as percentage of RBCs (Figure 4B). Reticulocyte counts were elevated in MyD88−/− and Tlr4−/− mice infected with the P86-24 stx2+ (Strr) strain: 19% ± 1.5% and 4,9% ± 0.3%, respectively (compared with the corresponding control groups in which the reticulocyte counts were below 0.1% P < 0.05). The elevated reticulocyte counts suggest hemolysis.
Figure 4.
Laboratory parameters in infected mice and controls. A: Red blood cell counts in mice infected with strains P86-24 stx2+ (Strr) (black), P87-23 stx2− (Strr) (hatched), and PBS controls (white). B: The red blood cell counts are presented as a percentage of the PBS control group for each mouse genotype due to variability between counts in the PBS control groups as presented in Panel A. C: Platelet counts. D: Creatinine levels. Bars show medians and error bars show standard deviations. *, P < 0.05.
Reduced Platelet Counts in Sick Mice
Platelet counts were significantly reduced in MyD88−/− mice infected with the P86-24 stx2+ (Strr) strain compared with the MyD88−/− PBS control group and MyD88−/− mice infected with the P87-23 stx2− (Strr) strain (Figure 4C). Tlr4−/− mice infected with the P86-24 stx2+ (Strr) strain showed decreased platelet counts, but this reduction was not statistically significant compared with the Tlr4−/− control group. Trif Lps2/Lps2 mice infected with the P86-24 stx2+ (Strr) strain exhibited significantly reduced platelet counts compared with the Trif Lps2/Lps control group and Trif Lps2/Lps2 mice infected with the P87-23 stx2− (Strr) strain. WT mice inoculated with the P86-24 stx2+ (Strr) strain also showed significantly decreased platelet counts compared with the WT PBS control group (Figure 4C). This indicates that infection with the P86-24 stx2+ (Strr) strain but not with the P87-23 stx2− (Strr) strain reduced platelet counts.
Elevated Creatinine Levels in Infected Mice
Creatinine levels were elevated in MyD88−/− and WT mice infected with the P86-24 stx2+ (Strr) strain compared with the corresponding control groups (Figure 4D). MyD88−/− mice showed a median of 0.85 mg/dl and WT mice showed a median of 0.93 mg/dl, which was significantly elevated, compared with the MyD88−/− control group (median 0.21 mg/dl) and the WT control group (median 0.25 mg/dl) respectively. Tlr4−/− and Trif Lps2/Lps2 mice infected with the P86-24 stx2+ (Strr) strain did not exhibit significantly elevated values compared with their PBS control groups (Figure 4D).
Sick Mice Developed Renal and Intestinal Pathology
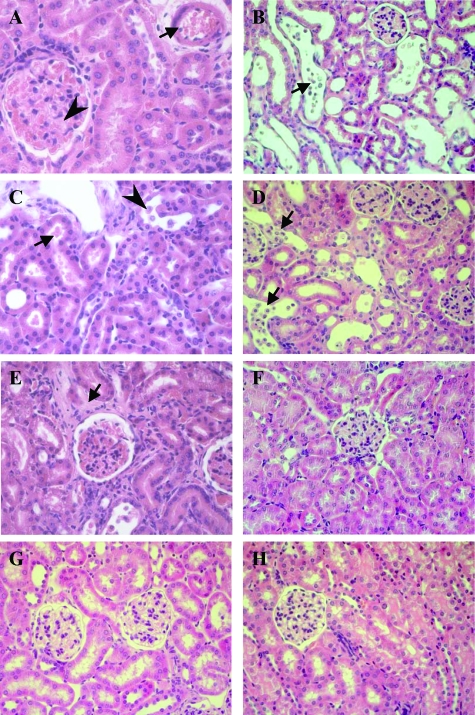
Kidneys from mice infected with the P86-24 stx2+ (Strr) strain were examined by light microscopy. The findings are summarized in Table 3. The most severe renal changes consisted of vascular congestion of cortical arteriolae and glomerular capillaries as well as tubular epithelial desquamation. These findings were mostly found in specimens from MyD88−/− and Tlr4−/− mice (Figure 5, A–E). Trif Lps2/Lps and WT mice inoculated with the P86-24 stx2+ (Strr) strain that did not develop disease exhibited mostly normal histology (Figure 6F). Mice inoculated with the P87-23 stx2− (Strr) strain (n = 12) and PBS control mice (n = 5) exhibited normal histology (Figure 5, G–H).
Table 3.
Pathological Findingsa in Mice Infected with P86-24 stx2+ (Strr)
| Tissue | Mice
|
|||
|---|---|---|---|---|
| MyD88−/− | Tlr4−/− | TrifLps2/Lps2 | WT | |
| Kidneys | n = 7b | n = 5c | n = 5d | n = 6e |
| Vascular congestion in glomerular capillaries and arteriolae | +++ e | +++ | + | + |
| Mesangial proliferation | + | − | − | + |
| Tubular epithelial desquamation | +++ | +++ | + | + |
| RBC in tubular lumen | − | + | − | − |
| Interstitial edema | − | + | − | − |
| Interstitial infiltrates | − | + | − | − |
| Intestines | n = 6b | n = 5c | n = 5f | n = 5f |
| Hyperemia | +++ | ++ | ++ | + |
| Edema | ++ | + | − | + |
| Erosions of the epithelial lining | ++ | + | − | − |
| Thickening of the submucosa | ++ | + | − | + |
| Damaged crypts | ++ | + | − | − |
| Inflammatory infiltrates | + | ++ | + | +++ |
The degree of pathological findings was defined as: (−) not observed, mild (+), moderate (++), or severe (+++).
All these mice were severely ill.
All mice had moderate-severe disease.
Milder pathological findings were found in two mice with disease scores 1 and 2, minimal vascular congestion or normal histology in asymptomatic mice (n = 3).
Pathology was found in mice with disease score 1 to 3 (n = 4), asymptomatic mice (n = 2) exhibited normal histology.
Pathology was only found in symptomatic mice (n = 3), asymptomatic mice (n = 2) exhibited normal histology or minimal inflammatory infiltrates.
Figure 5.
Renal cortical histology in infected mice and controls. A: MyD88−/− mouse infected with the P86-24 stx2+ (Strr) strain showing vascular arteriole congestion (arrow), glomerular capillary congestion, and mesangial expansion (arrowhead). B: MyD88−/− mouse infected with the P86-24 stx2+ (Strr) strain showing extensive tubular cellular desquamation (arrow). C: Tlr4−/− mouse inoculated with the P86-24 stx2+ (Strr) strain showing red blood cells in tubular lumina (arrow) indicating hematuria and tubular cellular desquamation (arrowhead). D: Tlr4−/− mouse inoculated with the P86-24 stx2+ (Strr) strain showing extensive tubular cellular desquamation (arrows). E: Tlr4−/− mouse inoculated with the P86-24 stx2+ (Strr) strain showing interstitial edema (arrow). F: WT mouse inoculated with the P86-24 stx2+ (Strr) strain showing normal histology. G: Renal tissue from a mouse inoculated with the P87-23 stx2− (Strr) strain showing normal histology. H: Renal tissue from a PBS control mouse. Magnification = original × 400.
Figure 6.
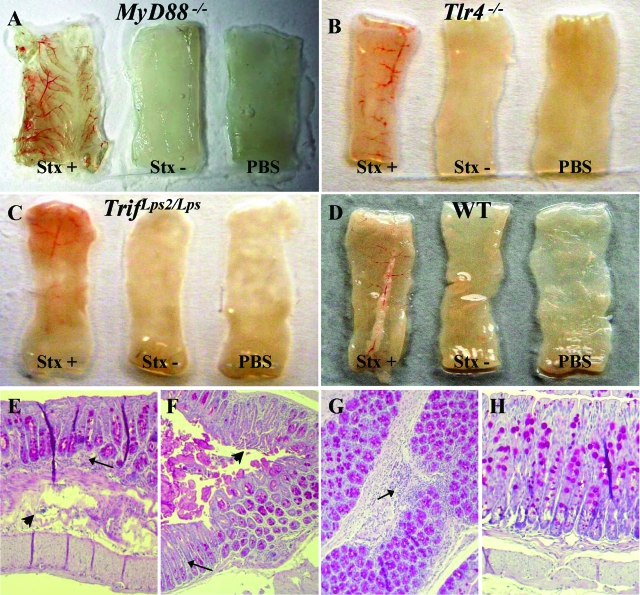
Intestinal pathology in infected mice. Macroscopic sections from the distal colon samples obtained from A: MyD88−/− mice. B: Tlr4−/− mice. C: Trif Lps2/Lps2 mice. D: WT mice. E: Distal colon from a MyD88−/− mouse infected with the P86-24 stx2+ (Strr) strain showing infiltrates (arrow) and damage and thickening of the submucosa (arrowhead). F: Distal colon from a MyD88−/− mouse infected with the P86-24 stx2+ (Strr) strain showing infiltrates (arrow) and surface epithelial erosions (arrowhead). G: Proximal colon from a WT mouse infected with the P86-24 stx2+ (Strr) strain showing infiltrates (arrow). H: Distal colon from a MyD88−/−PBS control mouse showing normal histology. Magnification = original × 100.
Intestines were subject to macroscopic examination showing that proximal and distal colons removed from sick mice were hyperemic with areas of punctiform bleeding, as shown in Figure 6A–D for distal colons. Proximal and distal colons from mice infected with the P86-24 stx2+ (Strr) strain were examined by light microscopy. The findings are summarized in Table 3 and demonstrated in Figure 6, E–H. Pathological findings in sick MyD88−/− and Tlr4−/− mice included mostly edema, surface epithelial erosions, thickening of the submucosa, damaged crypts, and minimal inflammatory infiltrates whereas intestines from WT mice showed larger areas of inflammatory infiltrates in mice with disease score 1 to 3 (n = 3) and normal histology in asymptomatic mice (n = 2). The control mice (n = 5) exhibited normal histology (Figure 6H). Intestines from mice inoculated with the P87-23 stx2− (Strr) strain were not examined.
Fibrinogen Deposition in Sick Mice
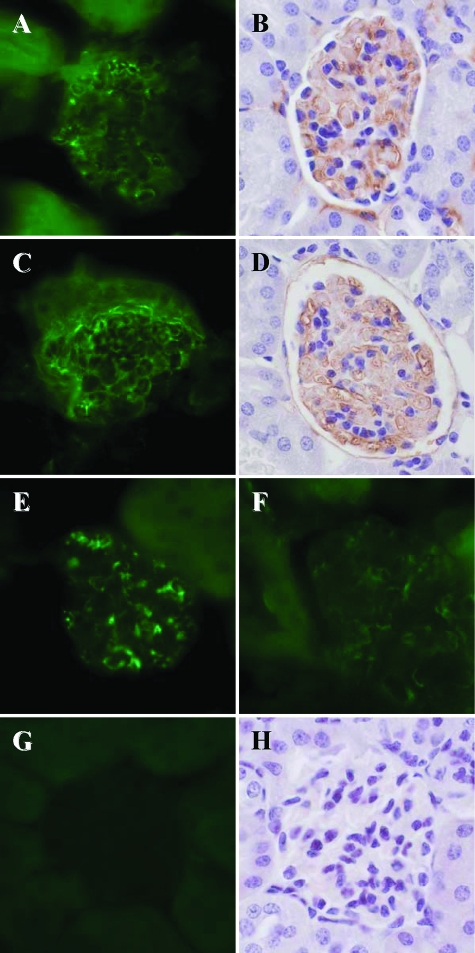
Fibrinogen deposition was detected by both immunofluorescence and immunohistochemistry in glomeruli and tubuli of MyD88−/− (n = 6 with severe clinical signs), Tlr4−/− (n = 5 with moderate-severe clinical signs), Trif Lps2/Lps2 (n = 5 with mild-moderate clinical signs), and WT mice (n = 5 with mild clinical signs) inoculated with the P86-24 stx2+ (Strr) strain (Figure 7, A–F). Renal tissues from PBS control mice (n = 5) were negative for fibrinogen (Figure 7, G and H) as were tissues from mice infected with the P86-24 stx2+ (Strr) strain labeled with the control antibody (not shown). Kidneys from mice inoculated with the P87-23 stx2− (Strr) strain were not examined.
Figure 7.
Immunofluorescence and immunohistochemistry for fibrinogen deposition in glomeruli of mice inoculated with the P86-24 stx2+ (Strr) strain. A, B: Renal tissues from MyD88−/− mice. C, D: Renal tissues from Tlr4−/− mice. E: Renal tissue from a Trif Lps2/Lps2 mouse. F: Renal tissue from a WT mouse. G, H: Renal tissues from a WT and a MyD88−/− PBS control mouse, respectively. Magnification = original × 600.
The Effect of Innate Immunity on Clinical Signs, Blood Counts, Renal Function, and Pathology
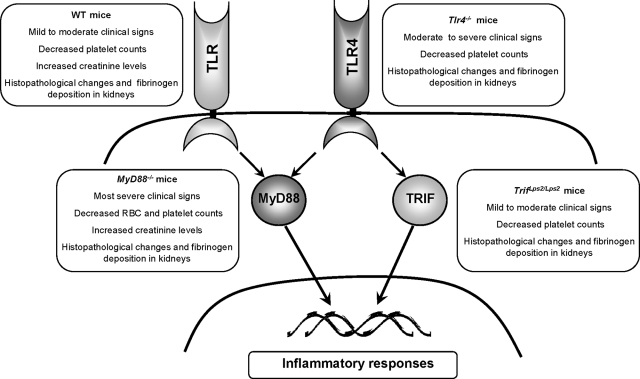
MyD88−/− mice exhibited the highest frequency and the most severe clinical signs after infection, with markedly elevated fecal bacterial counts, decreased RBC, and platelets counts and increased creatinine levels indicating decreased renal function. Severe histopathological changes were noted in the intestine and kidneys with fibrinogen deposition suggesting a prothrombotic state (Figure 8). Moderate to severe clinical signs, decreased platelet counts and histopathological changes were also noted in the Tlr4−/− mice, in comparison with the Trif Lps2/Lps2 and WT mice in which milder clinical signs were demonstrated as well as decreased platelet counts with milder renal pathology and no intestinal pathology. A decreased renal function (increased creatinine) was noted in WT mice as well.
Figure 8.
A schematic presentation of the role of TLR signaling through MyD88-dependent and independent pathways in Stx-induced disease.
The Effect of Purified Stx2 on Disease Development
The experiments with whole bacteria indicated that MyD88−/− mice were highly susceptible to Stx-induced disease. To discern if this was purely a toxin-mediated effect, or related to the mode of infection and host-pathogen interaction, Stx was injected intraperitoneally in MyD88−/− (n = 10) and WT (n = 10) mice. Administration of purified Stx2 induced disease in both groups of mice. All mice developed weight loss starting on day 2 after injection. The onset of clinical signs of disease was characterized by sporadic and isolated neurological signs (ataxia, paralysis, convulsions) and all mice developed severe clinical signs (disease score 3) between days 3 and 6 (data not shown). The results suggest that MyD88−/− mice are not more sensitive to the toxin than WT mice.
Discussion
Intestinal pathogens activate the transmembrane receptor TLR and thus prime the epithelial response in the intestinal mucosa. Failure to activate TLR signaling will lead to impaired inflammatory responses and defective bacterial clearance.17 The role of TLR4 and the intracellular adaptor proteins MyD88 and TRIF in E. coli O157 infection were investigated in a mouse model of intestinal bacterial infection showing that the highest fecal bacterial counts as well as the most severe clinical signs, hematological derangements and histopathology were noted in MyD88-deficient mice, moderate clinical signs in TLR4-deficient mice and milder clinical signs in the Trif Lps2/Lps2 and WT mice. Only mice infected with the Stx-producing strain developed signs of disease. This suggests that MyD88, which is common to all TLR receptors, is involved in the response to intestinal E. coli O157:H7 and that, in its absence, bacterial clearance is defective, allowing systemic spread of Stx leading to hemolysis, thrombocytopenia, and renal pathology resembling human HUS.
Various microbial ligands may bind TLRs on epithelial and blood cells. In the case of E. coli O157 known TLR ligands are LPS8 and flagellin.28 LPS binds to the extracellular domain of TLR4 as well as its co-receptors CD14 and MD2 leading to dimerization of TLR4.29 Intestinal epithelial cells express low levels of CD14 and release soluble CD1430 allowing this interaction to occur. Following dimerization intracellular adaptor proteins are recruited. TLR4 signaling may activate the MyD88-dependent pathway leading to activation of the transcription factor nuclear factor-κB or the MyD88-independent pathway involving TRIF resulting in activation of the transcription factors nuclear factor-κB and IRF-3.17,20 Flagellin binds to TLR528 and will thus activate only via the MyD88-dependent pathway.
All mice deficient in MyD88 exhibited severe disease and death within the shortest time after inoculation with the Stx-producing strain. This increased susceptibility was not due to increased sensitivity to the toxin as purified Stx caused the same clinical signs in WT and MyD88−/− mice. MyD88−/− mice have recently been shown to be deficient in RegIII gamma, a bactericidal lectin in the intestinal lumen. RegIII gamma is expressed in the small intestine of WT mice and enhances clearance of infection.31 We have shown that these mice had an increased intestinal bacterial burden suggesting that persistence of the bacteria in the gut may have contributed to enhanced virulence. Others have shown that MyD88−/− mice have a leakier intestinal barrier (even under control conditions),32 which may facilitate uptake of bacterial virulence factors. In addition, MyD88 is required for neutrophil recruitment during intestinal bacterial infection.33 Absence of MyD88 signaling in epithelial cells was shown to be associated with decreased production of cytoprotective factors such as interleukin-6 and KC-1.34 MyD88 has been suggested to be involved in the initial steps of mucosal inflammation35 and, although excessive inflammation may be deleterious, an initial response to E. coli O157:H7 in the gut could activate cytoprotective mechanisms necessary for bacterial clearance.
The high prevalence and severity of clinical signs in Tlr4−/− mice infected with the Stx-producing strain suggests that TLR4-dependent responses mediate a protective role against this bacterial infection by mounting an inflammatory response to LPS. The role of TLR4 in E. coli O157:H7 infection was previously addressed using C3H/HeJ mice,11 which have a point mutation in the TLR4 receptor36 rendering them hyporesponsive to LPS, in which the mice developed severe systemic and neurological signs compared to C3H/HeN mice, suggesting that hyporesponsiveness to LPS may have influenced bacterial clearance. When comparing the severity of clinical signs and rate of infection between MyD88−/− and Tlr4−/− mice, MyD88−/− mice exhibited a higher disease score as well as a higher incidence of illness, suggesting that Tlr4−/− mice are capable of mounting a first line of defense through alternative TLRs by recognizing molecular motifs present in E. coli O157:H7 other than LPS, such as flagellin,28 while the deficiency of MyD88 has consequences in the signaling pathways triggered by all TLRs (except TLR317) as depicted in Figure 8.
To date animal models in which the whole bacterium was used for inoculation have not succeeded to fully reproduce the symptoms and pathology of HUS (reviewed in14). We have previously described a mouse model in which C3H/HeN mice were intragastrically inoculated with E. coli O157:H7 and developed certain aspects of disease resembling HUS including gastrointestinal, neurological, systemic manifestations as well as pathology of the intestine and renal cortex and red blood cell fragmentation.11 Renal tubular injury was also demonstrated in the streptomycin-treated mouse model12,26 and recently in germ-free mice.37 Keepers et al demonstrated that mice co-injected with Stx2 and LPS developed thrombocytopenia, hemolysis and renal failure.15 Similar results were obtained in large animal models such as baboons38 and dogs.39 These latter models circumvent the mucosal innate immune response to bacterial colonization, which was more specifically addressed in the current study.
The mouse model we describe mimics certain aspects of HUS, particularly in the severely ill MyD88−/− mice. Mice developed reduced red blood cell counts suggesting anemia, hemolysis (elevated reticulocyte counts), thrombocytopenia, renal failure and glomerular pathology with fibrin deposition suggesting a prothrombotic state. Since weight loss reflecting a state of dehydration may affect levels of hemoglobin and creatinine, weight changes were studied. In the face of weight loss (Figure 2) hemoglobin levels will rise due to hemoconcentration associated with dehydration rather than a factual increase. For this reason RBC counts were studied and showed a decrease in severely ill MyD88−/− and Tlr4−/− mice compared to the controls suggesting that the hematocrit decreased during infection. In addition, reticulocyte counts were highly elevated in MyD88−/− and Tlr4−/− mice infected with the Stx-producing strain, suggesting a response to hemolytic anemia in which RBC are prematurely destroyed and reticulocytes are released into the bloodstream in an attempt to compensate this loss. The increase in creatinine levels in all mice infected with the Stx-producing strain most probably reflects glomerular and tubular damage as well as a degree of pre-renal failure due to dehydration. Unexpectedly, creatinine levels were comparable between WT mice and MyD88−/− mice. This may reflect the variability of the assay. In the clinical setting creatinine varies highly, and levels may vary considerably in human samples even when taken repeatedly in the same healthy individual. The levels presented here demonstrate a tendency to higher levels in mice inoculated with the Shiga toxin-producing strain (all except the Trif Lps2/Lps2 group) and these mice also exhibited more weight loss (dehydration) and renal pathology than mice inoculated with the Shiga toxin negative strain and PBS controls.
Clinical disease and histopathology did not develop in mice inoculated with the Stx non-producing strain suggesting that Stx was strongly associated with disease and pathology. Stx as well as E. coli O157 LPS circulate bound to platelets8,40 and induce their activation and aggregation. In humans and mice O157LPS binds to platelets via TLR4 in complex with P-selectin.8 TLR4 is required for LPS-induced activation of platelets leading to thrombocytopenia.8 Thus, as Tlr4−/− mice inoculated with the Stx-producing strain exhibited reduced platelet counts we propose that thrombocytopenia in these mice was induced by Stx (as platelet activation has been shown in vitro40) and not by LPS. As susceptibility to purified Stx2 did not account for the differences in disease presentation between mice in the different groups, this study shows that dysfunctional innate immune responses mediated via MyD88 enhanced Stx-induced disease. The increased bacterial burden in MyD88−/− mice suggests that disease and pathology in these mice was related to increased exposure to the toxin.
Footnotes
Address reprint requests to Diana Karpman, Department of Pediatrics, Clinical Sciences Lund, Lund University, 22185 Lund. E-mail: diana.karpman@med.lu.se.
Supported by grants from The Swedish Research Council (K2007-64X-14008-07-3 to DK, 07934, 14577, 14578 to C.S.), SIDA/SAREC, Sven Jerring Foundation, The Fund for Renal Research, Swedish Renal Foundation, Crown Princess Lovisa’s Society for Child Care, The Maggie Stephen’s Foundation, The Magnus Bergvall Foundation, The Foundation for Fighting Blood Disease, Thelma Zoegas Foundation and King Gustav Vs 80th Birthday Fund (all to D.K.), The Crafoord Foundation (to D.K. and C.S.), The Royal Physiographic Society (to T.J.R. and C.S.), The Medical Faculty, Lund University; The Network of Excellence Europathogenomics (NoE “EPG”); The Österlund, Lundberg and Wallenberg Foundations (to C.S.). Diana Karpman is the recipient of a clinical-experimental research fellowship from the Royal Swedish Academy of Sciences.
A part of this work was presented in poster form at The 2nd Bergamo Workshop on Hemolytic Uremic Syndrome, November 30 to December 1 2006, Mario Negri Institute, Bergamo, Italy, and at the 3rd International Workshop Thrombotic Microangiopathies, Hans-Knöll Institute, Jena, Germany October 4 to 6, 2007.
References
- Griffin PM. Escherichia coli O157:H7 and other enterohemorrhagic Escherichia coli. Blaser MJ, Smith PD, Ravdin JI, Greenberg HB, Guerrant RL, editors. New York: Raven Press; Infections of the gastrointestinal tract. 1995:739–761. [Google Scholar]
- Arbus GS. Association of verotoxin-producing E. coli and verotoxin with hemolytic uremic syndrome. Kidney Int. 1997;58:S91–S96. [PubMed] [Google Scholar]
- Karmali MA, Petric M, Lim C, Fleming PC, Arbus GS, Lior H. The association between idiopathic uremic syndrome and infection by verotoxin-producing Escherichia coli. J Infect Dis. 1985;151:775–782. doi: 10.1093/infdis/151.5.775. [DOI] [PubMed] [Google Scholar]
- Kaper JB. Enterohemorrhagic Escherichia coli. Curr Opin Microbiol. 1998;1:103–108. doi: 10.1016/s1369-5274(98)80149-5. [DOI] [PubMed] [Google Scholar]
- Paton JC, Paton AW. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin Microbiol Rev. 1998;11:450–479. doi: 10.1128/cmr.11.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe CM, Hurley BP, Acheson DW. Shiga toxin interactions with the intestinal epithelium. Methods Mol Med. 2003;73:263–273. doi: 10.1385/1-59259-316-x:263. [DOI] [PubMed] [Google Scholar]
- van Setten PA, Monnens LA, Verstraten RG, van den Heuvel LP, van Hinsbergh VW. Effects of verocytotoxin-1 on nonadherent human monocytes: binding characteristics, protein synthesis, and induction of cytokine release. Blood. 1996;88:174–183. [PubMed] [Google Scholar]
- Ståhl AL, Svensson M, Mörgelin M, Svanborg C, Tarr PI, Mooney JC, Watkins SL, Johnson R, Karpman D. Lipopolysaccharide from enterohemorrhagic Escherichia coli binds to platelets through TLR4 and CD62 and is detected on circulating platelets in patients with hemolytic uremic syndrome. Blood. 2006;108:167–176. doi: 10.1182/blood-2005-08-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg AA, Brown JE, Strömberg N, Westling-Ryd M, Schultz JE, Karlsson KA. Identification of the carbohydrate receptor for Shiga toxin produced by Shigella dysenteriae type 1. J Biol Chem. 1987;262:1779–1785. [PubMed] [Google Scholar]
- Boyd B, Lingwood C. Verotoxin receptor glycolipid in human renal tissue. Nephron. 1989;51:207–210. doi: 10.1159/000185286. [DOI] [PubMed] [Google Scholar]
- Karpman D, Connell H, Svensson M, Scheutz F, Alm P, Svanborg C. The role of lipopolysaccharide and Shiga-like toxin in a mouse model of Escherichia coli O157:H7 infection. J Infect Dis. 1997;175:611–620. doi: 10.1093/infdis/175.3.611. [DOI] [PubMed] [Google Scholar]
- Wadolkowski EA, Sung LM, Burris JA, Samuel JE, O'Brien AD. Acute renal tubular necrosis and death of mice orally infected with Escherichia coli strains that produce Shiga-like toxin type II. Infect Immun. 1990;58:3959–3965. doi: 10.1128/iai.58.12.3959-3965.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren S, Melton A, O'Brien A. Virulence of enterohemorrhagic Escherichia coli O91:H21 clinical isolates in an orally infected mouse model. Infect Immun. 1993;61:3832–3842. doi: 10.1128/iai.61.9.3832-3842.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proulx F, Seidman EG, Karpman D. Pathogenesis of Shiga toxin-associated hemolytic uremic syndrome. Pediatr Res. 2001;50:163–171. doi: 10.1203/00006450-200108000-00002. [DOI] [PubMed] [Google Scholar]
- Keepers TR, Psotka MA, Gross LK, Obrig TG. A murine model of HUS: Shiga toxin with lipopolysaccharide mimics the renal damage and physiologic response of human disease. J Am Soc Nephrol. 2006;17:3404–3414. doi: 10.1681/ASN.2006050419. [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Akira S, Yamamoto M, Takeda K. Role of adapters in Toll-like receptor signalling. Biochem Soc Trans. 2003;31:637–642. doi: 10.1042/bst0310637. [DOI] [PubMed] [Google Scholar]
- Hoebe K, Du X, Georgel P, Janssen E, Tabeta K, Kim SO, Goode J, Lin P, Mann N, Mudd S, Crozat K, Sovath S, Han J, Beutler B. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature. 2003;424:743–748. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- Kawai T, Takeuchi O, Fujita T, Inoue J, Mühlradt PF, Sato S, Hoshino K, Akira S. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IRF-3 and the expression of a subset of LPS-inducible genes. J Immunol. 2001;167:5887–5894. doi: 10.4049/jimmunol.167.10.5887. [DOI] [PubMed] [Google Scholar]
- Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- O'Brien AD, Melton AR, Schmitt CK, McKee ML, Batts ML, Griffin DE. Profile of Escherichia coli O157:H7 pathogen responsible for hamburger-borne outbreak of hemorrhagic colitis and hemolytic uremic syndrome in Washington. J Clin Microbiol. 1993;31:2799–2801. doi: 10.1128/jcm.31.10.2799-2801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TJ, Paton JC, Wang H, Talbot UM, Paton AW. Reduced virulence of an fliC mutant of Shiga-toxigenic Escherichia coli O113:H21. Infect Immun. 2006;74:1962–1966. doi: 10.1128/IAI.74.3.1962-1966.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmali MA, Petric M, Lim C, Cheung R, Arbus GS. Sensitive method for detecting low numbers of verotoxin-producing Escherichia coli in mixed cultures by use of colony sweeps and polymyxin extraction of verotoxin. J Clin Microbiol. 1985;22:614–619. doi: 10.1128/jcm.22.4.614-619.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadolkowski EA, Burris JA, O'Brien AD. Mouse model for colonization and disease caused by enterohemorrhagic Escherichia coli O157:H7. Infect Immun. 1990;58:2438–2445. doi: 10.1128/iai.58.8.2438-2445.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan K, Maeda N, Kluckman K, Lord S. Synthesis of a mouse model of the dysfibrinogen Vlissingen/Frankfurt IV. Ann NY Acad Sci. 2001;936:117–121. doi: 10.1111/j.1749-6632.2001.tb03498.x. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Iimura M, Kaper JB, Torres AG, Kagnoff MF. Role of Shiga toxin versus H7 flagellin in enterohaemorrhagic Escherichia coli signalling of human colon epithelium in vivo. Cell Microbiol. 2006;8:869–879. doi: 10.1111/j.1462-5822.2005.00673.x. [DOI] [PubMed] [Google Scholar]
- Miyake K. Endotoxin recognition molecules. Toll-like receptor 4-MD-2. Semin Immunol. 2004;16:11–16. doi: 10.1016/j.smim.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Funda DP, Tucková L, Farré MA, Iwase T, Moro I, Tlaskalová-Hogenová H. CD14 is expressed and released as soluble CD14 by human intestinal epithelial cells in vitro: lipopolysaccharide activation of epithelial cells revisited. Infect Immun. 2001;69:3772–3781. doi: 10.1128/IAI.69.6.3772-3781.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl K, Plitas G, Schnabl B, DeMatteo RP, Pamer EG. MyD88-mediated signals induce the bactericidal lectin RegIII gamma and protect mice against intestinal Listeria monocytogenes infection. J Exp Med. 2007;204:1891–1900. doi: 10.1084/jem.20070563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DL, Ma C, Bergstrom KS, Huang JT, Man C, Vallance BA. MyD88 signalling plays a critical role in host defence by controlling pathogen burden and promoting epithelial cell homeostasis during Citrobacter rodentium-induced colitis. Cell Microbiol. 2008;10:618–631. doi: 10.1111/j.1462-5822.2007.01071.x. [DOI] [PubMed] [Google Scholar]
- Lebeis SL, Bommarius B, Parkos CA, Sherman MA, Kalman D. TLR signaling mediated by MyD88 is required for a protective innate immune response by neutrophils to Citrobacter rodentium. J Immunol. 2007;179:566–577. doi: 10.4049/jimmunol.179.1.566. [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Strober W. Epithelial cells pay a Toll for protection. Nat Med. 2004;10:898–900. doi: 10.1038/nm0904-898. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Eaton KA, Friedman DI, Francis GJ, Tyler JS, Young VB, Haeger J, Abu-Ali G, Whittam TS. The pathogenesis of renal disease due to enterohemorrhagic Escherichia coli in germ free mice. Infect Immun. 2008;76:3054–3063. doi: 10.1128/IAI.01626-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor FB, Jr, Tesh VL, DeBault L, Li A, Chang AC, Kosanke SD, Pysher TJ, Siegler RL. Characterization of the baboon responses to Shiga-like toxin: descriptive study of a new primate model of toxic responses to Stx-1. Am J Pathol. 1999;154:1285–1299. doi: 10.1016/S0002-9440(10)65380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzke DM, Cowan LA, Schoning P, Fenwick BW. Glomerular ultrastructural lesions of idiopathic cutaneous and renal glomerular vasculopathy of greyhounds. Vet Pathol. 1995;32:451–459. doi: 10.1177/030098589503200501. [DOI] [PubMed] [Google Scholar]
- Karpman D, Papadopoulou D, Nilsson K, Sjogren AC, Mikaelsson C, Lethagen S. Platelet activation by Shiga toxin and circulatory factors as a pathogenetic mechanism in the hemolytic uremic syndrome. Blood. 2001;97:3100–3108. doi: 10.1182/blood.v97.10.3100. [DOI] [PubMed] [Google Scholar]