Abstract
Matrix metalloproteinase (MMP)−3 is induced by multiple cell types in the skin during processes involved in both normal and pathological tissue remodeling. We previously demonstrated that MMP3-null animals have an increased sensitivity to the development of squamous cell carcinoma, suggesting that overall, MMP3 has a protective role in squamous cell carcinoma. However, not all cellular responses affected by a loss of MMP3 are tumor-protective, and tumor expression of MMP3 is co-incident with an invasive tumor phenotype. Transgenic mice were generated with MMP3 targeted to keratinocytes to examine the biological role of tumor-produced MMP3. Overexpression of MMP3 reduced tumor multiplicity in response to chemically induced squamous cell carcinoma. Vascular density was increased with MMP3 overexpression; however, other cellular processes, including tumor growth and leukocyte infiltration, were unaffected. In accordance with the change in tumor multiplicity, SP-1 murine papilloma cell lines that were generated to stably express MMP3 lost the capacity to establish palpable tumors following orthotopic injection into immunocompromised mice. Analysis of epidermal biopsies taken at 1 to 2 weeks postinjection revealed that these MMP3-expressing Sp-1 lines had reduced levels of proliferation and pronounced differentiation. These same cells demonstrated an increased ability to differentiate in vitro, an effect that was inhibited by broad-spectrum MMP and selective MMP3 inhibition. These studies suggest that keratinocyte expression of MMP3 promotes cellular differentiation, impeding tumor establishment during tumorigenesis.
Matrix metalloproteinases (MMPs) are a family of extracellular matrix-degrading proteinases implicated in a variety of normal and pathological cellular processes including embryogenesis, angiogenesis, wound healing and cancer.1,2 While key roles for these enzymes in regulating fundamental processes throughout tumor progression and tumor invasion have been delineated3,4,5; there has been a lack of efficacy with broad-spectrum inhibition of MMPs as anti-tumor therapy.6 During tumorigenesis, the expression of a number of MMPs are induced de novo.7 Recent work supports the idea that MMPs, themselves, may play beneficial, anti-tumor roles throughout tumorigenesis.8,9 As these same MMPs can regulate cellular processes required during normal tissue remodeling, it is difficult to distinguish by association between MMPs that have been selected for pro-tumorigenic impulse and those that reflect a counter response attempting to initiate tissue repair and maintain homeostasis.10 Thus, a renewed focus has begun on defining the role of individual proteinases throughout tumor progression to determine the distinct spatial and temporal functions of these enzymes and to better predict efficacious modulation of these proteinases for anti-tumor therapy.11,12
There is correlative evidence suggesting that epidermal expression of one MMP family member, MMP3 (Stromelysin-1/E.C.3.4.24.17), influences tumor invasion. Under resting conditions, the skin produces negligible amounts of MMP3. However, MMP3 is transiently up-regulated in both the dermis and epidermis during normal re-epithelialization and, pathologically, in chronic wounds, blistering skin diseases and squamous cell carcinoma (SCC).13,14,15,16,17 Whereas MMP3 expression is induced in the tumor stroma in the early stages of tumorigenesis, tumor expression of MMP3 is co-incident with the development of highly invasive SCC and progression to an epithelial-to-mesenchymal phenotype.17,18,19,20,21,22 In head-and-neck SCC, MMP3 expression is a prognostic indicator of invasion and lymph node metastasis.23 In vitro, MMP3 expression in tumorigenic keratinocytes correlates with experimental models of invasion, as well.17,18,19,20,21,22 Overexpression of MMP3 in mammary epithelium is associated, in vitro, with epithelial-mesenchymal transition and, in vivo, with tumor promotion.24,25,26 Furthermore, substrates for MMP3 include both a variety of matrix molecules and non-matrix molecules such as growth factors and adhesion receptors including pro-MMPs, heparin-binding epidermal growth factor, pro-interleukin1-β, insulin-like growth factor binding protein-3, and E-cadherin.3,4 Together, the co-incidence of tumor expression of MMP3 with invasive carcinoma and the prospective proteolytic targets with known pro-tumorigenic functions infer a potential role for tumor localized production of MMP3 in tumor cell invasion.
Despite the evidence for an MMP3-dependent pro-tumorigenic role, our previous study indicated that the complete absence of MMP3 results in mice with an enhanced sensitivity to chemically induced SCC suggesting that MMP3 is overall anti-tumorigenic during SCC progression.27 However, not all cellular responses affected by loss of MMP3 are associated with a tumor protective role. Furthermore, as MMP3 is expressed by a variety of cell types and primarily by cells residing in the tumor stroma at early stages of SCC, the relative contribution of MMP3 from discrete cell populations is not readily discernable.28 Due to the variety of potential targets, we anticipate that there are both distinct protective and pro-tumorigenic MMP3-dependent responses that will depend on the source and localization of MMP3 production. In view of the fact that tumor expression of MMP3 is co-incident with gain of epithelial-to-mesenchymal phenotype, the association of MMP3 expression with highly invasive tumorigenic keratinocytes and the potential for pro-invasive effects following proteolytic digestion of select targets, we predicted that tumor localized expression of MMP3 would be tumor promoting. To begin to understand the role of keratinocyte-expression of MMP3, we took an approach complementary to the MMP3 null study and generated a transgenic mouse with epidermal targeted expression of MMP3 and SCC cell lines with stable MMP3 introduction. We have compared, in vivo, the tumorigenic responses of wild-type (WT) and MMP3 transgenic animals and the tumorigenic properties of squamous cell lines with MMP3 overexpression.
Materials and Methods
Construction of Expression Vector and Generation of Transgenic Mice
Transgenic mice with targeted expression of MMP3 were generated using a vector containing the bovine cytokeratin 5 and 6 minilocus obtained from Manfred Blessing.29 This vector contains endogenous splice and Poly A addition sites. A construct, rMMP3301 containing the full length WT rat MMP3 cDNA with an amino acid substitution at position 93 (P→V) was inserted into a SalI site in the vector placing the cDNAs under the control of the K5 promoter. This amino acid substitution results in autoactivated form of MMP3.30 The vector sequences were isolated with a BamHI digestion, purified by CsCl centrifugation and microinjected into (C57Bl/6xDBA) F1 fertilized eggs as previously described.31 Embryos were transferred to pseudopregnant females and pups were analyzed for presence of the transgene by Southern blotting. Transgenic founder animals were identified by Southern blot analysis of EcoRI-digested tail DNA under high stringency conditions as previously described.31 The number of copies of transgene DNA that integrated into the genome was determined by comparing the relative intensity of the hybridization signal to control DNAs containing 1 and 10 genome equivalents of the same DNA that was injected. Transgenic lines were generated by mating founder animals to (C57Bl/6xDBA) F1 males and females. Tail DNA samples were harvested for genotyping using PCR. We used a sense oligonucleotide primer (5′-TGAAGGTCTGGGAGGAGGTGAC-3′) and an antisense primer (5′-TTCCAGGCCCATCAAAAGGGA-3′). The resulting amplified fragment spans exon 4 and exon 5 and results in a ∼260-bp fragment from the genomic DNA and a ∼160-bp fragment from the rMMP3 transgene.
Skin Excisional Wounding
Transgenic and litter matched control mice were anesthetized with ketamine/xylazine and had their dorsal fur shaved. Full thickness excisional wounds were made using 3-mm biopsy punches (Acuderm, Ft. Lauderdale Florida). Skin samples were harvested and fixed in 4% paraformaldehyde and paraffin embedded.
Chemical Induction of SCC
Chemically induced SCC experiments were performed on C57Bl/6xDBA heterozygous transgenic and litter matched controls as previously described.27 Mice were housed, fed and treated in accordance with the guidelines approved by the Committee for Protection of Animal Subjects at Vanderbilt University Medical Center. At 8 weeks of age, K5*-MMP3301.24 transgenic and WT litter mate control mice were shaved on the dorsal area 2 days before initiating chemical carcinogenesis treatments and thereafter as needed. Mice were subjected to a single topical application of a solution containing 25 μg 7,12-dimethylbenz [a] anthracene (DMBA; Sigma Chemical Co., St. Louis, MO) dissolved in 100 μl of acetone directly applied to shaved skin. One week after the first treatment, 5 μg 12-O-tetradecanoylphorbol-13-acetate; (TPA; LC Laboratories, Woburns, MA) dissolved in 100 μl of acetone was applied twice weekly for 25 weeks and terminated 48 hours before tumors were harvested. Mice were examined weekly for the presence of skin tumors and for tumor dimensions to be recorded. Tumor volumes were estimated according to the formula V = (L/2) × (W)2 where V = volume, L = length and W = width. At autopsy, lungs were inflated and fixed with Bouin’s fixative and visually examined for the presence of metastases. Squamous tumors were dissected and immediately fixed in 4% paraformaldehyde before paraffin embedding.32
Tumor Allografts
For orthotopic injections, 8-week-old Rag-2 null mice in the 129SvEv background (Taconic) were subjected to 3 Gy of irradiation by Cesium125 exposure as previously detailed to improve tumor take.33 Sp-1 cells were stably selected following transfection with an expression vector containing the neomycin resistance gene or a vector containing the rMMP3 cDNA as previously detailed.34 Sp-1 parental cells, empty vector control clones (Sp-1 Neoa1, Neoa2, or Neoa3) or MMP3 expressing clones(Sp-1MMP3a4, MMP3a6 or MMP3a8), 3 × 105 cells diluted in 50 μl of 1× PBS, were introduced by intradermal injection. For the initial tumorigenecity study, mice were examined weekly for presence of skin tumors up to 3 months postinjections. For short term experiments (1 to 2 weeks), mice were tattooed around the site of injection and tissue resected within the tattooed area. For 5-bromo-2′-deoxyuridine (BrdU) incorporation analysis, mice were injected with 75 mg/kg BrdU 2 hours before animals were sacrificed. Tumors were dissected and immediately fixed in 4% paraformaldehyde before paraffin embedding.32
In Situ Hybridization and Generation of Riboprobes
Paraformaldehyde fixed-paraffin embedded sections (5 μm) of normal, wounded and tumorigenic skin were analyzed by in situ hybridization for rat MMP3 transgene expression as previously described.31 A 492-bp BglII/HincII fragment corresponding to sequences in the 3′ region of the rat cDNA was used as template in the generation of S35-labeled sense and antisense riboprobes.
Histopathological and Immunohistochemical Analysis
Paraformaldehyde-fixed paraffin embedded sections (5 μm) were stained with Mayer’s H&E or with Trichrome by Gomori’s method or analyzed by immunohistochemistry as previously described.27 The following primary antibodies were used in conjunction with antigen retrieval by heat denaturation (10 minutes microwave, 10 mmol/L sodium citrate, pH 6.0): rabbit polyclonal anti-keratin 5 (1:1000; Covance Research Products Inc., Denver, PA); rabbit polyclonal anti-keratin 6 (1:1000; Covance Research Products Inc., Denver, PA); mouse ascites anti-pan keratin (1:400; Sigma-Aldrich Corp., St. Louis, MO); rabbit polyclonal anti-mouse involucrin (1:400; Covance Research Products Inc., Denver, PA); rabbit polyclonal anti-fillagrin (1:400; Covance Research Products Inc., Denver, PA); mouse monoclonal anti-proliferating cell nuclear antigen (PCNA, 1:400; Zymed Laboratories, San Francisco, CA); rat monoclonal anti-neutrophil (1:200; Serotec Inc., Raleigh, NC); rat monoclonal anti-CD3ε (1:200; Serotec Inc., Raleigh, NC); and mouse anti-smooth muscle actin (1:800; Sigma-Aldrich Corp., St. Louis, MO). The following primary antibodies were used in conjunction with antigen retrieval by proteolysis (10 minutes, 0.1% Trypsin in 10 mmol/L Tris-Cl, pH 7.4) rat monoclonal anti-F4/80 (1:200; Serotec Inc., Raleigh, NC); and rat monoclonal anti-CD31/Pecam (1:100; B.D. Biosciences/Pharmingen. San Jose, CA). For BrdU analysis, the rat anti-BrdU (1:800; Accurate Chemical and Scientific Corp) was used following acid denaturation and neutralization as the antigen retrieval method. Negative controls were performed using appropriate species and isotype matched immunoglobulins. Sections were then incubated with appropriate secondary antibody (Vector Laboratories, Burlingame, CA). For immunohistochemistry, antibody binding was detected using ABC Elite Method (Vector Laboratories, Burlingame, CA) with diaminobenzidine as the substrate and the sections were counter stained with Mayer’s Hematoxylin to visualize cells. For immunofluorescence, sections were then incubated with appropriate secondary antibody (Vector Laboratories, Burlingame, CA) and with Hoechst 30551 to visualize cell nuclei. Sections were evaluated for presence or absence of antigen. In the case of PCNA immunostaining, an average of 1000 nuclei were evaluated for 6 to 8 stage matched tumors/experimental group. Quantitation of PECAM/CD-31 immunostaining was performed morphometrically using Metamorph Imaging System software (MDS, Inc, Toronto, Canada) to determine the area of positive staining for 6 to 8 stage matched tumors/experimental group. Neutrophil immunostaining was assessed by counting neutrophil positive cells within multiple arbitrary areas defined by Metamorph Imaging System Software (Universal Imaging Corporation, Downingtown, PA) of 6 to 8 tumors/experimental group.
Apoptosis Analysis
Terminal dUTP nicked-end labeling (TUNEL) was performed on paraformaldehyde-fixed, paraffin embedded sections (5 μm) using the Apo-Tag Kit (Intergen, Purchase, NY) according to manufacturer’s directions as previously described.27 Antibody binding was detected using ABC Elite Method (Vector Laboratories, Burlingame, CA) with diaminobenzidine as the substrate, and sections were counter stained with contrast green to visualize cells. An average of 1500 nuclei were evaluated for TUNEL positivity for 6 to 8 stage-matched tumors/experimental group.
In Vitro Differentiation Assay
For growth of cells in a semisolid media to induce differentiation, cells were resuspended 1 × 105 cells/ml in complete media (10% fetal bovine serum, Dulbecco’s modified Eagle’s medium), containing 1.65% methylcellulose (Sigma-Aldrich Corp., St. Louis, MO).35 Cells were plated onto polyHEMA coated bacterial dishes (Sigma-Aldrich Corp., St. Louis, MO) and cultured for 24 to 48 hours as indicated in the presence or absence of MMP-3 inhibitor (10 μm N-isobutyl-N-(4-methoxyphenylsulfonyl)-glycylhyrdroxamic acid; Calbiochem). Cells were harvested by washing 3 times in 1× PBS. An aliquot of cells were recovered both before and following growth in suspension. These cells were attached to coverglass by cytospin, and fixed in 10% phosphate buffered formalin for 10 minutes, at room temperature. Cells were immunostained for involucrin to identify differentiation status and with Hoechst 30551 to visualize cell nuclei as described above.
Statistical Analysis
Kaplan-Maier plots of time to onset of first tumor were analyzed using the log-rank test. Tumor multiplicity was analyzed by multivariant regression of the type described by Prentice et al.36 Tumor growth was plotted as the change in volume weekly. The linear range was defined as the rate between first appearance of tumors and the week at which the first plateau of each curve was reached. Immunohistochemical results were analyzed by a non-parametrical (Mann-Whitney test) where indicated. All statistical analysis was performed using Statview software (SAS Institute).
Results
Generation of Transgenic Mice with Epidermal-Targeted MMP3
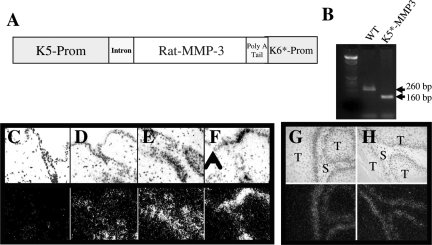
To assess the role of tumor localized expression of MMP3 during SCC in an autochthanous tumor model, we generated transgenic mice with keratinocyte-targeted MMP3 expression. To target expression of MMP3 to the mouse epidermis, we made a construct by inserting the rat MMP3301 cDNA into the bovine keratin (BK) 5 expression vector as shown in Figure 1A. This particular construct contains an amino acid substitution that results in autoactivated form of MMP3, which bypasses any requirement for endogenous factors for activation of MMP3 to its mature form30; furthermore, we previously used this MMP3 construct to successfully express MMP3 in mammary epithelium.37 A 9.5-kb BamHI recombination fragment was purified and microinjected into (C57Bl/6xDBA) F1 fertilized eggs. Two lines of mice, K5*-MMP3301.12 and K5*-MMP3301.24 were established from separate founders that were 1) identified to carry the transgene by Southern analysis and subsequently by PCR (Figure 1B), and 2) gave rise to progeny that expressed the transgene in the skin by Northern analysis. Although the BK5 promoter can drive expression of genes in other epithelial tissues,38 transgene expression was not detected by northern analysis of tissue from the mammary, uterus, tongue, or esophagus (data not shown). Additional analysis of transgene expression by in situ analysis in adult, dorsal skin yielded unexpected results with localization of the transgene expression to hair follicles, but not interfollicular skin (Figure 1, D and E). However, on wounding, the transgene was induced and present in the basal layer of the interfollicular epidermis (Figure 1F). This basally-located pattern of expression was subsequently detected in squamous tumors arising in these transgenic animals. The rMMP3 transgene expression was localized to tumor cells adjacent to the tumor stroma (Figure 1, G and H). The original expression cassette contained BK5 and BK6 in tandem, and the recombination fragment retained some 5′ upstream regions of the BK6 promoter region that have been shown to contain enhancer sequences.29,39,40 As the rMMP3 expression is induced on wounding or tumor induction, yet remains localized to the basal layer of the epidermis, both the BK5 and BK6 promoter elements appear to be regulating gene expression of the transgene and serendipitously have resulted in a transgenic model whereby MMP3 can be induced in interfollicular epidermis under certain conditions.
Figure 1.
Targeting MMP3 to murine epidermis. A: Construction of the expression vector. Transgenic mice with kerotinocytes targeted expression of MMP3 were generated using a vector containing the bovine cytokeratin 5 and 6 promoters. Construct containing a constitutively active construct that contains an amino acid substitution at position 93 (pro-val) was used. B: Genotyping for WT and K5-MMP3 mice. C–H: In situ hybridization for rat MMP3. Paraformaldehyde fixed-paraffin embedded sections (5 μm) from (C) WT or (D–H) K5-MMP3 animals under the indicated conditions were analyzed by in situ hybridization for rat MMP3 transgene expression. C–E: Resting skin (magnification = original ×20); (F) 1 day postwounded skin. Arrow head denotes wound edge. Magnification = original ×20; and (G–H) chemically induced SCC tumors T: tumor, S: tumor stroma (magnification = original ×20). Top panels: dark field; Bottom panels: bright field.
Keratinocyte Overexpression of MMP3 Reduces Tumor Multiplicity in Chemically Induced SCC
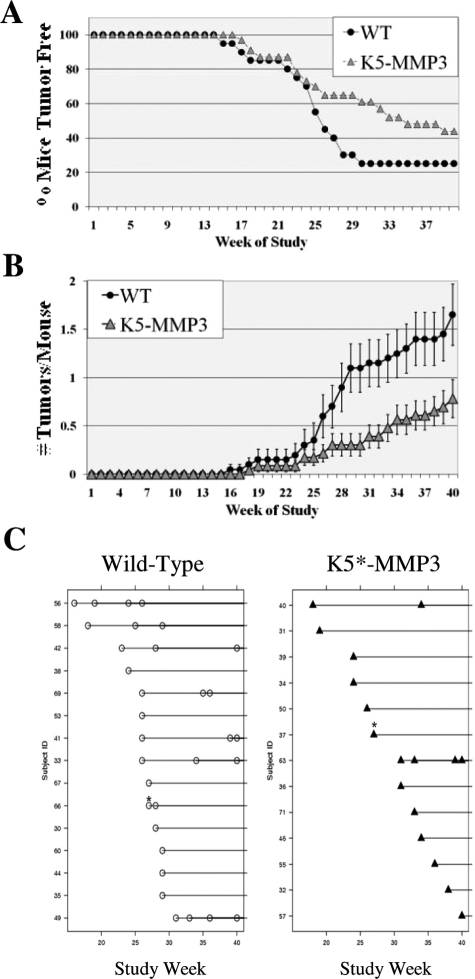
To determine whether tumor expression of MMP3 conferred a selective advantage for SCC, K5*-MMP3301.24 and litter matched WT mice were subjected to the classic two-stage chemically induced SCC regimen with DMBA initiation followed by 40 weeks of TPA promotion. We had previously detected changes early in tumorigenesis comparing SCC development in WT and MMP3 null mice, so we chose this procedure to extend the early stages of tumorigenesis and to directly compare our results with the previous study.27 Papillomas began to appear week 15 and week 17 in WT and K5*-MMP3301.24 mice, respectively (Figure 2A). By week 40, 75% of the WT mice had presented with tumors, whereas 56.5% of K5*-MMP3301.24 animals presented with tumors (Figure 2A). However, there was no significant difference between the two experimental groups with respect to time of development of the first tumor (Log Rank Test, P = 0.135). In contrast, the tumor overexpression of MMP3 significantly affected the mean number of tumors arising on the mice. The K5*-MMP3301.24 mice had significantly fewer tumors weeks 26 to 40 of the study (Figure 2B). Figure 2C shows the times of tumor development for all those mice that developed tumors in the two experimental groups. An analysis of the type described by Prentice et al, shows that there is a significant difference in the number of tumors between the K5*-MMP3301.24 and WT groups (P = 0.0231).36 These data demonstrating that tumor expression of MMP3-reduced tumor multiplicity suggests that MMP3 may affect early stages of tumor establishment.
Figure 2.
Tumorigenesis studies: (A) Tumor onset and (B,C) multiplicity of DMBA/TPA induced SCC in WT and K5- MMP3 mice. A: The onset of papillomas is depicted as the percentage of mice without tumors scored weekly during the course of DMBA/TPA treatment. (n = 20 WT and n = 23 K5-MMP3 mice. P = non-significant; Log Rank Sum). B: The average number of tumors/mouse (mean ± SEM) was calculated weekly during the course of study. C: Onset plots for individual tumor appearance on each study mice that developed tumors over the course of the study. Multiple tumors arising the same week are indicated by an asterisk (*). (P = 0.0231).
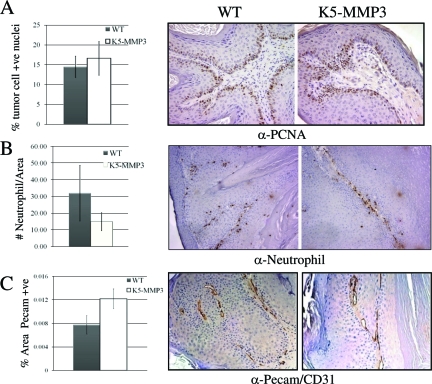
We determined whether tumor expression of MMP3 would affect tumor growth, as we previously noted enhanced tumor cell growth in the complete absence of MMP3.27 Tumor growth rates, as measured by the linear change of tumor volume assessed weekly from the time of tumor onset, were similar between tumors arising on WT and K5*-MMP3301.24 mice (data not shown). Likewise, papillomas induced by DMBA/TPA treatment in WT and K5*-MMP3301.24 animals had comparable levels of proliferating tumor cells as determined by the presence of nuclear PCNA (Figure 3A; p = NS). Neither were there any differences in tumor cell survival as determined by TUNEL positive cells between papillomas established in the two experimental groups (data not shown). Thus, tumor expression of MMP3 did not appear to influence tumor growth in established tumors.
Figure 3.
Tumor cell proliferation, tumor-associated neutrophil populations and vascular density. Tumors induced by DMBA/TPA treatment from K5-MMP31.24 and WT animals were evaluated following immunohistochemistry for (A) PCNA-positive nuclei, (B) the presence of neutrophils, or (C) % area positive for Pecam/CD31. A: Tumor cell proliferation is expressed as the proportion of tumor cells positive for PCNA (mean ± SE; P = non-significant). B: Neutrophil infiltration is represented as the average number of neutrophils/a defined tumor field (mean ± SE; P = non-significant). C: Vascular density was morphometrically quantitated using Metamorph software and is expressed as the percent tumor area positive for PECAM/CD-31 (mean ± SE; P ≤ 0.04). Magnification = original ×20.
Vascular Density is Increased in Tumors Arising on K5-MMP3 Mice, but Leukocyte Infiltration is Unaltered
MMP3 is a potential regulator of immune cell function, and has been shown to regulate leukocyte infiltration in a number of disease models.41,42,43 We previously reported that MMP-3 null animals have a reduction in neutrophil and monocyte populations in tumors from the papilloma stage and then subsequently throughout increased stages of disease progression.27 We, thus, investigated whether neutrophil infiltration was enhanced in tumors with MMP3 overexpression. In the present study, while there was a trend toward fewer neutrophils in the tumors arising on K5*-MMP3301.24 mice, the numbers of neutrophils in present in K5*-MMP3301.24 tumors as compared to WT tumors was not significantly different (Figure 3B; P = non-significant). In addition, tumors arising on both K5*-MMP3301.24 and WT mice had comparable numbers of macrophages. This suggested that MMP3 overexpression in the epidermis was not sufficient to enhance tumor-associated leukocyte infiltration. Furthermore, the absence of MMP3 had previously resulted in SCC tumors with reduced vascularity, and MMP3 is a potential regulator of angiogenesis.27,44 We, thus, compared tumors arising on WT and K5*-MMP3301.24 animals for any alterations in vascular density. Blood vessel density was examined using the cell surface endothelial marker PECAM/CD31 (Figure 3C). Tumors from K5*-MMP3301.24 animals had a significant elevation in blood vessel density compared to WT tumors. Thus, vascular density correlated with MMP-3 expression.
MMP3 Overexpression in Tumorigenic Keratinocytes Reduces Tumor Establishment in an Orthotopic Model
As the chemically induced SCC study revealed that overexpression of MMP3 led to a reduction in the number of tumors formed, we decided to explore the role of MMP3 on tumor establishment. The rMMP3301 construct was stably introduced into the tumorigenic keratinocyte Sp-1 cell line.33 We previously showed that rMMP3301 introduction into Sp-1 cells (Sp-1MMP3) modestly enhanced tumor cell invasion in vitro; however, the in vivo effects of MMP3 expression were unknown.45 Previous characterization of the tumorigenicity of the Sp-1, papilloma-derived cell line have shown the cells give rise to low grade, non-invasive tumors when introduced into immunocompromised mice.33 To focus more closely on early events in tumor formation, an orthotopic model was used where these cell lines were introduced intradermally into the dorsal region of mice. We predicted that if tumor expression of MMP3 was reducing tumor formation, then overexpression of MMP3 in Sp-1 cells should result in a reduction of tumor take. Surprisingly, three separate clones of MMP3-overexpressing cell lines, Sp-1MMP3a.4, Sp-1MMP3a.6, and Sp-1MMP3a.8, did not form tumors at all (Table 1). This is in contrast with the parental Sp-1 cell line and vector control (Sp-1neoa.1, Sp-1neoa.2, and Sp-1neoa.3) cell lines that maintained tumorigenicity. Thus, tumor expression of MMP3, whether assessed in a transgenic mouse model or an allograft model, appears to reduce tumor establishment.
Table 1.
Palpable Tumor Formation from MMP3-Expressing and Non-Expressing Sp-1 Squamous Cell Lines.
| Cell line | N | % Tumor take |
|---|---|---|
| Sp-1 (parental) | 10 | 100 |
| Sp-1 neo1 | 9 | 100 |
| Sp-1 neo2 | 9 | 100 |
| Sp-1 neo3 | 10 | 100 |
| Sp-1 MMP3a.4 | 10 | 0 |
| Sp-1 MMP3a.6 | 10 | 0 |
| Sp-1 MMP3a.8 | 6 | 0 |
MMP3-expressing (Sp-1MMP3a.4, Sp-1MMP3a.6, and Sp-1MMP3a.8)and non-expressing (Sp-1 parental cell line or vector control lines Sp-1neoa.1, Sp-1neoa.2, and Sp-1neoa.3) Sp-1 squamous cell lines were introduced intradermally into immunocompromised mice and evaluated for formation of palpable tumors.
Tumor MMP3 Expression Enhances Tumor Cell Differentiation in Vivo
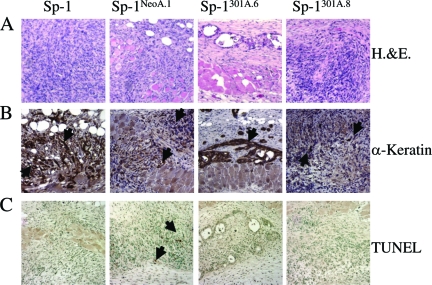
We followed Sp-1 parental, vector control (Sp-1neoa.1 and Sp-1neoa.2) and MMP3-overexpressing (Sp-1MMP3a.4, Sp-1MMP3a.6, and Sp-1MMP3a.8) cell lines 1 and 2 weeks after intradermal introduction to determine the cellular functions that were altered with MMP3 overexpression during the early stages of tumor formation. Cells were introduced intradermally as before, this time with the injection site demarcated with tattoos to allow for resection of the tissue 1 and 2 weeks postinjection. Neoplastic growth was apparent in the dermis by histological assessment 1 and 2 weeks postinjection independent of MMP3 expression status of the introduced cells (Figure 4A). In addition, the presence of squamous, epidermal cytokeratins confirmed that, even two weeks postinjection, Sp-1 cell line variants were present in the dermis (Figure 4B). One possibility for the lack of outgrowth of the Sp-1MMP3 cells into palpable tumors was that MMP3 was affecting cell survival either due to loss of survival and growth signals or through rejection of the cell lines due to their expression of a foreign protein, the rat MMP3. However, examination of immune cell types did not reveal any changes leukocyte infiltration in the Sp-1MMP3a.6 and Sp-1MMP3a.8 cell line allografts (data not shown). Furthermore, apoptosis, a general functional indicator of immune surveillance as well as of overall cell survival, was not enhanced and was primarily absent in the Sp-1MMP3 cell line allografts as compared to the Sp-1neo cell lines (Figure 4C). Together these results suggest that Sp-1MMP3 cell lines, like the parental and Sp-1neo cell lines, were surviving at least two weeks postintroduction into the dermis.
Figure 4.
Histological and Immunohistochemical detection and cell survival analysis of Sp-1 cell line allografts. Paraformaldehyde fixed-paraffin embedded sections (5 μm) were analyzed. A:1 week and 2 week postinjection samples (week 2 shown here) were assessed by H&E to identify allograft site. B: Sp-1 cells were present (α-pan keratin) up to 2 weeks post injection independent of genotype. C: TUNEL analysis of biopsies removed 1 week postinjection. Arrows indicate positive staining in (B) and (C). Panes left-right: Sp-1, Sp-1neoa.1, Sp-1MMP3a.6, Sp-1MMP3a.8. Magnification = original ×20.
To address whether MMP3 expression affected proliferation, allografts were examined for nuclear BrdU incorporation. Allografts generated from both MMP3 expressing and non-expressing tumor cells evidenced areas of active DNA synthesis. The Sp-1 parental and Sp-1neoa.1 cell line allografts had more cells positive for BrdU incorporation and additionally nuclear PCNA staining, suggesting enhanced tumor cell proliferation consistent with the larger areas of tumor cell number associated with these cell types 2 weeks postgrafting. However, when we costained tissue for epidermal specific markers (anti-pan keratin) along with examining the amount of de novo DNA synthesis (anti-BrdU), we could not detect any keratin positive cells that were also BrdU positive from 1 or 2 week allografts generated from the Sp-1MMP3a.6 and Sp-1MMP3a.8 cell lines (Figure 5A). These results suggested that one reason palpable tumors were not generated by the Sp-1MMP3 cell lines was a lack of tumor cell proliferation.
Figure 5.
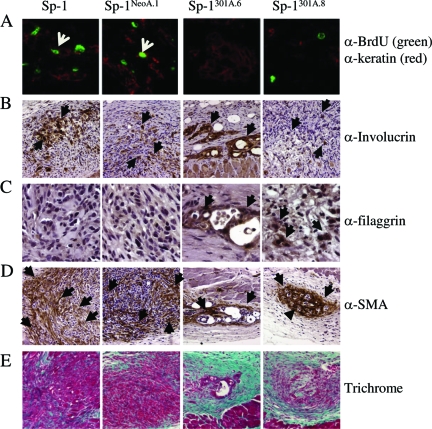
Tumor cell proliferation, tumor cell differentiation, and reactive stromal responses. A: Tumor cell proliferation. One-week samples were analyzed for tumor cell proliferation by examining cells for co-immunofluorescence (indicated by white arrowhead) that demonstrated BrdU incorporation (BrdU; green) and identified tumorigenic keratinocytes (pan-keratin; red) present in the dermis. B–C: Tumor cell differentiation. Allografts were analyzed by immunohistochemical detection for epidermal markers of differentiation 1 week postinjection for (B) involucrin (early stage differentiation marker), and 2 week postinjection for (C) filaggrin (later stage differentiation marker). Arrow- heads indicate positive staining. D–E: Stromal effects were analyzed in 2 week allografts by (D) presence of myofibroblasts by immunohistochemical detection of smooth muscle actin, with arrowheads indicating positive staining and (E) histological analysis with Gomori’s Trichrome (aqua-marine indicate collagen deposition). Panes left-right: Sp-1, Sp-1neoa.1, Sp-1MMP3a.6, Sp-1MMP3a.8. Original magnification: ×40 (A,C); ×20 (B,D); ×10 (E).
The lack of proliferation of the Sp-1MMP3 cells once introduced into the in vivo environment could be the result of a number of scenarios. In two-dimensional cell culture, growth curves of Sp-1neo and Sp-1MMP3 cells were similar. However, cells can respond to their normal in vivo 3D environment and begin to form differentiated structures typical of that individual cell type.46,47,48 Thus, we explored the possibility that the normal dermal microenvironment was inducing differentiation of the Sp-1MMP3 cell lines preferentially. To address changes in cell differentiation, Sp-1 allografts were examined for expression of early and late differentiation markers. All Sp-1 cell line variants expressed the early differentiation marker involucrin (Figure 5B), and the suprabasal Keratin 6, but not Keratin 10, week postinjection (data not shown). As these early genes can be induced experimentally within 1 day, this result following 7 days introduction into the dermis was not unexpected. Interestingly, at 2 weeks postintradermal injection, Sp-1MMP3a.6 and Sp-1MMP3a.8 allografts had detectable expression of the late differentiation marker filaggrin, unlike parental or Sp-1neo allografts that expressed only early differentiation markers (Figure 5C). In addition to tumor cell specific effects, the surrounding “stroma” of these orthotopically injected tumor cells was distinct between MMP3 expressing and non-expressing tumors as well. In contrast to the parental and Sp-1neo cells, myofibroblasts were tightly organized around the orthotopically introduced Sp-1MMP3 cell lines (Figure 5D). When these allograft sections were examined by histology with Gomori’s Trichrome, the orthotopically introduced Sp-1MMP3a.6 and Sp-1MMP3a.8 cells appeared surrounded by intact dermis by week 2 as compared with the Sp-1 and Sp-1neoa.1 cells that had patterns of staining more emblematic of activated dermis usually seen in proximity to tumor cells (Figure 5E). These results are consistent with tumor expression of MMP3 enhancing cellular differentiation and normal connective tissue remodeling.
Tumor MMP3 Expression Enhances Tumor Cell Differentiation in Vitro
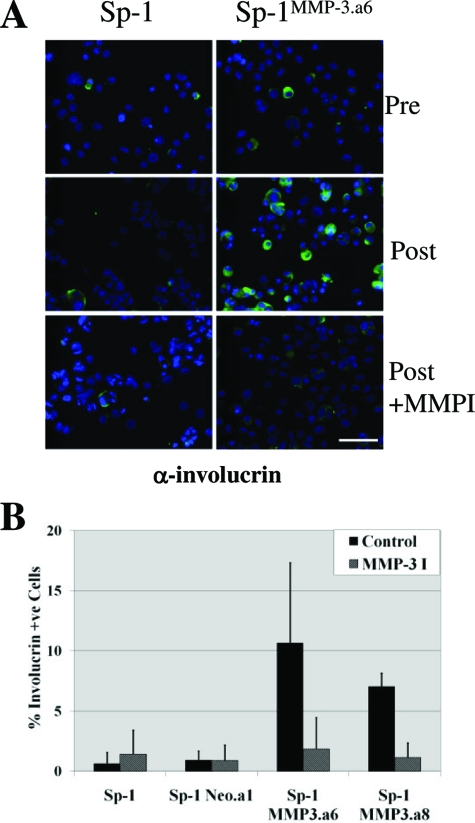
Tumor expression of MMP3 led to reduced tumor formation in vivo and to an enhancement of normal cellular differentiation apparent in tumor allografts. To determine whether this effect on differentiation was cell autonomous or dependent on the 3D organ microenvironment, we examined cellular differentiation induced in vitro by culturing cells in a semisolid medium containing 1.65% methylcellulose. The loss of cellular attachment by growing cells in suspension induces differentiation in keratinocytes.35 Cells were examined before and after 48 hours culture in suspension for expression of involucrin, one of the initial proteins induced during the keratinocyte terminal differentiation process (Figure 6). In contrast to the parental Sp-1 and Sp-1neo cell lines, Sp-1MMP3a.6, and Sp-1MMP3a.8 cell lines had significantly more involucrin expressing cells when grown in suspension, suggesting that there was an MMP3 mediated effect on keratinocyte differentiation (Figure 6). Selective inhibition of MMP3 activity with a synthetic MMP inhibitor, N-isobutyl-N-(4-methoxyphenylsulfonyl)-glycylhydroxamic acid, prevented this enhanced differentiation in the Sp-1MMP3a.6 and Sp-1MMP3a.8 cell lines (Figure 6). These results suggest that the MMP3 regulated effect on cellular differentiation is reversible and dependent on continued production of MMP3.
Figure 6.
Sp-1 cell line variants response to in vitro differentiation. A–B: Sp-1, Sp-1neoa.1, Sp-1MMP3a.6, and Sp-1MMP3a.8 cells lines were examined for involucrin positivity both pre- and postgrowth in suspension (medium containing 1.65% methylcellulose) in the presence and absence of selective MMP3 inhibition (MMP3 I; 10 μmol/L N-isobutyl-N-[4-methoxyphenylsulfonyl]-glycylhydroxamic acid). A: Representative images of Sp-1 and Sp-1MMP3a.6 cells collected pre-suspension and postgrowth in the presence and absence of MMPI and analyzed for involucrin positivity by immunofluorescence (green; involucrin positivity and blue: Hoechst 30551 nuclear stain; magnification = original ×10). Scale bar = 50 μm, B: Proportionate increase of tumor cells positive for involucrin following growth in suspension in the absence or presence of MMP3I (mean ± SE, n = 4). Sp-1MMP3a.6 or Sp-1MMP3a.8 versus Sp-1; P ≤ 0.05).
Discussion
Induction of MMP expression has been closely associated with invasive processes and linked to tumor-promoting and progressive effects. However, recent studies have demonstrated that MMPs can have both tumor-promoting and tumor-protective roles.8,9 We had previously shown that the absence of MMP3 resulted in mice with increased sensitivity to chemically induced SCC, which conferred an overall protective role for MMP3 in skin tumorigenesis.27 Mice that were null for MMP3 had faster growing tumors, and most strikingly, tumors from MMP3 null animals were much further progressed at the end of the study than WT animals with most tumor evidencing invasive or spindle-type of SCC characteristics.27 Furthermore, these tumors from MMP3 null animals demonstrated enhanced tumor cell proliferation. The predominant source of MMP3 is from cell types within the dermis and tumor stroma in the initial stages of SCC.17 Our prior study demonstrated that in the absence of MMP3, there were associated cellular differences such as reductions of infiltrating leukocytes in the stromal compartment at these early stages of SCC that correlated with the absence of MMP3 and the enhanced tumor growth and progression.32 As tumor expression of MMP3 has been associated with increased aggressiveness and epithelial-to-mesenchymal phenotype, we hypothesized that tumor expression of MMP3 would promote tumorigenesis.17,18,19,20,21,22,24,25 Thus, we predicted that stromal sources of MMP3 were the basis of the anti-tumorigenic aspects of MMP3 and hypothesized that tumor expression of MMP3 would be predominantly pro-tumorigenic. To examine the consequence of MMP3 expression in tumorigenic keratinocytes, we generated transgenic mice with keratinocyte-targeted MMP3 overexpression that we challenged with chemically induced SCC and compared the responses to littermate controls. Secondly, we compared the responses of allografts of tumorigenic keratinocytes with and without MMP3 overexpression. Overall, both of these in vivo murine models skin tumorigenesis resulted in a similar anti-tumorigenic phenotype, namely a reduction in tumor formation in the presence of tumor-generated MMP3. Furthermore, assessment of cellular functions at the initial stages of tumor establishment in the allograft model revealed a correlative increase in terminal differentiation and a reduction in cellular growth in tumorigenic keratinocytes with MMP3 overexpression that suggests that tumor produced MMP3 was promoting differentiation instead of tumor formation. These findings indicate that tumor derived expression of MMP3 is protective at early stages of tumorigenesis.
In addition to the protective, anti-tumor establishment aspect of MMP3 that was phenocopied with keratinocyte-targeted MMP3 overexpression in the chemically induced tumorigenesis study, there is a similar vascular effect that correlates with MMP3 expression. Tumors from K5*-MMP31.24 transgenic animals had an elevation in vascular density. This is consistent with the prior study where absence of MMP3 resulted in a reduction of tumor vascularity. The role of MMP3 in regulating tumor vascularization in the skin is unclear; however, a number of MMP3 substrates, such as vascular endothelial growth factor, have the potential to regulate vascularity.44 However, there were distinctions between cellular processes affected with absence of MMP3 versus MMP3 overexpression.27 For one, targeting rMMP3 to the epidermis did not affect tumor growth rates nor proliferation rates in the tumors that could establish in these animals. This suggests that MMP3 did not have further anti-proliferative effects in tumors that were able to escape the MMP3-impedence on tumor formation. Furthermore, while complete absence of MMP3 reduced the number if infiltrating leukocytes, we noted no significant changes in leukocyte populations in tumors from K5-MMP3 and WT mice.27 The absence of an MMP3 effect with keratinocyte-localized overexpression may mean that either the MMP3 mediated effect on proliferation and leukocyte infiltration is already maximized by endogenous production levels of MMP3 or that MMP3 localization to other cell types distinct from the epidermis are influencing these processes. However, while MMP3 overexpression does not effect proliferation rates in vitro, we did note an absence of proliferation in tumor cells with MMP3 overexpression that were grafted intradermally into immunocompromised animals. Intriguingly, in our earlier study, tumors from MMP3 null animals had an advanced tumor progression, thus the enhanced tumor cell proliferation apparent in the absence of MMP3 may be related to a loss of cellular differentiation signaling.
The tumor-produced, MMP3-dependent reduction in tumor formation is not limited to stratified epidermis. In our current study, we find that overexpression of MMP3 in Sp-1 cells reduces tumor establishment concomitant with an increase in differentiation in an allograft model. This tumor generated MMP3-dependent reduction in tumor formation was noted in a murine model of breast carcinoma as well.37 While targeted expression of MMP3 to mammary epithelium has varied effects of tumorigenesis depending on the genetic background of the mouse model26,27,37, we previously targeted glandular mammary epithelium for overexpression of MMP3, and noted that overexpression of MMP3 by glandular epithelium led to a reduction in tumor incidence in chemical induced mammary tumors.37 This MMP3 tumor-originated effect on mammary tumor formation was associated with a loss of basement membrane attachment and an increase in apoptosis. Thus, while targeted MMP3 overexpression resulted in individual cellular responses dependent on whether the expression was targeted to epidermal or glandular epithelium, the overall consequence of tumor-associated overexpression of MMP3 was a reduction in tumor formation.
While the MMP3-dependent reduction on mammary tumor establishment was associated with apoptosis and that on skin tumor formation was associated with differentiation, these cellular effects may be due to the distinct way that glandular epithelium and epidermal keratinocytes respond to lack of cellular attachments. The MMP regulation of cellular attachments can potentially be elicited by a variety of means including cleavage of matrix and loss of cellular matrix attachment sites, or loss of cellular attachments through down-regulation of expression or posttranslational proteolytic shedding of matrix receptors.3,49 We have previously shown a loss of extracellular matrix-surrounding areas of ectopic expression of MMP3 in glandular epithelium in the MMTV-MMP3 transgenic mouse.37 In agreement with these studies, Alexander et al has shown an increase in entactin degradation associated with mammary epithelium-targeted MMP3.50 Keratinocyte differentiation is controlled by a variety of factors including matrix and extracellular matrix interactions.51,52 Whereas loss of cellular attachment to the basement membrane results in a lethal deficit of survival signals for glandular epithelium and initiates programmed cell death, a reduction in cellular attachments and a loss of cellular contact with the basement membrane is associated with terminal differentiation in keratinocytes.51,52 Thus, loss of matrix attachment sites through proteolytic targeting in vivo should result in enhanced keratinocyte differentiation and remains a possible mechanism in the current study.
MMP3 has been associated with the differentiation of a variety of cell types. Overexpression of MMP3 in mammary epithelium results in virgin mice phenocopying features of pregnant animals such as enhanced branching, alveolar development and production of milk proteins.31,53 In the absence of MMP3, during mammary gland involution adipocytes demonstrate an increase in the rate of differentiation.54 The influence of MMP3 on epidermal terminal differentiation may be reflected in the localization pattern of MMP3 during normal tissue remodeling processes such as wound repair. In skin, there is negligible expression of MMP3 in the epidermis. However, on wound healing, MMP3 is rapidly up-regulated in basal keratinocytes adjacent to the migrating tongue and co-incident with proliferating keratinocytes.13,55 This association led to the idea that MMP3 could regulate proliferation in the epidermis. However, we detect no enhancement of proliferation in tumors that do establish in the K5*-MMP3301.24 mice as compared to WT controls. In addition, we do not detect any difference in keratinocyte proliferation in the K5*-MMP3301.24 mice during wound healing; however, there is a modest acceleration in the expression of differentiation factors present in the newly formed re-epithelialized skin (McCawley LJ, unpublished). Thus, perhaps MMP3 is induced coincident with proliferating keratinocytes during re-epithelialization to induce a portion of these cells to stratify and to terminally differentiate. Furthermore, in the absence of MMP3, chemically induced tumors grow faster and also have an accelerated disease progression, that is consistent with the notion that MMP3 expression up-regulates epidermal differentiation.27 As MMP3 is below detectable levels in skin it is difficult to discern whether MMP3 is coincident to triggering normal terminal differentiation of keratinocytes; however, MMP3 null animals appear phenotypically normal in that regard. On the other hand, while MMP3 is induced in numerous cell types both in the epidermis and dermis during wound repair, during skin tumorigenesis MMP3 is detected in the tumor stroma at early stages of disease progression, but tumorigenic keratinocyte expression of MMP3 is coincident only later during disease progression with the development of highly invasive SCC. During tumor progression, tumorigenic keratinocytes increasingly lose the ability to differentiate. Thus, we propose that if MMP3 is induced in tumorigenic keratinocytes at early stages of skin tumorigenesis, the expression induces terminal differentiation. If this were the case, we would predict that as tumorigenic keratinocytes lose the ability to terminally differentiate at late stages of disease progression, MMP3 expression is induced and the cell population expressing MMP3 increases as these cells are no longer lost to stratification of the epidermis. Thus, we propose that tumor expression of MMP3 is an indicator of the loss of ability to terminally differentiate.
Despite the bulk of experimental evidence documenting pro-tumorigenic roles of MMPs in cancer, these enzymes are induced and participate in the maintenance of normal tissue homeostasis.56 Individual MMP family members have demonstrated both distinct pro- and anti-tumorigenic functions during SCC.8,9,57,58,59 Thus, MMP inhibition will have to be selective against MMPs with identified pro-tumorigenic functions and used in the correct stage of progression to be efficacious as anti-tumor agents.11,12 The current study illuminates an anti-tumorigenic role for tumor induction of MMP3 that leads to differentiation of tumorigenic keratinocytes and the reduction of tumor formation.
Acknowledgments
We thank Barbara Fingleton and Mark Sinnamon for critical review of the manuscript.
Footnotes
Address reprint requests to Lisa McCawley, Vanderbilt University, Department of Cancer Biology, 771 PRB, 2220 Pierce Ave., Nashville, TN, 37232-6840. E-mail: Lisa.McCawley@vanderbilt.edu.
Supported in part by a Vanderbilt Skin Diseases Research Center Pilot and Feasibility Award (NIH P30 AR41943) to L.J.M.
Current address of B.J.L.: Department of Pediatrics, University of Utah, Salt Lake City, UT; Current address of H.C.C.: Department of Pharmacology, State University of New York, Stony Brook, NY.
References
- Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3:207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- McCawley LJ, Matrisian LM. Matrix metalloproteinases: they’re not just for matrix anymore! Curr Opin Cell Biol. 2001;13:534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Martin MD, Matrisian LM. The other side of MMPs: protective roles in tumor progression. Cancer Metastasis Rev. 2007;26:717–724. doi: 10.1007/s10555-007-9089-4. [DOI] [PubMed] [Google Scholar]
- Lopez-Otin C, Matrisian LM. Emerging roles of proteases in tumour suppression. Nat Rev Cancer. 2007;7:800–808. doi: 10.1038/nrc2228. [DOI] [PubMed] [Google Scholar]
- Radisky D, Hagios C, Bissell MJ. Tumors are unique organs defined by abnormal signaling and context. Semin Cancer Biol. 2001;11:87–95. doi: 10.1006/scbi.2000.0360. [DOI] [PubMed] [Google Scholar]
- Overall CM, Kleifeld O. Tumour microenvironment - opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer. 2006;6:227–239. doi: 10.1038/nrc1821. [DOI] [PubMed] [Google Scholar]
- Hu J, Van den Steen PE, Sang QX, Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov. 2007;6:480–498. doi: 10.1038/nrd2308. [DOI] [PubMed] [Google Scholar]
- Madlener M, Parks WC, Werner S. Matrix metalloproteinases (MMPs) and their physiological inhibitors (TIMPs) are differentially expressed during excisional skin wound repair. Exp Cell Res. 1998;242:201–210. doi: 10.1006/excr.1998.4049. [DOI] [PubMed] [Google Scholar]
- Saarialho-Kere UK, Vaalamo M, Airola K, Niemi KM, Oikarinen AI, Parks WC. Interstitial collagenase is expressed by keratinocytes that are actively involved in reepithelialization in blistering skin diseases. J Invest Dermatol. 1995;104:982–988. doi: 10.1111/1523-1747.ep12606231. [DOI] [PubMed] [Google Scholar]
- Saarialho-Kere UK, Pentland AP, Birkedal-Hansen H, Parks WC, Welgus HG. Distinct populations of basal keratinocytes express stromelysin-1 and stromelysin-2 in chronic wounds. J Clin Invest. 1994;94:79–88. doi: 10.1172/JCI117351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weckroth M, Vaheri A, Lauharanta J, Sorsa T, Konttinen YT. Matrix metalloproteinases, gelatinase and collagenase, in chronic leg ulcers. J Invest Dermatol. 1996;106:1119–1124. doi: 10.1111/1523-1747.ep12340167. [DOI] [PubMed] [Google Scholar]
- Kerkela E, Saarialho-Kere U. Matrix metalloproteinases in tumor progression: focus on basal and squamous cell skin cancer. Exp Dermatol. 2003;12:109–125. doi: 10.1034/j.1600-0625.2003.120201.x. [DOI] [PubMed] [Google Scholar]
- Nagase H. Stromelysins 1 and 2. Parks WC, Mecham RP, editors. San Diego: Academic Press,; Matrix metalloproteinases. 1998:pp. 43–84. [Google Scholar]
- Shima I, Sasaguri Y, Kusukawa J, Yamana H, Fujita H, Kakegawa T, Morimatsu M. Production of matrix metalloproteinase-2 and metalloproteinase-3 related to malignant behavior of esophageal carcinoma: a clinicopathologic study. Cancer. 1992;70:2747–2753. doi: 10.1002/1097-0142(19921215)70:12<2747::aid-cncr2820701204>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Kusukawa J, Sasaguri Y, Morimatsu M, Kameyama T. Expression of matrix metalloproteinase-3 in stage I and II squamous cell carcinoma of the oral cavity. J Oral Maxillofac Surg. 1995;53:530–534. doi: 10.1016/0278-2391(95)90065-9. [DOI] [PubMed] [Google Scholar]
- Airola K, Johansson N, Kariniemi AL, Kahari VM, Saarialhokere UK. Human collagenase-3 is expressed in malignant squamous epithelium of the skin. J Invest Dermatol. 1997;109:225–231. doi: 10.1111/1523-1747.ep12319441. [DOI] [PubMed] [Google Scholar]
- Kerkela E, Ala-aho R, Lohi J, Grenman R, Kahari V, Saarialho-Kere U. Differential patterns of stromelysin-2 (MMP-10) and MT1-MMP (MMP-14) expression in epithelial skin cancers. Br J Cancer. 2001;84:659–669. doi: 10.1054/bjoc.2000.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polette M, Clavel C, Muller D, Abecassis J, Binninger I, Birembaut P. Detection of mRNAs encoding collagenase I and stromelysin 2 in carcinomas of the head and neck by in situ hybridization. Invasion Metastasis. 1991;11:76–83. [PubMed] [Google Scholar]
- Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ. Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol. 1997;139:1861–1872. doi: 10.1083/jcb.139.7.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, Werb Z, Bissell MJ. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, Pinkel D, Bissell MJ, Werb Z. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137–146. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCawley LJ, Crawford HC, King LE, Jr, Mudgett J, Matrisian LM. A protective role for matrix metalloproteinase-3 in squamous cell carcinoma. Cancer Res. 2004;64:6965–6972. doi: 10.1158/0008-5472.CAN-04-0910. [DOI] [PubMed] [Google Scholar]
- Abramson SR, Conner GE, Nagase H, Neuhaus I, Woessner JF., Jr Characterization of rat uterine matrilysin and its cDNA Relationship to human pump-1 and activation of procollagenases. J Biol Chem. 1995;270:16016–16022. doi: 10.1074/jbc.270.27.16016. [DOI] [PubMed] [Google Scholar]
- Blessing M, Nanney LB, King LE, Jones CM, Hogan BL. Transgenic mice as a model to study the role of TGF-beta-related molecules in hair follicles. Genes Dev. 1993;7:204–215. doi: 10.1101/gad.7.2.204. [DOI] [PubMed] [Google Scholar]
- Park AJ, Matrisian LM, Kells AF, Pearson R, Yuan Z, Navre M. Mutational analysis of the transin (rat stromelysin) autoinhibitor region demonstrates a role for residues surrounding the “cysteine switch.”. J Biol Chem. 1991;266:1584–1590. [PubMed] [Google Scholar]
- Witty JP, Wright J, Matrisian LM. Matrix metalloproteinases are expressed during ductal and alveolar mammary morphogenesis, and misregulation of stromelysin-1 in transgenic mice induces unscheduled alveolar development. Mol Biol Cell. 1995;6:1287–1303. doi: 10.1091/mbc.6.10.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JH, McDonnell S, Portella G, Bowden GT, Balmain A, Matrisian LM. A switch from stromal to tumor cell expression of stromelysin-1 mRNA associated with the conversion of squamous to spindle carcinomas during mouse skin tumor progression. Mol Carcinog. 1994;10:207–215. doi: 10.1002/mc.2940100405. [DOI] [PubMed] [Google Scholar]
- Strickland JE, Greenhalgh DA, Koceva-Chylan A, Hennings H, Resterpo C, Balaschak M, Yuspa SH. Development of murine epidermal cell lines which contain an activated ras Ha oncogene and form papillomas in skin grafts on athymic nude mouse hosts. Cancer Res. 1988;48:165–169. [PubMed] [Google Scholar]
- McDonnell S, Matrisian LM. Stromelysin in tumor progression and invasion. Cancer Metastasis Rev. 1991;9:305–319. doi: 10.1007/BF00049521. [DOI] [PubMed] [Google Scholar]
- Li ER, Owens DM, Djian P, Watt FM. Expression of involucrin in normal, hyperproliferative and neoplastic mouse keratinocytes. Exp Dermatol. 2000;9:431–438. doi: 10.1034/j.1600-0625.2000.009006431.x. [DOI] [PubMed] [Google Scholar]
- Prentice RL, Williams BJ, Peterson AV. On the Regression-Analysis of Multivariate Failure Time Data. Biometrika. 1981;68:373–379. [Google Scholar]
- Witty JP, Lempka T, Coffey RJ, Jr, Matrisian LM. Decreased tumor formation in 7,12-dimethylbenzanthracene-treated stromelysin-1 transgenic mice is associated with alterations in mammary epithelial cell apoptosis. Cancer Res. 1995;55:1401–1406. [PubMed] [Google Scholar]
- Ramirez A, Bravo A, Jorcano JL, Vidal M. Sequences 5′ of the bovine keratin 5 gene direct tissue- and cell-type-specific expression of a lacZ gene in the adult and during development. Differentiation. 1994;58:53–64. doi: 10.1046/j.1432-0436.1994.5810053.x. [DOI] [PubMed] [Google Scholar]
- Ramirez A, Vidal M, Bravo A, Jorcano JL. Analysis of sequences controlling tissue-specific and hyperproliferation-related keratin 6 gene expression in transgenic mice. DNA Cell Biol. 1998;17:177–185. doi: 10.1089/dna.1998.17.177. [DOI] [PubMed] [Google Scholar]
- Ramirez A, Vidal M, Bravo A, Larcher F, Jorcano JL. A 5′-upstream region of a bovine keratin 6 gene confers tissue-specific expression and hyperproliferation-related induction in transgenic mice. Proc Natl Acad Sci USA. 1995;92:4783–4787. doi: 10.1073/pnas.92.11.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haro H, Crawford HC, Fingleton B, MacDougall JR, Shinomiya K, Spengler DM, Matrisian LM. Matrix metalloproteinase-3-dependent generation of a macrophage chemoattractant in a model of herniated disc resorption. J Clin Invest. 2000;105:133–141. doi: 10.1172/JCI7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner RL, Beltran L, Younkin EM, Lewis CS, Weiss SJ, Varani J, Johnson KJ. Role of stromelysin 1 and gelatinase B in experimental acute lung injury. Am J Respir Cell Mol Biol. 2001;24:537–544. doi: 10.1165/ajrcmb.24.5.4160. [DOI] [PubMed] [Google Scholar]
- Wang M, Qin X, Mudgett JS, Ferguson TA, Senior RM, Welgus HG. Matrix metalloproteinase deficiencies affect contact hypersensitivity: stromelysin-1 deficiency prevents the response and gelatinase B deficiency prolongs the response. Proc Natl Acad Sci USA. 1999;96:6885–6889. doi: 10.1073/pnas.96.12.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol. 2005;169:681–691. doi: 10.1083/jcb.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisian LM, McDonnell S, Miller DB, Navre M, Seftor EA, Hendrix MJC. The role of the matrix metalloproteinase stromelysin in the progression of squamous cell carcinomas. Am J Med Sci. 1991;302:157–162. doi: 10.1097/00000441-199109000-00008. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Rizki A, Mian IS. Tissue architecture: the ultimate regulator of breast epithelial function. Curr Opin Cell Biol. 2003;15:753–762. doi: 10.1016/j.ceb.2003.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Hayward SW, Wang YZ, Ricke WA. Role of the stromal microenvironment in carcinogenesis of the prostate. Int J Cancer. 2003;107:1–10. doi: 10.1002/ijc.11335. [DOI] [PubMed] [Google Scholar]
- Glick AB, Yuspa SH. Tissue homeostasis and the control of the neoplastic phenotype in epithelial cancers. Semin Cancer Biol. 2005;15:75–83. doi: 10.1016/j.semcancer.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Cauwe B, Van den Steen PE, Opdenakker G. The biochemical, biological, and pathological kaleidoscope of cell surface substrates processed by matrix metalloproteinases. Crit Rev Biochem Mol Biol. 2007;42:113–185. doi: 10.1080/10409230701340019. [DOI] [PubMed] [Google Scholar]
- Alexander CM, Howard EW, Bissell MJ, Werb Z. Rescue of mammary epithelial cell apoptosis and entactin degradation by a tissue inhibitor of metalloproteinase-1 transgene. J Cell Biol. 1996;135:1669–1667. doi: 10.1083/jcb.135.6.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM. Role of integrins in regulating epidermal adhesion, growth and differentiation. EMBO J. 2002;21:3919–3926. doi: 10.1093/emboj/cdf399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, Raghavan S. Getting under the skin of epidermal morphogenesis. Nat Rev Genet. 2002;3:199–209. doi: 10.1038/nrg758. [DOI] [PubMed] [Google Scholar]
- Sympson CJ, Talhouk RS, Alexander CM, Chin JR, Clift SM, Bissell MJ, Werb Z. Targeted expression of stromelysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression. J Cell Biol. 1994;125:681–693. doi: 10.1083/jcb.125.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander CM, Selvarajan S, Mudgett J, Werb Z. Stromelysin-1 regulates adipogenesis during mammary gland involution. J Cell Biol. 2001;152:693–703. doi: 10.1083/jcb.152.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilcher BK, Wang M, Qin XJ, Parks WC, Senior RM, Welgus HG. Role of matrix metalloproteinases and their inhibition in cutaneous wound healing and allergic contact hypersensitivity. Ann NY Acad Sci. 1999;878:12–24. doi: 10.1111/j.1749-6632.1999.tb07671.x. [DOI] [PubMed] [Google Scholar]
- Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Armiento J, DiColandrea T, Dalal SS, Okada Y, Huang MT, Conney AH, Chada K. Collagenase expression in transgenic mouse skin causes hyperkeratosis and acanthosis and increases susceptibility to tumorigenesis. Mol Cell Biol. 1995;15:5732–5739. doi: 10.1128/mcb.15.10.5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbin M, Fueyo A, Tester AM, Pendas AM, Pitiot AS, Astudillo A, Overall CM, Shapiro SD, Lopez-Otin C. Loss of collagenase-2 confers increased skin tumor susceptibility to male mice. Nat Genet. 2003;35:252–257. doi: 10.1038/ng1249. [DOI] [PubMed] [Google Scholar]