Abstract
Summary: Blastocystis is an unusual enteric protozoan parasite of humans and many animals. It has a worldwide distribution and is often the most commonly isolated organism in parasitological surveys. The parasite has been described since the early 1900s, but only in the last decade or so have there been significant advances in our understanding of Blastocystis biology. However, the pleomorphic nature of the parasite and the lack of standardization in techniques have led to confusion and, in some cases, misinterpretation of data. This has hindered laboratory diagnosis and efforts to understand its mode of reproduction, life cycle, prevalence, and pathogenesis. Accumulating epidemiological, in vivo, and in vitro data strongly suggest that Blastocystis is a pathogen. Many genotypes exist in nature, and recent observations indicate that humans are, in reality, hosts to numerous zoonotic genotypes. Such genetic diversity has led to a suggestion that previously conflicting observations on the pathogenesis of Blastocystis are due to pathogenic and nonpathogenic genotypes. Recent epidemiological, animal infection, and in vitro host-Blastocystis interaction studies suggest that this may indeed be the case. This review focuses on such recent advances and also provides updates on laboratory and clinical aspects of Blastocystis spp.
INTRODUCTION
Blastocystis is an unusual enteric protozoan parasite of humans and many animals (233, 250). It has a worldwide distribution and is often the most commonly isolated organism in parasitological surveys (6, 8, 21, 25, 179, 198). The parasite has been described since the early 1900s (12, 37), but only in the last decade or so have there been significant advances in our understanding of Blastocystis biology. However, the pleomorphic nature of the parasite and the lack of standardization in techniques have led to confusion and, in some cases, misinterpretation of data. This has hindered laboratory diagnosis and efforts to understand its mode of reproduction, life cycle, prevalence, and pathogenesis. Accumulating epidemiological, in vivo, and in vitro data strongly suggest that Blastocystis is a pathogen. Many genotypes exist in nature, and recent observations indicate that humans are in reality host to numerous zoonotic genotypes (1, 169). Such genetic diversity has led to a suggestion that previously conflicting observations on its pathogenesis are due to pathogenic and nonpathogenic genotypes (53). Recent epidemiological, animal infection, and in vitro host-Blastocystis interaction studies suggest that this may indeed be the case. This review will focus on such recent advances and also provide updates on laboratory and clinical aspects of Blastocystis spp. Excellent reviews on various topics in Blastocystis biology, including historical perspectives on parasite biology, animal isolates, and pathogenesis, were reported elsewhere previously (33, 233, 250, 256, 319).
CLASSIFICATION
Genetic Diversity
Blastocystis spp. from humans and animals have been reported to be morphologically similar. This is probably an oversimplification, as there have been reports describing distinct morphological differences among Blastocystis isolates (219, 236, 237, 303). However, it is nevertheless challenging to differentiate one isolate from another based on morphological criteria alone. Interestingly, extensive genetic variation has been observed among numerous isolates from both humans and animals. A number of molecular techniques to study the genetic diversity of Blastocystis spp. have been described. The techniques commonly employed are PCR-restriction fragment length polymorphism (RFLP) (2-4, 31, 53, 113, 202, 223, 271, 305), PCR followed by dideoxy sequencing (1, 17, 100, 169, 170, 214, 218, 231, 271, 304), and PCR with subtype-specific (sequence-tagged site [STS]) primers (2-4, 117, 119-121, 124, 128, 129). A few studies employed the use of arbitrary primed PCR (103, 125) or karyotyping (38, 99, 219, 276). Clark (52, 53), by PCR-RFLP of the entire small-subunit rRNA (ssrRNA) gene, revealed a remarkable amount of genetic variation that existed among 30 randomly selected human isolates. These RFLP profiles (riboprints) could be grouped into seven distinct genotypes (ribodemes). It was previously observed that there was a 7% divergence between ribodemes 1 and 2, which is approximately four times the genetic distance between homologous genes of Entamoeba histolytica and Entamoeba dispar (53). In the most extensive phylogenetic study to date, Noël et al. (169) analyzed the ssrRNA genes of 12 Blastocystis isolates from humans, rats, and reptiles together with 78 other Blastocystis sequences available in the GenBank database at the time of the study. They showed that Blastocystis spp. could be unambiguously placed within seven distinct clades, with six of the major groups comprising isolates from both humans and animals. Those authors concluded that numerous zoonotic isolates existed, with frequent animal-to-human and human-to-animal transmissions, and that animals represent a large potential reservoir for human infections. Thus, in the absence of genotype information and due to the extreme genetic diversity among Blastocystis isolates, caution is warranted when interpreting data or when extrapolating observations of morphology, drug sensitivity, and pathogenesis from one isolate to another.
Phylogenetic Studies and Identification of Isolates to the Species Level
The taxonomic classification of Blastocystis spp. has proven challenging and was only recently unambiguously placed within the stramenopiles despite the application of modern molecular phylogenetic approaches (18, 100, 218). The organism was initially classified as the cyst of a flagellate, vegetable, yeast, and fungus (319). It was subsequently reclassified as a protist by Zierdt and colleagues (319, 322) based on a number of protistan features, viz., one or more nuclei, smooth and rough endoplasmic reticulum, Golgi bodies, and mitochondrion-like organelles; it failed to grow on fungal media and was resistant to antifungal drugs but was sensitive to the antiprotozoal drugs metronidazole (Flagyl) and emetine (319, 322). The subsequent molecular analysis of Blastocystis ssrRNA and elongation factor 1α (EF-1α) gene sequences resulted in disparate conclusions on its taxonomic and phylogenetic affiliations. An earlier analysis of the ssRNA genes showed that Blastocystis sp. is not monophyletic with the yeasts, fungi, sarcodines, or sporozoans (108) and should be placed among the stramenopiles (100, 218). In contrast, studies involving EF-1α suggested that Blastocystis spp. diverged before the stramenopiles and may be a close relative of Entamoeba spp. (97, 158). This apparent discrepancy may be explained statistically by the low bootstrap value (58.1) used to group Blastocystis spp. with E. histolytica. Other possibilities for the variation in affinities exist. They may be due to the choice of genes for analysis. The ssrRNA genes have been known to possess drastic G+C content variation among species (93), which may give rise to misleading trees, and hence, other genes (e.g., EF-1α, EF-2, and RNA polymerase III large subunit) with less extreme biases have been suggested to be better candidates for phylogenetic studies. Other possible factors contributing to the discrepancy include inadequate species sampling, relatively few informative positions, mutational saturation, and long-branch attraction phenomena (186, 203, 204). A later study involving sequences from multiple conserved genes sought to resolve this discrepancy (18). The molecular analysis of Blastocystis ssrRNA, cytosolic-type 70-kDa heat shock protein, translation elongation factor 2, and the noncatalytic “B” subunit of vacuolar ATPase clearly demonstrated that Blastocystis is a stramenopile. The stramenopiles, synonymous with Heterokonta and Chromista (42), are a complex collection of “botanical” protists comprising heterotrophic and photosynthetic representatives. Molecular phylogenetic studies indicated that Blastocystis sp. is most closely related to Proteromonas lacertae (18, 100, 218), a flagellate of the hindgut of lizards and amphibians. Members of the stramenopile group are characterized by possessing flagella with mastigonemes (hair-like projections that extend laterally from the flagellum). Interestingly, Blastocystis sp. does not possess flagella, is nonmotile, and is therefore placed in a newly created class, class Blastocystea, subphylum Opalinata, infrakingdom Heterokonta, subkingdom Chromobiota, kingdom Chromista (41). A recent phylogenetic study showed that, based on the genetic distance between homologous genes, Blastocystis spp. from humans and animals can be potentially divided into 12 or more species (169). This and other studies have confirmed that Blastocystis genotypes are prevalent throughout the animal kingdom, with a number of genotypes comprising isolates from both humans and animals (1, 3, 4, 169, 214, 271, 295, 300, 305). In this regard, the practice of assigning Blastocystis species according to host origin poses a problem and has probably resulted in confusing reports regarding variations in pathogenesis and cell biology, since these differences could be attributed to distinct genotypes. Table 1 illustrates new designations for some well-studied human and animal isolates based on a recently published consensus terminology for Blastocystis sp. (229). Humans can be host to Blastocystis spp. from various mammals (subtype 1), primates and pigs (subtype 2), rodents (subtype 4), cattle and pigs (subtype 5), and birds (subtypes 6 and 7) (169, 295). Subtype 3 is the most frequently isolated genotype in epidemiological surveys and is probably the only genotype of human origin (31, 113, 291, 304). By phylogenetic analysis, subtypes 8 and 9 cluster most closely to subtypes 4 and 6, respectively (169, 214). We have very little information on both these subtypes except that subtype 8 has been reported in three studies (1, 214, 228), from monkeys, a pheasant, and humans, while subtype 9 was observed in 2 human isolates out of 102 isolates in an epidemiological study (304). These distributions of specific genotypes among various animal hosts should be viewed as tentative and would, in due course, become more representative as additional studies are performed. For example, subtype 5 is currently accepted as being the main Blastocystis genotype in pigs due the its high prevalence in this host (1, 295, 297). However, other studies (163, 271) showed that subtype 1 may dominates in pigs. Two recent reports (163, 201) revealed that pigs may harbor subtype 2, which, until those studies, comprised isolates only from humans and primates.
TABLE 1.
Old and new classification of commonly studied Blastocystis isolates based on consensus terminologya
| Species | Isolate(s) | Culture type | Host | New designation | References |
|---|---|---|---|---|---|
| B. hominis | Nand II | Axenic | Human | Blastocystis sp. subtype 1 | 169, 218 |
| B. hominis | Si | Axenic | Human | Blastocystis sp. subtype 1 | 164, 169 |
| B. hominis | B, C, E, G, H | Axenic | Human | Blastocystis sp. subtype 7 | 98, 169 |
| B. ratti | S1, WR1, WR2 | Axenic | Rat | Blastocystis sp. subtype 4 | 45, 169 |
| Blastocystis sp. | NIH:1295:1 | Xenic | Guinea pig | Blastocystis sp. subtype 4 | 169, 306 |
Terminology proposed by Stensvold et al. (229).
Blastocystis cells often possess one or two nuclei, and occasionally, quadrinucleate cells and cells possessing numerous nuclei have been reported (65, 142, 146, 319, 321). Whether these multinuclear states impact molecular and phylogenetic analyses is currently not known. Karyogamy and the exchange of genetic material have recently been demonstrated in the binucleate enteric protozoan parasite Giardia intestinalis (189). It is unknown if Blastocystis also undergoes such a process, and if so, the implications of such a phenomenon for the construction of phylogenetic trees should be ascertained. Future studies should aim to elucidate the ploidy of the Blastocystis genome and to understand if it is an asexual or sexual parasite, since such characteristics, which affect the extent to which genes evolve, can have major implications for how molecular phylogenetic data are interpreted (29, 174).
BIOLOGY
Morphological Forms
Blastocystis is a polymorphic protozoan, and four major forms have been described in the literature (Fig. 1 to 5). In reality, Blastocystis spp. can present with a bewildering array of forms within a single culture, and it may be difficult to assign a specific form to the cell in question (Fig. 1A and C). The extensive variation in Blastocystis forms has made studies of its cell biology challenging, resulting in misinterpretations of data from time to time. The central vacuole form, sometimes referred to as the central body form, is the most frequently observed form in laboratory culture and in stool samples. It is spherical and may display large size variations, ranging from 2 to 200 μm (average of 4 to 15 μm) (233). Extensive size variation can occur within and between isolates (65, 197). Dunn et al. (65) observed size variations of 4 to 63 μm among 10 human isolates; the mean diameters between stocks also varied significantly, with overlap between some isolates. A study of Blastocystis strains isolated from chickens revealed vacuolar forms ranging from 3 to 120 μm in diameter (132). The vacuolar form is characterized by a large central vacuole that occupies approximately 90% of the cell's volume (142, 233) (Fig. 1A, 2A, and 5C to E). This relegates the cytoplasm and organelles into a thin peripheral rim, which may sometimes be difficult to visualize under light microscopy (Fig. 5A and C). Nuclei and mitochondrion-like organelles are usually located within thickened cytoplasmic regions at opposite ends of the cell (Fig. 5E). Certain amphibian isolates possess thick cytoplasmic rims, which are easily discernible by conventional light microscopy (219). The central vacuole may appear empty or may contain fine to flocculant material. It was reported to contain carbohydrates, evidenced by positive staining with periodic acid-Schiff and Alcian blue staining (299), or lipids, evidenced by Sudan black B and Nile blue staining (302), suggesting a storage role for the organelle. The vacuole has also been suggested to play a role in schizogony-like reproduction (220, 241) by providing an environment for the development of minute parasite progeny. This is highly unlikely considering that these progeny appear strikingly similar to metabolic granules described previously and are therefore simply variants of the granular form (257, 290). Cytoplasmic contents, often containing organelles, may invaginate and deposit filament- or vesicle-like membrane-bound structures into the central vacuole (65, 159, 160, 181, 235, 239, 252). The exact significance of this process is unclear, although it has been postulated to be a mechanism of apoptotic body deposition in Blastocystis cells undergoing programmed cell death (254). The cytoplasm contains organelles typically observed in eukaryotes. The features observable by transmission electron microscopy (TEM) include one or more nuclei, Golgi apparatus, endosome-like vacuoles, microtubules, and mitochondrion-like organelles (Fig. 2 and 4). The organism is often surrounded by a surface coat (Fig. 2B), sometimes referred to as the fibrillar layer or capsule, of various thicknesses (65). The surface coat is often thicker in parasites freshly isolated from feces and gradually thins out during prolonged laboratory culture (40, 235), and cells without surface coats have been observed in vitro (65). The reason for this thinning out is unknown but may be due to the postulated role of the coat in trapping bacteria for nutritional purposes (311, 314), which is not possible during axenic culture, or may be unnecessary if nutrients provided in laboratory culture are sufficient for growth. The surface coat contains a variety of carbohydrates (127, 258) and has been postulated to play a role in trapping and degrading bacteria for nutrition (311, 314), protecting against osmotic shock (40), or to provide a mechanical barrier for functionally important plasma membrane proteins from the immune system (259).
FIG. 1.
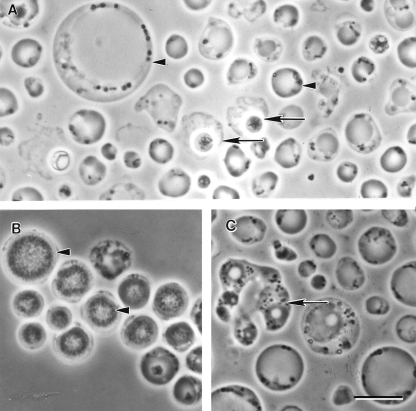
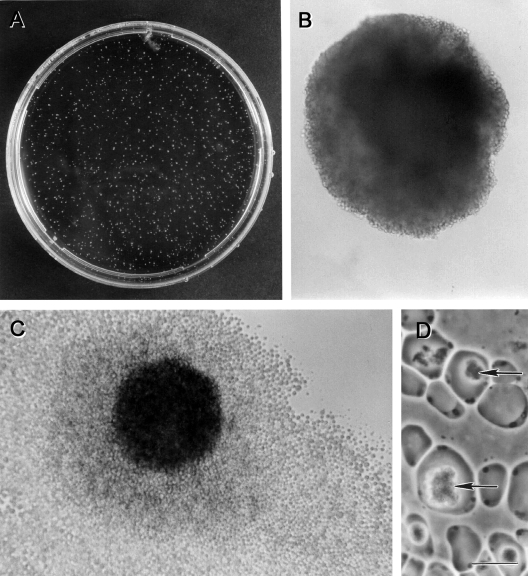
Morphological forms of Blastocystis sp. subtype 4 by phase-contrast microscopy. (A) Vacuolar and fecal cyst forms from in vitro axenic culture displaying extensive size variation (arrowheads). Note the refractile appearance and loose outer coat of cysts (arrows). (B) Granular form with distinct granular inclusions within the central vacuole (arrowhead). (C) Amoeboid forms occasionally seen in culture showing pseudopod-like cytoplasmic extensions (arrow). Bar, 10 μm.
FIG. 5.
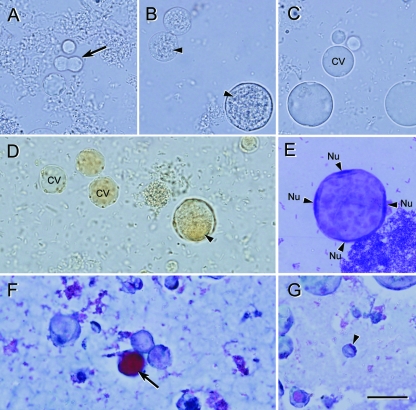
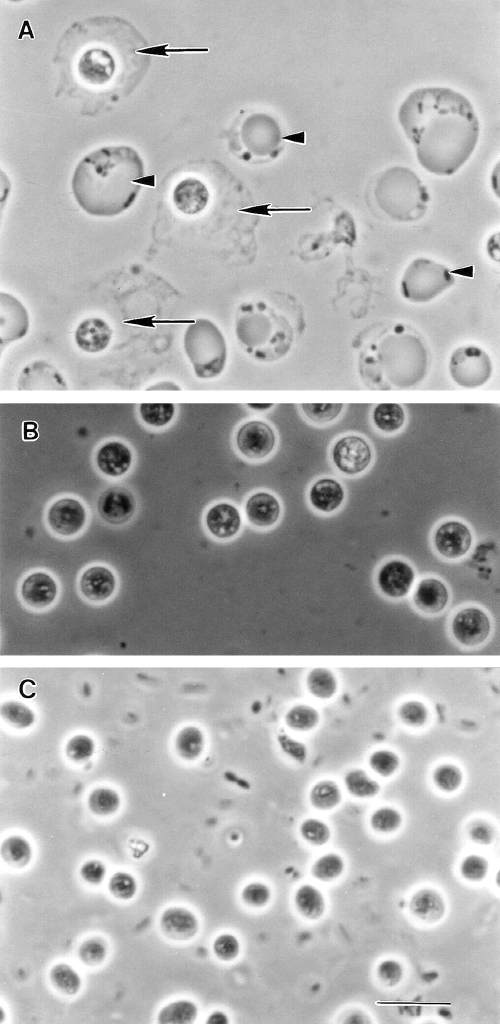
Light microscopy of nonaxenic Blastocystis sp. subtype 1 cultured in Jones’ medium. (A to C) Unstained wet mounts of various diagnostic forms of Blastocystis. (A) Cells undergoing binary fission (arrow). (B) Granular forms commonly seen in laboratory culture. (C) Vacuolar forms with virtually indiscernible thin cytoplasmic rims. (D) Iodine-stained wet mount revealing cells with distinct organelles, a cytoplasmic rim, and granular inclusions (arrowhead). (E) Giemsa-stained permanent smear of the large vacuolar form containing four nuclei (Nu) evenly distributed around the cytoplasmic rim. (F and G) Trichrome-stained permanent smear of vacuolar forms. The central vacuole of some cells stain strongly (arrow), while others stain less intensely; this may be due to the biochemical heterogeneity of the vacuolar contents. (G) Small vacuolar form (arrowhead) revealing organelles within the cytoplasmic rim. Bar, 20 μm.
FIG. 2.
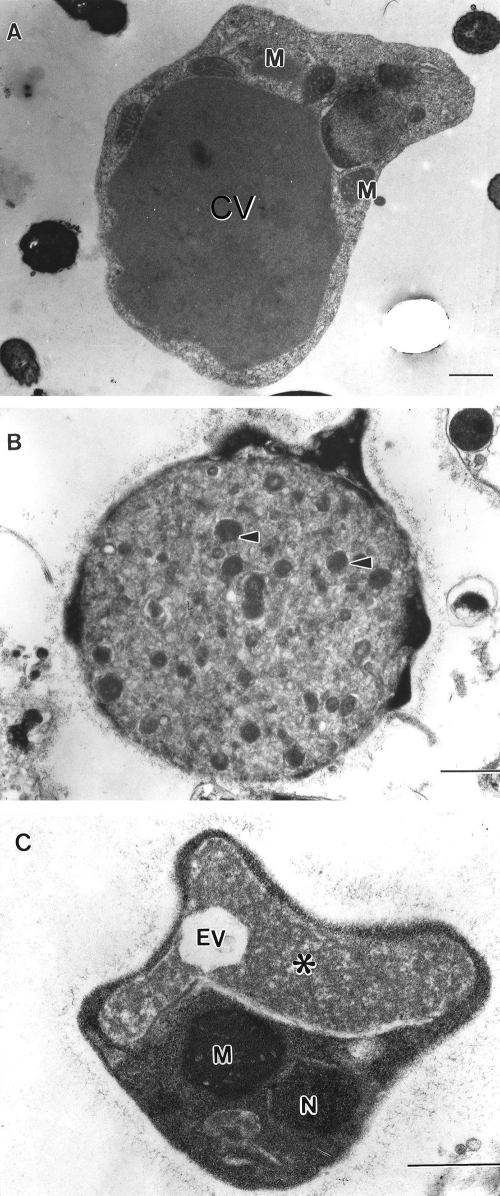
Transmission electron micrographs of Blastocystis sp. subtype 4. (A) Vacuolar form revealing large central vacuole (CV) resulting in a thin band of peripheral cytoplasm. (B) Granular form revealing electron-dense granules (arrowheads) occupying the entire central vacuole. Note the surface coat surrounding the parasite. (C) Irregular-shaped amoeboid form with central vacuole (asterisk) and empty vacuole (EV). M, mitochondrion-like organelle; N, nucleus. Bar, 1 μm.
FIG. 4.
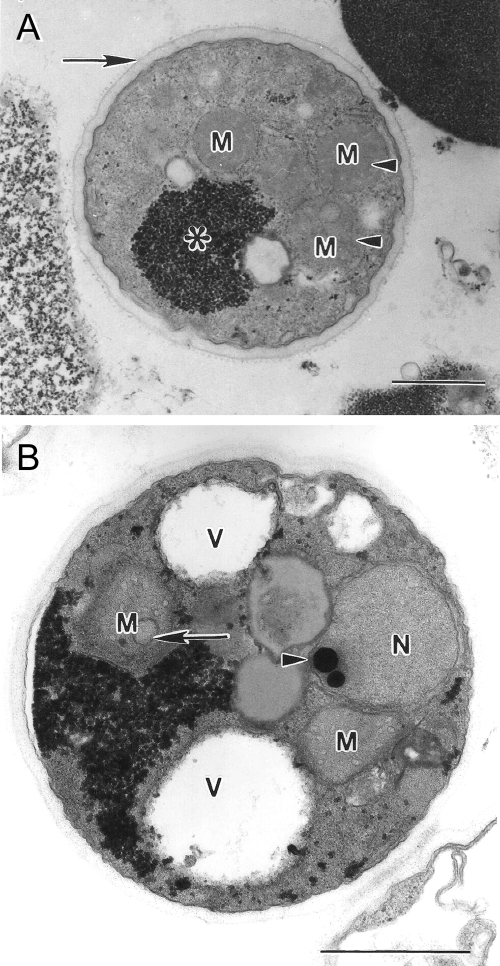
Transmission electron micrographs of Blastocystis sp. subtype 4 cysts. (A) Mature cyst with a distinct double-layered cyst wall (arrow) and reduced glycogen mass (asterisk). Mitochondrion-like organelles (M) contain faint saccate cristae (arrowhead). (B) Mature cyst containing large vacuoles (V), a nucleus (N) with a dense chromatin mass (arrowhead), and mitochondrion-like organelles (M) with saccate and circular (arrow) cristae. Bar, 1 μm.
The granular form resembles the vacuolar form except that granules are present within the cytoplasm or, more commonly, within the central vacuole of the organism (Fig. 1B and 2B and 5B). These are more frequently observed in nonaxenized, older, and antibiotic-treated cultures and have been described as being vacuolar forms containing granules rather than as being a distinct parasite stage (33, 233). The intracellular granules are heterogeneous and have been described as being myelin-like inclusions, small vesicles, crystalline granules, and lipid droplets (65). Reproductive granules within the central vacuole have been described and have been reported to function in schizogony-like division (220, 241, 319). Other authors argued that this cannot be accepted due to the lack of evidence that these granules are indeed viable and develop into Blastocystis cells (33, 233, 257, 290).
The amoeboid form (Fig. 1C and 2C) of Blastocystis spp. is rarely reported, and there are contradicting descriptions of what constitutes this morphological type. An early report described numerous amoeba-like forms in the diarrheal fluid of a patient who died of aspiration pneumonia (326). These cells were irregularly convoluted, and some cells possessed one or two large pseudopods. In another study, amoeboid forms from in vitro culture were observed to be 10 to 15 μm, possessing features typical of vacuolar forms, with the exception of one or two pseudopods (249). We have reported the presence of numerous amoeboid forms (Fig. 6) from Blastocystis colonies grown in soft agar (45, 252, 260, 261). Light microscopy and TEM showed cells with a central vacuole, a surface coat, and numerous Golgi bodies and mitochondria within the cytoplasmic extensions of pseudopods (252). Dunn et al. (65) previously described amoeboid cells ranging from 2.6 to 7.8 μm with extended pseudopodia and lysosome-like compartments containing ingested bacteria. In contrast to our studies, these forms lacked a central vacuole, a Golgi complex, a surface coat, and mitochondria (65). Considering the genetic diversity of the organism, it is plausible that the differing descriptions are due to genotypic variations among Blastocystis isolates. The presence of bacteria and bacterial remnants within the amoeboid form suggests a nutritional role for this form. The amoeboid form has been postulated to play a role in pathogenesis (115, 262, 264). However, the light and TEM micrographs in two of these reports (262, 264) were unconvincing for this form and appear more like irregularly shaped central vacuole forms, a common artifact of TEM processing. Despite the observation of pseudopod-like cytoplasmic extensions, the amoeboid form appears to be nonmotile. The identification of stage-specific molecular markers would be useful for studies of various developmental forms of the parasite and would obviate the problem of distinguishing the various forms by morphological criteria alone.
FIG. 6.
Colonies of Blastocystis sp. subtype 4 after 12 days of culture on an agar plate containing 0.3% Bacto agar in Iscove's modified Dulbecco's medium containing 10% horse serum. (A) Petri dish with numerous buff-colored colonies. (B and C) Magnified view of single colonies. (C) Occasionally, parasite cells spread out at the interface of the agar and the bottom surface of the plate. (D) Amoeboid cells isolated from a colony with large inclusions (arrows) within the central vacuole. Bar, 10 μm.
The cyst form (Fig. 1A and 3) is the most recently described form of the parasite, and the late discovery is due to its small size (2 to 5 μm), which can result in confusion with fecal debris, and the observation that cysts are infrequently seen in laboratory culture (46, 153, 154, 234, 312). The cysts are variable in shape but are mostly ovoid or spherical. The cyst is protected by a multilayered cyst wall (Fig. 4), which may or may not be covered by a loose surface coat (153, 312, 313). The cytoplasm of the cyst may contain one to four nuclei, mitochondria, glycogen deposits, and small vacuoles (5, 46, 153, 312). One report (237) described the presence of large multinucleate cysts from the stools of Macaca monkeys, which were 15 μm in size, and this was suggested to be an indication of differences among Blastocystis species. Blastocystis cysts were reportedly able to survive in water for up to 19 days at a normal temperature but are fragile at extreme temperatures and in common disinfectants (154). A later study (306) showed that cysts could survive up to 1 month at 25°C and 2 months at 4°C. The contrasting viabilities among the studies may be due to isolate variations. Vacuolar and granular forms, in contrast, are sensitive to temperature changes, osmotic shock, and exposure to air (146, 319). Experimental infectivity studies of BALB/c mice, Wistar rats, and a variety of bird species with the cyst form indicate that this form is undoubtedly the transmissible form of the parasite. The formation of in vitro-derived cysts by the incubation of vacuolar forms in encystation medium was reported previously (240, 241, 244, 282). These “cysts” appear to be curiously similar to the classical granular forms, and the granules within the central vacuole were reported to be reproductive in nature (240, 241, 243). It is likely that these are artifacts of culture induced by the encystation medium, as they bear no morphological similarity to the fecal cyst. Interestingly, in vitro-derived cysts are able to infect Wistar rats (244) and were apparently resistant to osmotic lysis (282).
FIG. 3.
Phase-contrast microscopy of Blastocystis cysts. (A) Spherical cysts of subtype 4 from an in vitro axenic culture displaying a loose outer coat (arrows) among vacuolar forms (arrowheads). (B) Enrichment of subtype 4 cysts and loss of outer coat are apparent after overnight incubation in distilled water. (C) Cysts from a human isolate revealing ovoid morphology distinct from the spherical cysts of subtype 4. Bar, 10 μm.
Other forms have also been described, and these forms include the avacuolar and multivacuolar forms. These forms reported from TEM studies of fresh stool samples were significantly smaller (5 to 8 μm) than culture forms and were suggested to be the form that occurs in vivo (233, 235). However, others observed typical vacuolar forms from fresh fecal samples (9, 64, 128, 132, 227, 232, 281, 318). The central vacuole was absent in the avacuolar form, while the multivacuolar forms contained multiple small vacuoles. The small size and distinct multivacuolar or avacuolar morphology may be due to strain variations, or they are possibly cells in various stages of encystation or excystation, as similar morphologies were described in TEM studies of cells undergoing excystation (46, 153).
Life Cycle
Numerous conflicting life cycles have been proposed (33, 217, 220, 233, 250, 319, 321), and these discrepancies are due largely to the belief that Blastocystis exhibits multiple reproductive processes (83, 220, 318). The suggestion that Blastocystis undergoes multiple fission has led to life cycles where schizogony is one of the modes of reproduction (220, 319). This and other proposed modes such as plasmotomy (budding) (263), endodyogeny (318), and sac-like pouches (83, 242) are more likely due to the pleomorphic nature of the organism and not true modes of reproduction. A life cycle comprising thick- and thin-walled cysts from multiple fission was proposed (220). Those authors hypothesized that the thick-walled cysts are important for external transmission, while the thin-walled cysts were autoinfectious. There is little scientific evidence to support such a proposal, although schizogony-like reproduction in Blastocystis has been perpetuated in a number of authoritative sources, including medical parasitology textbooks and the DPDx website of the Centers for Disease Control and Prevention (www.dpd.cdc.gov/dpdx/HTML/Blastocystis.htm). Until proven otherwise, the only accepted mode of reproduction is binary fission. The application of live-cell imaging technology should provide a better understanding of the modes of reproduction of Blastocystis spp.
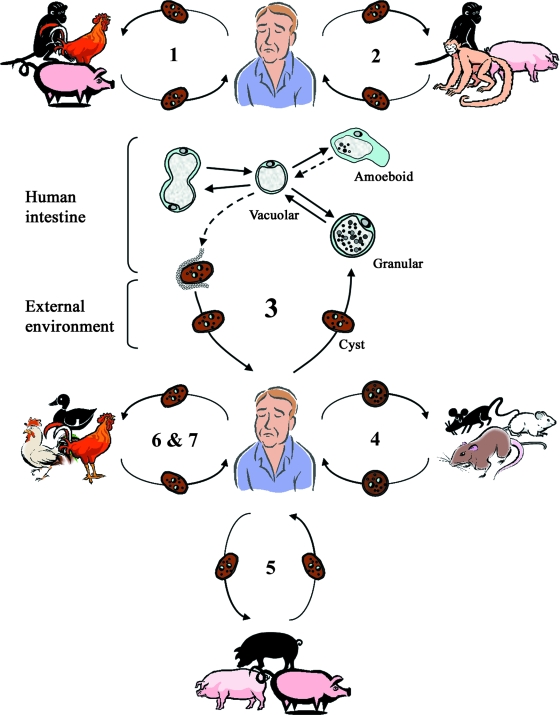
A revised life cycle (Fig. 7) must take into account the large reservoir of Blastocystis spp. among various animal populations and that humans are potential hosts to numerous zoonotic genotypes (subtypes). Upon ingestion of cysts, the parasite undergoes excystation in the large intestines and develops into vacuolar forms. Encystation occurs during passage along the large intestines and is deposited in the feces (152). The fecal cysts may be covered by a fibrillar layer that is gradually lost during cyst development (313).
FIG. 7.
Proposed life cycle for Blastocystis cells taking into account recent studies (163, 169, 201, 295) suggesting the existence of zoonotic genotypes (subtypes 1 to 7) with various host specificities. Humans and animals are infected by fecal cysts, which develop into vacuolar forms in the large intestines. In humans, vacuolar forms divide by binary fission and may develop into amoeboid or granular forms. Vacuolar forms undergo encystation in the host intestines, and intermediate cyst forms may be surrounded by a thick fibrillar layer that is subsequently lost during passage in the external environment. Information on the transition from the amoeboid to the vacuolar form and from the vacuolar to the cyst form is lacking. These hypothetical pathways are represented by dotted lines. Subtype 1 is cross-infective among mammalian and avian isolates; subtypes 2, 3, 4, and 5 comprise primate/pig, human, cattle/pig, and rodent isolates, respectively; and subtypes 6 and 7 include avian isolates. The proposed scheme suggests that humans are potentially infected by seven or more species of Blastocystis and that certain animals represent reservoirs for transmission to humans. (Adapted from reference 251 with permission from Taylor and Francis.)
Apart from a few studies, the transitions from one of the classically described forms to another are not well understood. TEM studies of the development of cysts to vacuolar forms were elegantly demonstrated with a human isolate and a rat isolate (46, 153). In those reports, fecal cysts from both humans and rat develop similarly and dramatically into vacuolar forms within 24 h of inoculation into growth medium. In one of those studies (153), cells undergoing excystation apparently developed from cysts into granular forms before becoming vacuolar in morphology. Whether these granular forms are similar to those from patient samples and laboratory culture is not known. In a separate study (317), Blastocystis cysts enriched from a patient sample were cultured in Jones’ medium and characterized by TEM at 24 h. The micrographs revealed that cell division of vacuolar forms occurs while the parasite is still within the cyst wall and that both granular and vacuolar forms were observed in the same sample. Because only one time point was performed, it is difficult to conclude the order in which these forms developed. Certain culture conditions were reported to induce the development of the granular form from the vacuolar form. These conditions include old cultures (250), axenization (327), transfer to a different culture medium (235), and increases in serum concentrations in the culture medium (65, 130, 217, 321). Amoeboid forms probably arise from vacuolar forms. Some evidence for this is seen when vacuolar forms are cultured in agar, and after incubation, the resultant colonies contain numerous amoeboid forms (260, 261).
LABORATORY DIAGNOSIS
Microscopy
Blastocystis poses considerable challenges for the diagnostic laboratory. Firstly, the uncertain pathogenesis of the parasite discourages many clinicians from considering Blastocystis to be the etiological agent of disease. Secondly, the polymorphic nature of the organism in wet mounts can result in confusion with yeast, Cyclospora sp., or fat globules. The classical vacuolar forms may not predominate in fresh fecal specimens (235), while the smaller fecal cyst, when present, may be difficult to identify. Direct microscopy is usually done with stained specimens. Multiple stool specimens should be examined, since the parasite may exhibit irregular shedding (88, 281). Morphological features that may aid in the laboratory diagnosis of Blastocystis infection are summarized in Table 2. Wet mounts with Lugol's iodine (Fig. 5D) and permanent-stained smears with acid-fast, Giemsa (Fig. 5E), Field's, and trichrome (Fig. 5F and G) stains have been described, with trichrome being the most popular stain employed (8, 171, 175, 179, 210, 227, 270, 289, 307). Trichrome is a routinely employed stain in many clinical microbiology laboratories, and studies have shown that it is more sensitive for the detection of intestinal protozoa than iodine-stained wet mounts (71, 78, 117), and this is also the case for Blastocystis spp. (179, 270). However, short-term (24 to 72 h) in vitro culture increases the sensitivity of detection compared to that of direct microscopy of fecal smears stained with Lugol's iodine or trichrome (135, 227, 270), although one study (245) did not mentioned the use of any staining method. However, in the case of mixed infections, in vitro culture may favor the preferential amplification of one subtype over another (183, 295), although this was not seen in another study involving a mixed infection (227). In contrast to in vitro culture, a number of reports indicated that the formol ethyl acetate concentration technique (FECT) results in very poor sensitivity for parasite detection (227, 231, 245). Subtype 3 was apparently associated with false-negative results associated with this method (227), although the reason for this bias is currently unknown. This may explain a study in Thailand, employing only formol ethyl acetate concentration, revealing a prevalence of 0.19% for Blastocystis isolates in primary school children in central Thailand (211). Another study using a number of diagnostic approaches (simple smear, formol ether concentration, Boeck and Drbohlav's Locke-egg-serum medium culture, and modified acid-fast and modified trichrome staining) showed the prevalence of Blastocystis isolation to be 45.2% among Thai children in the same region (210). Present-day diagnostic laboratories should also include the fecal cyst as an indicator of infection. If necessary, these cysts can be selectively concentrated by density gradient approaches to increase sensitivity (309, 315). This enrichment approach may be more practical in the research laboratory setting since it may be cumbersome to perform during routine diagnosis or in large-scale surveys. It was suggested that the intensity of infection should be reported, and the general criteria are whether five or more parasites are seen in a high-powered field (×400) for wet mounts (77, 133, 166, 188, 194, 215, 319) or under oil immersion (×1,000) (175, 179, 307) if permanent-stained smears are used. Using this criterion and in comparison with reports that do not use this criterion, accumulating studies suggest a correlation between infection density and symptoms (51, 60, 67, 82, 85, 111, 116, 155, 165, 167, 175, 177, 215, 307). There are, however, a number of studies that reported a lack of such a correlation (63, 86, 131, 145, 180, 216). The reason for this discrepancy is presently unclear but may be due to genotype differences among Blastocystis isolates or to host factors such as age and genetic background variations in the populations studied.
TABLE 2.
Diagnostic features of Blastocystisa
| Stage or form | Size (μm) | Presence of central vacuole | No. of nuclei | Area(s) of occurrence | Description | Reference(s) |
|---|---|---|---|---|---|---|
| Vacuolar | 2-200 (usually 5-15) | Present | 1-4 (usually 1-2) | Culture, feces | Central vacuole occupies 70-90% of cell vol; occasionally, giant cells with multiple nuclei are seen | 142, 233 |
| Granular | 6.5-8 | Present | 1-4 | Feces, culture | Granules within central vacuole | 233 |
| Multivacuolar | 5-8 | Present or absent | 1-2 | Feces, culture | Rarely seen | 233, 235 |
| Avacuolar | ∼5 | Absent | 1-2 | Intestine, feces | Rarely seen in feces | 233, 235 |
| Amoeboid | 2.6-7.8 | Present or absent | 1-2 | Feces, culture | Rarely reported; conflicting information on morphology | 65, 260, 326 |
| Cyst | 3-10 | Absent | 1-4 | Feces, culture | Rarely seen in culture; cyst wall present; may be surrounded by a fibrillar layer | 233, 309, 310 |
Adapted from reference 233 with permission.
Laboratory Culture
Xenic or monoxenic laboratory cultures of Blastocystis isolates, which are cultures of Blastocystis cells grown in association with nonstandardized or single known species of microorganisms, respectively, can be maintained in Jones’ (110) or Boeck and Drbohlav's inspissated egg (30) medium. Jones’ medium is the medium of choice in studies involving culture to identify the parasite in patient samples (135, 183, 227, 231, 245, 270, 291). There was one report (183) on the inability to grow Blastocystis isolates from Australian marsupials in Jones’ media, suggesting that this medium may not support fecal cultures from certain animal hosts. Other studies utilized diphasic agar slant medium, which was useful for the culture of Blastocystis isolates from cattle, pigs, and chickens (2-4, 109, 110). Axenized cultures, that is, cultures of Blastocystis cells not associated with any other living organism, display luxuriant growth in a variety of media such as Iscove's modified Dulbecco's medium, minimal essential medium, or biphasic inspissated egg slant overlaid with Locke's solution (98, 324). Cell densities of up to 2.5 × 107 cells/ml can be attained for monophasic medium (98), while slightly higher densities of approximately 6.0 × 107 cells/ml were reported for cells cultured in biphasic inspissated egg medium (324). The doubling time of axenic isolates can be variable, ranging from 6 to 23 h, depending on the isolate, study, and type of medium used (33, 324). Doubling times of approximately 50 h can be deduced from growth curves of Blastocystis isolates belonging to avian subtype 7 (98, 169), and this may be due to the nonoptimal incubation temperature, as avian hosts, particularly chickens, generally have higher body temperatures than mammals. The colony growth of Blastocystis cells can be established in soft agar (Fig. 6) using the pour plate method (260, 261, 277), and clonal growth was achieved with the addition of sodium thioglycolate as a reducing agent (260). The technique was useful as a step toward the axenization of Blastocystis isolates by physically isolating parasite colonies from bacterial ones (45, 164) and for screening surface-reactive antibodies for cytotoxic activity (259). Blastocystis cells are also able to grow on solid medium, and parasite clones appear to be macroscopically similar to bacterial colonies (255). These cultures were viable for up to 2 weeks and could be further expanded in liquid or solid medium. Interestingly, for the same isolate grown in liquid medium (98), cultures reach maximal cell densities around 4 days postinoculation, enter death phase at day 5, and are subsequently difficult to subculture. This indicates that the growth characteristics of the same Blastocystis isolate in solid medium are markedly different from those of the isolate in liquid medium.
Axenic cultures of Blastocystis isolates are important for molecular and biochemical studies. Axenization can be achieved by the addition of antibiotic cocktails to eliminate contaminating bacteria and yeasts, and a variety of antibiotic mixtures have been described, with various levels of success (45, 128, 164, 269, 319). The process is generally laborious and may take weeks to months, and the successful elimination of microbial contaminants is not guaranteed. It has been suggested that some isolates require the presence of bacteria to survive, and therefore, the removal of all bacteria results in the death of the parasite (319). Lanuza et al. (128) previously described an improved method for Blastocystis axenization and managed to axenize 25 out of 81 isolates using a combination of Ficoll-metrozoic acid gradient and the addition of antibiotics. Cultures were initially treated with a basic antibiotic solution comprising 0.4% ampicillin, 0.1% streptomycin, and 0.0006% amphotericin B, and in subsequent subcultures, resistant bacteria were isolated and antimicrobial assays performed. The final stages of axenization involved the addition of antibiotics against remaining bacteria with density gradient enrichment for Blastocystis spp. The time required for axenization was approximately 3 weeks. In addition to antibiotic treatment, some authors noted improved success when physical methods were employed during axenization to separate parasites from the bulk of the bacterial load. This includes differential centrifugation (269), density gradient separation (128), and colony growth (45, 164). Such methods enrich for the parasite and provide a growth advantage. Chen et al. (45) noted that the axenization of Blastocystis would not have been possible without parasite enrichment via colony growth. There was a report on the use of micropipette manipulation to isolate Blastocystis clones from nonaxenic cultures of turkey cecal contents (95), and it would be interesting to investigate if this technique could be exploited as a step toward axenization.
Serology
Blastocystis infections lead to immunoglobulin G (IgG) and IgA responses, as detected by indirect fluorescent antibody (IFA) testing and enzyme-linked immunosorbent assay (ELISA) (104, 112, 143, 323, 328). ELISA titers ranged from 1:50 to 1:1,600 (328), and previously reported studies revealed that high titers are associated with symptomatic infections (104, 143, 323, 328). Early ELISA studies showed that sera from patients harboring Blastocystis spp. had high IgG titers against parasite extracts (323, 328). In one of the studies (328), 30 sera from 28 patients were tested: 3 were negative at the 1/50 threshold dilution, 8 were positive at 1/50, 3 were positive at 1/100, 2 were positive at 1/200, 3 were positive at 1/400, 6 were positive at 1/800, and 5 were positive at 1/1,600. Normal sera (42 blood bank sera) were all negative at 1/50. Interestingly, IgA responses against Blastocystis spp. could not be detected in the symptomatic population. A recent study investigated the secretory IgA, serum IgA, and serum IgG levels in Blastocystis-positive individuals with and without symptoms by ELISA (143). This study showed that only sera from symptomatic patients had significantly higher Blastocystis-reactive secretory IgA, serum IgA, and serum IgG levels than did sera from asymptomatic carriers and healthy controls. In contrast, Kaneda et al. (112) showed by IFA that asymptomatic individuals harboring Blastocystis spp. possessed serum antibodies to the parasite, although antibody titers were very low, and serum dilutions greater than 1:60 failed to elicit a reaction. However, the strongest reaction was seen in an individual with chronic infection. It was suggested that constant exposure to the parasite was necessary to elicit a serological response. The 1/50 ELISA cutoff used in studies of symptomatic patients reported previously by Zierdt and colleagues (323, 328) is also rather low and suggests that the parasite induces a weak immune response. However, considering the genotypic (53, 169) and antigenic (122, 126, 156, 258, 298) diversity among morphologically identical isolates, the low values may be due to the choice of isolate used as the coating antigen. Such antigenic variations may also explain the discrepant observations among studies and must be taken into consideration should a serological test kit be developed. Monoclonal antibodies against Blastocystis spp. have been described (258, 298). The majority of antibodies were IgM and localized to surface coat antigens. These antibodies exhibited limited cross-reactivity against different genotypes, indicating antigenic diversity among Blastocystis isolates (253, 258). Although currently unavailable, monoclonal antibodies specific for human-infective genotypes would be useful for antigen detection studies, as was previously described for Entamoeba histolytica/E. dispar (283). Similarly, the application of genotype-specific antigens in ELISA or immunofluorescence formats should be useful for serological and epidemiological studies.
Blastocystis-associated symptoms are generally self-limiting and may last between 1 and 14 days (63, 131, 307, 320, 329). However, some infections persist for months if left untreated (90, 94, 112, 131, 165, 175), and it is currently unknown if chronic infections influence seropositivity. A single study (112) of the serological response of Blastocystis-positive asymptomatic individuals showed that the highest IFA titer was obtained from a healthy individual infected with Blastocystis for 2 years. An ELISA study reported previously by Zierdt et al. (328) included two patients who provided sera within the first 2 weeks postsymptom and subsequently another sample at convalescence, about 6 weeks after onset. Comparison of acute-phase sera with convalescent-phase sera revealed an eightfold increase for one patient and a threefold increase for the other patient. Although large-scale studies are needed to validate these observations, results of those studies suggested that Blastocystis sp. does elicit an immune response, and both chronic and acute infections can result in significantly higher antibody titers than asymptomatic infections. Currently, considering our limited knowledge of the host immune response to Blastocystis spp. and the apparent antigenic diversity of the parasite, it is not practical to include serology in the routine laboratory diagnosis of Blastocystis, and it should be limited to epidemiological and serological studies.
Molecular Approaches
Molecular PCR-based diagnostic approaches for Blastocystis identification have been described. Subtype-specific diagnostic primers, also referred to as STS primers, were developed from random amplification of polymorphic DNA analysis of Blastocystis isolates by Yoshikawa et al. (296, 297, 301), and these approaches amplified seven distinct subtypes, which corresponded to different clades inferred from ssrRNA. Such an approach has been shown to be useful for epidemiological studies, providing information on the distribution of various genotypes among human and animal populations (2, 4, 138, 297, 304) and on the zoonotic nature of certain genotypes.
Other groups characterized isolates by PCR of ssrRNA followed by RFLP analysis (2-4, 31, 53, 202, 271, 291, 295, 305), dideoxy sequencing (183, 201, 227, 230, 231), or nested amplification of intragenic regions (270). PCR-RFLP analysis of the Blastocystis ssrRNA gene is commonly employed for prevalence studies, and a variety of primers for its amplification have been described (31, 53, 227, 291). However, major limitations of this approach are the lack of standardization of the conditions and choice of primers, mutations at restriction sites, and the difficulty in interpreting RFLP profiles from mixed infections. A high-throughput pyrosequencing technique for the rapid sequencing of the Blastocystis ssrRNA gene was described (230). This approach detects nucleotide polymorphisms in the gene and was able to genotype 48/48 Danish isolates in approximately 1 h but was unable to detect mixed-subtype infections. This approach would be extremely useful for large-scale epidemiological studies and for the rapid identification of genotypes during outbreak situations (87, 114, 123). A recent study compared the relative performances of various diagnostic methods for the identification of Blastocystis isolates (227). The FECT, permanent trichrome staining of feces fixed in sodium acetate-acetic acid-formalin, in vitro culture, and PCR approaches were compared using 107 samples from 93 patients with suspected enteroparasitic disease. The PCR approach was shown to be superior to the other approaches, and detection of Blastocystis-specific DNA was as sensitive as the culture method. This is in contrast to data from a study reported previously by Termmathurapoj et al. (270) that suggested that in vitro culture expansion was superior to direct PCR from stool samples. Another study (183) revealed that direct PCR from stool samples was superior to the culture method, with PCR detecting Blastocystis spp. in 35% of the samples, while the culture method detected 19%. The discrepancies observed in studies reported previously by Termmathurapoj et al. (270) and Parkar et al. (183) could be attributed to the fact that the latter involved the culture of feces from various animals in Jones’ medium, which did not support parasite growth, and that their DNA extraction method was more efficient. Other possibilities include the different specificities of the PCR primers employed for these studies (227). A method for the detection of Blastocystis spp. directly from unpreserved stool samples was described and provides a rapid diagnostic tool for Blastocystis identification (231). Primers specific for Blastocystis ssrRNA were able to detect greater than 32 parasites/200 mg stool artificially spiked with cultured parasites. In the evaluation of 43 clinical specimens, the PCR approach was tested against FECT and a culture technique, proving 100% test specificity and a significantly higher sensitivity than FECT. In that study, there were instances where culture-negative samples were PCR positive. This was attributed to the degeneration of parasites in the stool or to low numbers that prevented growth in vitro. Jones et al. (109) recently reported a method for the real-time PCR detection of Blastocystis spp. from stools. Primers specific to an undefined 152-bp region of the parasite genome was used for the assay and was able to amplify 11 laboratory-cultured isolates from the ATCC belonging to subtypes 1, 3, and 4. Results could be obtained within 3 h, with a detection limit of 760 cells per 100 mg of stool. Oddly, only three clinical samples were used in that study, and only one ATCC strain was spiked into Blastocystis-negative stool samples to determine sensitivity. For specificity determinations, those authors excluded only cross-reactions with bacterial but not protozoal pathogens. The assay was not able to distinguish among Blastocystis subtypes.
In summary, a variety of methods for the laboratory diagnosis of Blastocystis spp. exist. The FECT should be discouraged due to low sensitivity. Trichrome staining of direct fecal smears is sensitive, provides a permanent record of the specimen, and should be supplemented with information on whether five or more parasites are visible per oil immersion (×1,000) field. Other authors included more detailed reporting on parasite abundance (11, 85, 131, 179, 180) and quantified parasite abundance using terms such as rare (one to two parasites in every 10 high-power fields), few to moderate (one parasite in every one to five high-power fields), or abundant (five or more parasites per high-power field) (131). Such detailed reporting, beyond whether five or more parasites are present, is unnecessary since there is some controversy regarding correlation between infection density and disease. In instances of low parasite levels or when fecal cysts predominate in stool or environmental samples, in vitro culture is a useful method for diagnosis (245, 246). Direct wet mounts stained with iodine do not seem to add additional value to the diagnostic process, since trichrome-stained permanent smears have been shown to be more sensitive (179, 270). Considering current data, trichrome staining of direct smears coupled with stool culture in Jones’ medium, cost permitting, is the best approach for diagnosing Blastocystis infection in terms of specificity and sensitivity. Future laboratory diagnosis may need to include genotype information once a link between genotype and parasite pathogenesis is firmly established. For screening and epidemiological studies, PCR amplification of Blastocystis DNA from fresh stools or stool cultures is a convenient alternative to microscopy, and genotyping should also be included in the analysis. The development of real-time PCR for the sensitive and rapid detection of Blastocystis spp. with the ability to discriminate between multiple genotypes within a sample would be similarly advantageous for screening and epidemiological studies.
CLINICAL ASPECTS
Epidemiology and Prevalence
Authors of early studies lamented the lack of epidemiological data on Blastocystis spp. (33, 233). However, recent years have shown a dramatic increase in prevalence studies, and these studies have shed light on the parasite's genotype distribution, mode of transmission, and pathogenesis.
Blastocystis is an extremely ubiquitous parasite with a worldwide distribution (107, 250). It is not uncommon for it to be the most frequently isolated parasite in epidemiological surveys (15, 21, 51, 72, 94, 185, 210, 248, 287, 289). Prevalence varies widely from country to country and within various communities of the same country. In general, developing countries have higher prevalences of the parasite than developed countries, and this has been linked to poor hygiene, exposure to animals, and consumption of contaminated food or water. Prevalence can be low in countries such as Japan (0.5 to 1%) (96, 101) and Singapore (3.3%) (291) and high in developing nations including Argentina (27.2%) (25), Brazil (40.9%) (6), Cuba (38.5%) (70), Egypt (33.3%) (198), and Indonesia (60%) (185). In some countries, the carriage rate can be rather variable, depending on the subpopulation studied. Prevalence ranges of 1.9 to 32.6%, 0.19 to 45.2%, and 1.04 to 18.3% in prevalence studies from China (137), Thailand (210, 211), and Turkey (7, 56), respectively, have been reported. Such variations within the same country could reflect true differences between communities, especially if the same techniques were employed to identify the parasite. However, variations are also likely due to the use of different diagnostic approaches and the inherent difficulty in identifying stages other than the vacuolar form.
Recent surveys incorporated genotype information by PCR of Blastocystis DNA from feces or from stool culture. Such studies are now shedding light on the distributions of genotypes among human populations (Table 3) and animal hosts and also provide information on transmission routes or sources. A study by Yoshikawa et al. (304) employed the use of PCR-based genotype classification to study the distribution of Blastocystis genotypes among isolates from Bangladesh, Germany, Japan, Pakistan, and Thailand. The most dominant subtype among four populations except Thailand was subtype 3 (41.7 to 92.3%), followed by either subtype 1 (7.7 to 25%) or subtype 6 (10 to 22.9%). Similar genotype distributions in Singapore (78% subtype 3 and 22% subtype 1) (291), China (60.4% subtype 3 and 24.5% subtype 1) (137), Greece (60% subtype 3 and 20% subtype 1) (147), Germany (54% subtype 3 and 21% subtype 1) (31), and Turkey (75.9% subtype 3) (180) were also reported. In most studies, other genotypes were identified at lower frequencies (Table 3). Collectively, those studies suggest that subtype 3 is the subtype of human origin and that there is no correlation between Blastocystis geographic origin and genotype. It may be worthwhile to note that avian subtypes 6 and 7 may grow optimally at 40°C instead of 37°C, as is the case for the avian protozoan flagellate Histomonas meleagridis (84, 279). Isolates belonging to subtype 7 have longer doubling times, about 50 h, when cultured at 37°C (98). In this case, these slow-growing subtypes may still be missed during in vitro culture expansion of stool samples, resulting in an underrepresentation of such subtypes in epidemiological surveys. Although surveys revealed that the majority of individuals are host to a particular Blastocystis subtype, mixed infections in a minority of individual have also frequently been reported (31, 62, 137, 138, 180, 183, 227, 271, 294, 295). Depending on the study, mixed subtypes have been seen among 1.1 to 14.3% of samples surveyed. Most are coinfections with subtype 1 and subtype 3 (137, 138, 271, 294) while subtype 1/subtype 2 (137, 138), subtype 2/subtype 3 (137), and subtype 3/subtype 5 (295) combinations were infrequently reported. Intra-subtype 1 and -subtype 2 variations in ssrRNA sequence were also reported for single isolates (180, 227). It may be difficult to ascertain the true distribution of mixed infections in a particular individual, as this depends on the method employed to determine the Blastocystis subtype. Studies that genotype parasites after in vitro propagation (31, 62, 137, 138, 271) risk underestimating mixed infections since certain subtypes may outgrow others, as was shown recently (183, 295). Hence, genotyping of Blastocystis DNA obtained directly from stools may be more accurate for identifying mixed infections if PCR conditions are optimal. PCR employing subtype-specific STS primers is visually more discriminatory for mixed infections than is PCR-RFLP or sequence analysis of a single ssrRNA amplicon. In the former approach, the presence of bands corresponding to specific subtypes in agarose gels is immediately indicative of a mixed infection (295, 304), while in the latter two methods, mixed infections result in complicated RFLP profiles or mixed peaks in sequencing chromatograms (31, 183), which may be difficult to interpret.
TABLE 3.
Distribution of Blastocystis subtypes infecting humans in different geographic regions
| Country/region and type of isolates (no. of infected individuals studied) | Subtype distribution (%)a
|
No. of positive isolates/total no. of isolates (%)b | Reference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Unknown/mixed | |||
| Bangladesh (26) | 7.7 | — | 92.3 | — | — | — | — | — | — | — | NA | 304 |
| Guangxi, China (35) | 37.1 | — | 40 | — | — | — | 5.7 | — | — | 17.2 | NA | 294 |
| Yunnan, China (78) | 20.5 | 1.3 | 70.5 | 1.3 | — | — | — | — | — | 6.5 | NA | 138 |
| Denmark (29) | 3.4 | 20.7 | 51.7 | 24.1 | — | — | — | — | — | — | NA | 231 |
| Denmark (28) | 17.9 | 32.1 | 46.6 | 3.8 | — | — | — | — | — | — | NA | 227 |
| Egypt (44) | 18.2 | — | 54.5 | — | — | 18.2 | 9.1 | — | — | — | NA | 105 |
| Germany (166) | 21 | 1 | 66 | 7 | — | — | — | — | — | 5 | NA | 31 |
| Germany (12) | 25 | 16.7 | 41.7 | 16.7 | — | — | — | — | — | — | 12/67 (17.9) | 304 |
| Greece (45) | 20 | 13.3 | 60 | 2.2 | — | 2.2 | 2.2 | — | — | — | NA | 147 |
| Japan (55) | 20 | 21.8 | 43.6 | 10.9 | — | — | — | — | — | 3.6 | NA | 113 |
| Japan (50) | 8 | — | 52 | 4 | — | 22 | 10 | — | 4 | — | 50/2,037 (2.45) | 304 |
| Pakistan (10) | 20 | — | 70 | — | — | 10 | — | — | — | — | NA | 304 |
| Singapore (9) | 22.2 | — | 77.8 | — | — | — | — | — | — | — | 9/276 (3.3) | 291 |
| Thailand (153) | 90.2 | — | 4.6 | — | — | — | 1.3 | — | — | 3.9 | 334/924 (36.1)c | 271 |
| Turkey (87) | 9.2 | 13.8 | 75.9 | — | — | — | 1.1 | — | — | — | NA | 180 |
| Turkey | ||||||||||||
| Isolates from pediatric patients (51) | 21.6 | 19.6 | 52.9 | — | — | — | — | — | — | 5.9 | 51/161 (31.7) | 62 |
| Isolates from adult patients (41) | 14.6 | 24.4 | 58.5 | — | — | — | — | — | — | 2.4 | 41/125 (32.8) | 62 |
—, subtype not detected.
NA, not available.
Three hundred thirty-four Blastocystis-positive samples were obtained by in vitro culture of stool specimens. Out of these 334 isolates, only 153 were amenable to PCR amplification.
Accumulating recent (2003 to 2008) epidemiological and other studies suggest that Blastocystis is pathogenic or associated with a variety of disorders (16, 22, 39, 47, 50, 67, 72, 85, 90, 91, 134, 143, 149, 150, 161, 168, 179, 184, 191-193, 196, 205, 264, 293, 308). This is in contrast to reports that suggested otherwise (43, 131, 180, 274). Certain populations may be susceptible to Blastocystis-associated disorders, and risk factors include immunocompromised health (36, 51, 79, 173, 176, 196, 267, 308), poor hygiene practices (32, 55, 57, 85, 168, 171, 200, 208, 225), immigrants from and travelers to developing tropical countries (48, 51, 107, 216, 224), and exposure to animals or consumption of contaminated food or water (10, 25, 55, 57, 111, 134, 138, 166, 167, 175, 183, 200, 207, 208, 211a, 248). In a number of recent surveys, Blastocystis was reported to be found with higher incidences in immunocompetent individuals suffering from intestinal disorders than in the asymptomatic group (67, 85, 116, 134, 149, 150, 168, 293). Interestingly, a recent survey showed a high prevalence of Blastocystis isolation in patients with allergic skin disease (179). Patients infected with human immunodeficiency virus have a higher incidence of harboring Blastocystis spp. (11, 36, 51, 72, 79, 91, 308). In a number of those studies (36, 51, 79, 308), the presence of the parasite was linked to nonspecific symptoms including abdominal pain, diarrhea, and flatulence (51), although a report indicated that there was no correlation between Blastocystis infection and disease in individuals infected with human immunodeficiency virus/AIDS (11). Higher incidences of Blastocystis isolation were observed in individuals under immunosuppressive therapy, such as renal transplant patients (176, 196) and children with nephrotic syndrome receiving corticosteroids (173). In a study of patients with hematological malignancies undergoing chemotherapy-induced neutropenia, Blastocystis was the most common parasite isolated, and infection was linked to abdominal pain, diarrhea, and flatulence (267). Blastocystis infection is commonly seen in children from various geographical settings, and accumulating epidemiological and case studies suggest that Blastocystis infection causes gastrointestinal disease in this cohort (13, 16, 35, 59, 85, 145, 149, 167, 221). Collectively, there is an increasing body of evidence suggesting that Blastocystis is pathogenic or is an opportunistic pathogen, with immunocompromised populations being more susceptible to infection and its associated symptoms.
Blastocystis infections are common among certain occupations that involve exposure to animals, again reinforcing the zoonotic nature of the organism. These include food handlers (14, 57, 119, 200, 208) and animal handlers such as zookeepers and abattoir workers (183, 211a).
Longitudinal epidemiological studies add an important characteristic to point prevalence studies by permitting the characterization of temporal changes in affected patients and in disease characteristics, such as the frequency, complications, and outcomes of a disease. There are only a few such studies involving Blastocystis spp. (49, 94). An earlier study (49) involving young (10 to 28 months of age) Kenyan children over a 10-month period revealed a significant association between Blastocystis infection with unformed stools and diarrhea, while a later study (94) of Peace Corps volunteers in Guatemala over a 2-year period showed no correlation between Blastocystis infection and gastrointestinal symptoms. The discrepancy may be attributed to the different age groups studied or to geographical differences in Blastocystis genotypes.
An increasing number of prevalence studies have implicated contaminated water as being a source of Blastocystis infections (24, 25, 34, 68, 114, 118, 138, 162, 166, 167, 175, 248, 273, 285). This is not surprising since the transmissible form of the parasite is the water-resistant cyst (154). In a study involving a Thai army population, Blastocystis was found to be the most common (21.9%) intestinal parasite (248). This high prevalence among the soldier population was significantly linked to the consumption of unfiltered or nonboiled water. A recent study (138) involving the use of STS primers on stool samples of 238 randomly selected individuals from a village in Yunnan province, China, revealed high infection rates (32.6%). It was observed that the consumption of raw water plants was associated with subtype 1 infections, while drinking unboiled water was associated with subtype 3 infections. This was the first study to investigate the association between subtypes and transmission routes, although more studies are needed before any firm associations can be made.
One of the key questions in Blastocystis biology is whether disease is genotype related. A few studies have been carried out to address this issue, although the results have been equivocal. A study by Kaneda et al. (113) employed PCR-RFLP ribotyping on Blastocystis spp. isolated from asymptomatic individuals and patients with gastrointestinal symptoms. Their results suggested that ribodemes I, III, and VI (subtypes 1, 4, and 2, respectively) were associated with symptoms, with colonoscopic evidence of inflammation in patients harboring ribodemes III and VI. Ribodeme II (subtype 3), which was the most commonly isolated genotype, was not associated with symptoms. In a similar study, genotyping was carried out with isolates of 28 patients with gastrointestinal disorders and 16 asymptomatic individuals (105). Subtype 1 was found exclusively in symptomatic patients, while subtypes 3 and 6 were found in both groups. Subtype 7 was found only in asymptomatic individuals. Those authors concluded that subtype 1 was the most virulent, while subtypes 3 and 6 consisted of pathogenic and nonpathogenic strains. A study of isolates from China revealed an association between subtype 1 and disease, while subtype 3 was isolated predominantly from asymptomatic individuals (294). Tan et al. (265) employed arbitrary primed PCR on Blastocystis DNA and were able to distinguish among isolates obtained from eight symptomatic and eight asymptomatic isolates. In contrast, other studies indicated no association between disease and parasite genotype (31, 304). Böhm-Gloning et al. (31) analyzed 158 isolates by PCR-RFLP and determined that the study population was infected by five subgroups (genotypes), none of which was significantly correlated with intestinal disease. A study involving isolates from asymptomatic and symptomatic individuals from Bangladesh revealed no association between genotypes and disease, although only 26 samples were analyzed (304). In a recent case study, Blastocystis sp. subtype 8 was isolated from a Danish woman suffering from diarrhea, abdominal pain, bloating, and flatulence. No other infectious cause was evident, and her symptoms subsided after a course of trimethoprim-sulfamethoxazole (TMP-SMX) therapy (228). A recent study among Blastocystis isolates from a Turkish hospital revealed the presence of subtypes 1, 2, and 3 among adult and pediatric patients (62). Only subtype 2 showed a statistically different distribution between asymptomatic and symptomatic patients, with a greater proportion within the asymptomatic group. One reason for the discrepant conclusions on subtype association with disease is how the data were interpreted. Most authors seek statistical differences in subtype distribution between asymptomatic and symptomatic groups (31, 62, 180, 294, 304), while others consider the possibility that pathogenic subtypes can be present in approximately equal numbers in either group, possibly due to intrasubtype variations (105) or the presence of pathological evidence within the symptomatic group (113). Due to these complications, Dogruman-Al et al. (62) suggested that it is clearer to identify nonpathogenic subtypes since these subtypes should consistently be found in greater proportions within the asymptomatic group. Indeed, more studies with larger sample sizes are needed before this issue is resolved. Genotyping of isolates during outbreak situations may provide a valuable opportunity to identify pathogenic subtypes. The possibility of intrasubtype variation in pathogenesis should also be considered, as was suggested previously (105, 113). Collectively, studies suggest that at least subtype 1 is associated with disease, while subtypes 2 and 3 may be nonpathogenic.
Infection and Disease
Signs and symptoms.
An earlier report by Clark (53) indicated that there are about equal numbers of studies that either implicate or exonerate Blastocystis spp. as a cause of disease. This balance has tipped dramatically over the last decade, with evidence accumulating from epidemiological, in vitro, and animal studies strongly suggesting the pathogenic potential of the parasite. Clinical features of illness that have been attributed to Blastocystis spp. are nonspecific and include nausea, anorexia, abdominal pain, bloating, flatulence, and acute or chronic diarrhea. Of these features, the most commonly recorded symptoms among patients are abdominal pain and diarrhea (67, 116, 245, 267). Symptoms can be variable, ranging from mild diarrheal illness (90) and chronic diarrhea (205) to acute gastroenteritis (16, 136, 161). A number of studies suggested that the finding of greater than five parasites per high-power field (×400) or, less commonly, oil immersion (×1,000) objective is associated with the acute presentation of gastrointestinal symptoms (51, 67, 82, 85, 111, 116, 155, 165, 167, 177, 215), while one study described an association between infection density and allergic cutaneous diseases (80). In a study of Blastocystis-positive patients from a Turkish hospital, the criteria for selection were the absence of any other coinfecting pathogens and the presence of more than five parasites per high-power field (116). The symptoms from this group were abdominal pain (76.9%), diarrhea (50%), distention (32.6%), and urticaria (5.7%), suggesting an association between parasite density and pathology. Other signs and symptoms associated with Blastocystis infections include fecal leukocytes (60, 89, 116, 206, 208, 280), eosinophilia (75, 124, 161, 215, 280, 307), and cutaneous rashes, particularly urticaria (19, 28, 278).
There are no reports of Blastocystis-associated dysentery, and it appears that the parasite is generally noninvasive, as indicated by endoscopy (43, 76, 329, 330) and histology of experimentally infected animals (5, 152, 187). An interesting case study described Blastocystis trophozoites present in the liver abscess aspirate of a 63-year-old man with a 5-day history of fever and blood-tinged watery diarrhea (102). Blastocystis was also present in stools, while E. histolytica could not be detected, suggesting an invasive extraintestinal Blastocystis infection. This was subsequently ruled out, as serology indicated high titers of E. histolytica antibodies, which subsided following metronidazole treatment, and because E. histolytica DNA was detected by PCR in the liver aspirate. Those authors concluded that the patient suffered amebic dysentery and liver abscess, with the latter being coinfected with Blastocystis. The findings of that study indicated that extraintestinal infections with Blastocystis spp. may occur, with invasion mediated by some other pathogen. Fecal leukocytes, when present, are indicative of inflammatory diarrhea, which may be due to infectious and noninfectious etiologies such as inflammatory bowel disease and infections with Clostridium difficile, Salmonella spp., and Shigella spp. (23, 172, 212, 213). Some studies reported the presence of fecal leukocytes in symptomatic patients suffering from Blastocystis-associated diarrhea (60, 89, 116, 206, 208, 280), while others did not observe such an association (81, 275, 329). There is currently little evidence to suggest that Blastocystis infection results in or is associated with inflammatory diarrhea (157), and if fecal leukocytes are present, other possible causes should be pursued. Two studies reported an association between Blastocystis infection and the presence of Charcot-Leyden crystals in stools (14, 161). Charcot-Leyden crystals are breakdown products of eosinophils, and their presence in stools is traditionally associated with E. histolytica infections (73). The significance of Charcot-Leyden crystals in Blastocystis infections is presently unclear, although an undetected E. histolytica infection could have resulted in such an observation, since mixed infections with Blastocystis and E. histolytica are not uncommon (102, 144, 171, 197, 215).
Accumulating reports also suggest an association between Blastocystis and irritable bowel syndrome (IBS), a functional bowel disorder in which abdominal pain is associated with a defect or a change in bowel habits (82, 104, 144, 293). In two studies (82, 293), Blastocystis was detected more frequently in IBS patients than in a control group consisting of IBS-negative patients with gastrointestinal symptoms. A serological study revealed significantly higher IgG2 levels against Blastocystis in IBS patients who were both stool positive and negative for the parasite (104). Those authors suggested that carbohydrate antigens of the parasite were responsible for the increase in IgG2 levels. In contrast, others found no correlation between Blastocystis infection in IBS patients and that in controls (274). The current data suggest an association between the parasite and IBS, although it cannot currently be concluded that it is the etiological agent of the disease, since an abnormal intestinal condition may provide an environment in which the parasite can thrive (275).
Parasites have been known to be associated with allergic cutaneous lesions, particularly urticaria. Helminths including Strongyloides stercoralis, Ascaris lumbricoides, Anisakis simplex, and Trichuris trichiura have been reported to exhibit a direct relationship with chronic urticaria (80). Among the protozoa, there is some evidence linking G. intestinalis with urticaria (80). Interestingly, accumulating case reports also suggest a causal link between Blastocystis and cutaneous lesions (19, 28, 39, 90, 115, 120, 148, 184, 278). In those studies, the presence of the parasite was seen concurrently in patients with acute or chronic urticaria (19, 28, 115, 278), delayed-pressure urticaria (39), angioedema (148), and palmoplantar pruritis (120). Treatment of the parasite leads to the resolution of both the infection and cutaneous lesions. The concept of luminal protozoa as being causative agents of allergy-like cutaneous lesions is interesting and has been suggested to be linked to the activation, by parasite molecules, of certain specific immune cell subsets such as interleukin 3 (IL-3)-, IL-4-, IL-5-, or IL-13-secreting Th2 cells, which mediate IgE allergic responses (184). It was also suggested that Blastocystis molecules may activate the complement pathway with the generation of anaphylotoxins C3a and C5a. The interactions of these molecules with mast cells and basophils induce histamine release and subsequent related skin disorders (278). Some studies suggested an association between Blastocystis infection and the use of nonsteroidal anti-inflammatory medications, although the significance of this association is unknown (28, 90). A recent study revealed that acute urticaria was associated with amoeboid forms belonging to subtype 3 (115). Future studies should investigate the presence of Blastocystis-specific IgE as an indication of parasite-specific allergic responses. The possibility of IgE-independent allergy mechanisms also exists and should be considered (278), since some studies showed that patients with Blastocystis-associated urticaria have IgE levels within the normal range (39, 80, 115, 184, 278). Eosinophils were recently postulated to play a direct role in the pathology of urticaria (226), and it may be interesting to investigate if there is any association between Blastocystis and eosinophil-mediated urticaria. Taken together, those studies suggested that Blastocystis can cause a variety of disorders not necessarily confined to the intestinal tract. In this regard, the presence of Blastocystis infection in patients with urticaria indicates a causal role of the parasite, and appropriate chemotherapy should be considered.
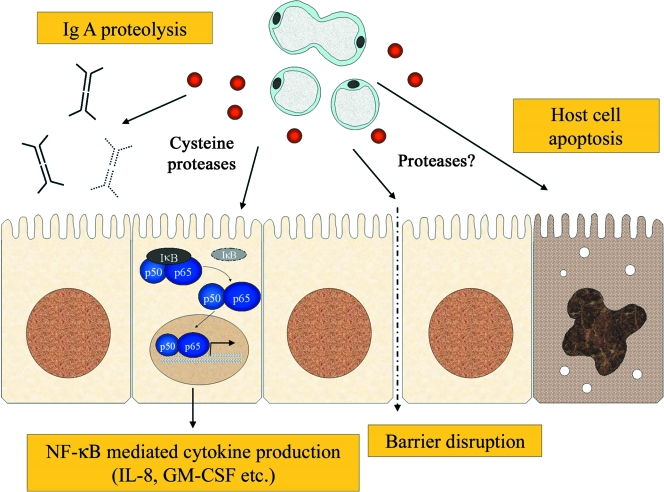
Pathogenic potential.
Among the studies that purported a lack of association between Blastocystis infection and intestinal disease, many of them focused on the distribution of the parasite between symptomatic and asymptomatic individuals (11, 43, 81, 94, 131, 216, 247, 275). Such studies indicated either no significant difference between the prevalences of the parasite in either group (11, 94, 131, 216, 275) or a higher prevalence in asymptomatic individuals (43, 81, 247). Leder et al. (131) conducted a case-controlled study in a cohort of 2,800 people and concluded that there was no correlation between clinical symptoms and Blastocystis infection in immunocompetent individuals. This finding was based on the following observations. In the absence of any enteric pathogen, Blastocystis was detected without significant differences between asymptomatic and symptomatic groups. Symptoms did not correlate with parasite abundance, and individuals were likely to harbor Blastocystis isolates during both symptomatic and asymptomatic periods. Such conclusions are based on the assumption that the parasite is biologically homogenous, and therefore, if pathogenic, more symptomatic individuals would harbor the parasite than asymptomatic ones. However, these assumptions do not imply that Blastocystis is nonpathogenic since infections with other established enteric protozoan pathogens do not always lead to disease. Most Giardia (approximately 60%) and E. histolytica (approximately 90%) infections are asymptomatic or mildly symptomatic in immunocompetent individuals, and only a minority of infections lead to intestinal disease (26, 103, 178). Furthermore, a number of asymptomatic E. histolytica carriers may subsequently develop intestinal disease (121), and this may also be true for Blastocystis. The asymptomatic state also appears to be common for Cryptosporidium infections based on the high frequency of seroconversion compared to that of clinically diagnosed disease (74, 125, 190). Studies of these pathogens revealed that clinical outcome is multifactorial, influenced by host and parasite factors, and it may therefore be difficult to predict pathogenic potential even from case-controlled studies. As such, studies that do not show a significant difference between the occurrence of Blastocystis spp. in asymptomatic populations and the occurrence in symptomatic populations cannot be used as an argument that the parasite is nonpathogenic. In recent years, a number of studies focused on Blastocystis infections in symptomatic individuals in the absence of coinfecting enteric pathogens (116, 140, 161, 165, 177, 205). All those studies revealed the resolution of symptoms upon antiprotozoal treatment with the concomitant eradication of the parasite (Table 4). Currently, there have been two reports on the placebo-controlled treatment of Blastocystis-positive symptomatic patients, with both studies showing that chemotherapy was successful in both clinical cure and eradication of the parasite (165, 205). Nigro et al. (165) conducted a placebo-controlled treatment trial of Blastocystis infection with metronidazole to evaluate the drug's efficacy in inducing clinical remission and parasite clearance in immunocompetent individuals with Blastocystis infection as the only cause of diarrhea. Out of 616 subjects with diarrhea, 76 patients were infected with Blastocystis spp. in the absence of other infectious or noninfectious causes of diarrhea. Of these patients, 40 were assigned to the treatment group and 36 were assigned to the placebo group. After therapy, 35 of 40 (88%) patients in the treatment group reported clinical cure, with parasitological clearance in 32 cases (80%), whereas only one case (3%) in the placebo group achieved intestinal clearance of Blastocystis. In the other placebo-controlled study, Rossignol et al. (205) investigated the efficacy of nitazoxanide for the treatment of diarrhea and enteritis with Blastocystis as the sole identified pathogen. The results indicated that in contrast to the placebo group, the drug was effective in the resolution of symptoms, with parasite eradication in the nitazoxanide-treated group. A recent study (116) investigated the effect of metronidazole on 52 individuals infected with Blastocystis spp. in the absences of other enteric parasites or bacterial pathogens. Intestinal symptoms were evident in 46 of 52 (88.4%) of these patients. Out of 41 individuals who submitted a second stool specimen after metronidazole therapy, 39 reported eradication of the parasite, with clinical cure in 36 of the 39 (92.3%) patients. Intestinal symptoms persisted in the remaining two individuals, who failed to respond to chemotherapy. A study by Ok et al. (177) investigated the effect of TMP-SMX on Blastocystis-positive patients. Fifty-three symptomatic patients harboring Blastocystis spp. as the sole evident cause of diarrhea were treated with TMP-SMX for 7 days. The parasite was eradicated in 50 of 53 (94.3%) patients, with clinical symptoms disappearing in 39 (73.6%) patients and decreasing in 10 (18.9%) patients, and no change was observed in one (1.9%) patient, whereas symptoms persisted in all three patients in whom Blastocystis infection could not be eradicated.
TABLE 4.
Studies of treatment and outcome of symptomatic individuals harboring Blastocystis as the sole identified pathogen
| Study type | Drug administered, dosagea | Duration between treatment and clinical evaluation | Outcome
|
Description | Reference | |
|---|---|---|---|---|---|---|
| No. of patients with clinical cure/total no. of patients (%) | No. of patients with total parasite eradication/total number of patients (%) | |||||
| Placebo controlled, single blind | Metronidazole,1.5 g single dose/day for 10 days | 1 mo | Drug, 35/40 (88); placebo, 5/36 (14) | Drug, 32/40 (80); placebo, 1/36 (3) | Excluded Clostridium difficile toxin, pathogenic parasites, bacteria, viruses, and noninfectious causes; follow-up at 6 mo suggested parasitological relapses | 165 |
| 6 mo | Drug, 30/40 (75); placebo, 12/36 (33) | Drug, 19/40 (48); placebo, 5/36 (14) | ||||
| Placebo controlled, double blind, randomized | Nitazoxanide, 500 mg bid for 3 days | 7 days | Drug, 36/42 (86); placebo, 16/42 (38) | Drug, 36/42 (86); placebo, 5/42 (12) | Excluded parasitic and bacterial but not viral causes of diarrhea | 205 |
| University-based survey | 177 | |||||
| Adults | 320 mg TMP and 1,600 mg SMX daily in two equal doses for 7 days | 7 days | 8/15 (53)b | 14/15 (93) | Excluded parasitic and bacterial but not viral causes of diarrhea | |
| Children | 6 mg/kg TMP and 30 mg/kg SMX daily in two equal doses for 7 days | 7 days | 31/38 (82) | 36/38 (95) | ||
| University-based survey | Metronidazolec | 14 days | 36/41 (88) | 39/41 (95) | Excluded parasitic and bacterial but not viral causes of diarrhea | 116 |
bid, twice a day.
Six out of 15 individuals in this group showed decreases in symptoms; collectively, 14 out of 15 individuals were cured or experienced alleviation of symptoms.
Information on the dose for metronidazole was not included in that study.
Studies of therapeutic improvement concomitant with parasite clearance in symptomatic patients with Blastocystis infection as the only evident cause of disease are compelling for a pathogenic role of the organism. However, there are a number of caveats to such a conclusion. The drugs used in these studies, metronidazole, nitazoxanide, and TMP-SMX, are all broad-spectrum antibiotics, and clinical cure may have been attributed to some as-yet-unidentified, missed, or occult microbial enteric pathogen. Secondly, some studies did not include a comprehensive exclusion of possible enteric pathogens, especially viruses or bacterial toxins (116, 177, 205), which may have contributed to the reported symptoms. A major gap in our understanding of Blastocystis pathogenesis is the inability to fulfill Koch's postulates. Recent studies suggested that rats and chickens are good candidates as animal models (105, 106, 266, 306), and future studies should focus on the pathology and transmissibility of Blastocystis spp. in these hosts. The use of Blastocystis-specific inhibitors such as cytotoxic monoclonal antibodies (253, 254) in these animal models would be an invaluable strategy to investigate if there is a correlation between infection and disease, as these would circumvent the broad-spectrum effects of drugs commonly administered. Taking into account that there are no reports that unequivocally prove that Blastocystis is nonpathogenic and that there are accumulating studies that indicate otherwise, it would be prudent to consider Blastocystis to be an emerging protozoan pathogen. Treatment should be administered for acute and chronic cases when all other possible infectious or noninfectious etiologies have been excluded.
Animal Studies
A major obstacle to our understanding of Blastocystis pathobiology is the absence of a good animal model in which to test Koch's postulates. A variety of experimental infection studies involving rats, mice, guinea pigs, and chickens have been described (5, 105, 106, 151, 152, 182, 187, 244, 266, 306). An early study showed that germ-free guinea pigs were susceptible to Blastocystis infections via oral and intracecal inoculations (187). Heavy infections led to diarrhea and gross cecal hyperemia. A later study involved the experimental infection of young (less than 8 weeks old) BALB/c mice with Blastocystis (152). Infections were generally self-limiting, although some mice showed weight loss, and histological examination revealed inflammatory responses and mucosal sloughing. The mild and self-limiting nature of this infection may be due to the low pathogenic potential of this particular isolate or the possibility that mice in general may not be good hosts for Blastocystis. Surveys have shown that laboratory mice generally do not harbor Blastocystis, while rats and domestic fowl, particularly chickens, are often infected with the parasite (44, 132). Subsequent experimental infection studies focused on rats and chickens as hosts and potential animal models (105, 106, 266, 306). Those studies showed that the cyst is the transmissible form of the parasite and that as few as 10 to 100 cysts are sufficient to establish an infection (266, 306). Studies have shown that isolates from a guinea pig and a laboratory rat can infect Wistar rats (306) and that isolates from chickens, quails, and geese easily infect chicks (266). More recently, the infectivity of various zoonotic Blastocystis genotypes from humans was tested in rats and chickens (106). There was variability in the infectivity of the isolates in relation to rodent subtype 4 and avian subtype 6. Interestingly, subtype 3, a possible human isolate, could not infect chickens and rats, while avian subtype 7 could infect only chickens, suggesting that these subtypes have a restricted host range. In another study, isolates from asymptomatic and symptomatic patients were genotyped and used for experimental infections in rats (105). Interestingly, isolates from symptomatic patients induced moderate to severe pathological changes in infected rats, while isolates from asymptomatic individuals induced mild pathology. Those authors concluded that subtype 1, which induced 25% mortality in rats, was pathogenic, while pathogenic and nonpathogenic variants exist among subtypes 3 and 4. This is the first study to correlate Blastocystis genotypes with virulence in a laboratory animal and suggests that rats are good animal models for the study of Blastocystis pathobiology. However, the observation that variations in host specificity may exist for different Blastocystis subtypes may limit experimental infection studies using rats (106). Hence, the lack of pathology may reflect resistance to colonization in the rat rather than a nonpathogenic role of the parasite. More studies are urgently needed to validate the roles of various genotypes in intestinal disease.
In Vitro Studies
Cytopathic effects on host cells.
A few studies have sought to investigate the effects of Blastocystis spp. on mammalian cell cultures (139, 191, 192, 286). Cells and lysates of Blastocystis spp. isolated from asymptomatic carriers and symptomatic patients induced cytopathic effects on Chinese hamster ovary cells but not in HT-29 colonic epithelial cells (286). The lack of cytopathic effects on HT-29 was validated in a later study, which was also absent in T-84 cell lines exposed to Blastocystis (139). However, it was observed that 24 h of incubation of these colonic epithelial cells lines with Blastocystis induced the production of proinflammatory cytokines IL-8 and granulocyte-macrophage colony-stimulating factor. At 6 h of incubation, Blastocystis cells did not induce IL-8 production but reduced the Escherichia coli- or lipopolysaccharide-induced secretion of IL-8. Those authors suggested that Blastocystis is able to modulate the host immune response, and at initial stages of infection, the parasite may downregulate the host immune response to improve survival, as was demonstrated for Toxoplasma gondii (58) and Cryptosporidium parvum (129). Our laboratory recently showed that Blastocystis ratti (subtype 4) cysteine proteases induced IL-8 production from T-84 cells in an NF-κB-dependent manner (191). Besides a proinflammatory role, the upregulation of NF-κB can also be a mechanism to enhance replication, survival, and dissemination of the parasite within the host (268). Sustained NF-κB activation has been shown to increase the expression of antiapoptotic molecules, hence preventing the death of host cells, which may allow the pathogen to replicate (268). Whether this phenomenon operates in Blastocystis-host interactions or if various subtypes modulate host responses differently is unknown, and this should be investigated. The exposure of intestinal epithelial cells to Blastocystis sp. subtype 4 led to limited apoptosis not associated with compromise in host cell barrier function (192), suggesting that the parasite may exert an antiapoptotic effect on host cells. We recently reported a systematic in vitro study to investigate the effect of Blastocystis ratti (subtype 4) on a rat intestinal epithelial cell line (IEC-6) (192). The assays were carried out using Millicell cell inserts, which facilitated the study of contact-independent effects on host cells via parasite secretions. The exposure of IEC-6 cells to Blastocystis induced apoptosis in a contact-independent fashion. Host cells also revealed F-actin distribution, a loss of transepithelial resistance (TER), and increased permeability. The inhibition of apoptosis did not rescue cells from TER loss and increased permeability, suggesting that apoptosis may not play a major role in barrier function compromise. Metronidazole was able to abrogate TER loss and increased permeability, indicating that live parasites were required to mediate these adverse effects and also suggesting the therapeutic role of the drug in vivo. Those authors proposed that barrier function compromise, evidenced by TER and permeability assays, is related to Blastocystis-associated diarrhea. Taking into account the genotypic and antigenic diversity of the parasite, more studies should be carried out on a range of Blastocystis subtypes and their effects on colonic cell lines.
Cysteine proteases as virulence factors.
Cysteine proteases of parasitic protozoa have been implicated in a number of important biological functions including the invasion of host cells, immune evasion, pathogenesis, and virulence (209). Like most other protozoan parasites, Blastocystis contains predominantly cysteine proteases, evidenced by sensitivity to the inhibitors iodoacetamide and E-64 in azocasein assays (191, 222), and these localize to the parasite central vacuole (191). Parasitic lysates of Blastocystis isolate B (subtype 7) were found to have high protease activity, and nine protease bands of low (20 to 33 kDa) and high (44 to 75 kDa) molecular masses were reported in sodium dodecyl sulfate-polyacrylamide gel electrophoresis gelatin assays. Proteases were found to be pH dependent, and the highest proteolytic activity was observed at neutral pHs (222). Blastocystis lysates and spent medium were able to degrade human secretory IgA, the predominant immunoglobulin defense at the mucosal surface (193). Two isolates were investigated, and it was observed that B. hominis isolate B (subtype 7) and B. ratti (subtype 4) cleaved secretory IgA with cysteine and aspartic protease activities, respectively. This suggests that Blastocystis proteases are virulence factors and contribute to parasite survival in vivo by degrading neutralizing mucosal antibodies. The in vivo role of IgA in mucosal defense against Blastocystis is unknown, although a serological study (143) showed significantly higher secretory IgA levels in symptomatic individuals with Blastocystis infections than in asymptomatic carriers and healthy individuals. Secretory IgA has been shown to be important for the control and host eradication of Giardia (66, 103), evidenced by the inability of IgA-deficient mice to clear experimental infections. Blastocystis does not readily colonize mice (44), and although rats can be experimentally infected with Blastocystis, IgA-deficient rats have not been described in the literature. However, IgA-deficient chickens have been described (141, 272), and it would be interesting to investigate the role of IgA using chickens, since chickens can be experimentally infected with Blastocystis (106). Additionally, longitudinal epidemiological studies focusing on the persistence of Blastocystis infection in individuals suffering from selective IgA deficiency may shed light on the pathogenesis of the parasite. Cysteine proteases from live Blastocystis parasites and lysates were shown to mediate IL-8 secretion from T-84 colonic epithelial cells in an NF-κB-dependent manner. This was evidenced by the protease-induced nuclear translocation of NF-κBp50 and the transcription, expression, and secretion of IL-8 (191). Those authors proposed that in vivo, Blastocystis-mediated intestinal epithelial cell production of IL-8 causes an influx of inflammatory cells into the intestinal mucosa with resultant tissue damage and gastrointestinal disturbances. It was reported that the invasion of the intestinal epithelium by pathogens is not necessary for the induction of inflammation (27), and since Blastocystis is a noninvasive parasite, secreted products from the parasite might initiate the inflammatory process by activating cell surface receptors. Pathogenic E. histolytica cells produce the extracellular release of 10- to 1,000-fold more cysteine proteases than the noninvasive E. dispar (199). It would be worthwhile to study the relative protease levels among the various Blastocystis subtypes and to investigate if there is a similar correlation between protease activity and virulence. Most parasite cysteine proteases that have been characterized fall into clan CA (papain-like). These are assigned to various clans by a number of characteristics including sequence homology directly spanning the catalytic cysteine and histidine (209). The cysteine protease of Blastocystis should accordingly be further classified into their respective clans and families to obtain a clearer picture of their biological functions.
Current in vitro studies support a pathogenic role for Blastocystis. Parasite secretory components, such as cysteine proteases, may exert a variety of detrimental effects on host cells, resulting in cytopathic effects, barrier compromise, and the production of proinflammatory cytokines (Fig. 8).
FIG. 8.
Model for pathogenesis of Blastocystis spp. Blastocystis infection may result in a variety of pathological outcomes such as secretory IgA degradation, barrier function compromise, host cell apoptosis, and induction of proinflammatory cytokines. IgA degradation and barrier disruption may promote the growth and invasion of neighboring pathogens. GM-CSF, granulocyte-macrophage colony-stimulating factor. (Adapted from reference 251 with permission from Taylor and Francis.)
Treatment
The need to treat individuals infected with Blastocystis is equivocal, considering the controversial pathogenesis of the organism and the apparent self-limiting nature of symptoms (11, 20, 63, 131, 238, 284, 307, 320, 329). In instances where treatment is warranted, metronidazole is the most frequently prescribed antibiotic (39, 155, 161, 165, 267, 278). Various drug regimens for metronidazole have been prescribed, ranging from 250 to 750 mg three times a day for 10 days (155, 161, 278) to 1.5 mg/day for 10 days (39, 165, 267), or used in combination with other drugs such as paromomycin (184) or cotrimoxazole (TMP-SMX) (16). A placebo-controlled treatment trial was carried out in order to evaluate the efficacy of metronidazole treatment in inducing clinical remission and parasitological eradication in immunocompetent individuals with Blastocystis infection as the only evident cause of diarrhea (165). The data suggested that Blastocystis induced intestinal disease and responded to metronidazole treatment. In another study involving 28 individuals severely infected with Blastocystis spp. (155), 12 were administered metronidazole at 250 to 750 mg three times/day for 10 days, while 9 patients were administered one tablet of cotrimoxazole (TMP-SMX) three times/day for 10 days. Eradication was observed in 4 of the 12 and 2 of the 9 patients treated with metronidazole and cotrimoxazole, respectively, indicating treatment failure in some patients with severe Blastocystis infections, possibly due to drug resistance. Studies have shown that Blastocystis strains isolated from patients may exhibit differences in sensitivity to metronidazole (92, 292, 316), ranging from 0.01 to 5 mg/ml, depending on the study. A report of isolates from IBS patients revealed 60% resistance to 0.1 mg/ml of metronidazole (292). Another study of isolates from different geographical locations reported an Indonesian isolate showing resistance at 1.0 mg/ml metronidazole (92). The cyst form has been shown to be resistant (up to 5 mg/ml) to the cytotoxic effect of the drug (316). Those observations, together with the extensive genetic heterogeneity of the organism, may offer explanations for the variability in drug susceptibilities and treatment failures.
Studies to investigate the usefulness of cotrimoxazole in Blastocystis infections have been carried out (155, 177). Ok et al. (177) observed that the drug eradicated Blastocystis infections and resolved or decreased symptoms in over 90% of the cohort. In contrast, a study by Moghaddam et al. (155) showed only 22% (two of nine patients) eradication of the parasite in Blastocystis-infected individuals. Nitazoxanide, a 5-nitrothiazole broad-spectrum antiparasitic agent, has been reported to be effective against Blastocystis (50, 61, 205). A placebo-controlled study revealed that 36 (86%) of the 42 Blastocystis-infected patients who received nitazoxanide showed resolution of symptoms, compared with 16 (38%) of 42 patients who received placebo, with a concurrent absence of the parasite in stool samples.
Paromomycin, a broad-spectrum antibiotic indicated for acute and chronic intestinal amoebiasis, was shown to successfully treat Blastocystis infections associated with cutaneous lesions, predominantly urticaria (19, 120, 184, 278). Treatment failure was suspected for one of three patients in one study, who was subsequently successfully treated with metronidazole (278). Curiously, an early in vitro study to investigate the susceptibilities of four axenic strains of Blastocystis to a variety of drugs revealed that paromomycin was not inhibitory to the parasites (325). This suggests that the drug acts by destroying bacterial flora necessary for the parasite's growth. Alternatively, this discrepancy may reflect genotype variations of Blastocystis in the drug's killing effects, and there is therefore a need to reevaluate all in vitro sensitivity studies, case studies, and treatment trials in relation to the actual genotype exposed to the drug.
In summary, a variety of drug treatment options are available for Blastocystis infections (Table 5), and metronidazole appears to be the most effective drug for Blastocystis chemotherapy despite some evidence for treatment failures (75, 155, 288). In such circumstances, cotrimoxazole and nitazoxanide may be considered as second-choice drugs. Treatment should be considered if diarrhea is persistent and no other pathogen apart from Blastocystis is identified in fecal specimens. Future studies should investigate if there is an association between genotype and variations in drug sensitivity and should also focus on the mechanism(s) of action of and resistance to metronidazole.
TABLE 5.
Treatment options and regimens for Blastocystis infections
| Drug | Dosagea | Side effects | Reference(s) |
|---|---|---|---|
| Metronidazole | 750 mg tid for 10 days | Metallic taste, transient nausea | 90, 115, 161 |
| Adult | 500 mg tid for 10 days | 140, 267 | |
| 1.5-g single dose/day for 10 days | 39, 165 | ||
| Pediatric | 15 mg/kg/day for 7 days | 307 | |
| 20-30 mg/kg/day for 10 days | 175 | ||
| TMP-SMX | |||
| Adult | 320 mg TMP and 1,600 mg SMX daily in 2 equal doses for 7 days | Hives, lack or loss of appetite, nausea, skin rash, vomiting | 177 |
| Pediatric | 6 mg/kg TMP and 30 mg/kg SMX daily in 2 equal doses for 7 days | 177 | |
| Nitazoxanide | 205 | ||
| Adult | 500 mg bid for 3 days | Yellowish urine, abdominal pain, headache, nausea | |
| Pediatric | |||
| 1-3 yr | 100 mg bid for 3 days | ||
| 4-11 yr | 200 mg bid for 3 days | ||
| Paromomycin | 25 mg/kg tid for 10 days | Nausea, abdominal cramps, diarrhea | 278 |
| Paromomycin-metronidazole combination | 1,000 mg paromomycin bid for 10 days and 750 mg metronidazole tid for 10 days | 184 |
tid, three times a day; bid, twice a day.
CONCLUSIONS
The authors of the last extensive review on Blastocystis, which was published in 1996 (233), wrote in their conclusion, “Our current knowledge of Blastocystis hominis and the putative disease it causes is insufficient to determine the significance of the parasite in humans.” In the 12 years since, our knowledge of this interesting parasite has increased tremendously. The term B. hominis is no longer applicable to all human isolates, since we now know that humans can be infected by numerous genotypes, many of which are zoonotic. Hence, laboratories should report the presence of the parasite from patient samples as being Blastocystis sp. instead of B. hominis and, in addition, include details on whether five or more parasites are observed per oil immersion (×1,000) field. A standardization of Blastocystis terminology to improve communication and correlate research results has been proposed. All mammalian and avian isolates are designated Blastocystis sp. and assigned to one of nine subtypes (229). Current data suggest that subtype 3 is the only subtype of human origin and, therefore, is the true B. hominis. Laboratory rats or chicks appear to be good candidates for the development of an animal model, and major efforts should be directed at this endeavor. Once this is achieved, issues pertaining to pathogenesis, life cycle, and roles of various stages can be better addressed. Our understanding of Blastocystis biology will accelerate once genome information is available, as has been the case for other parasitic protozoa (54, 69). There are limited studies of the molecular and cell biology of Blastocystis. Despite accumulating evidence indicating that the parasite is pathogenic and that proteases are involved in pathogenesis, not a single virulence factor gene has been identified, cloned, and characterized. As more studies are conducted, the roles of each Blastocystis subtype in disease will become clearer. Greater collaboration among research groups is needed. A key research priority is to elucidate the pathogenic potential of the parasite and to understand its pathogenesis. Once these are clarified, there will be a surge in interest in other relevant aspects of Blastocystis biology, including diagnosis and treatment.
Acknowledgments
I am especially grateful to Ng Geok Choo, N. P. Ramachandran, Eileen Chen, and Manoj Kumar for research input and laboratory support pertaining to our work mentioned in this review and Sylvie Alonso, Cynthia He, Georges Snounou, and Mark Taylor for helpful discussions and comments on various sections of this review.
Research from K. S. W. Tan's laboratory has been supported by generous grants from the Academic Research Fund, National Medical Research Council, and Biomedical Research Council.
REFERENCES
- 1.Abe, N. 2004. Molecular and phylogenetic analysis of Blastocystis isolates from various hosts. Vet. Parasitol. 120:235-242. [DOI] [PubMed] [Google Scholar]
- 2.Abe, N., Z. Wu, and H. Yoshikawa. 2003. Molecular characterization of Blastocystis isolates from birds by PCR with diagnostic primers and restriction fragment length polymorphism analysis of the small subunit ribosomal RNA gene. Parasitol. Res. 89:393-396. [DOI] [PubMed] [Google Scholar]
- 3.Abe, N., Z. Wu, and H. Yoshikawa. 2003. Molecular characterization of Blastocystis isolates from primates. Vet. Parasitol. 113:321-325. [DOI] [PubMed] [Google Scholar]
- 4.Abe, N., Z. Wu, and H. Yoshikawa. 2003. Zoonotic genotypes of Blastocystis hominis detected in cattle and pigs by PCR with diagnostic primers and restriction fragment length polymorphism analysis of the small subunit ribosomal RNA gene. Parasitol. Res. 90:124-128. [DOI] [PubMed] [Google Scholar]
- 5.Abou El Naga, I. F., and A. Y. Negm. 2001. Morphology, histochemistry and infectivity of Blastocystis hominis cyst. J. Egypt. Soc. Parasitol. 31:627-635. [PubMed] [Google Scholar]
- 6.Aguiar, J. I., A. Q. Goncalves, F. C. Sodre, R. Pereira Sdos, M. N. Boia, E. R. de Lemos, and R. R. Daher. 2007. Intestinal protozoa and helminths among Terena Indians in the State of Mato Grosso do Sul: high prevalence of Blastocystis hominis. Rev. Soc. Bras. Med. Trop. 40:631-634. [DOI] [PubMed] [Google Scholar]
- 7.Akdemir, C., and R. Helvaci. 2007. Evaluation of parasitology laboratory results of a group of people older than 15 years of age in Kutahya. Turkiye Parazitol. Derg. 31:129-132. (In Turkish.) [PubMed] [Google Scholar]
- 8.Aksoy, U., C. Akisu, S. Bayram-Delibas, S. Ozkoc, S. Sahin, and S. Usluca. 2007. Demographic status and prevalence of intestinal parasitic infections in schoolchildren in Izmir, Turkey. Turk. J. Pediatr. 49:278-282. [PubMed] [Google Scholar]
- 9.Al, F. D., and M. Hokelek. 2007. Is Blastocystis hominis an opportunist agent? Turkiye Parazitol. Derg. 31:28-36. (In Turkish.) [PubMed] [Google Scholar]
- 10.Al-Binali, A. M., C. S. Bello, K. El-Shewy, and S. E. Abdulla. 2006. The prevalence of parasites in commonly used leafy vegetables in South Western, Saudi Arabia. Saudi Med. J. 27:613-616. [PubMed] [Google Scholar]
- 11.Albrecht, H., H. J. Stellbrink, K. Koperski, and H. Greten. 1995. Blastocystis hominis in human immunodeficiency virus-related diarrhea. Scand. J. Gastroenterol. 30:909-914. [DOI] [PubMed] [Google Scholar]
- 12.Alexeieff, A. 1911. Sur la nature des formations dites kystes de Trichomonas intestinalis. C. R. Soc. Biol. 71:296-298. [Google Scholar]
- 13.al-Tawil, Y. S., M. A. Gilger, G. S. Gopalakrishna, C. Langston, and K. E. Bommer. 1994. Invasive Blastocystis hominis infection in a child. Arch. Pediatr. Adolesc. Med. 148:882-885. [DOI] [PubMed] [Google Scholar]
- 14.Amin, A. M. 1997. Blastocystis hominis among apparently healthy food handlers in Jeddah, Saudi Arabia. J. Egypt. Soc. Parasitol. 27:817-823. [PubMed] [Google Scholar]
- 15.Amin, O. M. 2002. Seasonal prevalence of intestinal parasites in the United States during 2000. Am. J. Trop. Med. Hyg. 66:799-803. [DOI] [PubMed] [Google Scholar]
- 16.Andiran, N., Z. C. Acikgoz, S. Turkay, and F. Andiran. 2006. Blastocystis hominis—an emerging and imitating cause of acute abdomen in children. J. Pediatr. Surg. 41:1489-1491. [DOI] [PubMed] [Google Scholar]
- 17.Arisue, N., T. Hashimoto, and H. Yoshikawa. 2003. Sequence heterogeneity of the small subunit ribosomal RNA genes among Blastocystis isolates. Parasitology 126:1-9. [DOI] [PubMed] [Google Scholar]
- 18.Arisue, N., T. Hashimoto, H. Yoshikawa, Y. Nakamura, G. Nakamura, F. Nakamura, T. A. Yano, and M. Hasegawa. 2002. Phylogenetic position of Blastocystis hominis and of stramenopiles inferred from multiple molecular sequence data. J. Eukaryot. Microbiol. 49:42-53. [DOI] [PubMed] [Google Scholar]
- 19.Armentia, A., J. Mendez, A. Gomez, E. Sanchis, A. Fernandez, R. de la Fuente, and P. Sanchez. 1993. Urticaria by Blastocystis hominis. Successful treatment with paromomycin. Allergol. Immunopathol. (Madrid) 21:149-151. [PubMed] [Google Scholar]
- 20.Babb, R. R., and S. Wagener. 1989. Blastocystis hominis—a potential intestinal pathogen. West. J. Med. 151:518-519. [PMC free article] [PubMed] [Google Scholar]
- 21.Baldo, E. T., V. Y. Belizario, W. U. De Leon, H. H. Kong, and D. I. Chung. 2004. Infection status of intestinal parasites in children living in residential institutions in Metro Manila, the Philippines. Korean J. Parasitol. 42:67-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barahona Rondon, L., C. Maguina Vargas, C. Naquira Velarde, I. A. Terashima, and R. Tello. 2003. Human blastocystosis: prospective study symptomatology and associated epidemiological factors. Rev. Gastroenterol. Peru 23:29-35. (In Spanish.) [PubMed] [Google Scholar]
- 23.Bartlett, J. G. 1997. Clostridium difficile infection: pathophysiology and diagnosis. Semin. Gastrointest. Dis. 8:12-21. [PubMed] [Google Scholar]
- 24.Basualdo, J., B. Pezzani, M. De Luca, A. Cordoba, and M. Apezteguia. 2000. Screening of the municipal water system of La Plata, Argentina, for human intestinal parasites. Int. J. Hyg. Environ. Health 203:177-182. [DOI] [PubMed] [Google Scholar]
- 25.Basualdo, J. A., M. A. Cordoba, M. M. de Luca, M. L. Ciarmela, B. C. Pezzani, M. S. Grenovero, and M. C. Minvielle. 2007. Intestinal parasitoses and environmental factors in a rural population of Argentina, 2002-2003. Rev. Inst. Med. Trop. Sao Paulo 49:251-255. [DOI] [PubMed] [Google Scholar]
- 26.Bercu, T. E., W. A. Petri, and J. W. Behm. 2007. Amebic colitis: new insights into pathogenesis and treatment. Curr. Gastroenterol. Rep. 9:429-433. [DOI] [PubMed] [Google Scholar]
- 27.Berkes, J., V. K. Viswanathan, S. D. Savkovic, and G. Hecht. 2003. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut 52:439-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biedermann, T., K. Hartmann, A. Sing, and B. Przybilla. 2002. Hypersensitivity to non-steroidal anti-inflammatory drugs and chronic urticaria cured by treatment of Blastocystis hominis infection. Br. J. Dermatol. 146:1113-1114. [DOI] [PubMed] [Google Scholar]
- 29.Birky, C. W., Jr. 1996. Heterozygosity, heteromorphy, and phylogenetic trees in asexual eukaryotes. Genetics 144:427-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boeck, W. C., and J. Drbohlav. 1925. The cultivation of Entamoeba histolytica. Am. J. Hyg. 5:371-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Böhm-Gloning, B., J. Knobloch, and B. Walderich. 1997. Five subgroups of Blastocystis hominis from symptomatic and asymptomatic patients revealed by restriction site analysis of PCR-amplified 16S-like rDNA. Trop. Med. Int. Health 2:771-778. [DOI] [PubMed] [Google Scholar]
- 32.Borda, C. E., M. J. Rea, J. R. Rosa, and C. Maidana. 1996. Intestinal parasitism in San Cayetano, Corrientes, Argentina. Bull. Pan. Am. Health Organ. 30:227-233. [PubMed] [Google Scholar]
- 33.Boreham, P. F., and D. J. Stenzel. 1993. Blastocystis in humans and animals: morphology, biology, and epizootiology. Adv. Parasitol. 32:1-70. [DOI] [PubMed] [Google Scholar]
- 34.Brandonisio, O., P. Maggi, M. A. Panaro, S. Lisi, A. Andriola, A. Acquafredda, and G. Angarano. 1999. Intestinal protozoa in HIV-infected patients in Apulia, South Italy. Epidemiol. Infect. 123:457-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bratt, D. E., and E. S. Tikasingh. 1990. Blastocystis hominis in two children of one family. West Indian Med. J. 39:57-58. [PubMed] [Google Scholar]
- 36.Brites, C., M. G. Barberino, M. A. Bastos, M. Sampaio Sa, and N. Silva. 1997. Blastocystis hominis as a potential cause of diarrhea in AIDS patients: a report of six cases in Bahia, Brazil. Braz. J. Infect. Dis. 1:91-94. [PubMed] [Google Scholar]
- 37.Brumpt, E. 1912. Blastocystis hominis n sp. et formes voisines. Bull. Soc. Pathol. Exot. 5:725-730. [Google Scholar]
- 38.Carbajal, J. A., L. del Castillo, M. D. Lanuza, J. Villar, and R. Borrás. 1997. Karyotypic diversity among Blastocystis hominis isolates. Int. J. Parasitol. 27:941-945. [DOI] [PubMed] [Google Scholar]
- 39.Cassano, N., B. M. Scoppio, M. C. Loviglio, and G. A. Vena. 2005. Remission of delayed pressure urticaria after eradication of Blastocystis hominis. Acta Derm. Venereol. 85:357-358. [DOI] [PubMed] [Google Scholar]
- 40.Cassidy, M. F., D. J. Stenzel, and P. F. Boreham. 1994. Electron microscopy of surface structures of Blastocystis sp. from different hosts. Parasitol. Res. 80:505-511. [DOI] [PubMed] [Google Scholar]
- 41.Cavalier-Smith, T. 1998. A revised six-kingdom system of life. Biol. Rev. Camb. Philos. Soc. 73:203-266. [DOI] [PubMed] [Google Scholar]
- 42.Cavalier-Smith, T. 1997. Sagenista and Bigyra, two phyla of heterotrophic heterokont chromists. Archiv. Protist. 148:253-267. [Google Scholar]
- 43.Chen, T. L., C. C. Chan, H. P. Chen, C. P. Fung, C. P. Lin, W. L. Chan, and C. Y. Liu. 2003. Clinical characteristics and endoscopic findings associated with Blastocystis hominis in healthy adults. Am. J. Trop. Med. Hyg. 69:213-216. [PubMed] [Google Scholar]
- 44.Chen, X. Q., M. Singh, L. C. Ho, K. T. Moe, S. W. Tan, and E. H. Yap. 1997. A survey of Blastocystis sp. in rodents. Lab. Anim. Sci. 47:91-94. [PubMed] [Google Scholar]
- 45.Chen, X. Q., M. Singh, L. C. Ho, S. W. Tan, G. C. Ng, K. T. Moe, and E. H. Yap. 1997. Description of a Blastocystis species from Rattus norvegicus. Parasitol. Res. 83:313-318. [DOI] [PubMed] [Google Scholar]
- 46.Chen, X. Q., M. Singh, J. Howe, L. C. Ho, S. W. Tan, and E. H. Yap. 1999. In vitro encystation and excystation of Blastocystis ratti. Parasitology 118:151-160. [DOI] [PubMed] [Google Scholar]
- 47.Cheng, H. S., Y. L. Guo, and J. W. Shin. 2003. Hematological effects of Blastocystis hominis infection in male foreign workers in Taiwan. Parasitol. Res. 90:48-51. [DOI] [PubMed] [Google Scholar]
- 48.Cheng, H. S., Z. F. Haung, W. H. Lan, T. C. Kuo, and J. W. Shin. 2006. Epidemiology of Blastocystis hominis and other intestinal parasites in a Vietnamese female immigrant population in southern Taiwan. Kaohsiung J. Med. Sci. 22:166-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chunge, R. N., N. Nagelkerke, P. N. Karumba, N. Kaleli, M. Wamwea, N. Mutiso, E. O. Andala, J. Gachoya, R. Kiarie, and S. N. Kinoti. 1991. Longitudinal study of young children in Kenya: intestinal parasitic infection with special reference to Giardia lamblia, its prevalence, incidence and duration, and its association with diarrhoea and with other parasites. Acta Trop. 50:39-49. [DOI] [PubMed] [Google Scholar]
- 50.Cimerman, S., M. C. Ladeira, and W. A. Iuliano. 2003. Blastocystosis: nitazoxanide as a new therapeutic option. Rev. Soc. Bras. Med. Trop. 36:415-417. (In Portugese.) [DOI] [PubMed] [Google Scholar]
- 51.Cirioni, O., A. Giacometti, D. Drenaggi, F. Ancarani, and G. Scalise. 1999. Prevalence and clinical relevance of Blastocystis hominis in diverse patient cohorts. Eur. J. Epidemiol. 15:389-393. [DOI] [PubMed] [Google Scholar]
- 52.Clark, C. G. 2000. Cryptic genetic variation in parasitic protozoa. J. Med. Microbiol. 49:489-491. [DOI] [PubMed] [Google Scholar]
- 53.Clark, C. G. 1997. Extensive genetic diversity in Blastocystis hominis. Mol. Biochem. Parasitol. 87:79-83. [DOI] [PubMed] [Google Scholar]
- 54.Coppel, R. L., and C. G. Black. 2005. Parasite genomes. Int. J. Parasitol. 35:465-479. [DOI] [PubMed] [Google Scholar]
- 55.Cruz Licea, V., A. Plancarte Crespo, C. Moran Alvarez, S. Valencia Rojas, G. Rodriguez Sasnchez, and L. Vega Franco. 2003. Blastocystis hominis among food vendors in Xochimilco markets. Rev. Latinoam. Microbiol. 45:12-15. [PubMed] [Google Scholar]
- 56.Culha, G. 2006. The distribution of patients with intestinal parasites presenting at the Parasitology Laboratory of the Mustafa Kemal University Medical Faculty. Turkiye Parazitol. Derg. 30:302-304. (In Turkish.) [PubMed] [Google Scholar]
- 57.Danchaivijitr, S., Y. Rongrungruang, U. Kachintorn, V. Techasathit, S. Pakaworavuthi, and K. Kachintorn. 2005. Prevalence and effectiveness of an education program on intestinal pathogens in food handlers. J. Med. Assoc. Thai. 88(Suppl. 10):S31-S35. [PubMed] [Google Scholar]
- 58.Denney, C. F., L. Eckmann, and S. L. Reed. 1999. Chemokine secretion of human cells in response to Toxoplasma gondii infection. Infect. Immun. 67:1547-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Devera, R. A., V. J. Velasquez, and M. J. Vasquez. 1998. Blastocystosis in preschool children from Bolivar City, Venezuela. Cad. Saude Publica 14:401-407. (In Spanish.) [PubMed] [Google Scholar]
- 60.Diaczok, B. J., and J. Rival. 1987. Diarrhea due to Blastocystis hominis: an old organism revisited. South. Med. J. 80:931-932. [DOI] [PubMed] [Google Scholar]
- 61.Diaz, E., J. Mondragon, E. Ramirez, and R. Bernal. 2003. Epidemiology and control of intestinal parasites with nitazoxanide in children in Mexico. Am. J. Trop. Med. Hyg. 68:384-385. [PubMed] [Google Scholar]
- 62.Dogruman-Al, F., H. Dagci, H. Yoshikawa, O. Kurt, and M. Demirel. 2008. A possible link between subtype 2 and asymptomatic infections of Blastocystis hominis. Parasitol. Res. 103:685-689. [DOI] [PubMed] [Google Scholar]
- 63.Doyle, P. W., M. M. Helgason, R. G. Mathias, and E. M. Proctor. 1990. Epidemiology and pathogenicity of Blastocystis hominis. J. Clin. Microbiol. 28:116-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duda, A., D. J. Stenzel, and P. F. Boreham. 1998. Detection of Blastocystis sp. in domestic dogs and cats. Vet. Parasitol. 76:9-17. [DOI] [PubMed] [Google Scholar]
- 65.Dunn, L. A., P. F. Boreham, and D. J. Stenzel. 1989. Ultrastructural variation of Blastocystis hominis stocks in culture. Int. J. Parasitol. 19:43-56. [DOI] [PubMed] [Google Scholar]
- 66.Eckmann, L. 2003. Mucosal defences against Giardia. Parasite Immunol. 25:259-270. [DOI] [PubMed] [Google Scholar]
- 67.El-Shazly, A. M., A. A. Abdel-Magied, S. N. El-Beshbishi, H. A. El-Nahas, M. A. Fouad, and M. S. Monib. 2005. Blastocystis hominis among symptomatic and asymptomatic individuals in Talkha Center, Dakahlia Governorate, Egypt. J. Egypt. Soc. Parasitol. 35:653-666. [PubMed] [Google Scholar]
- 68.Elshazly, A. M., H. M. Elsheikha, D. M. Soltan, K. A. Mohammad, and T. A. Morsy. 2007. Protozoal pollution of surface water sources in Dakahlia Governorate, Egypt. J. Egypt. Soc. Parasitol. 37:51-64. [PubMed] [Google Scholar]
- 69.Ersfeld, K. 2003. Genomes and genome projects of protozoan parasites. Curr. Issues Mol. Biol. 5:61-74. [PubMed] [Google Scholar]
- 70.Escobedo, A. A., R. Canete, and F. A. Nunez. 2007. Intestinal protozoan and helminth infections in the Municipality San Juan y Martinez, Pinar del Rio, Cuba. Trop. Doct. 37:236-238. [DOI] [PubMed] [Google Scholar]
- 71.Estevez, E. G., and J. A. Levine. 1985. Examination of preserved stool specimens for parasites: lack of value of the direct wet mount. J. Clin. Microbiol. 22:666-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Florez, A. C., D. A. Garcia, L. Moncada, and M. Beltran. 2003. Prevalence of microsporidia and other intestinal parasites in patients with HIV infection, Bogota, 2001. Biomedica 23:274-282. (In Spanish.) [PubMed] [Google Scholar]
- 73.Fotedar, R., D. Stark, N. Beebe, D. Marriott, J. Ellis, and J. Harkness. 2007. Laboratory diagnostic techniques for Entamoeba species. Clin. Microbiol. Rev. 20:511-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Frost, F. J., E. Fea, G. Gilli, F. Biorci, T. M. Muller, G. F. Craun, and R. L. Calderon. 2000. Serological evidence of Cryptosporidium infections in southern Europe. Eur. J. Epidemiol. 16:385-390. [DOI] [PubMed] [Google Scholar]
- 75.Garavelli, P. L. 1991. The therapy of blastocystosis. J. Chemother. 3(Suppl. 1):245-246. [PubMed] [Google Scholar]
- 76.Garavelli, P. L., L. Scaglione, A. Merighi, and M. Libanore. 1992. Endoscopy of blastocystosis (Zierdt-Garavelli disease). Ital. J. Gastroenterol. 24:206. [PubMed] [Google Scholar]
- 77.Garcia, L. S. 2007. Diagnostic medical parasitology, 5th ed. ASM Press, Washington, DC.
- 78.Gardner, B. B., D. J. Del Junco, J. Fenn, and J. H. Hengesbaugh. 1980. Comparison of direct wet mount and trichrome staining techniques for detecting Entamoeba species trophozoites in stools. J. Clin. Microbiol. 12:656-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gassama, A., P. S. Sow, F. Fall, P. Camara, A. Gueye-N′diaye, R. Seng, B. Samb, S. M'Boup, and A. Aidara-Kane. 2001. Ordinary and opportunistic enteropathogens associated with diarrhea in Senegalese adults in relation to human immunodeficiency virus serostatus. Int. J. Infect. Dis. 5:192-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Giacometti, A., O. Cirioni, L. Antonicelli, G. D'Amato, C. Silvestri, M. S. Del Prete, and G. Scalise. 2003. Prevalence of intestinal parasites among individuals with allergic skin diseases. J. Parasitol. 89:490-492. [DOI] [PubMed] [Google Scholar]
- 81.Giacometti, A., O. Cirioni, M. Balducci, D. Drenaggi, M. Quarta, M. De Federicis, P. Ruggeri, D. Colapinto, G. Ripani, and G. Scalise. 1997. Epidemiologic features of intestinal parasitic infections in Italian mental institutions. Eur. J. Epidemiol. 13:825-830. [DOI] [PubMed] [Google Scholar]
- 82.Giacometti, A., O. Cirioni, A. Fiorentini, M. Fortuna, and G. Scalise. 1999. Irritable bowel syndrome in patients with Blastocystis hominis infection. Eur. J. Clin. Microbiol. Infect. Dis. 18:436-439. [DOI] [PubMed] [Google Scholar]
- 83.Govind, S. K., A. A. Khairul, and H. V. Smith. 2002. Multiple reproductive processes in Blastocystis. Trends Parasitol. 18:528. [DOI] [PubMed] [Google Scholar]
- 84.Grabensteiner, E., D. Liebhart, H. Weissenbock, and M. Hess. 2006. Broad dissemination of Histomonas meleagridis determined by the detection of nucleic acid in different organs after experimental infection of turkeys and specified pathogen-free chickens using a mono-eukaryotic culture of the parasite. Parasitol. Int. 55:317-322. [DOI] [PubMed] [Google Scholar]
- 85.Graczyk, T. K., C. K. Shiff, L. Tamang, F. Munsaka, A. M. Beitin, and W. J. Moss. 2005. The association of Blastocystis hominis and Endolimax nana with diarrheal stools in Zambian school-age children. Parasitol. Res. 98:38-43. [DOI] [PubMed] [Google Scholar]
- 86.Grossman, I., L. M. Weiss, D. Simon, H. B. Tanowitz, and M. Wittner. 1992. Blastocystis hominis in hospital employees. Am. J. Gastroenterol. 87:729-732. [PubMed] [Google Scholar]
- 87.Guglielmetti, P., C. Cellesi, N. Figura, and A. Rossolini. 1989. Family outbreak of Blastocystis hominis associated gastroenteritis. Lancet ii:1394. [DOI] [PubMed] [Google Scholar]
- 88.Guimarães, S., and M. I. Sogayar. 1993. Blastocystis hominis: occurrence in children and staff members of municipal day-care centers from Botucatu, Sao Paulo State, Brazil. Mem. Inst. Oswaldo Cruz 88:427-429. [DOI] [PubMed] [Google Scholar]
- 89.Guirges, S. Y., and N. S. Al-Waili. 1987. Blastocystis hominis: evidence for human pathogenicity and effectiveness of metronidazole therapy. Clin. Exp. Pharmacol. Physiol. 14:333-335. [DOI] [PubMed] [Google Scholar]
- 90.Gupta, R., and K. Parsi. 2006. Chronic urticaria due to Blastocystis hominis. Australas. J. Dermatol. 47:117-119. [DOI] [PubMed] [Google Scholar]
- 91.Hailemariam, G., A. Kassu, G. Abebe, E. Abate, D. Damte, E. Mekonnen, and F. Ota. 2004. Intestinal parasitic infections in HIV/AIDS and HIV seronegative individuals in a teaching hospital, Ethiopia. Jpn. J. Infect. Dis. 57:41-43. [PubMed] [Google Scholar]
- 92.Haresh, K., K. Suresh, A. K. Anus, and S. Saminathan. 1999. Isolate resistance of Blastocystis hominis to metronidazole. Trop. Med. Int. Health 4:274-277. [DOI] [PubMed] [Google Scholar]
- 93.Hasegawa, M., and T. Hashimoto. 1993. Ribosomal RNA trees misleading? Nature 361:23. [DOI] [PubMed] [Google Scholar]
- 94.Herwaldt, B. L., K. R. de Arroyave, S. P. Wahlquist, A. M. de Merida, A. S. Lopez, and D. D. Juranek. 2001. Multiyear prospective study of intestinal parasitism in a cohort of Peace Corps volunteers in Guatemala. J. Clin. Microbiol. 39:34-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hess, M., T. Kolbe, E. Grabensteiner, and H. Prosl. 2006. Clonal cultures of Histomonas meleagridis, Tetratrichomonas gallinarum and a Blastocystis sp. established through micromanipulation. Parasitology 133:547-554. [DOI] [PubMed] [Google Scholar]
- 96.Hirata, T., H. Nakamura, N. Kinjo, A. Hokama, F. Kinjo, N. Yamane, and J. Fujita. 2007. Prevalence of Blastocystis hominis and Strongyloides stercoralis infection in Okinawa, Japan. Parasitol. Res. 101:1717-1719. [DOI] [PubMed] [Google Scholar]
- 97.Ho, L. C., A. Armiugam, K. Jeyaseelan, E. H. Yap, and M. Singh. 2000. Blastocystis elongation factor-1alpha: genomic organization, taxonomy and phylogenetic relationships. Parasitology 121:135-144. [DOI] [PubMed] [Google Scholar]
- 98.Ho, L. C., M. Singh, G. Suresh, G. C. Ng, and E. H. Yap. 1993. Axenic culture of Blastocystis hominis in Iscove's modified Dulbecco's medium. Parasitol. Res. 79:614-616. [DOI] [PubMed] [Google Scholar]
- 99.Ho, L. C., M. Singh, K. Suresh, G. C. Ng, and E. H. Yap. 1994. A study of the karyotypic patterns of Blastocystis hominis by pulsed-field gradient electrophoresis. Parasitol. Res. 80:620-622. [DOI] [PubMed] [Google Scholar]
- 100.Hoevers, J. D., and K. F. Snowden. 2005. Analysis of the ITS region and partial ssu and lsu rRNA genes of Blastocystis and Proteromonas lacertae. Parasitology 131:187-196. [DOI] [PubMed] [Google Scholar]
- 101.Horiki, N., M. Maruyama, Y. Fujita, T. Yonekura, S. Minato, and Y. Kaneda. 1997. Epidemiologic survey of Blastocystis hominis infection in Japan. Am. J. Trop. Med. Hyg. 56:370-374. [DOI] [PubMed] [Google Scholar]
- 102.Hu, K. C., C. C. Lin, T. E. Wang, C. Y. Liu, M. J. Chen, and W. H. Chang. 2008. Amoebic liver abscess or is it? Gut 57:627, 683. [DOI] [PubMed] [Google Scholar]
- 103.Huang, D. B., and A. C. White. 2006. An updated review on Cryptosporidium and Giardia. Gastroenterol. Clin. N. Am. 35:291-314. [DOI] [PubMed] [Google Scholar]
- 104.Hussain, R., W. Jaferi, S. Zuberi, R. Baqai, N. Abrar, A. Ahmed, and V. Zaman. 1997. Significantly increased IgG2 subclass antibody levels to Blastocystis hominis in patients with irritable bowel syndrome. Am. J. Trop. Med. Hyg. 56:301-306. [DOI] [PubMed] [Google Scholar]
- 105.Hussein, E. M., A. M. Hussein, M. M. Eida, and M. M. Atwa. 2008. Pathophysiological variability of different genotypes of human Blastocystis hominis Egyptian isolates in experimentally infected rats. Parasitol. Res. 102:853-860. [DOI] [PubMed] [Google Scholar]
- 106.Iguchi, A., A. Ebisu, S. Nagata, Y. Saitou, H. Yoshikawa, S. Iwatani, and I. Kimata. 2007. Infectivity of different genotypes of human Blastocystis hominis isolates in chickens and rats. Parasitol. Int. 56:107-112. [DOI] [PubMed] [Google Scholar]
- 107.Jelinek, T., G. Peyerl, T. Löscher, F. von Sonnenburg, and H. D. Nothdurft. 1997. The role of Blastocystis hominis as a possible intestinal pathogen in travellers. J. Infect. 35:63-66. [DOI] [PubMed] [Google Scholar]
- 108.Johnson, A. M., A. Thanou, P. F. Boreham, and P. R. Baverstock. 1989. Blastocystis hominis: phylogenetic affinities determined by rRNA sequence comparison. Exp. Parasitol. 68:283-288. [DOI] [PubMed] [Google Scholar]
- 109.Jones, M. S., II, R. D. Ganac, G. Hiser, N. R. Hudson, A. Le, and C. M. Whipps. 2008. Detection of Blastocystis from stool samples using real-time PCR. Parasitol. Res. 103:551-557. [DOI] [PubMed] [Google Scholar]
- 110.Jones, W. R. 1946. The experimental infection of rats with Entamoeba histolytica. Ann. Trop. Med. Parasitol. 40:130. [DOI] [PubMed] [Google Scholar]
- 111.Kain, K. C., M. A. Noble, H. J. Freeman, and R. L. Barteluk. 1987. Epidemiology and clinical features associated with Blastocystis hominis infection. Diagn. Microbiol. Infect. Dis. 8:235-244. [DOI] [PubMed] [Google Scholar]
- 112.Kaneda, Y., N. Horiki, X. Cheng, H. Tachibana, and Y. Tsutsumi. 2000. Serologic response to Blastocystis hominis infection in asymptomatic individuals. Tokai J. Exp. Clin. Med. 25:51-56. [PubMed] [Google Scholar]
- 113.Kaneda, Y., N. Horiki, X. J. Cheng, Y. Fujita, M. Maruyama, and H. Tachibana. 2001. Ribodemes of Blastocystis hominis isolated in Japan. Am. J. Trop. Med. Hyg. 65:393-396. [DOI] [PubMed] [Google Scholar]
- 114.Karanis, P., C. Kourenti, and H. Smith. 2007. Waterborne transmission of protozoan parasites: a worldwide review of outbreaks and lessons learnt. J. Water Health 5:1-38. [DOI] [PubMed] [Google Scholar]
- 115.Katsarou-Katsari, A., C. M. Vassalos, K. Tzanetou, G. Spanakos, C. Papadopoulou, and N. Vakalis. 2008. Acute urticaria associated with amoeboid forms of Blastocystis sp. subtype 3. Acta Derm. Venereol. 88:80-81. [DOI] [PubMed] [Google Scholar]
- 116.Kaya, S., E. S. Cetin, B. C. Aridogan, S. Arikan, and M. Demirci. 2007. Pathogenicity of Blastocystis hominis, a clinical reevaluation. Turkiye Parazitol. Derg. 31:184-187. [PubMed] [Google Scholar]
- 117.Kellogg, J. A., and C. J. Elder. 1999. Justification for use of a single trichrome stain as the sole means for routine detection of intestinal parasites in concentrated stool specimens. J. Clin. Microbiol. 37:835-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Khalifa, A. M., M. M. El Temsahy, and I. F. Abou El Naga. 2001. Effect of ozone on the viability of some protozoa in drinking water. J. Egypt. Soc. Parasitol. 31:603-616. [PubMed] [Google Scholar]
- 119.Khan, Z. A., and I. S. Alkhalife. 2005. Prevalence of Blastocystis hominis among “healthy” food handlers in Dammam, Saudi Arabia. J. Egypt. Soc. Parasitol. 35:395-401. [PubMed] [Google Scholar]
- 120.Kick, G., F. Rueff, and B. Przybilla. 2002. Palmoplantar pruritus subsiding after Blastocystis hominis eradication. Acta Derm. Venereol. 82:60. [DOI] [PubMed] [Google Scholar]
- 121.Knobloch, J., and E. Mannweiler. 1983. Development and persistence of antibodies to Entamoeba histolytica in patients with amebic liver abscess. Analysis of 216 cases. Am. J. Trop. Med. Hyg. 32:727-732. [DOI] [PubMed] [Google Scholar]
- 122.König, G., and H. E. Müller. 1997. Blastocystis hominis in animals: incidence of four serogroups. Zentralbl. Bakteriol. 286:435-440. [DOI] [PubMed] [Google Scholar]
- 123.Koutsavlis, A. T., L. Valiquette, R. Allard, and J. Soto. 2001. Blastocystis hominis: a new pathogen in day-care centres? Can. Commun. Dis. Rep. 27:76-84. [PubMed] [Google Scholar]
- 124.Krech, T., X. M. Nguyen, and H. Spicher. 1990. Blastocystis hominis in feces. An assessment of 56 cases. Schweiz. Med. Wochenschr. 120:742-744. (In German.) [PubMed] [Google Scholar]
- 125.Kuhls, T. L., D. A. Mosier, D. L. Crawford, and J. Griffis. 1994. Seroprevalence of cryptosporidial antibodies during infancy, childhood, and adolescence. Clin. Infect. Dis. 18:731-735. [DOI] [PubMed] [Google Scholar]
- 126.Kukoschke, K. G., and H. E. Müller. 1991. SDS-PAGE and immunological analysis of different axenic Blastocystis hominis strains. J. Med. Microbiol. 35:35-39. [DOI] [PubMed] [Google Scholar]
- 127.Lanuza, M. D., J. A. Carbajal, and R. Borrás. 1996. Identification of surface coat carbohydrates in Blastocystis hominis by lectin probes. Int. J. Parasitol. 26:527-532. [DOI] [PubMed] [Google Scholar]
- 128.Lanuza, M. D., J. A. Carbajal, J. Villar, and R. Borrás. 1997. Description of an improved method for Blastocystis hominis culture and axenization. Parasitol. Res. 83:60-63. [DOI] [PubMed] [Google Scholar]
- 129.Laurent, F., L. Eckmann, T. C. Savidge, G. Morgan, C. Theodos, M. Naciri, and M. F. Kagnoff. 1997. Cryptosporidium parvum infection of human intestinal epithelial cells induces the polarized secretion of C-X-C chemokines. Infect. Immun. 65:5067-5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lavier, G. 1952. Blastocystis spp. Ann. Parasitol. Hum. Comp. 27:339-356. (In French.) [PubMed] [Google Scholar]
- 131.Leder, K., M. E. Hellard, M. I. Sinclair, C. K. Fairley, and R. Wolfe. 2005. No correlation between clinical symptoms and Blastocystis hominis in immunocompetent individuals. J. Gastroenterol. Hepatol. 20:1390-1394. [DOI] [PubMed] [Google Scholar]
- 132.Lee, M. G., and D. J. Stenzel. 1999. A survey of Blastocystis in domestic chickens. Parasitol. Res. 85:109-117. [DOI] [PubMed] [Google Scholar]
- 133.Lee, M. J. 1991. Pathogenicity of Blastocystis hominis. J. Clin. Microbiol. 29:2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Leelayoova, S., R. Rangsin, P. Taamasri, T. Naaglor, U. Thathaisong, and M. Mungthin. 2004. Evidence of waterborne transmission of Blastocystis hominis. Am. J. Trop. Med. Hyg. 70:658-662. [PubMed] [Google Scholar]
- 135.Leelayoova, S., P. Taamasri, R. Rangsin, T. Naaglor, U. Thathaisong, and M. Mungthin. 2002. In-vitro cultivation: a sensitive method for detecting Blastocystis hominis. Ann. Trop. Med. Parasitol. 96:803-807. [DOI] [PubMed] [Google Scholar]
- 136.Levy, Y., J. George, and Y. Shoenfeld. 1996. Severe Blastocystis hominis in an elderly man. J. Infect. 33:57-59. [DOI] [PubMed] [Google Scholar]
- 137.Li, L. H., X. P. Zhang, S. Lv, L. Zhang, H. Yoshikawa, Z. Wu, P. Steinmann, J. Utzinger, X. M. Tong, S. H. Chen, and X. N. Zhou. 2007. Cross-sectional surveys and subtype classification of human Blastocystis isolates from four epidemiological settings in China. Parasitol. Res. 102:83-90. [DOI] [PubMed] [Google Scholar]
- 138.Li, L. H., X. N. Zhou, Z. W. Du, X. Z. Wang, L. B. Wang, J. Y. Jiang, H. Yoshikawa, P. Steinmann, J. Utzinger, Z. Wu, J. X. Chen, S. H. Chen, and L. Zhang. 2007. Molecular epidemiology of human Blastocystis in a village in Yunnan province, China. Parasitol. Int. 56:281-286. [DOI] [PubMed] [Google Scholar]
- 139.Long, H. Y., A. Handschack, W. König, and A. Ambrosch. 2001. Blastocystis hominis modulates immune responses and cytokine release in colonic epithelial cells. Parasitol. Res. 87:1029-1030. [DOI] [PubMed] [Google Scholar]
- 140.Lucia, J. F., C. Aguilar, and A. Betran. 2007. Blastocystis hominis colitis in a haemophilic patient as a cause of lower gastrointestinal bleeding. Haemophilia 13:224-225. [DOI] [PubMed] [Google Scholar]
- 141.Luster, M. I., G. A. Leslie, and R. K. Cole. 1976. Selective IgA deficiency in chickens with spontaneous autoimmune thyroiditis. Nature 263:331. [DOI] [PubMed] [Google Scholar]
- 142.MacPherson, D. W., and W. M. MacQueen. 1994. Morphological diversity of Blastocystis hominis in sodium acetate-acetic acid-formalin-preserved stool samples stained with iron hematoxylin. J. Clin. Microbiol. 32:267-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mahmoud, M. S., and W. A. Saleh. 2003. Secretory and humoral antibody responses to Blastocystis hominis in symptomatic and asymptomatic human infections. J. Egypt. Soc. Parasitol. 33:13-30. [PubMed] [Google Scholar]
- 144.Markell, E. K., and M. P. Udkow. 1986. Blastocystis hominis: pathogen or fellow traveler? Am. J. Trop. Med. Hyg. 35:1023-1026. [DOI] [PubMed] [Google Scholar]
- 145.Martin-Sanchez, A. M., A. Canut-Blasco, J. Rodriguez-Hernandez, I. Montes-Martinez, and J. A. Garcia-Rodriguez. 1992. Epidemiology and clinical significance of Blastocystis hominis in different population groups in Salamanca (Spain). Eur. J. Epidemiol. 8:553-559. [DOI] [PubMed] [Google Scholar]
- 146.Matsumoto, Y., M. Yamada, and Y. Yoshida. 1987. Light-microscopical appearance and ultrastructure of Blastocystis hominis, an intestinal parasite of man. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 264:379-385. [DOI] [PubMed] [Google Scholar]
- 147.Menounos, P. G., G. Spanakos, N. Tegos, C. M. Vassalos, C. Papadopoulou, and N. C. Vakalis. 2008. Direct detection of Blastocystis sp. in human faecal samples and subtype assignment using single strand conformational polymorphism and sequencing. Mol. Cell. Probes 22:24-29. [DOI] [PubMed] [Google Scholar]
- 148.Micheloud, D., J. Jensen, E. Fernandez-Cruz, and J. Carbone. 2007. Chronic angioedema and Blastocystis hominis infection. Rev. Gastroenterol. Peru 27:191-193. (In Spanish.) [PubMed] [Google Scholar]
- 149.Miller, S. A., C. L. Rosario, E. Rojas, and J. V. Scorza. 2003. Intestinal parasitic infection and associated symptoms in children attending day care centres in Trujillo, Venezuela. Trop. Med. Int. Health 8:342-347. [DOI] [PubMed] [Google Scholar]
- 150.Minvielle, M. C., B. C. Pezzani, M. A. Cordoba, M. M. De Luca, M. C. Apezteguia, and J. A. Basualdo. 2004. Epidemiological survey of Giardia spp. and Blastocystis hominis in an Argentinian rural community. Korean J. Parasitol. 42:121-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Moe, K. T., M. Singh, P. Gopalakrishnakone, L. C. Ho, S. W. Tan, X. Q. Chen, and E. H. Yap. 1998. Cytopathic effect of Blastocystis hominis after intramuscular inoculation into laboratory mice. Parasitol. Res. 84:450-454. [DOI] [PubMed] [Google Scholar]
- 152.Moe, K. T., M. Singh, J. Howe, L. C. Ho, S. W. Tan, X. Q. Chen, G. C. Ng, and E. H. Yap. 1997. Experimental Blastocystis hominis infection in laboratory mice. Parasitol. Res. 83:319-325. [DOI] [PubMed] [Google Scholar]
- 153.Moe, K. T., M. Singh, J. Howe, L. C. Ho, S. W. Tan, X. Q. Chen, and E. H. Yap. 1999. Development of Blastocystis hominis cysts into vacuolar forms in vitro. Parasitol. Res. 85:103-108. [DOI] [PubMed] [Google Scholar]
- 154.Moe, K. T., M. Singh, J. Howe, L. C. Ho, S. W. Tan, G. C. Ng, X. Q. Chen, and E. H. Yap. 1996. Observations on the ultrastructure and viability of the cystic stage of Blastocystis hominis from human feces. Parasitol. Res. 82:439-444. [DOI] [PubMed] [Google Scholar]
- 155.Moghaddam, D. D., E. Ghadirian, and M. Azami. 2005. Blastocystis hominis and the evaluation of efficacy of metronidazole and trimethoprim/sulfamethoxazole. Parasitol. Res. 96:273-275. [DOI] [PubMed] [Google Scholar]
- 156.Müller, H. E. 1994. Four serologically different groups within the species Blastocystis hominis. Zentralbl. Bakteriol. 280:403-408. [DOI] [PubMed] [Google Scholar]
- 157.Nagler, J., M. Brown, and R. Soave. 1993. Blastocystis hominis in inflammatory bowel disease. J. Clin. Gastroenterol. 16:109-112. [DOI] [PubMed] [Google Scholar]
- 158.Nakamura, Y., T. Hashimoto, H. Yoshikawa, T. Kamaishi, F. Nakamura, K. Okamoto, and M. Hasegawa. 1996. Phylogenetic position of Blastocystis hominis that contains cytochrome-free mitochondria, inferred from the protein phylogeny of elongation factor 1 alpha. Mol. Biochem. Parasitol. 77:241-245. [DOI] [PubMed] [Google Scholar]
- 159.Nasirudeen, A. M., Y. E. Hian, M. Singh, and K. S. Tan. 2004. Metronidazole induces programmed cell death in the protozoan parasite Blastocystis hominis. Microbiology 150:33-43. [DOI] [PubMed] [Google Scholar]
- 160.Nasirudeen, A. M., K. S. Tan, M. Singh, and E. H. Yap. 2001. Programmed cell death in a human intestinal parasite, Blastocystis hominis. Parasitology 123:235-246. [DOI] [PubMed] [Google Scholar]
- 161.Nassir, E., J. Awad, A. B. Abel, J. Khoury, M. Shay, and F. Lejbkowicz. 2004. Blastocystis hominis as a cause of hypoalbuminemia and anasarca. Eur. J. Clin. Microbiol. Infect. Dis. 23:399-402. [DOI] [PubMed] [Google Scholar]
- 162.Navarrete, N., and P. Torres. 1994. Prevalence of infection by intestinal helminths and protozoa in school children from a coastal locality in the province of Valdivia, Chile. Bol. Chil. Parasitol. 49:79-80. (In Spanish.) [PubMed] [Google Scholar]
- 163.Navarro, C., M. V. Domínguez-Márquez, M. M. Garijo-Toledo, S. Vega-García, S. Fernández-Barredo, M. T. Pérez-Gracia, A. García, R. Borrás, and M. T. Gómez-Muñoz. 2008. High prevalence of Blastocystis sp. in pigs reared under intensive growing systems: frequency of ribotypes and associated risk factors. Vet. Parasitol. 153:347-358. [DOI] [PubMed] [Google Scholar]
- 164.Ng, G. C., and K. S. Tan. 1999. Colony growth as a step towards axenization of Blastocystis isolates. Parasitol. Res. 85:678-679. [DOI] [PubMed] [Google Scholar]
- 165.Nigro, L., L. Larocca, L. Massarelli, I. Patamia, S. Minniti, F. Palermo, and B. Cacopardo. 2003. A placebo-controlled treatment trial of Blastocystis hominis infection with metronidazole. J. Travel. Med. 10:128-130. [DOI] [PubMed] [Google Scholar]
- 166.Nimri, L., and R. Batchoun. 1994. Intestinal colonization of symptomatic and asymptomatic schoolchildren with Blastocystis hominis. J. Clin. Microbiol. 32:2865-2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Nimri, L. F. 1993. Evidence of an epidemic of Blastocystis hominis infections in preschool children in northern Jordan. J. Clin. Microbiol. 31:2706-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nimri, L. F., and M. Meqdam. 2004. Enteropathogens associated with cases of gastroenteritis in a rural population in Jordan. Clin. Microbiol. Infect. 10:634-639. [DOI] [PubMed] [Google Scholar]
- 169.Noël, C., F. Dufernez, D. Gerbod, V. P. Edgcomb, P. Delgado-Viscogliosi, L. C. Ho, M. Singh, R. Wintjens, M. L. Sogin, M. Capron, R. Pierce, L. Zenner, and E. Viscogliosi. 2005. Molecular phylogenies of Blastocystis isolates from different hosts: implications for genetic diversity, identification of species, and zoonosis. J. Clin. Microbiol. 43:348-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Noël, C., C. Peyronnet, D. Gerbod, V. P. Edgcomb, P. Delgado-Viscogliosi, M. L. Sogin, M. Capron, E. Viscogliosi, and L. Zenner. 2003. Phylogenetic analysis of Blastocystis isolates from different hosts based on the comparison of small-subunit rRNA gene sequences. Mol. Biochem. Parasitol. 126:119-123. [DOI] [PubMed] [Google Scholar]
- 171.Noor Azian, M. Y., Y. M. San, C. C. Gan, M. Y. Yusri, Y. Nurulsyamzawaty, A. H. Zuhaizam, M. N. Maslawaty, I. Norparina, and I. Vythilingam. 2007. Prevalence of intestinal protozoa in an aborigine community in Pahang, Malaysia. Trop. Biomed. 24:55-62. [PubMed] [Google Scholar]
- 172.Nordlander, E., S. Phuphaisan, L. Bodhidatta, J. Arthur, and P. Echeverria. 1990. Microscopic examination of stools and a latex slide agglutination test for the rapid identification of bacterial enteric infections in Khmer children. Diagn. Microbiol. Infect. Dis. 13:273-276. [DOI] [PubMed] [Google Scholar]
- 173.Noureldin, M. S., A. A. Shaltout, E. M. El Hamshary, and M. E. Ali. 1999. Opportunistic intestinal protozoal infections in immunocompromised children. J. Egypt. Soc. Parasitol. 29:951-961. [PubMed] [Google Scholar]
- 174.Odds, F. C., M. E. Bougnoux, D. J. Shaw, J. M. Bain, A. D. Davidson, D. Diogo, M. D. Jacobsen, M. Lecomte, S. Y. Li, A. Tavanti, M. C. Maiden, N. A. Gow, and C. d'Enfert. 2007. Molecular phylogenetics of Candida albicans. Eukaryot. Cell 6:1041-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.O'Gorman, M. A., S. R. Orenstein, R. Proujansky, R. M. Wadowsky, P. E. Putnam, and S. A. Kocoshis. 1993. Prevalence and characteristics of Blastocystis hominis infection in children. Clin. Pediatr. (Philadelphia) 32:91-96. [DOI] [PubMed] [Google Scholar]
- 176.Ok, U. Z., M. Cirit, A. Uner, E. Ok, F. Akcicek, A. Basci, and M. A. Ozcel. 1997. Cryptosporidiosis and blastocystosis in renal transplant recipients. Nephron 75:171-174. [DOI] [PubMed] [Google Scholar]
- 177.Ok, U. Z., N. Girginkardesler, C. Balcioglu, P. Ertan, T. Pirildar, and A. A. Kilimcioglu. 1999. Effect of trimethoprim-sulfamethaxazole in Blastocystis hominis infection. Am. J. Gastroenterol. 94:3245-3247. [DOI] [PubMed] [Google Scholar]
- 178.Ortega, Y. R., and R. D. Adam. 1997. Giardia: overview and update. Clin. Infect. Dis. 25:545-549. [DOI] [PubMed] [Google Scholar]
- 179.Özcakir, O., S. Güreser, S. Ergüven, Y. A. Yilmaz, R. Topaloğlu, and G. Hasçelik. 2007. Characteristics of Blastocystis hominis infection in a Turkish university hospital. Turkiye Parazitol. Derg. 31:277-282. [PubMed] [Google Scholar]
- 180.Özyurt, M., O. Kurt, K. Mølbak, H. V. Nielsen, T. Haznedaroglu, and C. R. Stensvold. 2008. Molecular epidemiology of Blastocystis infections in Turkey. Parasitol. Int. 57:300-306. [DOI] [PubMed] [Google Scholar]
- 181.Pakandl, M. 1999. Blastocystis sp. from pigs: ultrastructural changes occurring during polyxenic cultivation in Iscove's modified Dulbecco's medium. Parasitol. Res. 85:743-748. [DOI] [PubMed] [Google Scholar]
- 182.Pakandl, M. 1992. An experimental transmission of porcine strains of Blastocystis sp. in the laboratory mice and gerbils. Folia Parasitol. (Prague) 39:383-386. [PubMed] [Google Scholar]
- 183.Parkar, U., R. J. Traub, S. Kumar, M. Mungthin, S. Vitali, S. Leelayoova, K. Morris, and R. C. Thompson. 2007. Direct characterization of Blastocystis from faeces by PCR and evidence of zoonotic potential. Parasitology 134:359-367. [DOI] [PubMed] [Google Scholar]
- 184.Pasqui, A. L., E. Savini, M. Saletti, C. Guzzo, L. Puccetti, and A. Auteri. 2004. Chronic urticaria and Blastocystis hominis infection: a case report. Eur. Rev. Med. Pharmacol. Sci. 8:117-120. [PubMed] [Google Scholar]
- 185.Pegelow, K., R. Gross, K. Pietrzik, W. Lukito, A. L. Richards, and D. J. Fryauff. 1997. Parasitological and nutritional situation of school children in the Sukaraja district, West Java, Indonesia. Southeast Asian J. Trop. Med. Public Health 28:173-190. [PubMed] [Google Scholar]
- 186.Philippe, H., and J. Laurent. 1998. How good are deep phylogenetic trees? Curr. Opin. Genet. Dev. 8:616-623. [DOI] [PubMed] [Google Scholar]
- 187.Phillips, B. P., and C. H. Zierdt. 1976. Blastocystis hominis: pathogenic potential in human patients and in gnotobiotes. Exp. Parasitol. 39:358-364. [DOI] [PubMed] [Google Scholar]
- 188.Pikula, Z. P. 1987. Blastocystis hominis and human disease. J. Clin. Microbiol. 25:1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Poxleitner, M. K., M. L. Carpenter, J. J. Mancuso, C. J. Wang, S. C. Dawson, and W. Z. Cande. 2008. Evidence for karyogamy and exchange of genetic material in the binucleate intestinal parasite Giardia intestinalis. Science 319:1530-1533. [DOI] [PubMed] [Google Scholar]
- 190.Priest, J. W., C. Bern, J. M. Roberts, J. P. Kwon, A. G. Lescano, W. Checkley, L. Cabrera, D. M. Moss, M. J. Arrowood, C. R. Sterling, R. H. Gilman, and P. J. Lammie. 2005. Changes in serum immunoglobulin G levels as a marker for Cryptosporidium sp. infection in Peruvian children. J. Clin. Microbiol. 43:5298-5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Puthia, M. K., J. Lu, and K. S. Tan. 2008. Blastocystis ratti contains cysteine proteases that mediate interleukin-8 response from human intestinal epithelial cells in an NF-κB-dependent manner. Eukaryot. Cell 7:435-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Puthia, M. K., S. W. Sio, J. Lu, and K. S. Tan. 2006. Blastocystis ratti induces contact-independent apoptosis, F-actin rearrangement, and barrier function disruption in IEC-6 cells. Infect. Immun. 74:4114-4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Puthia, M. K., A. Vaithilingam, J. Lu, and K. S. Tan. 2005. Degradation of human secretory immunoglobulin A by Blastocystis. Parasitol. Res. 97:386-389. [DOI] [PubMed] [Google Scholar]
- 194.Qadri, S. M., G. A. al-Okaili, and F. al-Dayel. 1989. Clinical significance of Blastocystis hominis. J. Clin. Microbiol. 27:2407-2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Reference deleted.
- 196.Rao, K., U. Sekar, K. T. Iraivan, G. Abraham, and P. Soundararajan. 2003. Blastocystis hominis—an emerging cause of diarrhoea in renal transplant recipients. J. Assoc. Physicians India 51:719-721. [PubMed] [Google Scholar]
- 197.Rapeeporn, Y., N. Warunee, W. Nuttapong, S. Sompong, and K. Rachada. 2006. Infection of Blastocystis hominis in primary schoolchildren from Nakhon Pathom province, Thailand. Trop. Biomed. 23:117-122. [PubMed] [Google Scholar]
- 198.Rayan, H. Z., O. A. Ismail, and E. K. El Gayar. 2007. Prevalence and clinical features of Dientamoeba fragilis infections in patients suspected to have intestinal parasitic infection. J. Egypt. Soc. Parasitol. 37:599-608. [PubMed] [Google Scholar]
- 199.Reed, S. L., W. E. Keene, and J. H. McKerrow. 1989. Thiol proteinase expression and pathogenicity of Entamoeba histolytica. J. Clin. Microbiol. 27:2772-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Requena, I., Y. Hernandez, M. Ramsay, C. Salazar, and R. Devera. 2003. Prevalence of Blastocystis hominis among food handlers from Caroni municipality, Bolivar State, Venezuela. Cad. Saude Publica 19:1721-1727. (In Spanish.) [DOI] [PubMed] [Google Scholar]
- 201.Rivera, W. L. 2008. Phylogenetic analysis of Blastocystis isolates from animal and human hosts in the Philippines. Vet. Parasitol 156:178-182. [DOI] [PubMed] [Google Scholar]
- 202.Rivera, W. L., and M. A. Tan. 2005. Molecular characterization of Blastocystis isolates in the Philippines by riboprinting. Parasitol. Res. 96:253-257. [DOI] [PubMed] [Google Scholar]
- 203.Roger, A. J., and L. A. Hug. 2006. The origin and diversification of eukaryotes: problems with molecular phylogenetics and molecular clock estimation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361:1039-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Roger, A. J., O. Sandblom, W. F. Doolittle, and H. Philippe. 1999. An evaluation of elongation factor 1 alpha as a phylogenetic marker for eukaryotes. Mol. Biol. Evol. 16:218-233. [DOI] [PubMed] [Google Scholar]
- 205.Rossignol, J. F., S. M. Kabil, M. Said, H. Samir, and A. M. Younis. 2005. Effect of nitazoxanide in persistent diarrhea and enteritis associated with Blastocystis hominis. Clin. Gastroenterol. Hepatol. 3:987-991. [DOI] [PubMed] [Google Scholar]
- 206.Russo, A. R., S. L. Stone, M. E. Taplin, H. J. Snapper, and G. V. Doern. 1988. Presumptive evidence for Blastocystis hominis as a cause of colitis. Arch. Intern. Med. 148:1064. [PubMed] [Google Scholar]
- 207.Sadaga, G. A., and H. H. Kassem. 2007. Prevalence of intestinal parasites among primary schoolchildren in Derna District, Libya. J. Egypt. Soc. Parasitol. 37:205-214. [PubMed] [Google Scholar]
- 208.Sadek, Y., A. F. el-Fakahany, A. H. Lashin, and F. A. el-Salam. 1997. Intestinal parasites among food-handlers in Qualyobia Governorate, with reference to the pathogenic parasite Blastocystis hominis. J. Egypt. Soc. Parasitol. 27:471-478. [PubMed] [Google Scholar]
- 209.Sajid, M., and J. H. McKerrow. 2002. Cysteine proteases of parasitic organisms. Mol. Biochem. Parasitol. 120:1-21. [DOI] [PubMed] [Google Scholar]
- 210.Saksirisampant, W., S. Nuchprayoon, V. Wiwanitkit, S. Yenthakam, and A. Ampavasiri. 2003. Intestinal parasitic infestations among children in an orphanage in Pathum Thani province. J. Med. Assoc. Thai. 86(Suppl. 2):S263-S270. [PubMed] [Google Scholar]
- 211.Saksirisampant, W., J. Prownebon, M. Kulkumthorn, S. Yenthakam, S. Janpla, and S. Nuchprayoon. 2006. Prevalence of intestinal parasitic infections among school children in the central region of Thailand. J. Med. Assoc. Thai. 89:1928-1933. [PubMed] [Google Scholar]
- 211a.Salim, H. R., G. S. Kumar, S. Vellayan, J. W. Mak, A. K. Anuar, I. Init, G. D. Vennila, R. Saminathan, and K. Ramakrishnan. 1999. Blastocystis in animal handlers. Parasitol. Res. 85:1032-1033. [DOI] [PubMed] [Google Scholar]
- 212.Schoepfer, A. M., M. Trummler, P. Seeholzer, D. H. Criblez, and F. Seibold. 2007. Accuracy of four fecal assays in the diagnosis of colitis. Dis. Colon Rectum 50:1697-1706. [DOI] [PubMed] [Google Scholar]
- 213.Schoepfer, A. M., M. Trummler, P. Seeholzer, B. Seibold-Schmid, and F. Seibold. 2008. Discriminating IBD from IBS: comparison of the test performance of fecal markers, blood leukocytes, CRP, and IBD antibodies. Inflamm. Bowel Dis. 14:32-39. [DOI] [PubMed] [Google Scholar]
- 214.Scicluna, S. M., B. Tawari, and C. G. Clark. 2006. DNA barcoding of Blastocystis. Protist 157:77-85. [DOI] [PubMed] [Google Scholar]
- 215.Sheehan, D. J., B. G. Raucher, and J. C. McKitrick. 1986. Association of Blastocystis hominis with signs and symptoms of human disease. J. Clin. Microbiol. 24:548-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Shlim, D. R., C. W. Hoge, R. Rajah, J. G. Rabold, and P. Echeverria. 1995. Is Blastocystis hominis a cause of diarrhea in travelers? A prospective controlled study in Nepal. Clin. Infect. Dis. 21:97-101. [DOI] [PubMed] [Google Scholar]
- 217.Silard, R. 1979. Contributions to Blastocystis hominis studies. Aspects of degenerescence. Arch. Roum. Pathol. Exp. Microbiol. 38:105-114. [PubMed] [Google Scholar]
- 218.Silberman, J. D., M. L. Sogin, D. D. Leipe, and C. G. Clark. 1996. Human parasite finds taxonomic home. Nature 380:398. [DOI] [PubMed] [Google Scholar]
- 219.Singh, M., L. C. Ho, A. L. Yap, G. C. Ng, S. W. Tan, K. T. Moe, and E. H. Yap. 1996. Axenic culture of reptilian Blastocystis isolates in monophasic medium and speciation by karyotypic typing. Parasitol. Res. 82:165-169. [DOI] [PubMed] [Google Scholar]
- 220.Singh, M., K. Suresh, L. C. Ho, G. C. Ng, and E. H. Yap. 1995. Elucidation of the life cycle of the intestinal protozoan Blastocystis hominis. Parasitol. Res. 81:446-450. [DOI] [PubMed] [Google Scholar]
- 221.Sinniah, B., and B. Rajeswari. 1994. Blastocystis hominis infection, a cause of human diarrhea. Southeast Asian J. Trop. Med. Public Health 25:490-493. [PubMed] [Google Scholar]
- 222.Sio, S. W., M. K. Puthia, A. S. Lee, J. Lu, and K. S. Tan. 2006. Protease activity of Blastocystis hominis. Parasitol. Res. 99:126-130. [DOI] [PubMed] [Google Scholar]
- 223.Snowden, K., K. Logan, C. Blozinski, J. Hoevers, and P. Holman. 2000. Restriction-fragment-length polymorphism analysis of small-subunit rRNA genes of Blastocystis isolates from animal hosts. Parasitol. Res. 86:62-66. [DOI] [PubMed] [Google Scholar]
- 224.Sohail, M. R., and P. R. Fischer. 2005. Blastocystis hominis and travelers. Travel Med. Infect. Dis. 3:33-38. [DOI] [PubMed] [Google Scholar]
- 225.Soriano, S. V., L. M. Barbieri, N. B. Pierangeli, A. L. Giayetto, A. M. Manacorda, E. Castronovo, B. C. Pezzani, M. Minvielle, and J. A. Basualdo. 2001. Intestinal parasites and the environment: frequency of intestinal parasites in children of Neuquen, Patagonia, Argentina. Rev. Latinoam. Microbiol. 43:96-101. [PubMed] [Google Scholar]
- 226.Staumont-Salle, D., D. Dombrowicz, M. Capron, and E. Delaporte. 2006. Eosinophils and urticaria. Clin. Rev. Allergy Immunol. 30:13-18. [DOI] [PubMed] [Google Scholar]
- 227.Stensvold, C. R., M. C. Arendrup, C. Jespersgaard, K. Mølbak, and H. V. Nielsen. 2007. Detecting Blastocystis using parasitologic and DNA-based methods: a comparative study. Diagn. Microbiol. Infect. Dis. 59:303-307. [DOI] [PubMed] [Google Scholar]
- 228.Stensvold, C. R., M. C. Arendrup, H. V. Nielsen, A. Bada, and S. Thorsen. 2008. Symptomatic infection with Blastocystis sp. subtype 8 successfully treated with trimethoprim-sulfamethoxazole. Ann. Trop. Med. Parasitol. 102:271-274. [DOI] [PubMed] [Google Scholar]
- 229.Stensvold, C. R., G. K. Suresh, K. S. Tan, R. C. Thompson, R. J. Traub, E. Viscogliosi, H. Yoshikawa, and C. G. Clark. 2007. Terminology for Blastocystis subtypes—a consensus. Trends Parasitol. 23:93-96. [DOI] [PubMed] [Google Scholar]
- 230.Stensvold, C. R., R. J. Traub, G. von Samson-Himmelstjerna, C. Jespersgaard, H. V. Nielsen, and R. C. Thompson. 2007. Blastocystis: subtyping isolates using pyrosequencing technology. Exp. Parasitol. 116:111-119. [DOI] [PubMed] [Google Scholar]
- 231.Stensvold, R., A. Brillowska-Dabrowska, H. V. Nielsen, and M. C. Arendrup. 2006. Detection of Blastocystis hominis in unpreserved stool specimens by using polymerase chain reaction. J. Parasitol. 92:1081-1087. [DOI] [PubMed] [Google Scholar]
- 232.Stenzel, D. J., and P. F. Boreham. 1994. Bacteria-like endosymbionts in Blastocystis sp. Int. J. Parasitol. 24:147-149. [DOI] [PubMed] [Google Scholar]
- 233.Stenzel, D. J., and P. F. Boreham. 1996. Blastocystis hominis revisited. Clin. Microbiol. Rev. 9:563-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Stenzel, D. J., and P. F. Boreham. 1991. A cyst-like stage of Blastocystis hominis. Int. J. Parasitol. 21:613-615. [DOI] [PubMed] [Google Scholar]
- 235.Stenzel, D. J., P. F. Boreham, and R. McDougall. 1991. Ultrastructure of Blastocystis hominis in human stool samples. Int. J. Parasitol. 21:807-812. [DOI] [PubMed] [Google Scholar]
- 236.Stenzel, D. J., M. F. Cassidy, and P. F. Boreham. 1994. Morphology of Blastocystis sp. from domestic birds. Parasitol. Res. 80:131-137. [DOI] [PubMed] [Google Scholar]
- 237.Stenzel, D. J., M. G. Lee, and P. F. Boreham. 1997. Morphological differences in Blastocystis cysts—an indication of different species? Parasitol. Res. 83:452-457. [DOI] [PubMed] [Google Scholar]
- 238.Sun, T., S. Katz, B. Tanenbaum, and C. Schenone. 1989. Questionable clinical significance of Blastocystis hominis infection. Am. J. Gastroenterol. 84:1543-1547. [PubMed] [Google Scholar]
- 239.Suresh, K., S. Y. Chong, J. Howe, L. C. Ho, G. C. Ng, E. H. Yap, and M. Singh. 1995. Tubulovesicular elements in Blastocystis hominis from the caecum of experimentally-infected rats. Int. J. Parasitol. 25:123-126. [DOI] [PubMed] [Google Scholar]
- 240.Suresh, K., J. Howe, S. Y. Chong, G. C. Ng, L. C. Ho, A. K. Loh, N. P. Ramachandran, E. H. Yap, and M. Singh. 1994. Ultrastructural changes during in vitro encystment of Blastocystis hominis. Parasitol. Res. 80:327-335. [DOI] [PubMed] [Google Scholar]
- 241.Suresh, K., J. Howe, G. C. Ng, L. C. Ho, N. P. Ramachandran, A. K. Loh, E. H. Yap, and M. Singh. 1994. A multiple fission-like mode of asexual reproduction in Blastocystis hominis. Parasitol. Res. 80:523-527. [DOI] [PubMed] [Google Scholar]
- 242.Suresh, K., J. W. Mak, L. S. Chuong, T. Ragunathan, and I. Init. 1997. Sac-like pouches in Blastocystis from the house lizard Cosymbotus platyurus. Parasitol. Res. 83:523-525. [DOI] [PubMed] [Google Scholar]
- 243.Suresh, K., G. C. Ng, L. C. Ho, E. H. Yap, and M. Singh. 1994. Differentiation of the various stages of Blastocystis hominis by acridine orange staining. Int. J. Parasitol. 24:605-606. [DOI] [PubMed] [Google Scholar]
- 244.Suresh, K., G. C. Ng, N. P. Ramachandran, L. C. Ho, E. H. Yap, and M. Singh. 1993. In vitro encystment and experimental infections of Blastocystis hominis. Parasitol. Res. 79:456-460. [DOI] [PubMed] [Google Scholar]
- 245.Suresh, K., and H. Smith. 2004. Comparison of methods for detecting Blastocystis hominis. Eur. J. Clin. Microbiol. Infect. Dis. 23:509-511. [DOI] [PubMed] [Google Scholar]
- 246.Suresh, K., H. V. Smith, and T. C. Tan. 2005. Viable Blastocystis cysts in Scottish and Malaysian sewage samples. Appl. Environ. Microbiol. 71:5619-5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Svenungsson, B., A. Lagergren, E. Ekwall, B. Evengård, K. O. Hedlund, A. Kärnell, S. Löfdahl, L. Svensson, and A. Weintraub. 2000. Enteropathogens in adult patients with diarrhea and healthy control subjects: a 1-year prospective study in a Swedish clinic for infectious diseases. Clin. Infect. Dis. 30:770-778. [DOI] [PubMed] [Google Scholar]
- 248.Taamasri, P., M. Mungthin, R. Rangsin, B. Tongupprakarn, W. Areekul, and S. Leelayoova. 2000. Transmission of intestinal blastocystosis related to the quality of drinking water. Southeast Asian J. Trop. Med. Public Health 31:112-117. [PubMed] [Google Scholar]
- 249.Tan, H. K., and C. H. Zierdt. 1973. Ultrastructure of Blastocystis hominis. Z. Parasitenkd. 42:315-324. [DOI] [PubMed] [Google Scholar]
- 250.Tan, K. S. 2004. Blastocystis in humans and animals: new insights using modern methodologies. Vet. Parasitol. 126:121-144. [DOI] [PubMed] [Google Scholar]
- 251.Tan, K. S. 2008. Blastocystis spp. In N. A. Khan (ed.), Emerging protozoan pathogens. Taylor and Francis, Oxford, United Kingdom.
- 252.Tan, K. S., J. Howe, E. H. Yap, and M. Singh. 2001. Do Blastocystis hominis colony forms undergo programmed cell death? Parasitol. Res. 87:362-367. [DOI] [PubMed] [Google Scholar]
- 253.Tan, K. S., M. Ibrahim, G. C. Ng, A. M. Nasirudeen, L. C. Ho, E. H. Yap, and M. Singh. 2001. Exposure of Blastocystis species to a cytotoxic monoclonal antibody. Parasitol. Res. 87:534-538. [DOI] [PubMed] [Google Scholar]
- 254.Tan, K. S., and A. M. Nasirudeen. 2005. Protozoan programmed cell death—insights from Blastocystis deathstyles. Trends Parasitol. 21:547-550. [DOI] [PubMed] [Google Scholar]
- 255.Tan, K. S., G. C. Ng, E. Quek, J. Howe, N. P. Ramachandran, E. H. Yap, and M. Singh. 2000. Blastocystis hominis: a simplified, high-efficiency method for clonal growth on solid agar. Exp. Parasitol. 96:9-15. [DOI] [PubMed] [Google Scholar]
- 256.Tan, K. S., M. Singh, and E. H. Yap. 2002. Recent advances in Blastocystis hominis research: hot spots in terra incognita. Int. J. Parasitol. 32:789-804. [DOI] [PubMed] [Google Scholar]
- 257.Tan, K. S., and D. J. Stenzel. 2003. Multiple reproductive processes in Blastocystis: proceed with caution. Trends Parasitol. 19:290-291, 291-292. [DOI] [PubMed] [Google Scholar]
- 258.Tan, S. W., L. C. Ho, K. T. Moe, X. Q. Chen, G. C. Ng, E. H. Yap, and M. Singh. 1996. Production and characterization of murine monoclonal antibodies to Blastocystis hominis. Int. J. Parasitol. 26:375-381. [DOI] [PubMed] [Google Scholar]
- 259.Tan, S. W., M. Singh, L. C. Ho, J. Howe, K. T. Moe, X. Q. Chen, G. C. Ng, and E. H. Yap. 1997. Survival of Blastocystis hominis clones after exposure to a cytotoxic monoclonal antibody. Int. J. Parasitol. 27:947-954. [DOI] [PubMed] [Google Scholar]
- 260.Tan, S. W., M. Singh, K. T. Thong, L. C. Ho, K. T. Moe, X. Q. Chen, G. C. Ng, and E. H. Yap. 1996. Clonal growth of Blastocystis hominis in soft agar with sodium thioglycollate. Parasitol. Res. 82:737-739. [DOI] [PubMed] [Google Scholar]
- 261.Tan, S. W., M. Singh, E. H. Yap, L. C. Ho, K. T. Moe, J. Howe, and G. C. Ng. 1996. Colony formation of Blastocystis hominis in soft agar. Parasitol. Res. 82:375-377. [DOI] [PubMed] [Google Scholar]
- 262.Tan, T. C., and K. G. Suresh. 2006. Amoeboid form of Blastocystis hominis—a detailed ultrastructural insight. Parasitol. Res. 99:737-742. [DOI] [PubMed] [Google Scholar]
- 263.Tan, T. C., and K. G. Suresh. 2007. Evidence of plasmotomy in Blastocystis hominis. Parasitol. Res. 101:1521-1525. [DOI] [PubMed] [Google Scholar]
- 264.Tan, T. C., and K. G. Suresh. 2006. Predominance of amoeboid forms of Blastocystis hominis in isolates from symptomatic patients. Parasitol. Res. 98:189-193. [DOI] [PubMed] [Google Scholar]
- 265.Tan, T. C., K. G. Suresh, K. L. Thong, and H. V. Smith. 2006. PCR fingerprinting of Blastocystis isolated from symptomatic and asymptomatic human hosts. Parasitol. Res. 99:459-465. [DOI] [PubMed] [Google Scholar]
- 266.Tanizaki, A., H. Yoshikawa, S. Iwatani, and I. Kimata. 2005. Infectivity of Blastocystis isolates from chickens, quails and geese in chickens. Parasitol. Res. 96:57-61. [DOI] [PubMed] [Google Scholar]
- 267.Tasova, Y., B. Sahin, S. Koltas, and S. Paydas. 2000. Clinical significance and frequency of Blastocystis hominis in Turkish patients with hematological malignancy. Acta Med. Okayama 54:133-136. [DOI] [PubMed] [Google Scholar]
- 268.Tato, C. M., and C. A. Hunter. 2002. Host-pathogen interactions: subversion and utilization of the NF-κB pathway during infection. Infect. Immun. 70:3311-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Teow, W. L., G. C. Ng, P. P. Chan, Y. C. Chan, E. H. Yap, V. Zaman, and M. Singh. 1992. A survey of Blastocystis in reptiles. Parasitol. Res. 78:453-455. [DOI] [PubMed] [Google Scholar]
- 270.Termmathurapoj, S., S. Leelayoova, P. Aimpun, U. Thathaisong, T. Nimmanon, P. Taamasri, and M. Mungthin. 2004. The usefulness of short-term in vitro cultivation for the detection and molecular study of Blastocystis hominis in stool specimens. Parasitol. Res. 93:445-447. [DOI] [PubMed] [Google Scholar]
- 271.Thathaisong, U., J. Worapong, M. Mungthin, P. Tan-Ariya, K. Viputtigul, A. Sudatis, A. Noonai, and S. Leelayoova. 2003. Blastocystis isolates from a pig and a horse are closely related to Blastocystis hominis. J. Clin. Microbiol. 41:967-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Tiwary, B. K., and M. C. Goel. 1984. Suppression of IgA synthesis in chickens. Vet. Immunol. Immunopathol. 7:305-313. [DOI] [PubMed] [Google Scholar]
- 273.Torres, P., J. C. Miranda, L. Flores, J. Riquelme, R. Franjola, J. Pérez, S. Auad, C. Hermosilla, and S. Riquelme. 1992. Blastocystosis and other intestinal protozoan infections in human riverside communities of the Valdivia River basin, Chile. Rev. Inst. Med. Trop. Sao Paulo 34:557-564. (In Portugese.) [PubMed] [Google Scholar]
- 274.Tungtrongchitr, A., S. Manatsathit, C. Kositchaiwat, J. Ongrotchanakun, N. Munkong, P. Chinabutr, S. Leelakusolvong, and W. Chaicumpa. 2004. Blastocystis hominis infection in irritable bowel syndrome patients. Southeast Asian J. Trop. Med. Public Health 35:705-710. [PubMed] [Google Scholar]
- 275.Udkow, M. P., and E. K. Markell. 1993. Blastocystis hominis: prevalence in asymptomatic versus symptomatic hosts. J. Infect. Dis. 168:242-244. [DOI] [PubMed] [Google Scholar]
- 276.Upcroft, J. A., L. A. Dunn, L. S. Dommett, A. Healey, P. Upcroft, and P. F. Boreham. 1989. Chromosomes of Blastocystis hominis. Int. J. Parasitol. 19:879-883. [DOI] [PubMed] [Google Scholar]
- 277.Valido, E. M., and W. L. Rivera. 2007. Colony growth of Philippine isolates of Blastocystis hominis in simplified, soft agar medium. Parasitol. Res. 101:213-217. [DOI] [PubMed] [Google Scholar]
- 278.Valsecchi, R., P. Leghissa, and V. Greco. 2004. Cutaneous lesions in Blastocystis hominis infection. Acta Derm. Venereol. 84:322-323. [DOI] [PubMed] [Google Scholar]
- 279.van der Heijden, H. M., and W. J. Landman. 2008. In vitro effect of herbal products against Histomonas meleagridis. Vet. Parasitol. 154:1-7. [DOI] [PubMed] [Google Scholar]
- 280.Vannatta, J. B., D. Adamson, and K. Mullican. 1985. Blastocystis hominis infection presenting as recurrent diarrhea. Ann. Intern. Med. 102:495-496. [DOI] [PubMed] [Google Scholar]
- 281.Vennila, G. D., G. S. Kumar, A. K. Anuar, S. Rajah, R. Saminathan, S. Sivanandan, and K. Ramakrishnan. 1999. Irregular shedding of Blastocystis hominis. Parasitol. Res. 85:162-164. [DOI] [PubMed] [Google Scholar]
- 282.Villar, J., J. A. Carbajal, M. D. Lanuza, C. Muñoz, and R. Borrás. 1998. In vitro encystation of Blastocystis hominis: a kinetics and cytochemistry study. Parasitol. Res. 84:54-58. [DOI] [PubMed] [Google Scholar]
- 283.Visser, L. G., J. J. Verweij, M. Van Esbroeck, W. M. Edeling, J. Clerinx, and A. M. Polderman. 2006. Diagnostic methods for differentiation of Entamoeba histolytica and Entamoeba dispar in carriers: performance and clinical implications in a non-endemic setting. Int. J. Med. Microbiol. 296:397-403. [DOI] [PubMed] [Google Scholar]
- 284.Waghorn, D. J., and P. Hancock. 1991. Clinical significance of Blastocystis hominis. Lancet 337:609. [DOI] [PubMed] [Google Scholar]
- 285.Waikagul, J., S. Krudsood, P. Radomyos, B. Radomyos, K. Chalemrut, P. Jonsuksuntigul, S. Kojima, S. Looareesuwan, and W. Thaineau. 2002. A cross-sectional study of intestinal parasitic infections among schoolchildren in Nan Province, Northern Thailand. Southeast Asian J. Trop. Med. Public Health 33:218-223. [PubMed] [Google Scholar]
- 286.Walderich, B., S. Bernauer, M. Renner, J. Knobloch, and G. D. Burchard. 1998. Cytopathic effects of Blastocystis hominis on Chinese hamster ovary (CHO) and adeno carcinoma HT29 cell cultures. Trop. Med. Int. Health 3:385-390. [DOI] [PubMed] [Google Scholar]
- 287.Wang, L. C. 2004. Changing patterns in intestinal parasitic infections among Southeast Asian laborers in Taiwan. Parasitol. Res. 92:18-21. [DOI] [PubMed] [Google Scholar]
- 288.Wilson, K. W., D. Winget, and S. Wilks. 1990. Blastocystis hominis infection: signs and symptoms in patients at Wilford Hall Medical Center. Mil. Med. 155:394-396. [PubMed] [Google Scholar]
- 289.Windsor, J. J., L. Macfarlane, G. Hughes-Thapa, S. K. Jones, and T. M. Whiteside. 2002. Incidence of Blastocystis hominis in faecal samples submitted for routine microbiological analysis. Br. J. Biomed. Sci. 59:154-157. [DOI] [PubMed] [Google Scholar]
- 290.Windsor, J. J., D. J. Stenzel, and L. Macfarlane. 2003. Multiple reproductive processes in Blastocystis hominis. Trends Parasitol. 19:289-290, 291-292. [DOI] [PubMed] [Google Scholar]
- 291.Wong, K. H., G. C. Ng, R. T. Lin, H. Yoshikawa, M. B. Taylor, and K. S. Tan. 2008. Predominance of subtype 3 among Blastocystis isolates from a major hospital in Singapore. Parasitol. Res. 102:663-670. [DOI] [PubMed] [Google Scholar]
- 292.Yakoob, J., W. Jafri, N. Jafri, M. Islam, and M. Asim Beg. 2004. In vitro susceptibility of Blastocystis hominis isolated from patients with irritable bowel syndrome. Br. J. Biomed. Sci. 61:75-77. [DOI] [PubMed] [Google Scholar]
- 293.Yakoob, J., W. Jafri, N. Jafri, R. Khan, M. Islam, M. A. Beg, and V. Zaman. 2004. Irritable bowel syndrome: in search of an etiology: role of Blastocystis hominis. Am. J. Trop. Med. Hyg. 70:383-385. [PubMed] [Google Scholar]
- 294.Yan, Y., S. Su, R. Lai, H. Liao, J. Ye, X. Li, X. Luo, and G. Chen. 2006. Genetic variability of Blastocystis hominis isolates in China. Parasitol. Res. 99:597-601. [DOI] [PubMed] [Google Scholar]
- 295.Yan, Y., S. Su, J. Ye, X. Lai, R. Lai, H. Liao, G. Chen, R. Zhang, Z. Hou, and X. Luo. 2007. Blastocystis sp. subtype 5: a possibly zoonotic genotype. Parasitol. Res. 101:1527-1532. [DOI] [PubMed] [Google Scholar]
- 296.Yoshikawa, H., N. Abe, M. Iwasawa, S. Kitano, I. Nagano, Z. Wu, and Y. Takahashi. 2000. Genomic analysis of Blastocystis hominis strains isolated from two long-term health care facilities. J. Clin. Microbiol. 38:1324-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Yoshikawa, H., N. Abe, and Z. Wu. 2004. PCR-based identification of zoonotic isolates of Blastocystis from mammals and birds. Microbiology 150:1147-1151. [DOI] [PubMed] [Google Scholar]
- 298.Yoshikawa, H., I. Katagiri, X. H. Li, M. BeckerHapak, and D. C. Graves. 1995. Antigenic differences between Blastocystis hominis and Blastocystis sp. revealed by polyclonal and monoclonal antibodies. J. Protozool. Res. 5:118-128. [Google Scholar]
- 299.Yoshikawa, H., N. Kuwayama, and Y. Enose. 1995. Histochemical detection of carbohydrates of Blastocystis hominis. J. Eukaryot. Microbiol. 42:70-74. [DOI] [PubMed] [Google Scholar]
- 300.Yoshikawa, H., K. Morimoto, Z. Wu, M. Singh, and T. Hashimoto. 2004. Problems in speciation in the genus Blastocystis. Trends Parasitol. 20:251-255. [DOI] [PubMed] [Google Scholar]
- 301.Yoshikawa, H., I. Nagano, Z. Wu, E. H. Yap, M. Singh, and Y. Takahashi. 1998. Genomic polymorphism among Blastocystis hominis strains and development of subtype-specific diagnostic primers. Mol. Cell. Probes 12:153-159. [DOI] [PubMed] [Google Scholar]
- 302.Yoshikawa, H., J. Satoh, and Y. Enose. 1995. Light and electron microscopic localization of lipids in Blastocystis hominis. J. Electron Microsc. (Tokyo) 44:100-103. [PubMed] [Google Scholar]
- 303.Yoshikawa, H., Z. Wu, J. Howe, T. Hashimoto, N. Geok-Choo, and K. S. Tan. 2007. Ultrastructural and phylogenetic studies on Blastocystis isolates from cockroaches. J. Eukaryot. Microbiol. 54:33-37. [DOI] [PubMed] [Google Scholar]
- 304.Yoshikawa, H., Z. Wu, I. Kimata, M. Iseki, I. K. Ali, M. B. Hossain, V. Zaman, R. Haque, and Y. Takahashi. 2004. Polymerase chain reaction-based genotype classification among human Blastocystis hominis populations isolated from different countries. Parasitol. Res. 92:22-29. [DOI] [PubMed] [Google Scholar]
- 305.Yoshikawa, H., Z. Wu, I. Nagano, and Y. Takahashi. 2003. Molecular comparative studies among Blastocystis isolates obtained from humans and animals. J. Parasitol. 89:585-594. [DOI] [PubMed] [Google Scholar]
- 306.Yoshikawa, H., K. Yoshida, A. Nakajima, K. Yamanari, S. Iwatani, and I. Kimata. 2004. Fecal-oral transmission of the cyst form of Blastocystis hominis in rats. Parasitol. Res. 94:391-396. [DOI] [PubMed] [Google Scholar]
- 307.Zaki, M., A. S. Daoud, R. N. Pugh, F. al-Ali, G. al-Mutairi, and Q. al-Saleh. 1991. Clinical report of Blastocystis hominis infection in children. J. Trop. Med. Hyg. 94:118-122. [PubMed] [Google Scholar]
- 308.Zali, M. R., A. J. Mehr, M. Rezaian, A. R. Meamar, S. Vaziri, and M. Mohraz. 2004. Prevalence of intestinal parasitic pathogens among HIV-positive individuals in Iran. Jpn. J. Infect. Dis. 57:268-270. [PubMed] [Google Scholar]
- 309.Zaman, V. 1996. The diagnosis of Blastocystis hominis cysts in human faeces. J. Infect. 33:15-16. [DOI] [PubMed] [Google Scholar]
- 310.Zaman, V. 1998. The differential identification of Blastocystis hominis cysts. Ann. Trop. Med. Parasitol. 92:233-235. [DOI] [PubMed] [Google Scholar]
- 311.Zaman, V., J. Howe, and M. Ng. 1997. Observations on the surface coat of Blastocystis hominis. Parasitol. Res. 83:731-733. [DOI] [PubMed] [Google Scholar]
- 312.Zaman, V., J. Howe, and M. Ng. 1995. Ultrastructure of Blastocystis hominis cysts. Parasitol. Res. 81:465-469. [DOI] [PubMed] [Google Scholar]
- 313.Zaman, V., J. Howe, and M. Ng. 1997. Variation in the cyst morphology of Blastocystis hominis. Parasitol. Res. 83:306-308. [DOI] [PubMed] [Google Scholar]
- 314.Zaman, V., J. Howe, M. Ng, and T. K. Goh. 1999. Scanning electron microscopy of the surface coat of Blastocystis hominis. Parasitol. Res. 85:974-976. [DOI] [PubMed] [Google Scholar]
- 315.Zaman, V., and K. Z. Khan. 1994. A concentration technique for obtaining viable cysts of Blastocystis hominis from faeces. J. Pak. Med. Assoc. 44:220-221. [PubMed] [Google Scholar]
- 316.Zaman, V., and M. Zaki. 1996. Resistance of Blastocystis hominis cysts to metronidazole. Trop. Med. Int. Health 1:677-678. [DOI] [PubMed] [Google Scholar]
- 317.Zaman, V., M. Zaki, M. Manzoor, J. Howe, and M. Ng. 1999. Postcystic development of Blastocystis hominis. Parasitol. Res. 85:437-440. [DOI] [PubMed] [Google Scholar]
- 318.Zhang, X., J. Y. Qiao, X. J. Zhou, F. R. Yao, and Z. C. Wei. 2007. Morphology and reproductive mode of Blastocystis hominis in diarrhea and in vitro. Parasitol. Res. 101:43-51. [DOI] [PubMed] [Google Scholar]
- 319.Zierdt, C. H. 1991. Blastocystis hominis—past and future. Clin. Microbiol. Rev. 4:61-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Zierdt, C. H. 1988. Blastocystis hominis, a long-misunderstood intestinal parasite. Parasitol. Today 4:15-17. [DOI] [PubMed] [Google Scholar]
- 321.Zierdt, C. H. 1973. Studies of Blastocystis hominis. J. Protozool. 20:114-121. [DOI] [PubMed] [Google Scholar]
- 322.Zierdt, C. H., C. T. Donnolley, J. Muller, and G. Constantopoulos. 1988. Biochemical and ultrastructural study of Blastocystis hominis. J. Clin. Microbiol. 26:965-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Zierdt, C. H., and B. Nagy. 1993. Antibody response to Blastocystis hominis infections. Ann. Intern. Med. 118:985-986. [DOI] [PubMed] [Google Scholar]
- 324.Zierdt, C. H., and J. C. Swan. 1981. Generation time and growth rate of the human intestinal parasite Blastocystis hominis. J. Protozool. 28:483-485. [DOI] [PubMed] [Google Scholar]
- 325.Zierdt, C. H., J. C. Swan, and J. Hosseini. 1983. In vitro response of Blastocystis hominis to antiprotozoal drugs. J. Protozool. 30:332-334. [DOI] [PubMed] [Google Scholar]
- 326.Zierdt, C. H., and H. K. Tan. 1976. Ultrastructure and light microscope appearance of Blastocystis hominis in a patient with enteric disease. Z. Parasitenkd. 50:277-283. [DOI] [PubMed] [Google Scholar]
- 327.Zierdt, C. H., and R. L. Williams. 1974. Blastocystis hominis: axenic cultivation. Exp. Parasitol. 36:233-243. [DOI] [PubMed] [Google Scholar]
- 328.Zierdt, C. H., W. S. Zierdt, and B. Nagy. 1995. Enzyme-linked immunosorbent assay for detection of serum antibody to Blastocystis hominis in symptomatic infections. J. Parasitol. 81:127-129. [PubMed] [Google Scholar]
- 329.Zuckerman, M. J., H. Ho, L. Hooper, B. Anderson, and S. M. Polly. 1990. Frequency of recovery of Blastocystis hominis in clinical practice. J. Clin. Gastroenterol. 12:525-532. [DOI] [PubMed] [Google Scholar]
- 330.Zuckerman, M. J., M. T. Watts, H. Ho, and F. V. Meriano. 1994. Blastocystis hominis infection and intestinal injury. Am. J. Med. Sci. 308:96-101. [DOI] [PubMed] [Google Scholar]