Abstract
Experimental autoimmune anterior uveitis (EAAU) serves as an animal model for human idiopathic AU, the most common form of intraocular inflammation of significant morbidity whose recurrence can lead to permanent vision loss. This study was undertaken to inhibit EAAU by inducing tolerance to melanin-associated antigen (MAA) and to investigate the underlying mechanisms responsible for tolerance induction. Intravenous administration of MAA both induced tolerance and inhibited EAAU in Lewis rats. Flow cytometric analysis revealed that the proliferation of lymph node cells in response to antigenic stimulation was drastically reduced in the state of tolerance both in vivo and in vitro. Our results from co-culture experiments demonstrated that intravenous administration of MAA led to the generation of T-regulatory cells that suppress T-cell proliferative responses and induce tolerance. Expression levels of both interleukin-10 and transforming growth factor-β2 were elevated whereas reduced levels of tumor necrosis factor-α, interferon-γ, and interleukin-2 were detected in tolerance-induced animals. Tolerance was reversed by replenishing these animals with recombinant interleukin-2. Tolerance could be adoptively transferred by removing lymph node cells from tolerance-induced donors and giving them to recipient rats. Interestingly, adoptive transfer of tolerance failed when lymph nodes cells were depleted of CD4+CD25+ T cells. In conclusion, T-cell nonresponsiveness because of active suppression mediated by T-regulatory cells facilitates the development of tolerance to MAA in EAAU.
Uveitis is broadly defined as inflammation of the uvea and is responsible for more than 2.8% of blindness in the US. Each year, 17.6% of active uveitis patients experience a transient or permanent loss of vision. A recent report from the Northern California Epidemiology Study suggested a higher disease rate for the older population in the US.1,2 Anterior uveitis (AU) is a term that refers to inflammation within the anterior segment of the eye; retinal involvement is not a component of AU.1,2,3 Idiopathic AU is the most common form of intraocular inflammation in humans and the recurrent nature of the disease can lead to permanent visual loss.1,2,3 Idiopathic AU is a disease that is associated with significant morbidity and can only be treated symptomatically, but not cured. Treatment options available to uveitis patients have limitations because uveitis is generally treated symptomatically with topical, periocular, or systemic administration of corticosteroids. Uveitis patients with severe unresponsiveness to corticosteroid may need immunosuppressive therapy.1,2,3 Unfortunately, these therapies are associated with serious side effects. Therefore, continuous efforts and future investigations are needed so that uveitis could be prevented and efficient and safe therapies for the future could be developed.
Antigen-specific immunological tolerance presents the opportunity to avoid complications associated with nonspecific therapies and offers a potential therapeutic alternative.4 In the current study we have used an experimental autoimmune anterior uveitis (EAAU) animal model to inhibit intraocular inflammation by inducing antigen-specific immunological tolerance. EAAU, a clinically relevant animal model of human idiopathic AU, is a CD4+ T-cell-mediated autoimmune disease of the eye.5,6,7,8,9,10,11 We have extensively characterized this model and have shown that severe inflammation occurs in the anterior segment of the eye of Lewis rats after the foot-pad injection of melanin-associated antigen (MAA) emulsified in Freund’s complete adjuvant.6,7,8,9,10,11
Immunological tolerance is a phenomenon by which the autoreactive cells are either eliminated or neutralized to provide the immune system the ability to distinguish between self and nonself.12 Breakdown of tolerance can lead to autoimmune disorder in which the immune system mounts an inappropriate attack on self-tissue. Suppression of immunological responsiveness by inducing antigen-specific immune tolerance has been reported to prevent and treat a number of autoimmune diseases.4,13
The state of antigen-specific immunological tolerance can be produced by administration of an antigen via various routes including oral, nasal, and intravenous.14,15,16,17,18,19,20,21,22,23,24 Tolerance induction is a complex process and may involve various mechanisms such as induction of anergy in antigen-specific T cells,23,24 physical elimination of effector T cells via apoptosis,25 suppression of immune responses through the secretion of anti-inflammatory molecules, such as transforming growth factor (TGF)-β and interleukin (IL)-10,26,27 and induction of CD4+CD25+ T-regulatory cells (Tregs).28 These mechanisms may operate alone or simultaneously during the course of tolerance development.
The present study was undertaken to inhibit EAAU by intravenously-induced immune tolerance to MAA. Additionally, in vivo and in vitro methods were used to elucidate the underlying mechanism(s) of intravenously-induced immune tolerance to MAA that leads to the inhibition of EAAU. Inhibition of EAAU by inducing immunological tolerance to MAA via oral and intranasal routes is currently being explored in our laboratory.
Materials and Methods
Animals
Pathogen-free male Lewis rats (5 to 6 weeks old) were obtained from Harlan Sprague Dawley (Indianapolis, IN). This study was approved by the Institutional Animal Care and Use Committee, University of Arkansas for Medical Sciences, Little Rock, AR.
Induction and Evaluation of EAAU
MAA was purified from bovine iris and ciliary body as previously described by us.11 Male Lewis rats were immunized with 100 μl of stable emulsion containing 75 μg of MAA emulsified (1:1) in CFA (Sigma-Aldrich, St. Louis, MO) using a single-dose induction protocol in the hind footpad as previously described by us.11 Depending on the experiment, animals were examined daily between days 3 and 30 after injection for the clinical signs of uveitis using slit lamp biomicroscopy. EAAU was scored by an observer unaware of the experimental design. Intensity of uveitis was scored in a masked manner on the arbitrary scale of 0 to 6, as follows: 0, normal; 1, dilated iris vessels and thickened iris and ciliary body; exudates in the anterior chamber with protein, a few scattered inflammatory cells, or both; 2, moderate infiltration of inflammatory cells in the iris, ciliary body, or both; moderate number of inflammatory cells within the anterior chamber; 3, heavy infiltration of inflammatory cells within the iris and ciliary body and within the anterior chamber; 4, heavy exudation of cells with dense protein aggregation in the anterior chamber; inflammatory cell deposits on the corneal endothelium; 5, presence of hemorrhage with extremely heavy infiltration of inflammatory cells within the iris, the ciliary body, and the anterior chamber as well as dense inflammatory cell deposits on the corneal endothelium; 6, extreme hemorrhage with very dense deposits on corneal endothelium. Eyes were also harvested at various time points for histological analysis to assess the course and severity of inflammation using the criteria previously reported.6,29
Induction of Immune Tolerance
To induce tolerance, each Lewis rat received a total of three intravenous injections of MAA at the following three time points: 7 days before (day −7), 4 days before (day −4), and 1 day before (day −1) immunization with MAA to induce EAAU. MAA was dissolved 400 μl of phosphate-buffered saline (PBS) and three different doses (100, 200, and 400 μg) of MAA were used separately at these time points. Lewis rats injected similarly with 400 μg of ovalbumin (Sigma-Aldrich) or PBS (400 μl) were used as control. Twenty-four hours after the last intravenous injection (day 0), Lewis rats were immunized subcutaneously with MAA to induce EAAU as described above. A separate group of animals tolerized by intravenous administration of MAA (400 μg in 400 μl of PBS) received a single injection of recombinant IL-2 (rIL-2; R&D Systems, Minneapolis, MN) intravenously (2 μg/rat in 400 μl of reconstitution solution) on days 8, 10, and 12 after immunization with MAA. Control animals received 400 μl of reconstitution solution. Reconstitution solution was prepared according to the manufacturer’s specifications (R&D Systems). Animals were monitored for the development and severity of EAAU as described above.
Histology
Freshly enucleated rat eyes were fixed in neutral buffered 10% formalin solution (Sigma-Aldrich) for 24 hours at room temperature, dehydrated in ethanol through ascending series of ethanol concentrations and embedded in paraffin. Four-μm sections were stained with hematoxylin and eosin (H&E) purchased from Fisher Scientific (Fair Lawn, NJ). Sections were examined using an Axioskop microscope (Carl Zeiss Meditec, Inc., Thornwood, NY).
Cell Preparation from Popliteal Lymph Nodes, Spleen, and Eye
Single cell suspension from spleen, popliteal lymph nodes (LNs), and eye was prepared by mashing the tissues with frosted slides (Fisher Scientific) followed by filtration through the cell strainer (BD Biosciences, San Jose, CA). Red blood cells were removed from LN cells by treating the cells with red blood cell lysis buffer (2.07 g NH4Cl, 0.25 g NaHCO3, 9.3 mg ethylenediaminetetraacetic acid, in 100 ml H2O). Total lymphocytes were purified by passing the cells through Histopaque gradient (Sigma Aldrich) according to the manufacturer’s protocol. The cells were suspended in complete RPMI 1640 culture medium with l-glutamine (Mediatech, Herndon, VA) containing 1% (v/v) minimum essential medium (Life Technologies, Rockville, MD), NEAA (BioWhittaker, Allendale, NJ), mixture of antibiotics (100 U/ml penicillin, 100 U/ml streptomycin, and 0.25 μg/ml Amphotericin B) purchased from BioWhittaker, and 10% (v/v) fetal calf serum (Mediatech).
Cell Separation
Total lymphocytes harvested from the popliteal LNs of tolerized and nontolerized animals were plated with MAA (10 μg/ml) for 3 days and nonadherent cells were collected. For depletion of CD25+ cells nonadherent cells were labeled with biotin-labeled CD25 antibody (BD Biosciences). CD25+ cells were then removed using anti-biotin microbeads (Miltenyi Biotec, Auburn, CA) according to the manufacturer’s instructions.
Co-Culture Experiments
For co-culture experiments, the cells were separated using a BD FACSAria cell sorter (BD Biosciences). Briefly, total lymphocytes from LNs of tolerized and nontolerized animals were labeled with anti-CD4-APC and anti-CD25-PE. The CD4+CD25−, CD4+CD25+, and CD4− cells were sorted in different tubes using BD FACSAria. CD4+CD25− cells were labeled with carboxyfluorescein succinimidyl ester (CFSE). The cells were cultured with MAA (10 μg/ml) for 6 days as follows: well 1 received CD4− (rest of the cells after separation of CD4+ cells), CD4+CD25+ and CFSE-labeled CD4+CD25− cells from the popliteal LNs of the tolerized animal. Well 2 received CD4− (rest of the cells after separation of CD4+ cells) and CFSE-labeled CD4+CD25− cells from tolerized animal along with CD4+CD25+ cells from the nontolerized animal. Well 3 received CD4− (rest of the cells after separation of CD4+ cells), CD4+CD25+ cells and CFSE-labeled CD4+ CD25− cells from the popliteal LNs of the nontolerized animal. Well 4 received CD4− (rest of the cells after separation of CD4+ cells) and CFSE-labeled CD4+ CD25− cells from popliteal LNs of nontolerized animal along with CD4+CD25+ cells from the tolerized animal. In a separate set of experiment wells 1 and 2 received CD4− (rest of the cells after separation of CD4+ cells) and CFSE-labeled CD4+CD25− cells from the popliteal LNs of the tolerized animal. Wells 3 and 4 received CD4− (rest of the cells after separation of CD4+ cells) and CFSE-labeled CD4+CD25− cells from the popliteal LNs of the nontolerized animal. MAA (10 μg/ml) was added to wells 1 and 3 whereas wells 2 and 4 did not receive MAA. In this set of experiment wells 1, 2, 3, and 4 did not receive CD4+CD25+ cells. On the 7th day the cells were collected and CFSE-positive cells were analyzed on FACSCalibur. The peaks for each generation and data were obtained by analyzing the raw data on the ModFit Proliferation Wizard program Verity Software, Topsham, ME.
Flow Cytometry
Surface Stain
One million cells were treated with anti-rat CD32 antibody (BD Biosciences) for 15 minutes at 4°C to block the Fc receptors. Appropriately diluted primary antibody (anti- rat CD25-PE, anti-rat CD4-APC, and anti-rat CD8a-APC from Biolegend (San Diego, CA) was added and incubated for 30 minutes at 4°C in dark. The cells were then washed with stain buffer (BD Biosciences). Cells were resuspended in 500 μl of stain buffer for flow analysis on BD FACSCalibur (BD Biosciences). The data were then analyzed on WinMDI 2.8 J. Trotter, The Scripps Research Institute, La Jolla, CA.
Intracellular Staining
Total lymphocyte harvested from popliteal LNs were cultured overnight with MAA (10 μg/ml) and Golgi stop (BD Biosciences). The Fc receptor was blocked and stained for surface markers as described above. The cells were washed two times with stain buffer and were resuspended in 100 μl of cytofix/cytoperm solution (BD Biosciences) containing optimal concentration of primary antibody IL-2 (R&D Systems) for 20 minutes at 4°C. The cells were then washed two times with Perm/Wash solution (BD Biosciences) and incubated with secondary antibody for 20 minutes in the dark at 4°C. The cells were washed again two times with Perm/Wash solution (BD Biosciences) and resuspended in 500 μl of stain buffer (BD Biosciences) for flow analysis.
For FoxP3 intracellular staining, total lymphocyte cell suspension was prepared from the popliteal LNs harvested at different time points during EAAU. The cells were labeled with surface markers and were then labeled intracellularly with fluorescein isothiocyanate-conjugated FoxP3 antibody (eBiosciences, San Diego, CA) as described above.
Cell Proliferation Analysis
In Vitro Cell Proliferation Assay
Total lymphocytes from popliteal LNs were labeled with CFSE using the Cell Trace CFSE cell proliferation kit according to the manufacturer’s protocol (Molecular Probes, Invitrogen, Carlsbad, CA) and were cultured with MAA (10 μg/ml) for 6 days. CFSE-labeled total lymphocytes from tolerized and nontolerized rats cultured in the absence of MAA served as control. The cells were then collected, labeled for surface markers, and used for flow analysis. The raw data from FACSCalibur was analyzed using the ModFit Proliferation Wizard Program.
In Vivo Cell Proliferation Assay
An equal number of CFSE-labeled total lymphocytes from popliteal LNs of tolerized and nontoleralized animal were intravenously injected into naïve Lewis rats. The rats were immunized with MAA 24 hours after the cell transfer. CFSE-positive cells harvested from spleen, popliteal LNs, and eyes at day 12 after immunization were analyzed using FACSCalibur. The data were further analyzed using the ModFit Proliferation Wizard program.
Enzyme-Linked Immunosorbent Assay (ELISA)
Total lymphocytes harvested from popliteal LNs were cultured in the absence and presence of MAA (10 μg/ml) for 24 hours. Supernatants were collected after 24 hours for quantitative ELISA for interferon (IFN)-γ, tumor necrosis factor (TNF)-α, IL-10 (BD PharMingen, San Diego, CA), and IL-2 (R&D Systems). ELISA was performed using paired mAbs according to the manufacturer’s recommendations. The concentration of each cytokine was calculated by computer software using the standard curves obtained from known concentrations. The values were then normalized by the amount of total protein present in the culture supernatant.
Semiquantitative Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) Analysis
Equal amounts of the total RNA were used to detect the mRNA levels of β-actin, TNF-α, IFN-γ, IL-10, TGF-β2, and IL-2 by RT-PCR using a RNA-PCR kit (Bio-Rad, Hercules, CA). Total RNA was extracted using the SV total RNA isolation kit (Promega, Madison, WI) according to the manufacturer’s protocol. The sense and anti-sense oligonucleotide primers for rat proteins were synthesized at IDT (Coralville, IA). The primer sequences as well as the predicted sizes of amplified cDNA are presented in the Table 1. The cDNA was synthesized using MuLV reverse transcriptase (Bio-Rad) with 1 μg of total RNA in a total volume of 20 μl (pH 8.3). Reverse transcription reaction was performed according to the manufacturer’s manual. The cDNA (2 μl per reaction) was then amplified by PCR using 20, 25, 30, and 35 cycles. The thermal cycle profile used in this study is as follows: i) an initial denaturing at 95°C for 5 minutes, then 30 seconds in each cycle; ii) annealing the primer with DNA at 55°C for 30 seconds; and iii) extending the primer at 72°C for 30 seconds. All reactions were normalized for β-actin expression. The negative controls consisted of omission of RNA template or reverse transcriptase from the reaction mixture. PCR products were analyzed on a 2% agarose gel and visualized by using the Gel Doc XR and Quantity One program (Bio-Rad). These experiments were repeated three times with similar results.
Table 1.
Primer Sequences Used in RT-PCR for Each Protein
| Gene | Primer | Sequence | Amplified PCR product size (bp) |
|---|---|---|---|
| β-Actin | Forward | 5′-GCGCTCGTCGTCGACAACGG-3′ | 335 |
| Reverse | 5′-GTGTGGTGCCAAATCTTCTCC-3′ | ||
| IFN-γ | Forward | 5′-ATCTGGAGGAACTGGCAAAAGGACG-3′ | 288 |
| Reverse | 5′-CCTTAGGCTAGATTCTGGTGACAGC-3′ | ||
| TNF-α | Forward | 5′-ATGATCCGAGATGTGGAACTGGCA-3′ | 295 |
| Reverse | 5′-GCTCCTCTGCTTGGTGGTTTGCTA-3′ | ||
| IL-10 | Forward | 5′-AAGGACCAGCTGGACAACAT-3′ | 292 |
| Reverse | 5′-AGACACCTTTGTCTTGGAGCTTA-3′ | ||
| TGF-β2 | Forward | 5′-ATCGTCCGCTTCGATGTCTCAACA-3′ | 274 |
| Reverse | 5′-ATCCCAGGTTCCTGTCTTTGTGGT-3′ | ||
| IL-2 | Forward | 5′-TTGCACTGACGCTTGTCCTCCTT-3′ | 398 |
| Reverse | 5′-CCATCTCCTCAGAAATTCCACCAC-3′ |
The sequences of all oligonucleotides are shown in 5′ to 3′ direction.
Statistical Analysis
The data are expressed as the mean ± SD. Data were analyzed and compared using the Student’s t-test, and differences were considered statistically significant with P < 0.05.
Results
Effect of Intravenous MAA Administration on the Induction of EAAU
MAA was administered intravenously to induce tolerance against MAA in the EAAU animal model. Three intravenous injections of MAA before the induction of the disease (ie, 7, 4, and 1 day before immunization with MAA) resulted in suppression of EAAU and development of tolerance to MAA in a dose-dependent manner (Table 2). Complete protection (tolerance) was observed both clinically and histologically with 400 μg of MAA (Table 2, Figure 1A). However, the animals that received 200 μg of MAA intravenously, were not protected completely (Table 2). Five of eight eyes developed mild EAAU whereas two of eight eyes developed severe disease (Table 2). In contrast, intravenous administration of 100 μg of MAA did not induce tolerance against MAA (Table 2). Similarly, intravenous injection of OVA or PBS (Table 2) did not induce tolerance and all animals developed the normal course of EAAU with massive cellular infiltration in the iris, ciliary body, and the anterior segment of the eye (Figure 1B). Based on these results 400 μg of MAA were used in subsequent experiments.
Table 2.
Effect of Intravenous Administration of MAA on EAAU
| MAA i.v. (μg) | OVA i.v. (μg) | PBS i.v. (μl) | Eyes with EAAU
|
Day of onset | Duration of disease (days) | ||
|---|---|---|---|---|---|---|---|
| Incidence | Mild | Severe | |||||
| 100 | 0 | 400 | 8/8 | 0/8 | 8/8 | 14 ± 1 | 12 ± 1 |
| 200 | 0 | 400 | 7/8 | 5/8 | 2/8 | 15 ± 1 | 10 ± 1 |
| 400 | 0 | 400 | 0/24 | ||||
| 0 | 400 | 400 | 24/24 | 0/24 | 24/24 | 14 ± 1 | 12 ± 1 |
| 0 | 0 | 400 | 24/24 | 0/24 | 24/24 | 14 ± 1 | 12 ± 0.5 |
EAAU, Experimental autoimmune anterior uveitis; MAA, melanin-associated antigen; OVA, ovalbumin; PBS, phosphate buffered saline; i.v., intravenous.
Incidence of EAAU given as positive/total eyes after clinical examination. Severity of inflammation on histopathological examination was grouped as normal (0), mild (1+ to 2+), or severe (3+ to 4+).
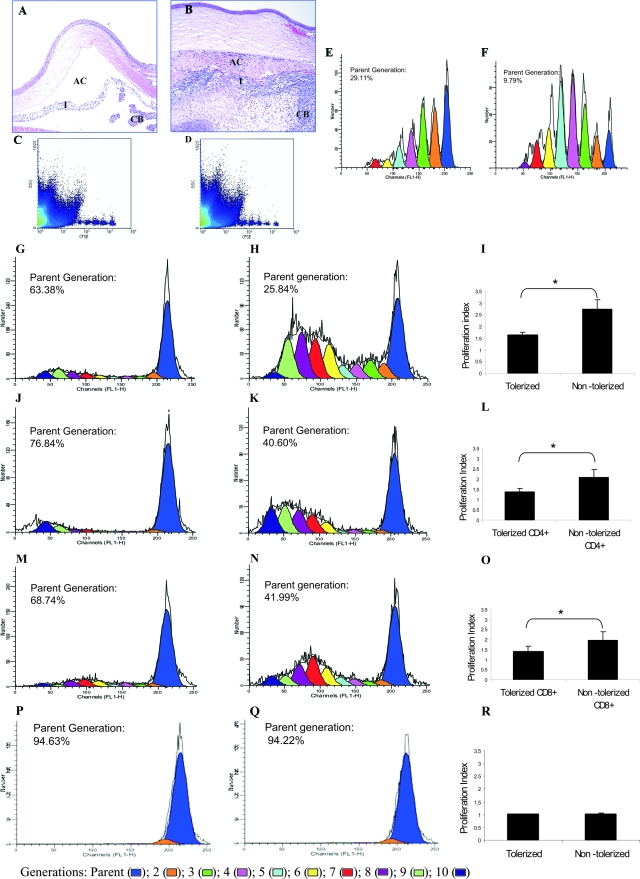
Figure 1.
Induction of tolerance to MAA in EAAU by intravenous injection of MAA. Histopathological changes in the eyes of Lewis rats injected with MAA (tolerized, A) and PBS (nontolerized, B) intravenously. At the peak of EAAU (day 19 after immunization) eyes were harvested and stained with H&E. B: Note the heavy infiltration of inflammatory cells within the iris (I), the ciliary body (CB), and the anterior chamber (AC) of nontolerized rats. A: No inflammation was observed in the eyes of tolerized rats. C–F: In vivo proliferation of total lymphocytes derived from the popliteal LNs. Representative raw data (obtained by FACS analysis) for in vivo proliferation of CFSE-labeled donor-derived total lymphocytes obtained from tolerized (C) and nontolerized (D) rats. E–F: Donor population was gated and analyzed from the spleen samples of recipient rats and CFSE histograms were obtained by the analysis of the raw data using ModFit proliferation wizard software. Each peak represents a different generation and channel FL1-H represents CFSE signal. In vivo proliferation of CFSE-labeled total lymphocytes from tolerized donor (E) was markedly suppressed compared to nontolerized donor (F) in the spleen of recipient Lewis rats. G–R: In vitro proliferation in response to MAA. Total lymphocytes (G), CD4+ T cells (J), and CD8+ T cells (M) from popliteal LNs (LNs) of tolerized animals have impaired proliferative response to MAA in vitro. A strong proliferative response for total lymphocytes (H), CD4+ T cells (K), and CD8+ T cells (N) from nontolerized animals was observed when the cells were cultured in the presence of MAA. I, L, and O: Cumulative proliferation index data from three independent experiments for total lymphocyte, CD4+ T cells, and CD8+ T cells, respectively. Popliteal LN lymphocytes derived from tolerized (P) and nontolerized (Q) animals cultured in the absence of MAA served as control. P and Q are representative CFSE histograms whereas R is cumulative proliferation index data from three independent control experiments. *P < 0.05. Original magnifications, ×10.
Effect of Intravenous MAA Administration on Proliferation of LN Cells
Because EAAU is induced by foot-pad injection of MAA,5,6 experiments were performed to investigate the contribution of draining LN (ie, popliteal LN) cells in the development of tolerance to MAA. For this we studied the proliferation (both in vivo and in vitro) of total lymphocytes harvested from the popliteal LNs of tolerized and nontolerized animals.
In Vivo Proliferation
Tolerized and nontolerized Lewis rats were sacrificed at day 14 after immunization (onset of EAAU) with MAA. An equal number of carboxyfluorescein diacetate succinimidyl ester-labeled total lymphocytes harvested from popliteal LN of donor-tolerized and donor-nontolerized animals were intravenously injected into naïve Lewis rats (recipients) separately. The recipient rats were immunized with MAA 24 hours after lymphocyte transfer. At day 12 after immunization total lymphocytes were harvested from the spleen, popliteal LNs, and eyes of recipient animals and were analyzed on FACSCalibur. CFSE-labeled cells were undetectable in the eye and few cells were detected in the LNs of the recipient rats (data not shown). The majority of CFSE-labeled cells obtained from both tolerized and nontolerized animals were detected in the spleen of the recipient rats (Figure 1, C and D). Equal numbers of CFSE-positive cells were gated and their proliferation was analyzed using the ModFit Proliferation Wizard Program. CFSE is an intracellular stain that passively diffuses inside the cells and is passed on to the next generation. The daughter cells are half as fluorescent as the parent cells. The ModFit Proliferation Wizard Program detects each generation in the form of individual peak based on the intensity of CFSE stain. Each peak corresponds to one generation and up to 10 generation can be monitored with the right most peak (blue) representing the parent generation (Figure 1, E and F). Furthermore, the percentage of cells in each generation including the cells remaining in the parent generation as well as proliferation index can also be calculated using this program. Our results demonstrated that intravenously injected CFSE-labeled lymphocytes proliferated in vivo at a higher rate in the recipient animals that received CFSE-labeled lymphocytes from nontolerized animals (proliferation index = 4.35; Figure 1, D and F) compared to those that received cells from tolerized animal (proliferation index = 2.10; Figure 1, C and E).
In Vitro Proliferation
In a separate experiment CFSE-labeled total lymphocytes from popliteal LNs of tolerized and nontolerized Lewis rats sacrificed at day 14 after immunization with MAA were cultured separately in the presence of MAA (10 μg/ml) for 6 days in vitro. The results of this in vitro experiment were similar to those of the in vivo experiment described above. Total lymphocytes from the LNs of the tolerized animal had a reduced ability to proliferate (proliferation index = 1.6 ± 0.11; Figure 1, G and I) compared to total lymphocytes from nontolerized animal (proliferation index = 2.74 ± 0.41; Figure 1, H and I) and these differences were statistically significant (P < 0.05, Figure 1I). CD4+ and CD8+ T cells in these in vitro cultured lymphocytes from popliteal LNs were labeled with anti-rat CD4-APC and anti-rat CD8-APC separately and in vitro proliferation was analyzed using flow cytometry and the ModFit Proliferation Wizard program. The CD4+ cells from the LNs of the tolerized animals proliferated at a significantly lower rate (proliferation index = 1.38 ± 0.18, P < 0.05; Figure 1, J and L) compared to the CD4+ cells from nontolerized animal (proliferation index = 2.06 ± 0.38; Figure 1, K and L) in response to the antigen. Similarly, the CD8+ cells from the LNs of the tolerized animal proliferated at a significantly lower rate (proliferation index = 1.43 ± 0.25, P < 0.05; Figure 1, M and O) compared to the CD8+ cells from nontolerized animal (proliferation index = 1.96 ± 0.41; Figure 1, N and O). As expected, in the absence of MAA total lymphocytes from both tolerized (Figure 1, P and R) and nontolerized (Figure 1, Q and R) animals proliferated at a low rate with a proliferation index of 1.02 ± 0.01 and 1.03 ± 0.02, respectively. Taken together these results suggest that tolerance induced by intravenous administration of MAA is associated with diminished MAA induced T-cell proliferative response in popliteal LNs.
Effect of Tolerance on Cytokine Expression
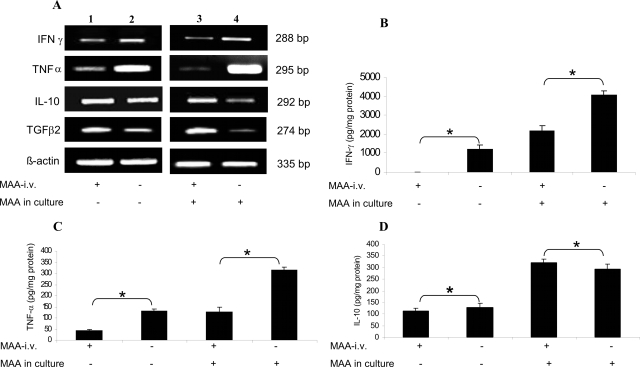
We next investigated if there is a difference in the cytokine profile between tolerized and nontolerized animals. We analyzed the expression of IFN-γ, TNF-α, IL-10, and TGF-β2 at mRNA (IFN-γ, TNF-α, IL-10 and TGF-β2) and protein (IFN-γ, TNF-α, and IL-10) level using semiquantitative RT-PCR and ELISA, respectively. These cytokines were selected because they play an important role in EAAU pathogenesis9,10 and tolerance induction.26,27 Rats were tolerized by intravenous administration of MAA and EAAU was induced as described above. The animals were sacrificed at day 14 after immunization (the onset of disease) and popliteal LN cells were harvested. Total lymphocytes were purified and cultured for 24 hours in the absence and presence of MAA (10 μg/ml). Cells were collected for RNA preparation for RT-PCR and the supernatant was collected for ELISA as described in the Materials and Methods. The RT-PCR analysis revealed that the expression of IFN-γ and TNF-α was profoundly decreased in the culture of lymphocytes derived from LNs of tolerized animals and cultured in absence of MAA (Figure 2A, lane 1) compared to those harvested from nontolerized animals (Figure 2A, lane 2). Interestingly, the reduction in the expression of TNF-α mRNA was more drastic in total lymphocytes harvested from LNs of tolerized animals and cultured overnight in the presence of MAA (Figure 2A, lane 3) compared to those from nontolerized animals (Figure 2A, lane 4). The levels of IL-10 mRNA were slightly increased in total lymphocytes harvested from LNs of tolerized animals when cultured in the absence of MAA (Figure 2A, lane 1) compared to those harvested from nontolerized animals (Figure 2A, lane 2). In marked contrast, the IL-10 expression increased dramatically in lymphocytes harvested from LNs of tolerized animals when cultured overnight in the presence of MAA (Figure 2A, lane 3) compared to those from nontolerized animals (Figure 2A, lane 4). Levels of TGF-β2 mRNA were profoundly elevated in total lymphocytes derived from MAA intravenously injected animals (Figure 2, lane 3) compared to PBS intravenously injected (Figure 2, lane 4) rats when cultured in the presence of MAA compared to when these cells were cultured in the absence of MAA (Figure 2, lanes 1 and 2). These results were further corroborated at the protein level using ELISA for IFN-γ (Figure 2B), TNF-α (Figure 2C), and IL-10 (Figure 2D).
Figure 2.
Analysis of cytokine expression after intravenous MAA-induced tolerance in EAAU. Total lymphocytes from popliteal LNs of tolerized and nontolerized animals were cultured for 24 hours in the absence and presence of MAA (10 μg/ml). Cells were collected for RNA preparation for RT-PCR (A) and the supernatant was used for ELISA (B–D). A: Effect of intravenously induced tolerance to MAA on IFN-γ, TNF-α, IL-10, and TGF-β2 transcripts is shown. Samples from cells cultured in the absence of MAA were loaded in lanes 1 and 2 whereas lanes 3 and 4 contain samples cultured in the presence of MAA. In tolerized animals (lanes 1 and 3) mRNA levels of IFN-γ and TNF-α, were down-regulated compared to nontolerized rats (lanes 2 and 4). In contrast IL-10 and TGF-β2 transcripts were up-regulated in tolerized animals (lanes 1 and 3) compared to nontolerized rats (lanes 2 and 4). A strong band at 335 bp for β-actin indicated equal amounts of RNA in each lane. The figure shows ethidium-bromide-stained bands for PCR products after UV exposure. Results are representative of three independent experiments. Protein levels of IFN-γ (B), TNF-α (C), and IL-10 (D) in culture supernatants were determined by ELISA. Data are reported as mean ± SD for triplicate determinations. *P < 0.05.
Role of CD4+CD25+ Tregs in Tolerance to MAA
Number of CD4+CD25+ Tregs
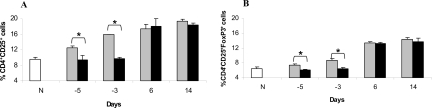
We first investigated whether tolerance induced by intravenous administration of MAA is associated with an increase in the number of CD4+CD25+ Tregs. Lewis rats were tolerized by the intravenous administration of MAA as described above. The rats that received the same volume of PBS (intravenously) instead of MAA were used as control (nontolerized). EAAU was induced by immunization with MAA (day 0). Popliteal LNs were harvested from the rats sacrificed 5 days before immunization (day −5), 3 days before immunization (day −3), and at days 6 and 14 after immunization. Total lymphocytes were stained for CD4, CD25, and Foxp3 were analyzed by flow cytometry to obtain the percentage of CD4+CD25+ and CD4+CD25+FoxP3+ T cells in the total CD4+ cell population. A basal level of CD4+CD25+ (9.3 ± 0.58%) and CD4+CD25+FoxP3+ (6.4 ± 0.53%) T cells was detected in LNs of naïve animals (Figure 3). An increased number of CD25+CD4+ T cells (12.4 ± 0.5%) were found in LNs of tolerized animals on day −5 that received MAA intravenously (Figure 3A). These numbers further increased in LNs of tolerized animals on days −3 (16%, Figure 3A), 6 (17.3 ± 1.15%, Figure 3A), and day 14 (19.3 ± 0.57%, Figure 3A). In contrast, the number of CD4+CD25+ T cells in PBS intravenously treated nontolerized Lewis rats at days −5 (9.3 ± 1.15%, Figure 3A) and −3 (9.75 ± 0.35%, Figure 3A) were similar to naïve rats. However, the percentage of CD4+CD25+ T cells in the popliteal LNs of nontolerized animals (ie, animals with EAAU) increased at day 6 (18 ± 2%, Figure 3A) and day 14 (18.33 ± 0.57%, Figure 3A) after immunization, similar to that observed in tolerized rats at these time points. Interestingly, at days −5 and −3 the differences in the percentage of CD4+CD25+ T cells between tolerized and nontolerized animals were statistically significant (P < 0.05).
Figure 3.
CD4+CD25+ (A) and CD4+CD25+ FoxP3+ (B) Tregs in total lymphocytes harvested from the popliteal LNs of tolerized (gray) and nontolerized (black) animals at 5 and 3 days before immunization with MAA and at days 6 and 14 after immunization. N represents naïve rats. Percentage of CD4+CD25+ (A) and CD4+CD25+ FoxP3+ (B) Tregs was determined by flow cytometry and cumulative data from three independent experiments is shown as bar graph. *P < 0.05.
Next we analyzed the number of CD4+CD25+FoxP3+ Tregs (Figure 3B) at the above-mentioned time points and observed a similar pattern as noted for CD4+CD25+ Tregs (Figure 3A). In tolerized animals statistically significant (P < 0.05) increase in the percentage of CD4+CD25+FoxP3+ Tregs at days −5 (7.42 ± 0.28%) and −3 (8.66 ± 0.57%) was observed compared with nontolerized animals at days −5 (6.12 ± 0.17%) and −3 (6.49 ± 0.26%). Interestingly, the percentage of CD4+CD25+FoxP3+ Tregs in nontolerized animals was similar to that in naïve animals (6.2 ± 0.1%) at these time points (Figure 3B). A similar increase in the percentage of CD4+CD25+FoxP3+ Tregs was observed in the popliteal LNs of both tolerized and nontolerized animals at days 6 and 14 after immunization (Figure 3B).
Co-Culture of Lymph Node Cells from Tolerized and Nontolerized Animals
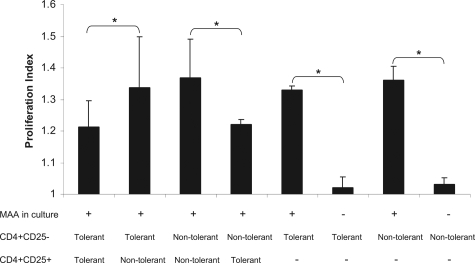
We next analyzed the function of CD4+CD25+ T cells using a co-culture system. Tolerized and nontolerized Lewis rats were sacrificed at day 14 after immunization (at the onset of EAAU). For co-culture experiments, total lymphocytes from popliteal LNs of these animals were prepared separately and CD4+CD25+, CD4+CD25−, and CD4− cells were sorted using BD FACSAria. CD4+CD25− cells were labeled with CFSE. The cells were cultured with MAA (10 μg/ml) for 6 days as described in the Material and Methods.
These experiments were repeated three times and all results from replicate are summarized in Figure 4. CFSE-labeled CD4+CD25− cells from the popliteal LNs of the tolerized animal proliferated at a higher rate when plated with CD4+CD25+ cells from the popliteal LNs of the nontolerized animal (proliferation index = 1.34 ± 0.16, Figure 4) compared with CFSE-labeled CD4+CD25− cells from the popliteal LNs of the tolerized animal plated with CD4+CD25+ cells from the popliteal LNs of the tolerized (proliferation index = 1.2 ± 0.08, Figure 4) and these differences were statistically significant (P < 0.05, Figure 4). Furthermore, CFSE-labeled CD4+CD25− from the LNs of nontolerized animal proliferated at a higher rate (proliferation index = 1.37 ± 0.12, Figure 4) when plated with CD4+CD25+ Tregs of the nontolerized animal. In contrast, CFSE-labeled CD4+CD25− from the LNs of nontolerized animal proliferated at a lower rate when plated with CD4+CD25+ Tregs of the tolerized animal (proliferating index = 1.22 ± 0.02, Figure 4) and these differences were statistically significant (P < 0.05, Figure 4).
Figure 4.
Proliferation of CD4+CD25− T cells in co-culture system measured by CFSE signal using flow cytometry. Data from three independent experiments is shown as bar graph. *P < 0.05.
To investigate if CD4+CD25− T cells are anergic, CD4+CD25− T cells from popliteal LNs of tolerized and nontolerized animals were cultured in the presence and absence of MAA. CD4+CD25+ Tregs were not added to the culture in this experiment. In the presence of MAA CFSE-labeled CD4+CD25− cells from the popliteal LNs of tolerized animals proliferated at a high rate (proliferation index = 1.33 ± 0.013, Figure 4). Similarly, CFSE-labeled CD4+CD25− cells from the popliteal LNs of nontolerized animals proliferated at a high rate (proliferation index = 1.36 ± 0.04, Figure 4) in the presence of MAA. In contrast, almost negligible proliferation was detected when CFSE-labeled CD4+CD25− cells from the popliteal LNs of tolerized (proliferation index = 1.02 ± 0.34, Figure 4) and nontolerized (proliferation index = 1.03 ± 0.023, Figure 4) were cultured in the absence of MAA.
Taken together these results demonstrated that the CD4+CD25+ Tregs from tolerized animals have the ability to not only suppress the proliferation of effector T cells in tolerized animals but also are capable of suppressing the proliferation of effector T cells of nontolerized animals. Additionally, our results suggest that active suppression by CD4+CD25+ Tregs may be the major mechanism responsible for tolerance induction by intravenous injection MAA in EAAU.
Role of IL-2
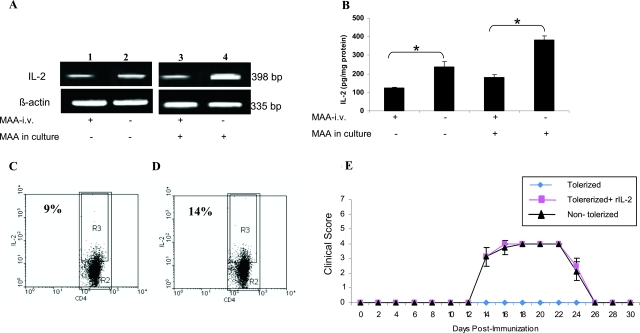
The role of IL-2 in intravenous MAA tolerance in EAAU was explored. Tolerance was induced in Lewis rats as described above and total lymphocytes from popliteal LNs of tolerized and nontolerized animals sacrificed at day 14 after immunization were cultured overnight in the absence or presence of MAA (10 μg/ml). The supernatant was collected for ELISA and the cells were harvested for semiquantitative RT-PCR analysis. Our results of RT-PCR analysis demonstrated that the IL-2 expression moderately decreased in lymphocytes from LNs of tolerized animals (Figure 5A, lane 1) as compared to nontolerized animals (Figure 5A, lane 2) when cultured in the absence of MAA. The decrease in IL-2 mRNA expression was more drastic in lymphocytes from LNs of tolerized animals cultured in presence of MAA (Figure 5A, lane 3) compared to those from nontolerized animals and cultured in the presence of MAA (Figure 5A, lane 4). Similar results were observed in ELISA analysis in which the IL-2 protein levels were significantly (P < 0.005) lower in the supernatant of lymphocytes harvested from the LNs of tolerized animals compared to nontolerized animals (Figure 5B).
Figure 5.
Relationship between IL-2 and MAA intravenously induced tolerance. Total lymphocytes obtained from popliteal LNs of tolerized and nontolerized Lewis rats were cultured for 24 hours with or without MAA for RT-PCR (A) and ELISA (B). A: Expression of IL-2 transcript using RT-PCR. Low levels of IL-2 mRNA were observed in tolerized animals (lanes 1 and 3) compared to nontolerized rats (lanes 2 and 4). A strong band at 335 bp for β-actin indicated equal amounts of RNA in each lane. B: Levels of IL-2 protein were quantitated by ELISA. Compared with nontolerized rats the levels of IL-2 protein were significantly reduced in tolerized rats. Results shown are the mean ± SD of results in triplicate independent analyses. *P < 0.005. C and D: FACS analysis of intracellular IL-2. Total lymphocytes harvested from popliteal LNs at the onset of EAAU (day 14 after immunization) were stained with anti-IL-2 and anti-CD4. Dot plots show IL-2 content of gated CD4+ T cells in tolerized (C) and nontolerized (D) animals. R2 represents the CD4+ cells in total lymphocyte population and R3 represents the population of IL-2-expressing CD4+ cells. A decrease in IL-2-expressing CD4+ cells could be seen in tolerized animals compared to nontolerized animals. Recombinant IL-2 (rIL-2) reverses tolerance by reversing the suppressive effect of intravenous MAA. E: Lewis rats were divided into three groups. The first and second groups were tolerized with intravenous administration of MAA as described in the Materials and Methods. The rats in group 3 received the same volume of PBS intravenously. EAAU was induced by immunization with MAA (day 0). Lewis rats in group 2 were treated with rIL-2 intravenously on days 8, 10, and 12 after immunization. All animals in group 1 were tolerized and did not develop EAAU. All Lewis rats in group 2 that received rIL-2 failed to develop tolerance to intravenous MAA and developed disease. The clinical course and severity of EAAU in these animals was similar to that observed in intravenous PBS rats in group 3. All of the experiments were repeated three times with similar results.
Next we analyzed if there is a reduction in IL-2-producing cells in the LNs of tolerized animals. Total lymphocytes prepared from popliteal LNs harvested from tolerized and nontolerized Lewis rats were cultured with MAA (10 μg/ml) overnight. These cells were then stained with anti-IL-2 (intracellular staining) and anti-CD4 (cell surface staining) for flow cytometry analysis (Figure 5, C and D). At day 14 after immunization a low percentage of IL-2-producing CD4+ T cells was detected in the LNs of tolerized animals (9%, Figure 5C) compared to nontolerized animals (14%, Figure 5D).
Because our above-mentioned results demonstrated that IL-2 expression was drastically reduced in tolerized animals, we investigated if the state of tolerance can be reverted by replenishing tolerant rats with recombinant IL-2 (r-IL2). Lewis rats were divided into three groups (six rats per group). Lewis rats in the first and second group were tolerized with intravenous administration of MAA before the induction of disease as described above. The rats in the third group received the same volume of PBS (intravenously) instead of MAA and were used as control (nontolerized). EAAU was induced by immunization with MAA (day 0). The Lewis rats in first group received a single intravenous injection of rIL-2 (2 μg/rat) intravenously on days 8, 10, and 12 after immunization. All rats that received PBS intravenously (nontolerized) developed EAAU on day 14 ± 1 with the maximum average clinical score of 4 at the peak of the disease (Figure 5E). The rats that received intravenous MAA treatment (tolerized) before induction of the disease (no rIL-2 treatment) did not develop EAAU (Figure 5E). In contrast, Lewis rats that were tolerized with MAA and received rIL-2 on days 8, 10, and 12 after immunization developed severe EAAU (Figure 5E). The clinical (Figure 5E) and histological (data not shown) pattern of uveitis in these animals was similar to that observed in the nontolerized animals. These results indicated that the administration of exogenous recombinant IL-2 can reverse the tolerance in EAAU. Taken together our results suggest that IL-2 plays a critical role in the development of tolerance against MAA in EAAU animal model.
Adoptive Transfer of Tolerance
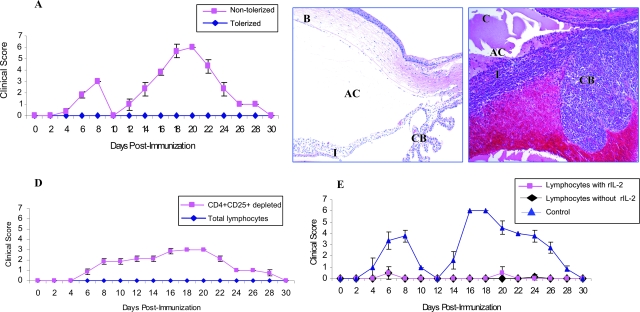
Total lymphocytes from popliteal LNs of tolerized and nontolerized animals were cultured in the presence of MAA (10 μg/ml) for 3 days as previously described by us.8,11 Nonadherent cells (2 × 107 cells) were transferred to naïve Lewis rats via the tail vein and these animals were immunized with a subcutaneous injection of MAA (together with complete Freund’s adjuvant) 24 hours after cell transfer. The animals were examined daily from days 3 to 30 for clinical signs of uveitis. Animals were also sacrificed at various time points and the eyes were harvested for histological analysis. In the animals that received nonadherent cells from nontolerized Lewis rats the disease developed in two phases. The first phase starting at day 5 was induced by adoptively transferred MAA-specific lymphocytes whereas the second phase that started at day 11 was because of the foot-pad injection of MAA in the presence of adjuvant (Figure 6A). These animals developed severe disease in both the phases with the resolution on day 10 for the first phase and on day 30 for the second phase (Figure 6A). The clinical and histological pattern of uveitis in the first phase was similar to our previous result of adoptive transfer EAAU.8,11 Similarly, the clinical course of EAAU in the second phase was similar to that observed after active immunization with MAA.6,7,11 However, these animals developed extremely severe EAAU (clinical score = 6 at the peak of disease) because in these animal primed MAA-specific T cells were already present as a result of adoptive transfer and were hypersensitized against the uveitogenic antigen (Figure 6, A and C). Interestingly, intravenous administration of MAA lead to complete abrogation of the ability of popliteal LN lymphocytes to transfer tolerance. Both the first and second phases of EAAU were completely inhibited in the animals that received total lymphocytes from popliteal LNs harvested from tolerized animals (Figure 6, A and B).
Figure 6.
Adoptive transfer of tolerance. Total lymphocytes harvested from popliteal LNs of tolerized and nontolerized animals were cultured in the presence of MAA for 3 days. Nonadherent cells (2 × 107) were adoptively transferred to naïve Lewis rats and the animals were immunized with MAA 24 hours after the cell transfer. A: EAAU developed in two phases in Lewis rats that received LN cells from nontolerized rats whereas adoptive transfer of mixed lymphocytes from tolerized Lewis rats induced tolerance in the recipient rats. B: No inflammation could be seen at day 19 in the animals that received adoptively transferred mixed lymphocytes from tolerized animals. C: Ocular histopathology at day 19 after immunization revealed extremely severe (clinical score 6) EAAU in the animals that received total lymphocytes from the LNs of nontolerized animals. I, Iris; CB, ciliary body; AC, anterior chamber. D: Effect of CD4+CD25+ T-cell depletion on the adoptive transfer of tolerance. Adoptive transfer of lymphocytes harvested from popliteal LNs transferred tolerance to the recipient Lewis rats. Depletion of CD4+CD25+ T cell from the total lymphocytes harvested from popliteal LNs of tolerized animals impaired the ability of these cells to transfer tolerance. E: Effect of recombinant IL-2 on adoptive transfer of tolerance. Presence or absence of recombinant IL-2 in culture of total lymphocytes harvested from the popliteal LNs did not affect their ability to transfer tolerance to naïve rats. Total lymphocytes from nontolerized Lewis rats were used as control. Original magnifications, ×10.
In an another set of experiments, total lymphocytes from LNs harvested from tolerized animal were cultured with MAA for 3 days followed by depletion of CD4+CD25+ Tregs. The animals were divided in two groups. The first group received total lymphocytes and the second group received lymphocytes depleted of CD4+CD25+ Tregs (Figure 6D). As expected, the animals in the first group that received total lymphocytes from popliteal LNs of tolerized animals did not develop disease in both the phases and the recipient animals were tolerized. Interestingly, animals in the second group that received lymphocytes devoid of CD4+CD25+ Tregs from tolerized Lewis rats failed to develop tolerance and developed EAAU on day 5 after cell transfer. However, in these animals EAAU did not develop in two phases (Figure 6D) as observed with the animals that received unfractionated lymphocytes from nontolerized animals (Figure 6, A and C). In these animals the severity of EAAU increased at day 8 and plateaued until day 14. This was followed by more severe EAAU on days 16 to 20, severity of EAAU declined thereafter, and the disease resolved by day 30 in these animals. Taken together our results demonstrated that the presence of CD4+CD25+ Tregs is also essential for adoptive transfer of tolerance in EAAU.
Effects of IL-2 on Tregs
As mentioned above administration of exogenous IL-2 reversed tolerance and tolerized animals that received exogenous IL-2 developed EAAU. This clearly established that IL-2 plays a critical role in induction of tolerance. However, these results did not indicate if the reversal of tolerance is attributable to increased IL-2 levels as a result of administration of exogenous IL-2 or attributable to suppression of functions of Tregs by IL-2 as reported by others.30 To examine the exact mechanism of tolerance reversal by exogenous IL-2, total lymphocytes from LN cells obtained from tolerized animals were cultured with MAA (10 μg/ml) in the presence (20 ng/ml IL-2) or absence of recombinant IL-2 for 3 days. After this, nonadherent cells were washed thoroughly and 2 × 107 cells were adoptively transferred to naïve Lewis rats via the tail vein as previously described by us.8,11 Twenty-four hours after cell transfer these animals were immunized with MAA to induce EAAU. All animals that received IL-2-treated lymphocytes or lymphocytes not treated with IL-2 developed tolerance and did not develop EAAU (Figure 6E). Animals that received lymphocytes from the popliteal LNs of nontolerized animals served as controls. As expected, EAAU developed in two phases in these animals (Figure 6E). These results indicated that presence of exogenous IL-2 did not suppress the activity of Tregs. The function of Tregs remained unaltered even in presence of IL-2 indicating thereby that exogenous IL-2 resulted in effector T-cell proliferation instead of Treg suppression.
Discussion
Idiopathic AU is the most common form of intraocular inflammation in humans.1,2 EAAU serves as an animal model for human idiopathic AU and this model of ocular autoimmunity has been extensively investigated in our laboratory.6,7,8,9,10,11,29 Work from our laboratory throughout the past decade has established that in EAAU the ocular inflammatory process is driven by MAA-specific CD4+ T cells.7,8,11 Our unpublished results suggest that cellular responsiveness to MAA was associated with idiopathic AU in humans. In the present study the EAAU animal model was used to inhibit autoimmune AU by inducing immune tolerance against MAA. MAA was injected intravenously to induce tolerance and the underlying mechanisms were investigated. The evidence derived from our study suggests that active suppression mediated by CD4+CD25+ Tregs leads to tolerance induction to MAA in EAAU. To the best of our knowledge this is the first report describing the inhibition of idiopathic AU by inducing antigen-specific immune tolerance.
We found that intravenous administration of MAA before induction of EAAU inhibited the development of AU and induced tolerance in a dose-dependent manner. In contrast, all rats that received a similar treatment with OVA or PBS were not tolerized and developed EAAU. Thus, our results demonstrate that intravenous administration of MAA leads to the development of antigen-specific peripheral tolerance that results in the protection against EAAU, an organ-specific autoimmune disease of the eye. Several reports in the literature have suggested that immune tolerance can be induced by intravenous administration of specific antigens in a wide variety of animal models of autoimmunity.22,23,24,28,31,32 In addition, intravenous injection of the pathogenic antigen has been reported to suppress multiple sclerosis in humans.13,33
We studied in vivo and in vitro proliferative response of lymphocytes from popliteal LNs and observed a strong proliferative response in nontolerized rats, but not in intravenously MAA-tolerized animals. We also observed that CD4+ and CD8+ cells from the popliteal LNs of tolerized rats also proliferated at a dramatically reduced rate on antigenic stimulation. These results clearly show that T cells were tolerized to MAA after intravenous administration and implicate T-cell nonresponsiveness as one of the possible mechanisms of tolerance in the EAAU animal model. Immune cells including T cells have been reported to be tolerized to the autoantigen after intravenous administration of specific antigens in various animal models of autoimmunity.31,32
In the present study ∼10% of CD4+CD25+ Tregs were detected in the draining LNs of naïve animals as well as in the LNs of PBS intravenously treated nontolerized Lewis rats before immunization with MAA to induce EAAU. These cells may represent naturally occurring Tregs. CD4+CD25+ T cells have been reported to constitute ∼5 to 10% of the CD4+ T-cell pool in normal humans and normal animals.34 In contrast, the number of MAA-specific CD4+CD25+ Tregs in tolerized (MAA intravenously) rats increased before MAA immunization. Although a similar increase in the number of these cells was observed in both tolerized and nontolerized rats after immunization with MAA emulsified (1:1) in Freund’s complete adjuvant (to induce EAAU); the suppressive activity of CD4+CD25+ Tregs deferred between these two groups of animals. It was observed that CD4+CD25+ Tregs purified from nontolerized animals did not suppress the proliferation of CD4+CD25− T cells from tolerized or nontolerized animals. On the other hand CD4+CD25+ Tregs purified from tolerized animals suppressed the proliferation of CD4+CD25− T cells from tolerized as well as nontolerized animals; in response to antigenic stimulation. These results clearly demonstrate that intravenous administration of MAA leads to generation of CD4+CD25+ Tregs that suppress T-cell responses and induce tolerance. The majority of CD4+CD25+ T cells in tolerized and nontolerized animals were positive for Forkhead box P3 (FoxP3) at all time points studied. FoxP3 is a Treg-specific transcription factor.34 Thus, on the basis of these results it is reasonable to conclude that the suppressive activity and not the number of CD4+CD25+ T cells is critical for the induction of tolerance against MAA in EAAU. In the present study the number of CD4+CD25+ Tregs in nontolerized animals (animals with EAAU) increased after MAA immunization. This increased number of Tregs may be needed to set the stage for the resolution of EAAU. Experiments are currently underway in our laboratory to address this issue. Similar to our observation an increased number of CD4+CD25+ T cells were observed in the animals with experimental autoimmune encephalomyelitis and rheumatoid arthritis and Tregs were reported to be involved in the natural resolution of these diseases.35,36
CD4+CD25+ Tregs play a central role in the maintenance of naturally occurring self-tolerance.37,38 Studies using a wide variety of animal models have documented a critical role for CD4+CD25+ Tregs in the induction of immune tolerance to self antigens in vivo.34 The absence or defective function of these CD4+CD25+ T cells could lead to the development of autoimmune disorders37 such as thyroiditis,39 gastritis,40 systemic lupus erythematosus,41 hepatitis,42 and rheumatoid arthritis.43
Tregs have been shown to mediate their suppressive function through cell-to-cell contact as well as via soluble mediators, such as IL-10 and TGF-β.26,27,44,45,46 In the present study we demonstrated that compared to nontolerized animals (animals with EAAU) the LN cells from tolerized animals expressed increased levels of IL-10 and TGF-β2. Expression of IFN-γ and TNF-α was down-regulated in these animals. A similar shift in cytokine profile as a result of tolerance induction has been reported in other inflammatory diseases including adjuvant-induced arthritis47 and experimental autoimmune encephalomyelitis.48
Using the adoptive transfer model of EAAU,7,11 we observed that total lymphocytes from popliteal LNs of tolerized rats could adoptively transfer tolerance to MAA. As expected, total lymphocytes from popliteal LNs of tolerized animals that are devoid of CD4+CD25+ cells, failed to transfer tolerance in this model. These results conclusively demonstrated that CD4+CD25+ Treg-mediated suppression is the major mechanism for tolerance induction against MAA in EAAU. Furthermore, our results clearly demonstrated that CD4+CD25− T cells in tolerized animals are not anergic because when cultured with MAA in the absence of CD4+CD25+ Tregs they proliferated at a high rate that was similar to those harvested from nontolerized animals. Our results are in contrast with those reported in the animal model of collagen-induced arthritis. In this report Brand and colleagues49 demonstrated that antigen-specific T cells harvested from tolerized transgenic mice did not respond to the antigenic stimulation. Our results further demonstrated that within the LN of tolerized and nontolerized animals there was no difference in the number of cells undergoing apoptosis (data not shown). These observations suggest that the clonal deletion of uveitogenic T cells may not be the mechanism involved in tolerance induction to MAA in EAAU.
Studies by other investigators have implicated different mechanisms including clonal deletion, anergy, and Tregs to be involved in the induction of immune tolerance.28,49,50,51 Hilliard and colleagues28 reported that all three mechanisms may be simultaneously involved in the induction of tolerance in experimental autoimmune encephalomyelitis by intravenous administration of myelin basic protein. Intravenous injection of the autoantigen before immunization has been reported to suppress arthritis in different animal models.49,51 Although several mechanisms have been proposed, the role of CD4+CD25+ Tregs in the tolerance induction in arthritis is not clear.52 Our results presented here clearly showed that CD4+CD25+ Treg-mediated active suppression is the major mechanism responsible for induction of tolerance in EAAU. Clonal deletion and anergy do not appear to play a role in induction of tolerance in EAAU. This observation is clinically relevant because it identifies a single mechanism for tolerance induction and may lead to the development of more specific therapeutic strategy for the treatment of autoimmune uveitis.
The results from the present study demonstrated that IL-2-expressing CD4+ cells were reduced in the state of tolerance in EAAU animal model. IL-2 is produced primarily by activated CD4+ T cells and is an important growth factor for activated CD4+ effector T cells.53,54 In our study the IL-2 expression (detected by RT-PCR, ELISA, and intracellular staining) was dramatically down-regulated in lymphocytes harvested from LNs of tolerized animals compared to nontolerized animals. This decrease in IL-2 expression can be attributed to the suppressive effect of CD4+CD25+ Tregs. The reversal of tolerance to MAA observed by replenishing tolerized rats with recombinant IL-2 further suggested that MAA-specific T-effector cells were present in the tolerized rat, but were actively suppressed.
On the basis of above-mentioned results we can speculate that the presence of a high number of CD4+CD25+ Tregs in the animals that were tolerized before the induction of EAAU versus nontolerized rats in our study might be responsible for the low IL-2 levels in tolerized animals at the onset of EAAU. Additionally, increased IL-2 production by MAA-specific CD4+ T cells after immunization to induce MAA may increase the suppressive activity of Tregs. It is known that CD4+ T cells produce IL-2 very rapidly in vivo and competition among different subsets of T cells determines the course of an immune response.55 Our results of adoptive transfer experiments demonstrate that IL-2 does not alter the suppressive activity of Tregs because these cells treated with IL-2 in vitro were able to induce tolerance. Thus, on the basis of these results we can conclude that tolerance is caused by reduced IL-2 levels and reversal of tolerance by exogenous administration of IL-2 is attributable to proliferation of T-effector cells and not attributable to suppression of Tregs.
CD4+CD25+ Tregs do not produce IL-2 and are dependent on the IL-2 produced by CD4+CD25− T cells.54 Previous reports in the literature have shown that CD4+CD25+ Tregs compete for IL-2 with the responder/effector T cells and IL-2 is essential for the suppressive activity of CD4+CD25+ Tregs.30,56 In the presence of IL-2, these cells actively suppress other effector T cells by inhibiting their IL-2 production.53 CD4+CD25+ Tregs reduce the levels of IL-2 not only by scavenging the existing IL-2 but also by down-regulating the production of IL-2 at mRNA and protein level by effector T cells.30,31,34,54,56,57,58 IL-2 knockout mice have reduced numbers of Tregs and they have been reported to develop lethal spontaneous autoimmunity.59,60
In summary, intravenous administration of MAA effectively prevented the development of EAAU in Lewis rats by inducing the state of immune tolerance against MAA. This tolerance to MAA is the result of T-cell nonresponsiveness (functional inactivation) caused by the suppressive activities of Tregs. Experiments are currently underway in our laboratory to explore the effect of MAA-specific immune tolerance on the progression and severity of already existing (on going) EAAU. It is hoped that these studies will lead to the development of effective therapy based on selective antigen-specific modulation of the immune response in the treatment of idiopathic autoimmune anterior uveitis that is now only treated symptomatically.
Acknowledgments
We thank Ruslana G. Tytarenko for histology and Andrea M. Fox for her help with the use of the flow cytometer.
Footnotes
Address reprint requests to Nalini S. Bora, Ph.D., Department of Ophthalmology, Jones Eye Institute, 4301 West Markham, Mail Slot 523, Little Rock, AR 72205. E-mail: nbora@uams.edu.
Supported by the National Institutes of Health (grants EY016205 and EY 014623); the Pat and Willard Walker Eye Research Center; the Jones Eye Institute; and the Arkansas Master Tobacco Settlement and Arkansas Biosciences Institute, University of Arkansas for Medical Sciences, Little Rock, AR.
References
- Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111:491–500. doi: 10.1016/j.ophtha.2003.06.014. [DOI] [PubMed] [Google Scholar]
- Bora NS, Kaplan HJ. Intraocular diseases—anterior uveitis. Chem Immunol Allergy. 2007;92:213–220. doi: 10.1159/000099272. [DOI] [PubMed] [Google Scholar]
- Bloch-Michel E, Nussenblatt RB. International Uveitis Study Group recommendations for the evaluation of intraocular inflammatory disease. Am J Ophthalmol. 1987;103:234–235. doi: 10.1016/s0002-9394(14)74235-7. [DOI] [PubMed] [Google Scholar]
- von Herrath MG, Harrison LC. Antigen-induced regulatory T cells in autoimmunity. Nat Rev Immunol. 2003;3:223–232. doi: 10.1038/nri1029. [DOI] [PubMed] [Google Scholar]
- Broekhuyse RM, Kuhlman ED, Winkens HJ, Van Vugt AHM. Experimental autoimmune anterior uveitis (EAAU), a new form of experimental uveitis: I: induction by a detergent-insoluble, intrinsic protein fraction of the retina pigment epithelium. Exp Eye Res. 1991;52:465–474. doi: 10.1016/0014-4835(91)90044-f. [DOI] [PubMed] [Google Scholar]
- Bora NS, Kim MC, Kabeer NH, Simpson SC, Tandhasetti MT, Cirrito TP, Kaplan AD, Kaplan HJ. Experimental autoimmune anterior uveitis. Induction with melanin-associated antigen from the iris and ciliary body. Invest Ophthalmol Vis Sci. 1995;36:1056–1066. [PubMed] [Google Scholar]
- Kim MC, Kabeer NH, Tandhasetti MT, Kaplan HJ, Bora NS. Immunohistochemical studies in melanin associated antigen (MAA) induced experimental autoimmune anterior uveitis (EAAU). Curr Eye Res. 1995;14:703–710. doi: 10.3109/02713689508998498. [DOI] [PubMed] [Google Scholar]
- Bora NS, Woon MD, Tandhasetti MT, Cirrito TP, Kaplan HJ. Induction of experimental autoimmune anterior uveitis by a self-antigen: melanin complex without adjuvant. Invest Ophthalmol Vis Sci. 1997;38:2171–2175. [PubMed] [Google Scholar]
- Woon MD, Kaplan HJ, Bora NS. Kinetics of cytokine production in experimental autoimmune anterior uveitis (EAAU). Curr Eye Res. 1998;17:955–961. doi: 10.1076/ceyr.17.10.955.5246. [DOI] [PubMed] [Google Scholar]
- Jha P, Sohn JH, Xu Q, Nishihori H, Wang Y, Nishihori S, Manickam B, Kaplan HJ, Bora PS, Bora NS. The complement system plays a critical role in the development of experimental autoimmune anterior uveitis. Invest Ophthalmol Vis Sci. 2006;47:1030–1038. doi: 10.1167/iovs.05-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora NS, Sohn JH, Kang SG, Cruz JM, Nishihori H, Suk HJ, Wang Y, Kaplan HJ, Bora PS. Type I collagen is the autoantigen in experimental autoimmune anterior uveitis. J Immunol. 2004;172:7086–7094. doi: 10.4049/jimmunol.172.11.7086. [DOI] [PubMed] [Google Scholar]
- Kamradt T, Mitchison NA. Tolerance and autoimmunity. N Engl J Med. 2001;344:655–664. doi: 10.1056/NEJM200103013440907. [DOI] [PubMed] [Google Scholar]
- Miller SD, Turley DM, Podojil JR. Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat Rev Immunol. 2007;7:665–677. doi: 10.1038/nri2153. [DOI] [PubMed] [Google Scholar]
- Gregerson DS, Obritsch WF, Donoso LA. Oral tolerance in experimental autoimmune uveoretinitis. Distinct mechanisms of resistance are induced by low dose vs high dose feeding protocols. J Immunol. 1993;151:5751–5761. [PubMed] [Google Scholar]
- Faria AM, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232–259. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner HL, Friedman A, Miller A, Khoury SJ, Al-Sabbagh A, Santos L, Sayegh M, Nussenblatt RB, Trentham DE, Hafler DA. Oral tolerance: immunologic mechanisms and treatment of animal and human organ-specific autoimmune diseases by oral administration of autoantigens. Annu Rev Immunol. 1994;12:809–837. doi: 10.1146/annurev.iy.12.040194.004113. [DOI] [PubMed] [Google Scholar]
- Whitacre CC, Gienapp IE, Meyer A, Cox KL, Javed N. Treatment of autoimmune disease by oral tolerance to autoantigens. Clin Immunol Immunopathol. 1996;80(Suppl 3):S31–S39. doi: 10.1006/clin.1996.0139. [DOI] [PubMed] [Google Scholar]
- Bai XF, Link H. Nasal tolerance induction as a potential means of immunotherapy for autoimmune diseases: implications for clinical medicine. Clin Exp Allergy. 2000;30:1688–1696. doi: 10.1046/j.1365-2222.2000.00972.x. [DOI] [PubMed] [Google Scholar]
- Vrabec TR, Gregerson DS, Dua HS, Donoso LA. Inhibition of experimental autoimmune uveoretinitis by oral administration of S-antigen and synthetic peptides. Autoimmunity. 1992;12:175–184. doi: 10.3109/08916939209148457. [DOI] [PubMed] [Google Scholar]
- Tsitoura DC, DeKruyff RH, Lamb JR, Umetsu DT. Intranasal exposure to protein antigen induces immunological tolerance mediated by functionally disabled CD4+ T cells. J Immunol. 1999;163:2592–2600. [PubMed] [Google Scholar]
- Liu JQ, Bai XF, Shi FD, Xiao BG, Li HL, Levi M, Mustafa M, Wahren B, Link H. Inhibition of experimental autoimmune encephalomyelitis in Lewis rats by nasal administration of encephalitogenic MBP peptides: synergistic effects of MBP 68-86 and 87-99. Int Immunol. 1998;10:1139–1148. doi: 10.1093/intimm/10.8.1139. [DOI] [PubMed] [Google Scholar]
- Verbeek R, van der Mark K, Wawrousek EF, Plomp AC, van Noort JM. Tolerization of an established alphaB-crystallin-reactive T-cell response by intravenous antigen. Immunology. 2007;121:416–426. doi: 10.1111/j.1365-2567.2007.02592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorian SK, Clark L, Heber-Katz E, Amento EP, Rostami A. Induction of peripheral tolerance with peptide-specific anergy in experimental autoimmune neuritis. Cell Immunol. 1993;150:298–310. doi: 10.1006/cimm.1993.1198. [DOI] [PubMed] [Google Scholar]
- Jacobs MJ, van den Hoek AE, van de Putte LB, van den Berg WB. Anergy of antigen-specific T lymphocytes is a potent mechanism of intravenously induced tolerance. Immunology. 1994;82:294–300. [PMC free article] [PubMed] [Google Scholar]
- Sun JB, Czerkinsky C, Holmgren J. Sublingual ‘oral tolerance’ induction with antigen conjugated to cholera toxin B subunit generates regulatory T cells that induce apoptosis and depletion of effector T cells. Scand J Immunol. 2007;66:278–286. doi: 10.1111/j.1365-3083.2007.01975.x. [DOI] [PubMed] [Google Scholar]
- Taylor A, Verhagen J, Blaser K, Akdis M, Akdis CA. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: the role of T regulatory cells. Immunology. 2006;117:433–442. doi: 10.1111/j.1365-2567.2006.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckmann D, Bruett CH, Ploettner H, Lutz MB, Schuler G. Human CD4(+)CD25(+) regulatory, contact-dependent T cells induce interleukin 10-producing, contact-independent type 1-like regulatory T cells [corrected]. J Exp Med. 2002;196:247–253. doi: 10.1084/jem.20020642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard BA, Kamoun M, Ventura E, Rostami A. Mechanisms of suppression of experimental autoimmune encephalomyelitis by intravenous administration of myelin basic protein: role of regulatory spleen cells. Exp Mol Pathol. 2000;68:29–37. doi: 10.1006/exmp.1999.2290. [DOI] [PubMed] [Google Scholar]
- Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha P, Sohn JH, Xu Q, Wang Y, Kaplan HJ, Bora PS, Bora NS. Suppression of complement regulatory proteins (CRPs) exacerbates experimental autoimmune anterior uveitis (EAAU). J Immunol. 2006;176:7221–7231. doi: 10.4049/jimmunol.176.12.7221. [DOI] [PubMed] [Google Scholar]
- Thorstenson KM, Khoruts A. Generation of anergic and potentially immunoregulatory CD25+CD4 T cells in vivo after induction of peripheral tolerance with intravenous or oral antigen. J Immunol. 2001;167:188–195. doi: 10.4049/jimmunol.167.1.188. [DOI] [PubMed] [Google Scholar]
- Riemekasten G, Langnickel D, Enghard P, Undeutsch R, Humrich J, Ebling FM, Hocher B, Humaljoki T, Neumayer H, Burmester GR, Hahn BH, Radbruch A, Hiepe F. Intravenous injection of a D1 protein of the Smith proteins postpones murine lupus and induces type 1 regulatory T cells. J Immunol. 2004;173:5835–5842. doi: 10.4049/jimmunol.173.9.5835. [DOI] [PubMed] [Google Scholar]
- Warren KG, Catz I, Ferenczi LZ, Krantz MJ. Intravenous synthetic peptide MBP8298 delayed disease progression in an HLA class II-defined cohort of patients with progressive multiple sclerosis: results of a 24-month double-blind placebo-controlled clinical trial and 5 years of follow-up treatment. Eur J Neurol. 2006;13:887–895. doi: 10.1111/j.1468-1331.2006.01533.x. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- Kao JK, Hsue YT, Lin CY. Role of new population of peripheral CD11c(+)CD8(+) T cells and CD4(+)CD25(+) regulatory T cells during acute and remission stages in rheumatoid arthritis patients. J Microbiol Immunol Infect. 2007;40:419–427. [PubMed] [Google Scholar]
- McGeachy MJ, Stephens LA, Anderton SM. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. J Immunol. 2005;175:3025–3032. doi: 10.4049/jimmunol.175.5.3025. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- Bala KK, Moudgil KD. Induction and maintenance of self tolerance: the role of CD4+CD25+ regulatory T cells. Arch Immunol Ther Exp (Warsz) 2006;54:307–321. doi: 10.1007/s00005-006-0035-x. [DOI] [PubMed] [Google Scholar]
- Morris GP, Kong YC. Interference with CD4+CD25+ T-cell-mediated tolerance to experimental autoimmune thyroiditis by glucocorticoid-induced tumor necrosis factor receptor monoclonal antibody. J Autoimmun. 2006;26:24–31. doi: 10.1016/j.jaut.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Zwar TD, Read S, van Driel IR, Gleeson PA. CD4+CD25+ regulatory T cells inhibit the antigen-dependent expansion of self-reactive T cells in vivo. J Immunol. 2006;176:1609–1617. doi: 10.4049/jimmunol.176.3.1609. [DOI] [PubMed] [Google Scholar]
- Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007;178:2579–2588. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- Longhi MS, Hussain MJ, Mitry RR, Arora SK, Mieli-Vergani G, Vergani D, Ma Y. Functional study of CD4+CD25+ regulatory T cells in health and autoimmune hepatitis. J Immunol. 2006;176:4484–4491. doi: 10.4049/jimmunol.176.7.4484. [DOI] [PubMed] [Google Scholar]
- Kelchtermans H, De Klerck B, Mitera T, Van Balen M, Bullens D, Billiau A, Leclercq G, Matthys P. Defective CD4+CD25+ regulatory T cell functioning in collagen-induced arthritis: an important factor in pathogenesis, counter-regulated by endogenous IFN-gamma. Arthritis Res Ther. 2005;7:R402–R415. doi: 10.1186/ar1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Puijvelde GH, van Es T, van Wanrooij EJ, Habets KL, de Vos P, van der Zee R, van Eden W, van Berkel TJ, Kuiper J. Induction of oral tolerance to HSP60 or an HSP60-peptide activates T cell regulation and reduces atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2677–2683. doi: 10.1161/ATVBAHA.107.151274. [DOI] [PubMed] [Google Scholar]
- Zhang X, Koldzic DN, Izikson L, Reddy J, Nazareno RF, Sakaguchi S, Kuchroo VK, Weiner HL. IL-10 is involved in the suppression of experimental autoimmune encephalomyelitis by CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:249–256. doi: 10.1093/intimm/dxh029. [DOI] [PubMed] [Google Scholar]
- Li MO, Sanjabi S, Flavell RA. Transforming growth factor-β controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:455–471. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Prakken BJ, Roord S, van Kooten PJ, Wagenaar JP, van Eden W, Albani S, Wauben MH. Inhibition of adjuvant-induced arthritis by interleukin-10-driven regulatory cells induced via nasal administration of a peptide analog of an arthritis-related heat-shock protein 60 T cell epitope. Arthritis Rheum. 2002;46:1937–1946. doi: 10.1002/art.10366. [DOI] [PubMed] [Google Scholar]
- Khoury SJ, Hancock WW, Weiner HL. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor beta, interleukin 4, and prostaglandin E expression in the brain. J Exp Med. 1992;176:1355–1364. doi: 10.1084/jem.176.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand DD, Myers LK, Whittington KB, Latham KA, Stuart JM, Kang AH, Rosloniec EF. Detection of early changes in autoimmune T cell phenotype and function following intravenous administration of type II collagen in a TCR-transgenic model. J Immunol. 2006;168:490–498. doi: 10.4049/jimmunol.168.1.490. [DOI] [PubMed] [Google Scholar]
- Treschow AP, Bäcklund J, Holmdahl R, Issazadeh-Navikas S. Intrinsic tolerance in autologous collagen-induced arthritis is generated by CD152-dependent CD4+ suppressor cells. J Immunol. 2005;174:6742–6750. doi: 10.4049/jimmunol.174.11.6742. [DOI] [PubMed] [Google Scholar]
- Kresina TF, Moskowitz RW. Adoptive transfer of suppression of arthritis in the mouse model of collagen-induced arthritis. Evidence for a type II collagen-specific suppressor T cell. J Clin Invest. 1985;75:1990–1998. doi: 10.1172/JCI111917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londei M. Role of regulatory T cells in experimental arthritis and implications for clinical use. Arthritis Res Ther. 2005;7:118–120. doi: 10.1186/ar1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony PA, Paulos CM, Ahmadzadeh M, Akpinarli A, Palmer DC, Sato N, Kaiser A, Hinrichs CS, Klebanoff CA, Tagaya Y, Restifo NP. Interleukin-2-dependent mechanisms of tolerance and immunity in vivo. J Immunol. 2006;176:5255–5266. doi: 10.4049/jimmunol.176.9.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4:665–674. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- Sojka DK, Bruniquel D, Schwartz RH, Singh NJ. IL-2 secretion by CD4+ T cells in vivo is rapid, transient, and influenced by TCR-specific competition. J Immunol. 2004;172:6136–6143. doi: 10.4049/jimmunol.172.10.6136. [DOI] [PubMed] [Google Scholar]
- de la Rosa M, Rutz S, Dorninger H, Scheffold A. Interleukin-2 is essential for CD4+CD25+ regulatory T cell function. Eur J Immunol. 2004;34:2480–2488. doi: 10.1002/eji.200425274. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- Maloy KJ, Powrie F. Fueling regulation: IL-2 keeps CD4+ Treg cells fit. Nat Immunol. 2005;6:1071–1072. doi: 10.1038/ni1105-1071. [DOI] [PubMed] [Google Scholar]
- Hwang KW, Sweatt WB, Mashayekhi M, Palucki DA, Sattar H, Chuang E, Alegre ML. Transgenic expression of CTLA-4 controls lymphoproliferation in IL-2-deficient mice. J Immunol. 2004;173:5415–5424. doi: 10.4049/jimmunol.173.9.5415. [DOI] [PubMed] [Google Scholar]
- Papiernik M, de Moraes ML, Pontoux C, Vasseur F, Penit C. Regulatory CD4 T cells: expression of IL-2R alpha chain, resistance to clonal deletion and IL-2 dependency. Int Immunol. 1998;10:371–378. doi: 10.1093/intimm/10.4.371. [DOI] [PubMed] [Google Scholar]