Abstract
Progressive synaptic degeneration and neuron loss are major structural correlates of cognitive impairment in Alzheimer’s disease (AD). The mechanisms by which synaptic degeneration in AD occurs have not been established. The activation of proteins within the caspase family has been implicated in AD-associated neurodegeneration, and synaptically localized caspase activity could play a role in the synaptic degeneration and loss found in AD. We used synaptosomal fractionation with Western blotting and immunohistochemistry to examine the anatomical, subcellular, and subsynaptic expression patterns of caspase 3 in both the anterior cingulate cortex and hippocampus of control and AD patients. In both control and AD cases, there was a selective enrichment of caspase- 3 at synapses, particularly in the postsynaptic density (PSD) fractions. Compared with controls, AD patients exhibited significant increases in synaptic procaspase- 3 and active caspase-3 expression levels that were most evident in the PSD fractions. These data demonstrate for the first time the preferential localization and increase of caspase-3 in the PSD fractions in AD and suggest an important role for caspase 3 in synapse degeneration during disease progression.
Alzheimer’s disease (AD), the leading cause of dementia in the aging population in the US, is a neurodegenerative disease characterized by progressive deposition of amyloid β-peptide (Aβ) in senile plaques, aggregation of paired helical filament tau (PHFtau) in neurofibrillary tangles and neuritic plaques, progressive synaptic degeneration and neuron loss, and activation of astroglia and microglia. Among these pathological changes, synapse loss has been described as the most highly correlated to severity of cognitive impairment.1,2 Synaptic degeneration in AD is characterized by dendritic spine loss,3 loss of axon terminals,4 and decreased expression of presynaptic and postsynaptic proteins.5,6,7,8,9,10,11
Elucidating the mechanisms by which synaptic degeneration occurs is crucial to achieving a better understanding of AD dementia. Neurodegeneration in AD occurs with activation of various proteases including caspases, calpains, and cathepsins, as well as lysosomal and ubiquitin-proteasome system proteases.12,13,14 Apoptosis-related caspases have been proposed to contribute significantly to progressive neuron death in AD.15,16,17,18,19,20,21 In cultured hippocampal neurons, Aβ causes caspase activation leading to apoptosis.22 Abnormal expression levels of initiator caspases 8 and 9 and effector caspases 3 and 6 have been reported in postmortem brain tissues from patients with AD.23,24,25,26,27,28 Additionally, the caspases have a role in amyloid precursor protein (APP) processing and the biogenesis of amyloidogenic Aβ peptide species.18 Both caspase-cleaved APP and activated caspase-9 have been found to be present in brains of AD patients but not in controls.20 Active caspases and the resulting cleaved caspase substrates, including fodrin, actin, tau, and APP, have been detected in postmortem AD brain tissue and AD animal models, which further supports the hypothesis that caspases contribute to AD neurodegeneration.18,20,29,30,31,32,33,34
Caspase-3 is a principal effector caspase in apoptotic cascades leading to neuronal apoptosis, as caspase-3-null mice lose developmental neuronal apoptosis, whereas other effector caspase knockout models, for example caspase-6, do not.35,36 Recent work has also implicated caspase-3 in various mechanisms of synaptic plasticity.37 There is relatively little information on caspase-3 in the AD brain in general and at the synapse in particular. Caspase-3 has been implicated in the processing of APP into amyloidogenic fragments and the accumulation of caspase-cleaved APP may be an early neurodegenerative event in the progression of AD.18,20,38 Immunohistochemical analysis has demonstrated increased levels of active caspase-3 in hippocampal neuron somata and neurites of AD brains with a high degree of co-localization with neurofibrillary tangles and plaques.39 Caspase-3 also has been reported to be present in synapses in response to apoptotic insults,39,40,41 but its local effects at the synapse have not been described.
To better understand the synaptic degeneration associated with AD, we examined the anatomical, subcellular, and subsynaptic expression patterns of caspase-3 in the anterior cingulate cortex and hippocampus of control and AD patients. We describe a selective enrichment of caspase-3 at synapses, especially in postsynaptic density fractions (PSDs), and a significant increase in synaptic procaspase-3 and active caspase-3 expression in AD.
Materials and Methods
Cases and Controls
Human brain tissues used in this study were obtained from the Center for Neurodegenerative Disease Research collection at the University of Pennsylvania (Philadelphia, PA) and the Rush Alzheimer Disease Center at Rush University (Chicago, IL). All tissues were obtained in accord with institutional review board-approved protocols at the respective institutions. Summary case information is presented in Table 1. For the biochemical fractionation and Western blotting experiments, fresh frozen anterior cingulate cortex gray matter from 22 cases were used (12 AD and 10 normal controls). Anterior cingulate cortex was chosen as a region of interest because of extensive metabolic and molecular neuroimaging data highlighting its abnormality in AD and its role in the clinically relevant psychiatric, social, and executive behavioral abnormalities in AD.42,43,44,45 For immunohistochemistry and image analysis, fixed paraffin-embedded tissues from anterior cingulate (15 AD, 12 controls) and mid-hippocampus (22 AD, 23 controls) were used. Cases and controls were well matched for age, sex, and postmortem interval and there were no significant differences between groups for these variables.
Table 1.
Case Information
| Group | No. | Age (SD) | Sex (F/M) | PMI (SD) |
|---|---|---|---|---|
| Fractionation and Western blotting—anterior cingulate | ||||
| C | 10 | 83.6 (8.8) | 5/5 | 8.6 (5.7) |
| AD | 12 | 83.6 (6.4) | 6/6 | 8.1 (7.1) |
| Immunohistochemistry—anterior cingulated | ||||
| C | 12 | 80.9 (9.3) | 5/7 | 9.8 (6.6) |
| AD | 15 | 80.6 (9.8) | 10/5 | 9.1 (6.7) |
| Immunohistochemistry—hippocampus | ||||
| C | 23 | 74.0 (13.6) | 13/10 | 12.3 (5.9) |
| AD | 22 | 74.9 (11.8) | 12/10 | 11.6 (5.9) |
Groups: C, control; AD, Alzheimer’s disease.
Tissue Preparation and Biochemical Fractionation of Synaptosomes
Nuclear and cytoplasmic extracts were prepared with the NXTRACT CelLytic nuclear extraction kit according to the manufacturer’s instructions (Sigma-Aldrich, St. Louis, MO). Synaptosomes were prepared using a one-step synaptosome preparation based on the method of Phillips and colleagues.46 For each sample, 0.5 g of tissue was homogenized in a sucrose solution (0.32 mol/L sucrose, 0.1 mmol/L CaCl2, 1 mmol/L MgCl2) with a protease inhibitor cocktail (P8340, Sigma). The homogenate was brought to a final sucrose concentration of 1.25 mol/L by the addition of 2 mol/L sucrose and 0.1 mmol/L CaCl2. The homogenate was overlaid with 1.0 mol/L sucrose and 0.1 mmol/L CaCl2 and centrifuged at 100,000 × g for 3 hours at 4°C. The synaptosomal fraction, which forms a band at the 1.25/1.0 mol/L sucrose interface, was collected and stored at −80°C until use.
Synaptosomal extracts were further fractionated to separate pre- and postsynaptic material using the method of Phillips and colleagues46 Briefly, to obtain synaptic vesicle, presynaptic, and PSD fractions, synaptosomes were washed two times in 0.1 mmol/L CaCl2 and pelleted at 40,000 × g. Synaptosomes were then solubilized in 20 mmol/L Tris-HCl, pH 6.0, 1% Triton X-100, 0.1 mmol/L CaCl2, incubated on ice for 30 minutes, and centrifuged at 40,000 × g with the resultant supernatant being the synaptic vesicle fraction. The pellet contains the synaptic junction, consisting of pre- and postsynaptic membranes. This material was solubilized in 20 mmol/L Tris-HCl, pH 8.0, 1% Triton X-100, 0.1 mmol/L CaCl2, incubated on ice for 30 minutes, and centrifuged at 40,000 × g resulting in the supernatant containing the presynaptic fraction and the pellet containing the PSD fraction. Synaptic vesicle and presynaptic fractions were processed with acetone precipitation, and all fractions were dissolved in 5% SDS. Protein concentration was determined by the BCA method (Pierce, Rockford, IL).
Western Blot Analysis
For the Western blot analysis validating the specificity of the subsynaptic fractions obtained by biochemical fractionation, 10 μg protein of synaptic vesicle, presynaptic, and PSD fractions from normal human brain tissue were separated on 10% Tris-glycine gels (Novex Invitrogen, Carlsbad, CA) and transferred to nitrocellulose-supported membranes. In all other Western blot analyses, 20 μg of protein of each sample was separated on 12% Tris-glycine gels (Novex) and transferred to polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA). Membranes were blocked with 5% milk and incubated with primary antibody overnight at 4°C. Primary antibodies were used at the following dilutions in 3% milk in Tris-buffered saline with 0.1% Tween-20:caspase-3, 1:250 (IMG-144A; Imgenex, San Diego, CA); synaptophysin, 1:3000 (MSB5258; Chemicon, Millipore, Billerica, MA); syntaxin-1, 1:1000 (sc-12736; Santa Cruz Biotechnology, Santa Cruz, CA), and NMDAR-1, 1:1000 (sc-9058, Santa Cruz Biotechnology); PSD-95, 1:1000 (US Biological, Swampscott, MA), and β-actin (A3853; Sigma, St. Louis, MO). After primary antibody incubation, membranes were incubated with a horseradish peroxidase-coupled secondary antibody (HRP-conjugated mouse IgG, NA931; Amersham, Piscataway, NJ) for 1 hour at room temperature and processed with the ECL Plus chemiluminescence system (Amersham). Band density was quantified by densitometric analysis with the GS-800 calibrated densitometer (Bio-Rad) using Quantity One 1-D analysis software (Bio-Rad).
Immunohistochemistry and Image Analysis
Fixed, paraffin-embedded tissues were cut in the coronal plane at 6 μm on a rotary microtome that had been tested to assure invariant section thickness, and mounted on APES-coated slides. Dewaxed sections were immersed in 5% hydrogen peroxide dissolved in absolute methanol for 30 minutes to quench endogenous peroxidase activity. For antigen retrieval, the sections were boiled in 1 mmol/L ethylenediaminetetraacetic acid in 0.1 mol/L Tris buffer, pH 8.0, for 10 minutes. After cooling for 20 minutes. and rinsing in water, followed by two changes of Tris-Triton (0.01% Triton X-100 in 0.1 mol/L Tris-HCL buffer, pH 7.6), sections were blocked for 45 minutes in 2% horse serum dissolved in Tris-Triton and incubated in the primary antibody, anti-cleaved caspase-3, 1:200 for hippocampal sections, 1:8000 for anterior cingulate sections (Asp-175; Cell Signaling Technology, Danvers, MA) overnight (14 to 18 hours) at 4°C. After Tris-Triton rinses, sections were incubated in a biotinylated secondary antibody (Vector Laboratories, Burlingame, CA) for an hour at room temperature. Hippocampal sections were then treated for another hour at room temperature with an avidin-biotin-peroxidase complex made from a Vectastain Elite ABC kit (Vector Laboratories) and developed for 10 minutes in a Biogenex (San Ramon, CA) solution containing 0.05% diaminobenzidine and 0.03% hydrogen peroxide in Tris-Triton, supplemented with 0.25% NiSO4·6H2O to amplify the immunohistochemical signal. Anterior cingulate sections, which benefited from additional antigen retrieval, were treated for 30 minutes with a streptavidin-horseradish peroxidase complex from a TSA biotin system kit (Perkin Elmer, Waltham, MA) and incubated for 10 minutes with a biotinyl tyramide solution. These sections were again treated for 30 minutes with a streptavidin-horseradish peroxidase complex and then developed in a diaminobenzidine reaction, as described for the hippocampal sections. After clearing in xylenes, all tissue sections were coverslipped under Cytoseal 60 (Richard-Allan Scientific, Kalamazoo, MI). Specificity of the primary antibody for its antigen of interest was confirmed with a blocking experiment with recombinant activated caspase-3 (CASP3F, Sigma-Aldrich).
Immunolabeled slides were first qualitatively examined, and then immunoreactivity was semiquantitatively measured in extracellular neuropil regions of interest in anterior cingulate and the molecular layer of the dentate gyrus of the hippocampal formation by net optical density (OD), defined as the OD of the region of interest minus the OD of the background (obtained from blood vessel walls in the regions of interest). OD analysis was performed on high resolution, gray-scale photomontages captured on Leica DMRBE microscope equipped with a motorized microscope stage with Image-Pro Plus software (Media Cybernetics, Silver Spring, MD). OD comparisons were performed on sections photographed at the same, verified light intensity. The operator was blind to any identifying information throughout data accrual and analysis.
Statistics
Statistical significance of differences between diagnostic groups was assessed with two-tailed Student’s t-test. Statistical significance was defined as P < 0.05.
Results
Biochemical Localization of Caspase-3 in Nuclear, Cytosolic, and Synaptic Compartments
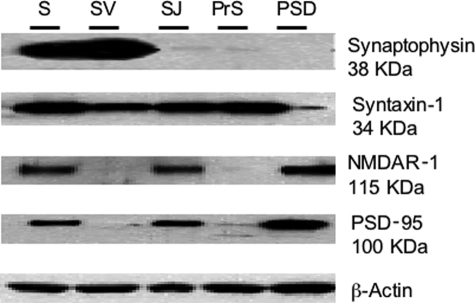
Western blotting demonstrated that caspase-3, in its procaspase form, was present in cytosolic, nuclear, and synaptosomal extracts (Figure 1). In all cases, expression levels were highest in the synaptosomal extracts, followed by cytosolic and then nuclear extracts. Thus, a significant proportion of caspase-3 is localized at the synapse. Next, synaptosomal extracts were fractionated into subsynaptic compartments, and Western blotting was used to validate the efficiency of the method (Figure 2).46 The proteins used as markers for the different subsynaptic fractions included synaptophysin for synaptic vesicles, syntaxin-1 for presynaptic plasma membrane and vesicle fusion protein, and NMDAR-1 and PSD-95 for postsynaptic density. The synaptic vesicle fractions showed a positive signal for both synaptophysin and syntaxin-1, although the signal for synaptophysin was much stronger. There was no evidence of NMDAR-1 or PSD-95 in the synaptic vesicle fraction. The presynaptic fraction showed a positive signal exclusively for syntaxin-1, and the PSD fraction displayed a positive signal for NMDAR-1 and PSD-95, as expected.
Figure 1.
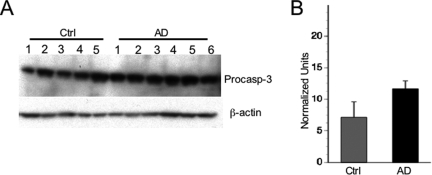
Caspase-3 distribution in cytosolic extracts (cyt), nuclear extracts (nucl), and synaptosomes (syn) from normal and AD brain tissue. The caspase-3 antibody recognizes both procaspase-3 (∼32 kDa) and the large subunit of active/cleaved caspase-3 (∼14 to 21 kDa). β-Actin was used as a loading control for normalization purposes.
Figure 2.
Western blots demonstrate synaptic fractionation efficiency in whole human brain. Five lanes were loaded with equal protein amounts from whole synaptosome preparation (S, lane 1), synaptic vesicle (SV, lane 2), synaptic junction (SJ, lane 3), presynaptic (PrS, lane 4), and postsynaptic density (PSD, lane 5) fractions. Blots were immunolabeled with antibodies to specific, compartmentalized synaptic protein constituents, including synaptophysin (synaptic vesicles), syntaxin-1 [presynaptic plasma membrane (SJ) for vesicle fusion], and NMDAR-1 and PSD-95, which are components of the postsynaptic density.
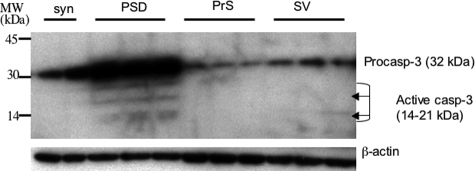
Caspase-3, in its procaspase form, was present in the synaptosomal extracts of all samples (Figure 3), and its distribution in the various fractions of synaptosomal extracts was determined (Figure 4). There was a high level of expression of procaspase-3 in the PSD fractions and low levels of expression in the synaptic vesicle and presynaptic fractions. Active caspase-3 was observed only in PSD fractions and could be detected neither in synaptic vesicle and presynaptic membrane fractions nor in unfractionated synaptosomes in any case (Figure 4).
Figure 3.
A: Caspase-3 expression in synaptosomes from normal and AD brain tissue. β-Actin was used as a loading control for normalization purposes. B: Densitometric analysis of procaspase-3 expression in synaptosomes in control (Ctrl) and AD groups (t = 3.8, P < 0.01).
Figure 4.
Relative distribution of caspase-3 in synaptosomal extracts and subsynaptic fractions of human brain tissue. A control case was used to prepare synaptosomes (S) and all subsynaptic fractions (PSD, postsynaptic density; PrS, presynaptic; SV, synaptic vesicle). All subsynaptic fractions were loaded in triplicate, the synaptosomes in duplicate. Left margin, molecular sizes.
Samples from well-matched cases were divided into two groups (males and females), each with five control and six AD samples to measure differences in pro- and active caspase-3 expression levels between control and AD cases (Figure 3B and Figure 5). Extracts from the 11 cases in each group were run together on single blots. Caspase-3 band densities were normalized to β-actin. There were no differences in normalized density readings between males and females, so all cases were combined without adjustment for statistical analysis of diagnostic group differences. In synaptosomal fractions, procaspase-3 expression was significantly higher in AD by an average of 39% compared to controls (Figure 3). In PSD fractions, procaspase-3 was significantly higher in AD by an average of 84%, whereas active caspase-3 was significantly increased in AD by 147% (Figure 5). No significant differences in caspase-3 expression were found in the nuclear fraction between groups.
Figure 5.
Caspase-3 expression in PSD subsynaptic fractions from control and AD brain tissue. The caspase-3 antibody recognizes both procaspase-3 (∼32 kDa) and the large subunit of active/cleaved caspase-3 (∼14 to 21 kDa). β-Actin was used as a loading control for normalization purposes. Only images of group 1 (N1 to N5 and AD1 to AD6) results are shown, but D includes data from both groups 1 and 2 (N6 to N10 and AD7 to AD13). A: Both pro- and active caspase-3 bands are displayed. B: Procaspase-3 bands are displayed more clearly with a shorter exposure time than in A. C: A longer exposure time than in A displays clearly the stack of bands (∼20 kDa, 17 kDa, and 14 kDa) corresponding to active caspase-3. D: Densitometric analysis show increased expression levels in AD for procaspase-3 (t = 3.7, P < 0.002) and activated caspase-3 (t = 3.5, P < 0.004).
Immunohistochemical Expression of Active Caspase-3 in Anterior Cingulate and Hippocampal Formation
The cellular, laminar, and neuropil expression of active caspase-3 was examined in hippocampal formation and anterior cingulate cortex of control and AD cases with immunohistochemistry (Figure 6). Labeling was visually greater in AD than control cases in almost all instances. In both brain regions, variable numbers of neurons and glia exhibited immunostaining of varying intensities in cytoplasm, and infrequently in nuclei.
Figure 6.
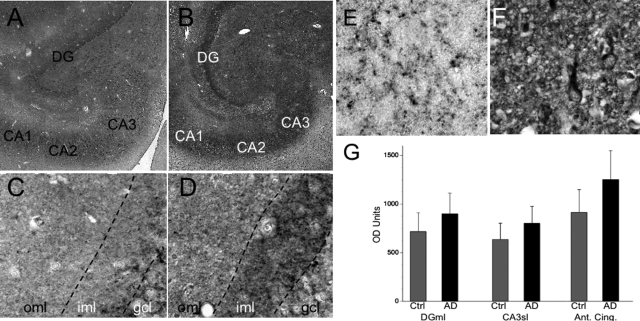
Immunohistochemical expression of activated caspase-3. A to D: Hippocampal formation of nonneuropsychiatric control case (A, 78-year-old female) and AD case (B, 81-year-old female). Note the especially intense labeling of synaptic terminal fields in the molecular layer of the dentate gyrus (DG) as well as neuropil of the ammonic subfields (CA1 to CA3) in the AD case. High magnification of dentate gyrus with the outer molecular layer (oml), the inner molecular layer (iml), and the granule cell layer (gcl) designated in control case (C, 64-year-old female) and AD case (D, 68-year-old male). Note the increased punctate and diffuse immunolabeling, especially in the inner molecular layer (iml) and less so in the outer molecular layer (oml). E and F: Anterior cingulate cortex, layer II in control (E, 86-year-old male) and AD (F, 56-year-old female). Increased expression of activated caspase-3 is evident in AD (F) in neuropil and somatodendritic portions of neurons, but not nuclei. G: Optical densitometry (mean + SD bars) shows significance between group differences in dentate gyrus molecular layer (DGml, t[1,43] = 3.0, P < 0.005), CA3 stratum lucidum (CA3sl, t[1,43] = 2.3, P < 0.03), and anterior cingulate (Ant. Cing., t[1,25] = 3.1, P < 0.005).
In anterior cingulate cortex, occasional neurons exhibited active caspase-3 immunoreactivity in cytoplasm that was clearly above background. This was chiefly observed in deeper layers. Background neuropil labeling was relatively homogeneous except for layer I and the superficial portion of layer II, where it was more intense. Numerous caspase-3-positive puncta and thread-like processes were present in the neuropil, suggestive of neuritic processes and spines. This was especially evident in the AD cases.
As in the anterior cingulate, occasional neurons in the hippocampal formation expressed caspase-3, both among granule cells in the dentate gyrus and pyramidal neurons in the ammonic subfields. In addition, most of these cells exhibited somatodendritic labeling without nuclear labeling. In the dentate gyrus of the hippocampal formation, distinct neuropil labeling was present in both the inner and outer molecular layers, representing, respectively, the synaptic terminal fields of the hilar mossy cells and the perforant pathway of the entorhinal cortex, with especially high expression levels evident in the inner molecular layer. In the ammonic subfields, labeling was prominent in the neuropil of stratum pyramidale, stratum radiatum, and stratum lucidum (CA3). Neuropil labeling had a fine mesh appearance in the dentate gyrus molecular layer and was accompanied by puncta and thread-like processes in the hilus and ammonic subfields. Semiquantitative analyses of optical density of active caspase immunoreactivity (minus background, measured in local blood vessel walls, which were unstained) in AD compared to control cases found significant increases in active caspase-3 expression in neuropil in AD compared to controls, with a mean 26% increase in the dentate gyrus molecular layer and a 37% increase in the extracellular neuropil of the anterior cingulate.
Discussion
The aims of this study were twofold: to compare the localization of caspase-3 in nuclear, cytosolic, synaptic, and subsynaptic compartments in human brain tissue; and to determine whether there are differences in the levels of synaptically localized caspase-3 between AD and control patients. Western blotting experiments demonstrated that a significant proportion of caspase-3 is localized at the synapse relative to the distribution levels in the nuclear and cytosolic extracts and that active caspase-3 is predominantly localized to the PSD fraction of synapses. In anterior cingulate cortex, Western blotting showed significantly higher levels of caspase-3 (in its procaspase form) in the synaptosomes of AD cases compared to controls and significantly higher levels of both procaspase-3 and active caspase-3 specifically in the PSD fractions in AD. Likewise, immunohistochemistry data indicated a greater degree of labeling for active caspase-3 in neuropil of synaptic fields in both the anterior cingulate and hippocampus of AD cases. To our knowledge, this study provides the first demonstration of subsynaptic localization of procaspase-3 or active caspase-3.
Our immunohistochemical data shows expression of active caspase-3 in synaptic fields and some neurons, whereas the biochemical data indicated presence of active caspase-3 only in the highly purified subsynaptic PSD fraction. That active caspase-3 is detectable only in the PSD fraction by Western blotting indicates the highly enriched concentration of active caspase-3 in this subsynaptic compartment relative to other subsynaptic compartments and the neuronal cell body. Our inability to detect active caspase-3 in these other fractions does not exclude its presence there; rather, it indicates that active caspase-3 was not expressed at a sufficiently high concentration to be detected by our Western blotting protocol. Our immunohistochemical and biochemical data complement one another, and particularly the biochemical studies emphasize the prominence of active caspase-3 in the postsynaptic densities of AD patients.
To understand the potential role of caspase-3 in AD-related synaptic degeneration, it is worthwhile to consider the relationships between caspase-3, APP, and Aβ. Caspase-3 has been shown to cleave APP giving rise to amyloidogenic fragments including Aβ,18,20 although not all have found this.47 In turn, exposure of cultured cortical neurons to Aβ or infection of rat hippocampal neurons with APP-expressing adenovirus (with associated increased Aβ accumulation) causes activation of caspase-3 and neuron-specific apoptosis. In accordance with the prevailing amyloid hypothesis, the accumulation and toxicity of either fibrillar or oligomeric Aβ are thought to cause both neuronal and synaptic loss in AD. To a lesser degree, there has been some research examining Aβ as a trigger for synaptic degeneration and loss in AD.6,48,49,50,51
Caspase-3 has also been implicated in mechanisms of tau-mediated neurodegeneration in AD. Tau is a substrate of caspase-3,31,33,52 and a mechanism linking Aβ deposition and neurofibrillary tangles in AD has been proposed: Aβ peptides promote neuronal pathological tau filament assembly by triggering caspase activation, leading to caspase cleavage of tau, which generates a proteolytic product that assembles more rapidly and extensively into pathological tau filaments.33,53 The fact that the rTg4510 tau transgenic mouse shows caspase-3 activation provides additional supporting evidence linking caspase-3 and tau-mediated neurodegeneration.54 Furthermore, it has been shown in the P301S tauopathy mouse model that synaptic loss occurs 3 months before fibrillary tau tangles appear.55 The occurrence of synaptic loss thus coincides with the conversion of normal tau to hyperphosphorylated insoluble tau, which destabilizes microtubules and impairs axonal and dendritic transport.56 Thus, it is possible that the abnormal accumulation of activated caspase-3 in synapses, particularly in the PSD fraction, reflects abnormal tau-mediated disruption of intraneuronal transport.
Previous immunohistochemical studies have shown immunoreactivity of active caspase-3 and caspase-3-cleaved substrates in neurons, senile plaques, and neurofibrillary tangles in AD,17,18,38,39,57,58,59,60 although not all investigators have reported these findings.61 Of note is that none of these previous studies have focused on caspase-3 in synaptic terminal fields or neuropil. The results of our study further highlight caspase-3 as a significant effector caspase involved in AD and contribute to a greater understanding of the role of synaptic degeneration as a correlate for mental decline in AD.2,19,62,63,64,65 The fact that active caspase-3 is especially localized in the synapse, specifically postsynaptically, suggests that pro-apoptotic signaling is occurring at the synapse leading to synaptic degeneration. It also is consistent with the hypothesis that apoptotic signaling can be initiated at the synapse and be communicated to the cell body, which could contribute to neuronal cell death by apoptosis.41 Because caspase-3 activation has been proposed as an early neurodegenerative event in the progression of AD,38 it is important to elaborate the mechanisms underlying the synaptic loss and degeneration linked to active caspase-3.
Acknowledgments
We thank the study participants at the University of Pennsylvania Alzheimer’s Disease Center, the Rush Memory and Aging Project, the Rush Religious Orders Study; and the staff of the University of Pennsylvania Center for Neurodegenerative Disease Research and the Rush Alzheimer’s Disease Center.
Footnotes
Address reprint requests to Steven E. Arnold, M.D., Center for Neurobiology and Behavior, University of Pennsylvania, 2205 Translational Research Laboratories, 125 South 31st St., Philadelphia, PA 19104. E-mail: sarnold@exchange.upenn.edu.
Supported by the National Institutes of Health (grants AG10124, AG15819, AG024871, and AG17917).
N.L. and J.W.C. contributed equally to this work and should be considered co-first authors of this article.
References
- Terry RD. Cell death or synaptic loss in Alzheimer disease. J Neuropathol Exp Neurol. 2000;59:1118–1119. doi: 10.1093/jnen/59.12.1118. [DOI] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Blanpied TA, Ehlers MD. Microanatomy of dendritic spines: emerging principles of synaptic pathology in psychiatric and neurological disease. Biol Psychiatry. 2004;55:1121–1127. doi: 10.1016/j.biopsych.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Schmitt FA, DeKosky ST, Mufson EJ. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology. 2007;68:1501–1508. doi: 10.1212/01.wnl.0000260698.46517.8f. [DOI] [PubMed] [Google Scholar]
- Almeida CG, Tampellini D, Takahashi RH, Greengard P, Lin MT, Snyder EM, Gouras GK. Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol Dis. 2005;20:187–198. doi: 10.1016/j.nbd.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Gylys KH, Fein JA, Yang F, Wiley DJ, Miller CA, Cole GM. Synaptic changes in Alzheimer’s disease: increased amyloid-beta and gliosis in surviving terminals is accompanied by decreased PSD-95 fluorescence. Am J Pathol. 2004;165:1809–1817. doi: 10.1016/s0002-9440(10)63436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harigaya Y, Shoji M, Shirao T, Hirai S. Disappearance of actin-binding protein, drebrin, from hippocampal synapses in Alzheimer’s disease. J Neurosci Res. 1996;43:87–92. doi: 10.1002/jnr.490430111. [DOI] [PubMed] [Google Scholar]
- Hatanpää K, Isaacs KR, Shirao T, Brady DR, Rapoport SI. Loss of proteins regulating synaptic plasticity in normal aging of the human brain and in Alzheimer disease. J Neuropathol Exp Neurol. 1999;58:637–643. doi: 10.1097/00005072-199906000-00008. [DOI] [PubMed] [Google Scholar]
- Masliah E, Iwai A, Mallory M, Ueda K, Saitoh T. Altered presynaptic protein NACP is associated with plaque formation and neurodegeneration in Alzheimer’s disease. Am J Pathol. 1996;148:201–210. [PMC free article] [PubMed] [Google Scholar]
- Mukaetova-Ladinska EB, Garcia-Siera F, Hurt J, Gertz HJ, Xuereb JH, Hills R, Brayne C, Huppert FA, Paykel ES, McGee M, Jakes R, Honer WG, Harrington CR, Wischik CM. Staging of cytoskeletal and beta-amyloid changes in human isocortex reveals biphasic synaptic protein response during progression of Alzheimer’s disease. Am J Pathol. 2000;157:623–636. doi: 10.1016/s0002-9440(10)64573-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim KS, Lubec G. Drebrin, a dendritic spine protein, is manifold decreased in brains of patients with Alzheimer’s disease and Down syndrome. Neurosci Lett. 2002;324:209–212. doi: 10.1016/s0304-3940(02)00210-0. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynaud F, Marcilhac A. Implication of calpain in neuronal apoptosis. A possible regulation of Alzheimer’s disease. FEBS J. 2006;273:3437–3443. doi: 10.1111/j.1742-4658.2006.05352.x. [DOI] [PubMed] [Google Scholar]
- Nixon RA, Cataldo AM. Lysosomal system pathways: genes to neurodegeneration in Alzheimer’s disease. J Alzheimers Dis. 2006;9:277–289. doi: 10.3233/jad-2006-9s331. [DOI] [PubMed] [Google Scholar]
- Cribbs DH, Poon WW, Rissman RA, Blurton-Jones M. Caspase-mediated degeneration in Alzheimer’s disease. Am J Pathol. 2004;165:353–355. doi: 10.1016/S0002-9440(10)63302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert A, Keil U, Marques CA, Bonert A, Frey C, Schussel K, Muller WE. Mitochondrial dysfunction, apoptotic cell death, and Alzheimer’s disease. Biochem Pharmacol. 2003;66:1627–1634. doi: 10.1016/s0006-2952(03)00534-3. [DOI] [PubMed] [Google Scholar]
- Eckert A, Marques CA, Keil U, Schussel K, Muller WE. Increased apoptotic cell death in sporadic and genetic Alzheimer’s disease. Ann NY Acad Sci. 2003;1010:604–609. doi: 10.1196/annals.1299.113. [DOI] [PubMed] [Google Scholar]
- Gervais FG, Xu D, Robertson GS, Vaillancourt JP, Zhu Y, Huang J, LeBlanc A, Smith D, Rigby M, Shearman MS, Clarke EE, Zheng H, Van Der Ploeg LH, Ruffolo SC, Thornberry NA, Xanthoudakis S, Zamboni RJ, Roy S, Nicholson DW. Involvement of caspases in proteolytic cleavage of Alzheimer’s amyloid-beta precursor protein and amyloidogenic A beta peptide formation. Cell. 1999;97:395–406. doi: 10.1016/s0092-8674(00)80748-5. [DOI] [PubMed] [Google Scholar]
- LeBlanc AC. The role of apoptotic pathways in Alzheimer’s disease neurodegeneration and cell death. Curr Alzheimer Res. 2005;2:389–402. doi: 10.2174/156720505774330573. [DOI] [PubMed] [Google Scholar]
- Lu DC, Rabizadeh S, Chandra S, Shayya RF, Ellerby LM, Ye X, Salvesen GS, Koo EH, Bredesen DE. A second cytotoxic proteolytic peptide derived from amyloid beta-protein precursor. Nat Med. 2000;6:397–404. doi: 10.1038/74656. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol. 2000;1:120–129. doi: 10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- Chan SL, Mattson MP. Caspase and calpain substrates: roles in synaptic plasticity and cell death. J Neurosci Res. 1999;58:167–190. [PubMed] [Google Scholar]
- Guo H, Albrecht S, Bourdeau M, Petzke T, Bergeron C, LeBlanc AC. Active caspase-6 and caspase-6-cleaved tau in neuropil threads, neuritic plaques, and neurofibrillary tangles of Alzheimer’s disease. Am J Pathol. 2004;165:523–531. doi: 10.1016/S0002-9440(10)63317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Mallory M, Alford M, Tanaka S, Hansen LA. Caspase dependent DNA fragmentation might be associated with excitotoxicity in Alzheimer disease. J Neuropathol Exp Neurol. 1998;57:1041–1052. doi: 10.1097/00005072-199811000-00007. [DOI] [PubMed] [Google Scholar]
- Raina AK, Hochman A, Zhu X, Rottkamp CA, Nunomura A, Siedlak SL, Boux H, Castellani RJ, Perry G, Smith MA. Abortive apoptosis in Alzheimer’s disease. Acta Neuropathol. 2001;101:305–310. doi: 10.1007/s004010100378. [DOI] [PubMed] [Google Scholar]
- Rohn TT, Head E, Nesse WH, Cotman CW, Cribbs DH. Activation of caspase-8 in the Alzheimer’s disease brain. Neurobiol Dis. 2001;8:1006–1016. doi: 10.1006/nbdi.2001.0449. [DOI] [PubMed] [Google Scholar]
- Rohn TT, Rissman RA, Davis MC, Kim YE, Cotman CW, Head E. Caspase-9 activation and caspase cleavage of tau in the Alzheimer’s disease brain. Neurobiol Dis. 2002;11:341–354. doi: 10.1006/nbdi.2002.0549. [DOI] [PubMed] [Google Scholar]
- Shimohama S, Tanino H, Fujimoto S. Changes in caspase expression in Alzheimer’s disease: comparison with development and aging. Biochem Biophys Res Commun. 1999;256:381–384. doi: 10.1006/bbrc.1999.0344. [DOI] [PubMed] [Google Scholar]
- Calon F, Lim GP, Yang F, Morihara T, Teter B, Ubeda O, Rostaing P, Triller A, Salem N, Jr, Ashe KH, Frautschy SA, Cole GM. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer’s disease mouse model. Neuron. 2004;43:633–645. doi: 10.1016/j.neuron.2004.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Anderson AJ. A potential role for apoptosis in neurodegeneration and Alzheimer’s disease. Mol Neurobiol. 1995;10:19–45. doi: 10.1007/BF02740836. [DOI] [PubMed] [Google Scholar]
- Chung CW, Song YH, Kim IK, Yoon WJ, Ryu BR, Jo DG, Woo HN, Kwon YK, Kim HH, Gwag BJ, Mook-Jung IH, Jung YK. Proapoptotic effects of tau cleavage product generated by caspase-3. Neurobiol Dis. 2001;8:162–172. doi: 10.1006/nbdi.2000.0335. [DOI] [PubMed] [Google Scholar]
- Fasulo L, Ugolini G, Visintin M, Bradbury A, Brancolini C, Verzillo V, Novak M, Cattaneo A. The neuronal microtubule-associated protein tau is a substrate for caspase-3 and an effector of apoptosis. J Neurochem. 2000;75:624–633. doi: 10.1046/j.1471-4159.2000.0750624.x. [DOI] [PubMed] [Google Scholar]
- Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, Guillozet AL, Lu M, Fu Y, Garcia-Sierra F, LaPointe N, Miller R, Berry RW, Binder LI, Cryns VL. Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer’s disease. Proc Natl Acad Sci USA. 2003;100:10032–10037. doi: 10.1073/pnas.1630428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman J, Rissman RA, Sarsoza F, Kim RC, Dick M, Bennett DA, Cotman CW, Rohn TT, Head E. Caspase-cleaved tau accumulation in neurodegenerative diseases associated with tau and alpha-synuclein pathology. Acta Neuropathol. 2005;110:135–144. doi: 10.1007/s00401-005-1027-3. [DOI] [PubMed] [Google Scholar]
- Zheng TS, Flavell RA. Divinations and surprises: genetic analysis of caspase function in mice. Exp Cell Res. 2000;256:67–73. doi: 10.1006/excr.2000.4841. [DOI] [PubMed] [Google Scholar]
- Zheng TS, Hunot S, Kuida K, Flavell RA. Caspase knockouts: matters of life and death. Cell Death Differ. 1999;6:1043–1053. doi: 10.1038/sj.cdd.4400593. [DOI] [PubMed] [Google Scholar]
- Kudryashova IV, Kudryashov IE, Gulyaeva NV. Long-term potentiation in the hippocampus in conditions of inhibition of caspase-3: analysis of facilitation in paired-pulse stimulation. Neurosci Behav Physiol. 2006;36:817–824. doi: 10.1007/s11055-006-0092-y. [DOI] [PubMed] [Google Scholar]
- Zhao M, Su J, Head E, Cotman CW. Accumulation of caspase cleaved amyloid precursor protein represents an early neurodegenerative event in aging and in Alzheimer’s disease. Neurobiol Dis. 2003;14:391–403. doi: 10.1016/j.nbd.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Su JH, Zhao M, Anderson AJ, Srinivasan A, Cotman CW. Activated caspase-3 expression in Alzheimer’s and aged control brain: correlation with Alzheimer pathology. Brain Res. 2001;898:350–357. doi: 10.1016/s0006-8993(01)02018-2. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Duan W. “Apoptotic” biochemical cascades in synaptic compartments: roles in adaptive plasticity and neurodegenerative disorders. J Neurosci Res. 1999;58:152–166. [PubMed] [Google Scholar]
- Mattson MP, Keller JN, Begley JG. Evidence for synaptic apoptosis. Exp Neurol. 1998;153:35–48. doi: 10.1006/exnr.1998.6863. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Hashimoto H, Kawabe J, Higashiyama S, Kai T, Kataoka K, Shimada A, Inoue K, Shiomi S, Kiriike N. The relationship between depressive symptoms and prefrontal hypoperfusion demonstrated by eZIS in patients with DAT. Neurosci Lett. 2008;441:328–331. doi: 10.1016/j.neulet.2008.06.053. [DOI] [PubMed] [Google Scholar]
- Grimmer T, Henriksen G, Wester HJ, Forstl H, Klunk WE, Mathis CA, Kurz A, Drzezga A: Clinical severity of Alzheimer’s disease is associated with PIB uptake in PET. Neurobiol Aging (in press) [DOI] [PubMed] [Google Scholar]
- Levy-Cooperman N, Burhan AM, Rafi-Tari S, Kusano M, Ramirez J, Caldwell C, Black SE. Frontal lobe hypoperfusion and depressive symptoms in Alzheimer disease. J Psychiatry Neurosci. 2008;33:218–226. [PMC free article] [PubMed] [Google Scholar]
- Li Y, Rinne JO, Mosconi L, Pirraglia E, Rusinek H, Desanti S, Kemppainen N, Nagren K, Kim BC, Tsui W, de Leon MJ: Regional analysis of FDG and PIB-PET images in normal aging, mild cognitive impairment, and Alzheimer’s disease. Eur J Nucl Med Mol Imaging (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips GR, Huang JK, Wang Y, Tanaka H, Shapiro L, Zhang W, Shan WS, Arndt K, Frank M, Gordon RE, Gawinowicz MA, Zhao Y, Colman DR. The presynaptic particle web: ultrastructure, composition, dissolution, and reconstitution. Neuron. 2001;32:63–77. doi: 10.1016/s0896-6273(01)00450-0. [DOI] [PubMed] [Google Scholar]
- Soriano S, Lu DC, Chandra S, Pietrzik CU, Koo EH. The amyloidogenic pathway of amyloid precursor protein (APP) is independent of its cleavage by caspases. J Biol Chem. 2001;276:29045–29050. doi: 10.1074/jbc.M102456200. [DOI] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, Viola KL, Klein WL. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HG, Moreira PI, Zhu X, Smith MA, Perry G. Staying connected: synapses in Alzheimer disease. Am J Pathol. 2004;165:1461–1464. doi: 10.1016/S0002-9440(10)63404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Zhao L, Ma QL, Calon F, Harris-White ME, Yang F, Lim GP, Morihara T, Ubeda OJ, Ambegaokar S, Hansen JE, Weisbart RH, Teter B, Frautschy SA, Cole GM. Role of p21-activated kinase pathway defects in the cognitive deficits of Alzheimer disease. Nat Neurosci. 2006;9:234–242. doi: 10.1038/nn1630. [DOI] [PubMed] [Google Scholar]
- Fasulo L, Ugolini G, Cattaneo A. Apoptotic effect of caspase-3 cleaved tau in hippocampal neurons and its potentiation by tau FTDP-mutation N279K. J Alzheimers Dis. 2005;7:3–13. doi: 10.3233/jad-2005-7102. [DOI] [PubMed] [Google Scholar]
- Cho JH, Johnson GV. Glycogen synthase kinase 3 beta induces caspase-cleaved tau aggregation in situ. J Biol Chem. 2004;279:54716–54723. doi: 10.1074/jbc.M403364200. [DOI] [PubMed] [Google Scholar]
- Ramalho RM, Viana RJ, Castro RE, Steer CJ, Low WC, Rodrigues CM. Apoptosis in transgenic mice expressing the P301L mutated form of human tau. Mol Med. 2008;14:309–317. doi: 10.2119/2007-00133.Ramalho. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, Maeda J, Suhara T, Trojanowski JQ, Lee VM. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci. 2007;8:663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- Ayala-Grosso C, Ng G, Roy S, Robertson GS. Caspase-cleaved amyloid precursor protein in Alzheimer’s disease. Brain Pathol. 2002;12:430–441. doi: 10.1111/j.1750-3639.2002.tb00460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala-Grosso C, Tam J, Roy S, Xanthoudakis S, Da Costa D, Nicholson DW, Robertson GS. Caspase-3 cleaved spectrin colocalizes with neurofilament-immunoreactive neurons in Alzheimer’s disease. Neuroscience. 2006;141:863–874. doi: 10.1016/j.neuroscience.2006.04.041. [DOI] [PubMed] [Google Scholar]
- Bader Lange ML, Cenini G, Piroddi M, Mohmmad Abdul H, Sultana R, Galli F, Memo M, Butterfield DA. Loss of phospholipid asymmetry and elevated brain apoptotic protein levels in subjects with amnestic mild cognitive impairment and Alzheimer disease. Neurobiol Dis. 2008;29:456–464. doi: 10.1016/j.nbd.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SL, Griffin WS, Mattson MP. Evidence for caspase-mediated cleavage of AMPA receptor subunits in neuronal apoptosis and Alzheimer’s disease. J Neurosci Res. 1999;57:315–323. doi: 10.1002/(SICI)1097-4547(19990801)57:3<315::AID-JNR3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- LeBlanc A, Liu H, Goodyer C, Bergeron C, Hammond J. Caspase-6 role in apoptosis of human neurons, amyloidogenesis, and Alzheimer’s disease. J Biol Chem. 1999;274:23426–23436. doi: 10.1074/jbc.274.33.23426. [DOI] [PubMed] [Google Scholar]
- Coleman PD, Yao PJ. Synaptic slaughter in Alzheimer’s disease. Neurobiol Aging. 2003;24:1023–1027. doi: 10.1016/j.neurobiolaging.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Masliah E. Mechanisms of synaptic dysfunction in Alzheimer’s disease. Histol Histopathol. 1995;10:509–519. [PubMed] [Google Scholar]
- Masliah E, Crews L, Hansen L. Synaptic remodeling during aging and in Alzheimer’s disease. J Alzheimers Dis. 2006;9:91–99. doi: 10.3233/jad-2006-9s311. [DOI] [PubMed] [Google Scholar]
- Masliah E, Miller A, Terry RD. The synaptic organization of the neocortex in Alzheimer’s disease. Med Hypotheses. 1993;41:334–340. doi: 10.1016/0306-9877(93)90078-5. [DOI] [PubMed] [Google Scholar]