Abstract
The T-cell-derived, pleiotropic cytokine interferon (IFN)-γ is believed to play a key regulatory role in immune-mediated demyelinating disorders of the central nervous system, including multiple sclerosis and experimental autoimmune encephalomyelitis. Our previous work has demonstrated that the endoplasmic reticulum (ER) stress response modulates the response of oligodendrocytes to this cytokine. The ER stress response activates the pancreatic ER kinase, which coordinates an adaptive program known as the integrated stress response by phosphorylating translation initiation factor 2α (eIF2α). In this study, we found that growth arrest and DNA damage 34 (GADD34), a stress-inducible regulatory subunit of a phosphatase complex that dephosphorylates eIF2α, was selectively up-regulated in myelinating oligodendrocytes in mice that ectopically expressed IFN-γ in the central nervous system. We also found that a GADD34 mutant strain of mice displayed increased levels of phosphorylated eIF2α (p-eIF2α) in myelinating oligodendrocytes when exposure to IFN-γ, as well as diminished oligodendrocyte loss and hypomyelination. Furthermore, treatment with salubrinal, a small chemical compound that specifically inhibits protein phosphatase 1(PP1)-GADD34 phosphatase activity, increased the levels of p-eIF2α and ameliorated hypomyelination and oligodendrocyte loss in cultured hippocampal slices exposed to IFN-γ. Thus, our data provide evidence that an enhanced integrated stress response could promote oligodendrocyte survival in immune-mediated demyelination diseases.
In response to cellular stresses, a family of protein kinases phosphorylate the α subunit of the eukaryotic translation initiation factor 2 (eIF2α) on serine 51 to adapt cells to the stresses, which has been referred to as the integrated stress response (ISR).1,2,3 Phosphorylation of eIF2α attenuates global protein translation, which allows cells to conserve resources, and activates a transcriptional program that promotes the expression of numerous cytoprotective genes. Endoplasmic reticulum (ER) stress, which is elicited by the accumulation of unfolded or misfolded proteins in the ER, activates adaptive coordinated responses including the activation of pancreatic ER kinase (PERK), an ER-localized eIF2α kinase. PERK-eIF2α-mediated ISR protects cells against ER stress and other cellular stresses.1,3,4 Nevertheless, ER stress that cannot be resolved by the adaptive responses ultimately leads to the apoptotic death of the cells.4,5
To recover from inhibition of global protein biosynthesis induced by the ISR, eIF2α is quickly dephosphorylated by a complex containing the enzyme phosphatase (PP1) and its essential nonenzymatic co-factor, growth arrest and DNA damage 34 (GADD34).6,7,8 Interestingly, the expression of GADD34 is regulated by induction of the cytosolic transcription factor ATF4, the translation of which is up-regulated in the presence of eIF2α phosphorylation, creating a tight autofeedback loop.8,9 Importantly, it has been demonstrated that GADD34 inactivation increases the levels of phosphorylated eIF2α (p-eIF2α) in ER-stressed cells and protects cells from the stress.10,11 Salubrinal (sal), a small chemical compound, has been identified that specifically inhibits PP1-GADD34 phosphatase activity; resulting in sustained eIF2α phosphorylation in ER-stressed cells. Treatment with sal protects cells from ER stress and viral infection.12,13
Studies have suggested that myelinating cells, oligodendrocytes in the central nervous system (CNS), and Schwann cells in the peripheral nervous system, are highly sensitive to the disruption of homeostasis in the ER.14,15,16 During active phases of myelination these cells produce a vast amount of myelin as an extension of their plasma membrane, which may contribute to their sensitivity to disruptions in the secretory pathway. For example, Southwood and colleagues14 have demonstrated that oligodendrocyte apoptosis in proteolipid protein mutant mice is associated with ER stress in these cells. Moreover, a recent report has indicated that ER stress in Schwann cells contributes to demyelination in the peripheral nervous system of P0 glycoprotein mutant mice.16 We have also shown that the ER stress response in oligodendrocytes is a factor in immune-mediated demyelinating disorders.15
The T-cell-derived pleiotropic cytokine interferon (IFN)-γ, which becomes detectable in the CNS in the symptomatic phase of multiple sclerosis (MS) and experimental autoimmune encephalomyelitis (EAE), is believed to play a crucial role in these immune-mediated demyelinating diseases.17,18,19 Nevertheless, the data concerning its roles, beneficial or detrimental, in these disorders are at times contradictory.20,21 In previous reports, we have shown that myelinating oligodendrocyte death during development or remyelinating oligodendrocyte death in cuprizone-induced demyelinated lesions elicited by IFN-γ is associated with a severe ER stress response, and that PERK is essential for oligodendrocyte survival during ER stress.15,22 In contrast, we have also demonstrated that the protective effect of IFN-γ on EAE-induced demyelination is associated with a modest ER stress response in mature oligodendrocytes, and PERK is also essential for the protective role of IFN-γ in EAE.23 Here we demonstrate that GADD34 blockage, via genetic mutation or sal treatment, ameliorated IFN-γ-induced oligodendrocyte loss and hypomyelination.
Materials and Methods
Mouse Breeding
Line110 GFAP/tTA mice and line184 TRE/IFN-γ mice on the C57BL/6 background24 were mated with GADD34 mutant mice11 on the C57BL/6 background, and the resulting progeny were intercrossed to obtain GFAP/tTA; TRE/IFN-γ; GADD34 WT mice, and GFAP/tTA; TRE/IFN-γ; GADD34 mutant mice. To prevent transcriptional activation of the TRE/IFN-γ transgene by tTA, 0.05 mg/ml of doxycycline was added to the drinking water and was provided ad libitum. All animal procedures were conducted in complete compliance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Chicago.
Hippocampal Slice Cultures
Hippocampal slice cultures were prepared from postnatal day 6 rat pups and maintained until their use as previously described25,26,27 except that they were transferred to serum-free media at 4 days in vitro. Serum-free media consisted of Neurobasal (96%; Invitrogen, Carlsbad, CA) supplemented to 42 mmol/L glucose, 1 mmol/L glutamine (Sigma-Aldrich, St. Louis, MO), B27 supplement (2%, Invitrogen), gentamicin (10 μg/ml), and Fungizone (250 μg/ml, Invitrogen). Cultures were fed twice a week with freshly prepared serum-free media containing rat IFN-γ (EMB Biosciences, San Diego, CA) and sal (EMB Biosciences) as indicated.
Real-Time Polymerase Chain Reaction (PCR)
RNA samples were isolated from mouse brains using TRIzol reagent (Invitrogen) and were treated with DNaseI (Invitrogen) to eliminate genomic DNA. Reverse transcription was performed using a SuperScript first-strand synthesis system for RT-PCR kit (Invitrogen). Real-time PCR was performed with iQ Supermix (Bio-Rad Laboratories, Hercules, CA) on a real-time PCR detection system (model iQ, Bio-Rad Laboratories) as described.22 The following primers and probes for real-time PCR were used: glyceraldehyde phosphate dehydrogenase (GAPDH) sense primer (5′-CTCAACTACATGGTCTACATGTTCCA-3′); GAPDH antisense primer (5′-CCATTCTCGGCCTTGACTGT-3′); GAPDH probe (5′-TGAC TCCACTCACGGCAAATTCAACG-3′); GADD34 sense primer (5′-CCCTCCAACTCTCCTTCTTCAG-3′); GADD34 antisense primer (5′-CAGCCTCAGCATTCCGACAA-3′); GADD34 probe (vCGTGCCCTGCCCTGTGTGCCTCTA-3′); tumor necrosis factor-α (TNF-α) sense primer (5′-GGCAGGTTCTGTCCCTTTCA-3′); TNF-α antisense primer (5′-ACCGCCTGGAGTTCTGGAA); TNF-α probe (5′-CCCAAGGCGCCACATCTCCCT-3′); inducible nitric oxide synthase (iNOs) sense primer (5′-GCTGGGCTGTACAAACCTTCC-3′); iNOs antisense primer (5′-TTGAGGTCTAAAGGCTCCGG-3′); iNOs probe (5′-TGTCCG AAGCAAACATCACATTCAGATCC-3′); IFN-γ sense primer (5′-GATATCTGGAGGAACTGGCAAAA-3′); IFN-γ antisense primer (5′-CTTCAAAGAGTCTGAGGTAGAAAGAGATA AT-3′); IFN-γ probe (5′-TGGTGACATGAAAATCCTGC AGAGCCA-3′).
Immunohistochemistry
Anesthetized mice were perfused through the left cardiac ventricle with 4% paraformaldehyde in phosphate-buffered saline (PBS). The half saggital brains were removed, postfixed with paraformaldehyde, cryopreserved in 30% sucrose, embedded in optimal cutting temperature compound, and frozen on dry-ice. Frozen sections were cut in a cryostat at a thickness of 10 μm. For immunohistochemistry, the sections were treated with −20°C acetone, blocked with PBS containing 10% goat serum and 0.1% Triton X-100, and incubated overnight with the primary antibody diluted in blocking solution. Fluorescein, Texas Red, or enzyme-labeled secondary antibodies (Vector Laboratories, Burlingame, CA) were used for detection. Immunohistochemistry for CC1 (APC7, 1:50; EMB Biosciences), GADD34 (1:50; Santa Cruz Biotechnology, Santa Cruz, CA), p-eIF-2α (1:50; Cell Signaling Technology, Beverly, MA), myelin basic protein (MBP, 1:1000; Sternberger Monoclonals, Lutherville, MD), CD3 (1:50, Santa Cruz Biotechnology), CD11b (1:50; Chemicon, Temecula, CA), and Rip (1:1000, Chemicon) was performed and analyzed as previously described.22,23 Hippocampal slice cultures were prepared and processed for immunohistochemistry as described previously.25,26
Electron Microscopy
Mice were anesthetized and perfused with 4% paraformaldehyde and 2.5% glutaraldehyde. White matter of the corpus callosum was processed. Thin sections were cut, stained with uranyl acetate and lead citrate, and analyzed as previously described.22,28
Western Blot Analysis
Six cultured hippocampal slices per each condition were rinsed in ice-cold PBS, pooled, and homogenized using a motorized homogenizer as previously described.22 After incubating on ice for 15 minutes, the extracts were cleared by centrifugation at 14,000 rpm twice for 30 minutes each. The protein content of each extract was determined by protein assay (Bio-Rad Laboratories). The extracts (40 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and were transferred to nitrocellulose. The blots were incubated with primary antibody (see below), and the signal was revealed by chemiluminescence after reacting with horseradish peroxidase-conjugated second antibody. The following primary antibodies were used: eIF-2α (1:500, Santa Cruz Biotechnology), p-eIF-2α (1:1000, Cell Signaling Technology), ATF4 (1:500, Santa Cruz Biotechnology), MBP (1:5000, Sternberger Monoclonals), and actin (1:1000, Sigma-Aldrich). The density of immunoblotting was quantified with Kodak 1D Image Analysis software (Kodak, Hercules, New Haven, CT).
Statistics
Data are expressed as mean ± SD. Multiple comparisons were statistically evaluated by one-way ANOVA test followed by Holm-Sidak test using Sigmastat 3.1 software (Hearne Scientific Software, Chicago, IL). Differences were considered statistically significant if P < 0.05.
Results
GADD34 Inactivation Increased the Levels of p-eIF2α in Oligodendrocytes in Mice Expressing IFN-γ in the CNS
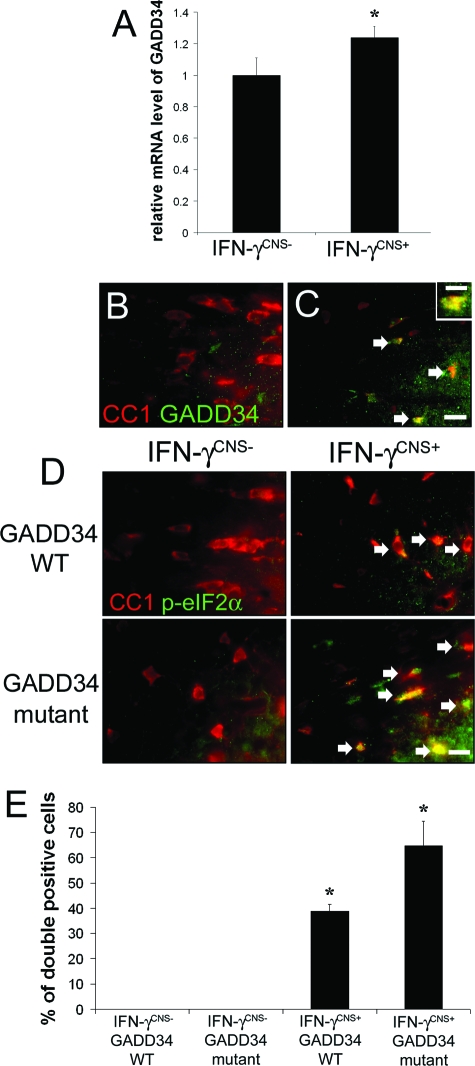
GADD34, which is expressed at very low levels in unstressed cells, is a stress-inducible regulatory subunit of a phosphatase complex that dephosphorylates eIF2α.7,8 We have generated transgenic mice that allow for the temporally controlled delivery of IFN-γ to the CNS using the tetracycline-controllable system.24 The expression of the IFN-γ transgene is repressed in GFAP/tTA; TRE/IFN-γ double-transgenic mice treated with doxycycline solution from conception (IFN-γCNS− mice). The levels of IFN-γ become detectable at approximately postnatal day 10 (P10) in the CNS of GFAP/tTA; TRE/IFN-γ double-transgenic mice that are released from doxycycline solution at embryonic day 14 (E14, IFN-γCNS+ mice).15 We have shown that activation of PERK-eIF2α pathway plays a crucial role in oligodendrocyte survival in IFN-γCNS+ mice.15 Real-time PCR analysis revealed that the presence of IFN-γ in the CNS significantly increased the expression of GADD34 in the brain (1.24 ± 0.07 versus 1.0 ± 0.11, P < 0.05; Figure 1A). We further determined the cellular expression pattern of GADD34 in the CNS using CC1, a marker for oligodendrocytes, and GADD34 double immunostaining. We found that GADD34 was undetectable in oligodendrocytes in the CNS of 21-day-old control IFN-γCNS− mice (Figure 1B), but became detectable in these cells primarily in the corpus callosum of IFN-γCNS+ mice (Figure 1C). Moreover, 48 ± 7% of the CC1-positive oligodendrocytes in the corpus callosum were GADD34-positive, and 89 ± 11% of GADD34-positive cells were CC1-positive cells. Myelination in the corpus callosum begins at approximately P14, and becomes robust at approximately P21, a time point when myelination in other areas of the CNS is largely complete.29 Thus, these data are consistent with our previous report that oligodendrocytes at the active stage of myelination are most sensitive to IFN-γ.15
Figure 1.
GADD34 inactivation increased the levels of p-eIF2α in oligodendrocytes in mice expressing IFN-γ in the CNS. A: Real-time PCR analysis revealed increased levels of GADD34 mRNA in the brain of mice expressing IFN-γ in the CNS. GADD34 mRNA levels are relative to the housekeeping gene GAPDH. N = 3 animals, error bars represent SD, *P < 0.05. B and C: CC1 and GADD34 double labeling showed elevated levels of GADD34 in oligodendrocytes (arrows, double-positive cells) in the corpus callosum of 21-day-old mice expressing IFN-γ in the CNS. Inset: High-magnification image showed the distribution of GADD34 in oligodendrocytes. D: CC1 and p-eIF2α double labeling showed modest activation of eIF2α in oligodendrocytes (arrows, double-positive cells) in the corpus callosum of 21-day-old mice expressing IFN-γ in the CNS, and GADD34 inactivation further increased the level of p-eIF2α in oligodendrocytes (arrows, double-positive cells). B, C, and D: n = 3 animals. E: Quantitative analysis showed that the presence of IFN-γ significantly increased the percentage of CC1/p-eIF2α double-positive cells in the corpus callosum of 21-day-old mice, and GADD34 inactivation further increased the percentage. N = 3 animals, error bars represent SD, *P < 0.05. Scale bars: 4 μm (C, inset); 10 μm (C, D).
We pursued a genetic approach to assess the protective effects of GADD34 blockage on oligodendrocyte death elicited by IFN-γ. GADD34 mutant mice, whose inactive GADD34 allele encodes a C-terminally truncated protein that lacks the phosphatase domain, appear healthy and display resistance to cell death induced by ER stress.11 We crossed GFAP/tTA and TRE/IFN-γ mice with GADD34 mutant mice, and the resulting progeny were intercrossed to obtain GFAP/tTA; TRE/IFN-γ; GADD34 WT mice and GFAP/tTA; TRE/IFN-γ; GADD34 mutant mice. GFAP/tTA; TRE/IFN-γ; GADD34 WT mice released from doxycycline at E14 (IFN-γCNS+ GADD34 WT mice) showed tremor and ataxia as previously described.15 In contrast, the tremoring phenotype was much milder in GFAP/tTA; TRE/IFN-γ; GADD34 mutant mice released from doxycycline at E14 (IFN-γCNS+ GADD34 mutant mice; Table 1). Nevertheless, a number of GFAP/tTA; TRE/IFN-γ; GADD34 mutant mice died by P 28. In previous reports, we have shown that the presence of IFN-γ in the CNS during development induces cerebellar dysplasia or medulloblastoma.24,30,31 Our preliminary data suggest that the premature death of these mice might be attributable to enhanced medulloblastoma formation (unpublished data).
Table 1.
GADD34 Inactivation Alleviated Tremoring Phenotype in Mice Expressing IFN-γ in the CNS
| No tremor (percentage) | Minor tremor (percentage) | Tremor (percentage) | |
|---|---|---|---|
| IFN-γCNS−GADD34 WT mice (n = 20) | 100 | 0 | 0 |
| IFN-γCNS−GADD34 mutant mice (n = 20) | 100 | 0 | 0 |
| IFN-γCNS+GADD34 WT mice (n = 20) | 10 | 20 | 70 |
| IFN-γCNS+GADD34 mutant mice (n = 24) | 33 | 42 | 25 |
We next examined the correlation between the severities of the tremoring phenotype and the levels of p-eIF-2α in oligodendrocytes in these animals. CC1 and p-eIF2α double labeling showed modest activation of eIF2α in oligodendrocytes in the corpus callosum of 21-day-old IFN-γCNS+ GADD34 WT mice, and GADD34 inactivation further increased the levels of p-eIF2α in these cells in IFN-γCNS+ GADD34 mutant mice (Figure 1, D and E). Collectively, these data indicate that GADD34 inactivation elevated the levels of p-eIF2α in oligodendrocytes in response to IFN-γ and attenuated the severity of the tremoring phenotype.
GADD34 Inactivation Diminished Oligodendrocyte Loss and Hypomyelination Elicited by IFN-γ
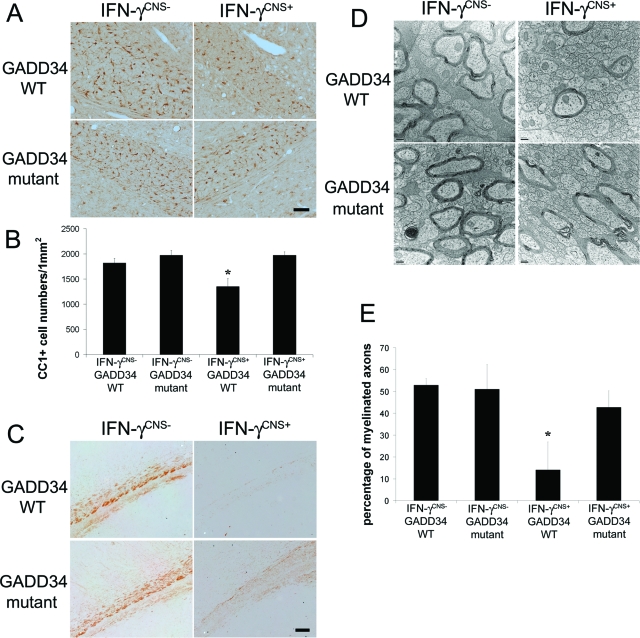
In our previous report, we showed that the tremoring phenotype in mice expressing IFN-γ in the CNS during development was primarily caused by the death of myelinating oligodendrocytes and subsequent hypomyelination.15 Importantly, in the absence of IFN-γ, GADD34 inactivation did not significantly change oligodendrocyte numbers or the myelination process in the CNS (Figure 2). We next determined whether the GADD34 mutation promoted oligodendrocyte survival in response to IFN-γ. Oligodendrocytes, identified by CC1 immunostaining, in the corpus callosum of 21-day-old IFN-γCNS+; GADD34 WT mice were significantly reduced compared to control IFN-γCNS−; GADD34 WT mice (Figure 2, A and B). In contrast, oligodendrocyte numbers in IFN-γCNS+; GADD34 mutant mice were comparable to control IFN-γCNS−; GADD34 WT mice or IFN-γCNS−; GADD34 mutant mice (Figure 2, A and B). Thus, these data indicate that GADD34 inactivation diminished the detrimental effects of IFN-γ on oligodendrocyte survival.
Figure 2.
GADD34 inactivation ameliorated oligodendrocyte loss and hypomyelination elicited by IFN-γ. A and B: CC1 immunostaining showed that oligodendrocytes in the corpus callosum of 21-day-old IFN-γCNS+; GADD34 WT mice were significantly reduced compared to control IFN-γCNS−; GADD34 WT mice (B, *P < 0.05). In contrast, oligodendrocyte numbers in IFN-γCNS+; GADD34 mutant mice were comparable to control IFN-γCNS−; GADD34 WT mice or IFN-γCNS−; GADD34 mutant mice. N = 3 animals. C: MBP immunostaining showed severe hypomyelination in the corpus callosum of 21-day-old IFN-γCNS+; GADD34 WT mice, and GADD34 inactivation protected against hypomyelination elicited by IFN-γ. N = 3 animals. D: Electron microscope analysis revealed that the presence of IFN-γ reduced the number of myelinated axons in the corpus callosum of 21-day-old mice on a GADD34 WT background and that GADD34 inactivation ameliorated the reduction in myelinated axons in mice expressing IFN-γ in the CNS. N = 3 animals. E: Electron microscope analysis revealed that the percentage of myelinated axons in the corpus callosum of 21-day-old IFN-γCNS+; GADD34 WT mice is significantly reduced compared to IFN-γCNS−; GADD34 WT mice or IFN-γCNS−; GADD34 mutant mice (*P < 0.05). Nevertheless, the percentage of myelinated axons in the corpus callosum of IFN-γCNS+; GADD34 mutant mice are comparable to IFN-γCNS−; GADD34 mutant mice. N = 3 animals. B and E: Error bars represent SD. Scale bars: 25 μm (A); 50 μm (C); 200 nm (D).
We further examined the myelinating function of oligodendrocytes in these animals. Immunostaining for MBP was notably enhanced in the corpus callosum of 21-day-old IFN-γCNS+; GADD34 mutant mice compared with IFN-γCNS+; GADD34 WT mice (Figure 2C). Moreover, electron microscope analysis revealed that the myelinated axons in the corpus callosum of 21-day-old IFN-γCNS+; GADD34 mutant mice (42.75 ± 7.5%) were significantly increased compared to IFN-γCNS+; GADD34 WT mice (14.01 ± 12.8%; Figure 2, D and E). Taken together, these data confirm that the ER stress response modulates the response of myelinating oligodendrocytes to IFN-γ and suggest that an enhanced ISR prevents oligodendrocyte death and hypomyelination elicited by this cytokine.
GADD34 Inactivation Did Not Significantly Affect the Immune Response Induced by IFN-γ
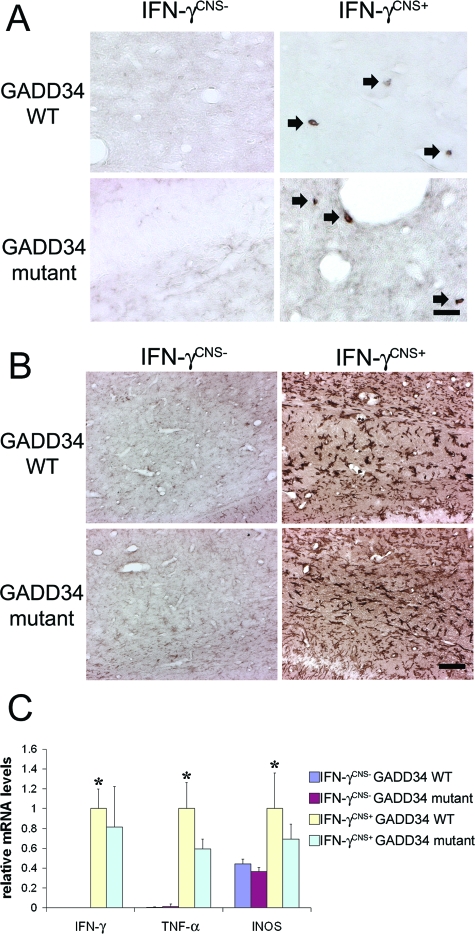
IFN-γ modulates the immune responses by promoting Th1 T-cell differentiation, activating microglia/macrophages and up-regulating the expression of many inflammatory mediators such as TNF-α and iNOs.17,32 Several lines of evidence have suggested that IFN-γ may induce myelin abnormalities via the activation of microglia/macrophages.17,33 We tested whether GADD34 inactivation affects the immune response induced by IFN-γ in the CNS. CD3 immunostaining showed few infiltrated T cells in the corpus callosum of 21-day-old IFN-γCNS+; GADD34 WT mice (Figure 3A), which is consistent with previous studies.30 Moreover, GADD34 inactivation did not significantly alter T-cell infiltration induced by IFN-γ (Figure 3A). We further found that the presence of IFN-γ in the CNS dramatically activated microglia/macrophages (Figure 3B) and significantly stimulated the expression of TNF-α and iNOs in the CNS (Figure 3C). Nevertheless, GADD34 inactivation did not significantly affect the activation of microglia/macrophages or the up-regulation of TNF-α and iNOs (Figure 3, B and C). Therefore, it is unlikely that GADD34 inactivation attenuates oligodendrocyte loss and hypomyelination induced by IFN-γ through the modulation of the immune response.
Figure 3.
GADD34 inactivation did not significantly affect immune response induced by IFN-γ. A: CD3 immunostaining showed few infiltrated T cells (arrow, CD3-positive T cells) in the corpus callosum of 21-day-old IFN-γCNS+; GADD34 WT mice compared to control IFN-γCNS−; GADD34 WT mice or IFN-γCNS−; GADD34 mutant mice. Moreover, the numbers of infiltrated T cells (arrow, CD3-positive T cells) in IFN-γCNS+; GADD34 mutant mice were comparable to IFN-γCNS+; GADD34 WT mice. N = 3 animals. B: CD11b immunostaining showed that the presence of IFN-γ dramatically activated microglia/macrophages in the corpus callosum of 21-day-old IFN-γCNS+; GADD34 WT mice compared to control IFN-γCNS−; GADD34 WT mice or IFN-γCNS−; GADD34 mutant mice. Nevertheless, the activation of microglia/macrophages in IFN-γCNS+; GADD34 mutant mice was comparable to IFN-γCNS+; GADD34 WT mice. N = 3 animals. C: Real-time PCR analysis revealed significantly increased levels of IFN-γ, TNF-α, and iNOS mRNA in the brain of 21-day-old IFN-γCNS+; GADD34 WT mice compared to control IFN-γCNS−; GADD34 WT mice, or IFN-γCNS−; GADD34 mutant mice (*P < 0.05). Importantly, GADD34 inactivation did not change the levels of IFN-γ, TNF-α, and iNOS mRNA in the brain mice expressing IFN-γ in the CNS. N = 3 animals, error bars represent SD. Scale bars: 10 μm (A); 25 μm (B).
Sal Treatment Ameliorated Hypomyelination and Oligodendrocyte Loss Elicited by IFN-γ
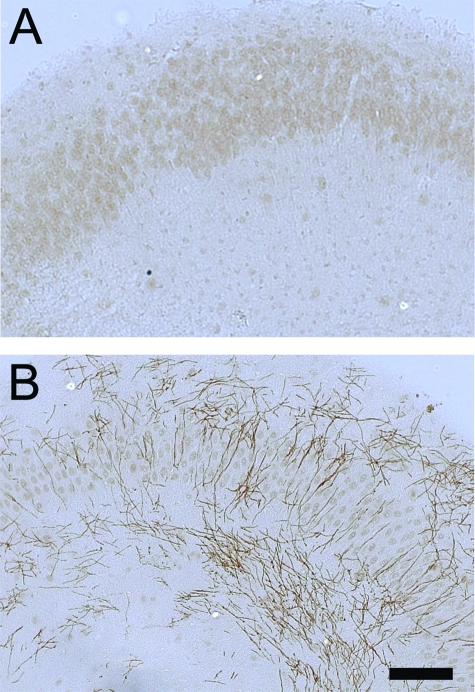
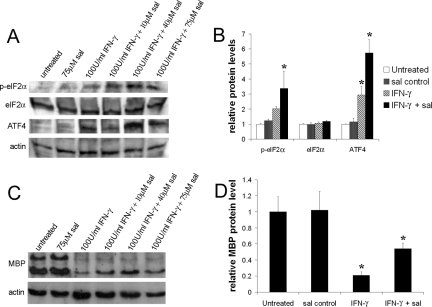
Sal specifically blocks eIF2α dephosphorylation, resulting in elevated levels of p-eIF2α in ER-stressed cells.12 Importantly, it has been demonstrated that sal treatment protects cells from ER stress.12 To test the beneficial potential of sal in the hypomyelination elicited by IFN-γ, we exploited an in vitro myelination model, the hippocampal slice culture. Myelination in hippocampal slice cultures begins ∼7 days of culture and myelin amounts become abundant by 21 days (Figure 4). After 7 days of culture, the hippocampal slices were treated with 100 U/ml IFN-γ for up to 7 days. The cultured hippocampal slices exposed to IFN-γ displayed notably increased levels of p-eIF2α and its downstream transcription factor ATF4 (Figure 5, A and B), suggesting an activation of the ER stress response. The treated cultures contained decreased levels of MBP and a reduction of Rip-positive oligodendrocyte numbers (Figure 5, C and D; and Figure 6), suggesting hypomyelination and oligodendrocyte loss. Although treatment with 75 μmol/L sal alone did not change the levels of p-eIF2α and ATF4 in cultured hippocampal slices, treatment with 10, 40, or 75 μmol/L sal combined with 100 U/ml of IFN-γ markedly elevated the levels of p-eIF2α compared to treatment with IFN-γ alone (Figure 5A). Nevertheless, the most effective concentration for sal appeared to be 40 μmol/L (Figure 5, A and B; and Supplemental Figure S1, see http://ajp.amjpathol.org), suggestive that the higher concentration of sal in combination with IFN-γ may cause cell or tissue damage.34 The 40 μmol/L sal treatment also further increased the levels of ATF4 in cultured hippocampal slices exposed to IFN-γ (Figure 5, A and B; and Supplemental Figure S1, see http://ajp.amjpathol.org). Moreover, treatment with 40 μmol/L sal notably decreased the reduction of MBP levels and oligodendrocyte numbers in cultured hippocampal slices exposed to IFN-γ (Figure 5, C and D; and Figure 6). Thus, these findings further support the suggestion that an enhanced ISR protects against hypomyelination and oligodendrocyte loss elicited by IFN-γ.
Figure 4.
The myelination process in hippocampal slice cultures. A: MBP immunostaining showed that myelin was undetectable in hippocampal slice cultures at 7 days of culture. B: MBP immunostaining showed abundant myelin in hippocampal slice cultures at 21 days of culture. N = 3 cultured slices. Scale bar = 50 μm.
Figure 5.
Sal treatment increased the levels of p-eIF2α in the cultured hippocampal slices exposed to IFN-γ. A: Western blot analysis showed that IFN-γ slightly elevated the levels of p-eIF2α and ATF4 in cultured hippocampal slices and that sal treatment further increased the levels of p-eIF2α and ATF4. B: Densitometry analysis of Western blot results showed that 100 U/ml of IFN-γ treatment increased the levels of p-eIF2α and ATF4 in cultured hippocampal slices compared to untreated slices or sal-treated slices (*P < 0.05), and that 40 μmol/L sal combined with 100 U/ml of IFN-γ treatment further elevated the levels of p-eIF2α and ATF4 in cultured hippocampal slices compared to 100 U/ml of IFN-γ-treated slices (*P < 0.05). The relative protein levels are relative to actin. The experiments have been repeated three times, error bars represent SD. C: Western blot analysis revealed that IFN-γ dramatically reduced the levels of MBP in cultured hippocampal slices and that sal treatment ameliorated the reduction. D: Densitometry analysis of Western blot results showed that 100 U/ml of IFN-γ significantly reduced the levels of MBP in cultured hippocampal slices (*P < 0.05) and that 40 μmol/L sal treatment significantly ameliorated the reduction (*P < 0.05). The relative protein levels are relative to actin. The experiments have been repeated three times, error bars represent SD.
Figure 6.
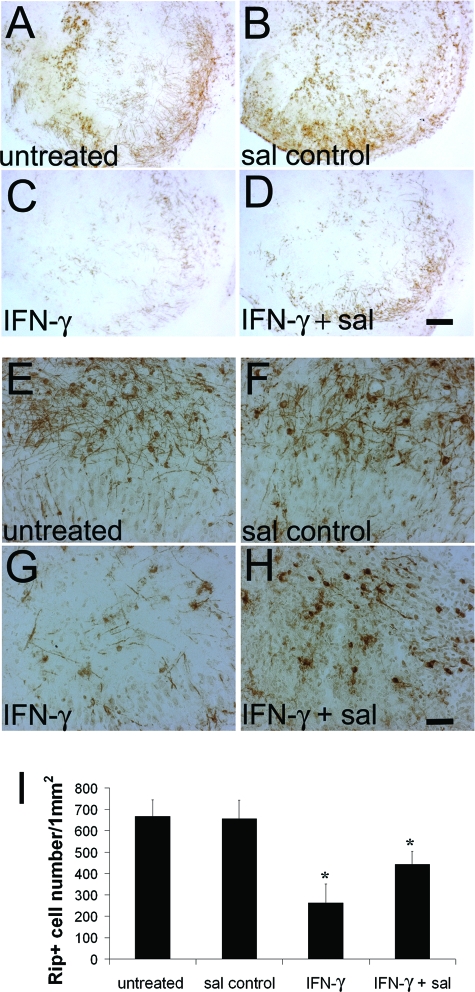
Sal treatment decreased hypomyelination and oligodendrocyte loss elicited by IFN-γ. A–D: MBP immunostaining revealed that 100 U/ml of IFN-γ markedly inhibited myelination in cultured hippocampal slices and that 40 μmol/L sal treatment reduced the inhibition (A: untreated; B: sal control; C: IFN-γ; D: IFN-γ + sal). N = 3 cultured slices. E–I: Rip immunostaining showed that oligodendrocytes in cultured hippocampal slices exposed to IFN-γ were significantly reduced compared to the untreated slices (I: *P < 0.05) and that 40 μmol/L sal treatment significantly attenuated the severity of oligodendrocyte loss (I: *P < 0.05) (E: untreated; F: sal control; G: IFN-γ; H: IFN-γ + sal). N = 3 cultured slices, error bars represent SD. Scale bars: 150 μm (D); 25 μm (H).
Discussion
The immune cytokine IFN-γ is regarded as a major pro-inflammatory cytokine involved in the pathogenesis of MS/EAE, although its effects in these disorders are highly controversial.17,19,20,21 IFN-γ or its receptor knockout mice develop a significantly worse EAE disease course compared to wild-type mice.35,36 It has also been shown that CNS delivery of IFN-γ before disease onset protects against EAE-induced demyelination and oligodendrocyte loss.23,37 In contrast, transgenic mice that ectopically express IFN-γ in the CNS during development display a tremoring phenotype and myelin abnormalities.15,30,38 Administration of IFN-γ to MS patients and EAE mice exacerbates clinical symptoms and suppresses myelin repair.22,39,40Although a number of reports have suggested that the immune modulation functions of IFN-γ contribute to its controversial roles in MS/EAE,20,21 our previous data suggest that the dual, beneficial or detrimental, roles of IFN-γ are mediated, at least in part, by ER stress in oligodendrocytes.15,19,22,23
PERK-mediated ISR activated by the ER stress response protects cells against ER stress, reactive oxidative/nitrative stress and immune-mediated damage.1,3,23 Nevertheless, ER stress that cannot be resolved by the adaptive responses ultimately leads to cell death.4,5 We have found that the modest ER stress response elicited by IFN-γ in mature oligodendrocytes of adult mice, which does not cause cell death, protects against EAE-induced demyelination, oligodendrocyte death, and axonal damage. We have also demonstrated that the PERK-mediated ISR is essential for the protective effects of IFN-γ in EAE.23 In contrast, the severe ER stress response elicited by IFN-γ in myelinating oligodendrocytes in young, developing mice eliminates those cells whose ER stress cannot be resolved by the adaptive response. Moreover, the PERK mutation markedly exacerbates apoptosis of myelinating oligodendrocytes and hypomyelination in transgenic mice that express IFN-γ in the developing CNS.15
Here we show that GADD34, a stress-inducible regulatory subunit of a phosphatase complex that dephosphorylates eIF2α, is selectively up-regulated in myelinating oligodendrocytes in mice expressing IFN-γ in the CNS. Using a genetic approach, we find that GADD34 inactivation increased the levels of p-eIF2α in myelinating oligodendrocytes, and diminished oligodendrocyte loss and hypomyelination in mice expressing IFN-γ in the CNS. Furthermore, we find that sal, a small chemical compound that specifically inhibits PP1-GADD34 phosphatase activity, elevated the levels of p-eIF2α and ATF4 and ameliorated hypomyelination and oligodendrocyte loss in cultured hippocampal slices exposed to IFN-γ. Taken together, our previous and current findings indicate that an enhanced PERK-mediated ISR could potentiate the beneficial effects of IFN-γ on oligodendrocytes and suppress its detrimental effects on these cells in immune-mediated demyelination diseases.
Although a recent report has shown that the ISR plays an important role in Th2 T-cell differentiation and Th2 cytokine IL-4 production,41 we show here that GADD34 inactivation does not significantly alter the level of total IFN-γ in the CNS of transgenic mice (Figure 3C). We also find that GADD34 inactivation does not significantly affect the infiltration of T cells, the activation of microglia/macrophages, or the up-regulation of TNF-α and iNOs in transgenic mice that ectopically express IFN-γ in the CNS. Thus, it is unlikely that GADD34 inactivation significantly changes the immune response induced by IFN-γ in the CNS. Moreover, we have previously shown that the enforced expression of the suppressor of cytokine signaling 1 (SOCS1) specifically in oligodendrocytes in transgenic mice is sufficient to block oligodendrocyte loss and hypomyelination induced by IFN-γ.42 Since it has been demonstrated that SOCS1 is capable of blocking the intracellular Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling pathway,43 our previous data provide strong evidence that IFN-γ exerts a direct deleterious effect on oligodendrocytes through the JAK/STAT signaling pathway. Taken together, these data indicate that GADD34 blockage protects against oligodendrocyte loss and hypomyelination induced by IFN-γ through a direct cytoprotective effect in oligodendrocytes.
The pathological hallmarks of MS include inflammation, oligodendrocytes loss, demyelination, and axonal degeneration.33,44,45 Regeneration of oligodendrocytes and subsequent remyelination would likely restore neurological function and protect against axonal degeneration in MS patients.46,47 Evidence is accumulating that there are sufficient oligodendrocyte precursors in MS demyelinated lesions, and that the remyelination failure is primarily attributable to the insufficient regeneration of myelinating oligodendrocytes.48,49,50 We have previously shown that remyelinating oligodendrocyte apoptosis elicited by IFN-γ, which is known to be present in the MS demyelinated lesions,51,52 might be a major contributing factor to poor remyelination in individuals with MS.22 We have also found that the ER stress response is associated with apoptosis of remyelinating oligodendrocytes elicited by IFN-γ in the cuprizone-induced demyelination model, and that PERK is essential for remyelinating oligodendrocyte survival during ER stress.22 Although a recent report showed that sal treatment exacerbated fatty acid-induced ER stress and pancreatic β-cell apoptosis,53 the beneficial effects of sal treatment on hypomyelination elicited by IFN-γ suggest that chemical compounds that enhance the ISR could ameliorate ER stress and remyelinating oligodendrocyte death elicited by IFN-γ in MS demyelinated lesions.
On the other hand, evidence from the human genetic disease leukoencephalopathy with vanishing white matter suggests that proper regulation of translation initiation is essential for the myelinating function of oligodendrocytes.54,55 Vanishing white matter is a fatal hypomyelinating disorder caused by mutations in the β subunit of the translation initiation factor eIF2 (eIF2B).55,56 Although vanishing white matter oligodendrocytes have an abnormal appearance, there does not appear to be a reduction of oligodendrocyte numbers in this disorder.57 Thus, it seems that eIF2B mutations primarily disrupt the myelinating function of oligodendrocytes.56 Although we show here that GADD34 blockage promotes developmental myelination in the presence of IFN-γ, elevated levels of p-eIF2α by GADD34 inactivation, which correlates with eIF-2B inactivation in vanishing white matter patients, might result in dysfunction of oligodendrocytes. Thus, the potential beneficial effects of GADD34 blockage on the remyelination process in immune-mediated demyelination diseases needs to be carefully evaluated in remyelination models in vitro and in vivo.
Currently approved treatments for MS have no clear impact on myelin repair in demyelinated lesions.47 Importantly, ER stress-responsive genes such as ATF4 and heat shock protein 70 (HSP70) have been demonstrated to be up-regulated in MS demyelinated lesions.58,59 We show here that GADD34 blockage using a genetic approach or a small chemical compound protects against immune cytokine IFN-γ-induced oligodendrocyte death and myelin abnormalities. Thus, our data provides evidence that therapeutic strategies that enhance PERK-eIF2α-mediated IRS in oligodendrocyte could promote the remyelination process in patients with MS.
Footnotes
Address reprint requests to Brian Popko, The Jack Miller Center for Peripheral Neuropathy, Department of Neurology, The University of Chicago, 5841 South Maryland Ave. MC2030, Chicago, IL 60637. E-mail: bpopko@uchicago.edu.
Supported by the National Institutes of Health (grants NS34939 to B.P., NS19108 to R.P.K., and DK47119 and ES08681 to D.R.), the American Heart Association (to R.P.K.), the White Foundation (to R.P.K.), the Myelin Repair Foundation (to B.P.), and the National Multiple Sclerosis Society (career transition fellowship Grant TA 3026-A-1 to W.L.).
Supplemental material for this article can be found on http://ajp. amjpathol.org.
Present address of W.L.: Department of Cell Biology and Neuroscience, University of South Alabama, Mobile, AL.
References
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- Proud CG. eIF2 and the control of cell physiology. Semin Cell Dev Biol. 2005;16:3–12. doi: 10.1016/j.semcdb.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Lu PD, Jousse C, Marciniak SJ, Zhang Y, Novoa I, Scheuner D, Kaufman RJ, Ron D, Harding HP. Cytoprotection by pre-emptive conditional phosphorylation of translation initiation factor 2. EMBO J. 2004;23:169–179. doi: 10.1038/sj.emboj.7600030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce M, Yuan J. Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell Death Differ. 2006;13:363–373. doi: 10.1038/sj.cdd.4401817. [DOI] [PubMed] [Google Scholar]
- Schröder M, Kaufman RJ. Divergent roles of IRE1alpha and PERK in the unfolded protein response. Curr Mol Med. 2006;6:5–36. doi: 10.2174/156652406775574569. [DOI] [PubMed] [Google Scholar]
- Connor JH, Weiser DC, Li S, Hallenbeck JM, Shenolikar S. Growth arrest and DNA damage-inducible protein GADD34 assembles a novel signaling complex containing protein phosphatase 1 and inhibitor 1. Mol Cell Biol. 2001;21:6841–6850. doi: 10.1128/MCB.21.20.6841-6850.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol. 2001;153:1011–1022. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novoa I, Zhang Y, Zeng H, Jungreis R, Harding HP, Ron D. Stress-induced gene expression requires programmed recovery from translational repression. EMBO J. 2003;22:1180–1187. doi: 10.1093/emboj/cdg112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang HY, Wek SA, McGrath BC, Lu D, Hai T, Harding HP, Wang X, Ron D, Cavener DR, Wek RC. Activating transcription factor 3 is integral to the eukaryotic initiation factor 2 kinase stress response. Mol Cell Biol. 2004;24:1365–1377. doi: 10.1128/MCB.24.3.1365-1377.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jousse C, Oyadomari S, Novoa I, Lu P, Zhang Y, Harding HP, Ron D. Inhibition of a constitutive translation initiation factor 2alpha phosphatase, CReP, promotes survival of stressed cells. J Cell Biol. 2003;163:767–775. doi: 10.1083/jcb.200308075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, Yuan J. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- Wiseman RL, Balch WE. A new pharmacology—drugging stressed folding pathways. Trends Mol Med. 2005;11:347–350. doi: 10.1016/j.molmed.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Southwood CM, Garbern J, Jiang W, Gow A. The unfolded protein response modulates disease severity in Pelizaeus-Merzbacher disease. Neuron. 2002;36:585–596. doi: 10.1016/s0896-6273(02)01045-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Harding HP, Ron D, Popko B. Endoplasmic reticulum stress modulates the response of myelinating oligodendrocytes to the immune cytokine interferon-gamma. J Cell Biol. 2005;169:603–612. doi: 10.1083/jcb.200502086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennuto M, Tinelli E, Malaguti M, Del Carro U, D'Antonio M, Ron D, Quattrini A, Feltri ML, Wrabetz L. Ablation of the UPR-mediator CHOP restores motor function and reduces demyelination in Charcot-Marie-Tooth 1B mice. Neuron. 2008;57:393–405. doi: 10.1016/j.neuron.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popko B, Corbin JG, Baerwald KD, Dupree J, Garcia AM. The effects of interferon-gamma on the central nervous system. Mol Neurobiol. 1997;14:19–35. doi: 10.1007/BF02740619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imitola J, Chitnis T, Khoury SJ. Cytokines in multiple sclerosis: from bench to bedside. Pharmacol Ther. 2005;106:163–177. doi: 10.1016/j.pharmthera.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Lees JR, Cross AH. A little stress is good: IFN-gamma, demyelination, and multiple sclerosis. J Clin Invest. 2007;117:297–299. doi: 10.1172/JCI31254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühl H, Pfeilschifter J. Anti-inflammatory properties of pro-inflammatory interferon-gamma. Int Immunopharmacol. 2003;3:1247–1255. doi: 10.1016/S1567-5769(03)00131-0. [DOI] [PubMed] [Google Scholar]
- Wheeler RD, Owens T. The changing face of cytokines in the brain: perspectives from EAE. Curr Pharm Des. 2005;11:1031–1037. doi: 10.2174/1381612053381657. [DOI] [PubMed] [Google Scholar]
- Lin W, Kemper A, Dupree JL, Harding HP, Ron D, Popko B. Interferon-gamma inhibits central nervous system remyelination through a process modulated by endoplasmic reticulum stress. Brain. 2006;129:1306–1318. doi: 10.1093/brain/awl044. [DOI] [PubMed] [Google Scholar]
- Lin W, Bailey SL, Ho H, Harding HP, Ron D, Miller SD, Popko B. The integrated stress response prevents demyelination by protecting oligodendrocytes against immune-mediated damage. J Clin Invest. 2007;117:448–456. doi: 10.1172/JCI29571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Kemper A, McCarthy KD, Pytel P, Wang JP, Campbell IL, Utset MF, Popko B. Interferon-gamma induced medulloblastoma in the developing cerebellum. J Neurosci. 2004;24:10074–10083. doi: 10.1523/JNEUROSCI.2604-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkler PE, Kraig RP. Reactive astrocytosis from excitotoxic injury in hippocampal organ culture parallels that seen in vivo. J Cereb Blood Flow Metab. 1997;17:26–43. doi: 10.1097/00004647-199701000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkler PE, Hulse RE, Schmitt MW, Nicholson C, Kraig RP. Optical current source density analysis in hippocampal organotypic culture shows that spreading depression occurs with uniquely reversing currents. J Neurosci. 2005;25:3952–3961. doi: 10.1523/JNEUROSCI.0491-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkler PE, Briant J, Hulse RE, Kraig RP. Neural activity-dependent modulation of myelination. (abstract) Soc Neurosci. 2006;32:87.5. [Google Scholar]
- Coetzee T, Fujita N, Dupree J, Shi R, Blight A, Suzuki K, Suzuki K, Popko B. Myelination in the absence of galactocerebroside and sulfatide: normal structure with abnormal function and regional instability. Cell. 1996;86:209–219. doi: 10.1016/s0092-8674(00)80093-8. [DOI] [PubMed] [Google Scholar]
- Vincze A, Mázló M, Seress L, Komoly S, Abrahám H. A correlative light and electron microscopic study of postnatal myelination in the murine corpus callosum. Int J Dev Neurosci. 2008;26:575–584. doi: 10.1016/j.ijdevneu.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Corbin JG, Kelly D, Rath EM, Baerwald KD, Suzuki K, Popko B. Targeted CNS expression of interferon-gamma in transgenic mice leads to hypomyelination, reactive gliosis, and abnormal cerebellar development. Mol Cell Neurosci. 1996;7:354–370. doi: 10.1006/mcne.1996.0026. [DOI] [PubMed] [Google Scholar]
- Wang J, Lin W, Popko B, Campbell IL. Inducible production of interferon-gamma in the developing brain causes cerebellar dysplasia with activation of the Sonic hedgehog pathway. Mol Cell Neurosci. 2004;27:489–496. doi: 10.1016/j.mcn.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Young HA, Bream JH. IFN-gamma: recent advances in understanding regulation of expression, biological functions, and clinical applications. Curr Top Microbiol Immunol. 2007;316:97–117. doi: 10.1007/978-3-540-71329-6_6. [DOI] [PubMed] [Google Scholar]
- Buntinx M, Stinissen P, Steels P, Ameloot M, Raus J. Immune-mediated oligodendrocyte injury in multiple sclerosis: molecular mechanisms and therapeutic interventions. Crit Rev Immunol. 2002;22:391–424. [PubMed] [Google Scholar]
- Calabrese EJ. Dose-response features of neuroprotective agents: an integrative summary. Crit Rev Toxicol. 2008;38:253–348. doi: 10.1080/10408440801981965. [DOI] [PubMed] [Google Scholar]
- Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- Willenborg DO, Fordham S, Bernard CC, Cowden WB, Ramshaw IA. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J Immunol. 1996;157:3223–3227. [PubMed] [Google Scholar]
- Furlan R, Brambilla E, Ruffini F, Poliani PL, Bergami A, Marconi PC, Franciotta DM, Penna G, Comi G, Adorini L, Martino G. Intrathecal delivery of IFN-gamma protects C57BL/6 mice from chronic-progressive experimental autoimmune encephalomyelitis by increasing apoptosis of central nervous system-infiltrating lymphocytes. J Immunol. 2001;167:1821–1829. doi: 10.4049/jimmunol.167.3.1821. [DOI] [PubMed] [Google Scholar]
- LaFerla FM, Sugarman MC, Lane TE, Leissring MA. Regional hypomyelination and dysplasia in transgenic mice with astrocyte-directed expression of interferon-gamma. J Mol Neurosci. 2000;15:45–59. doi: 10.1385/JMN:15:1:45. [DOI] [PubMed] [Google Scholar]
- Panitch HS, Hirsch RL, Schindler J, Johnson KP. Treatment of multiple sclerosis with gamma interferon: exacerbations associated with activation of the immune system. Neurology. 1987;37:1097–1102. doi: 10.1212/wnl.37.7.1097. [DOI] [PubMed] [Google Scholar]
- Renno T, Taupin V, Bourbonniere L, Verge G, Tran E, De Simone R, Krakowski M, Rodriguez M, Peterson A, Owens T. Interferon-gamma in progression to chronic demyelination and neurological deficit following acute EAE. Mol Cell Neurosci. 1998;12:376–389. doi: 10.1006/mcne.1998.0725. [DOI] [PubMed] [Google Scholar]
- Scheu S, Stetson DB, Reinhardt RL, Leber JH, Mohrs M, Locksley RM. Activation of the integrated stress response during T helper cell differentiation. Nat Immunol. 2006;7:644–651. doi: 10.1038/ni1338. [DOI] [PubMed] [Google Scholar]
- Balabanov R, Strand K, Kemper A, Lee JY, Popko B. Suppressor of cytokine signaling 1 expression protects oligodendrocytes from the deleterious effects of interferon-gamma. J Neurosci. 2006;26:5143–5152. doi: 10.1523/JNEUROSCI.0737-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander WS, Starr R, Fenner JE, Scott CL, Handman E, Sprigg NS, Corbin JE, Cornish AL, Darwiche R, Owczarek CM, Kay TW, Nicola NA, Hertzog PJ, Metcalf D, Hilton DJ. SOCS1 is a critical inhibitor of interferon gamma signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell. 1999;98:597–608. doi: 10.1016/s0092-8674(00)80047-1. [DOI] [PubMed] [Google Scholar]
- Frohman EM, Racke MK, Raine CS. Multiple sclerosis—the plaque and its pathogenesis. N Engl J Med. 2006;354:942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Bo L, Mork S, Chang A. Pathogenesis of tissue injury in MS lesions. J Neuroimmunol. 1999;98:49–56. doi: 10.1016/s0165-5728(99)00081-8. [DOI] [PubMed] [Google Scholar]
- Brück W, Kuhlmann T, Stadelmann C. Remyelination in multiple sclerosis. J Neurol Sci. 2003;206:181–185. doi: 10.1016/s0022-510x(02)00191-0. [DOI] [PubMed] [Google Scholar]
- Franklin RJ. Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci. 2002;3:705–714. doi: 10.1038/nrn917. [DOI] [PubMed] [Google Scholar]
- Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. A quantitative analysis of oligodendrocytes in multiple sclerosis lesions. A study of 113 cases. Brain. 1999;122:2279–2295. doi: 10.1093/brain/122.12.2279. [DOI] [PubMed] [Google Scholar]
- Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J Neurosci. 2000;20:6404–6412. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y, Solanky M, Menonna J, Chapin J, Li W, Dowling P. Platelet-derived growth factor-alpha receptor-positive oligodendroglia are frequent in multiple sclerosis lesions. Ann Neurol. 2001;49:776–785. doi: 10.1002/ana.1015. [DOI] [PubMed] [Google Scholar]
- Panitch HS. Interferons in multiple sclerosis. A review of the evidence. Drugs. 1992;44:946–962. doi: 10.2165/00003495-199244060-00004. [DOI] [PubMed] [Google Scholar]
- Vartanian T, Li Y, Zhao M, Stefansson K. Interferon-gamma-induced oligodendrocyte cell death: implications for the pathogenesis of multiple sclerosis. Mol Med. 1995;1:732–743. [PMC free article] [PubMed] [Google Scholar]
- Cnop M, Ladriere L, Hekerman P, Ortis F, Cardozo AK, Dogusan Z, Flamez D, Boyce M, Yuan J, Eizirik DL. Selective inhibition of EIF2alpha dephosphorylation potentiates fatty acid-induced ER stress and causes pancreatic beta-cell dysfunction and apoptosis. J Biol Chem. 2007;282:3989–3997. doi: 10.1074/jbc.M607627200. [DOI] [PubMed] [Google Scholar]
- Leegwater PA, Vermeulen G, Konst AA, Naidu S, Mulders J, Visser A, Kersbergen P, Mobach D, Fonds D, van Berkel CG, Lemmers RJ, Frants RR, Oudejans CB, Schutgens RB, Pronk JC, van der Knaap MS. Subunits of the translation initiation factor eIF2B are mutant in leukoencephalopathy with vanishing white matter. Nat Genet. 2001;29:383–388. doi: 10.1038/ng764. [DOI] [PubMed] [Google Scholar]
- Richardson JP, Mohammad SS, Pavitt GD. Mutations causing childhood ataxia with central nervous system hypomyelination reduce eukaryotic initiation factor 2B complex formation and activity. Mol Cell Biol. 2004;24:2352–2363. doi: 10.1128/MCB.24.6.2352-2363.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Knaap MS, Pronk JC, Scheper GC. Vanishing white matter disease. Lancet Neurol. 2006;5:413–423. doi: 10.1016/S1474-4422(06)70440-9. [DOI] [PubMed] [Google Scholar]
- Wong K, Armstrong RC, Gyure KA, Morrison AL, Rodriguez D, Matalon R, Johnson AB, Wollmann R, Gilbert E, Le TQ, Bradley CA, Crutchfield K, Schiffmann R. Foamy cells with oligodendroglial phenotype in childhood ataxia with diffuse central nervous system hypomyelination syndrome. Acta Neuropathol (Berl) 2000;100:635–646. doi: 10.1007/s004010000234. [DOI] [PubMed] [Google Scholar]
- Mycko MP, Papoian R, Boschert U, Raine CS, Selmaj KW. Microarray gene expression profiling of chronic active and inactive lesions in multiple sclerosis. Clin Neurol Neurosurg. 2004;106:223–229. doi: 10.1016/j.clineuro.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Cwiklinska H, Mycko MP, Luvsannorov O, Walkowiak B, Brosnan CF, Raine CS, Selmaj KW. Heat shock protein 70 associations with myelin basic protein and proteolipid protein in multiple sclerosis brains. Int Immunol. 2003;15:241–249. doi: 10.1093/intimm/dxg022. [DOI] [PubMed] [Google Scholar]