Abstract
Summary: In 1984, children presented to the emergency department of a hospital in the small town of Promissão, São Paulo State, Brazil, with an acute febrile illness that rapidly progressed to death. Local clinicians and public health officials recognized that these children had an unusual illness, which led to outbreak investigations conducted by Brazilian health officials in collaboration with the U.S. Centers for Disease Control and Prevention. The studies that followed are an excellent example of the coordinated and parallel studies that are used to investigate outbreaks of a new disease, which became known as Brazilian purpuric fever (BPF). In the first outbreak investigation, a case-control study confirmed an association between BPF and antecedent conjunctivitis but the etiology of the disease could not be determined. In a subsequent outbreak, children with BPF were found to have bacteremia caused by Haemophilus influenzae biogroup aegyptius (H. aegyptius), an organism previously known mainly to cause self-limited purulent conjunctivitis. Molecular characterization of blood and other isolates demonstrated the clonal nature of the H. aegyptius strains that caused BPF, which were genetically distant from the diverse strains that cause only conjunctivitis. This led to an intense effort to identify the factors causing the unusual invasiveness of the BPF clone, which has yet to definitively identify the virulence factor or factors involved. After a series of outbreaks and sporadic cases through 1993, no additional cases of BPF have been reported.
The average time from the kids arriving in the hospital until death was 2 hours.
David Fleming, Epidemic Intelligence Service Officer, Centers for Disease Control and Prevention
He got more purple … . I was in a room with him and the doctor asked me to leave so I wouldn't see. And right after the doctor came out and said he had died. It was really fast. The disease didn't even last 24 hours. It was really fast.
Mother of a child with BPF
—What's Killing the Children?, NOVA documentary, WGBH-TV, Boston, MA, 18 December 1990.
INTRODUCTION
The sudden appearance of a new and fatal pediatric disease in Brazil in the 1980s alarmed Brazilian clinicians and public health officials and led to a series of collaborative investigations between the São Paulo Secretariat of Health and the U.S. Centers for Disease Control and Prevention (CDC). As a result of these investigations, a new syndrome characterized by epidemic purpura fulminans preceded by purulent conjunctivitis was identified. This new disease was eventually found to be caused by Haemophilus influenzae biogroup aegyptius (H. aegyptius) bacteremia and was descriptively named Brazilian purpuric fever (BPF) or, in Portuguese, febre purpúrica brasileira. Extensive laboratory investigations over more than 20 years have attempted to identify the virulence factors for the H. aegyptius strains causing BPF because this organism had previously been known to cause mostly uncomplicated purulent conjunctivitis. The purpose of this paper is to summarize what has been learned about the epidemiology and microbiology of BPF during the more than 20 years since the first reported outbreaks were identified.
CHRONOLOGY OF BPF
In 1984, Claire Broome, then the Chief of the Meningitis and Special Pathogens Branch at the CDC, received a telephone call from Emil Gotschlich of Rockefeller University about a possible variant meningococcal outbreak. Gotschlich had been contacted by a colleague in Brazil about an unusual cluster of cases of a highly fatal disease among children in the small town of Promissão, São Paulo State. These discussions resulted in an invitation to the CDC from the Brazilian Ministry of Health to assist in the investigation of this outbreak.
Promissão and Londrina Outbreaks
During October through December 1984, 10 children aged 3 months to 10 years presented to a hospital over a 2-month period in the small, agricultural town of Promissão, São Paulo State, Brazil, with fatal purpura fulminans following purulent conjunctivitis (9, 16). Attempts to isolate the etiologic agent, serologic tests, and extensive autopsies failed to identify the etiology of the disease. Eleven less severe cases occurred during the same time in Promissão, presenting with fever, vomiting, and, for two patients, limited purpuric lesions. A number of different hypotheses for the outbreak were considered, including adverse reaction to a drug or vaccine, exposure to an environmental toxin, or transmission of infection from a vector found in the nearby sugarcane fields. Other hypotheses included meningococcal disease, a viral hemorrhagic fever virus, and dengue hemorrhage fever, but the presentation was atypical (41).
BPF was presumed to be infectious and there were clues to the possible role of H. aegyptius, including ongoing outbreaks of H. aegyptius conjunctivitis in Promissão during the BPF outbreak and the isolation of the organism from an aseptically obtained petechial scraping from a child with BPF (9, 25). However, the etiology of BPF could not be definitively determined during the investigation of the Promissão outbreak.
During the Promissão investigation, officials were made aware of an outbreak consisting of 13 children with a similar disease that had occurred during the period between February and July of 1984 in Londrina, in the adjacent state of Paraná (Table 1). These cases were initially thought to be part of a concomitant meningococcal disease outbreak (4, 25). However, the lack of meningitis and the occurrence of preceding conjunctivitis in many cases led to the recognition that this outbreak was also BPF.
TABLE 1.
Characteristics of BPF outbreaks, Brazil, 1984 to 1991
| Brazilian state | Town(s) | Total Population | Time period | Age range | No. of cases | Incidence in children <10 yr old | Reference(s) |
|---|---|---|---|---|---|---|---|
| Paraná | Londrina | 390,100 | February-July 1984 | 6 mo-10 yr | 13 | 0.2 per 1,000 | 9 |
| São Paulo | Promissão | 20,430 | October-December 1984 | 3 mo-8 yr | 10 | 2.3 per 1,000 | 9 |
| São Paulo | Serrana | 18,552 | March-June 1986 | 1-6 yr | 11 | 2.4 per 1,000 | 10 |
| Mato Grosso | Cuiabá, Alto Taquari, Várzea Grande, Boa Ventura, Nortelândia, Lucas do Rio Verde | 618,748 | December 1989-April 1990 | 5 mo-9 yr | 10 | 6 per 100,000 | 8, 15 |
| Mato Grosso do Sul | Campo Grande, Maracaju, Nivarai | 579,795 | May 1991 | <10 yr | 19 | 0.14 per 1,000 | 53 |
Six sporadic cases also occurred in Promissão and Londrina separated in time by 1 to 6 months from the outbreaks (9). Nine other children meeting the BPF case definition were identified between October 1984 and February 1985 in six towns that were within 60 miles of Promissão.
Serrana Outbreak
As a result of the Promissão and Londrina investigations, investigators at Instituto Adolfo Lutz, the reference laboratory of the São Paulo Secretariat of Health, prepared blood culture media, consisting of tryptic soy broth with sodium polyanethol sulfonate, to be dispatched in preparation for a possible subsequent BPF outbreak. The next outbreak, involving 11 children, occurred during March through May 1986 in Serrana, another small town in São Paulo State (Table 1) (10). Most of the cases were admitted to a teaching hospital of the Universidade de São Paulo in the nearby city of Ribeirão Preto, which facilitated the laboratory investigation of cases. Cultures of normally sterile body fluids were positive for a clone of H. aegyptius (which subsequently became known as the BPF clone) in 10 of the Serrana cases, confirming this organism as the etiology of BPF.
Sporadic Cases during 1987 and 1988
Based on these outbreaks, BPF became reportable in the São Paulo State notifiable disease surveillance system. During 1987 and 1989, 29 BPF cases were reported from four cities in São Paulo State: Nova Granada, Serra Azul, Serrana, and Votuporanga (22). Of these, two were definite cases (one with the BPF clone isolated from blood and another that met the case definition) and nine were possible cases (defined below), including one with the BPF clone isolated from a brother and another with a BPF clone strain isolated from a sister.
Mato Grosso
During late 1989 and early 1990, an outbreak occurred in multiple small cities in Mato Grosso State, as well as in the capital of Cuiabá (8, 15) (Table 1). Of a total of 26 children with acute illness, 10 cases from six geographically separated cities were confirmed as definite (3 children) or possible cases (7 children). Of these 10 cases, 6 died and one underwent autoamputation of portions of toes and fingers as a complication of septic shock. Although the 16 other cases could not be confirmed, some of these were believed to be BPF because no other etiology of the illness was identified and the BPF clone was isolated from the conjunctivae of two of the case children.
Mato Grosso do Sul
In May of 1991, 19 BPF cases were reported in two small cities, Maracaju and Nivarai in the state of Mato Grosso do Sul, as well as in the capital of Campo Grande, where one case was confirmed with the isolation of H. aegyptius from blood (1, 53, 57). In Maracaju, investigators noted a large epidemic of purulent conjunctivitis that rapidly spread throughout the city and a high density of Hippelates flies in the conjunctival secretions of children with conjunctivitis, which were believed to have contributed to the dissemination of the conjunctivitis epidemic (Fig. 1).
FIG. 1.
Hippelates flies in face and eyes of a child during a conjunctivitis epidemic in Maracaju, Mato Grosso do Sul State, which also had concomitant BPF cases. (Photo courtesy of Maria Cristina Brandileone and Maria Lúcia Tondella.)
BPF Cases Reported outside Brazil
The first report of invasive disease caused by H. aegyptius was in 1986. The case occurred in a Bedouin pastor in Israel with a history of aortic stenosis who presented with complicated H. aegyptius aortic valve endocarditis and died despite valve replacement and antimicrobial therapy (47). There was neither a reported rash nor a reported history of antecedent conjunctivitis in this case. In 1986, two cases consistent with BPF were reported from Australia (39, 67). One case was a 3-year-old boy from Alice Springs with an illness consistent with BPF, including antecedent conjunctivitis 2 days previously, and H. aegyptius bacteremia (39). A similar case with H. aegyptius bacteremia was reported from western Australia (67). In that patient, the preceding conjunctivitis was found to be caused by Haemophilus sp. but the conjunctival isolate was not identified to the species level. Extensive hemorrhagic rashes were reported for both Australian cases.
The only case consistent with BPF reported in the United States occurred in 1996 (60) (James Hadler, personal communication). The case involved a 17-month-old child with upper respiratory infection and purulent conjunctivitis followed about 4 weeks later by fever, rigors, icterus, and a lower extremity morbilliform rash. A sibling and a cousin also had a recent history of conjunctivitis. Blood cultures were positive for H. aegyptius. A peripheral blood sample had a high Epstein-Barr virus (EBV) load, in situ hybridization of several tissues revealed the presence of EBV, and there was serologic evidence of EBV infection. Despite antibiotic therapy, the child died and an autopsy revealed massive hepatic and bone marrow necrosis. Since the patient appeared to have died from overwhelming EBV infection, the relative roles of H. aegyptius and EBV in this case are not clear. In addition, the BPF case definition requires the absence of serologic evidence for known pathogens other than H. aegyptius.
CLINICAL FEATURES OF BPF
The first recognized cases of BPF in Londrina and Promissão were initially confused with meningococcemia because of the similar clinical picture and the presence of a concomitant outbreak of meningococcal disease (4). However, the occurrence only in children, the lack of meningitis cases, the extraordinarily high case fatality rate, and the lack of identification of Neisseria meningitidis or other agents that cause purpura fulminans suggested the occurrence of a new disease.
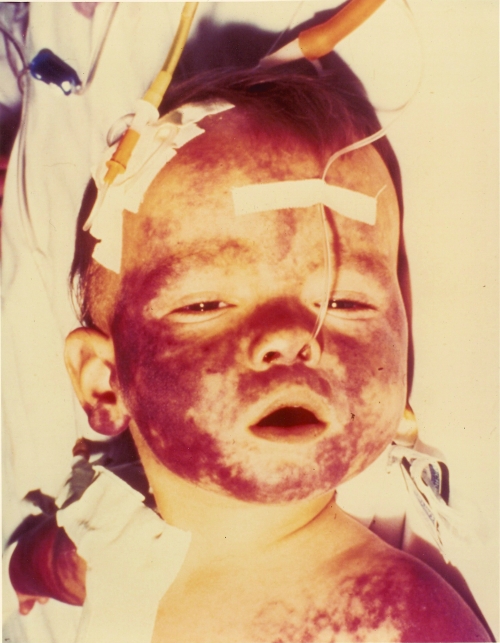
The clinical presentation of BPF is characterized by high fever; hemorrhagic skin lesions; abdominal pain, nausea, and vomiting; septic shock; and death in an otherwise healthy child, consistent with the syndrome of purpura fulminans (Fig. 2). The most salient features on physical examination were purpuric skins lesions affecting primarily the face and extremities; cyanosis; and rapid necrosis of soft tissues, particularly of the hands, feet, nose, and ears (41).
FIG. 2.
Extensive purpura in a child with BPF. (Reproduced from reference 9 with permission of the publisher.)
The conjunctivitis that preceded BPF was purulent and not mucoid or hemorrhagic. In most cases, the conjunctivitis had resolved before the onset of BPF. The median intervals between the onset of conjunctivitis and BPF in the Promissão and Serrana outbreaks were 7 and 16 days, respectively (Table 2).
TABLE 2.
Association of BPF with preceding conjunctivitisa
| Location | No. BPF patients with conjunctivitis/total no. with BPF (%) | No. controls with conjunctivitis/total no. (%) | P value | Mean (range) interval (days)b |
|---|---|---|---|---|
| Londrina | 13/15 (87) | 1/8 (12) | 0.0001 | NA |
| Promissão | 18/20 (90) | 9/20 (45) | 0.006 | 7 (1-45) |
| Serrana | 10/11 (91) | 6/20 (30) | 0.002 | 16 (1-60) |
Adapted from reference 22.
Interval between onset of conjunctivitis and onset of fever in patients with BPF. NA, not available.
Before the etiology of BPF was known, determining the spectrum of illness was limited by the clinical case definition which, because of the need for specificity, included only children with severe illness. However, once the etiology of BPF was determined to be H. aegyptius bacteremia, a clearer picture of the spectrum of illness emerged. In the Serrana outbreak, only half of the children positive by blood culture met the clinical case definition, which underscored the definition's lack of sensitivity. Among the five children with H. aegyptius bacteremia who did not meet the case definition, illness ranged from mild fever alone to fever with systemic symptoms and nonpetechial rash. Laboratory parameters of BPF cases from 1984 and 1985 are shown in Table 3.
TABLE 3.
Summary of clinical laboratory evaluations of children with BPF in Brazil in 1984 and 1985a
| Laboratory test | No. of BPF patients tested | Mean; median (range) |
|---|---|---|
| Cerebrospinal fluid | ||
| Leukocytes (no. per μl) | 22 | 26; 19 (1-57) |
| Polymorphonuclear leukocytes (no. per μl) | 18 | 6; 5 (0-19) |
| Lymphocytes (no. per μl) | 18 | 18; 19 (2-38) |
| Glucose (mg/dl) | 21 | 54; 56 (21-108) |
| Protein (mg/dl) | 22 | 28; 28 (12-61) |
| Blood | ||
| Hemoglobin (mg/dl) | 7 | 11.1; 11.4 (9.1-12.7) |
| Hematocrit (%) | 17 | 35.9; 35.8 (29.0-48.0) |
| Leukocytes (103/μl) | 19 | 13.2; 12.0 (3.5-34.0) |
| Bands (%) | 14 | 14; 13 (2-32) |
| Polymorphonuclear leukocytes (%) | 16 | 56; 55 (21-90) |
| Lymphocytes (%) | 12 | 29; 31 (1-57) |
| Platelets (103/μl) | 14 | 77; 50 (12-247) |
| Fibrinogen (mg/dl) | 10 | 312; 310 (30-573) |
| Prothrombin time (s) | 11 | 36; 27 (16-98) |
Adapted from reference 9 with permission of the publisher.
Histopathologies from both outbreak and sporadic cases were consistent with purpura fulminans and characterized by diffuse hemorrhage, including of the skin, lungs, and adrenal glands; intravascular microthrombi; and tissue necrosis (3, 5, 9). There was substantial central nervous system edema without meningitis or neuronal or glial cell changes. There was no evidence for vasculitis.
The pathogenesis of BPF is not well established but presumably patients become pharyngeal or conjunctival carriers of H. aegyptius, with subsequent seeding of the bloodstream. This hypothesis is supported by the isolation of H. aegyptius from both the conjunctiva and oropharynx of two Serrana BPF cases with H. aegyptius bacteremia (10).
The BPF clone was susceptible to many commonly used antibiotics, including ampicillin, cefuroxime, cefotaxime, rifampin, and chloramphenicol (11). In the Serrana outbreak, the initiation of parenteral antibiotics before the development of hemorrhagic skins lesions was associated with better survival (10). In the absence of treatment or in the presence of septic shock, the prognosis of BPF is poor. Of the 276 outbreak and sporadic cases reported as part of BPF surveillance from 1984 to 1993, 106 (38%) resulted in death. However, because some of these cases may not have had BPF, this may represent an underestimate of the true case fatality rate. Of 38 children with well-documented BPF by the end of 1985, 27 died, for a case fatality rate of 71% (9). Permanent sequelae, including soft tissue necrosis and amputation of the extremities, occurred in the Serrana and Mato Grosso outbreaks but the precise frequency of these events is not known (8, 15, 17).
For the eradication of conjunctival carriage H. aegyptius, oral rifampin has been shown to be more effective (100% eradication at 21 days) than topical chloramphenicol (50%) among 28 children with BPF clone conjunctivitis (45). This study suggests that the use of rifampin for treatment of BPF clone conjunctivitis could potentially prevent the development of BPF.
EPIDEMIOLOGIC FEATURES OF BPF
The investigation of BPF outbreaks required the development of a case definition that could be used to classify cases and study the disease. Because there initially was no specific diagnostic test, the first BPF case definition was based on clinical criteria (Table 4). Once the etiology of BPF was determined to be H. aegyptius bacteremia, the definition of a definite BPF case was modified to incorporate this new information (Table 5). A possible case was defined by fever, hemorrhagic skin lesions, and a recent history of conjunctivitis (22).
TABLE 4.
Original clinical case definition for BPFa
| Definition |
|---|
| (i) Acute illness in a child aged between 3 mo and 10 yr that is characterized by: |
| (a) Fever of ≥38.5°C |
| (b) Abdominal pain and/or vomiting |
| (c) Development of petechiae or purpura within ≤72 h of onset of fever |
| (d) No evidence of meningitis |
| (ii) History of conjunctivitis ≤15 days before onset of fever |
| (iii) One (or both) of the following tests negative for N. meningitidis: |
| (a) Blood cultures taken before antibiotic administration |
| (b) Serum or urine antigen detection |
| (iv) In fatal cases, postmortem examination negative for microorganisms |
| (v) Other laboratory data, if obtained: |
| (a) Cerebrospinal fluid with ≤100 leukocytes/μl and negative by culture or antigen detection for pathogenic bacteria |
| (b) Blood cultures negative for known pathogenic bacteria |
| (c) Negative serologic studies for known pathogens |
Adapted from reference 9 with permission of the publisher.
TABLE 5.
Revised case definition to reflect the knowledge that the etiology of BPF was H. aegyptius bacteremia fevera
| Factor |
|---|
| (i) Febrile illness in a child with isolation of H. aegyptius from a normally sterile body site (e.g., blood or CSFb) or |
| (ii) Acute illness in a child aged between 3 mo and 10 yr characterized by: |
| (a) Fever of ≥38.5°C |
| (b) Abdominal pain and/or vomiting |
| (c) Development of petechiae or purpura |
| (d) No evidence of meningitis |
| (iii) History of conjunctivitis ≤30 days before onset of fever |
| (iv) One (or both) of the following tests negative for N. meningitidis: |
| (a) Blood cultures taken before antibiotic administration |
| (b) Serum or urine antigen detection |
| (v) Other laboratory data, if obtained: |
| (a) CSFb with ≤100 or fewer leukocytes/μl and negative by culture or antigen detection for pathogenic bacteria other than H. aegyptius |
| (b) Blood cultures negative for known pathogenic bacteria other than H. aegyptius |
| (c) Negative serologic studies for known pathogens other than H. aegyptius |
Based on definitions from reference 22.
CSF, cerebrospinal fluid.
The first case-control study, conducted in Promissão, was used to look broadly at possible risk factors for BPF, with the hopes of identifying the etiology of this new disease. There were no differences between case children and controls with regard to history of chronic medical conditions, exposure to other children with BPF, recent vaccinations, socioeconomic status, source of water and type of sewage disposal, and animal exposure (9). Among the deceased Promissão children, there was no evidence of exposure to toxic substances or recent vaccination and there were no secondary household cases.
However, a history of recent conjunctivitis was more common among case children than among controls, an association that was found in the Londrina, Promissão, and Serrana outbreaks (Table 2) (22). In all three outbreaks, approximately 90% of BPF case children had a history of preceding conjunctivitis a mean of 1 to 2 weeks before the onset of BPF. This finding was in contrast to the 12% to 40% of controls for whom preceding conjunctivitis was reported, which resulted in very strong associations between conjunctivitis and BPF. In the Promissão and Serrana outbreaks, healthy controls were used to identify risk factors for BPF. However, there were concerns about recall bias in comparing ill BPF case children with well control children. To address this issue, in the Londrina investigation, children with meningococcal disease were used as controls and the strong association between preceding conjunctivitis and BPF remained.
There were reports of epidemics of purulent conjunctivitis during BPF outbreaks. For example, a survey of households with children of <10 years of age indicated that an outbreak of purulent conjunctivitis had occurred in low-income sections of Promissão. H. aegyptius was the most common etiologic agent isolated from children with conjunctivitis during this survey. This organism was already known as the principal agent of purulent conjunctivitis in Brazil (42). In contrast to the epidemics of viral hemorrhagic conjunctivitis that were widespread throughout Brazil during the period from 1984 to 1986 and that affected all age groups, the purulent conjunctivitis outbreaks that preceded BPF affected children primarily (9, 61).
Tondella et al. isolated BPF clone strains of H. aegyptius associated with BPF from two pools of Hippelates and Liohippelates flies that were collected from around the eyes of children with conjunctivitis (58). These results suggested the participation of these species in the mechanical transmission of the BPF clone of H. aegyptius.
The median age of BPF cases in the Londrina, Promissão, and Serrana outbreaks was 30 to 36 months, and all patients were under 10 years old. Despite the fact that bacterial conjunctivitis generally occurs during hot months (January and February in BPF areas in Brazil), based on a limited number of outbreaks and sporadic cases, there did not appear to be any major seasonal tendency: the Londrina outbreak occurred between February and July 1984, the Promissão outbreak occurred during the period from October to December 1984, and the Serrana outbreak occurred from March to June 1986.
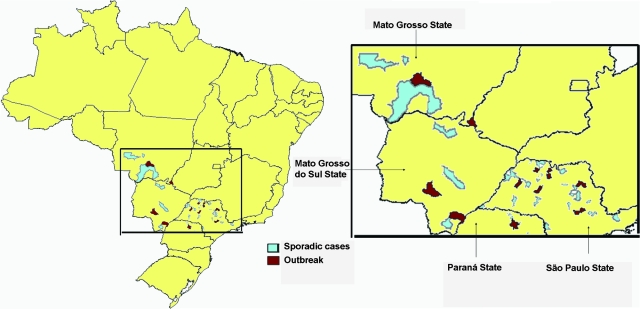
From 1984 to 1993, a total of 276 outbreak and sporadic cases were reported, 210 from São Paulo State, 32 from Mato Grosso, 21 from Mato Grosso do Sul, and 13 from Paraná (53). These states, though contiguous, are spread over a large geographic area (Fig. 3). The fact that some regions of Brazil were experiencing high rates of meningococcal disease during this period and the clinical similarities of BPF to this disease may have led to an underestimate of the number of cases of BPF, particularly sporadic cases.
FIG. 3.
Geographic distribution of sporadic cases and outbreaks of Brazilian purpuric fever in Brazil, 1984 to 1993. Indicated areas are municipalities with sporadic BPF cases or BPF outbreaks. Data sources: Center for Epidemiologic Surveillance, São Paulo Secretariat of Health and Brazilian Ministry of Health.
However, there are several limitations of these surveillance data. Only 28 (10%) of the 276 cases were confirmed by the isolation of H. aegyptius from blood and, of these, 10 were from the Serrana outbreak. Despite the fact that 46% of cases had conjunctivitis at the time of hospital admission, the difficulties in making a clinical diagnosis of BPF would suggest that the positive predictive value of a BPF case in this surveillance system may not be high. In addition, the lack of widespread use of blood cultures in all geographic areas with BPF cases, in addition to the passive nature of this surveillance system, makes substantial underreporting of true BPF cases likely.
LABORATORY ASPECTS OF BPF
Nomenclature and Taxonomy
Haemophilus species are non-spore-forming gram-negative coccobacilli (26). They lack motility and are aerobic or facultatively anaerobic. They require preformed growth factors present in blood, principally X factor (protoporphyrin IX or protoheme) and/or V factor (nicotinamide dinucleotide or nicotinamide dinucleotide phosphate). Haemophilus species are obligate parasites and are part of the normal flora of the human upper respiratory tract. Humans are the only natural host of H. influenzae and many other Haemophilus species.
H. influenzae was first described for cases of endemic influenza in the early 1890s. The organism can produce a polysaccharide capsule that is serologically classified into serotypes a to f. The polysaccharide capsule is the major virulence factor that allows it to cause invasive disease. Nontypeable strains produce no capsule.
H. influenzae can be classified into biotypes I to VII according to its ability to produce indole, urease, and ornithine decarboxylase. Before the widespread use of H. influenzae type b (Hib) conjugate vaccines in the United States, over 90% of invasive H. influenzae infections were caused by a limited number of clones of serotype b, most of which were of biotype I (27). Most strains of H. influenzae that cause conjunctivitis and upper respiratory tract infections and conjunctivitis are nontypeable and generally belong to biotypes II and III (26).
H. aegyptius was firstly described by Koch in 1883, who observed the organism in eye secretions from Egyptian patients with conjunctivitis (29). Weeks made a similar observation 3 years later in the United States (62). The organism subsequently became known as the Koch-Weeks bacillus and then, after being assigned a variety of other names, as H. aegyptius (22, 46).
Weeks subsequently described many of the microbiological, clinical, and epidemiological characteristics of H. aegyptius, including the highly contagious nature of this organism and its propensity to cause large epidemics of conjunctivitis (63, 64). The first studies identifying H. aegyptius as an agent of purulent conjunctivitis reported that the seasonality of conjunctivitis in tropical regions coincided with the highest density of some fly species of the genera Hippelates and Liohippelates, which are commonly found in Brazil (7, 12, 18, 22, 38, 44). The transmission of H. aegyptius to a culture dish from a gnat taken from the eye of a young child with conjunctivitis was subsequently described by Pittman et al. (22). During the effort to eradicate trachoma in the 1930s in the southern United States, there was substantial interest in these eye gnats because they served as mechanical vectors not just of H. aegyptius but also of Chlamydia trachomatis (7, 58).
Because of its propensity to cause a highly contagious epidemic conjunctivitis that occurs seasonally in hot climates, H. aegyptius was for many years considered to be a separate species from H. influenzae. In addition, there are differentiating phenotypic characteristics between the two organisms. H. aegyptius was distinguishable from H. influenzae by serological tests, growth characteristics in semifluid media, the absence of xylose fermentation, the lack of indole production, and the ability to hemagglutinate (37, 46). It was also confirmed that H. aegyptius and H. influenzae biotype III have distinguishing phenotypic properties: H. aegyptius has a considerably poorer growth on chocolate agar, no growth in tryptic soy agar containing V + X factor, distinct bacillary morphology, and susceptibility to troleandomycin (37). However, none of these characteristics unequivocally differentiate H. aegyptius from H. influenzae. In addition, DNA hybridization studies indicated that the two organisms are phylogenetically a single species (2). To account for both the clinical and phenotypic differences, as well as the phylogenetic similarities, the name H. influenzae biogroup aegyptius has been assigned to what was previously known as H. aegyptius (11). For both simplicity and historical reasons, however, we refer to the organism as H. aegyptius throughout this review.
The BPF Clone and the Development of Diagnostic Tests
The strong epidemiologic association between H. aegyptius conjunctivitis and BPF identified during the Promissão outbreak led to an intense investigation of BPF-associated H. aegyptius, even before the etiology of BPF was definitively determined. BPF-associated H. aegyptius isolates were found to be highly clonal and could be distinguished from other H. aegyptius isolates based on a variety of phenotypic and genotypic characteristics. Initial studies sought to further characterize what became known as the BPF clone, which has the following properties (11, 23, 36): (i) The presence of an approximately 32-kb plasmid with an AccI restriction endonuclease pattern referred to as 3031 (because the isolate in which it was first identified had the laboratory identification number F3031), (ii) a characteristic whole-cell sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) profile referred to as 3031, (iii) a characteristic multilocus enzyme electrophoresis (MLEE) pattern (type 2), (iv) one of two closely related EcoRI rRNA gene restriction patterns (ribotype patterns 3 and 4), (v) the presence of a 25-kDa protein (putative pilin), and (vi) resistance to trimethoprim-sulfamethoxazole.
Some of these characteristics are markers for the BPF clone but are not responsible for the organism's virulence. For example, MLEE was used to determine the genetic relatedness of bacterial strains by use of housekeeping genes. However, some of these markers were attractive candidates as potential virulence factors and were therefore extensively investigated, as discussed below.
By use of MLEE, the two Australian BPF isolates from blood were reported to be genetically closely related to each other but not related to BPF clone isolates from Brazil (28, 56). In addition, the Australian isolates did not have any of the typical BPF clone characteristics, including the 3031 plasmid (56). The one reported case from the United States was dissimilar to the Australian strains and also did not share the BPF clone characteristics (60).
The identification of characteristics that differentiated the BPF clone from other strains of H. aegyptius led to the development of tests that could quickly identify the clone. Polyclonal antiserum raised in rabbits against the BPF clone was used in a slide agglutination test. The primary cell surface antigen detected in this assay was determined to be the 25-kDa protein. The utility of this test was that it could be used to rapidly identify the BPF clone with a sensitivity and specificity of 100% and 85%, respectively (11). However, to improve on the specificity of this assay, a sandwich enzyme immunoassay was developed using BPF clone-specific immunoglobulin G2b (IgG2b) and IgM monoclonal antibodies. This assay also had a sensitivity of 100% for detecting the BPF clone and a specificity of 94%. These monoclonal antibodies are also believed to recognize the 25-kDa protein (56). Latex agglutination assays using both polyclonal and monoclonal BPF clone antibodies were also developed (56).
BPF Clone Virulence Factors
The emergence of a clone of H. aegyptius that was capable of causing dramatic outbreaks of fatal bacteremia in previously healthy children suggested the acquisition of an unidentified virulence factor or factors in H. aegyptius. This hypothesis led to a series of studies to identify the virulence factors responsible for the virulence of the BPF clone.
Infant rat model of BPF.
The infant rat model had previously been shown to be useful for studying Hib pathogenesis, making it attractive for studying the BPF clone (54). Initial observations that the BPF clone was more virulent in this model than were control strains of H. aegyptius supported the notion that the BPF clone had unidentified virulence factors (13, 52). For example, bacteremia was detected at 24 h in 66% (38 of 58) of infant rats inoculated intraperitoneally with one of three BPF clone isolates compared to 2% (1 of 59) animals inoculated with a non-BPF clone isolate. The estimated 50% infectious doses for a BPF clone isolate and a control H. aegyptius strain were 103.4 and 106.9, respectively. The two Australian case isolates, not part of the BPF clone but associated with clinical BPF, also caused bacteremia in this animal model more frequently than did control isolates. Interestingly, unlike children with BPF, rats with bacteremia caused by the BPF clone frequently developed meningitis.
Studies were also performed to determine the ability of immune adult rat serum to protect infant rats challenged intraperitoneally with a BPF clone isolate (Table 6) (14, 51, 52). The efficacy of immune serum prepared using BPF clone isolates in providing protection against bacteremia was complete, whereas normal adult rat serum and immune serum prepared using a non-BPF clone control isolate of H. aegyptius provided minimal protection. Immune serum prepared using an Australian BPF isolate did not protect against bacteremia following challenge with BPF clone isolates. These data suggested that the BPF clone has unique antigens that elicit an antibody response that protects infant rats.
TABLE 6.
Protective effect of immune serum measured after subcutaneous and intraperitoneal injection of adult rats with whole H. aegyptius isolatesa
| Pretreatment adult rat serum | Challenge isolate | No. of infant rats tested | No. (%) with bacteremia |
|---|---|---|---|
| Normal serum | BPF clone | 25 | 22 (88) |
| Immune serum prepared using non-BPF control H. aegyptius isolates | BPF clone | 24 | 22 (94) |
| Immune serum prepared using BPF clone isolates | BPF clone | 33 | 0 (0) |
| Immune serum prepared using Australian BPF strain | BPF clone | 15 | 14 (93) |
The 3031 plasmid.
Early in the BPF outbreak investigations, it became clear that the 3031 plasmid was present in BPF strains but not other strains of H. aegyptius (9). To determine the association of this plasmid with BPF, isolates from the following three groups of patients were compared: BPF case patients (group A), patients with conjunctivitis but not BPF but who were epidemiologically associated with BPF cases (group B), and patients with conjunctivitis that were not associated with BPF (group C) (11). The group A strains had been isolated from blood, cerebrospinal fluid, conjunctiva, and oropharynx. All of the group A isolates, 72% of the group B isolates, and 11% of the group C isolates had a 24-MDa plasmid, which were classified into seven restriction pattern (patterns 3031, 1947, 3055, 3125, 8135, 3097, and 3331). All group A isolates had plasmid pattern 3031, which was absent from 92% of group C isolates. Based on these data, the 3031 plasmid was used as an epidemiologic marker of the BPF clone. Because of the role of plasmids in the pathogenesis of other bacterial diseases, it was also hypothesized to play a role in BPF virulence (32).
The entire 3031 plasmid has been sequenced and found to have 32,379 bp, which potentially includes 34 open reading frames encoding proteins of over 50 amino acids (30). Eight of these open reading frames were unlike other sequences found in public bacterial DNA databases. The plasmid was organized into two main domains, the first consisting mostly of DNA housekeeping and plasmid transfer genes. The second domain, while also containing these types of genes, contained a variety of putative genes encoding products of unknown function. Although this study does not rule out the 3031 plasmid as involved in BPF clone pathogenesis, no known bacterial virulence factor was identified. In addition, the absence of this plasmid in BPF-associated H. aegyptius strains from Valparaíso and Pradópolis, São Paulo State, suggests that it could not be responsible for the virulent nature of the BPF clone, although one cannot be certain that the plasmid could have been present but lost during laboratory manipulation (59). Although no sterile site isolates were obtained from Pradópolis cases, the three Valparaíso isolates were obtained from blood (two isolates) and hemorrhagic cerebrospinal fluid (one isolate) cultures. The lack of the 3031 plasmid in BPF isolates from Australia does not provide evidence for or against its role in virulence because these isolates do not belong to the BPF clone and could have other virulence mechanisms.
Capsule.
There were several lines of evidence suggesting that the BPF clone might have a polysaccharide capsule. First, bacteria that were thought to be encapsulated were identified in the bone marrow of BPF patients (5). Second, BPF clone strains appeared to have surface carbohydrate, as evidenced by ruthenium red staining on electron microscopy. However, this carbohydrate-reactive material was evident only after the BPF clone had been passaged in mice and the amount of staining was substantially less than what was observed with a Hib control strain. In addition, ruthenium red staining is not specific for the presence of polysaccharide capsule (13). Third, the H. influenzae cap probe was found to hybridize with BPF clone DNA but not with the DNA of non-BPF clone strains, suggesting the presence of H. influenzae cap genes (13). Invasive H. influenzae strains are encapsulated and are of one of six capsular serotypes (a to f); these strains share the cap genes, which are required for encapsulation. However, it was subsequently shown that selective hybridization of the H. influenzae cap probe to the BPF clone strains was due to the presence of insertion element IS1016, which is situated at the cap loci of some Hib strains (19, 24, 31). Despite the presence of this insertion element, the BPF clone was found to not have the capsular genes that are found in H. influenzae serotypes a to f. In another study, attempts to isolate acidic polysaccharide were unsuccessful (13). Therefore, at present there is no firm evidence that the BPF clone is an encapsulated organism. If so, this would make H. aegyptius fairly unique among invasive bacterial pathogens, which generally require a polysaccharide capsule to cause severe invasive disease in apparently healthy children.
Pilin protein.
The BPF clone was found to contain a 25-kDa protein that was absent from other H. aegyptius isolates (65). The protein exhibited an unusual charcoal gray color in silver-stained sodium dodecyl sulfate-polyacrylamide gel electrophoresis profiles of whole BPF clone strain cells. The protein was extensively characterized because of its potential role in virulence, its role in diagnostic tests, and of its potential value as an immunogen. It appears to be the key cell surface antigen that is detected in the agglutination reaction with polyclonal anti-H. aegyptius antiserum, which, as indicated above, has been used as a rapid test for identifying the BPF clone. It also contains a heat-labile epitope that is recognized by the monoclonal antibodies used in the enzyme-linked immunosorbent assay, which was demonstrated using colloidal gold-enhanced immunoelectron microscopy (65).
Sequencing of the BPF 25-kDa protein gene revealed a 191-amino-acid protein with 71% amino acid homology to the Hib pilin protein (66). The presence of pilin in the BPF clone was also supported by electron microscopy studies (65). The BPF clone pilus system was characterized and found to be generally similar to Hib, with significant differences being a second pilus locus in the BPF clone that appeared to have occurred through a recent duplication event, as well as a novel sequence in an adhesin (HafE) (48). Expression of the 25-kDa pilin protein appears to be regulated by phase variation, a phenomenon also observed for Hib.
The role of the pilin protein in attachment to various human cells has been studied. For human epithelial cells, no differences in attachment were found between BPF clone strain 3031 that did express the protein and the same strain that did not (21). However, the number of CFU attaching to human conjunctival cells (Chang cells) was approximately 1 log greater for a BPF clone isolate than for a non-BPF strain. Both BPF clone and non-BPF strains invaded human nasopharyngeal epithelial cells after attachment (21), similar to what is seen with N. meningitidis. These data suggest that the BPF clone pilin protein may enhance the attachment of H. aegyptius to the conjunctiva. Whether it plays other roles in BPF pathogenesis is not clear.
Other Potential Virulence Factors
Given the striking clinical similarities between BPF and invasive meningococcal disease, as well as the propensity for Haemophilus species to acquire DNA via horizontal gene transfer, attempts have been made to determine whether the BPF clone could have acquired genes from N. meningitidis. In one study, a 3031 plasmid probe failed to hybridize with the chromosomal DNA of four meningococcal strains representing serogroups A to C (30). A similar lack of hybridization was seen with H. influenzae serotypes a to f.
In another study using subtractive DNA hybridization between a BPF clone strain and a Brazilian conjunctivitis strain of H. aegyptius, 46 chromosomal loci that were unique to the BPF clone were identified, some of which were further characterized (33). One gene, bpf001, was found to encode a 195-amino-acid protein that is homologous to the Legionella pneumophila epithelial cell entry-enhancing protein EnhC and the product of the meningococcal gene NMB0419, which was previously of unknown function. The investigators were unable to genetically manipulate the BPF clone strain and therefore focused their investigations on the meningococcal homologue NMB0419 as a possible way of inferring the function of the bpf001 gene product by use of a gene knockout approach. The wild-type meningococcal strain was found to invade epithelial cells to an extent significantly greater than that seen for the knockout strain. The authors concluded that NMB0419 is involved in meningococcal invasiveness and therefore speculated that the finding could be extrapolated to Bpf001. An additional element that was unique to the BPF clone in this study was bacteriophage HP1 of H. influenzae.
Another subtractive DNA hybridization study using BPF clone strain F3031 and H. aegyptius conjunctivitis isolate F1947 identified F3031-specific chromosomal regions, which appeared to have been acquired mostly from unrelated bacterial species (55). Twenty-one tester-specific clones were identified, including those possessing iga, which encodes an Ig protease; hmcD, which encodes hemocin; various phage-related genes; and hypothetical genes of unknown function.
A 145-kDa conserved phase-variable surface protein present in BPF clone strains did not contribute to virulence in an infant rat model (49, 50). There are genetic and antigenic differences between the IgA1 protease produced by the BPF clone and those produced by non-BPF clone strains of H. aegyptius (13, 34). Although this supports the clonal nature of BPF-causing strains, there are no data to suggest that differences in IgA1 protease contribute to BPF pathogenesis. More recently, the BPF clone was found to have a 60-kDa molecule that was absorbable by human erythrocytes. Intravenous injection of rabbits with either the whole bacterial extracellular product or the purified 60-kDa fraction resulted in purpura, congestion, and fibrin thrombi in the organs or rabbits, suggesting that this protein could contribute to the pathogenesis of BPF (6).
Origin of the BPF Clone
To identify the origin of the BPF clone, the genetic relationships between the BPF clone and other strains of H. influenzae have been investigated. One study used MLEE to determine the relationships between encapsulated H. influenzae, the BPF clone isolates from Promissão, and non-BPF clone H. aegyptius isolates from Brazil and other countries. BPF clone isolates were found to be only distantly related to the non-BPF clone H. aegyptius isolates (43). Although the BPF clone isolates were most closely related to two H. influenzae serotype c isolates, the genetic distance between the two groups was only approximately 50%.
More-recent studies used multilocus sequence typing (MLST), a more objective and portable DNA sequence-based method that has replaced MLEE for determining the genetic relatedness of bacteria (35). MLST of H. influenzae involves DNA sequencing of portions of seven housekeeping genes. In one study of 131 encapsulated and unencapsulated H. influenzae isolates, a BPF clone isolate (F3028) was found to be of sequence type 65 (ST 65) and genetically unrelated to the other isolates (40). In another study, 656 H. influenzae isolates, about half of which were unencapsulated, were also analyzed by MLST (20). Among the encapsulated and nontypeable strains, 98% and 65%, respectively, could be classified into 1 of 13 clades. BPF clone isolates (including isolate F3028), all found to be ST 65, were not in any of the 13 clades. Epidemiologically unrelated conjunctivitis strains were found to be of a variety of STs that were clustered in or near clade 4.
There are four ST 65 isolates that have been submitted to the H. influenzae MLST website, all BPF clone isolates from São Paulo State, including one blood isolate from Valparaíso that lacks the 3031 plasmid (http://haemophilus.mlst.net/, last accessed August 8, 2008) (11, 59) (Table 7 and Fig. 4). There are an additional 12 non-BPF clone conjunctival isolates from various towns in São Paulo State that were obtained during the 1980s and that comprise STs 70 to 76. In an analysis restricted to these 16 BPF clone and non-BPF clone isolates from São Paulo State, the BPF clone isolates were seven-locus (of seven loci) variants of all of the non-BPF clone isolates, with the exception of ST 70, against which they were six-locus variants. These data indicate that the BPF clone is not substantially related to non-BPF clone H. aegyptius isolates from São Paulo State. In contrast, the non-BPF clone conjunctival isolates differed from each other at only one to four loci. Based on the lack of close genetic relatedness between the BPF clone and other strains of H. influenzae that have been analyzed to date, including non-BPF clone conjunctivitis isolates from the same time period and Brazilian state as the BPF outbreaks, the origin of the BPF clone remains a mystery.
TABLE 7.
H. aegyptius isolates analyzed by MLST on H. influenzae MLST websitea
| BPF clone | Specimen type | City | Year | CDC isolate no. | MLST alleles
|
ST | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| adk | atpG | frdB | fucK | mdh | pgi | recA | ||||||
| Yes | CSF | Fartura | 1986 | F3028 | 24 | 26 | 24 | 21 | 36 | 33 | 21 | 65 |
| Yesb | Blood | Valparaíso | 1990 | G1100 | 24 | 26 | 24 | 21 | 36 | 33 | 21 | 65 |
| Yes | Conjunctiva | Serrana | 1986 | F3099 | 24 | 26 | 24 | 21 | 36 | 33 | 21 | 65 |
| Yes | Conjunctiva | Serrana | 1986 | F3050 | 24 | 26 | 24 | 21 | 36 | 33 | 21 | 65 |
| No | Conjunctiva | Guariba | 1986 | F3043 | 25 | 20 | 24 | 24 | 38 | 34 | 25 | 70 |
| No | Conjunctiva | Restinga | 1986 | F3089 | 25 | 20 | 24 | 24 | 38 | 34 | 25 | 70 |
| No | Conjunctiva | Guariba | 1986 | F3093 | 25 | 20 | 24 | 24 | 38 | 34 | 25 | 70 |
| No | Conjunctiva | Serrana | 1986 | F3125 | 25 | 20 | 28 | 23 | 39 | 34 | 25 | 71 |
| No | Conjunctiva | Promissão | 1984 | F1951 | 25 | 20 | 28 | 14 | 40 | 34 | 25 | 72 |
| No | Conjunctiva | Promissão | 1984 | F1947 | 25 | 20 | 28 | 24 | 39 | 35 | 25 | 73 |
| No | Conjunctiva | Garça | 1985 | F2068 | 25 | 20 | 28 | 24 | 39 | 35 | 25 | 73 |
| No | Conjunctiva | Baurú | 1985 | F2029 | 25 | 20 | 24 | 18 | 38 | 34 | 25 | 74 |
| No | Conjunctiva | Ribeirão Preto | 1986 | F3098 | 25 | 20 | 29 | 24 | 39 | 35 | 25 | 75 |
| No | Conjunctiva | Ribeirão Preto | 1986 | F3119 | 26 | 20 | 30 | 24 | 36 | 36 | 25 | 76 |
| No | Conjunctiva | Ribeirão Preto | 1986 | F3118 | 26 | 20 | 30 | 24 | 36 | 36 | 25 | 76 |
| No | Conjunctiva | Ribeirão Preto | 1986 | F3121 | 26 | 20 | 30 | 24 | 36 | 36 | 25 | 76 |
http://haemophilus.mlst.net/ (references 1 and 11). Also see Fig. 4. All isolates are from São Paulo State. CSF, cerebrospinal fluid.
Has BPF clone characteristics except that it lacks the 3031 plasmid.
FIG. 4.
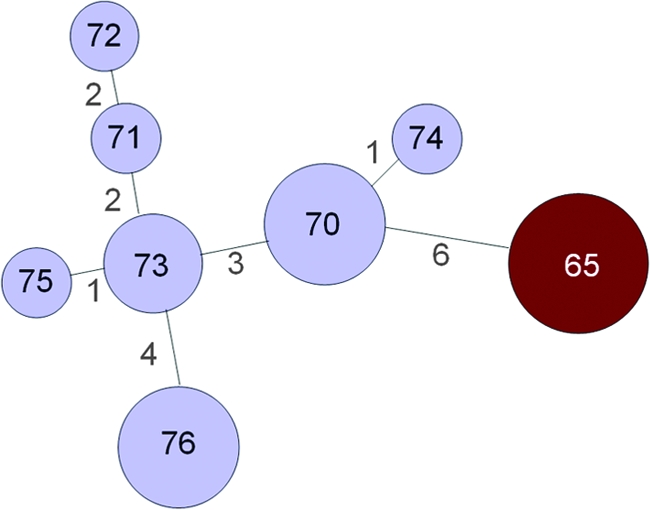
Minimum spanning tree analysis of STs of H. aegyptius isolates from Brazil. Lines between the circles are proportional to the number of locus differences, which are indicated by the numbers between circles. Circle sizes are proportional to the number of isolates with each ST. Numbers in circles represent the STs. Sources of information about the isolates are http://haemophilus.mlst.net/ (references 1 and 11); also see text and Table 7. BPF clone isolates are shown in red; all other H. aegyptius isolates are shown in light blue.
ENIGMAS SURROUNDING BPF
There are many questions about BPF that remain unanswered. Perhaps the most intriguing question is how the BPF clone originated. The lack of substantial genetic relatedness of the BPF clone to encapsulated H. influenzae or unencapsulated strains from patients with conjunctivitis, including those from the same geographic location and time period in Brazil as BPF cases, suggests the possibility of an unrecognized, nonhuman source (43). Another possible explanation for the failure to identify a strain of H. influenzae that is closely related to the BPF clone is that not all H. influenzae lineages causing human disease have been analyzed by MLST. If true, it is conceivable that as additional MLST data accumulate, a human source of the BPF clone will become apparent.
Another unanswered question is why BPF seems to have disappeared. The emergence and persistence of new infectious diseases is unpredictable. Human immunodeficiency virus, which clearly became a new human pathogen only decades ago, rapidly spread worldwide and has persisted. Likewise, West Nile virus, initially introduced into the United States in 1999, rapidly spread nationwide and has persisted as a cause of viral encephalitis. On the other hand, the severe acute respiratory syndrome coronavirus emerged suddenly in 2002, spread worldwide, and then subsequently disappeared within several years for unknown reasons. Similarly, BPF emerged in Brazil in the mid-1980s, caused disease for around 10 years, and subsequently has not been confirmed to have occurred since then.
The reasons for the rapid disappearance of BPF are not clear. One hypothesis is that the BPF clone had novel antigens to which the pediatric population had no immunity, accounting for the burst of disease over several years. Over time, with its rapid spread and propensity to cause outbreaks of conjunctivitis, in addition to BPF, the population could eventually have become immune to the BPF clone. However, considering that meningococcal disease is endemic in Brazil, it is conceivable that sporadic cases still occur and are being misdiagnosed as meningococcemia or other infectious illnesses. It is unlikely that outbreaks still occur in areas previously affected by BPF, given the relatively advanced level of health care and the general knowledge about BPF among Brazilian physicians in those regions.
Other questions about BPF also remain unanswered. Why did it affect primarily small, agricultural cities, without affecting large nearby urban centers? For example, Promissão and Serrana, towns with populations at the time of around 20,000, are approximately 250 and 175 miles, respectively, from the city of São Paulo, which had a population of around 15 million inhabitants. Also, why was it apparently restricted to a relatively limited area of Brazil, despite a very large, mobile population and the common occurrence of purulent conjunctivitis outbreaks throughout the country?
The genome of BPF clone isolate F3031 has recently been sequenced. A preliminary analysis does not suggest the origin of the organism. Although additional analyses are under way, no new insights have yet emerged about the microbial factors responsible for the virulence of the BPF clone (J. Simon Kroll, personal communication, 9 August 2008). It is unclear whether the remaining questions about BPF will ever be answered.
Acknowledgments
We thank Larry Klein for the transcript of What's Killing the Children?; Maria Lúcia Tondella and Maria Cristina Brandileone for Fig. 1; Kathleen Shutt for the MLST analysis; and Claire Broome, Leonard Mayer, Claudio Sacchi, and Maria Lúcia Tondella for their thoughtful review of the manuscript.
Financial support for this review was provided in part by a Research Career Award, National Institute of Allergy and Infectious Diseases (K24 AI52788, to Lee H. Harrison); by a grant from the Fogarty International Center Global Infectious Diseases Research Training Program, National Institutes of Health (5 D43 TW006592, to the University of Pittsburgh); and Conselho Nacional de Pesquisas (CNPq) (contract number 309502/2003-9, to Eliseu A. Waldman).
REFERENCES
- 1.Ajello, G. W., G. M. Matar, B. Swaminathan, W. F. Bibb, L. O. Helsel, and B. A. Perkins. 1995. A rapid dot immunoassay for detecting the Brazilian purpuric fever clone of Haemophilus influenzae biogroup aegyptius with a “flow through” device. Curr. Microbiol. 30:345-349. [DOI] [PubMed] [Google Scholar]
- 2.Albritton, W. L., J. K. Setlow, M. Thomas, F. Sottnek, and A. G. Steigerwalt. 1984. Heterospecific transformation in the genus Haemophilus. Mol. Gen. Genet. 193:358-363. [DOI] [PubMed] [Google Scholar]
- 3.Alves, V. A. F., M. F. Araújo, R. S. Costa, M. F. Franco, E. L. P. Lancellotti, L. Gorelkin, L. P. Mecellis, M. Ottoboni, M. P. S. Lopes, M. A. S. L. Velludo, J. Barbieri Neto, M. C. C. Brandileone, M. L. C. Tondella, C. T. Sacchi, S. M. G. Thomé, and M. R. Montenegro. 1987. Febre purpúrica brasileira: padrões histopatológicos em necrópsias, abstr. 267. Rev. Soc. Bras. Med. Trop. 20(Suppl.):128. [Google Scholar]
- 4.Baldy, J. L. S., E. T. Anzai, A. Jabur, A. M. Bonametti, J. N. Passos, P. K. Takata, and R. M. B. Quesada. 1987. Febre purpúrica brasileira: relato de oito casos prováveis, observados em Londrina-PR, no primeiro semestre de 1984, abstr. 268. Rev. Soc. Bras. Med. Trop. 20(Suppl.):128. [Google Scholar]
- 5.Barbieri Neto, J. 1988. Brazilian purpuric fever. Lancet i:883-884. [DOI] [PubMed] [Google Scholar]
- 6.Barbosa, S. F., S. Hoshino-Shimizu, M. G. Alkmin, and H. Goto. 2003. Implications of Haemophilus influenzae biogroup aegyptius hemagglutinins in the pathogenesis of Brazilian purpuric fever. J. Infect. Dis. 188:74-80. [DOI] [PubMed] [Google Scholar]
- 7.Bengston, I. A. 1933. Seasonal acute conjunctivitis occurring in the Southern states. Public Health Rep. 48:917-926. [Google Scholar]
- 8.Brazilian Purpuric Fever Study Group. 1992. Brazilian purpuric fever identified in a new region of Brazil. J. Infect. Dis. 165(Suppl. 1):S16-S19. [DOI] [PubMed] [Google Scholar]
- 9.Brazilian Purpuric Fever Study Group. 1987. Brazilian purpuric fever: epidemic purpura fulminans associated with antecedent purulent conjunctivitis. Lancet ii:757-761. [PubMed] [Google Scholar]
- 10.Brazilian Purpuric Fever Study Group. 1987. Haemophilus aegyptius bacteraemia in Brazilian purpuric fever. Lancet ii:761-763. [PubMed] [Google Scholar]
- 11.Brenner, D. J., L. W. Mayer, G. M. Carlone, L. H. Harrison, W. F. Bibb, M. C. Brandileone, F. O. Sottnek, K. Irino, M. W. Reeves, J. M. Swenson, et al. 1988. Biochemical, genetic, and epidemiologic characterization of Haemophilus influenzae biogroup aegyptius (Haemophilus aegyptius) strains associated with Brazilian purpuric fever. J. Clin. Microbiol. 26:1524-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buehler, J. W., J. T. Holloway, R. A. Goodman, and R. K. Sikes. 1983. Gnat sore eyes: seasonal, acute conjunctivitis in a southern state. South. Med. J. 76:587-589. [PubMed] [Google Scholar]
- 13.Carlone, G. M., L. Gorelkin, L. L. Gheesling, A. L. Erwin, S. K. Hoiseth, M. H. Mulks, S. P. O'Connor, R. S. Weyant, J. Myrick, L. Rubin, et al. 1989. Potential virulence-associated factors in Brazilian purpuric fever. J. Clin. Microbiol. 27:609-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlone, G. M., L. Gorelkin, L. L. Gheesling, S. K. Hoiseth, M. H. Mulks, S. P. O'Connor, R. S. Weyant, J. E. Myrick, L. W. Mayer, R. J. Arko, et al. 1989. Potential virulence factors of Haemophilus influenzae biogroup aegyptius in Brazilian purpuric fever. Pediatr. Infect. Dis. J. 8:245-247. [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. 1990. Brazilian purpuric fever-Mato Grosso, Brazil. MMWR Morb. Mortal. Wkly. Rep. 39:903-905. [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. 1985. Preliminary report: epidemic fatal purpuric fever among children-Brazil. MMWR Morb. Mortal. Wkly. Rep. 34:217-219. [PubMed] [Google Scholar]
- 17.Cervi, M. C., G. M. Rocha, and L. G. Rubin. 2005. Febre purpúrica brasileira, p. 911-918. In R. Focaccia (ed.), Veronesi. Tratado de infectologia. Atheneu, São Paulo, Brazil.
- 18.Dawson, C. R. 1960. Epidemic Koch-Weeks conjunctivitis and trachoma in the Coachella Valley of California. Am. J. Ophthalmol. 49:801-808. [PubMed] [Google Scholar]
- 19.Dobson, S. R., J. S. Kroll, and E. R. Moxon. 1992. Insertion sequence IS1016 and absence of Haemophilus capsulation genes in the Brazilian purpuric fever clone of Haemophilus influenzae biogroup aegyptius. Infect. Immun. 60:618-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erwin, A. L., S. A. Sandstedt, P. J. Bonthuis, J. L. Geelhood, K. L. Nelson, W. C. Unrath, M. A. Diggle, M. J. Theodore, C. R. Pleatman, E. A. Mothershed, C. T. Sacchi, L. W. Mayer, J. R. Gilsdorf, and A. L. Smith. 2008. Analysis of genetic relatedness of Haemophilus influenzae isolates by multilocus sequence typing. J. Bacteriol. 190:1473-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farley, M. M., A. M. Whitney, P. Spellman, F. D. Quinn, R. S. Weyant, L. Mayer, and D. S. Stephens. 1992. Analysis of the attachment and invasion of human epithelial cells by Haemophilus influenzae biogroup aegyptius. J. Infect. Dis. 165(Suppl. 1):S111-S114. [DOI] [PubMed] [Google Scholar]
- 22.Harrison, L. H., G. A. da Silva, M. Pittman, D. W. Fleming, A. Vranjac, C. V. Broome, et al. 1989. Epidemiology and clinical spectrum of Brazilian purpuric fever. J. Clin. Microbiol. 27:599-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irino, K., F. Grimont, I. Casin, and P. A. Grimont. 1988. rRNA gene restriction patterns of Haemophilus influenzae biogroup aegyptius strains associated with Brazilian purpuric fever. J. Clin. Microbiol. 26:1535-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapogiannis, B. G., S. Satola, H. L. Keyserling, and M. M. Farley. 2005. Invasive infections with Haemophilus influenzae serotype a containing an IS1016-bexA partial deletion: possible association with virulence. Clin. Infect. Dis. 41:e97-e103. [DOI] [PubMed] [Google Scholar]
- 25.Kerr-Pontes, L. R., and A. Ruffino-Netto. 1991. Epidemiological study of Brazilian purpuric fever. Epidemic in a locality of São Paulo state (Brazil), 1986. Rev. Saúde Pública 25:375-380. (In Portuguese.) [DOI] [PubMed] [Google Scholar]
- 26.Kilian, M. 2005. Genus III. Haemophilus, p. 883-918. In D. J. Brenner, N. R. Krieg, and J. T. Staley (ed.), Bergey's manual of systematic bacteriology, 2nd ed., vol. 2. The proteobacteria. Springer, New York, NY. [Google Scholar]
- 27.Kilian, M. 1976. A taxonomic study of the genus Haemophilus, with the proposal of a new species. J. Gen. Microbiol. 93:9-62. [DOI] [PubMed] [Google Scholar]
- 28.Kilian, M., K. Poulsen, and H. Lomholt. 2002. Evolution of the paralogous hap and iga genes in Haemophilus influenzae: evidence for a conserved hap pseudogene associated with microcolony formation in the recently diverged Haemophilus aegyptius and H. influenzae biogroup aegyptius. Mol. Microbiol. 46:1367-1380. [DOI] [PubMed] [Google Scholar]
- 29.Koch, R. 1883. Bericht über die Thätigkeit der deutschen Cholerakommisionen in Aegypten und Ostindien. Wien Med. Wochenschr. 33:1548-1551. [Google Scholar]
- 30.Kroll, J. S., J. L. Farrant, S. Tyler, M. B. Coulthart, and P. R. Langford. 2002. Characterisation and genetic organisation of a 24-MDa plasmid from the Brazilian purpuric fever clone of Haemophilus influenzae biogroup aegyptius. Plasmid 48:38-48. [DOI] [PubMed] [Google Scholar]
- 31.Kroll, J. S., I. Hopkins, and E. R. Moxon. 1988. Capsule loss in H. influenzae type b occurs by recombination-mediated disruption of a gene essential for polysaccharide export. Cell 53:347-356. [DOI] [PubMed] [Google Scholar]
- 32.Lan, R., and P. R. Reeves. 2002. Escherichia coli in disguise: molecular origins of Shigella. Microbes Infect. 4:1125-1132. [DOI] [PubMed] [Google Scholar]
- 33.Li, M. S., J. L. Farrant, P. R. Langford, and J. S. Kroll. 2003. Identification and characterization of genomic loci unique to the Brazilian purpuric fever clonal group of H. influenzae biogroup aegyptius: functionality explored using meningococcal homology. Mol. Microbiol. 47:1101-1111. [DOI] [PubMed] [Google Scholar]
- 34.Lomholt, H., and M. Kilian. 1995. Distinct antigenic and genetic properties of the immunoglobulin A1 protease produced by Haemophilus influenzae biogroup aegyptius associated with Brazilian purpuric fever in Brazil. Infect. Immun. 63:4389-4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maiden, M. C. 2006. Multilocus sequence typing of bacteria. Annu. Rev. Microbiol. 60:561-588. [DOI] [PubMed] [Google Scholar]
- 36.Mayer, L. W., W. F. Bibb, K. A. Birkness, K. Irino, R. S. Weyant, M. W. Reeves, J. M. Swenson, et al. 1989. Distinguishing clonal characteristics of the Brazilian purpuric fever-producing strain. Pediatr. Infect. Dis. J. 8:241-243. [PubMed] [Google Scholar]
- 37.Mazloum, H. A., M. Kilian, Z. M. Mohamed, and M. D. Said. 1982. Differentiation of Haemophilus aegyptius and Haemophilus influenzae. Acta Pathol. Microbiol. Immunol. Scand. Sect. B 90:109-112. [DOI] [PubMed] [Google Scholar]
- 38.McGuire, C. D., and R. C. Durant. 1957. The role of flies in the transmission of eye disease in Egypt. Am. J. Trop. Med. Hyg. 6:569-575. [DOI] [PubMed] [Google Scholar]
- 39.McIntyre, P., G. Wheaton, J. Erlich, and D. Hansman. 1987. Brasilian purpuric fever in central Australia. Lancet ii:112. [DOI] [PubMed] [Google Scholar]
- 40.Meats, E., E. J. Feil, S. Stringer, A. J. Cody, R. Goldstein, J. S. Kroll, T. Popovic, and B. G. Spratt. 2003. Characterization of encapsulated and noncapsulated Haemophilus influenzae and determination of phylogenetic relationships by multilocus sequence typing. J. Clin. Microbiol. 41:1623-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Milstein, T. 1985. An indeterminate disease in the city of Promissão: preliminary report. Rev. Paul. Med. 103:95-98. (In Portuguese.) [PubMed] [Google Scholar]
- 42.Monteiro Salles, F. J. 1941. Bacterioscopia das secreções conjuntivais. Rev. Med. Cir. Sao Paulo 1:105-129. [Google Scholar]
- 43.Musser, J. M., and R. K. Selander. 1990. Brazilian purpuric fever: evolutionary genetic relationships of the case clone of Haemophilus influenzae biogroup aegyptius to encapsulated strains of Haemophilus influenzae. J. Infect. Dis. 161:130-133. [DOI] [PubMed] [Google Scholar]
- 44.Paganelli, C. H., and C. W. Sabrosky. 1993. Hippelates flies (Diptera: Chloropidae) possibly associated with Brazilian purpuric fever. Proc. Entomol. Soc. Wash. 95:165-174. [Google Scholar]
- 45.Perkins, B. A., M. L. Tondella, I. M. Bortolotto, O. A. Takano, G. A. da Silva, K. Irino, M. C. Brandileone, L. H. Harrison, J. D. Wenger, C. V. Broome, et al. 1992. Comparative efficacy of oral rifampin and topical chloramphenicol in eradicating conjunctival carriage of Haemophilus influenzae biogroup aegyptius. Pediatr. Infect. Dis. J. 11:717-721. [DOI] [PubMed] [Google Scholar]
- 46.Pittman, M., and D. J. Davis. 1950. Identification of the Koch-Weeks bacillus (Hemophilus aegyptius). J. Bacteriol. 59:413-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Porath, A., K. Wanderman, A. Simu, B. Vidne, and M. Alkan. 1986. Endocarditis caused by Haemophilus aegyptius. Am. J. Med. Sci. 292:110-111. [DOI] [PubMed] [Google Scholar]
- 48.Read, T. D., M. Dowdell, S. W. Satola, and M. M. Farley. 1996. Duplication of pilus gene complexes of Haemophilus influenzae biogroup aegyptius. J. Bacteriol. 178:6564-6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rubin, L. G. 1995. Phase-variable expression of the 145-kDa surface protein of Brazilian purpuric fever case-clone strains of Haemophilus influenzae biogroup aegyptius. J. Infect. Dis. 171:713-717. [DOI] [PubMed] [Google Scholar]
- 50.Rubin, L. G. 1995. Role of the 145-kilodalton surface protein in virulence of the Brazilian purpuric fever clone of Haemophilus influenzae biogroup aegyptius for infant rats. Infect. Immun. 63:3555-3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rubin, L. G., G. M. Carlone, et al. 1989. An infant rat model of bacteremia with Brazilian purpuric fever isolates of Haemophilus influenzae biogroup aegyptius (Haemophilus aegyptius). Pediatr. Infect. Dis. J. 8:247-248. [PubMed] [Google Scholar]
- 52.Rubin, L. G., E. S. Gloster, G. M. Carlone, et al. 1989. An infant rat model of bacteremia with Brazilian purpuric fever isolates of Hemophilus influenzae biogroup aegyptius. J. Infect. Dis. 160:476-482. [DOI] [PubMed] [Google Scholar]
- 53.Silva, G. A. 1997. Febre purpúrica brasileira: uma contribuição aos conhecimentos clínicos epidemiológicos de uma doença recém identificada. Universidade de São Paulo, São Paulo, Brazil.
- 54.Smith, A. L., D. H. Smith, D. R. Averill, Jr., J. Marino, and E. R. Moxon. 1973. Production of Haemophilus influenzae b meningitis in infant rats by intraperitoneal inoculation. Infect. Immun. 8:278-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smoot, L. M., D. D. Franke, G. McGillivary, and L. A. Actis. 2002. Genomic analysis of the F3031 Brazilian purpuric fever clone of Haemophilus influenzae biogroup aegyptius by PCR-based subtractive hybridization. Infect. Immun. 70:2694-2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Swaminathan, B., L. W. Mayer, W. F. Bibb, G. W. Ajello, K. Irino, K. A. Birkness, C. F. Garon, M. W. Reeves, M. C. de Cunto Brandileone, F. O. Sottnek, et al. 1989. Microbiology of Brazilian purpuric fever and diagnostic tests. J. Clin. Microbiol. 27:605-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tondella, M. L., M. C. C. Brandileone, V. S. D. Vieira, R. C. Zanella, and A. E. Taunay. 1992. Atuação do Instituto Adolfo Lutz na investigação da febre purpúrica brasileira, p. 255-271. In J. L. Antunes (ed.), Instituto Adolfo Lutz: 100 anos do Laboratório de Saúde Pública. Letras & Letras, São Paulo, Brazil.
- 58.Tondella, M. L., C. H. Paganelli, I. M. Bortolotto, O. A. Takano, K. Irino, M. C. Brandileone, B. Mezzacapa Neto, V. S. Vieira, and B. A. Perkins. 1994. Isolation of Haemophilus aegyptius associated with Brazilian purpuric fever, of Chloropidae (Diptera) of the genera Hippelates and Liohippelates. Rev. Inst. Med. Trop. Sao Paulo 36:105-109. (In Portugese.) [PubMed] [Google Scholar]
- 59.Tondella, M. L., F. D. Quinn, and B. A. Perkins. 1995. Brazilian purpuric fever caused by Haemophilus influenzae biogroup aegyptius strains lacking the 3031 plasmid. J. Infect. Dis. 171:209-212. [DOI] [PubMed] [Google Scholar]
- 60.Virata, M., N. E. Rosenstein, J. L. Hadler, N. L. Barrett, M. L. Tondella, L. W. Mayer, R. S. Weyant, B. Hill, and B. A. Perkins. 1998. Suspected Brazilian purpuric fever in a toddler with overwhelming Epstein-Barr virus infection. Clin. Infect. Dis. 27:1238-1240. [DOI] [PubMed] [Google Scholar]
- 61.Waldman, E. A., S. Takimoto, M. A. Ishida, C. Kitamura, and L. I. Mendonca. 1990. Enterovirus 70 in the metropolitan region of São Paulo, Brazil, from 1984 to 1987: infection aspects in endemic and epidemic periods. Rev. Inst. Med. Trop. Sao Paulo 32:221-228. (In Portuguese.) [PubMed] [Google Scholar]
- 62.Weeks, J. E. 1886. The bacillus of acute conjunctival catarrh, or ‘pink eye.’ Arch. Ophthalmol. 15:441-451. [DOI] [PubMed] [Google Scholar]
- 63.Weeks, J. E. 1887. The pathogenic microbe of “acute catarrhal conjunctivitis.” N. Y. Med. Rec. 31:571-579. [Google Scholar]
- 64.Weeks, J. E. 1895. The status of our knowledge of the aetiological factor in acute contagious conjunctivitis. N. Y. Eye Infirmary Rep. January: 24-36.
- 65.Weyant, R. S., W. F. Bibb, D. S. Stephens, B. P. Holloway, W. F. Moo-Penn, K. A. Birkness, L. O. Helsel, and L. W. Mayer. 1990. Purification and characterization of a pilin specific for Brazilian purpuric fever-associated Haemophilus influenzae biogroup aegyptius (H. aegyptius) strains. J. Clin. Microbiol. 28:756-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Whitney, A. M., and M. M. Farley. 1993. Cloning and sequence analysis of the structural pilin gene of Brazilian purpuric fever-associated Haemophilus influenzae biogroup aegyptius. Infect. Immun. 61:1559-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wild, B. E., J. W. Pearman, P. B. Campbell, P. K. Swan, and D. L. Garry. 1989. Brazilian purpuric fever in Western Australia. Med. J. Aust. 150:344-346. [DOI] [PubMed] [Google Scholar]