Abstract
Early cancer cell migration and invasion of neighboring tissues are mediated by multiple events, including activation of focal adhesion signaling. Key regulators include the focal adhesion kinase (FAK) and FAK-related proline-rich tyrosine kinase 2 (Pyk2), whose distinct functions in cancer progression remain unclear. Here, we compared Pyk2 and FAK expression in breast cancer and their effects on ErbB-2-induced tumorigenesis and the potential therapeutic utility of targeting Pyk2 compared with FAK in preclinical models of breast cancer. Pyk2 is overexpressed in tissues from early and advanced breast cancers and overexpressed with both FAK and epidermal growth factor receptor-2 (ErbB-2) in a subset of breast cancer cases. Down-regulation of Pyk2 in ErbB-2-positive, FAK-proficient, and FAK-deficient cells reduced cell proliferation, which correlated with reduced mitogen-activated protein kinase (MAPK) activity. In contrast, Pyk2 silencing had little impact on cell migration and invasion. In vivo, Pyk2 down-regulation reduced primary tumor growth induced by a metastatic variant of ErbB-2-positive MDA 231 breast cancer cells but had little effect on lung metastases in contrast to FAK down-regulation. Dual reduction of Pyk2 and FAK expression resulted in strong inhibition of both primary tumor growth and lung metastases. Together, these data support the cooperative function of Pyk2 and FAK in breast cancer progression and suggest that dual inhibition of FAK and Pyk2 is an efficient therapeutic approach for targeting invasive breast cancer.
Malignant tumor cells invade surrounding normal tissue and disseminate to distant organs through a multistep and multifactorial process. In general, the acquisition of early autonomous motile property involves cell polarization toward blood and lymphatic vessels in response to chemotactic signals, cell cytoskeleton remodeling, and formation of cell plasma membrane protrusions. The latter are the site of dynamic turnover of multiple focal adhesion molecules, which allow formation of stable cell-matrix attachments near the leading edge of the protrusions, forward movement of the cell body, and disassembly of focal-matrix adhesions and retraction at the trailing edge. These processes are the driving force for early cancer cell migration and invasion.
Central to the regulation of the cell migration cycle is the focal adhesion kinase (FAK) and its homologous FAK-related proline-rich tyrosine kinase 2 (Pyk2).1 FAK and Pyk2 share 45% amino acid sequence homology with a highly conserved central catalytic kinase domain. The noncatalytic regions of both proteins contain proline-rich residues with binding motifs for Src homology (SH) 3 domain-containing proteins, such as Crk-associated substrate, GTPase regulator associated with FAK, and Pleckstrin homology, and SH3 domain containing Arf-GAP proteins, along with a focal adhesion-targeting domain that is critical for FAK recruitment to focal adhesions and for its association with paxillin and talin.2 In addition, both Pyk2 and FAK contain several tyrosine autophosphorylation sites, including an autophosphorylation site (Y397 for FAK and Y402 for Pyk2), which acts as a binding site for the SH2 site of Src tyrosine kinases.
Pyk2 and FAK are activated after integrin clustering in response to various extracellular matrix components, such as fibronectin, and by a number of growth factor receptors, including ErbB tyrosine kinases.3 After activation, both Pyk2 and FAK undergo multiple phosphorylations and engage in protein-protein interactions with several cytoskeletal proteins, such as paxillin; tyrosine kinases, such as Src and C-terminal src-kinase; serine/threonine kinases, such as integrin-linked kinase and p21-activated kinase; and modulators of small GTPases of the Rho family.4 These multiple interactions have been established as critical regulators of early cell invasion signaling, promoting cancer cell migration, and the initiation of metastasis formation.5 However, increasing evidence points toward distinct and possibly antagonistic functions for Pyk2 and FAK. For instance, FAK is reported to be predominantly localized to focal adhesions,6 whereas Pyk2 is largely cytosolic,6,7,8,9,10 and unlike FAK, its phosphorylation is independent of cell adhesion.10 FAK and Pyk2 contain conserved sites within their COOH-terminal domain for paxillin binding.11 However, FAK but not Pyk2 binds to talin.8 Pyk2 but not FAK associates with Hic-5, Nir (Pyk2 N-terminal domain-interacting receptors), and pancreatic associated protein (Pap) proteins.6,12,13 Similarly, Pyk2 but not FAK binds to PSGAP, a Pleckstrin homology and SH3 domain containing Rho GTPase activating protein (RhoGAP) protein capable of stimulating Cdc42 and RhoA, and inhibits its effect on Cdc42.14 Moreover, expression of Pyk2 induces apoptosis in several cell lines15,16 and negatively regulates cell cycle progression,17,18 whereas FAK can protect against apoptosis and promotes cell cycle progression.17,19 These distinct mechanisms could explain the discrepant functional assignments and biological implications reported for FAK versus Pyk2.20,21,22,23,24,25
In this study, we investigated the functional role of Pyk2 versus FAK in ErbB-2-positive breast cancer cell proliferation, migration, and invasion and its correlation to human breast cancer progression. We report that both Pyk2 and FAK are co-overexpressed in early-stage breast cancer and in ErbB-2-positive human breast cancers, supporting a function in breast cancer progression. In contrast to FAK, we demonstrate that Pyk2 has a distinct function in the regulation of cell proliferation of ErbB2-positive cells but little impact on cell invasion; this mechanism involves at least in part modulation of the mitogen-activated protein kinase (MAPK) pathway. We demonstrate that dual down-regulation of Pyk2 and FAK results in a potent inhibition of breast cancer progression in a preclinical invasive breast cancer model.
Materials and Methods
Reagents
The following antibodies were used: Monoclonal anti-ErbB-2 (Ab-3, clone 3B5), polyclonal anti-ErbB-2 (Ab-1), and polyclonal anti-ErbB-3 (clone C-17) were from Oncogene Science (Cambridge, MA); anti-FAK (clone 4.47) was from Biosource International (Camarillo, CA); anti-phosphotyrosine antibody (4G10) and anti-extracellular signal-regulated kinase 2 (Erk2; clone B3B9) were obtained from Upstate (Lake Placid, NY); anti-Pyk2 was obtained from BD Bioscience Transduction Laboratories (Lexington, KY); anti-phospho-MAPK was obtained from New England Biolabs Inc. (Ipswich, MA); rhodamine conjugated to phalloidin was from Sigma-Aldrich (St. Louis, MO), and anti-glyceraldehyde-3-phosphate dehydrogenase was from Cedarlane (Burlington, ON, Canada). Heregulin (HRG) was from Neomarkers (Fremont, CA), and the mitogen-activated protein kinase kinase 1 inhibitor UO126 was from Alexis Biochemicals (Lausen, Switzerland).
Cell Lines and Cell Culture
Mouse embryonic fibroblast (FAK+/+ or FAK−/−) cells were kindly provided by Dr. Dusko Ilic (University of California, San Francisco, CA). The breast adenocarcinoma cell lines MDA 231-AP2, MDA 231-ErbB-2, and MDA 231-M were described previously.26 FAK+/+ or FAK−/− cells were cultured in DMEM (Life Technologies, Rockville, MD) supplemented with 10% fetal bovine serum, 1 mmol/L sodium pyruvate, 1% (v/v) nonessential amino acids, 100 μmol/L 2-mercaptoethanol, and penicillin/streptomycin. Human breast adenocarcinoma cells were maintained in RPMI 1640 (Mediatech, Washington, DC) supplemented with 10% fetal bovine serum and penicillin/streptomycin.
Stable Overexpression of ErbB Receptors in FAK+/+ and FAK−/− Cells
ErbB-2 and ErbB-3 receptors were expressed in polyclonal cell populations using bicistronic retrovectors that express each of the receptors with the enhanced green fluorescent protein (EGFP) as described previously.27
Generation of Cells Expressing Stable FAK and Pyk2 siRNA
A specific 21-nucleotide sequence of mouse Pyk2 gene (GenBank accession no. NM_172498) (5′-GACCUGUAAGAAAGACUGU-3′), human Pyk2 gene (5′-GTTGCTATAGAAGCAGACC-3′),20 and human FAK gene (5′-GCATGTGGCCTGCTATGGA-3′)26 were cloned as inverted repeats into pSuper-retro puro vector according to the manufacturer’s instructions (Oligoengine, Seattle, WA). Control retroviral vector pRetro-Super puro alone or expressing target siRNA was transfected into Phoenix cells using Genejuice (Novagen, Darmstadt, Germany). Forty-eight hours after transfection, the supernatant of phoenix cells was filtered through 0.45-μm filter and used to infect target cell lines twice, 24 hours apart, in the presence of 8 μg/ml polybrene. Forty-eight hours after infection, polyclonal populations were selected for resistance to 1 μg/ml puromycin for 2 weeks to generate stable siRNA-expressing cells and matched controls.
Cell Proliferation Assay
Exponentially growing cells were seeded in 96-well plates at a density of 1 × 103 cells per 100 μl completed well and left undisturbed for 96 hours. Cell proliferation was evaluated 96 hours later using the 3-(4,5-dimethylthiazo-2-yl)-2,5-diphenyltetrazolium bromide metabolic assay as described previously.27
Cell Motility
Cell motility was examined using the phagokinetic track assay. Briefly, sterile coverslips coated with 1% bovine serum albumin and then a uniform carpet of gold colloidal solution containing 150 μmol/L HAuCl4-4H2O and 10 mmol/L Na2CO3 was applied. Serum-starved cells were plated at low density (0.5 × 103 per coverslip), allowed to attach for 1 hour, and then placed in 35-mm tissue culture dishes. Cells were then allowed to migrate for 8 hours in presence of serum-free medium and in the absence and presence of 10 ng of heregulin. Cells were then fixed by 0.1% formaldehyde, and the areas cleared of gold particles were examined under microscope and quantified by NIH image processing; 50 cells were examined per condition, and the results are presented as the average area free of gold colloidal particles in square millimeters ±SD. After digitization, color images were processed using specialized functions from Photoshop imaging software (Adobe Systems, Mountain View, CA), and results are reported as an area per square millimeter.
Invasion Assay
Cell invasion experiments were performed with 8-μm porous chambers coated with Matrigel (Becton Dickinson, Franklin Lakes, NJ) according to the manufacturer’s recommendations. Serum-starved cells were placed into the upper compartment (30,000 cells), and the chambers were placed into 24-well culture dishes containing 400 μl of DMEM 0.2% BSA with or without 20 ng/ml HRG (lower compartment). Cells were allowed to invade through the Matrigel membrane for 48 hours. The invasive cells underneath the membrane were fixed and stained. Filters were viewed under bright-field 40X objective and the counting was performed for three fields in each sample. Each experiment was done at least three times, and results are expressed as means ± SEM.
Western Blot and Immunoprecipitation Assays
Cells were grown to approximately 70 to 80% confluence after 24 hours of serum-starvation and treatment with HRG when indicated. Total cell lysates were prepared, blotted, detected using appropriate antibody, and visualized using enhanced chemiluminescence detection. Antibodies used are the following: ErbB-2 (antibody-3, clone 3B5; Oncogene Science), ErbB-3 (clone C-17; Santa Cruz Biotechnology, Santa Cruz, CA), Pyk2 (BD Bioscience Transduction Laboratories), glyceraldehyde-3-phosphate dehydrogenase (Cell Signaling Technology, Danvers, MA), FAK (clone 4.47; Biosource International), Erk1/2 (p44/42 mitogen-activated protein kinase), and phospho-Erk1/2-Y202/204 (Cell Signaling Technology). For immunoprecipitation, 200 μg of protein was immunoprecipitated with anti-ErbB-2 (antibody-3) and anti-ErbB-3 (clone C-17). Immunoprecipitated samples were then blotted on nitrocellulose and detected with antibody against phosphotyrosine (4G10; Upstate Biotechnology). Blots were then stripped and immunoblotted with antibodies specific for each immunoprecipitated receptor, as described above. To determine Erk activation, protein from cells was stimulated with HRG for 10 minutes and then immunoblotted with antibody specific for phosphorylated Erk1/2. Blots were then stripped and reprobed to recognize total Erk1/2. A preliminary Western assay was performed at 15 seconds, 30 seconds, 5 minutes, 10 minutes, 30 minutes, 1 hour, and 2 hours after HRG stimulation of serum-starved cells. Ten minutes was chosen as the optimal time point for ERK activation (not shown).
Immunofluorescence Labeling
Cells overexpressing ErbB receptors were processed for immunofluorescence based on similar conditions described above.27 ErbB-2 (antibody-3, clone 3B5; Oncogene Science) antibody was used. After labeling, the cells were viewed in a Zeiss Axiophot fluorescent microscope (Carl Zeiss, Thornwood, NY) equipped with a 63× Plan Apochromat objective and selective filters. Images were acquired from a cooled CCD camera and displayed on a high-resolution monitor. Images were analyzed by Northern Eclipse Image analysis system (Carl Zeiss).
In Vivo Tumorigenic and Invasion Studies
In vivo studies were approved by the McGill Animal Care Committee (protocol no. 4101) and were conducted in accordance with institutional and Canadian federal guidelines. Female Scid mice were obtained from Charles River Laboratories (St. Zotique, PQ, Canada). For primary tumors, ErbB-FAK+/+ and ErbB-FAK−/− cells (1 × 106 cells) were implanted subcutaneously in the flank of female Scid mice. Parental cells expressing empty retroviral particles were used as controls. MDA 231-M cells expressing siRNA against FAK, Pyk2, or both were injected into the mammary fat pad of mice. Control cells expressed empty pSuper-retro puro vector. These cells maintained the same growth rate as the parental cells. Tumor volumes were measured every 2nd or 3rd day using a caliper, and tumor volumes were calculated as volume = π/6 (length × width2). For tumor invasion, the lungs were fixed in 10% Bouin’s fixative, and lung surface metastases were counted using a stereomicroscope. In all cases, eight mice were used per condition.
Tissue Microarray Construction and Immunohistochemistry
Tissue microarrays (TMAs) from breast cancer patients cohort were assembled using a manual tissue arrayer (Beecher Instruments, Silver Spring, MD) as previously described.28 All TMA cores were assigned a diagnosis (ie, benign, carcinoma in situ, invasive, and lymph node metastatic breast cancer) by the study pathologist. Tissue cores from circled areas were targeted for transfer to a recipient array paraffin block. Three to five replicate tissue cores on average were sampled from each patient for the tissue microarray. TMA cores (0.6 mm in diameter) were each spaced at 0.8 mm from core center to core center. Three TMA blocks with a total of 202 cores belonging to 75 patients were constructed. All blocks contained benign breast tissue, ductal in situ carcinoma (DCIS), invasive carcinoma, and lymph node metastatic tumors. After construction, 4-μm sections were cut and stained with H&E to verify initial diagnosis. The study was approved by the Jewish General Hospital institutional review board.
Immunohistochemistry was performed using the NexES immunostainer (Ventana Medical Systems, Tuscon, AZ). Immunostaining was performed on 4-μm silane-coated slides (Sigma, St. Louis, MO), dried overnight at 37°C, and then dewaxed, rehydrated, and boiled (microwave) in EDTA (pH 7.0) or citrate buffer (pH 6.0) for antigen retrieval. Slides were incubated for 32 minutes at 37°C using the primary antibodies. Primary antibodies [Pyk2 (1:25; 610548; BD Bioscience Transduction Laboratories); and FAK (1:25; clone 4.47; catalog no. 05-537; Biosource International)] were incubated for 40 minutes at room temperature using the NexES automated immunostainer (Ventana Medical Systems). Antibodies were first titrated using test arrays of several samples representing various tissue types and various antigen retrieval methods. The titration showing the best staining with no background in epithelial breast tissue was used. The automated Ventana system then uses an indirect biotin-avidin system with a universal biotinylated immunoglobulin secondary antibody. Diaminobenzidine was used as a chromogen. Slides were subsequently counterstained with hematoxylin before mounting. Staining procedures were performed according to the manufacturer’s recommendations. Negative controls were obtained by omitting the specific primary antibodies. Protein expression was assessed using a four-tiered system (0, negative; 1, weak; 2, moderate; and 3, high expression).
Statistical Analysis
To analyze tumor growth, unpaired Student’s t-test was used to compare significance between groups. Data were then analyzed using analysis of variance comparing all groups, with group as an independent variable and volume as a repeated measure as a function of time, and Dunnett’s and Bonferroni’s tests. All statistical tests were two-sided and were considered to be significant at the 0.05 level. For human tissue data, analyses were performed using a computer-based statistical package of Statistical Product and Service Solution version 5.1 for Windows (SPSS, Chicago, IL). Results are reported as means ± SE. Differences among the four groups (benign, DCIS, invasive, and lymph node metastases) were compared with one-way analysis of variance and the post hoc Tukey honestly significantly different test for multiple comparisons. A value of P < 0.05 was considered significant. Only significant correlation coefficients are reported.
Results
Pyk2 Is Overexpressed with FAK in Early-Stage Breast Cancer
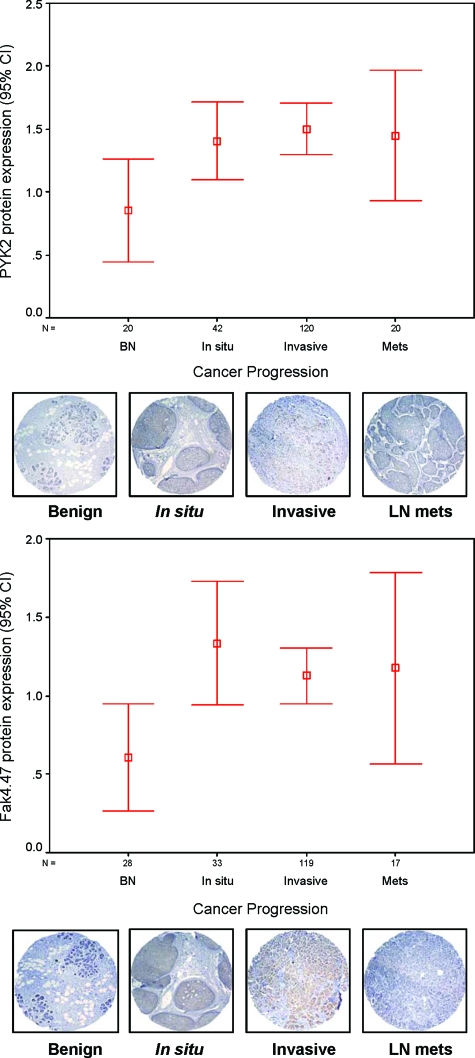
To examine the significance of Pyk2 expression compared with FAK for human breast disease, we performed immunohistochemical study on a high-density tissue microarray from a total of more than 200 breast cancer cases of various stages of progression. The pathology of each tissue microarray element was confirmed by an experienced pathologist and scored for staining (scale of 1 to 4) per cell type considered (epithelial, myoepithelial, and stromal). As shown in Figure 1, Pyk2 protein expression was significantly increased in the various stages of breast cancer progression compared with benign (P < 0.05). The average expression was highest in in situ cancer and invasive cancer compared with benign tissue (P < 0.05). Lymph node metastases showed a wider expression range compared with invasive breast cancer (Figure 1; see Supplemental Table S1 at http://ajp. amjpathol.org). Protein expression for FAK showed similar trends, with increased expression in in situ, invasive ,and a wider expression in lymph nodes metastasis versus benign breast tissue (Figure 1; see Supplemental Table S2 at http://ajp.amjpathol.org). Moreover, co-expression of Pyk2 and FAK was observed in more than 70% of cases (Table 1). In ErbB-2-positive breast cancer tissues (>grade 1), Pyk2/FAK co-overexpression was seen in 14 to 25% of cases (Table 1).
Figure 1.
Summary of Pyk2 and FAK expression in progressive breast cancer tissue array specimens. Pyk2 and FAK protein expression in a TMA of benign breast tissues versus carcinoma in situ, invasive, and lymph node metastasis, expressed in percentages. Confidence intervals (95%) show normalized mean protein intensity units of Pyk2 and FAK as determined by quantitative evaluation of immunohistochemistry; scale bars, ±SE. BN, benign; In situ, in situ breast carcinoma; Invasive, invasive breast cancer; Mets, lymph node breast metastasis. Error bars are representative cores of immunostaining of Pyk2 and FAK expression in various stages of breast cancer progression using TMA. Benign breast epithelium showed negative or very low intensity compared with different stages of breast cancer. *P < 0.05 in situ, invasive versus benign.
Table 1.
Percent Co-expression of FAK, Pyk2, and ErbB-2 in TMA Specimens with Grade >1
| Tissue type | FAK/ErbB-2 (%) | Pyk2/ErbB-2 (%) | FAK/Pyk2 (%) |
|---|---|---|---|
| Benign | 0/29 (0) | 0/23 (0) | 0/23 (0) |
| In situ | 6/33 (18.2) | 6/42 (14.3) | 24/33 (72.7) |
| Invasive | 23/119 (19.3) | 27/120 (22.5) | 76/119 (63.9) |
| Lymph node metastasis | 4/17 (23.5) | 5/20 (25) | 13/17 (76.5) |
Overexpression of ErbB-2/3 Receptors Rescues Tumorigenesis Defects of FAK-Deficient Cells
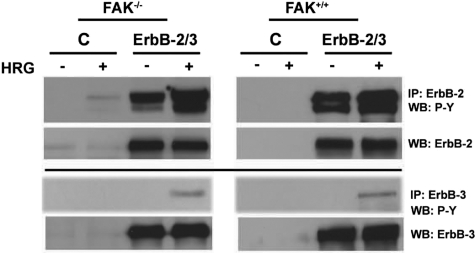
To investigate the role of Pyk2 versus FAK in ErbB-induced cancer progression, we initially used paired FAK-proficient and -deficient mouse embryonic fibroblasts transformed by overexpression of the ErbB-2/3 tyrosine kinase receptors. We have reported that co-expression of ErbB-2 and ErbB-3 in these cells promotes potent oncogenic transformation, tumor growth, and progression to metastases.26 ErbB-2 is an orphan receptor that is preferentially transactivated through receptor heterodimerization and transphosphorylation after ligand activation and in particular with the kinase-deficient ErbB-3.29,30 A polyclonal population of cells was stably transduced with a bicistronic retrovirus, expressing ErbB-2 or ErbB-3, and EGFP. Control cells expressed retroviral particles expressing EGFP alone. The phosphorylation status of ErbB-2/3 receptors in FAK−/− and FAK+/+ cells transduced with empty retroviral particles expressing EGFP alone (control) or particles encoding EGFP and ErbB-2/3 receptors was examined using immunoprecipitation assay coupled to Western blotting (Figure 2). As noted, control cells exhibit no detectable or weak labeling for ErbB-2/ErbB-3, whereas cells transduced with ErbB-2 or ErbB-3 retroviral particles overexpress the receptors. Overexpression of ErbB-2 receptor leads to receptor autophosphorylation, and activation of ErbB-3 with HRG further enhances ErbB-2 trans-phosphorylation. Moreover, enhanced basal phosphorylation was seen for the kinase-deficient ErbB-3 receptor as a result of cross-phosphorylation via ErbB-2 in agreement with our previous studies with these cells.26,27
Figure 2.
Overexpression and activation of ErbB-2 receptors in FAK-proficient and -deficient cells. Cells at 70% confluence were serum-starved for 24 hours and kept unstimulated [control (C)] or stimulated with 5 ng/ml HRG for 10 minutes. Cell lysates were immunoprecipitated with anti-ErbB, and the blots were probed using anti-phosphotyrosine antibody and reprobed with the corresponding ErbB-specific antibody. Note the constitutive autophosphorylation of ErbB-2 in ErbB-2 expressors and the enhanced ErbB-2 and ErbB-3 phosphorylation after stimulation with HRG.
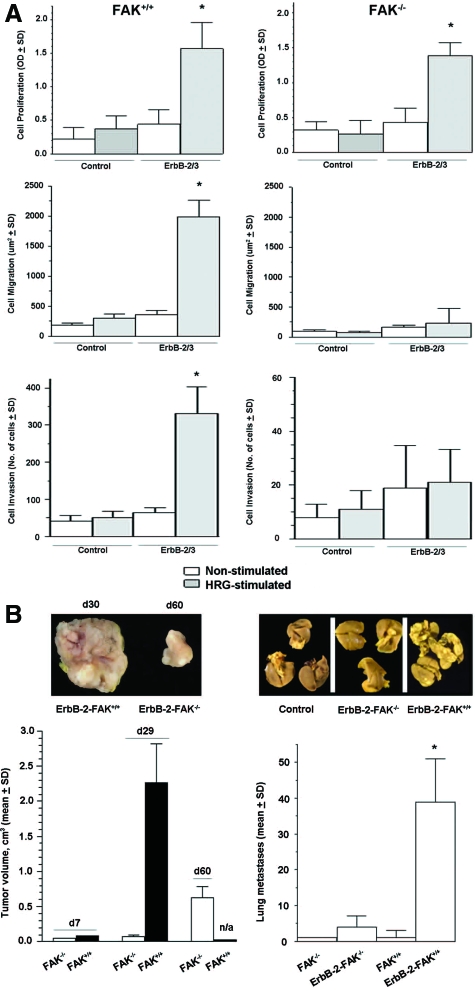
Control FAK−/− and FAK+/+ cells do not form colonies in soft agar and are unable to form tumors in vivo, but overexpression of ErbB receptors induces oncogenic transformation that is dependent on FAK.26 Here, we demonstrate that stimulation of serum-starved ErbB-2/3-FAK−/− cells with HRG significantly induced cell proliferation (P < 0.01) but had a minor effect on cell migration and cell invasion (Figure 3A). In ErbB-2/3-FAK+/+ cells, HRG significantly stimulated cell proliferation, cell migration, and cell invasion (P < 0.001). In contrast, none of these phenotypic features was induced by HRG in ErbB-2/3-negative FAK−/− or FAK+/+ cells. In a similar manner, when ErbB-transformed FAK−/− cells were implanted into the mammary fat pad or subcutaneously (not shown), tumors formed after a long latency period compared with ErbB-2/3-FAK+/+ cells (eg, tumor became palpable approximately 1 month after inoculation compared with 5 days for ErbB-2/3-FAK+/+). The latter were sacrificed on day 30 because of debilitating large tumors, whereas tumors induced by ErbB-2/3-FAK−/− cells became measurable only from day 40 and continued to grow, although slowly compared with ErbB-2/3-FAK+/+ cells (Figure 3B). In the same animals, lung metastases were detected at a later stage, and the incidence was significantly lower (P < 0.005) for ErbB-2/3-FAK−/− compared with ErbB-2/3-FAK+/+ (Figure 3B). These data support both FAK-dependent and -independent mechanisms for ErbB-induced tumor growth and invasion.
Figure 3.
ErbB-2/3 receptor overexpression partially rescues tumorigenesis defects of FAK−/− cells. A: Cell proliferation was measured using 3-(4,5-dimethylthiazo-2-yl)-2,5-diphenyltetrazolium bromide metabolic assay on exponentially growing cells after 48 hours of HRG stimulation and nonstimulated controls. Cell migration was determined by the phagokinetic track assay, where cells were allowed to migrate on gold colloidal particle-covered coverslips in the presence or absence of HRG. Total cell motility was quantified by processing the average area free of gold colloidal particles in square millimeters ± SD. Cell invasion was evaluated by culturing cells on Boyden chambers coated with matrigel and stimulating them for 48 hours using HRG as chemotactic agent in the lower chamber as described in Materials and Methods. The number of invading and hematoxylin stained cells were counted, and the percentage of invading cells was determined. Values are means ± SD from three independent experiments (P < 0.05, ErbB-2/3 versus control). *P < 0.01, ErbB-2/3 FAK−/− versus control FAK−/−; P < 0.001, ErbB-2/3 FAK+/+ versus control FAK+/+ B: Exponentially growing cells (1.0 × 106) were injected subcutaneously into the flank of SCID mice (left). Tumor growth was monitored over time as indicated in Materials and Methods. Each point represents the average of 8 animals ± SEM. Tumor volumes are not applicable for FAK+/+ at day 60 because animals were sacrificed on day 29 because of debilitating large tumors. Exponentially growing cells (1.0 × 106) were inoculated intravenously to SCID mice (right). Mice were sacrificed on day 42 after inoculation, and lungs were fixed in 10% Bouin’s fixative. Lung surface metastases were counted using a stereomicroscope. Each bar represents the average lung metastases (n = 8) ± SEM. *P < 0.005, ErbB-2/3 FAK−/− versus ErbB-2/3 FAK+/+.
Pyk2 Regulates ErbB-Induced Cell Proliferation in Part via Modulation of MAPK Activity but Has Minimal Impact on Cell Migration and Cell Invasion
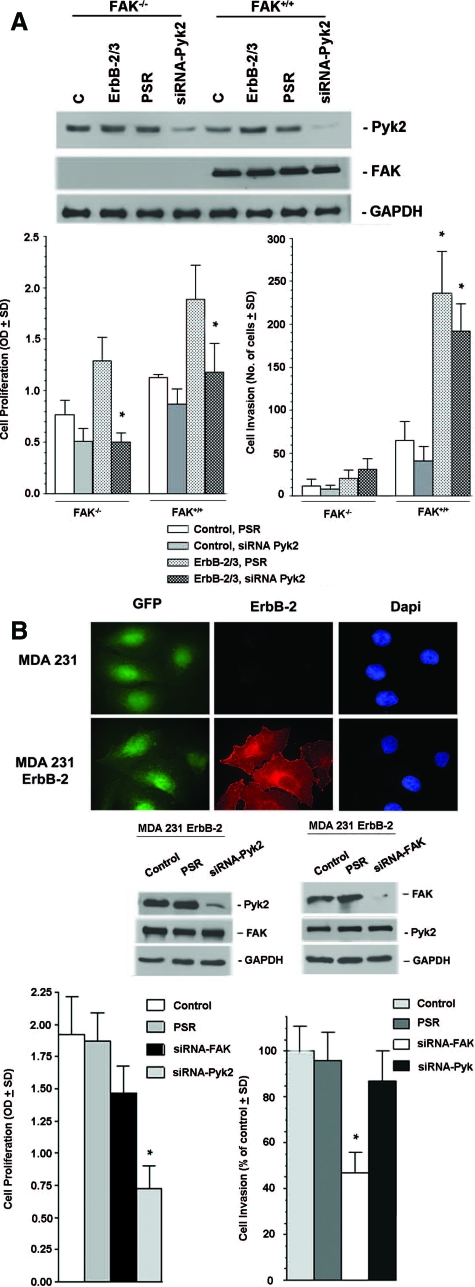
To establish the role of Pyk2 in ErbB-induced tumorigenesis versus invasiveness, we compared cell proliferation and cell invasion in ErbB-2/3-FAK−/− cells and their matched pairs where Pyk2 expression was down-regulated by siRNA (Figure 4A). Cell proliferation assay revealed that the proliferation of both serum-starved ErbB-2/3-FAK−/− and ErbB-2/3-FAK+/+ cells was increased in the presence of HRG, whereas cell invasion was only increased in ErbB-2/3-FAK+/+ cells (Figure 4A). Reduction of Pyk2 expression inhibited cell growth in both ErbB2/3-FAK−/− and ErbB2/3-FAK+/+ cells compared with matched control cells expressing pSUPER retro vector. Moreover, the anti-proliferative effect of Pyk2 siRNA was stronger in ErbB-2/3-FAK−/− compared with Erb-B2/3-FAK+/+ cells (P < 0.001). In contrast, only a small decrease in cell invasion was observed in Pyk2 siRNA-expressing ErbB-2/3 cells compared with controls (P < 0.01). Similar results were observed in MDA 231-M invasive breast cancer cells (Figure 4B), where down-regulation of Pyk2 expression results in a larger inhibition of cell proliferation than reduction of FAK expression compared with matched controls. However, MDA 231-M cells expressing Pyk2 siRNA demonstrate a very minor decrease in cell invasion compared with controls, whereas those expressing FAK siRNA show significant inhibitory effect on invasion.
Figure 4.
Impact of Pyk2 on ErbB-induced cell proliferation and cell invasion. A: A representative Western blot analysis on cells stably expressing Pyk2 siRNA showing more than 80% inhibition compared with control cells. Relative FAK expression is also shown. Shown are parental FAK−/− cells, ErbB-2/3 FAK−/− cells, ErbB-2/3 FAK−/− cells expressing PSR, and ErbB-2/3 FAK−/− cells expressing Pyk2 siRNA. Control and ErbB-2/3 FAK+/+ cells are shown as positive controls. Glyceraldehyde-3-phosphate dehydrogenase was used as internal control. Cell proliferation of control and ErbB-expressing FAK−/− cells and their matched pairs where Pyk2 was inhibited by siRNA (top). Cells were seeded on 96 wells, serum-starved for 24 hours and then stimulated with 5 ng/ml HRG. Cell proliferation was examined on day 5 using the 3-(4,5-dimethylthiazo-2-yl)-2,5-diphenyltetrazolium bromide assay as described in Materials and Methods (*P < 0.001, ErbB-2/3 FAK−/− siRNA Pyk2 versus ErbB-2/3 FAK+/+ siRNA Pyk2). Cell invasion was determined on the Boyden chamber of control and ErbB-expressing FAK−/− cells and their matched pair where Pyk2 was inhibited by siRNA (bottom). Cells were seeded on Boyden chambers coated with matrigel and stimulated for 48 hours using heregulin as chemotactic agent in the lower chamber as described in Materials and Methods. Values reported are means ± SD from at least three independent experiments (*P < 0.01, ErbB-2/3 FAK+/+ PSR versus ErbB-2/3 FAK+/+ siRNA Pyk2). B: Immunofluorescence staining showing effective overexpression of ErbB-2 in control and ErbB-2-transformed MDA 231 cells (top). Green fluorescent protein (GFP) and 4,6-diamidino-2-phenylindole (Dapi) staining are used as cellular controls. Knockdown of Pyk2 and FAK expression in MDA 231 ErbB-2 cells demonstrated in a representative Western blot analysis (middle). Cells stably expressed specific Pyk2 siRNA and FAK siRNA resulting in effective down-regulation of Pyk2 and FAK expression, respectively, compared with control cells and cells expressing PSR empty vector. Glyceraldehyde-3-phosphate dehydrogenase was used as internal control. Cell proliferation and cell invasion experiments were performed on control cells, cells expressing PSR, cells expressing FAK siRNA, and cells expressing Pyk2 siRNA (bottom). Cell proliferation was evaluated after 5 days using the 3-(4,5-dimethylthiazo-2-yl)-2,5-diphenyltetrazolium bromide metabolic assay. Cell invasion was determined using the Boyden chamber invasion assay and HRG as a chemoattractant for 48 hours as described in Materials and Methods. *P < 0.005, compared with PSR controls.
A major ErbB mitogenic mechanism occurs via activation of the MAPK-Erk pathway, and both FAK and Pyk2 are coupled to MAPK-Erk in part via interaction with Grb2 through Grb2 SH3-binding site.31 We therefore examined the impact of Pyk2 on ErbB-induced Erk activation. As shown in Figure 5A, overexpression of ErbB in FAK−/− cells slightly increased the basal Erk activity compared with parental control cells, as determined by phospho-Erk blotting; this is in agreement with our previous studies.26 Down-regulation of Pyk2 in ErbB-2/3 FAK−/− cells reduced the basal Erk activation (P < 0.005). This reduction was dependent on Pyk2 siRNA concentration and was not seen with cells expressing the empty siRNA plasmid (PSR) control (Figure 5B) (P < 0.01, for siRNA Pyk2 25 and 50 nmol/L compared with PSR-FAK−/− control cells; P < 0.005, siRNA Pyk2 100 nmol/L compared with PSR-FAK−/− control cells).
Figure 5.
Pyk2 regulates ErbB-induced activation of MAPKs in FAK-deficient cells. A: Cell extracts from parental FAK−/− cells, ErbB-2/3-FAK−/− cells, and their matched pairs expressing PSR or siRNA Pyk2. ErbB-2/3 FAK+/+ cells are shown as a control. Cells were serum-starved for 24 hours and then stimulated with HRG for 10 minutes. Total cell extracts were then collected and used for Western blot analysis to monitor the levels of phospho-Erk1/2. *P < 0.005, compared with control FAK−/−. Total Erk1/2 was used as internal control. Pyk2 expression is also shown to demonstrate efficiency of siRNA knockdown. B: Cell extracts from cells transiently transfected with increasing amount of siRNA Pyk2 were used to examine the levels of phospho-Erk following the same conditions as in A. Western blot analysis for Pyk2 expression shows efficiency of dose-dependent knockdown. Each experiment was repeated at least in triplicate. Error bars represent the average phosphor-Erk/Erk ± SD. *P < 0.01, siRNA Pyk2 25 nmol/L/50 nmol/L FAK−/− compared with PSR control; **P < 0.005, siRNA Pyk2 100 nmol/L FAK−/− compared with PSR control.
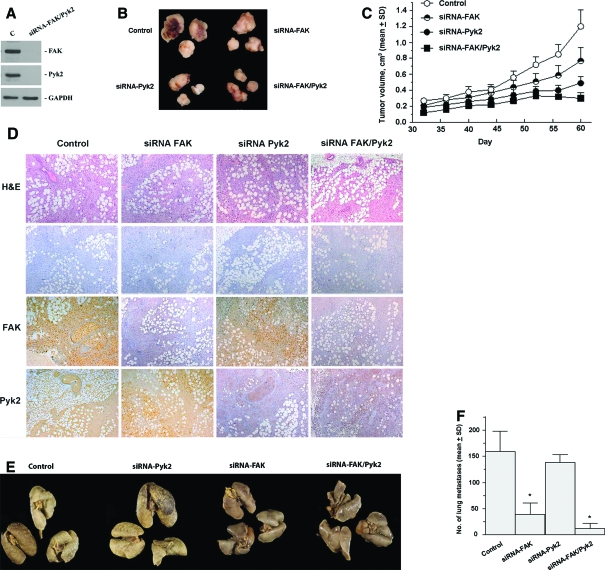
Dual Down-Regulation of Pyk2 and FAK Is Required for Prevention of Her2+-Induced Tumor Growth and Metastasis Formation
To examine the preclinical impact of Pyk2 down-regulation compared with FAK on breast cancer growth and metastasis formation, we investigated tumor growth and the incidence of lung metastases induced by the MDA 231-M breast cancer cells expressing siRNA against Pyk2, FAK (previously shown in Figure 4B), or Pyk2 and FAK (Figure 6A). Control cells expressed empty retroviral particles (PSR). All cells were implanted into the mouse mammary fat pad. Efficiency of FAK and Pyk2 down-regulation in tumor tissues was confirmed by immunohistochemical staining for FAK and Pyk2 proteins (Figure 6D). As shown in Figure 6, B, C, E, and F, down-regulation of Pyk2 has a great impact on tumor growth inhibition (P < 0.01) compared with lung metastasis formation. In contrast, FAK down-regulation was more potent in inhibiting lung metastasis (P < 0.001). Dual reduction of Pyk2 and FAK expression drastically inhibited tumor growth and lung metastases (P < 0.001). These data support dual inhibition of Pyk2 and FAK as a potential therapeutic approach for targeting invasive breast cancer.
Figure 6.
Inhibition of Pyk2 and FAK in the MDA 231-M breast cancer model leads to distinct inhibition of tumor growth versus lung metastases. A: A representative Western blot analysis of cells demonstrating dual inhibition of both FAK and Pyk2 through stable expression of FAK and Pyk2 siRNA. Cells expressing siRNA show more than 90% inhibition compared with control cells. B: Representative appearance of primary tumors after implantation of cells into the mammary fat pad of Scid mice. C: Tumor growth kinetics monitored over time as indicated in Materials and Methods. Each point represents the mean of eight mice ± SD. D: Immunohistochemistry on primary tumor tissue from control, siRNA FAK, siRNA Pyk2, and siRNA FAK/Pyk2. Control represents tumors induced by cells expressing PSR plasmid. Tumors were fixed in formalin, embedded in paraffin, and used for immunohistochemical analysis for FAK and Pyk2 or stained with H&E as described in Materials and Methods. −, negative control where primary antibodies were omitted. E: Incidence of lung metastases from the same group of animals. Representative lungs from mice inoculated with the various cell types. F: Quantification of lung surface metastases was determined after tissue fixation with Bouin. Results are mean surface lung metastases (n = 8) ± SD. *P < 0.001, compared with control.
Discussion
The homologous focal adhesion tyrosine kinases FAK and Pyk2 have been implicated in integrin signaling, cell survival, and cell migration and invasion, and both proteins are emerging as potential therapeutic targets for cancer. Elevated FAK levels were reported previously to occur in a subset of early-stage cancers such as DCIS of breast32,33 and advanced breast cancer and metastatic nodules.34,35,36,37 However, other studies failed to observe such a correlation between FAK levels and the invasive phenotype in breast cancer.38 In a similar manner, elevated Pyk2 expression was found to correlate with the progression of hepatocellular carcinoma,39 gastric carcinoma,40 and astrocytomas.41 In contrast, other studies reported rather decreased Pyk2 expression in advanced osteosacroma42 and in high-grade prostate cancer compared with normal epithelial prostate tissue and benign prostatic hyperplasia.23 These reports used small numbers of tissue cases and did not address the context of overexpression of the prognostic marker ErbB-2.
In the present study, both Pyk2 and FAK protein expression was found to be significantly increased in early and advanced breast cancer, including DCIS, compared with benign and normal breast tissues. Equally important, co-overexpression of Pyk2 with ErbB-2 was seen in early-stage DCIS and invasive breast cancer, whereas co-overexpression of FAK with ErbB2 was high in early-stage and invasive breast carcinomas. Therefore, the TMA data indicate that both FAK and Pyk2 are potential markers of aggressiveness, which, based on in vitro data, can function in a concerted manner during progression of primary breast cancer to invasive forms.
We demonstrate that down-regulation of Pyk2 in ErbB-transformed FAK-deficient cells using the siRNA approach prevented ErbB-induced cell proliferation but had a little impact on cell migration and invasion. In contrast, we reported earlier that restoration of FAK in ErbB-FAK-deficient cells can restore cell migration, cell invasion, and both tumorigenesis and invasiveness in vivo.26 This finding contrasts with a previous study in which overexpression of Pyk2 in MDA MB435 was observed,43 although the ErbB overexpression and signaling in relation to cancer cell invasion was not addressed. However, our results are in agreement with a study by Lim et al44 demonstrating a compensatory increase of Pyk2 expression on FAK inactivation and the ability of Pyk2 to rescue cell proliferation of FAK−/− mouse fibroblastic cells; this was found to occur via activation of distinct Rho signaling molecules. Moreover, in FAK-deficient bone marrow macrophage cells, the residual invasive capacity was reported to be independent from Pyk2.45
Inhibition of cell proliferation by Pyk2 siRNA in ErbB-expressing cells correlated with a reduction in MAPK activation after ErbB activation by heregulin. ErbB-mediated MAPK activation is a major oncogenic event that regulates cancer cell proliferation in ErbB-transformed cells.46,47,48,49,50,51,52,53 As well, MAPK-Erk signaling after FAK/Pyk2 activation has been reported to be necessary for cell proliferation.7,18,26,54,55,56,57,58,59 In FAK−/− cells overexpressing ErbB-2/3, we demonstrated that down-regulation of Pyk2 prevented MAPK-Erk activation. These data substantiate the hypothesis that Pyk2 can compensate for ErbB-induced cell proliferation and tumorigenesis in FAK−/− cells, at least in part, via enhanced activation of MAPK-Erk, a major signaling pathway required for the function of the Pyk2 homolog FAK in mitogenesis.7,26,60,61
Numerous studies demonstrate FAK overexpression in several types of carcinomas in vitro; and a recent study by Lahlou et al62 shows direct in vivo evidence of FAK implication in mammary tumor progression; targeted ablation of FAK resulted in decreased mammary tumor cell proliferation and blocked progression of hyperplasia to advanced carcinoma. These findings further stress the potential importance of targeting FAK to prevent primary breast cancer progression. A number of selective inhibitors of FAK have been identified and show promising results in preclinical models.63,64 At least one molecule that targets both FAK and Pyk2 is undergoing early clinical trials.65,66 To examine the relevance of cooperative signaling between Pyk2 and FAK for breast cancer progression, we compared therapeutic efficacy after down-regulation of Pyk2, FAK, and both Pyk-2 and FAK, using an orthotopic ErbB-2+ MDA 231 metastatic breast carcinoma model grown into the mammary fat pad. Our results indicate a strong impact of Pyk2 down-regulation on the growth of primary tumors but little impact on lung metastasis compared with FAK down-regulation. Dual reduction of both Pyk2 and FAK expression resulted in a strong therapeutic effect on preventing both tumor growth and lung metastases, highlighting the therapeutic potential of FAK and Pyk2 dual inhibitors.
In summary, this study supports distinct biological and therapeutic implications of FAK/Pyk2 in ErbB-2-positive breast cancer. Because ErbB-2 is a significant player in breast cancer progression, targeting ErbB downstream FAK/Pyk2 pathway is a promising therapeutic alternative and could have significant clinical implication for the management of ErbB-2-positive metastatic breast cancer, possibly Herceptin-refractory breast cancer.
Footnotes
Address reprint requests to Moulay A. Alaoui-Jamali, Lady Davis Institute for Medical Research and, Segal Comprehensive Cancer Center, Room E534, 3755 Chemin de la Côte-Ste-Catherine, Montreal, QC, Canada H3T 1E2; or Tarek A. Bismar, Department of Pathology and Laboratory Medicine, Rockyview General Hospital, Department of Medicine, University of Calgary, 7007 14 St. SW, Calgary, Canada T2V 1P9. E-mail: moulay.alaoui-jamali@mcgill.ca and tarek.bismar@cls.ab.ca.
Supported by the Canadian Institutes for Health Research and the Canadian Breast Cancer Research Alliance and by a CIHR Training Award (to Y.X.). T.A.B. is an FRSQ Clinical Scientist. M.A.A.-J. is a Fonds de la Recherche en Sante du Quebec (FRSQ) Scholar and a recipient of Dundi and Lyon Sachs Distinguished Scientist Award.
Supplemental material for this article can be found on http://ajp. amjpathol.org.
References
- Du QS, Ren XR, Xie Y, Wang Q, Mei L, Xiong WC. Inhibition of PYK2-induced actin cytoskeleton reorganization: PYK2 autophosphorylation and focal adhesion targeting by FAK. J Cell Sci. 2001;114:2977–2987. doi: 10.1242/jcs.114.16.2977. [DOI] [PubMed] [Google Scholar]
- Chen HC, Appeddu PA, Parsons JT, Hildebrand JD, Schaller MD, Guan JL. Interaction of focal adhesion kinase with cytoskeletal protein talin. J Biol Chem. 1995;270:16995–16999. doi: 10.1074/jbc.270.28.16995. [DOI] [PubMed] [Google Scholar]
- Vadlamudi RK, Adam L, Nguyen D, Santos M, Kumar R. Differential regulation of components of the focal adhesion complex by heregulin: role of phosphatase SHP-2. J Cell Physiol. 2002;190:189–199. doi: 10.1002/jcp.10054. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, Schlaepfer DD. FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol. 2000;2:249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- Andreev J, Simon JP, Sabatini DD, Kam J, Plowman G, Randazzo PA, Schlessinger J. Identification of a new Pyk2 target protein with Arf-GAP activity. Mol Cell Biol. 1999;19:2338–2350. doi: 10.1128/mcb.19.3.2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieg DJ, Ilic D, Jones KC, Damsky CH, Hunter T, Schlaepfer DD. Pyk2 and Src-family protein-tyrosine kinases compensate for the loss of FAK in fibronectin-stimulated signaling events but Pyk2 does not fully function to enhance FAK- cell migration. EMBO J. 1998;17:5933–5947. doi: 10.1093/emboj/17.20.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C, Xing Z, Bian ZC, Guo C, Akbay A, Warner L, Guan JL. Differential regulation of Pyk2 and focal adhesion kinase (FAK): the C-terminal domain of FAK confers response to cell adhesion. J Biol Chem. 1998;273:2384–2389. doi: 10.1074/jbc.273.4.2384. [DOI] [PubMed] [Google Scholar]
- Sabri A, Govindarajan G, Griffin TM, Byron KL, Samarel AM, Lucchesi PA. Calcium- and protein kinase C-dependent activation of the tyrosine kinase PYK2 by angiotensin II in vascular smooth muscle. Circ Res. 1998;83:841–851. doi: 10.1161/01.res.83.8.841. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Nagura K, Ishino M, Tobioka H, Kotani K, Sasaki T. Cloning and characterization of cell adhesion kinase beta, a novel protein-tyrosine kinase of the focal adhesion kinase subfamily. J Biol Chem. 1995;270:21206–21219. doi: 10.1074/jbc.270.36.21206. [DOI] [PubMed] [Google Scholar]
- Liu S, Thomas SM, Woodside DG, Rose DM, Kiosses WB, Pfaff M, Ginsberg MH. Binding of paxillin to alpha4 integrins modifies integrin-dependent biological responses. Nature. 1999;402:676–681. doi: 10.1038/45264. [DOI] [PubMed] [Google Scholar]
- Lev S, Hernandez J, Martinez R, Chen A, Plowman G, Schlessinger J. Identification of a novel family of targets of PYK2 related to Drosophila retinal degeneration B (rdgB) protein. Mol Cell Biol. 1999;19:2278–2288. doi: 10.1128/mcb.19.3.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuya M, Sasaki H, Aoto H, Mitaka T, Nagura K, Ohba T, Ishino M, Takahashi S, Suzuki R, Sasaki T. Cell adhesion kinase beta forms a complex with a new member: Hic-5, of proteins localized at focal adhesions. J Biol Chem. 1998;273:1003–1014. doi: 10.1074/jbc.273.2.1003. [DOI] [PubMed] [Google Scholar]
- Ren XR, Du QS, Huang YZ, Ao SZ, Mei L, Xiong WC. Regulation of CDC42 GTPase by proline-rich tyrosine kinase 2 interacting with PSGAP, a novel Pleckstrin homology and Src homology 3 domain containing rhoGAP protein. J Cell Biol. 2001;152:971–984. doi: 10.1083/jcb.152.5.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Parsons JT. Induction of apoptosis after expression of PYK2, a tyrosine kinase structurally related to focal adhesion kinase. J Cell Biol. 1997;139:529–539. doi: 10.1083/jcb.139.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch SM, Vuori K, Ruoslahti E, Chan-Hui PY. Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol. 1996;134:793–799. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Pestell R, Guan JL. Transcriptional activation of cyclin D1 promoter by FAK contributes to cell cycle progression. Mol Biol Cell. 2001;12:4066–4077. doi: 10.1091/mbc.12.12.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Zheng C, Guan J. Pyk2 and FAK differentially regulate progression of the cell cycle. J Cell Sci. 2000;113:3063–3072. doi: 10.1242/jcs.113.17.3063. [DOI] [PubMed] [Google Scholar]
- Lunn JA, Jacamo R, Rozengurt E. Preferential phosphorylation of focal adhesion kinase tyrosine 861 is critical for mediating an anti-apoptotic response to hyperosmotic stress. J Biol Chem. 2007;282:10370–10379. doi: 10.1074/jbc.M607780200. [DOI] [PubMed] [Google Scholar]
- Lipinski CA, Tran NL, Menashi E, Rohl C, Kloss J, Bay RC, Berens ME, Loftus JC. The tyrosine kinase pyk2 promotes migration and invasion of glioma cells. Neoplasia. 2005;7:435–445. doi: 10.1593/neo.04712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogenrieder T, Herlyn M. Axis of evil: molecular mechanisms of cancer metastasis. Oncogene. 2003;22:6524–6536. doi: 10.1038/sj.onc.1206757. [DOI] [PubMed] [Google Scholar]
- de Amicis F, Lanzino M, Kisslinger A, Cali G, Chieffi P, Ando S, Mancini FP, Tramontano D. Loss of proline-rich tyrosine kinase 2 function induces spreading and motility of epithelial prostate cells. J Cell Physiol. 2006;209:74–80. doi: 10.1002/jcp.20709. [DOI] [PubMed] [Google Scholar]
- Stanzione R, Picascia A, Chieffi P, Imbimbo C, Palmieri A, Mirone V, Staibano S, Franco R, De Rosa G, Schlessinger J, Tramontano D. Variations of proline-rich kinase Pyk2 expression correlate with prostate cancer progression. Lab Invest. 2001;81:51–59. doi: 10.1038/labinvest.3780211. [DOI] [PubMed] [Google Scholar]
- Yano H, Mazaki Y, Kurokawa K, Hanks SK, Matsuda M, Sabe H. Roles played by a subset of integrin signaling molecules in cadherin-based cell-cell adhesion. J Cell Biol. 2004;166:283–295. doi: 10.1083/jcb.200312013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano H, Uchida H, Iwasaki T, Mukai M, Akedo H, Nakamura K, Hashimoto S, Sabe H. Paxillin alpha and Crk-associated substrate exert opposing effects on cell migration and contact inhibition of growth through tyrosine phosphorylation. Proc Natl Acad Sci USA. 2000;97:9076–9081. doi: 10.1073/pnas.97.16.9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benlimame N, He Q, Jie S, Xiao D, Xu YJ, Loignon M, Schlaepfer DD, Alaoui-Jamali MA. FAK signaling is critical for ErbB-2/ErbB-3 receptor cooperation for oncogenic transformation and invasion. J Cell Biol. 2005;171:505–516. doi: 10.1083/jcb.200504124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen L, Benlimame N, Nie ZR, Xiao D, Wang T, Al Moustafa AE, Esumi H, Milanini J, Hynes NE, Pages G, Alaoui-Jamali MA. Differential regulation of tumor angiogenesis by distinct ErbB homo- and heterodimers. Mol Biol Cell. 2002;13:4029–4044. doi: 10.1091/mbc.E02-02-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin MA, Dunn R, Strawderman M, Pienta KJ. Tissue microarray sampling strategy for prostate cancer biomarker analysis. Am J Surg Pathol. 2002;26:312–319. doi: 10.1097/00000478-200203000-00004. [DOI] [PubMed] [Google Scholar]
- Alimandi M, Romano A, Curia MC, Muraro R, Fedi P, Aaronson SA, Di Fiore PP, Kraus MH. Cooperative signaling of ErbB3 and ErbB2 in neoplastic transformation and human mammary carcinomas. Oncogene. 1995;10:1813–1821. [PubMed] [Google Scholar]
- Pinkas-Kramarski R, Soussan L, Waterman H, Levkowitz G, Alroy I, Klapper L, Lavi S, Seger R, Ratzkin BJ, Sela M, Yarden Y. Diversification of Neu differentiation factor and epidermal growth factor signaling by combinatorial receptor interactions. EMBO J. 1996;15:2452–2467. [PMC free article] [PubMed] [Google Scholar]
- Cary LA, Guan JL. Focal adhesion kinase in integrin-mediated signaling. Front Biosci. 1999;4:D102–D113. doi: 10.2741/cary. [DOI] [PubMed] [Google Scholar]
- Oktay MH, Oktay K, Hamele-Bena D, Buyuk A, Koss LG. Focal adhesion kinase as a marker of malignant phenotype in breast and cervical carcinomas. Hum Pathol. 2003;34:240–245. doi: 10.1053/hupa.2003.40. [DOI] [PubMed] [Google Scholar]
- Watermann DO, Gabriel B, Jager M, Orlowska-Volk M, Hasenburg A, zur Hausen A, Gitsch G, Stickeler E. Specific induction of pp125 focal adhesion kinase in human breast cancer. Br J Cancer. 2005;93:694–698. doi: 10.1038/sj.bjc.6602744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agochiya M, Brunton VG, Owens DW, Parkinson EK, Paraskeva C, Keith WN, Frame MC. Increased dosage and amplification of the focal adhesion kinase gene in human cancer cells. Oncogene. 1999;18:5646–5653. doi: 10.1038/sj.onc.1202957. [DOI] [PubMed] [Google Scholar]
- Cance WG, Harris JE, Iacocca MV, Roche E, Yang X, Chang J, Simkins S, Xu L. Immunohistochemical analyses of focal adhesion kinase expression in benign and malignant human breast and colon tissues: correlation with preinvasive and invasive phenotypes. Clin Cancer Res. 2000;6:2417–2423. [PubMed] [Google Scholar]
- Judson PL, He X, Cance WG, Van Le L. Overexpression of focal adhesion kinase, a protein tyrosine kinase, in ovarian carcinoma. Cancer. 1999;86:1551–1556. doi: 10.1002/(sici)1097-0142(19991015)86:6<1551::aid-cncr23>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Owens LV, Xu L, Craven RJ, Dent GA, Weiner TM, Kornberg L, Liu ET, Cance WG. Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res. 1995;55:2752–2755. [PubMed] [Google Scholar]
- Madan R, Smolkin MB, Cocker R, Fayyad R, Oktay MH. Focal adhesion proteins as markers of malignant transformation and prognostic indicators in breast carcinoma. Hum Pathol. 2006;37:9–15. doi: 10.1016/j.humpath.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Sun CK, Ng KT, Sun BS, Ho JW, Lee TK, Ng I, Poon RT, Lo CM, Liu CL, Man K, Fan ST. The significance of proline-rich tyrosine kinase2 (Pyk2) on hepatocellular carcinoma progression and recurrence. Br J Cancer. 2007;97:50–57. doi: 10.1038/sj.bjc.6603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo HJ, Wang X, Liu YC, Wan YL, Yin HF, Li T, Zhu J. [Expression of proline-rich tyrosine kinase-2 (Pyk2) in gastric carcinoma and its significance]. Beijing Da Xue Xue Bao. 2005;37:261–264. [PubMed] [Google Scholar]
- Gutenberg A, Bruck W, Buchfelder M, Ludwig HC. Expression of tyrosine kinases FAK and Pyk2 in 331 human astrocytomas. Acta Neuropathol (Berl) 2004;108:224–230. doi: 10.1007/s00401-004-0886-3. [DOI] [PubMed] [Google Scholar]
- Schröder A, Delling G, Kaiser EA. [Expression analysis of protein tyrosine kinases of the FAK (focal adhesion kinase) family in osteosarcoma]. Pathologe. 2002;23:361–366. doi: 10.1007/s00292-002-0557-x. [DOI] [PubMed] [Google Scholar]
- Zrihan-Licht S, Fu Y, Settleman J, Schinkmann K, Shaw L, Keydar I, Avraham S, Avraham H. RAFTK/Pyk2 tyrosine kinase mediates the association of p190 RhoGAP with RasGAP and is involved in breast cancer cell invasion. Oncogene. 2000;19:1318–1328. doi: 10.1038/sj.onc.1203422. [DOI] [PubMed] [Google Scholar]
- Lim Y, Lim ST, Tomar A, Gardel M, Bernard-Trifilo JA, Chen XL, Uryu SA, Canete-Soler R, Zhai J, Lin H, Schlaepfer WW, Nalbant P, Bokoch G, Ilic D, Waterman-Storer C, Schlaepfer DD. PyK2 and FAK connections to p190Rho guanine nucleotide exchange factor regulate RhoA activity, focal adhesion formation, and cell motility. J Cell Biol. 2008;180:187–203. doi: 10.1083/jcb.200708194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen KA, Pixley FJ, Thomas KS, Vicente-Manzanares M, Ray BJ, Horwitz AF, Parsons JT, Beggs HE, Stanley ER, Bouton AH. Regulation of lamellipodial persistence, adhesion turnover, and motility in macrophages by focal adhesion kinase. J Cell Biol. 2007;179:1275–1287. doi: 10.1083/jcb.200708093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daub H, Weiss FU, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature. 1996;379:557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- Hendriks BS, Orr G, Wells A, Wiley HS, Lauffenburger DA. Parsing ERK activation reveals quantitatively equivalent contributions from epidermal growth factor receptor and HER2 in human mammary epithelial cells. J Biol Chem. 2005;280:6157–6169. doi: 10.1074/jbc.M410491200. [DOI] [PubMed] [Google Scholar]
- Jackson JG, St Clair P, Sliwkowski MX, Brattain MG. Blockade of epidermal growth factor- or heregulin-dependent ErbB2 activation with the anti-ErbB2 monoclonal antibody 2C4 has divergent downstream signaling and growth effects. Cancer Res. 2004;64:2601–2609. doi: 10.1158/0008-5472.can-03-3106. [DOI] [PubMed] [Google Scholar]
- Kumagai T, Davis JG, Horie T, O'Rourke DM, Greene MI. The role of distinct p185neu extracellular subdomains for dimerization with the epidermal growth factor (EGF) receptor and EGF-mediated signaling. Proc Natl Acad Sci USA. 2001;98:5526–5531. doi: 10.1073/pnas.071060598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkas-Kramarski R, Shelly M, Guarino BC, Wang LM, Lyass L, Alroy I, Alimandi M, Kuo A, Moyer JD, Lavi S, Eisenstein M, Ratzkin BJ, Seger R, Bacus SS, Pierce JH, Andrews GC, Yarden Y. ErbB tyrosine kinases and the two neuregulin families constitute a ligand-receptor network. Mol Cell Biol. 1998;18:6090–6101. doi: 10.1128/mcb.18.10.6090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, O'Rourke DM, Fei Z, Zhang HT, Kao CC, Greene MI. Domain-specific interactions between the p185(neu) and epidermal growth factor receptor kinases determine differential signaling outcomes. J Biol Chem. 1999;274:574–583. doi: 10.1074/jbc.274.2.574. [DOI] [PubMed] [Google Scholar]
- Seton-Rogers SE, Lu Y, Hines LM, Koundinya M, LaBaer J, Muthuswamy SK, Brugge JS. Cooperation of the ErbB2 receptor and transforming growth factor beta in induction of migration and invasion in mammary epithelial cells. Proc Natl Acad Sci USA. 2004;101:1257–1262. doi: 10.1073/pnas.0308090100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikhomirov O, Carpenter G. Ligand-induced, p38-dependent apoptosis in cells expressing high levels of epidermal growth factor receptor and ErbB-2. J Biol Chem. 2004;279:12988–12996. doi: 10.1074/jbc.M311655200. [DOI] [PubMed] [Google Scholar]
- Almeida EA, Ilic D, Han Q, Hauck CR, Jin F, Kawakatsu H, Schlaepfer DD, Damsky CH. Matrix survival signaling: from fibronectin via focal adhesion kinase to c-Jun NH(2)-terminal kinase. J Cell Biol. 2000;149:741–754. doi: 10.1083/jcb.149.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck CR, Hsia DA, Schlaepfer DD. Focal adhesion kinase facilitates platelet-derived growth factor-BB-stimulated ERK2 activation required for chemotaxis migration of vascular smooth muscle cells. J Biol Chem. 2000;275:41092–41099. doi: 10.1074/jbc.M005450200. [DOI] [PubMed] [Google Scholar]
- Klingbeil CK, Hauck CR, Hsia DA, Jones KC, Reider SR, Schlaepfer DD. Targeting Pyk2 to beta 1-integrin-containing focal contacts rescues fibronectin-stimulated signaling and haptotactic motility defects of focal adhesion kinase-null cells. J Cell Biol. 2001;152:97–110. doi: 10.1083/jcb.152.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawhney RS, Cookson MM, Omar Y, Hauser J, Brattain MG. Integrin alpha2-mediated ERK and calpain activation play a critical role in cell adhesion and motility via focal adhesion kinase signaling: identification of a novel signaling pathway. J Biol Chem. 2006;281:8497–8510. doi: 10.1074/jbc.M600787200. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Hou S, Lim ST, Tomar A, Yu H, Lim Y, Hanson DA, Uryu SA, Molina J, Mitra SK. Tumor necrosis factor-alpha stimulates focal adhesion kinase activity required for mitogen-activated kinase-associated interleukin 6 expression. J Biol Chem. 2007;282:17450–17459. doi: 10.1074/jbc.M610672200. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Jones KC, Hunter T. Multiple Grb2-mediated integrin-stimulated signaling pathways to ERK2/mitogen-activated protein kinase: summation of both c-Src- and focal adhesion kinase-initiated tyrosine phosphorylation events. Mol Cell Biol. 1998;18:2571–2585. doi: 10.1128/mcb.18.5.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun P, Baron V, Hauck CR, Schlaepfer DD, Van Obberghen E. Cell adhesion and focal adhesion kinase regulate insulin receptor substrate-1 expression. J Biol Chem. 2000;275:38371–38377. doi: 10.1074/jbc.M006162200. [DOI] [PubMed] [Google Scholar]
- Renshaw MW, Price LS, Schwartz MA. Focal adhesion kinase mediates the integrin signaling requirement for growth factor activation of MAP kinase. J Cell Biol. 1999;147:611–618. doi: 10.1083/jcb.147.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahlou H, Sanguin-Gendreau V, Zuo D, Cardiff RD, McLean GW, Frame MC, Muller WJ. Mammary epithelial-specific disruption of the focal adhesion kinase blocks mammary tumor progression. Proc Natl Acad Sci USA. 2007;104:20302–20307. doi: 10.1073/pnas.0710091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack-Davis JK, Martin KH, Tilghman RW, Iwanicki M, Ung EJ, Autry C, Luzzio MJ, Cooper B, Kath JC, Roberts WG, Parsons JT. Cellular characterization of a novel focal adhesion kinase inhibitor. J Biol Chem. 2007;282:14845–14852. doi: 10.1074/jbc.M606695200. [DOI] [PubMed] [Google Scholar]
- Halder J, Lin YG, Merritt WM, Spannuth WA, Nick AM, Honda T, Kamat AA, Han LY, Kim TJ, Lu C, Tari AM, Bornmann W, Fernandez A, Lopez-Berestein G, Sood AK. Therapeutic efficacy of a novel focal adhesion kinase inhibitor TAE226 in ovarian carcinoma. Cancer Res. 2007;67:10976–10983. doi: 10.1158/0008-5472.CAN-07-2667. [DOI] [PubMed] [Google Scholar]
- Roberts WG, Ung E, Whalen P, Cooper B, Hulford C, Autry C, Richter D, Emerson E, Lin J, Kath J, Coleman K, Yao L, Martinez-Alsina L, Lorenzen M, Berliner M, Luzzio M, Patel N, Schmitt E, LaGreca S, Jani J, Wessel M, Marr E, Griffor M, Vajdos F. Antitumor activity and pharmacology of a selective focal adhesion kinase inhibitor, PF-562271. Cancer Res. 2008;68:1935–1944. doi: 10.1158/0008-5472.CAN-07-5155. [DOI] [PubMed] [Google Scholar]
- Siu LL, Burns HA, Mileshkin L, Camidge DR, Rischin D, Chen EX, Jones S, Yin D, Fingert H. Phase 1 study of a focal adhesion kinase (FAK) inhibitor PF-00562271 in patients (pts) with advanced solid tumors. ASCO Annual Meeting Proceedings Part I. J Clin Oncol. 2007 Abstract 3527. [Google Scholar]