Abstract
Desmoid fibromatosis is a rare, nonmetastatic neoplasm marked by local invasiveness and relentless recurrence. Molecular determinants of desmoid recurrence remain obscure. β-Catenin deregulation has been commonly identified in sporadic desmoids although the incidence of CTNNB1 (the gene encoding β-catenin) mutations is uncertain. Consequently, we evaluated the prevalence of CTNNB1 mutations in a large cohort of sporadic desmoids and examined whether mutation type was relevant to desmoid outcome. Desmoid specimens (195 tumors from 160 patients, 1985 to 2005) and control dermal scars were assembled into a clinical data-linked tissue microarray. CTNNB1 genotyping was performed on a 138-sporadic desmoid subset. Immunohistochemical scoring was performed per standard criteria and data were analyzed using Kaplan-Meier and other indicated methods. CTNNB1 mutations were observed in 117 of 138 (85%) of desmoids. Three discrete mutations in two codons of CTNNB1 exon 3 were identified: 41A (59%), 45F (33%), and 45P (8%, excluded from further analysis because of rarity). Five-year recurrence-free survival was significantly poorer in 45F-mutated desmoids (23%, P < 0.0001) versus either 41A (57%) or nonmutated tumors (65%). Nuclear β-catenin expression was observed in 98% of specimens and intensity was inversely correlated with incidence of desmoid recurrence (P < 0.01). In conclusion, CTNNB1 mutations are highly common in desmoid tumors. Furthermore, patients harboring CTNNB1 (45F) mutations are at particular risk for recurrence and therefore may especially benefit from adjuvant therapeutic approaches.
Desmoid fibromatosis is a rare soft tissue monoclonal neoplasm demonstrating fibroblastic to myofibroblastic differentiation. With an estimated incidence of two to four desmoid tumors arising per million population per year, ∼1000 new desmoid diagnoses are made annually in the US.1 Desmoids predominantly affect young adults, especially females, and primarily involve the extremities (including proximal points of attachment at the pelvic and shoulder girdles), the trunk, and the intestinal mesenteries.2 Desmoid tumors can infrequently occur as part of familial syndromes such as familial adenomatous polyposis (FAP) and familial infiltrative fibromatosis, both identified as caused by a germ-line mutation in the adenomatosis polyposis gene (APC).3,4,5,6,7,8 However, most desmoids are sporadic and there is a global lack of knowledge regarding risk factors associated with their etiology and development.
Diagnosis is made on the basis of clinical, radiological, and histological parameters. However, distinguishing desmoid tumors from reactive processes such as scar or other benign fibroblastic neoplasms, and even differentiating from low-grade sarcomas can be difficult at times, especially when diagnosis is based on a needle biopsy.9,10 Although unable to metastasize, these highly infiltrative and locally destructive lesions have a significant tendency to recur, with recurrence rates ranging from 19 to 38% in multiple studies.2,11,12,13,14,15,16,17,18,19,20,21,22,23 Currently there are no reliable methods to identify those tumors with a higher risk for recurrence; the molecular determinants of desmoid behavior remain primarily unknown.23 Surgical resection remains the mainstay of desmoid therapy; however, these tumors frequently require multiple and at times debilitating surgical interventions for their control, resulting in significant treatment-related morbidity such as amputation or loss of significant portions of foregut. Anti-inflammatory agents, hormonal blockade, and cytotoxic chemotherapy also may be useful in selected patients, but overall response rates to these modalities remains modest at best.23,24,25,26
Investigation of FAP-related desmoids has led to the identification of a potential role for β-catenin deregulation in desmoid tumorigenesis.27 β-Catenin is a cadherin-binding protein involved in cell-cell adhesion, but also functions as a transcriptional activator when complexed in the nucleus with members of the T-cell factor/lymphocyte enhancer factor family of proteins.28 Control of cytosolic levels of β-catenin occurs via a multiprotein complex, in which APC, axin/conductin, and glycogen synthetase kinase-3β (GSK-3β) negatively regulate β-catenin expression. Once bound to this protein complex, β-catenin is sequentially phosphorylated on four critical amino acids (serines 45, 37, and 33 and threonine at 41), ultimately resulting in the targeting of β-catenin for ubiquitination and proteosomal degradation.29 Mutations in the CTNNB1 gene coding for β-catenin have been described in a variety of epithelial-originating malignancies, in which deregulated β-catenin has been identified as contributing to their protumorigenic phenotype.30,31,32,33,34,35 Most CTNNB1 mutations occur within exon 3 of the gene in the region encoding for the β-catenin protein sequence that contains the serine/threonine residues described above. These mutations result in β-catenin stabilization, increased nuclear accumulation, and transcriptional activation of target genes such as c-MYC, c-JUN, and cyclin D.28,32,33
Recently, mutations in exon 3 of the CTNNB1 gene have also been identified in sporadic desmoid tumors.27,36,37,38,39,40,41 Summarizing all desmoid tumors analyzed in the several initial published series (n = 106 total desmoid specimens) suggests that CTNNB1 mutations occur in ∼50% of sporadic tumors. However, a recent publication evaluating the β-catenin status in the largest single cohort analyzed to date (n = 76) identified a mutational prevalence of 87%, potentially rendering sporadic desmoid tumors as a neoplasm possessing among the highest rate of β-catenin mutations yet described.37 This incidence discrepancy remains to be resolved. In the current study we attempted to evaluate the prevalence and spectra of β-catenin mutations in a large clinically annotated cohort of sporadic human desmoid tumor specimens. Furthermore, we correlated genotyping results with clinical and immunohistochemical features, including time to recurrence from the original tumor resection and β-catenin protein expression pattern and intensity.
Materials and Methods
Patients and Tumor Tissues
With institutional review board approval, formalin-fixed, paraffin-embedded desmoid tumor specimens accrued between 1985 and 2005 were retrieved from The University of Texas M.D. Anderson Cancer Center (UTMDACC) pathology archives amounting to 195 specimens from 160 patients. When available, matching normal tissue was also retrieved. For tissue microarray (TMA) construction, dermal scar tissue specimens (n = 18) to be used as controls were also retrieved. All specimens were further screened and evaluated by an experienced soft tissue pathologist (A.J.L.), and only those with confirmed desmoid tumor histology and also containing tissue adequate for analytic purposes were included. Normal tissue and scar specimens were likewise confirmed as per these criteria as well. Clinical information including demographic, therapeutic, tumor, and clinical outcome variables were retrieved from patient medical records and from the UTMDACC soft tissue tumor database and were tabulated for correlative analyses. For all patients, analysis of time to recurrence was calculated from initial surgery for the primary desmoids to the first recurrence, regardless of whether the surgery was performed at UTMDACC or elsewhere. Site was categorized as superficial trunk (to include the abdominal wall, chest wall, and back), extremity, deep trunk/mesentery, and head and neck. All patients were treated surgically with curative intent; this was a criteria for inclusion in the study. Postoperative radiotherapy was given to ∼20% of patients. Cases in which patients were diagnosed with FAP or had a family history of FAP were excluded; therefore, only sporadic desmoid tumors were ultimately evaluated.
CTNNB1 Genotyping
DNA was extracted from two 20-μm-thick formalin-fixed, paraffin-embedded tissue rolls cut from blocks with at least 70% tumor using the QIAamp DNA mini kit DNA isolation kit (n = 160, including 151 sporadic desmoids tumors; Qiagen Valencia, CA). Polymerase chain reaction (PCR) used primers (BCAT-DES-F: 5′-AGTCACTGGCAGCAACAGTC-3′ and BCAT-DES-R: 5′-TCTTCCTCAGGATTGCCTT-3′) and thermocycling conditions previously reported to amplify exon 3 of CTNNB1 (phosphorylation domain, codons 30 to 48) with appropriate controls.42 PCR products were detected by gel electrophoresis in 2% agarose, and amplicon bands were purified using the QIAquick gel extraction kit (Qiagen). Direct sequencing used the above primers (forward and reverse), ABI Prism dye terminator cycle sequencing ready reaction kit, and ABI Prism 3100-Avant genetic analyzer (Applied Biosystems, Foster City, CA). Both strands were analyzed by the NCBI Blast Alignment Tool (http://www. ncbi.nlm.nih.gov/blast/Blast.cgi) to identify mutations.
TMA Construction
For TMA construction, hematoxylin and eosin (H&E)-stained sections were reviewed to define areas of desmoid tumor; dermal scars served as controls. Using an automated TMA apparatus (ATA-27; Beecher Instruments, Sun Prairie, WI), 0.6-mm punch samples (two per case; 408 total) were obtained from the donor desmoid (n = 195) and scar (n = 18) blocks and formatted into three recipient blocks. H&E-staining of 4-μm TMA sections was used to verify all samples.
Immunohistochemistry
The polymeric biotin-free horseradish peroxide method on a Microsystems Bond Max stainer (Beecher Instruments, Bannockburn, IL) was used for immunohistochemistry. Four-μm-thick sections were prepared from formalin-fixed paraffin-embedded tissue blocks and were dried in a 60-degree oven for 20 minutes. These sections were placed in the automated Bond Max stainer that pretreated the slides with enzyme-induced epitope retrieval for 2 minutes followed by incubation with antibodies against β-catenin (clone 14, dilution 1:500; BD Biosciences, San Jose, CA). Anti-mouse secondary antibody and the Refine polymer detection kit (Leica) was used for immunostaining, with 3,3-diaminobenzidine serving as chromagen. Positive and negative controls were run in parallel. Labeling intensity was graded by a soft tissue pathologist (A.J.L.) as none (=0), weak (=1), moderate (=2), or strong (=3) for both the cytosolic and nuclear compartments, and the percentage of positive tumor cells was estimated from the two paired TMA samples for each case. For nuclear staining, weak (=1) was defined functionally as having to view nuclei at ×400 to confirm nuclear accumulation. Moderate (=2) staining could be viewed at ×200 and the blue hematoxylin nuclear counterstain was apparent. In strong staining, the nuclear counterstain was no longer visible in the majority of nuclei. The staining on the entire array was performed in the UTMDACC clinical immunohistochemistry facility on two occasions and each was scored independently.
Statistical Methods
Associations among the possible predictors of time from primary surgery to initial recurrence were examined using Fisher’s exact test, Kruskal-Wallis test, or Spearman’s correlation coefficient as appropriate. Variables considered included CTNNB1 genotype, tumor size, tumor site, gender, and age at initial diagnosis. Time-to-recurrence was examined using Cox proportional hazards regression and the Kaplan-Meier method. Strata were compared using the log-rank test. Multivariate models were constructed by introducing all variables simultaneously into the model and then eliminating variables using the backward selection method. P values were two-tailed and considered significant at α <0.05. Analyses were conducted using SAS for Windows (release 9.1; SAS Institute, Cary, NC).
Results
CTNNB1 Mutations Are Highly Prevalent in Sporadic Desmoid Tumors
We first sought to evaluate the prevalence of CTNNB1 mutations in a relatively large cohort of clinically annotated sporadic desmoid tumors treated at a single institution. Initially, 160 tumors from as many patients were included. All cases had sufficient DNA extracted for CTNNB1 exon 3 genotyping; the results were definitive in 154 (96%) of cases. After excluding cases with a personal or family history of FAP (n = 9, none with CTNNB1 mutations) and seven cases lacking sufficient clinical information, a collection of 138 sporadic desmoid tumors remained and were included in this analysis (Table 1). Of these, 86 patients were female (62%) and 52 were male (38%). Median age was 32 years and ranged from 0.2 to 78 years. The following sites were involved: 54 in superficial trunk (39%), 53 in extremities (38%), 18 in deep trunk/mesentery (13%), and 13 in head and neck (9%). Size was known in 108 cases and ranged from 1.1 to 30 cm (median, 6 cm). The primary tumors were available for 89 patients and the clinical details of this subset are presented in Table 1. A mutation in exon 3 of the CTNNB1 gene was identified in 117 (85%) of the 138 cases. No mutations were identified in adjacent normal tissue in all cases in which this was tested (n = 25). Correlating the presence of CTNNB1 gene mutation with patient and tumor variables, female patients were much more likely than male patients to lack a mutation (22% versus 4%, P = 0.0032); associations with age at diagnosis, tumor sites, and desmoid size were not noted (Table 2).
Table 1.
Desmoid Tumor Cohort Data
| Total, n = 138
|
Primary, n = 89
|
|||
|---|---|---|---|---|
| n | % | n | % | |
| Gender | ||||
| Female | 86 | 62 | 53 | 60 |
| Male | 52 | 38 | 36 | 40 |
| Age, years (median, range) | 32 (0.2 to 78) | 33 (1 to 78) | ||
| Site | ||||
| Superficial trunk | 54 | 39 | 33 | 37 |
| Extremity | 53 | 38 | 31 | 35 |
| Deep trunk/mesentery | 18 | 13 | 14 | 16 |
| Head and neck | 13 | 10 | 11 | 12 |
| Size, cm (median, range) (total) | 6 (1.1 to 30) | (n = 108) | 6.8 (1.1 to 30) | (n = 76) |
Clinical data for the desmoid cohort. There were 138 desmoid patients with sufficient clinical and molecular data for analysis. The entire cohort of 138 was used for the analysis of clinical variables associated with CTNNB1 mutation genotype. The 89 patients with primary desmoid tumors on the array were used for the β-catenin immunohistochemical study.
Table 2.
Patient and Tumor Properties with CTNNB1 Genotype
| Total
|
WT
|
41A
|
45F
|
45P
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | n | % | n | % | n | % | n | % | n | % |
| Gender | ||||||||||
| Female | 86 | 62 | 19 | 22 | 36 | 42 | 25 | 29 | 6 | 7 |
| Male | 52 | 38 | 2 | 4 | 33 | 63 | 14 | 27 | 3 | 6 |
| Age at diagnosis | ||||||||||
| <30 years | 61 | 44 | 12 | 20 | 31 | 51 | 14 | 23 | 4 | 7 |
| >30 years | 77 | 56 | 9 | 12 | 38 | 49 | 25 | 32 | 5 | 7 |
| Tumor site | ||||||||||
| Superficial trunk | 54 | 39 | 10 | 19 | 27 | 50 | 11 | 20 | 6 | 11 |
| Extremity | 53 | 38 | 5 | 9 | 27 | 51 | 20 | 38 | 1 | 2 |
| Deep trunk/viscera | 18 | 13 | 3 | 17 | 11 | 61 | 2 | 11 | 2 | 11 |
| Head and neck | 13 | 10 | 3 | 23 | 4 | 31 | 6 | 46 | 0 | 0 |
| Tumor size* | ||||||||||
| <6 cm | 56* | 52 | 11 | 20 | 24 | 43 | 16 | 29 | 5 | 9 |
| >6 cm | 52* | 48 | 6 | 11 | 30 | 58 | 14 | 27 | 2 | 4 |
| Total | 138 | 21 | 15 | 69 | 50 | 39 | 28 | 9 | 7 | |
Distribution of CTNNB1 mutation types tabulated with patient clinical and tumor data. Females were more likely to lack a mutation in CTNNB1 and males were more likely to have a 41A mutation. There was a trend toward association of the 45F mutation with tumors involving an extremity.
Tumor size was available only for 108 of the total of 138 patients in this cohort. The total row gives the percentage of each tumor type in the population. WT, wild type (not mutated).
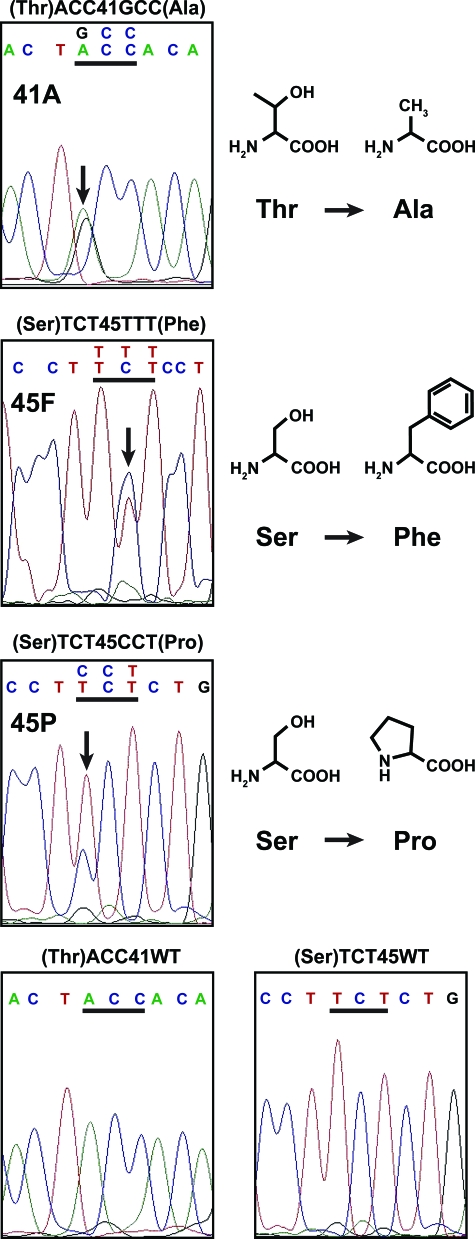
Next, we determined the CTNNB1 mutational spectra. Interestingly, only three different point mutations in two different codons (41 and 45) could be identified in all mutated samples (n = 117 of 138 total) (Figure 1): ACC to GCC in codon 41 (41A), resulting in the replacement of threonine by alanine, was identified in 69 samples (59%); TCT to TTT in codon 45 (45F), resulting in the replacement of serine by phenylalanine, was identified in 39 cases (33%); and TCT to CCT in codon 45 (45P), resulting in the replacement of serine with proline, was identified in nine samples (8%). When analyzing the incidence of the specific CTNNB1 gene mutations as a correlate of patient and tumor variables, we identified that males have a higher incidence of the 41A mutation than females (63% versus 42%, P = 0.0109) and in terms of tumor site there was a trend toward tumors involving the extremities having a higher incidence of the 45F mutation (38%, P = 0.0640) versus all other sites. No other associations were noted.
Figure 1.
CTNNB1 exon 3 sequencing of desmoid tumor was available from 154 of the 160 patients (96%) on the TMA. Of these, 138 had sufficient clinical data (and no evidence of FAP) for inclusion and 85% showed one of three specific mutations in CTNNB1. Examples of Sanger sequencing for each mutational type encountered (abbreviated 41A, 45F, and 45P, respectively) and lack of mutation are displayed. In the top three panels, the mutated bp is denoted by a black arrow on the DNA sequence tracing and the codon change is shown directly above the affected codon (underlined in black). In the bottom two panels, unaltered codons 41 and 45 are displayed. Additional data are presented in Table 1. The chemical structures of the amino acids in the normal and mutated codons are depicted on the right. A, adenine; C, cytosine; G, guanine; T, thymine; Thr, threonine; Ala, alanine; Ser, serine; Phe, phenylalanine; Pro, proline; WT, wild type.
CTNNB1 45F Mutations Significantly Correlate with Increased Desmoid Tumor Recurrence
We sought to evaluate whether any specific CTNNB1 gene mutation correlated with clinical outcome. Time to first recurrence was calculated from date of the initial attempt at complete surgical resection irrespective of whether this occurred at UTMDACC or elsewhere. In that the study cohort was representative of the referral pattern to a large tertiary center, it therefore included a large proportion of patients (34%) presenting with recurrent desmoid tumors, and the 5-year recurrence rate from time of diagnosis of initial tumor was 49% (95% CI 39 to 58%) (Figure 2A), however, it should be noted that recurrence was calculated from the time of the initial complete surgical resection.
Figure 2.
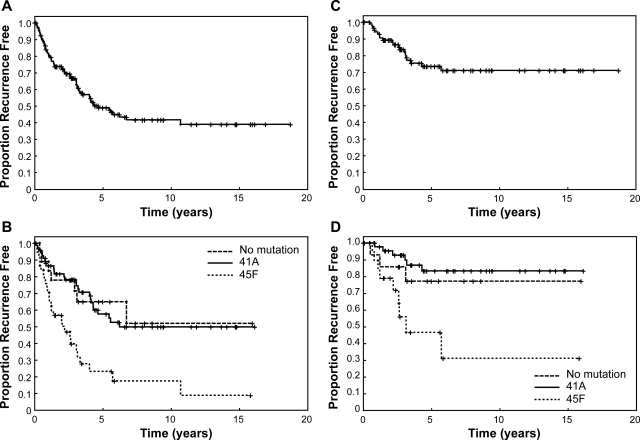
A: Kaplan-Meier plot of time from first surgery on the primary tumor to initial local recurrence. The entire cohort (n = 133) is shown in A. B: Patients with the 45F (Ser-TCT45TTT-Phe) mutation showed significantly greater hazard of recurrence compared to that of the 41A (Thr-ACC41GCC-Ala) mutation or no mutation groups. The 45P (Ser-TCT45CCT-Pro mutation) had too few patients for definitive analysis (n = 9), but recurrence experience resembled that of the 41A and no mutation groups. C and D: The equivalent data for the tumors of the patients who presented for surgery to UTMDACC with primary tumors (n = 88).
Recurrence-free survival differed among the different CTNNB1 gene mutational cohorts, and was significantly inferior for patients with the 45F genotype (Figure 2B, P < 0.0001). The estimated 5-year recurrence-free survival rate was 23% (95% CI, 10 to 40%) for patients with 45F mutation compared to 57% (95% CI, 43 to 69%), and 65% (95% CI, 38 to 83%) for 41A mutation and nonmutated CTNNB1, respectively. Only nine samples were found to harbor a 45P CTNNB1 gene mutation, and because of this low number these tumors were excluded from analysis, but showed a recurrence course similar to 41A and cases lacking mutation. Univariate analysis further identified an extremity location (P = 0.0178) and a younger age at diagnosis (P = 0.0209) to be significantly associated with an increased propensity for local recurrence. In multivariate analysis, only CTNNB1 gene mutational type (P = 0.0036) and patient age (P = 0.0059) were significant predictors of time to recurrence (Table 3).
Table 3.
Cox Proportional Hazards Regression Modeling: Time to First Recurrence
| Independent variable | Frequency
|
Univariate analysis
|
Multivariate reduced model
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | HR | 95% CI | P | HR | 95% CI | P | |||
| Mutation | ||||||||||
| 41A | 68 | 51 | 1.03 | 0.45 | 2.37 | 0.9423 | 1.11 | 0.48 | 2.56 | 0.8064 |
| 45F | 37 | 28 | 3.05 | 1.32 | 7.04 | 0.0092 | 3.50 | 1.51 | 8.14 | 0.0036 |
| 45P | 9 | 7 | 1.15 | 0.34 | 3.93 | 0.8259 | 1.13 | 0.33 | 3.86 | 0.8499 |
| WT (no mutation) | 19 | 14 | 1.00 | |||||||
| Gender | ||||||||||
| Female | 81 | 61 | 1.29 | 0.78 | 2.14 | 0.3234 | ||||
| Male | 52 | 39 | 1.00 | |||||||
| Site | ||||||||||
| Extremity | 53 | 40 | 3.52 | 1.24 | 9.95 | 0.0178 | ||||
| Superficial trunk | 52 | 39 | 2.50 | 0.87 | 7.18 | 0.0900 | ||||
| Head and neck | 11 | 8 | 1.50 | 0.34 | 6.72 | 0.5956 | ||||
| Deep trunk/viscera | 17 | 13 | 1.00 | |||||||
| Age at diagnosis (years) | 0.98 | 0.96 | 1.00 | 0.0209 | 0.98 | 0.96 | 0.99 | 0.0059 | ||
| Tumor size (cm) | 0.95 | 0.90 | 1.02 | 0.1505 | ||||||
Cox proportional hazards univariate and multivariate reduced models. This time to recurrence analysis is from the initial surgical procedure (whether at UTMDACC or elsewhere) to the first recurrence. In the multivariate model, both mutation type and age were significant independent predictors of time to recurrence with a 3.5-fold increase in hazard among those with mutation 45T versus no mutation and a 2% decrease in hazard for each year increase in age at diagnosis. Of the 138 patients 133 were used in this cohort; 5 had missing data and were excluded. n, number of patients; HR, hazard ratio; CI, confidence interval; WT, wild type, not mutated.
To further validate that these results were not affected by the inherent referral patterns of patients to a large national referral center such as UTMDACC by the inclusion of a large group of recurrent tumors, we evaluated recurrence-free survival analyzing only the subgroup of patients (n = 89) treated at UTMDACC for primary sporadic desmoids. The overall recurrence analysis of this entire population is presented in Figure 2C. The estimated 5-year recurrence-free survival rate for CTNNB1 gene 41A mutation-harboring tumors was 83% (95% CI, 66 to 92%), and the median time to recurrence has not yet been achieved. Tumors lacking a mutation followed an equivalent course. In remarkable contrast, 45F CTNNB1 gene mutation-harboring tumors demonstrated a 5-year recurrence-free survival rate of only 47% (95% CI, 19 to 70%), and the median time to recurrence of 3.16 years. The multivariate analysis of this cohort included the same variables as for the complete cohort plus the margin status of the surgical resection performed at UTMDACC. In the reduced model for this cohort, only the presence of the 45F mutation remained as a significant indicator of increased propensity to recurrence (Table 4).
Table 4.
Cox Proportional Hazards Regression Modeling: Time to First Recurrence Primaries Treated at UTMDACC Only
| Independent variable | Frequency
|
Univariate analysis
|
Multivariate reduced model
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | HR | 95% CI | P | HR | 95% CI | P | |||
| Mutation | ||||||||||
| 45F | 19 | 22 | 4.28 | 1.75 | 10.48 | 0.0015 | 4.28 | 1.75 | 10.48 | 0.0015 |
| Not 45F | 69 | 78 | 1.00 | 1.00 | ||||||
| Gender | ||||||||||
| Female | 52 | 59 | 1.15 | 0.47 | 2.82 | 0.7559 | ||||
| Male | 36 | 41 | 1.00 | |||||||
| Site | ||||||||||
| Extremity | 31 | 35 | 5.87 | 0.76 | 45.52 | 0.0905 | ||||
| Superficial trunk | 33 | 37 | 2.99 | 0.36 | 24.87 | 0.3117 | ||||
| Head and neck | 10 | 11 | 3.60 | 0.32 | 39.82 | 0.2969 | ||||
| Deep trunk/viscera | 14 | 16 | 1.00 | |||||||
| Age at diagnosis (years) | 88 | 100 | 0.98 | 0.95 | 1.01 | 0.1596 | ||||
| Tumor size (cm) | 75 | 100 | 0.98 | 0.89 | 1.07 | 0.6564 | ||||
| Margins | ||||||||||
| Positive | 36 | 44 | 2.33 | 0.92 | 5.93 | 0.0755 | ||||
| Negative | 46 | 56 | 1.00 | |||||||
Cox proportional hazards univariate and multivariate reduced models including only the 89 primaries treated surgically at UTMDACC. In this model, the status of margins (as determined by histopathological examination) was included and all patients were known to be treated surgically with intent for complete resection. Approximately 20% of patients were treated with adjuvant radiation therapy. Neither radiation therapy nor margin status was associated with mutation type on contingency analysis (data not shown). Although site and margins showed trends towards a significant relationship with recurrence, only mutational type was significantly related in both the univariate and reduced multivariate models. n, number of patients; HR, hazard ratio; CI, confidence interval; WT, wild type, not mutated.
Desmoid Tumors Express Nuclear β-Catenin; Lower Nuclear β-Catenin Intensity in Primary Sporadic Tumors Correlates with Increased Recurrence and 45F Mutations
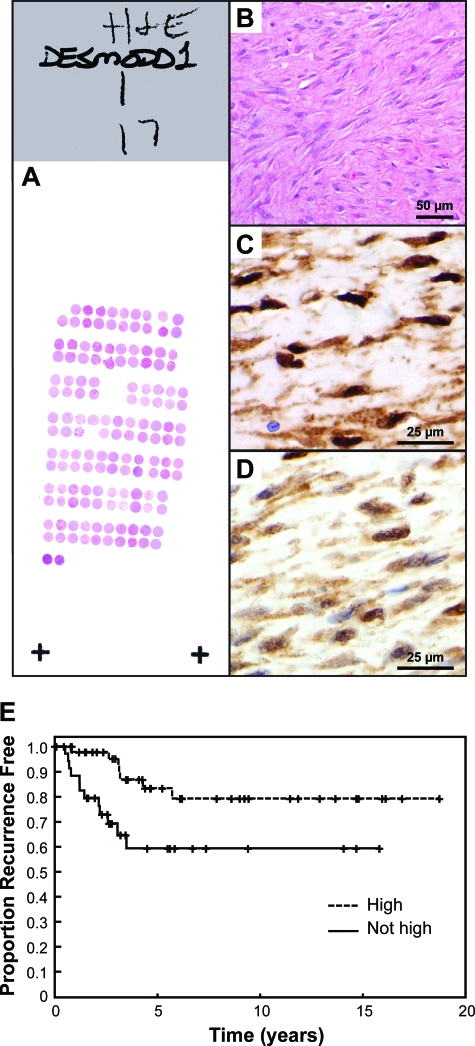
To evaluate further the β-catenin protein expression in the sequenced desmoid tumors, we have constructed a TMA consisting of all of the sequenced desmoids above as well as additional specimens so as to constitute a TMA consisting of 195 desmoid specimens and 18 scars (Figure 3, A and B). Desmoid tissues included 107 primary and 84 recurrent tumors (in four cases this information was not available). Of the 25 sets of samples comprising matched autologous primary/recurrent tumors (two to five samples each) retrieved from the same patients, 21 were sporadic tumors and 4 were FAP-related. TMA slides were immunostained with antibodies against β-catenin (Figure 3, C and D). Staining scores (localization, distribution, and intensity) were assigned by a soft tissue tumor pathologist (A.J.L.) in a blinded manner for two independent staining runs on separate days in the UTMDACC clinical immunohistochemistry laboratory. The independent scoring of the two sets of immunostained TMA slides were equivalent. Nuclear β-catenin was observed in 98% of desmoid samples. Compared to scars (n = 18) in which only two cases showed very focal, weak nuclear reactivity, desmoids demonstrated significantly greater percentages of nuclear reactivity and intensity (P < 0.0001). In desmoid tumors, more than 90% of tumors showed cytoplasmic activity that was at least moderate in intensity. Nuclear reactivity was seen in at least 50% of nuclei of at least intermediate intensity in greater than 90% of cases.
Figure 3.
A: Representative H&E-stained slide from one of the three TMA blocks. B: H&E of desmoid tumor on the TMA. Immunohistochemistry shows high (C) and moderate (D) levels of nuclear β-catenin. Nuclear reactivity was considered high (=3) when the immunoreactivity completely precluded visualization of the nuclear hematoxylin counterstain whereas moderate (=2) and weak (=1) staining allowed such visualization to varying degrees. This determination was made at ×200 magnification with advancement to ×400 when necessary for cases with minimal reactivity. E: Kaplan-Meier analysis of time to recurrence from primary surgery for desmoid tumor in patients (n = 84) in which the actual primary tumors were available for TMA immunohistochemistry. Desmoid tumors showing attenuated nuclear β-catenin intensity levels [moderate (=2), minimal (=1), or rarely, negative (=0)] demonstrated by immunohistochemistry increased propensity to recurrence when compared to desmoids with high (=3) levels (P = 0.0406). Moderate, minimal, and negative staining groups were combined (not high) because they behaved similarly and because there were few patients in the minimal and negative groups. Original magnifications: ×200 (B); ×400 (C, D).
To evaluate further a potential correlation between β-catenin protein expression intensity and CTNNB1 gene mutational type, we analyzed staining scores in available sporadic tumor specimens (primary or recurrent) harboring either 41A (n = 67) or 45F (n = 37) mutations. Desmoids bearing the 41A CTNNB1 gene mutation exhibited a more intense β-catenin nuclear expression (score 1 or 2 = 33%, 3 = 66%) compared to 45F CTNNB1-mutated desmoids (score 1 or 2 = 70%, 3 = 30%; P < 0.0001). No correlation was noted between cytoplasmic β-catenin or percentage of nuclei reactive for β-catenin.
Based on these findings we sought to evaluate whether staining intensity independently correlated with desmoid tumor recurrence. Only primary desmoids resected at UTMDACC (n = 89) were evaluated; desmoids exhibiting a less intense β-catenin nuclear staining (score 1 or 2) were associated with a significantly increased hazard of recurrence (Figure 3E, P = 0.0115) compared to those expressing higher intensity scores (=3). The 5-year recurrence-free survival of high-nuclear β-catenin expressors (n = 49) was 83% (95% CI, 66 to 92%) versus 59% (95% CI, 38 to 75%) in low to intermediate expressors (n = 35). No associations between recurrence and cytoplasmic β-catenin and or percentage nuclear β-catenin were noted.
Discussion
Dysregulation of the β-catenin signaling pathway is a common molecular event in desmoid tumors.3,4,5,6,7,8,37,39,40,43,44 However, evaluation of the actual prevalence of such mutations has been hampered by the rarity of these tumors, resulting in relatively small numbers of cases available for analysis (Table 4). Our current study, using the largest single cohort of sporadic desmoids from a single institution evaluated to date, identified CTNNB1 gene mutations in 85% of these cases. This finding further validates the results of a recently published series37; taken together, these investigations strongly suggest that CTNNB1 mutations are highly prevalent in desmoid tumors. Mutations stabilizing β-catenin have been found in many cancers at various incidence levels; however, the markedly elevated rate of such mutations in sporadic desmoids, as identified by us and others, potentially renders this disease as one of the most likely to harbor this specific genetic deregulation.
An important clinical application stemming from our results is the potential use of β-catenin sequencing as a diagnostic tool for desmoid tumors. These lesions have a strong resemblance to scar tissue or a reactive fibroblastic proliferation on both the macro- and microscopic levels; consequently, an accurate specific diagnosis can sometimes be difficult to establish, particularly in cases of potential desmoid local recurrence. β-Catenin sequencing can be easily and reproducibly conducted using paraffin-embedded tissue extracted via needle retrieval. Negative results of such an assay would not preclude the diagnosis of desmoid. However, given that most desmoids harbor CTNNB1 gene mutations, a positive result for such mutation would serve to confirm a diagnosis of desmoid; other benign lesions included in the differential diagnosis are not known to have CTNNB1 gene mutations or even show nuclear expression of β-catenin on immunohistochemistry.9,10,37,45 At UTMDACC we are currently evaluating this hypothesis by prospectively examining the sensitivity and specificity of β-catenin sequencing as an objective means of resolving difficult situations such as recurrent desmoid versus postsurgical scarring.
The clinical course of desmoid tumors is quite variable. Although some tumors exhibit a significant tendency for local recurrence even in the setting of negative microscopic margins, others do not recur after R1 resection, and some have been observed to spontaneously regress even without therapeutic intervention.2,46 This interdesmoid tumor heterogeneity suggests the possibility of underlying biological differences; however, relevant molecular prognostic markers are essentially unknown. As has also been observed by others37,39,40,44 (Table 5), we have shown that desmoid CTNNB1 mutational spectra are quite limited. Indeed, it is very unusual among solid tumors that only three specific mutations are noted: two involving codon 45 and one involving codon 41. In contrast, most other neoplasms harboring exon 3 CTNNB1 mutations exhibit a variety of mutations at multiple critical codons.28,30,31,33,35 This finding may indicate that these specific CTNNB1 mutations are perhaps crucial in desmoid development, and that the type of mutation observed in the CTNNB1 gene may affect or even alter the signaling properties of β-catenin in ways that are specifically conducive to desmoid formation.
Table 5.
CTNNB1 Mutational Spectrum in Previous Studies of Desmoid Tumors
| Total
|
WT
|
41A
|
45F
|
45P
|
Other
|
APC
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Article | n | % | n | % | n | % | n | % | n | % | n | % | n | % |
| Miyoshi et al, 199840 | 13 | 54 | 6 | 46 | 4 | 31 | 3 | 23 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tejpar et al, 199939 | 42 | 52 | 20* | 48 | 10 | 24 | 12 | 28 | 0 | 0 | 0 | 0 | 9 | 21 |
| Saito et al, 200144 | 18 | 39 | 11 | 61 | 0 | 0 | 2 | 11 | 0 | 0 | 5 | 28 | ND | ND |
| Abraham et al, 200245 | 33 | 45 | 18* | 55 | 11 | 33 | 3 | 9 | 0 | 0 | 1 | 3 | 10 | 30 |
| Amary et al, 200737 | 76 | 87 | 10 | 13 | 27 | 35 | 34 | 45 | 5 | 7 | 0 | 0 | ND | ND |
Proportion of desmoid tumors showing mutations in CTNNB1 in selected prior studies. The total column indicates the number of cases (n) in the study with the total percentage mutation in CTNNB1. The WT (wild type), 41A, 45F, and 45P columns indicate cases lacking mutations in CTNNB1 or of the specific types listed, respectively. Other indicates CTNNB1 mutations not of these three specific types. APC shows the numbers and percentages of mutations in the APC gene in the two studies in which this was performed for comparison; these cases are tabulated in the WT column (marked with an asterisk) and thus the percentages do not sum to 100 in these two rows. n, number of cases; WT, wild type; ND, not done.
In our study we identified that risk of recurrence in desmoids is strongly associated with a specific CTNNB1 mutation in codon 45 (45F). Such mutation confers a larger than threefold increased risk of recurrence that begins to be manifest at a significantly earlier time point in the desmoid tumor-host encounter. To the best of our knowledge, this is the first study to correlate desmoid mutation type with desmoid clinical outcome. Although other factors may be involved, this finding suggests that the biological consequences of the three different CTNNB1 mutations in exon 3 are not equivalent if only because one of the three candidate mutations confers a markedly more ominous clinical prognosis. There may be bona fide differences in the ability of the three mutant proteins as well as the stable wild-type protein to act as transcription factors, to gain entry into the nucleus, to achieve stability in the cellular environment, or perhaps to perform other oncologically relevant functions.
Perhaps most importantly, our study supports a novel and heretofore unappreciated role for 45F mutation as a prognostic factor strongly associated with recurrence in patients suffering from desmoid tumors. Because desmoid tumors lack the ability to metastasize, their morbidity and even mortality is a function of propensity for recurrence juxtaposed against the morbidity of therapeutic approaches to address such recurrence. Use of nonsurgical approaches as adjuvant strategies (eg, external beam radiotherapy, estrogen receptor blockade, traditional chemotherapy, imatinib mesylate, COX-2 inhibitors, and so forth) may be particularly applicable in 45F mutated desmoid tumor patients. Such treatments may only nonspecifically impact on underlying 45F-associated desmoid tumor recurrence behavior, and definitive mechanism-elucidating studies are yet to be conducted. Nonetheless, such nonspecific adjuvant treatments may still be useful in forestalling or preventing recurrence, especially in recurrence-prone populations such as those harboring a 45F mutation, and could be prospectively evaluated in clinical trials.
Multiple studies have demonstrated significant nuclear β-catenin expression in desmoid tumors as evaluated by immunohistochemistry, with an overall prevalence ranging from 82 to 100%.9,10,39,44,45,47,48 Similarly, in our cohort of desmoids, nuclear β-catenin was identifiable in 98% of cases; most normal scar tissues exhibited cytoplasmic but only minimal (two cases) or no nuclear β-catenin expression. It has been recently demonstrated that β-catenin expression is of value in the differential diagnosis of fibroblastic mesenchymal tumors and benign fibroblastic proliferations.9,10,45 However, little is known concerning the utility of β-catenin expression as a prognostic marker for desmoid tumors or the possible association between β-catenin expression levels and the different underlying molecular/genetic alterations resulting in β-catenin stabilization. A recent publication indicates that desmoids exhibiting increased nuclear expression (defined as >20% of the tumor cells expressing β-catenin) had a significantly higher recurrence rate than desmoids lacking nuclear β-catenin expression.49 In our study no correlation could be demonstrated between percentage of positive staining nuclear β-catenin tumor cells and desmoid recurrence and desmoids negative for β-catenin staining were very rare. However, it is intriguing that decreased rather than increased nuclear β-catenin staining intensity was significantly associated with a more aggressive disease phenotype in our study. In addition, the present study indicates that lower β-catenin intensity levels are associated with the CTNNB1 45F mutation type, which independently correlated with a higher tendency for recurrence. Further mechanistic experiments to evaluate the potential causal relationship between the 45F mutation, β-catenin expression, and desmoid recurrence are needed.
In summary, our study suggests that genotyping of CTNNB1 exon 3 could be useful as a diagnostic test in equivocal situations such as small core needle biopsies or in differentiating postsurgical scar from desmoid recurrence. Such genotyping may also provide important prognostic insight regarding risk of recurrence, and may therefore have bearing on selection of adjuvant therapeutic approaches. Although prospective trials are needed to test such possibilities, molecular insights such as these may ultimately lead to novel therapeutics for a devastating disease in which no new efficacious treatments have been introduced for the past several decades.50,51
Acknowledgments
We thank Kim Vu for expert assistance in figure production.
Footnotes
Address reprint requests to Dina Lev, Department of Cancer Biology, Unit 1104, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX 77030-4001. E-mail: dlev@mdanderson.org.
Supported in part by the Desmoid Tumor Research Foundation (to D.L.) and The University of Texas M.D. Anderson Cancer Center Physician-Scientist Program (to A.J.F.L.).
References
- Reitamo JJ, Hayry P, Nykyri E, Saxen E. The desmoid tumor. I. Incidence, sex-, age- and anatomical distribution in the Finnish population. Am J Clin Pathol. 1982;77:665–673. doi: 10.1093/ajcp/77.6.665. [DOI] [PubMed] [Google Scholar]
- Lev D, Kotilingam D, Wei C, Ballo MT, Zagars GK, Pisters PW, Lazar AA, Patel SR, Benjamin RS, Pollock RE. Optimizing treatment of desmoid tumors. J Clin Oncol. 2007;25:1785–1791. doi: 10.1200/JCO.2006.10.5015. [DOI] [PubMed] [Google Scholar]
- Eccles DM, van der Luijt R, Breukel C, Bullman H, Bunyan D, Fisher A, Barber J, du Boulay C, Primrose J, Burn J, Fodde R. Hereditary desmoid disease due to a frameshift mutation at codon 1924 of the APC gene. Am J Hum Genet. 1996;59:1193–1201. [PMC free article] [PubMed] [Google Scholar]
- Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, Smith KJ, Preisinger AC, Hedge P, McKechnie D, Finner R, Markham A, Groffen J, Boguski MS, Altschul SF, Horii A, Andoh H, Miyoshi Y, Miki Y, Nishisho I, Nakamura Y. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- Giardiello FM, Petersen GM, Piantadosi S, Gruber SB, Traboulsi EI, Offerhaus GJ, Muro K, Krush AJ, Booker SV, Luce MC, Laken SJ, Kinzler KW, Vogelstein B, Hamilton SR. APC gene mutations and extraintestinal phenotype of familial adenomatous polyposis. Gut. 1997;40:521–525. doi: 10.1136/gut.40.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan MJ, McCarthy TV, Doyle CT. Familial adenomatous polyposis: from bedside to benchside. Am J Clin Pathol. 1998;109:521–526. doi: 10.1093/ajcp/109.5.521. [DOI] [PubMed] [Google Scholar]
- Latchford A, Volikos E, Johnson V, Rogers P, Suraweera N, Tomlinson I, Phillips R, Silver A. APC mutations in FAP-associated desmoid tumours are non-random but not ‘just right’. Hum Mol Genet. 2007;16:78–82. doi: 10.1093/hmg/ddl442. [DOI] [PubMed] [Google Scholar]
- Miyaki M, Yamaguchi T, Iijima T, Takahashi K, Matsumoto H, Yasutome M, Funata N, Mori T. Difference in characteristics of APC mutations between colonic and extracolonic tumors of FAP patients: variations with phenotype. Int J Cancer. 2008;122:2491–2497. doi: 10.1002/ijc.23390. [DOI] [PubMed] [Google Scholar]
- Carlson JW, Fletcher CD. Immunohistochemistry for b-catenin in the differential diagnosis of spindle cell lesions: analysis of a series and review of the literature. Histopathology. 2007;51:509–514. doi: 10.1111/j.1365-2559.2007.02794.x. [DOI] [PubMed] [Google Scholar]
- Montgomery E, Folpe AL. The diagnostic value of b-catenin immunohistochemistry. Adv Anat Pathol. 2005;12:350–356. doi: 10.1097/01.pap.0000194628.58501.71. [DOI] [PubMed] [Google Scholar]
- Leibel SA, Wara WM, Hill DR, Bovill EG, Jr, de Lorimier AA, Beckstead JH, Phillips TL. Desmoid tumors: local control and patterns of relapse following radiation therapy. Int J Radiat Oncol Biol Phys. 1983;9:1167–1171. doi: 10.1016/0360-3016(83)90175-x. [DOI] [PubMed] [Google Scholar]
- Bataini JP, Belloir C, Mazabraud A, Pilleron JP, Cartigny A, Jaulerry C, Ghossein NA. Desmoid tumors in adults: the role of radiotherapy in their management. Am J Surg. 1988;155:754–760. doi: 10.1016/s0002-9610(88)80037-0. [DOI] [PubMed] [Google Scholar]
- McKinnon JG, Neifeld JP, Kay S, Parker GA, Foster WC, Lawrence W., Jr Management of desmoid tumors. Surg Gynecol Obstet. 1989;169:104–106. [PubMed] [Google Scholar]
- McCollough WM, Parsons JT, van der Griend R, Enneking WF, Heare T. Radiation therapy for aggressive fibromatosis. The Experience at the University of Florida. J Bone Joint Surg Am. 1991;73:5717–5725. [PubMed] [Google Scholar]
- Acker JC, Bossen EH, Halperin EC. The management of desmoid tumors. Int J Radiat Oncol Biol Phys. 1993;26:851–858. doi: 10.1016/0360-3016(93)90501-l. [DOI] [PubMed] [Google Scholar]
- Catton CN, O'Sullivan B, Bell R, Cummings B, Fornasier V, Panzarella T. Aggressive fibromatosis: optimisation of local management with a retrospective failure analysis. Radiother Oncol. 1995;34:17–22. doi: 10.1016/0167-8140(94)01483-j. [DOI] [PubMed] [Google Scholar]
- Ballo MT, Zagars GK, Pollack A, Pisters PW, Pollack RA. Desmoid tumor: prognostic factors and outcome after surgery, radiation therapy, or combined surgery and radiation therapy. J Clin Oncol. 1999;17:158–167. doi: 10.1200/JCO.1999.17.1.158. [DOI] [PubMed] [Google Scholar]
- Jelinek JA, Stelzer KJ, Conrad E, Bruckner J, Kliot M, Koh W, Laramore GE. The efficacy of radiotherapy as postoperative treatment for desmoid tumors. Int J Radiat Oncol Biol Phys. 2001;50:121–125. doi: 10.1016/s0360-3016(00)01570-4. [DOI] [PubMed] [Google Scholar]
- Gronchi A, Casali PG, Mariani L, Lo Vullo S, Colecchia M, Lozza L, Bertulli R, Fiore M, Olmi P, Santinami M, Rosai J. Quality of surgery and outcome in extra-abdominal aggressive fibromatosis: a series of patients surgically treated at a single institution. J Clin Oncol. 2003;21:1390–1397. doi: 10.1200/JCO.2003.05.150. [DOI] [PubMed] [Google Scholar]
- Abbas AE, Deschamps C, Cassivi SD, Nichols FC, III, Allen MS, Schleck CD, Pairolero PC. Chest-wall desmoid tumors: results of surgical intervention. Ann Thorac Surg. 2004;78:1219–1223. doi: 10.1016/j.athoracsur.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Duggal A, Dickinson IC, Sommerville S, Gallie P. The management of extra-abdominal desmoid tumours. Int Orthop. 2004;28:252–256. doi: 10.1007/s00264-004-0571-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SR, A'Hern R, Thomas JM. Aggressive fibromatosis of the abdominal wall, limbs and limb girdles. Br J Surg. 2004;91:1624–1629. doi: 10.1002/bjs.4792. [DOI] [PubMed] [Google Scholar]
- Kotiligam D, Lazar AJ, Pollock RE, Lev D. Desmoid tumor: a disease opportune for molecular insights. Histol Histopathol. 2008;23:117–126. doi: 10.14670/HH-23.117. [DOI] [PubMed] [Google Scholar]
- Gega M, Yanagi H, Yoshikawa R, Noda M, Ikeuchi H, Tsukamoto K, Oshima T, Fujiwara Y, Gondo N, Tamura K, Utsunomiya J, Hashimoto-Tamaoki T, Yamamura T. Successful chemotherapeutic modality of doxorubicin plus dacarbazine for the treatment of desmoid tumors in association with familial adenomatous polyposis. J Clin Oncol. 2006;24:102–105. doi: 10.1200/JCO.2005.02.1923. [DOI] [PubMed] [Google Scholar]
- Patel SR, Benjamin RS. Desmoid tumors respond to chemotherapy: defying the dogma in oncology. J Clin Oncol. 2006;24:11–12. doi: 10.1200/JCO.2005.03.6566. [DOI] [PubMed] [Google Scholar]
- Patel SR, Evans HL, Benjamin RS. Combination chemotherapy in adult desmoid tumors. Cancer. 1993;72:3244–3247. doi: 10.1002/1097-0142(19931201)72:11<3244::aid-cncr2820721118>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Alman BA, Li C, Pajerski ME, Diaz-Cano S, Wolfe HJ. Increased b-catenin protein and somatic APC mutations in sporadic aggressive fibromatoses (desmoid tumors). Am J Pathol. 1997;151:329–334. [PMC free article] [PubMed] [Google Scholar]
- Willert K, Jones KA. Wnt signaling: is the party in the nucleus? Genes Dev. 2006;20:1394–1404. doi: 10.1101/gad.1424006. [DOI] [PubMed] [Google Scholar]
- Xu W, Kimelman D. Mechanistic insights from structural studies of b-catenin and its binding partners. J Cell Sci. 2007;120:3337–3344. doi: 10.1242/jcs.013771. [DOI] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Polakis P. The oncogenic activation of b-catenin. Curr Opin Genet Dev. 1999;9:15–21. doi: 10.1016/s0959-437x(99)80003-3. [DOI] [PubMed] [Google Scholar]
- Willert K, Nusse R. b-Catenin: a key mediator of Wnt signaling. Curr Opin Genet Dev. 1998;8:95–102. doi: 10.1016/s0959-437x(98)80068-3. [DOI] [PubMed] [Google Scholar]
- Polakis P. The many ways of Wnt in cancer. Curr Opin Genet Dev. 2007;17:45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Lazar AJ, Calonje E, Grayson W, Dei Tos AP, Mihm MC, Jr, Redston M, McKee PH. Pilomatrix carcinomas contain mutations in CTNNB1, the gene encoding beta-catenin. J Cutan Pathol. 2005;32:148–157. doi: 10.1111/j.0303-6987.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- Gavert N, Ben-Ze'ev A. Beta-catenin signaling in biological control and cancer. J Cell Biochem. 2007;102:820–828. doi: 10.1002/jcb.21505. [DOI] [PubMed] [Google Scholar]
- Shitoh K, Konishi F, Iijima T, Ohdaira T, Sakai K, Kanazawa K, Miyaki M. A novel case of a sporadic desmoid tumour with mutation of the b-catenin gene. J Clin Pathol. 1999;52:695–696. doi: 10.1136/jcp.52.9.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amary MF, Pauwels P, Meulemans E, Roemen GM, Islam L, Idowu B, Bousdras K, Diss TC, O'Donnell P, Flanagan AM. Detection of b-catenin mutations in paraffin-embedded sporadic desmoid-type fibromatosis by mutation-specific restriction enzyme digestion (MSRED): an ancillary diagnostic tool. Am J Surg Pathol. 2007;31:1299–1309. doi: 10.1097/PAS.0b013e31802f581a. [DOI] [PubMed] [Google Scholar]
- Saito T, Oda Y, Kawaguchi K, Tanaka K, Matsuda S, Tamiya S, Iwamoto Y, Tsuneyoshi M. Possible association between higher b-catenin mRNA expression and mutated beta-catenin in sporadic desmoid tumors: real-time semiquantitative assay by TaqMan polymerase chain reaction. Lab Invest. 2002;82:97–103. doi: 10.1038/labinvest.3780399. [DOI] [PubMed] [Google Scholar]
- Tejpar S, Nollet F, Li C, Wunder JS, Michils G, dal Cin P, Van Cutsem E, Bapat B, van Roy F, Cassiman JJ, Alman BA. Predominance of b-catenin mutations and b-catenin dysregulation in sporadic aggressive fibromatosis (desmoid tumor). Oncogene. 1999;18:6615–6620. doi: 10.1038/sj.onc.1203041. [DOI] [PubMed] [Google Scholar]
- Miyoshi Y, Iwao K, Nawa G, Yoshikawa H, Ochi T, Nakamura Y. Frequent mutations in the b-catenin gene in desmoid tumors from patients without familial adenomatous polyposis. Oncol Res. 1998;10:591–594. [PubMed] [Google Scholar]
- Jilong Y, Jian W, Xiaoyan Z, Xiaoqiu L, Xiongzeng Z. Analysis of APC/b-catenin genes mutations and Wnt signalling pathway in desmoid-type fibromatosis. Pathology. 2007;39:319–325. doi: 10.1080/00313020701329823. [DOI] [PubMed] [Google Scholar]
- Koch A, Denkhaus D, Albrecht S, Leuschner I, von Schweinitz D, Pietsch T. Childhood hepatoblastomas frequently carry a mutated degradation targeting box of the b-catenin gene. Cancer Res. 1999;59:269–273. [PubMed] [Google Scholar]
- Saito T, Oda Y, Tanaka K, Matsuda S, Tamiya S, Iwamoto Y, Tsuneyoshi M. Beta-catenin nuclear expression correlates with cyclin D1 overexpression in sporadic desmoid tumours. J Pathol. 2001;195:222–228. doi: 10.1002/path.942. [DOI] [PubMed] [Google Scholar]
- Abraham SC, Reynolds C, Lee JH, Montgomery EA, Baisden BL, Krasinskas AM, Wu TT. Fibromatosis of the breast and mutations involving the APC/b-catenin pathway. Hum Pathol. 2002;33:39–46. doi: 10.1053/hupa.2002.30196. [DOI] [PubMed] [Google Scholar]
- Ng TL, Gown AM, Barry TS, Cheang MC, Chan AK, Turbin DA, Hsu FD, West RB, Nielsen TO. Nuclear b-catenin in mesenchymal tumors. Mod Pathol. 2005;18:68–74. doi: 10.1038/modpathol.3800272. [DOI] [PubMed] [Google Scholar]
- Lewis JJ, Boland PJ, Leung DH, Woodruff JM, Brennan MF. The enigma of desmoid tumors. Ann Surg. 1999;229:863–872. doi: 10.1097/00000658-199906000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery E, Torbenson MS, Kaushal M, Fisher C, Abraham SC. b-Catenin immunohistochemistry separates mesenteric fibromatosis from gastrointestinal stromal tumor and sclerosing mesenteritis. Am J Surg Pathol. 2002;26:1296–1301. doi: 10.1097/00000478-200210000-00006. [DOI] [PubMed] [Google Scholar]
- Bhattacharya B, Dilworth HP, Iacobuzio-Donahue C, Ricci F, Weber K, Furlong MA, Fisher C, Montgomery E. Nuclear b-catenin expression distinguishes deep fibromatosis from other benign and malignant fibroblastic and myofibroblastic lesions. Am J Surg Pathol. 2005;29:653–659. doi: 10.1097/01.pas.0000157938.95785.da. [DOI] [PubMed] [Google Scholar]
- Gebert C, Hardes J, Kersting C, August C, Supper H, Winkelmann W, Buerger H, Gosheger G. Expression of b-catenin and p53 are prognostic factors in deep aggressive fibromatosis. Histopathology. 2007;50:491–497. doi: 10.1111/j.1365-2559.2007.02619.x. [DOI] [PubMed] [Google Scholar]
- Luo J, Chen J, Deng ZL, Luo X, Song WX, Sharff KA, Tang N, Haydon RC, Luu HH, He TC. Wnt signaling and human diseases: what are the therapeutic implications? Lab Invest. 2007;87:97–103. doi: 10.1038/labinvest.3700509. [DOI] [PubMed] [Google Scholar]
- Herbst A, Kolligs FT. Wnt signaling as a therapeutic target for cancer. Methods Mol Biol. 2007;361:63–91. doi: 10.1385/1-59745-208-4:63. [DOI] [PubMed] [Google Scholar]