Abstract
The biological significance of protein and lipid glycosylation is well established. For example, cells respond to environmental stimuli by altering glycan structures on their surfaces, and cancer cells evade normal growth regulation in part by remodeling their surface glycans. In general, glycan chemical properties differ significantly from those of proteins, lipids, nucleic acids and small molecule metabolites. Thus, advances in glycomics, a comprehensive study to identify all glycans in an organism, rely on the development of specialized analytical methods. Mass spectrometry (MS) is emerging as an enabling technology in the field of glycomics. This review summarizes recent developments in mass spectrometric analysis methods for protein-based glycomics and glycoproteomics workflows.
1. Introduction
Glycosylation is the most common post-translational modification, occurring to up to half of all gene products (Apweiler, et al., 1999). As illustrated in Figure 1A, all cells are coated with a dense layer of glycans, a fact that implicates the importance of glycobiology in multicellular life. Glycan expression figures prominently in cell-cell and cell-matrix interactions. Glycans are expressed in a cell-type specific and temporally regulated manner to allow cell phenotypes to change dynamically in response to environmental stimuli (Varki, 2006). Their biosynthesis occurs in the lumen of the Golgi apparatus in a non-template controlled manner and mature structures arise through the activities of a series of enzymatic reactions specific to each glycoconjugate class. The mature structures are cell-type specific and depend on multiple factors, including enzyme and nucleotide sugar availability and kinetics of glycoconjugate transport. As a result, glyconjugate glycans are mixtures of variants (glycoforms) on a core structure. This serves to diversify the biological functions of the limited number of genes in higher animal genomes to create tissue structures with defined boundaries and controlled functions.
Figure 1.
(A). Glycoconjugates form key structural elements that mediate cell-cell and cell-matrix interactions. The structures of glycoconjugate glycans reflect enzymatic biosynthetic reactions that are under complex control. Biosynthetic reactions do not go to completion, giving rise to distributions of glycoforms built on a core structure. Glycoconjugates mediate the antigenic character of cell surfaces and add a level of plasticity to the functions of the genetically coded protein gene products. NCP = collagenous glycoprotein. Modified after (Varki, et al., 1999). (B) Common core structures for protein glycoconjugate glycans. Symbolic glycan structures were drawn using GlycoWorkbench (Ceroni, et al., 2008) using the symbolic nomenclature proposed by the Consortium for Functional Glycomics (Stevens, et al., 2006). (C) Common glycan derivatizations. Glycans released by enzymatic means and non-reducing chemical conditions exist as an equilibrium between close-ring hemiacetal and open ring aldehyde forms (shown for example with N-acetylglucosamine). Aldehyde forms may be reduced to alditols. Permethylation converts all OH groups to O-methyl and NH groups to N-methyl. Reductive amination entails reaction of the aldehyde with a primary amine to form a glycosyl imine (also known as a Schiff base). The imine is reduced to a secondary amine to increase its stability. R = glycan moiety, R ‘ = primary amine alkyl group.
Glycosylation has the potential to affect all stages of protein lifetimes, from folding and delivery to the cell surface, to their interactions with binding partners, their degradation and turnover (Varki, 2007). As illustrated in Figure 1B, glycans modifying proteins in animal cells include N-linked (bound to an asparagine side chain in an NXT or NXS amino acid consensus sequence with X ≠ proline), O-linked (with glycans attached to via serine or threonine side chains), and glycosaminoglycans (bound to a serine side chain).
All N-glycans contain a conserved GlcNAc2Man3 core structure, termed the chitobiose core. High mannose N-glycans contain only mannose attached to the core. Complex N-glycans have had all mannose residues removed by enzymatic processing and replaced to form antenna structures. Hybrid N-glycans have been incompletely processed and have at least one antenna containing only mannose residues. Many cytoplasmic and nuclear proteins are modified by β-O-GlcNAc at Ser/Thr side chains. Secreted and cell surface proteins may be modified by O-linked glycans generated by elaboration of GalNAc residues at serine or threonine side chains. Several O-glycan core structures exist and there is considerable structural diversity in this glycan class. Glycosaminoglycans are linear sulfated glycans that are bound to serine residues through a characteristic tetrasaccharide linker. In some cases N-glycans may be elaborated with glycosaminoglycan chains.
Glycomics is the study of the significance of glycoconjugate expression in biological systems. Because glycosylation involves a series of metabolic events, some of the concepts in lipidomics (German, et al., 2007) and metabolomics (Dettmer, et al., 2007) are common to glycomics. All three fields lend themselves to database searching algorithms that reference compound libraries rather than the genomic databases used in proteomics. In all three, the mass spectrometry (MS) dimension defines the chemical composition of observed biomolecules in a given sample and tandem MS is useful for determining structural details and defining isomeric mixture compositions. Complex glycans have masses that exceed the range typically used for metabolomics datasets (<1000 Da). Their chemistry differs significantly from those of proteins, lipids, and small molecule metabolites, and different sample preparation, chromatography, and ionization methods must be used.
The purpose of this review is to summarize recent developments in mass spectrometric glycomics related to protein-based glycoconjugates. To illustrate the methods used in emerging glycomics workflows, mass spectrometric ionization and chromatographic interfaces and their application in quantitative studies are summarized. Particular attention is paid to the uses of tandem MS for analysis of released glycan mixtures. Lastly, the review summarizes emerging glycoproteomics methods. Due to space limitation and the vastness of the filed, it is not feasible to discuss all the publications and contributions, and the understanding of authors whose work may have been omitted is requested.
2. Mass spectrometry of glycans
Sample preparation and ionization are critical components of MS glycomics workflow. Thus this section will briefly discuss different ionization methods used, as well as sample preparation and separation strategies that lead to improved analytical results.
a. Derivatization of glycans for mass spectrometry
Glycans released by enzymes or non-reducing chemical methods have reducing ends that are an equilibrium between cyclic hemiacetals and open ring aldehydes. Carbohydrates are derivatized to increase their volatility and stability for MS analysis. As shown in Figure 1C, glycans are often reduced to alditols to prevent separation of anomeric forms of the reducing end residue. Permethylation of glycan alditols results in the conversion of all hydrogen atoms bound to oxygen and nitrogen atoms to methyl groups and serves to render glycans hydrophobic. Permethylated carbohydrates are considerably more stable than native glycans and produce more informative tandem mass spectra. Permethylation is not compatible with some glycan modification groups (sulfate and phosphate). In addition, sample losses occur during the derivatization workup.
Reductive amination is widely used for derivatizing reducing glycans (Anumula, 2006), as shown in Figure 1C. The advantage to this reaction is that it is reliable and may be used to attach a chromophore, fluorophore, or stable isotope tag to a single position of a glycan. Reductive amination works for N-linked glycans released enzymatically from proteins and to any glycan class that contains a reducing end. It is not applicable to O-glycans released under reductive conditions, under which the aldehyde group is reduced to an alditol. Glycans reductively aminated with hydrophobic groups produce stronger mass spectrometric signals than do native glycans (Harvey, 2000b).
Permethylation and reductive amination are very effective means of derivatization for mass spectral analysis of glycans. There remains substantial interest in analysis of native glycans, however, primarily due to the desire to avoid losses during the derivatization workup procedures.
b. Ionization Methods
Mass spectrometric ionization of carbohydrates has been reviewed recently (Zaia, 2006). Therefore, this section will provide a brief description of two main ionization methods, matrix-assisted laser desorption/ionization (MALDI) and electrospray ionization (ESI) and highlight some specific requirements for glycan sample preparation that improve overall efficiency of the MS ionization process.
The analysis of complex mixtures by mass spectrometry is limited by the phenomenon of ion suppression, in which the most abundant molecules suppress ionization of those in lower abundance. Ion suppression is minimized when analyzing mixtures with equivalent acidic and hydrophobic properties. Since native released carbohydrates are hydrophilic and range from neutral to acidic, it is best to separate them according to acidity prior to MS to minimize ion suppression.
MALDI entails mixing analyte molecules with an organic matrix such as 2,5-dihydroxybenzoic acid. A small volume of the mixture (1-2 microliters) is dried on a metal target, allowing crystals to form. The target is placed in the vacuum source of a mass spectrometer. See reviews for more information on MALDI matrices for glyconjugates (Harvey, 2003, Harvey, 1999). Singly charged ions predominate using MALDI. It is typically used for analysis of chromatographic fractions collected off line. MALDI MS has the advantage of ease of sample preparation and relatively high tolerance to salts and other contaminants. However, acidic (sialylated, sulfated or phosphorylated) native carbohydrates undergo fragmentation during MALDI ionization. As indicated above, permethylation protects glycans from fragmentation during the MALDI process.
ESI entails spraying a solution containing the analyte through a needle to which is applied electrical potential. The electrical potential creates a condition in which very fine droplets are formed. Droplets are either repelled or attracted toward the needle according to their charge. In this way, appropriately charged droplets move toward the mass spectrometer orifice while undergoing solvent evaporation. This process results in formation of multiply charged gas phase ions that are analyzed in the mass spectrometer. ESI is used with many types of mass spectrometers and is particularly well suited to analysis of liquid chromatographic effluents.
The extent of fragmentation of acidic glycans and other fragile ions is much lower than observed using MALDI. ESI produces inherently better resolved peaks for glycoconjugates due to the absence of matrix adduct peaks (Satterfield and Welch, 2005) but has the disadvantage that sample preparation is more labor-intensive than in MALDI. Samples must be free of salts prior to introduction into the ion source to avoid the problem of multiple cation adduction. This entails use of liquid chromatography (LC), either off- or on-line prior to ESI MS.
In conclusion, both MALDI and ESI can be used to ionize glycan samples for large scale glycomics analysis. The performance of either depends on the sample composition and can be substantially enhanced by using chromatography to separate sample components prior to ionization.
c. Chromatographic interfaces for mass spectral glycomics
The combination of LC with MS provides a powerful analytical tool for both qualitative and quantitative glycan analysis and dramatically improves the amount of obtainable information. Although the development of glycan on-line LC/MS has lagged behind that for proteins, a number of practical approaches are emerging, as discussed below.
In order to use reversed phase LC/MS, reductive amination (Anumula, 2000, Anumula, 2006) is applied to increase the hydrophobicity of glycans. Aminopyridine (Kuraya and Hase, 1996), 2-aminobenzamide (Chen and Flynn, 2007) and 1-phenyl-3-methyl pyrazolone (Mason, et al., 2006) are widely used for derivatization of N-glycans prior to on-line LC/MS. Under these conditions, the compound with the longest retention time is the unmodified reductive amination reagent. As the size and polarity of the glycan increases, the degree to which it binds the stationary phase decreases.
Acidic glycans can also be induced to interact with reversed stationary phases through the use of amines such as dibutyl amine, tributyl amine or triethyl amine as ion pairs in the mobile phase. This approach has been used with on-line LC/MS of glycosaminoglycans (Thanawiroon, et al., 2004). Reductive amination with 8-aminonaphthalene-1,3,6-trisulfonate has been used to increase the acidity of N-glycans to obtain isomeric resolution using reversed phase ion pairing LC/MS (Gennaro, et al., 2003). Here, the advantages of high resolution using the reversed phase ion pairing mechanism are balanced against the disadvantages of the need to infuse millimolar concentrations of amines continuously into the ion source.
Graphitized carbon chromatography has been used for on-line LC/MS analysis of N- and O-glycans using packed capillary (Kawasaki, et al., 2003) and chip-based (Niñonuevo, et al., 2005) chromatography systems. Graphitized carbon chromatography can be used to separate structural isomers. Therefore, to maximize its performance, it is best to reduce or reductively aminate glycans to prevent splitting of peaks based on reducing end anomers. Mobile phases containing formic acid modifier are appropriate for analysis of native or reductively aminated neutral and sialylated glycans and permethylated glycans (Costello, et al., 2007).
Hydrophilic interaction chromatography (HILIC), used to separate glycans based on their size and hydrophilicity, is an effective chromatography mode for on-line LC/MS. HILIC chromatographic retention time information may be correlated with glycan structure using chromatographic standards (Guile, et al., 1996). Its use with a mass spectral detector facilitates the development of a retention time libraries against which unknown glycan compositions may be referenced (Pabst, et al., 2007). Capillary amide-HILC has been used for on-line LC/MS of N-glycans (Wuhrer, et al., 2004) glycopeptides (Wuhrer, et al., 2005) and glycosaminoglycans (Naimy, et al., 2008, Hitchcock, et al., 2008) and for off-line MALDI MS of glycosphingolipids (Zarei, et al., 2008). A disadvantage of HILIC is that resolution of structural isomers is generally not observed.
In conclusion there are several effective options for glycan chromatography that have been demonstrated with LC/MS in glycomics.
d. Ion mobility MS applied to glycomics
One of the challenges for LC is achieving separation of glycan structural isomers. Gas phase ion mobility separations (IMS) have the potential to overcome this obstacle and resolve glycan structural isomers based on differences in the three dimensional shape of such ions. (Clowers, et al., 2005, Clowers and Hill, 2005). In IMS, ions are moved through a chamber of relatively high gas pressure so as to provide a degree of separation of structural isomers prior to MS or tandem MS. Profiling of glycans released from biological sources often results in ions that have overlapping isotopic clusters. As shown in Figure 2, glycans isolated from the urine of a patient with a congenital disorder of glycosylation were analyzed by nano-scale ESI using an MS instrument in which a mobility separation chamber preceded the TOF-MS analyzer (Vakhrushev, et al., 2008). This instrument permits ions exiting the mobility chamber to be detected directly using MS or fragmented in a collision cell prior to MS detection. Figure 2A shows that two glycan compositions are observed in the m/z 1007-1012 window. Charge states are segregated into separate windows defined by ion mobility and m/z. As a result, it is possible to extract the two glycans, as shown in (b, c). It is also possible to visualize the differing product ion profile resulting from dissociation of the two compositions, as shown in (d). The m/z values for the monoisotopic ion for the two glycans have distinct mobility chromatograms, as shown in (e).
Figure 2.
Ion mobility spectrometry of human urine glycans from a congenital disorders of glycosylation patient. (a) Expansion of the mass range at m/z 1007-1012 of the negative ion mode nano-ESI Q-TOF MS of the urine fraction, acquired from the total ion chromatogram over all mobility drift times. (b) Expansion of the mass range at m/z 1007-1012 of the spectrum acquired from extracted ion chromatogram of doubly charged distributed area after ion mobility separation. (c) Expansion of the mass range at m/z 1007-1012 of the spectrum acquired from extracted ion chromatogram of triply charged distributed area after IM separation. (d) Plot of fragment ions obtained by CID of overlapped precursor ions at m/z 1007.354 (left) and 1008.827 (right) vs. m/z values. (e) Total ion current chromatogram with retained drift time of overlapped precursor ions at m/z 1007.354 (left) and 1008.827 (right). Selected areas indicate extracted ion current chromatogram A for the precursor ions at m/z 1008.827 and chromatogram B for the precursor ions at m/z 1007.354. XIC = extracted ion chromatogram. © 2008, American Chemical Society (Vakhrushev, et al., 2008).
In conclusion, mobility separations demonstrate clear potential as a means of analyzing complex released glycan mixtures in conjunction with MS detection. Although the mobility resolution is modest at present, the technology is advancing rapidly.
3. Quantitative glycomics
Glycan quantification is essential for determination for both fundamental studies of their biological activities and biomarker identification efforts. As in proteomics, quantitative methods for glycomics have been developed using label free and stable isotope labeled approaches. These trends are summarized below.
a. Label free approaches
i. N-linked glycomics
Since N-glycosylation represents one of the most common posttranslational modifications in eukaryotes, developing methodology to accurately and efficiently perform glycomics scale analysis of N-glycans is critical. A recent multi-institutional study of quantitative profiling of glycoprotein glycans highlights the strengths and weaknesses of methods in widespread use at present (Wada, et al., 2007). The study revealed that there was a significant variance among laboratories using chromatographic quantitation due to differences in reductive amination methods used. On the other hand, laboratories using permethylation of the released N-glycans followed by MALDI-TOF MS produced consistently good results. Those using graphitized carbon LC/MS with negative ion ESI MS detection gave consistent results with underivatized oligosaccharide alditols. For analysis of underivatized sialylated N-glycans, the ESI LC/MS approach was found to be superior to MALDI since it did not lead to fragmentation during ionization. However, the ionization responses depended on the degree of sialylation, somewhat complicating the task of obtaining quantitative results.
Further efforts are likely to refine the methods for recovery of sub-microgram quantities of glycans from the permethylation reaction. LC/MS approaches will be useful for N-glycans modified by groups not compatible with the permethylatioin chemistry, including sulfation and phosphorylation.
ii. O-linked glycomics
Mucin oligosaccharides represent the most common type of O—linked glycosylation and have high value as potential disease biomarkers. For example, mucin O-glycans have been profiled from gastric biopsies of Rhesus monkeys as a function of Helicobacter pylori infection (Cooke, et al., 2007). Such glycans are typically released as alditols by in alkaline β-elimination the presence of a reducing agent. The O-glycans released by β-elimination were analyzed by ESI and MALDI Fourier transform (FT) MS. The data demonstrate that there is a significant difference in composition of core O-glycan structures between samples from infected and uninfected animals, suggesting that the overall hallmark of infection is the loss of mucin-type core structures. A related glycomics approach has been developed to identify breast cancer glycan biomarkers based on the analysis of released O-glycans from cancer cell lines and serum samples (Kirmiz, et al., 2007). In addition, an on-line graphitized carbon LC/MS approach was used to analyze the composition of mucins in sputum of cystic fibrosis patients (Schulz, et al., 2007). The study revealed clear differences in glycan structures between cystic fibrosis patients and healthy controls.
In summary, MALDI and ESI-based methods for O-glycans are similar to those for N-glycans. Several groups are developing non-reductive release strategies for O-glycans that may prove effective.
iii. Glycosaminoglycan glycomics
The ability to measure the mass of glycosaminoglycan oligosaccharides forms the core of glycomics approaches aimed at correlation of structure with biological function (Sasisekharan, et al., 2006). Glycosaminoglycans can also be analyzed using MALDI MS when paired with basic peptides (Venkataraman, et al., 1999). Glycosaminoglycan oligosaccharides can also be analyzed using LC/MS setups, including size exclusion (Henriksen, et al., 2004), reversed phase ion pairing (Kuberan, et al., 2002) and hydrophilic interaction chromatography approaches (Naimy, et al., 2008, Hitchcock, et al., 2008). The on-line LC/MS approaches facilitate comparative profiling of glycosaminoglycan oligosaccharides as a function of tissue type, protein binding or other biological variables.
The challenging chemistry of these and other sulfated glycan classes is no longer a barrier since effective analytical techniques have been developed.
b. Stable isotope labels for glycomics
Stable isotope labels are used commonly in glycomics as a mean to improve determination of relative amounts of glycans in the sample. Depending on the sample and extent of the information needed, several different labels can be used, as described below.
i. Permethylation labeling
Use of deuterated methyl iodide is a straightforward means of introducing stable isotope labels into permethylated N-glycans (Viseux, et al., 1999). Labeled and unlabeled permethylated N-glycans would likely be resolved by graphitized carbon chromatography. Additionally, it is important to keep in mind that mass shift varies according to the glycan composition. An example of this labeling approach is demonstrated using a comparative glycoform mapping method (C-GlycoMAP), developed based on differential stable isotope labeling (CH3I/CD3I) and permethylation (Kang, et al., 2007). The differentially isotope labeled samples are combined prior to MALDI MS analysis to minimize sample-to-sample variation in peak abundances and maximize the ability to perform comparison of two samples. Stable isotope labels have also been introduced using 13CH3I (Aoki, et al., 2007).
Recently, the concept of quantification by isobaric labeling was introduced (Atwood, et al., 2008) under which glycans are permethylated with 13CH3I or CH2DI. These groups have exact masses differing by 0.002922 Da and permethylated glyans modified with these reagents have the same nominal mass but their exact masses differ by multiples of this value. The resolution required to separate the exact masses of typical N-and O-glycans differentially labeled with these reagents is approximately 30,000, well within the operating range of FTMS and Orbitrap mass spectrometers. Thus, it is possible to analyze the mixtures directly in the MS mode, or to select the nominal masses for subsequent tandem mass spectrometric analysis of isobaric glycoform mixtures.
ii. Reductive amination labeling
A number of reductive amination reagents are commercially available in deuterated form, allowing easy incorporation of stable isotope labels through reductive amination (Yuan, et al., 2005, Hitchcock, et al., 2006a). In addition, the reduction step may be conducted with a deuterated reducing agent to introduce a 1 Da mass shift (Hsu, et al., 2006). For many applications, however, a larger mass shift is desired so as to separate the glycan isotopic clusters. Mono- and oligosaccharides differentially isotope labeled with d0/d4pyridyl amine (PA) can be analyzed using graphitized carbon LC/MS, and the isotopes are partially separated chromatographically (Yuan, et al., 2005). Here, N-glycans labeled with the heavy form of PA are used as internal standards to facilitate quantitative comparison among glycan samples. Glycosaminoglycan oligosaccharides can be differentially labeled with d0/d42-anthranilic acid and analyzed by size exclusion LC/MS/MS (Hitchcock, et al., 2006a) or amide-HILIC LC/MS/MS (Hitchcock, et al., 2008). A reductive amination tag has been synthesized in four stable isotope forms (d0, d4, d8 and d12) and applied to MS analysis of N-glycans and glycosaminoglycans (Bowman and Zaia, 2007). The advantage to the tetraplex tags is that four samples may be mixed and analyzed simultaneously.
In summary, isotopic labeling techniques have the advantage over label-free methods that a stable isotope-labeled internal standard can be used to compensate for changes in instrument performance over time. For extremely complex samples, such internal standards may not be appropriate since they increase spectral complexity.
4. Tandem MS and the analysis of isomeric glycan mixtures
a. Principles of glycan tandem MS
The use of tandem MS for glycomics is driven by the need to produce structural information on glycans expressed in serum/plasma or tissue relevant to understanding of disease processes and biomarker discovery (Packer, et al., 2008). The challenge of this task resides in the fact that glycoconjugates are expressed as a distribution of glycosylation variants relative to a core structure. Thus, a glycan composition, indicated by its mass, typically reflects a mixture of positional isomers. One approach is to purify the glycans to homogeneity before tandem MS analysis and interpret obtained mass spectra directly or in comparison to the spectra of synthetic standards. In many cases such purification is not possible. Thus, another option for the tandem MS experiment is to analyze a mixture of positional isomers directly. In such an experiment, sequential stages of tandem MS are performed in series. Stages of MS are abbreviated MSn, where n = the stage. For MS measurement, n = 1, for the first stage of tandem MS, n = 2. Subsequent dissociation of a product ion from MS2 is termed MS3, and so on. The masses of the product ions indicate compositions of glycan sub-structures, any of which may be selected for dissociation in further stages of tandem MS.
Most glycan tandem mass spectra are produced by collisional induced dissociation (CAD), a technique in which selected precursor ions are dissociated by collision with gas atoms in a collision cell. The collisions increase the vibrational energy of the ions to the point that bond rupture occurs. Typically, the weakest bonds rupture to produce the most abundant product ions. It is possible to dissociate permethylated glycans using high energy CAD using a MALDI TOF/TOF instrument, under which conditions bond rupture is kinetically controlled and cross-ring cleavage ions are more abundant. This approach is not generally applicable to native and reductively aminated glycans due to fragmentation during the MALDI process.
The nomenclature for glycan and glycoconjugate product ions (Domon and Costello, 1988) is shown in
Figure 3A and it will be used throughout this section. Product ions containing a non-reducing terminus are labeled as A, B, C and those containing the non-reducing end or aglycon are labeled X, Y, Z. Cleavages across residue rings (A and X type ions) are particularly useful for determining linkages. In addition, as shown in Figure 3B, the D-ion (not part of the original nomenclature) consists of combined C- and Z-type fragmentation about a Hex or HexNAc residue that is 3-linked and may be used to differentiate N-acetyllactosamine linkages and mannose branching structure (Chai, et al., 2001).
Figure 3.
(A) Nomenclature for tandem mass spectrometric product ions of glycans and glycoconjugates from (Domon and Costello, 1988). (B) Illustration of use of D-type ions to differentiate N-acetyllactosamine linkages, mannose branching structure and core fucosylation.
b. Differentiation of glycan isomers using tandem MS
Tandem MS of glycan classes has been reviewed (Zaia, 2004). The following section summarizes how key glycan sub-structures may be differentiated using tandem MS, highlighting recent developments in the field. A detailed discussion is beyond the scope of this review, but relevant references are given in Table 1.
Table 1.
Summary of tandem mass spectrometric methods for differentiating isomeric glycan epitopes
| Epitope | Symbol | Glycan | Dissociation | Precursor ion | Stage | Product ion | References |
|---|---|---|---|---|---|---|---|
| Differentiation of N-acetyllactosamine isomers Type 1 Chain (neo-lactosamine)  Type 2 Chain (lactosamine) 
|
Native or reductively aminated |
CAD | [M-nH]n- | MS2 | D, 0,2A | (Chai, et al., 2001, Pfenninger, et al., 2002b, Pfenninger, et al., 2002a) |
|
| Permethylated | CAD | [M+nNa]n+ | MSn | →B2→ | (Ashline, et al., 2005) | ||
| Differentiation of neuraminic acid linkage isomers (α2-3)  (α2-6) 
|
Native or reductively aminated |
CAD | [M(X)+nH]n+ X = transition metal |
MS2 | X, Y, Z | (Leavell and Leary, 2001) | |
| Native or reductively aminated |
CAD | [M-nH]n- | MS2 MSn |
0,4A2-CO2, →Cn→, →Dn→ |
(Froesch, et al., 2003, Wheeler and Harvey, 2000, Deguchi, et al., 2006, Ito, et al., 2007) |
||
| Permethylated | CAD | [M+nNa]n+ | MS2 MSn |
A2 →211→ |
(Lemoine, et al., 1991, Mechref, et al., 2006, Anthony, et al., 2008) |
||
| Differentiation of branching isomers 3-, 4-, 6- antennae 
|
Native or reductively aminated |
CAD | [M(Na)+nH]n+ | MS2 | A- | (Harvey, 2000b, Harvey, 2000a) |
|
| Native or reductively aminated |
CAD | [M-nH]n- | MS2 | A- and D- | (Chai, et al., 2002, Sagi, et al., 2002, Takegawa, et al., 2005, Harvey, et al., 2008a, Harvey, et al., 2008b) |
||
| Native or reductively aminated |
EDD | [M-nH]n- | MS2 | A-, B-,C-, X-, Y-, Z- |
(Adamson and Hakansson, 2007b) | ||
| Permethylated | [M+nNa]n+ | MS2 | 0,4A-, 3,5A- | (Costello, et al., 2007, Zhao, et al., 2008) |
|||
| Permethylated | Hot ECD | [M+nNa]n+ | MS2 | 3,5A | (Zhao, et al., 2008) | ||
| Differentiation of fucosylated positional isomers Core vs antenna |
Native or reductively aminated |
CAD | [M(Na)+nH]n+ | MS2 | Core 0,2A, B, Y ion |
(Vakhrushev, et al., 2004, Suzuki, et al., 2005) |
|
| Native or reductively aminated |
[M-nH]n- | MS2 | C-, A-, D-, | (Sagi, et al., 2002, Harvey, et al., 2008b) |
|||
| Permethylated | [M+nNa]n+ | MS2 | B-, Y-, A- | (Viseux, et al., 1997) | |||
| Determination of disaccharide linkage (1,6) vs (1-4) vs (1-3) |
Permethylated | CAD | [M+nNa]n+ | MSn | →B2→ | (Ashline, et al., 2005, Prien, et al., 2008) |
|
| Quantification of glycosaminoglycan disaccharides (sulfation positional isomers) |
Native | CAD | [M-nH]n- | MS2, MS3 | B-, Y-, X- | (Saad, et al., 2005, Saad and Leary, 2003, Desaire, et al., 2001, Desaire and Leary, 2000, Behr, et al., 2005) |
|
| Quantification of chondroitin sulfate oligosaccharide positional isomers in mixtures CS type A  CS type B  CS type C 
|
Native | CAD | [M-nH]n- | MS2 ion abundances |
B-, Y-, X- | (Miller, et al., 2006, McClellan, et al., 2002, Zaia, et al., 2001) |
|
| Reductively aminated |
CAD | [M-nH]n- | MS2 ion abundances |
Y-, X- | (Hitchcock, et al., 2008, Hitchcock et al., 2006b, Hitchcock, et al., 2006a, Zaia, et al., 2003) |
||
| Determination of heparin/heparan sulfate tetrasaccharide absolute positions of sulfation and uronic acid epimerization |
Native | EDD | [M-nH]n- | MS2 | A- and X- | (Wolff, et al., 2007a, Wolff, et al., 2007b, Chi, et al., 2008) |
|
Symbols 
i. Permethylated glycans
Strategies for acquisition and interpretation of multistage MS have been most fully developed for permethylated glycans (Ashline, et al., 2005). The advantage to this approach is that tandem mass spectrometric dissociation of a glycosidic bond leaves a site that lacks a methyl group that is clearly indicated by mass. Thus, the linkage position is indicated by the mass of cross-ring cleavage ions (A or X types). It is possible to differentiate some types of positional isomers based on the formation of specific product ion types. Tandem MS of permethylated glycans produces more structural detail than does that of native and reductively aminated glycans.
ii. Native and reductively aminated glycans
Tandem MS of native and reductively aminated glycans may be acquired using either positive or negative ions. Generally speaking, positive ion tandem MS results in abundant glycosidic bond product ions, with those occurring adjacent to HexNAc, sialic acid, and Fuc particularly abundant. It must be noted that glycans must be cationized with sodium or other metals rather than analyzed as protonated ions. Protonated ions have been observed to undergo rearrangements during tandem MS. For N-glycans, A-type cross-ring cleavages to the core HexNAc residues are also abundant. The problem is that cross-ring cleavages to branching residues are typically low in abundances, as are D-type ions that similarly differentiate glycan branches. As a result, tandem MS of positive ions produces very limited detail on the branching structure of glycans and glycosidic linkages.
The structural detail produced from dissociation of negative ions is greater than in the positive mode. For neutral glycan classes, an abundant series of C-ions is often observed. Such ions readily form A-type ions for monosaccharide residues that have an unmodified hydroxyl group in the 3-position. In the event that this position is occupied (i.e. by a glycan branch) a D-type ion is formed. This pattern forms the basis of differentiation of lactosamine isomers and antenna branches, as detailed in Table 1.
iii. Activated ion dissociation of glycans
Electron capture dissociation (ECD) is an activated electron fragmentation method for positive ions using FTMS instruments that has been applied to glycans recently (Adamson and Hakansson, 2007a). Electron detachment dissociation (EDD) generates dissociation in the negative mode and has been shown to produce relatively abundant cross-ring cleavage ions for N-glycans and milk oligosaccharides (Adamson and Hakansson, 2007b) and glycosaminoglycans (Wolff, et al., 2007b, Wolff, et al., 2007a). As shown in Table 1, EDD generates product ions that differentiate glycan isomers. This approach therefore has great potential as a glycan analysis method.
In summary, tandem MS produces the greatest structural detail on permethylated glycans. Negative ion tandem MS is effective for producing useful structural information on native and reductively aminated glycans, including those classes not compatible with permethylation. Acitivated electron dissociation techniques, EDD in particular, show potential for incorporation into strategies for glycomics analysis.
5. Glycoproteomics - analysis of glycopeptides
a. MS analysis of the β-O-GlcNAc glycopeptides
Mass spectrometric analysis of glycopeptides is made challenging by the differing chemical properties of glycans and peptides. For example, although the β-O-GlcNAc modification occurs to Ser/Thr residues of many nuclear and cytoplasmic proteins it was not detected until fairly recently because it is both uncharged and labile (Hart, et al., 2007).
b. MS analysis of N- and O-glycopeptides
The ultimate goal of glycoproteomics is to quantify the site occupancy of glycosylation in the proteome and the structure of the distribution of glycoforms at each site. Glycoproteomics schemes typically involve enrichment of glycoproteins or glycopeptides using affinity techniques. Enrichment approaches for glycoproteomics have been reviewed recently (Xin, et al., 2008, Wuhrer, et al., 2007a) and only a few approaches will be mentioned here. Several groups have published workflows for serum glycoproteomics based on use of lectins for affinity capture of glycoproteins or glycopeptides (Geng, et al., 2001, Wang, et al., 2006, Madera, et al., 2006). Glycopeptides may also be isolated by hydrophilic interaction solid phase extraction (Wada, et al., 2004) or chromatography (Wuhrer, et al., 2005) and graphitized carbon solid phase extraction (Larsen, et al., 2005). They may also be enriched based on their high molecular weight using size exclusion chromatography (Alvarez-Manilla, et al., 2006).
c. Tandem MS of glycopeptides
One of the challenges to glycopeptidomics is that the glycan moiety has different chemical properties than the peptide backbone and dissociates under different mass spectrometric conditions. Since tandem MS for glycoproteomics has been reviewed recently (Wuhrer, et al., 2007a), only an outline of methodology will be given below, describing key aspects of fragmentation procedures routinely applied.
i. Collision-induced dissociation (CID) and infrared multiphoton dissociation (IRMPD)
IRMPD is a technique for dissociation that involves absorption of infrared light by the ions, causing vibrational excitation and subsequent bond fragmentation. Dissociation of glycopeptides using CID or IRMPD tends to produce abundant ions from cleavage of glycosidic bonds and low abundance ions from peptide backbone scission (Wuhrer, et al., 2007a). The balance of glycosidic versus peptide backbone dissociation types depends on the glycopeptide structure. In particular, high mannose N-glycans do not undergo glycosidic bond cleavage as readily as complex N-glycans, and produce relatively abundant ions from peptide backbone cleavage using IRMPD (Adamson and Hakansson, 2006).
ii. Electron capture dissociation (ECD)
Whereas IRMPD and CID, by contrast, tend to produce abundant glycosidic bond cleavages for glycopeptides (Wuhrer, et al., 2007a), ECD selectively fragments the peptide backbone of glycopeptides, allowing the site of glycosylation to be determined (Håkansson, et al., 2001). Thus, a combination of ECD and dissociation based on increasing internal bond energy (CID or IRMPD) produces the greatest amount of information on the glycopeptide structure and commercial mass spectrometers with these capabilities are now available. The ECD/IRMPD combination was used to analyze a xylosylated neutral N-linked glycopeptide (Håkansson, et al., 2001), a high mannose N-linked glycopeptide (Adamson and Hakansson, 2006), and a monosialylated N-linked glycopeptide that also carried an O-glycosylation site (Kjeldsen, et al., 2003). There do not appear to be any reports demonstrating the incorporation of ECD into an applied N-glycan glycoproteomics work flow.
iii. Electron transfer dissociation (ETD)
ETD is a dissociation method in which an electron is transferred between a reagent gas and an analyte ion inside an ion trap mass spectrometer, creating an activated electron species that undergoes dissociation similar to those observed in ECD (Coon, et al., 2005). The capability of ETD in an ion trap instrument has been demonstrated using the same glycopeptide used with earlier ECD work (Hogan, et al., 2005). In summary, abundant peptide backbone dissociation is observed for glycopeptides using ETD. CAD results in preferential fragmentation of the glycan moiety of glycopeptides. The combination of ETD and CAD has been used with on-line LC/MS was used to analyze a high mannose N-glycosylated peptide from human epidermal growth factor receptor (Wu, et al., 2007). Nano-LC/MS with a combination of CID and ETD dissociation was used to analyze complex immunoglobulin N-glycopeptides from human serum (Wuhrer, et al., 2007b). Sialylated glycopeptides have not yet been analyzed by ETD.
In summary, incorporation of ETD into glycoproteomic workflows has the advantage of the wide availability of ion trap MS instrumentation. The incorporation of ETD into such workflows has advanced faster than those using ECD at the time of this writing.
6. Conclusions
It has been the intention of this review to summarize the convergence of mass spectral methods into glycomics workflows to study glycan expression in areas of fundamental biochemistry and biomarker development. The structural information obtained from tandem mass spectrometric studies is greatest when the glycans are released from the peptide or protein backbone. For glycan classes compatible with the derivatization conditions, permethylation remains the clear methodological choice. Using permethylation, ionization responses are increased over those of underivatized glycans, and the chemical stability improved. Multistage tandem mass spectrometric dissociation of permethylated glycans produces the greatest level of detail possible using mass spectral techniques. Glycan classes modified with sulfate or other fragile substituents are not compatible with permethylation, but may be reductively aminated. Native and reductively aminated glycans produce the most informative tandem mass spectra for negatively charged ions. A number of LC/MS approaches have been incorporated into glycomics workflows for permethylated, native, and reductively aminated glycans. Glycopeptides may be analyzed using a combination of activated electron dissociation (ECD/ETD) and CID or IRMPD.
Supplementary Material
Figure 4.
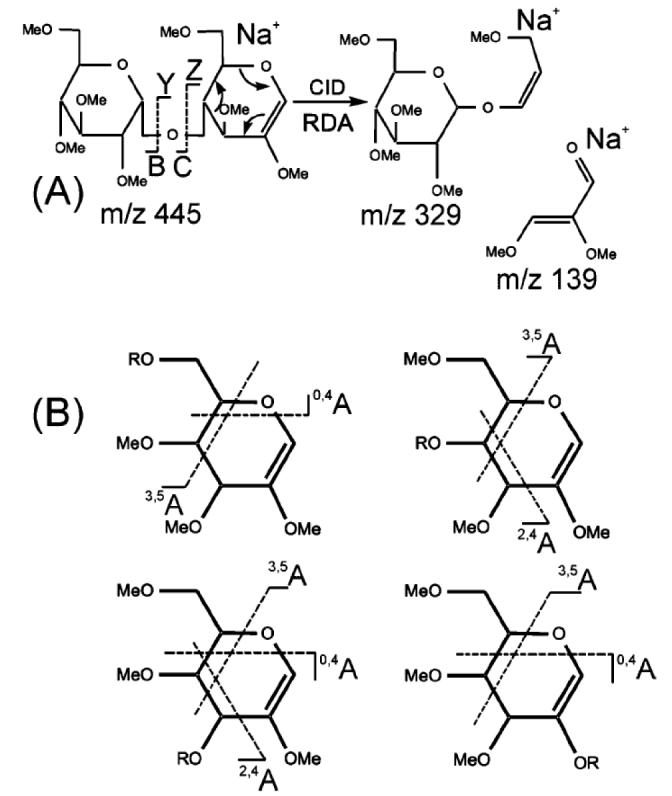
Example of the utility of multistage fragmentation of B-ions generated from permethylated glycans. A retro-Diels-Alder reaction in a 1,4-linked B2-type Hex-Hex disaccharide, shows formation of an m/z 329 ion from an m/z 445 ion (A). The m/z 139 ion is generally of low abundance or absent, presumably due to the stronger affinity for the metal ion charge carrier by the larger fragments. (B) shows the generic cross-ring cleavages that may be formed from B-type ions of various linkages. R indicates the location of mono- or oligosaccharide substituents. (Ashline, et al., 2005). ©2005, American Chemical Society, used with permission.
7. List of abbreviations used
- CAD
collisional activated dissociation
- ECD
electron capture dissociation
- EDD
electron detachment dissociation
- ETD
electron transfer dissociation
- ESI
electrospray ionization
- FTMS
Fourier transform mass spectrometry
- IRMPD
Infrared multi-photon dissociation
- LC/MS
liquid chromatography/mass spectrometry
- MALDI
matrix-assisted laser desorption/ionization
- MS
mass spectrometry
- QIT
quadrupole ion trap
- QQQ
triple quadrupole
- QTOF
quadrupole time-of-flight
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
10. References
- Adamson JT, Hakansson K. Infrared multiphoton dissociation and electron capture dissociation of high-mannose type glycopeptides. J Proteome Res. 2006;5:493–501. doi: 10.1021/pr0504081. [DOI] [PubMed] [Google Scholar]
- Adamson JT, Hakansson K. Electron capture dissociation of oligosaccharides ionized with alkali, alkaline earth, and transition metals. Anal Chem. 2007a;79:2901–2910. doi: 10.1021/ac0621423. [DOI] [PubMed] [Google Scholar]
- Adamson JT, Hakansson K. Electron detachment dissociation of neutral and sialylated oligosaccharides. J Am Soc Mass Spectrom. 2007b;18:2162–2172. doi: 10.1016/j.jasms.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Alvarez-Manilla G, Atwood J, 3rd, Guo Y, Warren NL, Orlando R, Pierce M. Tools for glycoproteomic analysis: size exclusion chromatography facilitates identification of tryptic glycopeptides with N-linked glycosylation sites. J Proteome Res. 2006;5:701–708. doi: 10.1021/pr050275j. [DOI] [PubMed] [Google Scholar]
- Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC, Ravetch JV. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 2008;320:373–376. doi: 10.1126/science.1154315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anumula KR. High-sensitivity and high-resolution methods for glycoprotein analysis. Anal Biochem. 2000;283:17–26. doi: 10.1006/abio.2000.4645. [DOI] [PubMed] [Google Scholar]
- Anumula KR. Advances in fluorescence derivatization methods for high-performance liquid chromatographic analysis of glycoprotein carbohydrates. Anal Biochem. 2006;350:1–23. doi: 10.1016/j.ab.2005.09.037. [DOI] [PubMed] [Google Scholar]
- Aoki K, Perlman M, Lim JM, Cantu R, Wells L, Tiemeyer M. Dynamic developmental elaboration of N-linked glycan complexity in the Drosophila melanogaster embryo. J Biol Chem. 2007;282:9127–9142. doi: 10.1074/jbc.M606711200. [DOI] [PubMed] [Google Scholar]
- Apweiler R, Hermjakob H, Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochimica et biophysica acta. 1999;1473:4–8. doi: 10.1016/s0304-4165(99)00165-8. [DOI] [PubMed] [Google Scholar]
- Ashline D, Singh S, Hanneman A, Reinhold V. Congruent Strategies for Carbohydrate Sequencing. 1. Mining Structural Details by MSn. Anal Chem. 2005;77:6250–6262. doi: 10.1021/ac050724z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood JA, 3rd, Cheng L, Alvarez-Manilla G, Warren NL, York WS, Orlando R. Quantitation by isobaric labeling: applications to glycomics. J Proteome Res. 2008;7:367–374. doi: 10.1021/pr070476i. [DOI] [PubMed] [Google Scholar]
- Behr JR, Matsumoto Y, White FM, Sasisekharan R. Quantification of isomers from a mixture of twelve heparin and heparan sulfate disaccharides using tandem mass spectrometry. Rapid Commun Mass Spectrom. 2005;19:2553–2562. doi: 10.1002/rcm.2079. [DOI] [PubMed] [Google Scholar]
- Bowman M, Zaia J. Novel tags for the stable isotopic labeling of carbohydrates and quantitative analysis by mass spectrometry. Anal Chem. 2007;76:5777–5784. doi: 10.1021/ac070581b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceroni A, Maass K, Geyer H, Geyer R, Dell A, Haslam SM. GlycoWorkbench: A Tool for the Computer-Assisted Annotation of Mass Spectra of Glycans. J Proteome Res. 2008 doi: 10.1021/pr7008252. [DOI] [PubMed] [Google Scholar]
- Chai W, Piskarev V, Lawson AM. Negative-Ion electrospray mass spectrometry of neutral underivatized oligosaccharides. Anal Chem. 2001;73:631–657. doi: 10.1021/ac0010126. [DOI] [PubMed] [Google Scholar]
- Chai W, Piskarev V, Lawson AM. Branching pattern and sequence analysis of underivatized oligosaccharides by combined MS/MS of singly and doubly charged molecular ions in negative-ion electrospray mass spectrometry. J Am Soc Mass Spectrom. 2002;13:670–679. doi: 10.1016/S1044-0305(02)00363-X. [DOI] [PubMed] [Google Scholar]
- Chen X, Flynn GC. Analysis of N-glycans from recombinant immunoglobulin G by on-line reversed-phase high-performance liquid chromatography/mass spectrometry. Anal Biochem. 2007;370:147–161. doi: 10.1016/j.ab.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Chi L, Wolff JJ, Laremore TN, Restaino OF, Xie J, Schiraldi C, Toida T, Amster IJ, Linhardt RJ. Structural Analysis of Bikunin Glycosaminoglycan. J Am Chem Soc. 2008 doi: 10.1021/ja0778500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowers BH, Dwivedi P, Steiner WE, Hill HH, Jr., Bendiak B. Separation of sodiated isobaric disaccharides and trisaccharides using electrospray ionization-atmospheric pressure ion mobility-time of flight mass spectrometry. J Am Soc Mass Spectrom. 2005;16:660–669. doi: 10.1016/j.jasms.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Clowers BH, Hill HH. Mass analysis of mobility-selected ion populations using dual gate, ion mobility, quadrupole ion trap mass spectrometry. Anal Chem. 2005;77:5877–5885. doi: 10.1021/ac050700s. [DOI] [PubMed] [Google Scholar]
- Cooke CL, An HJ, Kim J, Solnick JV, Lebrilla CB. Method for profiling mucin oligosaccharides from gastric biopsies of rhesus monkeys with and without Helicobacter pylori infection. Anal Chem. 2007;79:8090–8097. doi: 10.1021/ac071157d. [DOI] [PubMed] [Google Scholar]
- Coon JJ, Ueberheide B, Syka JE, Dryhurst DD, Ausio J, Shabanowitz J, Hunt DF. Protein identification using sequential ion/ion reactions and tandem mass spectrometry. Proc Natl Acad Sci U S A. 2005;102:9463–9468. doi: 10.1073/pnas.0503189102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello CE, Contado-Miller JM, Cipollo JF. A glycomics platform for the analysis of permethylated oligosaccharide alditols. J Am Soc Mass Spectrom. 2007;18:1799–1812. doi: 10.1016/j.jasms.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi K, Takegawa Y, Ito H, Miura N, Yoshioka S, Nagai S, Nakagawa H, Nishimura S. Structural assignment of isomeric 2-aminopyridine-derivatized monosialylated biantennary N-linked oligosaccharides using negative-ion multistage tandem mass spectral matching. Rapid Commun Mass Spectrom. 2006;20:412–418. doi: 10.1002/rcm.2320. [DOI] [PubMed] [Google Scholar]
- Desaire H, Leary J. Detection and quantification of the sulfated disaccharides in chondroitin sulfate by electrospray tandem mass spectrometry. J Am Soc Mass Spectrom. 2000;11:916–920. doi: 10.1016/S1044-0305(00)00168-9. [DOI] [PubMed] [Google Scholar]
- Desaire H, Sirich TL, Leary JA. Evidence of block and randomly sequenced chondroitin polysaccharides: sequential enzymatic digestion and quantification using ion trap tandem mass spectrometry. Anal Chem. 2001;73:3513–3520. doi: 10.1021/ac010385j. [DOI] [PubMed] [Google Scholar]
- Dettmer K, Aronov PA, Hammock BD. Mass spectrometry-based metabolomics. Mass Spectrom Rev. 2007;26:51–78. doi: 10.1002/mas.20108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domon B, Costello CE. A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconjugate J. 1988;5:397–409. [Google Scholar]
- Froesch M, Bindila L, Zamfir A, Peter-Katalinić J. Sialylation analysis of O-glycosylated sialylated peptides from urine of patients suffering from Schindler’s disease by Fourier transform ion cyclotron resonance mass spectrometry and sustained off-resonance irradiation collision-induced dissociation. Rapid Commun Mass Spectrom. 2003;17:2822–2832. doi: 10.1002/rcm.1273. [DOI] [PubMed] [Google Scholar]
- Geng M, Zhang X, Bina M, Regnier F. Proteomics of glycoproteins based on affinity selection of glycopeptides from tryptic digests. J Chromatogr B Biomed Sci Appl. 2001;752:293–306. doi: 10.1016/s0378-4347(00)00550-8. [DOI] [PubMed] [Google Scholar]
- Gennaro LA, Harvey DJ, Vouros P. Reversed-phase ion-pairing liquid chromatography/ion trap mass spectrometry for the analysis of negatively charged, derivatized glycans. Rapid Commun Mass Spectrom. 2003;17:1528–1534. doi: 10.1002/rcm.1079. [DOI] [PubMed] [Google Scholar]
- German JB, Gillies LA, Smilowitz JT, Zivkovic AM, Watkins SM. Lipidomics and lipid profiling in metabolomics. Curr Opin Lipodol. 2007;18:66–71. doi: 10.1097/MOL.0b013e328012d911. [DOI] [PubMed] [Google Scholar]
- Guile GR, Rudd PM, Wing DR, Prime SB, Dwek RA. A rapid high-resolution high-performance liquid chromatographic method for separating glycan mixtures and analyzing oligosaccharide profiles. Anal Biochem. 1996;240:210–226. doi: 10.1006/abio.1996.0351. [DOI] [PubMed] [Google Scholar]
- Håkansson K, Cooper HJ, Emmett MR, Costello CE, Marshall AG, Nilsson CL. Electron capture dissociation and Infrared multiphoton dissociation MS/MS of an N-glycosylated tryptic peptide to yield complementary sequence information. Anal Chem. 2001;73:4530–4536. doi: 10.1021/ac0103470. [DOI] [PubMed] [Google Scholar]
- Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–1022. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- Harvey DJ. Matrix-assisted laser desorption/ionization mass spectrometry of carbohydrates. Mass Spectrom Rev. 1999;18:349–450. doi: 10.1002/(SICI)1098-2787(1999)18:6<349::AID-MAS1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Harvey DJ. Collision-induced fragmentation of underivatized N-linked carbohydrates ionized by electrospray. J Mass Spectrom. 2000a;35:1178–1190. doi: 10.1002/1096-9888(200010)35:10<1178::AID-JMS46>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Harvey DJ. Electrospray mass spectrometry and fragmentation of N-linked carbohydrates derivatized at the reducing terminus. J Am Soc Mass Spectrom. 2000b;11:900–915. doi: 10.1016/S1044-0305(00)00156-2. [DOI] [PubMed] [Google Scholar]
- Harvey DJ. Matrix-assisted laser desorption/ionization mass spectrometry of carbohydrates and glycoconjugates. Mass Spectrom Rev. 2003;226:1–35. [Google Scholar]
- Harvey DJ, Crispin M, Scanlan C, Singer BB, Lucka L, Chang VT, Radcliffe CM, Thobhani S, Yuen CT, Rudd PM. Differentiation between isomeric triantennary N-linked glycans by negative ion tandem mass spectrometry and confirmation of glycans containing galactose attached to the bisecting (beta1-4-GlcNAc) residue in N-glycans from IgG. Rapid Commun Mass Spectrom. 2008a;22:1047–1052. doi: 10.1002/rcm.3470. [DOI] [PubMed] [Google Scholar]
- Harvey DJ, Royle L, Radcliffe CM, Rudd PM, Dwek RA. Structural and quantitative analysis of N-linked glycans by matrix-assisted laser desorption ionization and negative ion nanospray mass spectrometry. Anal Biochem. 2008b doi: 10.1016/j.ab.2008.01.025. [DOI] [PubMed] [Google Scholar]
- Henriksen J, Ringborg LH, Roepstorrf P. On-line size-exclusion chromatography/mass spectrometry of low molecular mass heparin. J Mass Spectrom. 2004;39:1305–1312. doi: 10.1002/jms.723. [DOI] [PubMed] [Google Scholar]
- Hitchcock A, Yates KE, Costello C, Zaia J. Comparative Glycomics of Connective Tissue Glycosaminoglycans Proteomics. 2008;8:1384–1397. doi: 10.1002/pmic.200700787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock AM, Costello CE, Zaia J. Glycoform quantification of chondroitin/dermatan sulfate using an LC/MS/MS platform. Biochemistry. 2006a;45:2350–2361. doi: 10.1021/bi052100t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock AM, Yates KE, Shortkroff S, Costello CE, Zaia J. Optimized extraction of glycosaminoglycans from normal and osteoarthritic cartilage for glycomics profiling. Glycobiology. 2006b;17:25–35. doi: 10.1093/glycob/cwl046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan JM, Pitteri SJ, Chrisman PA, McLuckey SA. Complementary structural information from a tryptic N-linked glycopeptide via electron transfer ion/ion reactions and collision-induced dissociation. J Proteome Res. 2005;4:628–632. doi: 10.1021/pr049770q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu J, Chang SJ, Franz AH. MALDI-TOF and ESI-MS analysis of oligosaccharides labeled with a new multifunctional oligosaccharide tag. J Am Soc Mass Spectrom. 2006;17:194–204. doi: 10.1016/j.jasms.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Ito H, Yamada K, Deguchi K, Nakagawa H, Nishimura S. Structural assignment of disialylated biantennary N-glycan isomers derivatized with 2-aminopyridine using negative-ion multistage tandem mass spectral matching. Rapid Commun Mass Spectrom. 2007;21:212–218. doi: 10.1002/rcm.2824. [DOI] [PubMed] [Google Scholar]
- Kang P, Mechref Y, Kyselova Z, Goetz JA, Novotny MV. Comparative glycomic mapping through quantitative permethylation and stable-isotope labeling. Anal Chem. 2007;79:6064–6073. doi: 10.1021/ac062098r. [DOI] [PubMed] [Google Scholar]
- Kawasaki N, Itoh S, Ohta M, Hayakawa T. Microanalysis of N-linked oligosaccharides in a glycoprotein by capillary liquid chromatography/mass spectrometry and liquid chromatography/tandem mass spectrometry. Anal Biochem. 2003;316:15–22. doi: 10.1016/s0003-2697(03)00031-9. [DOI] [PubMed] [Google Scholar]
- Kirmiz C, Li B, An HJ, Clowers BH, Chew HK, Lam KS, Ferrige A, Alecio R, Borowsky AD, Sulaimon S, Lebrilla CB, Miyamoto S. A serum glycomics approach to breast cancer biomarkers. Mol Cell Proteomics. 2007;6:43–55. doi: 10.1074/mcp.M600171-MCP200. [DOI] [PubMed] [Google Scholar]
- Kjeldsen F, Haselmann KF, Budnik BA, Sorensen ES, Zubarev RA. Complete characterization of posttranslational modification sites in the bovine milk protein PP3 by tandem mass spectrometry with electron capture dissociation as the last stage. Anal Chem. 2003;75:2355–2361. doi: 10.1021/ac026295b. [DOI] [PubMed] [Google Scholar]
- Kuberan B, Lech M, Zhang L, Wu ZL, Beeler DL, Rosenberg R. Analysis of Heparan Sulfate Oligosaccharides with Ion Pair-Reverse Phase Capillary High Performance Liquid Chromatography-Microelectrospray Ionization Time-of-Flight Mass Spectrometry. J Am Chem Soc. 2002;124:8707–8718. doi: 10.1021/ja0178867. [DOI] [PubMed] [Google Scholar]
- Kuraya N, Hase S. Analysis of pyridylaminated O-linked sugar chains by two-dimensional sugar mapping. Anal Biochem. 1996;233:205–211. doi: 10.1006/abio.1996.0029. [DOI] [PubMed] [Google Scholar]
- Larsen MR, Hojrup P, Roepstorff P. Characterization of gel-separated glycoproteins using two-step proteolytic digestion combined with sequential microcolumns and mass spectrometry. Mol Cell Proteomics. 2005;4:107–119. doi: 10.1074/mcp.M400068-MCP200. [DOI] [PubMed] [Google Scholar]
- Leavell MD, Leary JA. Stabilization and linkage analysis of metal-ligated sialic acid containing oligosaccharides. J Am Soc Mass Spectrom. 2001;12:528–536. doi: 10.1016/S1044-0305(01)00238-0. [DOI] [PubMed] [Google Scholar]
- Lemoine J, Strecker G, Leroy Y, Fournet B, Ricart G. Collisional-activation tandem mass spectrometry of sodium adduct ions of methylated oligosaccharides: sequence analysis and discrimination between alpha-NeuAc-(2-3) and alpha-NeuAc-(2-6) linkages. Carbohydr Res. 1991;221:209–217. doi: 10.1016/0008-6215(91)80057-t. [DOI] [PubMed] [Google Scholar]
- Madera M, Mechref Y, Klouckova I, Novotny MV. Semiautomated high-sensitivity profiling of human blood serum glycoproteins through lectin preconcentration and multidimensional chromatography/tandem mass spectrometry. J Proteome Res. 2006;5:2348–2363. doi: 10.1021/pr060169x. [DOI] [PubMed] [Google Scholar]
- Mason KE, Meikle PJ, Hopwood JJ, Fuller M. Characterization of sulfated oligosaccharides in mucopolysaccharidosis type IIIA by electrospray ionization mass spectrometry. Anal Chem. 2006;78:4534–4542. doi: 10.1021/ac052083d. [DOI] [PubMed] [Google Scholar]
- McClellan JM, Costello CE, O’Connor PB, Zaia J. Influence of charge state on product ion mass spectra and the determination of 4S/6S sulfation sequence of chondroitin sulfate oligosaccharides. Anal Chem. 2002;74:3760–3771. doi: 10.1021/ac025506+. [DOI] [PubMed] [Google Scholar]
- Mechref Y, Kang P, Novotny MV. Differentiating structural isomers of sialylated glycans by matrix-assisted laser desorption/ionization time-of-flight/time-of-flight tandem mass spectrometry. Rapid Commun Mass Spectrom. 2006;20:1381–1389. doi: 10.1002/rcm.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MJC, Costello CE, Malmström A, Zaia J. A tandem mass spectrometric approach to determination of chondroitin/dermatan sulfate oligosaccharide glycoforms. Glycobiology. 2006;16:502–513. doi: 10.1093/glycob/cwj093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naimy H, Leymarie N, Bowman M, Zaia J. Characterization of heparin oligosaccharides binding specifically to antithrombin III using mass spectrometry. Biochemistry. 2008;47:3155–3161. doi: 10.1021/bi702043e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niñonuevo M, An H, Yin H, Killeen K, Grimm R, Ward R, German B, Lebrilla C. Nanoliquid chromatography-mass spectrometry of oligosaccharides employing graphitized carbon chromatography on microchip with a high-accuracy mass analyzer. Electrophoresis. 2005;26:3641–3649. doi: 10.1002/elps.200500246. [DOI] [PubMed] [Google Scholar]
- Pabst M, Bondili JS, Stadlmann J, Mach L, Altmann F. Mass + retention time = structure: a strategy for the analysis of N-glycans by carbon LCESI-MS and its application to fibrin N-glycans. Anal Chem. 2007;79:5051–5057. doi: 10.1021/ac070363i. [DOI] [PubMed] [Google Scholar]
- Packer NH, von der Lieth CW, Aoki-Kinoshita KF, Lebrilla CB, Paulson JC, Raman R, Rudd P, Sasisekharan R, Taniguchi N, York WS. Frontiers in glycomics: bioinformatics and biomarkers in disease. An NIH White Paper prepared from discussions by the focus groups at a workshop on the NIH campus, Bethesda MD (September 11-13, 2006) Proteomics. 2008;8:8–20. doi: 10.1002/pmic.200700917. [DOI] [PubMed] [Google Scholar]
- Pfenninger A, Karas M, Finke B, Stahl B. Structural analysis of underivatized neutral human milk oligosaccharides in the negative ion mode by nano-electrospray MSn (part 1: methodology) J Am Soc Mass Spectrom. 2002a;13:1331–1340. doi: 10.1016/S1044-0305(02)00645-1. [DOI] [PubMed] [Google Scholar]
- Pfenninger A, Karas M, Finke B, Stahl B. Structural analysis of underivatized neutral human milk oligosaccharides in the negative ion mode by nano-electrospray MSn (part 2: application to isomeric mixtures) J Am Soc Mass Spectrom. 2002b;13:1341–1348. doi: 10.1016/S1044-0305(02)00646-3. [DOI] [PubMed] [Google Scholar]
- Prien JM, Huysentruyt LC, Ashline DJ, Lapadula AJ, Seyfried TN, Reinhold VN. Differentiating N-linked glycan structural isomers in metastatic and nonmetastatic tumor cells using sequential mass spectrometry. Glycobiology. 2008;18:353–366. doi: 10.1093/glycob/cwn010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad OM, Ebel H, Uchimura K, Rosen SD, Bertozzi CR, Leary JA. Compositional profiling of heparin/heparan sulfate using mass spectrometry: assay for specificity of a novel extracellular human endosulfatase. Glycobiology. 2005;15:818–826. doi: 10.1093/glycob/cwi064. [DOI] [PubMed] [Google Scholar]
- Saad OM, Leary JA. Compositional analysis and quantification of heparin and heparan sulfate by electrospray ionization ion trap mass spectrometry. Anal Chem. 2003;75:2985–2995. doi: 10.1021/ac0340455. [DOI] [PubMed] [Google Scholar]
- Sagi D, Peter-Katalinić J, Conradt HS, Nimtz M. Sequencing of tri- and tetraantennary N-glycans containing sialic acid by negative mode ESI QTOF tandem MS. J Am Soc Mass Spectrom. 2002;13:1138–1148. doi: 10.1016/S1044-0305(02)00412-9. [DOI] [PubMed] [Google Scholar]
- Sasisekharan R, Raman R, Prabhakar V. Glycomics approach to structure-function relationships of glycosaminoglycans. Annu Rev Biomed Eng. 2006;8:181–231. doi: 10.1146/annurev.bioeng.8.061505.095745. [DOI] [PubMed] [Google Scholar]
- Satterfield MB, Welch MJ. Comparison by LC-MS and MALDI-MS of prostate-specific antigen from five commercial sources with certified reference material 613. Clin Biochem. 2005;38:166–174. doi: 10.1016/j.clinbiochem.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Schulz BL, Sloane AJ, Robinson LJ, Prasad SS, Lindner RA, Robinson M, Bye PT, Nielson DW, Harry JL, Packer NH, Karlsson NG. Glycosylation of sputum mucins is altered in cystic fibrosis patients. Glycobiology. 2007;17:698–712. doi: 10.1093/glycob/cwm036. [DOI] [PubMed] [Google Scholar]
- Stevens J, Blixt O, Paulson JC, Wilson IA. Glycan microarray technologies: tools to survey host specificity of influenza viruses. Nat Rev Micro. 2006;4:857–864. doi: 10.1038/nrmicro1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Suzuki M, Ito E, Ishii H, Miseki K, Suzuki A. Convenient and rapid analysis of linkage isomers of fucose-containing oligosaccharides by matrix-assisted laser desorption/ionization quadrupole ion trap time-of-flight mass spectrometry. Glycoconj J. 2005;22:427–431. doi: 10.1007/s10719-005-4173-x. [DOI] [PubMed] [Google Scholar]
- Takegawa Y, Deguchi K, Ito S, Yoshioka S, Nakagawa H, Nishimura S. Structural assignment of isomeric 2-aminopyridine-derivatized oligosaccharides using negative-ion MSn spectral matching. Rapid Commun Mass Spectrom. 2005;19:937–946. doi: 10.1002/rcm.1872. [DOI] [PubMed] [Google Scholar]
- Thanawiroon C, Rice KG, Toida T, Linhardt RJ. LC/MS sequencing approach for highly sulfated heparin-derived oligosaccharides. J Biol Chem. 2004;279:2608–2615. doi: 10.1074/jbc.M304772200. [DOI] [PubMed] [Google Scholar]
- Vakhrushev SY, Langridge J, Campuzano I, Hughes C, Peter-Katalinic J. Ion mobility mass spectrometry analysis of human glycourinome. Anal Chem. 2008;80:2506–2513. doi: 10.1021/ac7023443. [DOI] [PubMed] [Google Scholar]
- Vakhrushev SY, Zamfir A, Peter-Katalinić J. 0,2An cross-ring cleavage as a general diagnostic tool for glycan assignment in glycoconjugate mixtures. J Am Soc Mass Spectrom. 2004;15:1863–1868. doi: 10.1016/j.jasms.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Varki A. Nothing in glycobiology makes sense, except in the light of evolution. Cell. 2006;126:841–845. doi: 10.1016/j.cell.2006.08.022. [DOI] [PubMed] [Google Scholar]
- Varki A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature. 2007;446:1023–1029. doi: 10.1038/nature05816. [DOI] [PubMed] [Google Scholar]
- Varki A, Cummings R, Esko J, Freeze H, Hart G, Marth G. Essentials of Glycobiology. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1999. [PubMed] [Google Scholar]
- Venkataraman G, Shriver Z, Raman R, Sasisekharan R. Sequencing complex polysaccharides. Science. 1999;286:537–542. doi: 10.1126/science.286.5439.537. [DOI] [PubMed] [Google Scholar]
- Viseux N, Costello CE, Domon B. Post-source decay mass spectrometry: optimized calibration procedure and structural characterization of permethylated oligosaccharides. J Mass Spectrom. 1999;34:364–376. doi: 10.1002/(SICI)1096-9888(199904)34:4<364::AID-JMS787>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Viseux N, de Hoffmann E, Domon B. Structural analysis of permethylated oligosaccharides by electrospray tandem mass spectrometry. Anal Chem. 1997;69:3193–3198. doi: 10.1021/ac961285u. [DOI] [PubMed] [Google Scholar]
- Wada Y, Azadi P, Costello CE, Dell A, Dwek RA, Geyer H, Geyer R, Kakehi K, Karlsson NG, Kato K, Kawasaki N, Khoo KH, Kim S, Kondo A, Lattova E, Mechref Y, Miyoshi E, Nakamura K, Narimatsu H, Novotny MV, Packer NH, Perreault H, Peter-Katalinic J, Pohlentz G, Reinhold VN, Rudd PM, Suzuki A, Taniguchi N. Comparison of the methods for profiling glycoprotein glycans: HUPO HGPI (Human Proteome Organisation Human Disease Glycomics/Proteome Initiative) multi-institutional study. Glycobiology. 2007 doi: 10.1093/glycob/cwl086. [DOI] [PubMed] [Google Scholar]
- Wada Y, Tajiri M, Yoshida S. Hydrophilic affinity isolation and MALDI multiple-stage tandem mass spectrometry of glycopeptides for glycoproteomics. Anal Chem. 2004;76:6560–6565. doi: 10.1021/ac049062o. [DOI] [PubMed] [Google Scholar]
- Wang YH, Wu SL, Hancock WS. Approaches to the study of N-linked glycoproteins in human plasma using lectin affinity chromatography and nano-HPLC coupled to electrospray linear ion trap-Fourier transform mass spectrometry. Glycobiology. 2006;16:514–523. doi: 10.1093/glycob/cwj091. [DOI] [PubMed] [Google Scholar]
- Wheeler SF, Harvey DJ. Negative ion mass spectrometry of sialylated carbohydrates: discrimination of N-acetylneuraminic acid linkages by MALDI-TOF and ESI- TOF mass spectrometry. Anal Chem. 2000;72:5027–5039. doi: 10.1021/ac000436x. [DOI] [PubMed] [Google Scholar]
- Wolff JJ, Amster IJ, Chi L, Linhardt RJ. Electron detachment dissociation of glycosaminoglycan tetrasaccharides. J Am Soc Mass Spectrom. 2007a;18:234–244. doi: 10.1016/j.jasms.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, Chi L, Linhardt RJ, Amster IJ. Distinguishing glucuronic from iduronic acid in glycosaminoglycan tetrasaccharides by using electron detachment dissociation. Anal Chem. 2007b;79:2015–2022. doi: 10.1021/ac061636x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SL, Huhmer AF, Hao Z, Karger BL. On-line LC-MS approach combining collision-induced dissociation (CID), electron-transfer dissociation (ETD), and CID of an isolated charge-reduced species for the trace-level characterization of proteins with post-translational modifications. J Proteome Res. 2007;6:4230–4244. doi: 10.1021/pr070313u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuhrer M, Catalina MI, Deelder AM, Hokke CH. Glycoproteomics based on tandem mass spectrometry of glycopeptides. J Chromatogr B Analyt Technol Biomed Life Sci. 2007a;849:115–128. doi: 10.1016/j.jchromb.2006.09.041. [DOI] [PubMed] [Google Scholar]
- Wuhrer M, Koeleman CA, Hokke CH, Deelder AM. Protein glycosylation analyzed by normal-phase nano-liquid chromatography--mass spectrometry of glycopeptides. Anal Chem. 2005;77:886–894. doi: 10.1021/ac048619x. [DOI] [PubMed] [Google Scholar]
- Wuhrer M, Koeleman CAM, Deelder AM, Hokke CN. Normal-phase nanoscale liquid chromatography - Mass spectrometry of underivatized oligosaccharides at low-femtomole sensitivity. Anal Chem. 2004;76:833–838. doi: 10.1021/ac034936c. [DOI] [PubMed] [Google Scholar]
- Wuhrer M, Stam JC, van de Geijn FE, Koeleman CA, Verrips CT, Dolhain RJ, Hokke CH, Deelder AM. Glycosylation profiling of immunoglobulin G (IgG) subclasses from human serum. Proteomics. 2007b;7:4070–4081. doi: 10.1002/pmic.200700289. [DOI] [PubMed] [Google Scholar]
- Xin L, Li M, Jianjun L. Recent developments in the enrichment of glycopeptides for glycoproteomics. Anal Lett. 2008;41:268–277. [Google Scholar]
- Yuan J, Hashii N, Kawasaki N, Itoh S, Kawanishi T, Hayakawa T. Isotope tag method for quantitative analysis of carbohydrates by liquid chromatography-mass spectrometry. J Chromatogr. 2005;1067:145. doi: 10.1016/j.chroma.2004.11.070. [DOI] [PubMed] [Google Scholar]
- Zaia J. Mass spectrometry of oligosaccharides. Mass Spectrom Rev. 2004;23:161–227. doi: 10.1002/mas.10073. [DOI] [PubMed] [Google Scholar]
- Zaia J. Mass Spectrometric Ionization of Carbohydrates. In: Gross ML, Caprioli RM, editors. Ionization Methods. Vol. 6. Elsevier; Amsterdam: 2006. pp. 889–903. [Google Scholar]
- Zaia J, Li X-Q, Chan S-Y, Costello CE. Tandem mass spectrometric strategies for determination of sulfation positions and uronic acid epimerization in chondroitin sulfate oligosaccharides. J Am Soc Mass Spectrom. 2003;14:1270–1281. doi: 10.1016/S1044-0305(03)00541-5. [DOI] [PubMed] [Google Scholar]
- Zaia J, McClellan JE, Costello CE. Tandem mass spectrometric determination of the 4S/6S sulfation sequence in chondroitin sulfate oligosaccharides. Anal Chem. 2001;73:6030–6039. doi: 10.1021/ac015577t. [DOI] [PubMed] [Google Scholar]
- Zarei M, Kirsch S, Muthing J, Bindila L, Peter-Katalinic J. Automated normal phase nano high performance liquid chromatography/matrix assisted laser desorption/ionization mass spectrometry for analysis of neutral and acidic glycosphingolipids. Anal Bioanal Chem. 2008;391:289–297. doi: 10.1007/s00216-008-1932-0. [DOI] [PubMed] [Google Scholar]
- Zhao C, Xie B, Chan SY, Costello CE, O’Connor PB. Collisionally activated dissociation and electron capture dissociation provide complementary structural information for branched permethylated oligosaccharides. J Am Soc Mass Spectrom. 2008;19:138–150. doi: 10.1016/j.jasms.2007.10.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







