Abstract
We developed a radiation cross-linked UHMWPE stabilized with a-tocopherol (Vitamin E) as a bearing material in total joint replacements. The stabilizing effect of a-tocopherol on free radical reactions in UHMWPE is not well understood. We investigated the effect of a-tocopherol on the oxidation and transformation of residual free radicals during real-time aging of a-tocopherol-doped, irradiated UHMWPE (aTPE) and irradiated UHMWPE (control). Samples were aged at 22° C (room temperature) in air, at 40°C in air, and at 40°C in water for seven months. During the first month, aTPE showed some oxidation at the surface, which stayed constant thereafter. Control exhibited substantial oxidation in the subsurface region, which increased with time. The alkyl/allyl free radicals transformed to oxygen centered ones in both materials; this transformation occurred faster in a-TPE. In summary, the real-time oxidation behavior of a-TPE was consistent with that observed using accelerated aging methods. This new UHMWPE is oxidation resistant and is expected to maintain its properties in the long-term.
Keywords: Total hip arthroplasty, highly cross-linked polyethylene, oxidation resistance, free radicals, biomaterials
Introduction
One of the major factors limiting the long-term performance of total hip replacement is the peri-prosthetic osteolysis secondary to the wear of the ultra-high molecular weight polyethylene (UHMWPE) used in the fabrication of acetabular components. Radiation cross-linking and heat treatment of UHMWPE has proven to be an important advance for improved wear resistance (1-4). Some of these improved UHMWPEs have been in clinical use since 1998 with early clinical studies showing markedly reduced wear rates with the highly crosslinked UHMWPEs in comparison with conventional UHMWPEs (5, 6).
Cross-linking of UHMWPE is achieved through the use of ionizing radiation (7), which forms free radicals through the radiolytic cleavage of C-H and C-C bonds. These free radicals recombine with each other and form cross-links in the amorphous portion of the polymer. The free radicals generated during irradiation in the crystalline phase, however, become trapped (8-10). These trapped free radicals are the precursors to oxidative embrittlement of irradiated UHMWPE in the long term.
Typically, a post-irradiation treatment step follows the radiation cross-linking of UHMWPE used in total joint applications to decrease the concentration of the residual free radicals and to minimize or eliminate the adverse effects of residual free radicals on the properties of UHMWPE (1, 2, 4). The most effective method of stabilization is to melt the irradiated UHMWPE, which reduces the concentration of the residual free radicals to undetectable levels (3). The method of irradiation and melting improves the wear resistance and does not compromise the oxidation resistance of UHMWPE. However, the post-irradiation melting step further reduces the mechanical properties and fatigue strength of irradiated UHMWPE, which are already decreased by cross-linking (11) due to a decrease in crystallinity that accompanies post-irradiation melting.
We reported a new type of highly cross-linked UHMWPE, where post-irradiation melting is replaced by stabilization of the residual free radicals by diffusing a-tocopherol, an antioxidant, into irradiated UHMWPE (12, 13). We have shown that irradiated UHMWPE stabilized in this manner has improved fatigue properties over post-irradiation melted UHMWPE (12). Our aim in the present study was to understand the mechanisms by which a-tocopherol protects UHMWPE against oxidation subsequent to exposure to radiation. Mechanisms of reactions of polyethylene chains with each other and with oxygen in the presence and absence of a-tocopherol subsequent to irradiation can be deduced by determining free radical and non-free radical species and their evolution over time.
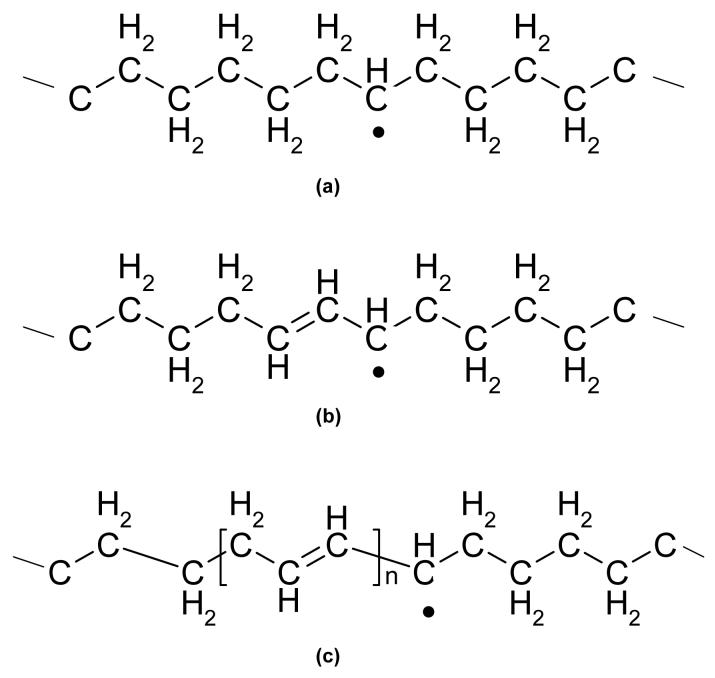
In irradiated UHMWPE, the prevalent free radicals are the carbon free radicals resulting from the breakage of the C-H bonds (14) and these radicals are found in three forms: the alkyl, allyl and polyenyl free radicals (15-17), the latter being formed especially at higher radiation doses ((18), Fig 1). Most of these free radicals recombine in the amorphous portion of the polymer (19), where the chains are highly mobile. For polyethylene irradiated at 77K, Waterman and Dole (20) found that free radicals decayed to 3.6% of the original concentration when the sample was heated to room temperature. The remaining free radicals are trapped in the highly ordered crystalline lamellae (8, 21). Since the interchain distance is fixed at 4.1Å between polyethylene chains in a crystal and the C-C bond distance is 1.52Å (22), interchain or intrachain cross-linking is highly unlikely. Therefore, free radicals travel along the crystal chains and encounter other free radicals to form a double bond or encounter the crystalline/amorphous interface. The decay of free radicals in the crystalline phase, therefore, is extremely slow, taking as long as years (23).
Figure 1. The chemical structure of primary free radicals formed in polyethylene by irradiation: alkyl (a), allyl (b) and polyenyl (c).
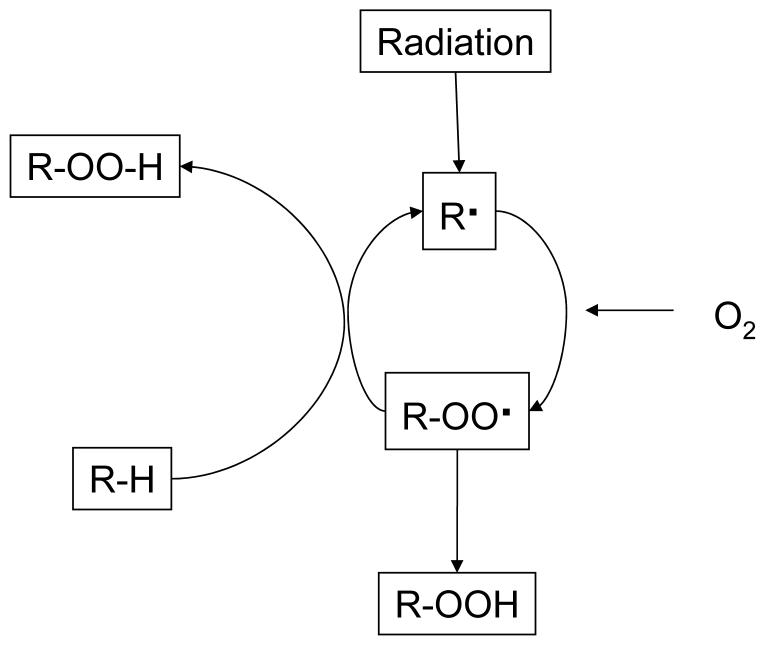
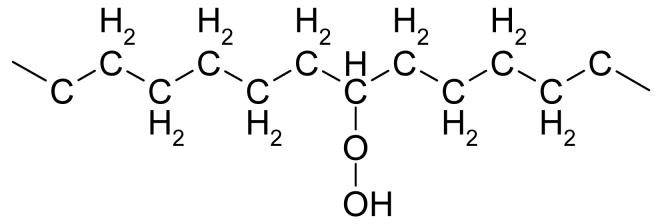
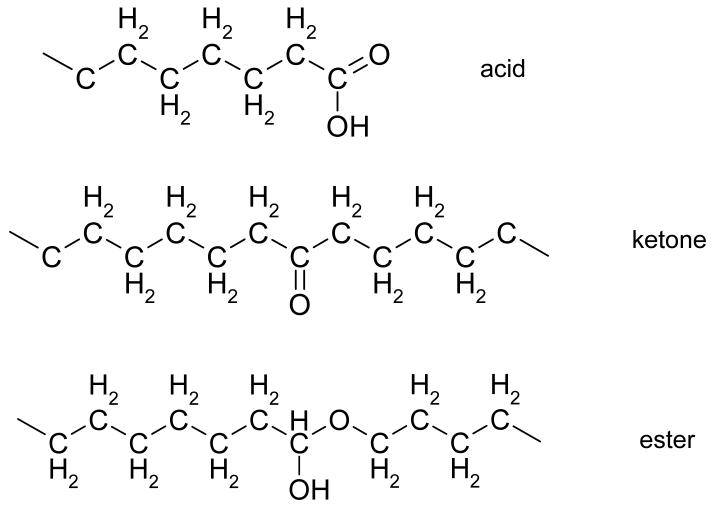
When oxygen is present in irradiated polyethylene, it reacts with the primary free radicals to form peroxy free radicals ((14, 24-27), Fig 2). These peroxy radicals, in the absence of an antioxidant like a-tocopherol, abstract a hydrogen atom from other polyethylene chains, creating primary free radicals, which can then react with oxygen to further this cascade ((28, 29), Fig 3). When peroxy free radicals get hydrogenated, they form hydroperoxides (Fig 4), which are not stable and over time degrade into oxidation products, mainly ketones, esters and acids ((30-34), Fig 5). The formation of these oxidation products is accompanied by chain scission and a decrease in the molecular weight of polyethylene, leading to deterioration of mechanical properties (35-37).
Figure 2. The chemical structure of peroxy free radicals formed in polyethylene by the reaction of primary free radicals with oxygen.
Figure 3. The oxidation cascade in polyethylene following irradiation in the absence of an antioxidant.
Figure 4. The chemical structure of hydroperoxides.
Figure 5. The chemical structure of the major oxidation products in irradiated polyethylene.
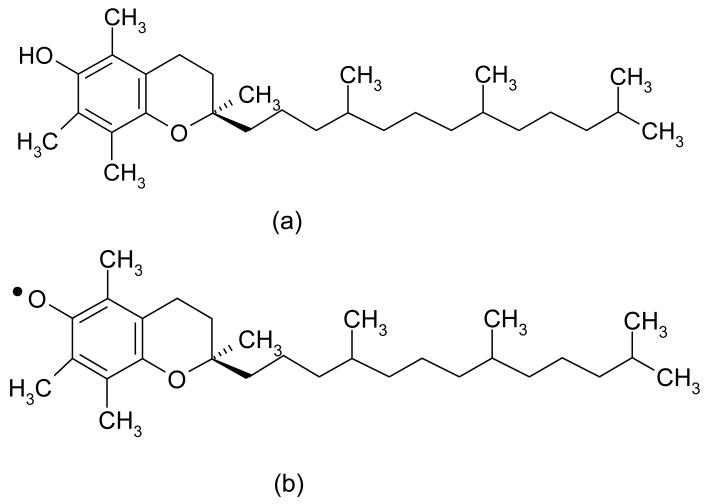
a-Tocopherol (vitamin E) is an antioxidant, whose main role in vivo is to stabilize the active free radicals (LOO·, LO·) resulting from the oxidation of polyunsaturated fatty acids in cell membranes. The antioxidant activity of a-tocopherol is due to hydrogen abstraction from the OH group on the chroman ring by a peroxyl free radical (Fig 6a). This results in a tocopheryl free radical (Fig 6b), which can combine with another peroxy free radical (38). Therefore, tocopherol can theoretically prevent two peroxy free radicals from attacking other fatty acid chains and producing more free radicals (38, 39). Oxidation reactions in polyethylene, which has a lipid-like molecular structure, also follow the same mechanism of oxidation as lipids do in vivo (29, 34, 40). In an irradiated polyethylene containing a-tocopherol, peroxy free radicals abstract a hydrogen from a-tocopherol forming hydroperoxides without the formation of new free radicals. a-Tocopherol is also effective in reacting with alkyl radicals (41), however the rate at which it reacts with peroxy free radicals is much faster than any other radical (38, 42). The oxidation cascade in irradiated polyethylene is hindered in the presence of a-tocopherol.
Figure 6. The chemical structure of α-tocopherol and α-tocopheryl free radical.
We have shown in several types of accelerated aging studies, carried out at elevated temperatures and/or in the presence of pure oxygen under high pressures, that a-tocopherol-doped, irradiated UHMWPE is more stable than gamma-sterilized or high-dose irradiated UHMWPE (11, 12). While accelerated aging is helpful in comparing the oxidation resistance and oxidation potential of different types of bearing materials, it cannot be used to predict the oxidation timeline or profile of a particular material in vivo. The mechanism of oxidation may be different in vivo than it is under accelerated aging oxidations. Therefore, it is crucial to determine the oxidative stability under more clinically relevant conditions to simulate more closely the aging behavior of this material on the shelf and in vivo.
In the present communication, we report on the oxidative changes, decay of free radicals, and transformation of free radicals during real-time aging of irradiated, a-tocopherol-stabilized and terminally gamma-sterilized UHMWPE and irradiated UHMWPE. The aging was carried out for seven months at room temperature, at 40°C, and in an aqueous bath at 40°C. The temperature of the human hip after total hip arthroplasty has been reported to be 40°C (43), which we used as a clinically relevant temperature for two of the aging conditions.
Experimental Section
Preparation of aTPE and control UHMWPE test samples
All specimens were manufactured from isostatically molded UHMWPE bar stock (Biomet Inc., Warsaw, IN). The UHMWPE resin utilized was GUR1050 (Ticona, Bishop TX).
For the preparation of aTPE samples, GUR1050 UHMWPE stock was machined into 30×30×10 mm blocks. The blocks were packaged under argon gas and the packages were gamma-irradiated to 85 kGy. The irradiated samples were then doped with D,L-a-tocopherol (Vitamin E, >98%, Fisher Scientific, Houston, TX) by immersion into a-tocopherol at 120 °C for 5 hours and subsequently annealed at 120 °C under argon flow for 64 hours for homogenization. The samples were then packaged in argon gas with an oxygen scavenger (Fresh Pax™, Multisorb Technologies Inc., Buffalo, NY) and gamma sterilized. The cumulative radiation dose received by the aTPE samples was over 100kGy (nearly 115kGy).
GUR1050 cubes (30 mm) that were packaged under argon gas and gamma-irradiated to 100kGy were used as control.
Real-time aging of aTPE and control UHMWPE test samples
All samples were stored in inert environment at room temperature until the start of the study. Baseline oxidation and free radical measurements were done at the start of the study. The aTPE and control blocks (n=3 each) were aged in real-time (i) in air at room temperature, (ii) in air at 40°C, and (iii) submerged in water in a stirred bath at 40°C. The blocks were removed from their respective testing environments at 1, 2, 3, 4 and 7 months for a length of time no longer than 5 hours. The free radical signals were analyzed by electron spin resonance and the oxidation profiles were determined using Fourier Transform Infrared Spectroscopy (FTIR, Bio-Rad FTS155, Natick MA).
Electron spin resonance (ESR) measurements
A 30×30×10 mm section was cut from the surface of each block. The sections (n=3) were analyzed by a Bruker EMX EPR system (Bruker BioSpin Corporation, Billerica, MA) at the Department of Physics at the University of Memphis (Memphis, TN) within 4 days of preparation. In addition to the real-time aged aTPE samples, one sample that was stored in sealed packaging in an inert atmosphere for 13 months was also analyzed for free radical content and type of free radicals. The free radical content of all samples was calculated by double integration of the ESR signal over the magnetic field and normalizing by the sample weight.
Determination of oxidation and a-tocopherol concentration profiles
A corner was cut from each block, exposing the interior surface of the block for analysis. Thin (∼150 μm) sections were cut from interior surface using a sledge microtome (Model 90-91-1177, LKB-Produkter AB, Bromma, Sweden). Infrared spectra were collected across the width of each thin section in 100 μm intervals, with each spectrum recorded as an average of 32 individual infrared scans. The infrared spectra were used to determine the a-tocopherol concentration profile and to ensure that a-tocopherol diffused across the thickness of the test samples. The α-tocopherol concentration was quantified by calculating an a-tocopherol index as the area under the a-tocopherol absorbance at 1245 cm-1 - 1275 cm-1 normalized to the polyethylene skeletal absorbance at 1850 cm-1 - 1985 cm-1.
Microtome-cut thin sections were boiled under reflux in hexane overnight and subsequently dried under vacuum before oxidation measurements to remove any species that are not bound to UHMWPE and might obscure the carbonyl absorbances of UHMWPE chains caused by oxidation. An oxidation index was calculated by normalizing the absorbance over 1680 cm-1 - 1780 cm-1 to the absorbance over 1330 cm-1 - 1390 cm-1. The surface oxidation index (SOI) was calculated as the average of the oxidation indices over the first 3 mm of the sample, while the bulk oxidation index (BOI) was calculated as the average of the oxidation indices of the central 500 μm of the sample as per ASTM F 2102-01e1.
Results
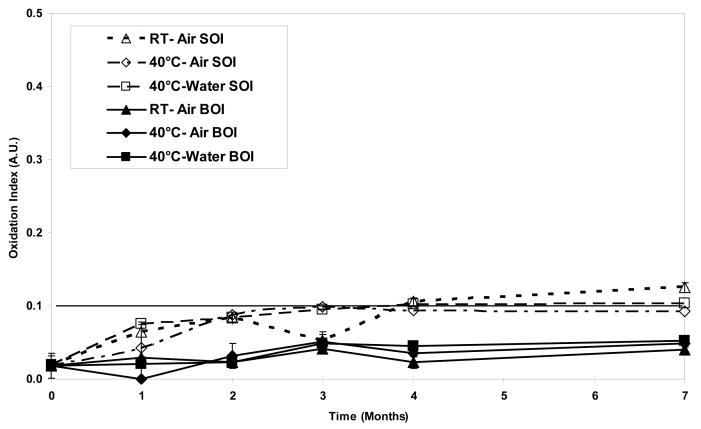
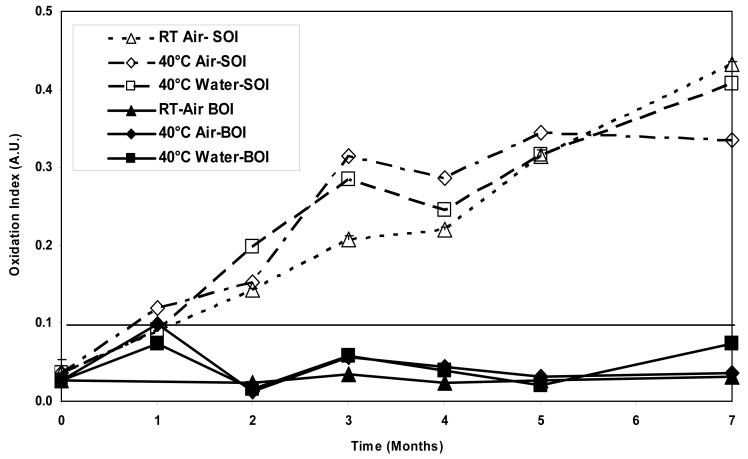
The average surface oxidation indices (SOI) in aTPE samples increased significantly (p<0.05) at each time step to approximately 0.12 for samples aged at room temperature in air. The SOI in the other two groups, namely the samples aged at 40°C in air, and at 40°C in water, increased in the first two months to approximately 0.1 and stayed relatively constant thereafter (p>0.05, Fig 7a). The resulting oxidation level at 7 months for room temperature aged samples was significantly higher than for samples aged at 40°C in air and 40°C in water (p=0.002 and 0.015, respectively). In contrast, the SOI in control UHMWPE increased steadily at each time point for all three environments (p<0.05) reaching oxidation levels of 0.4-0.5 in 7 months of real-time aging (Figs 7b). Similar to aTPE samples, the resulting oxidation levels at 7 months for room temperature aged control UHMWPE samples were significantly higher than those from samples aged at 40°C in air and 40°C in water (p=0.001 and 0.013, respectively). The average bulk oxidation indices (BOI) for both aTPE and control UHMWPE were below a baseline level of 0.1 in 7 months of real-time aging in all three aging environments.
Figure 7.
a. Average surface and bulk oxidation indices of irradiated, α-tocopherol-doped UHMWPE in air at room temperature, in air at 40°C and in water at 40°C as a function of time.
b. Average surface and bulk oxidation indices of irradiated control UHMWPE in air atroom temperature, in air at 40°C, and in water at 40°C as a function of time.
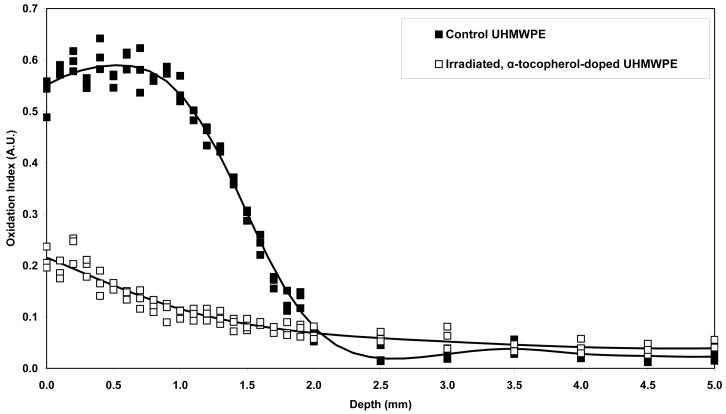
The oxidation profiles were different for real-time aged aTPE versus control UHMWPE (Fig 8). While the control samples showed a subsurface oxidation peak with a maximum at about 0.5-1.0 mm below the surface, the oxidation in the aTPE was maximum at the surface, which decreased and fell below baseline levels at about 1.0 mm below the surface.
Figure 8.
Oxidation profilesof irradiated, α-tocopherol-doped UHMWPE and 100-kGy irradiated UHMWPE after aging in air at room temperature for 7 months.
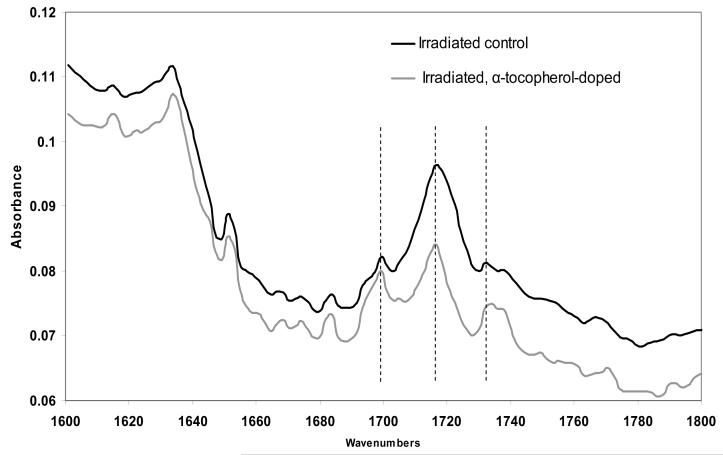
aTPE showed the same infrared absorbance peaks as control UHMWPE in all three aging environments. At the surface of the aTPE where maximum oxidation levels were observed (Fig 8), infrared spectra of the carbonyl region (1600-1800 cm-1) showed a small absorbance peak at 1695 cm-1, and two prominent absorbance peaks at 1718 cm-1 and 1738 cm-1 (Fig 9). At 400 μm below the surface of control UHMWPE where maximum oxidation was observed (Fig 8), the 1718 cm-1 was the prominent absorbance peak with smaller absorbance peaks at 1695 and 1738 cm-1 (Fig 9).
Figure 9.
The absorbance of oxidation products in irradiated, α-tocopherol-doped UHMWPE and irradiated control UHMWPE after aging in water at 40°C for 7 months.
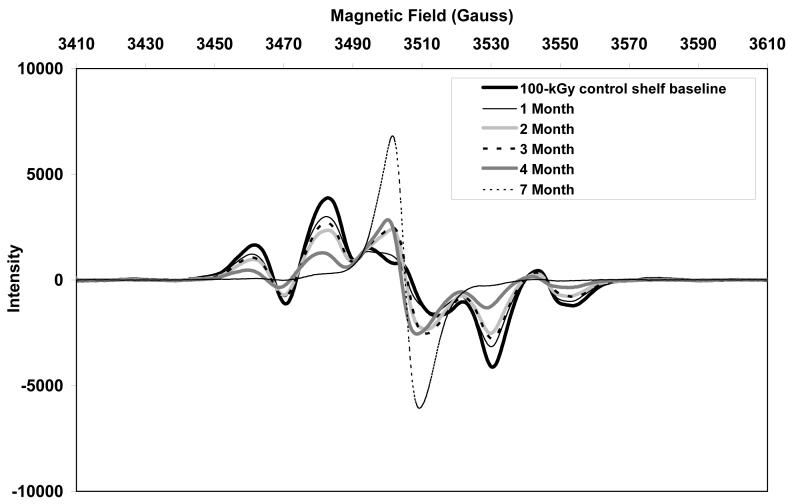
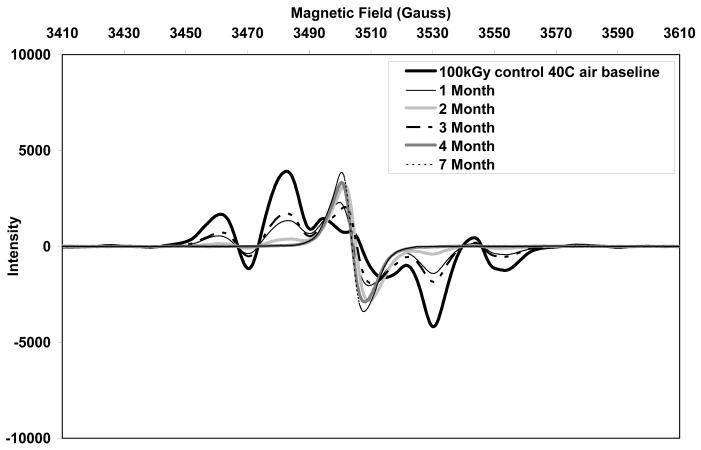
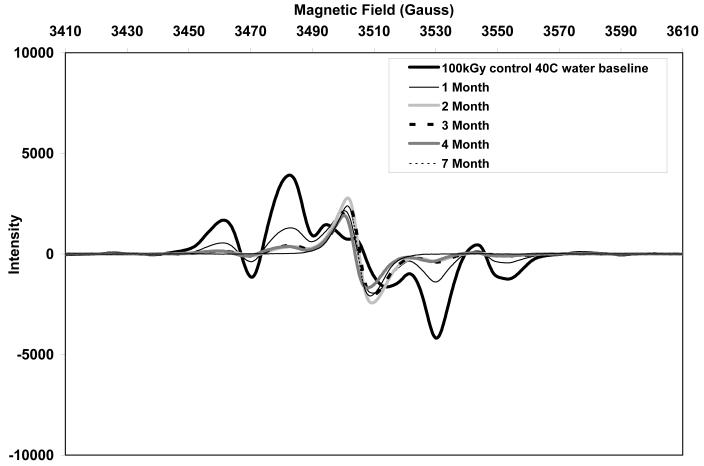
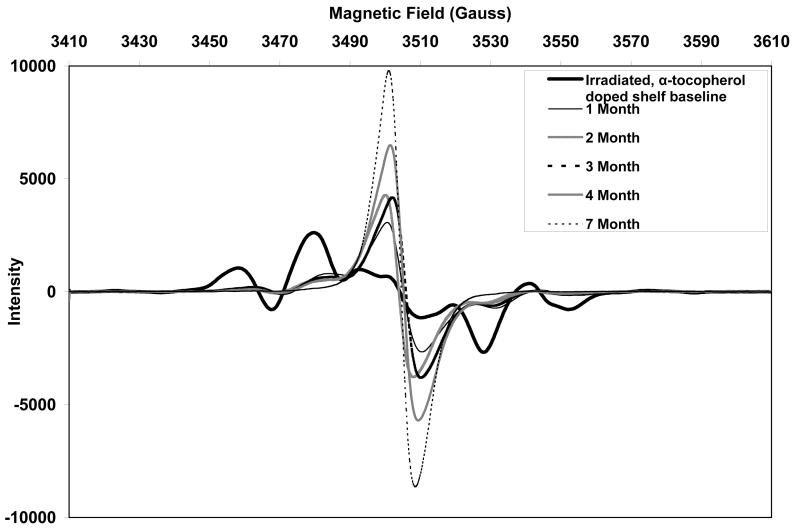
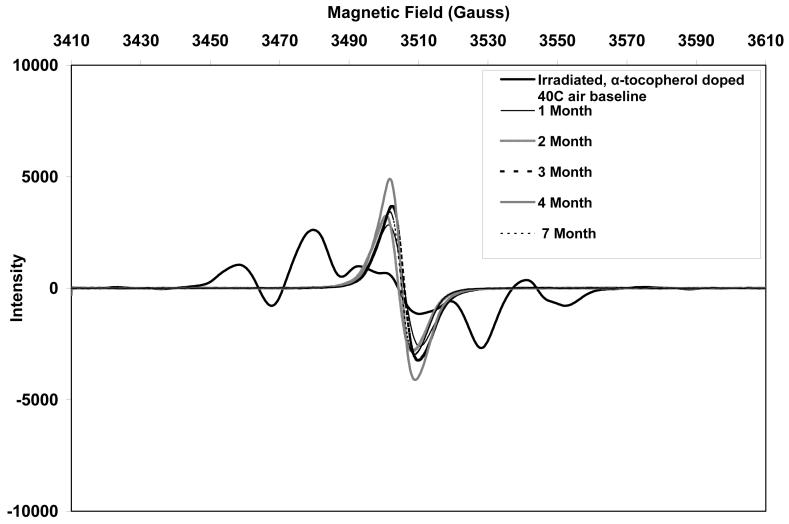
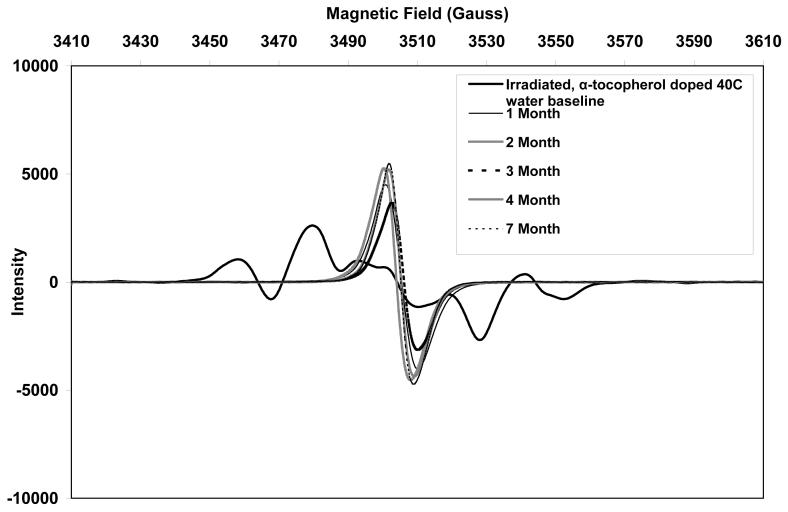
The initial ESR spectra of both aTPE and control UHMWPE showed a sextet free radical signal (Figs 10 and 11, respectively), which was eventually converted to a sharp singlet in all three aging environments. The alkyl free radicals transformed over time to oxygen centered radicals, observed as a single peak near 3505 Gauss. The rate of transformation was higher in aTPE versus the control UHMWPE. In aTPE samples, most of the transformation took place in one month for samples stored at 40°C in air or water, whereas in control UHMWPE, the transformation took four months at 40°C in air and seven months at 40°C in water. In both sets of samples, transformation was slower at room temperature than at 40°C.
Figure 10.
a. Free radical evolution of irradiated control UHMWPE in air at room temperature.
b. Free radical evolution of irradiated control UHMWPE in air at 40°C.
c. Free radical evolution of irradiated control UHMWPE in deionized water at 40°C.
Figure 11.
a. Free radical evolution of irradiated, α-tocopherol-doped UHMWPE in air at room temperature.
b. Free radical evolution of irradiated, α-tocopherol-doped UHMWPE in air at 40°C.
c. Free radical evolution of irradiated, α-tocopherol-doped UHMWPE in deionized water at 40°C.
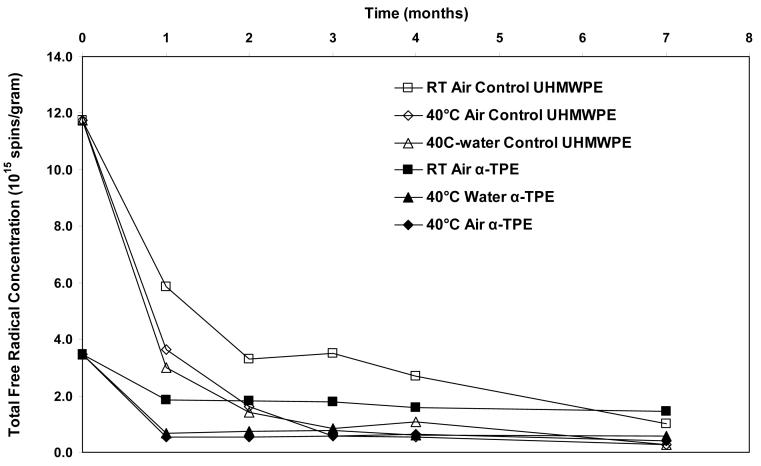
Total free radical content of both of the aTPE and control UHMWPE decayed rapidly (Fig 12). For the aTPE samples, the decay reached steady state in one month. The decay of the free radicals in control UHMWPE continued for 2 months for samples stored at room temperature and 3 months for samples stored at 40°C. A higher equilibrium concentration of free radicals remained in both samples stored at room temperature than those aged at 40°C. Also, the baseline free radical content of control UHMWPE was higher than that of α-tocopherol-doped, irradiated UHMWPE.
Figure 12.
Total free radicals as a function of time in irradiated control UHMWPE and irradiated, α-tocopherol-doped UHMWPE.
The ESR spectrum of irradiated, a-tocopherol-doped UHMWPE that had been aging in its inert gas package for 13 months showed mainly the sextet spectrum of alkyl/allyl free radicals (Fig 13).
Figure 13.
Free radical spectrumof irradiated, α-tocopherol-doped UHMWPE shelf-aged in inert packaging for 13 months.
Discussion
We investigated the stabilizing effect of a-tocopherol on the residual free radicals of irradiated UHMWPE during real-time aging. a-Tocopherol-stabilized, irradiated UHMWPE showed higher oxidative stability than unstabilized, irradiated UHMWPE during real-time aging. This was accompanied by a faster transformation of the allyl/alkyl free radicals to oxygen-centered free radicals in the former. The chain breaking antioxidant behavior of a-tocopherol was evidenced by the early cessation of oxidation in the a-tocopherol stabilized samples.
The aTPE samples that were aged at 40°C in air and water showed the formation of some carbonyl moieties during the first two months of real-time aging presumably as a result of the decay of hydroperoxides formed on polyethylene chains. There are two possible mechanisms for the formation of these hydroperoxides. One mechanism is the reaction of free radicals with the oxygen dissolved in UHMWPE during the initial 85 kGy electron beam irradiation prior to the doping with a-tocopherol. The other mechanism is the hydrogen abstraction from a-tocopherol by the peroxy free radicals formed on polyethylene. The fact that there was no further detectable oxidation after three months of aging indicated that the decay of the hydroperoxides formed by the former mechanism and the formation followed by decay of hydroperoxides by the latter mechanism were likely exhausted; and that a-tocopherol acted as an efficient chain breaking antioxidant. In contrast, the unstabilized, irradiated UHMWPE continued to oxidize on the surface as well as in the subsurface region (Fig 7b) because the residual free radicals were likely able to continue to form hydroperoxides and additional free radicals furthering the cascade of oxidation reactions.
In the presence of oxygen, the shift in the ESR spectrum of irradiated polyethylene from the multiple peaks of alkyl and allyl radicals to a sharp singlet (Figs 10 and 11) has previously been ascribed to the formation of oxygen centered radicals such as alkoxy (23, 44) and peroxy (23, 45, 46) radicals and the polyenyl radical (8, 18, 47). Most of the free radicals trapped in polyethylene in the long term are in the crystalline lamellae, which are not permeable to oxygen. Therefore, the accumulation of free radicals in the crystals is unlikely. More likely, the free radicals in the crystalline regions move along the crystal stems until they react with another free radical on the same chain or until they reach the crystal folds where they are able to abstract a hydrogen atom from nearby amorphous chains or react with diffused oxygen. This would lead to an increase in the number of polyenyl and peroxy free radicals and accumulation of hydroperoxides. Another contribution to the sharp singlet in the ESR spectra might be alkoxy radicals, which are formed by the breakdown of a hydroperoxide with the release of a hydroxy free radical. The reaction of these free radicals with other chains as well as α-tocopherol is slower than that of peroxy free radicals, so some accumulation of alkoxy radicals might have occurred.
The faster free radical decay in aTPE samples aged at 40°C compared to room temperature might have resulted in less oxidation in these samples. The oxidation of room temperature aged samples continued to increase after two months and resulted in a significantly different SOI between these samples at 7 months due to the combinatorial effects of the larger amount of free radicals in these samples and also to the slower reaction of these radicals with oxygen. The oxidation would be expected to stabilize when the hydroperoxides in these samples are exhausted.
The fact that aTPE showed the same transformation of free radical signature as the control UHMWPE suggested that the mechanism of the decay of the free radicals was similar. However, this evolution was not accompanied by oxidation in aTPE, supporting the chain-breaking antioxidant activity of a-tocopherol in irradiated polyethylene. These findings also corroborate our previous results comparing the oxidation behavior of aTPE to control UHMWPE using accelerated aging methods (12, 48). Based on those data, we had shown that the mechanical properties and pin-on-disc wear of a-tocopherol-containing, highly cross-linked UHMWPE were not changed when accelerated aged. Therefore, implant components made of aTPE that are aged on the shelf or used in vivo are expected to maintain their mechanical properties.
There were two phenomena of further interest. The total free radical content of a-tocopherol-stabilized, irradiated UHMWPE reached equilibrium at a lower value than the control UHMWPE (Fig 12), suggesting that the recombination of free radicals was more efficient in the former. Also, the rate of the change of alkyl/allyl type free radicals to polyenyl/alkoxy free radicals was faster in aTPE (Figs 10 and 11). We have previously shown that α-tocopherol stabilization did not significantly affect the crystallinity of UHMWPE. The aTPE was doped with α-tocopherol at 120°C, where 15% of the crystalline content had melted. It is possible that some α-tocopherol co-crystallized with UHMWPE and got incorporated into the crystalline lamellae or was primarily placed at the crystal/amorphous interface. This may have increased the mobility of the chains resulting in a plasticization effect and led to more efficient quenching of some of the free radicals. On the other hand, the absence of free radical transformation on the 13 month shelf-aged, a-tocopherol stabilized, irradiated UHMWPE suggest that oxygen plays an important role in the decay of free radicals. This shelf-aged sample was packaged with a barrier film shortly after a-tocopherol doping and homogenization in a nitrogen gas environment. This observation further strengthens the hypothesis that the formation of oxygen centered free radicals is responsible for the free radical decay and transformation and that a-tocopherol induced plasticization was an unlikely cause.
Conclusions
The real-time oxidation behavior of a-tocopherol-stabilized UHMWPE was consistent with that observed with accelerated aging methods. This new UHMWPE is oxidation resistant and is expected to maintain its mechanical properties in the long-term.
Acknowledgements
This work was funded by NIH R01 AR051142-02. We acknowledge with thanks the assistance of Dave Schroeder and Jordan Freedman at Biomet, Inc. in sample preparation and Dr. M. Shah Jahan at The Department of Physics at the University of Memphis for ESR measurements.
This work was funded by NIH R01 AR051142-02.
References
- 1.Muratoglu OK, Bragdon CR, O’Connor DO, Jasty M, Harris WH, Gul R, et al. Unified Wear Model for Highly Crosslinked Ultra-high Molecular Weight Polyethylenes (UHMWPE) Biomaterials. 1999;20(16):1463–1470. doi: 10.1016/s0142-9612(99)00039-3. [DOI] [PubMed] [Google Scholar]
- 2.Muratoglu OK, Bragdon CR, O’Connor DO, Jasty M, Harris WH. 1999 HAP Paul Award. A novel method of crosslinking UHMWPE to improve wear, reduce oxidation and retain mechanical properties. J Arthroplasty. 2001;16(2):149–160. doi: 10.1054/arth.2001.20540. [DOI] [PubMed] [Google Scholar]
- 3.Muratoglu OK, Merrill EW, Bragdon CR, O’Connor DO, Hoeffel D, Burroughs B, et al. Effect of Radiation, Heat, and Aging on In Vitro Wear Resistance of Polyethylene. Clinical Orthopaedics & Related Research. 2003;417:253–262. doi: 10.1097/01.blo.0000093004.90435.d1. [DOI] [PubMed] [Google Scholar]
- 4.McKellop H, Shen F-W, Lu B, Campbell P, Salovey R. Development of an extremely wear resistant ultra-high molecular weight polyethylene for total hip replacements. J Orthop Res. 1999;17(2):157–167. doi: 10.1002/jor.1100170203. [DOI] [PubMed] [Google Scholar]
- 5.Digas G, Karrholm J, Thanner J, Malchau H, Herberts P. Highly cross-linked polyethylene in total hip arthroplasty: randomized evaluation of penetration rate in cemented and uncemented sockets using radiostereometric analysis. Clin Orthop. 2004;(429):16. [PubMed] [Google Scholar]
- 6.Martell JM, Verner JJ, Incavo SJ. Clinical performance of a highly cross-linked polyethylene at two years in total hip arthroplasty: a randomized prospective trial. J Arthroplasty. 2003;18(7 Suppl 1):9. doi: 10.1016/s0883-5403(03)00341-3. [DOI] [PubMed] [Google Scholar]
- 7.Charlesby A. Cross-linking of polythene by pile radiation. Proc. Roy. Soc. Lond. 1952;A215:187–215. [Google Scholar]
- 8.Bhateja S, Duerst R, Aus E, Andrews E. Free radicals trapped in polyethylene crystals. Journal of Macromolecular Science-Physics. 1995;B34(3):263–272. [Google Scholar]
- 9.Keyser R, Tsuji K, Williams F. Trapped electrons in gmma-irradiated polyethylene identified by electron spin resonance spectroscopy. Macromolecules. 1968;1:289–290. [Google Scholar]
- 10.Tsuji K, Okamura S. ESR study of polyethylene irradiated with ultraviolet light and electron beams. In: Kinell P, Ranby B, Runnstrom-Reio V, editors. ESR Applications to Polymer Research. Almqvist & Wiksell Forlag AB; Stockholm: 1973. [Google Scholar]
- 11.Oral E, Malhi A, Muratoglu O. Mechanisms of decrease in fatigue crack propagation resistance in irradiated and melted UHMWPE. Biomaterials. 2006;27:917–925. doi: 10.1016/j.biomaterials.2005.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oral E, Wannomae KK, Hawkins NE, Harris WH, Muratoglu OK. α-Tocopherol Doped Irradiated UHMWPE for High Fatigue Resistance and Low Wear. Biomaterials. 2004;25(24):5515–5522. doi: 10.1016/j.biomaterials.2003.12.048. [DOI] [PubMed] [Google Scholar]
- 13.Oral E, Wannomae K, Muratoglu O. The effect of doping conditions on α-tocopherol stabilized UHMWPE. Transactions; 51st Annual Meeting of the Orthopaedic Research Society; Washington, D.C.. 2005; 2005. [Google Scholar]
- 14.Carlsson D, Chmela S, Lacoste J. On the structures and yields of the first peroxyl radicals in γ-irradiated polyolefins. Macromolecules. 1990;23:4934–4938. [Google Scholar]
- 15.Libby D, Ormerod M, Charlesby A. Electron spin resonance spectra of some polymers at 77-degrees-K. Polymer. 1960;1:212–218. [Google Scholar]
- 16.Charlesby A, Libby D, Ormerod M. Proceedings of the Royal Society (London) 1961;A262(130):207. [Google Scholar]
- 17.Ohnishi S, Sugimoto S, Nitta I. Temperature dependence of the ESR spectrum of polyethylene. Journal of Chemical Physics. 1962;37:1283–1288. [Google Scholar]
- 18.Lawton E, Balwit J, Powell R. Paramagnetic-resonance studies of irradiated high-density polyethylene. II. Effect of irradiation dose on the radical species trapped at room temperature. Journal of Chemical Physics. 1960;33(2):405–412. [Google Scholar]
- 19.Dole M. Free radicals in irradiated polyethylene. In: Dole M, editor. The radiation chemistry of macromolecules. Academic Press; New York: 1972. pp. 335–348. [Google Scholar]
- 20.Waterman D, Dole M. Radiation chemistry of polyethylene. 10. Kinetics of conversion of alkyl to allyl free radicals. Journal of Physical Chemistry. 1970;74(9):1913–1922. [Google Scholar]
- 21.Keller A, Ungar G. Radiation effects and crystallinity in polyethylene. Journal of Physics and Chemistry. 1983;22(12):155–181. [Google Scholar]
- 22.Peacock AJ. Handbook of Polyethylene. Marcel Dekker Inc.; New York: 2000. [Google Scholar]
- 23.Jahan MS, King MC, Haggard WO, Sevo KL, Parr JE. A study of long-lived free radicals in gamma-irradiated medical grade polyethlene. Radiat. Phys. Chem. 2001;62:141–144. [Google Scholar]
- 24.Kuzuya M, Kondo S, Sugito M, Yamashiro T. Peroxy radical formation from plasma-induced surface free radicals of polyethylene as studied by electron spin resonance. Macromolecules. 1998;31:3230–3234. [Google Scholar]
- 25.Seguchi T, Tamura N. Mechanism of decay of alkyl radicals in irradiated polyethylene on exposure to air as studied by electron spin resonance. J. Physical Chem. 1973;77(1):40–44. [Google Scholar]
- 26.Carlsson D, Dobbin C, Wiles D. Direct observations of macroperoxyl radical propagation and termination by electron spin resonance and infrared spectroscopies. Macromolecules. 1985;18:2092–2094. [Google Scholar]
- 27.Ohnishi S, Sugimoto S, Nitta I. Electron spin resonance study of radiation oxidation of polymers. IIIA. Results for polyethylene and some general remarks. Journal of Polymer Science: Part A: Polymer Chemistry. 1963;1:605–623. [Google Scholar]
- 28.Rabek J, Ranby B. Photochemical oxidation reactions of synthetic polymers. In: Kinell P, Ranby B, editors. ESR Applications to Polymer Research. Almqvist-Wiksell Forlag AB; Stockholm: 1973. [Google Scholar]
- 29.Al-Malaika S. Autoxidation. In: Scott G, editor. Atmospheric Oxidation and Antioxidants. Elsevier Science Publishers B.V.; Amsterdam: 1993. pp. 45–82. [Google Scholar]
- 30.Al-Malaika S. Perspectives in Stabilisation of Polyolefins. Advances in Polymer Science. 2004;169:121–150. [Google Scholar]
- 31.Assink R, Celina M, Dunbar T, Alam T, Clough R, Gillen K. Analysis of Hydroperoxides in Solid Polyethylene by MAS 13C NMR and EPR. Macromolecules. 2000;33:4023–4029. [Google Scholar]
- 32.Costa L, Luda MP, Trossarelli L. Ultra-high molecular weight polyethylene: I. Mechano-oxidative degradation. Polymer Degradation and Stability. 1997;55:329–338. [Google Scholar]
- 33.Costa L, Luda MP, Trossarelli L. Ultra high molecular weight polyethylene-II. Thermal- and photo-oxidation. Polymer Degradation and Stability. 1997;58:41–54. [Google Scholar]
- 34.Costa L, Luda MP, Trossarelli L, Brach del Prever EM, Crova M, Gallinaro P. Oxidation in orthopaedic UHMWPE sterilized by gamma radiation and ethylene oxide. Biomaterials. 1998;19:659–668. doi: 10.1016/s0142-9612(97)00160-9. [DOI] [PubMed] [Google Scholar]
- 35.Costa L, Jacobson K, Bracco P, Brach del Prever EM. Oxidation of orthopaedic UHMWPE. Biomaterials. 2002;23:1613–1624. doi: 10.1016/s0142-9612(01)00288-5. [DOI] [PubMed] [Google Scholar]
- 36.Kurtz S, Hozack WJ, Marcolongo M, Turner J, Rimnac CM, Edidin A. Degradation of mechanical properties of UHMWPE acetabular liners following long-term implantation. Journal of Arthroplasty. 2003;18(7 Supp 1):68–78. doi: 10.1016/s0883-5403(03)00292-4. [DOI] [PubMed] [Google Scholar]
- 37.Collier J, Sutula L, Currier B, John H, Wooding R, Williams I, et al. Overview of Polyethylene as a Bearing Material: Comparison of Sterilization Methods. Clinical Orthopaedics. 1996;333:76–86. [PubMed] [Google Scholar]
- 38.Kamal-Eldin A, Appelqvist L. The Chemistry and Antioxidant Properties of Tocopherols and Tocotrienols. Lipids. 1996;31(7):671–701. doi: 10.1007/BF02522884. [DOI] [PubMed] [Google Scholar]
- 39.Burton GW, Traber MG, Vitamin E. Antioxidant activity, biokinetics, and bioavailability. Annual Reviews in Nutrition. 1990;10:357–382. doi: 10.1146/annurev.nu.10.070190.002041. [DOI] [PubMed] [Google Scholar]
- 40.Esterbauer H, Gebicki J, Puhl H, Jurgens G. The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Radic Biol Med. 1992;13:341–390. doi: 10.1016/0891-5849(92)90181-f. [DOI] [PubMed] [Google Scholar]
- 41.Scott G. Antioxidants: Chain breaking mechanisms. In: Scott G, editor. Atmospheric Oxidation and Antioxidants. Elsevier Science Publishers B.V.; Amsterdam: 1993. pp. 121–160. [Google Scholar]
- 42.Burton G, Ingold K. Autoxidation of Biological Molecules. 1. The Antioxidant Activity of Vitamin E and Related Chain-Breaking Phenolic Antioxidants in Vitro. J. Am. Chem. Soc. 1981;103:6472–6477. [Google Scholar]
- 43.Bergmann G, Graichen F, Rohlmann A, Verdonschot N, van Lenthe GH. Frictional heating of total hip implants. Part I. measurements in patients. J. Biomechanics. 2001;34:421–428. doi: 10.1016/s0021-9290(00)00188-3. [DOI] [PubMed] [Google Scholar]
- 44.Jahan M, Stovall J, King M. Observation of a non-radical intermediate in the oxidation pathway of free radicals in gamma-irradiated medical grade polyethylene. Nuclear Instruments and Methods in Physics Research B. 2001;185:323–327. [Google Scholar]
- 45.Alam T, Celina M, Collier J, Currier B, Currier J, Jackson S, et al. γ-Irradiation of ultrahigh-molecular-weight polyethylene: Electron paramagnetic resonance and nuclear magnetic resonance spectroscopy and imaging studies of the mechanism of subsurface oxidation. J Polym Sci Part A: Polym Chem. 2004;42:5929–5941. [Google Scholar]
- 46.O’Neill P, Birkinshaw C, Leahy J, Barklie R. The role of long lived free radicals in the ageing of irradaited ultrahigh molecular weight polyethylene. Polymer Degradation and Stability. 1999;63:31–39. [Google Scholar]
- 47.Lawton E, Balwit J, Powell R. Paramagnetic-resonance studies of irradiated high-density polyethylene. I. Radical species and the effect of environment on their behavior. Journal of Chemical Physics. 1960;33(2):395–404. [Google Scholar]
- 48.Oral E, Christensen S, Malhi A, Wannomae K, Muratoglu O. Wear resistance and mechanical properties of highly crosslinked UHMWPE doped with vitamin E. Journal of Arthroplasty. 2006;21(4):580–591. doi: 10.1016/j.arth.2005.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]