Abstract
The organization of cellular niches has been shown to play a key role in regulating normal stem cell differentiation and regeneration, yet relatively little is known about the architecture of microenvironments that support malignant metastasis.1,2 Using dynamic in vivo confocal imaging, we show that the murine bone marrow (BM) contains unique anatomic regions defined by specialized endothelium. This vasculature expresses the adhesion molecule E-selectin and the chemoattractant SDF-1 in discrete, discontinuous areas that localize the homing of a variety of tumor cell lines. Disruption of SDF-1/CXCR4 interactions inhibits Nalm-6 cell (acute lymphoblastic leukaemia) homing to these vessels. Further studies revealed that circulating leukemic cells engraft surrounding these vessels, suggesting that this molecularly distinct vasculature denotes a microenvironment for early metastatic tumor spread in BM. Finally, purified hematopoietic stem/progenitor cells and lymphocytes also localize to the same microdomains, indicating that this vasculature may function in benign states to demarcate specific portals for entry of cells into the marrow space. Specialized vascular structures therefore appear to delineate a microenvironment with unique physiology that is exploited by circulating malignant cells.
It has been thought that tumor cells derive their ability to transit to specific organs by co-opting the same tissue-homing mechanisms used by benign leukocytes.3 Substantial in vitro and more limited in vivo data provide evidence that tumors depend on selectin-, integrin-, and chemokine-mediated vascular cell adhesion events in order to identify and bind to vascular beds at sites of tissue entry.4,5 These molecular mechanisms are thought to enable the efficient spread of malignancies to target organs. Differential expression of these endothelial signals among tissues is known to control the destination of cellular traffic, but the contributions of the vascular molecular framework to the regulation of complex cellular microenvironments remain to be fully elucidated.
The bone marrow (BM) is a frequent site for solid tumor spread. It can also be considered the most ubiquitous site for leukemic cell metastasis, as disease is seen to migrate from the initial birthplace of the leukemic clone to marrow spaces in distant sites throughout the body. These observations suggest that BM provides an avid environment for circulating tumor lodgement and growth. Moreover, the BM is commonly the source of latent or “minimal residual disease” following treatment, raising the possibility that specific anti-apoptotic “niches” for metastatic growth may exist. Understanding the biologic architecture of this host microenvironment therefore has significant implications for our approach to tumor treatment.
While a variety of in vitro and in vivo techniques exist to study cell transit through BM, these are limited in their ability to assess the spatial and temporal relationship of cells. To examine the dynamic interactions of intravenously-injected tumor cells with the BM microenvironment, we imaged fluorescently-labelled cells using in vivo confocal microscopy. Since the cortex of the mouse skull is relatively thin, imaging of the underlying BM can be performed on the skull with minimal manipulation.6 In addition, the calvarium and other flat bones represent a significant BM compartment, contributing approximately 45% of the hematopoietically-active marrow in the adult.7
In our initial experiments, we sought to test the hypothesis that leukemic cells could be observed to interact with the vascular endothelium in a fashion mimicking the the multi-step tissue-homing mechanisms of their benign leukocyte counterparts. To our surprise, progressive scanning and optical sectioning through the marrow revealed the existence of unique, spatially-restricted vascular domains to which the majority of marrow-homing Nalm-6 pre-B acute lymphoblastic leukaemia (ALL) cells arrested (Fig. 1a–b). Using video-rate imaging, we observed that leukemic cells rolled along and bound this endothelium minutes after injection (Supplementary Videos 1–4).8 Serial imaging of mice on days 3, 10 (Fig.1 c–d) and 14 (data not shown) demonstrated that Nalm-6 ALL diapedesed at these sites and increased in numbers in these perivascular locations. This localization of tumor cells to specific regions was not restricted to Nalm-6. Other cell lines including human (REH, RS4;11) and murine (300-19) leukemias as well as multiple myeloma (U266) and solid tumors (MatLyLu prostatic carcinoma) homed to the same vascular microdomains (see Supplementary Fig. 1).
Figure 1.
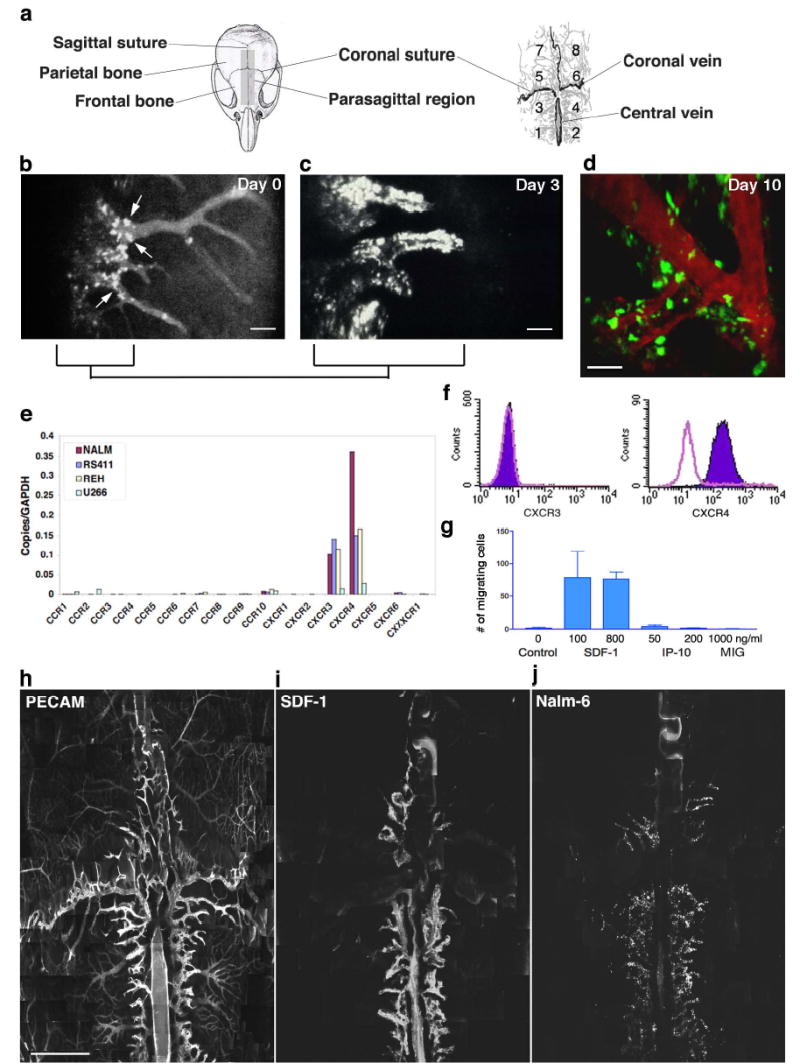
Leukemic cell homing/engraftment in mouse skull BM in vivo. a. Cartoons of the mouse skull and vascular anatomy, divided into quadrants for reference. b. Nalm-6 cells bound to parasagittal vascular segments (arrows) 1h post-injection (area 4). Scale bar = 200 μm. c–d. Peri-vascular cell growth 3 and 10 days post-injection. Scale bars = 100 μm. e. Quantitative RT-PCR of Nalm-6 and other tumor cell lines reveals abundant mRNA for CXCR4 and CXCR3. f. Flow cytometry of Nalm-6 demonstrates surface expression of CXCR4 only. g. In vitro chemotaxis of Nalm-6 toward the CXCR ligand SDF-1 but not toward the CXCR3 ligands IP-10 or MIG. Error bars represent standard error of the mean. h. BM vasculature labelled with anti-CD31 (assembled from approximately 500 images, scale bar = 1 mm). Signal dampening in lateral regions is due to variations in tissue optical properties (Supplementary Fig. 10). i. Montage image assembled from a separate mouse showing restricted SDF-1 expression (see also Supplementary Fig. 3, PECAM-1/SDF-1 co-label). j. Nalm-6 homing to SDF-1-expressing regions in this montage image from a third mouse.
Given these observations, we hypothesized that the endothelium in these BM regions expressed a unique combination of vascular cell adhesion molecules and/or chemokines capable of attracting a broad variety of tumor metastases. Because of the large number of chemokines defined to date, we narrowed our evaluation by first defining the chemokine receptors expressed in common by various cell lines that homed to these regions. Quantitative RT-PCR studies revealed that mRNA for the chemokine receptors CXCR3 and CXCR4 are produced in common by these cells (Fig. 1e). No cell surface CXCR3 receptor was detected by flow cytometry of Nalm-6, however, while CXCR4 receptors were abundant (Fig. 1f). In vitro transwell chemotaxis assays confirmed that Nalm-6 cells are not responsive to CXCR3 ligands, yet migrate effectively to the sole chemokine known to bind CXCR4, SDF-1. (Fig. 1g). These studies defined SDF-1 as a candidate chemokine mediating Nalm-6 homing to these vascular regions. This result was particularly compelling as SDF-1 has been shown to play a role in BM engraftment for a diversity of malignant tumors, including Nalm-6.9–15
To examine SDF-1 chemokine expression within BM, we performed immunoimaging of SDF-1 in vivo via in situ labelling of cell surface antigens with intravenously-injected fluorescent antibodies (J.M. Runnels et al., manuscript submitted). While SDF-1 is known to be produced by BM stromal cells, mediating B-lymphopoiesis and myelopoiesis in addition to functioning as chemoattractant, little is known about its localization within the marrow space.16,17 We found that SDF-1 was expressed in vascular “hot spots” corresponding to the regions that attracted leukemic cells (Fig. 1h–j, Supplementary Figs. 2 and 3). The majority of these SDF-1-positive vessels were located parasagittally within the frontal and parietal bones. This parasagittal SDF-1-positive vasculature consisted of sinusoidal networks as well as larger venules leading into these capillary beds. Conversely, bilateral para-coronal sinusoidal networks possessing similar size and flow characteristics as the parasagittal sinusoidal beds were SDF-1-negative. Therefore, SDF-1 expression was not strictly vessel size or flow dependent.
Using this immunoimaging approach, we then examined the regional distribution of various vascular cell adhesion molecules known to be expressed in BM.18 While ICAM-1, VCAM-1, PECAM-1 and P-selectin were expressed diffusely throughout the marrow vasculature (Fig. 2a–e), E-selectin expression corresponded to the pattern of SDF-1-positivity, and was distinctly limited to vessels that supported leukemic cell engraftment (Fig. 2f–l). Paralleling SDF-1 localization, E-selectin terminated abruptly along individual venules as one moved laterally away from the central axis of the sinusoidal vasculature. Interestingly, E-selectin upregulation was dramatically demarcated to the extent that E-selectin was expressed on only one wall of certain microvessels (Fig. 2g–i). These results were confirmed by repeating our in vivo immunoimaging studies of PECAM-1, SDF-1 and E-selectin expression using alternative antibody clones (see Methods for details).
Figure 2.
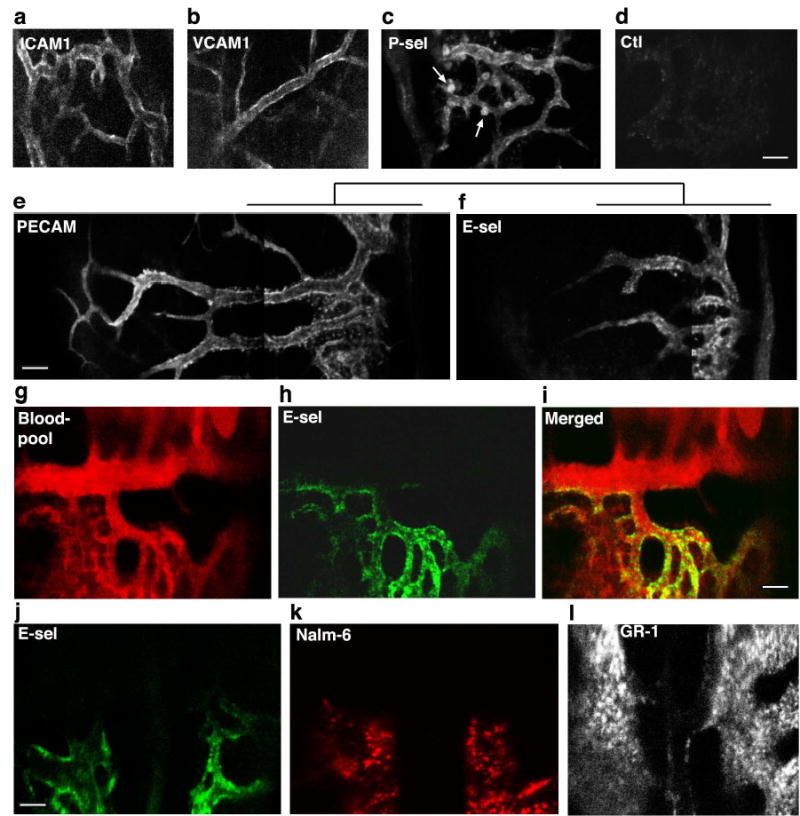
In vivo immunofluorescence microscopy of vascular CAM expression in BM. a–c. Representative images of ICAM-1 (area 6), VCAM-1 (area 8) and P-selectin (area 4). Each are expressed throughout the BM vasculature. Arrows indicate P-selectin+ megakaryocytes. d. Isotype control. e–f. Montage images of extended PECAM-1 expression vs. restricted E-selectin expression (both area 3). g–i. In this image series from area 4/6, the BM blood pool (red) is delineated by a non-specific fluorescent antibody signal. E-selectin expression (green) is restricted to one wall of a large collecting vein and to sinusoidal microvasculature in area 4. j–l. Correspondence of E-selectin expression (green) with Nalm-6 (red) homing patterns. This is contrasted with anti-GR1 (myeloid differentiation antigen) staining of the same region (midline, intersection of areas 3–6). Scale bars = 100 μm.
Together, the immunoimaging experiments suggested that specific microvascular domains in the BM express high levels of both E-selectin and SDF-1. In vivo co-localization studies confirmed that Nalm-6 binding was restricted to these same E-selectin+SDF-1+ vessel beds (Fig. 3a–f). These observations raised the possibility that E-selectin and/or SDF-1 regulated this restricted leukemic cell homing.
Figure 3.
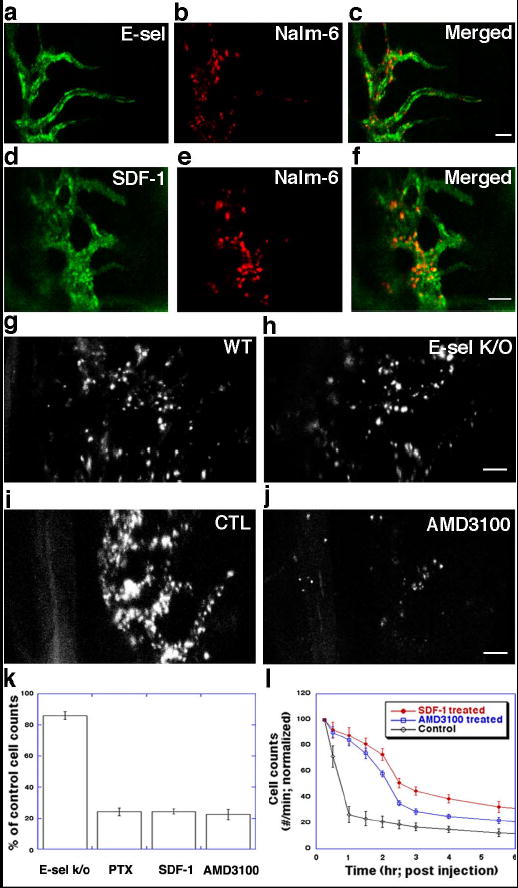
Leukemic cell homing to SDF-1+E-selectin+ microvascular domains is inhibited by SDF-1/CXCR4 blockade. a–c. Co-localization of E-selectin expression (green) and Nalm-6 cell homing (red, 30 minutes post-injection). (parasagittal region of area 4). d–f. Co-localization of SDF-1 vascular expression (green) and Nalm-6 cell homing (red, 30 minutes post-injection) (area 3). g–h. Cell homing is slightly decreased (<20%) in the E-selectin knockout mouse compared to wild-type control (1h post-injection). i–j. Nalm-6 homing is inhibited by AMD3100-CXCR4 blockade. k. Quantitation of effect of SDF-1/CXCR4 inhibition on cell homing to BM microdomains by pertussis toxin (PTX), SDF-1-desensitization and AMD3100. Error bars represent standard error of the mean. l. Enumeration of circulating leukemic cells by in vivo flow cytometry demonstrates that Nalm-6 blocked from BM homing by SDF-1-desensitization or single-dose AMD3100 remain in peripheral circulation. As the treatment effects wear off, cells correspondingly exit the circulation. Scale bars = 100 μm. Error bars represent standard error of the mean.
To test this hypothesis, we evaluated the functional role of E-selectin and CXCR4/SDF-1 interactions for leukemic homing to these specific regions. When Nalm-6 cells were injected in an E-selectin knockout mouse, we observed <20% reduction in homing to BM (Fig. 3g–h, k). In contrast, when cells were pre-treated with pertussis toxin, an inhibitor of chemokine receptor Gαi-mediated signaling, homing was decreased by almost 80% (Fig. 3k). CXCR4 down-regulation via SDF-1 desensitization (Fig. 3k, Supplementary Fig. 4) as well as highly specific CXCR4 receptor blockade by the small molecule inhibitor AMD3100 (Fig. 3i–k) yielded similar results.19 These data suggest that while E-selectin may serve to enhance homing, the majority of Nalm-6 vascular adhesion events can occur in its absence. Loss of SDF-1, however, does not appear to be compensated for by other adhesive molecules. These results also suggest that SDF-1/CXCR4 interactions, known to be important for cell retention in the marrow matrix (see also Supplementary Fig. 5), are pivotal at the earliest stage of tumor metastasis in the BM, i.e. recognition and binding to permissive vasculature.20 While this mechanism has been suggested by in vitro data, it has not previously been observed in vivo.21,22
We next examined whether cells that were inhibited from homing to BM spread to alternative tissue compartments or remained in the peripheral circulation. To quantify leukemia homing kinetics, we applied in vivo flow cytometry, a novel technology to detect and count individual fluorescently-labelled cells flowing through peripheral vessels as a function of time post-injection.23 As shown in Fig.3Q, >70% of Nalm-6 cells exit the circulation in control mice within the first hour post-injection, consistent with the rapid homing to BM observed with microscopy. In contrast, <20% of cells treated with AMD3100 exit the circulation at this same time point, suggesting that cells inhibited from BM homing remained in the peripheral circulation. In a time course consistent with when drug effect would be expected to decline, cells correspondingly exited the circulation.24
Lastly, we have explored the normal function of these vascular regions. As it is known that SDF-1 is a potent hematopoietic stem/progenitor cell (HSPC) and lymphocyte chemoattractant and that blood-borne HSPC and lymphocytes traffic to BM, we hypothesized that circulating HSPC and lymphocytes might preferentially home to these same microdomains.25,26 Fluorescently-labelled HSPC isolated from donor balb/c mice were injected into recipient mice and intravital BM imaging was performed at multiple time points. Representative images from 2h post-injection show HSPC adherent to BM microvasculature in the same restricted domains as SDF-1 vascular expression (Fig. 4a–c). 70 days post-injection (Fig. 4d), populations of labelled cells are still detected in the BM extravascular space within these microdomains. Although we cannot completely rule out the possibility that the observed fluorescence signal comes from cell debris that has been phagocytosed by other cells (e.g. macrophages), the morphology and persistence of dye are consistent with the engraftment of a primitive or stem cell pool with slow cell cycling. Flow cytometry studies of cultured cells confirmed the stability of membrane dye signal in slowly-dividing cells, while rapidly-dividing cells diluted dye to undetectable levels after 4–5 cell divisions (Supplementary Fig. 6). In another set of experiments, T lymphocytes isolated from the spleen and lymph nodes of a balb/c mouse were labelled and injected intravenously in a SCID recipient (Supplementary Fig. 7). Again, cells homed immediately to the same microdomains and were observed within these areas two weeks later. These observations suggest that benign circulating HSPC, mature T lymphocytes and leukemic cells use the same vascular subregions for localization in BM. Whether these unique vascular domains intersect or interact with the previously defined HSC BM endosteal cellular niche is not yet defined. In addition, whether benign and malignant cells utilize overlapping or distinct molecular features to identify these vascular sites is the subject of ongoing experiments, though our preliminary data demonstrate that benign T lymphocyte BM homing is only minimally inhibited by AMD3100/CXCR4 blockade. Moreover, results from other investigators suggest that HSPC are only modestly inhibited from homing to BM by AMD3100.27 Should such differences in how these and other benign and malignant cells recognize BM microenvironments be confirmed, these could be exploited to thwart metastasis while minimizing effects on normal BM function.
Figure 4.
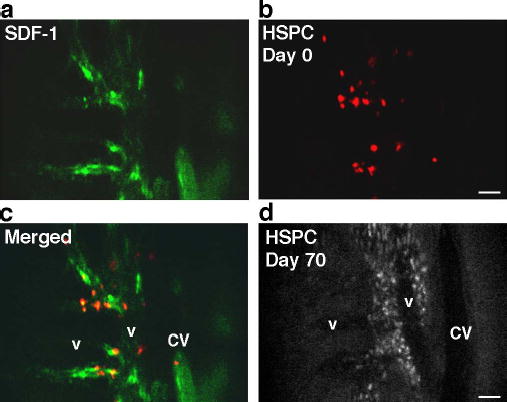
HSPC homing to SDF-1-positive vascular microdomains. a–c. Co-localization of SDF-1 (green) and HSPC (red) 2h post-injection (parasagittal region of area 3). d. The same mouse imaged 70 days post-HSPC injection (approximately the same area as in a–c). Although fluorescence signal intensity is diminished due to HSPC proliferation, engrafted cells are apparent in the perivascular spaces. Scale bars = 100 μm. CV=central vein, v=venule.
It has long been believed that early micrometastases from liquid and solid tumors occur in specific anatomic compartments of the BM, although no molecular basis for such regionalization has been established.28 While many investigations have explored the role of various cell adhesion molecules and chemokines in cell homing to bone marrow, studies based on traditional methods have been limited by spatial and temporal sampling restrictions. Elegant studies using bioluminescence imaging have provided temporal information, but are restricted by limitations of spatial resolution.29 Our approach has allowed us to combine a high degree of spatial and temporal resolution with an in vivo molecular labelling technique. Using this approach, we have demonstrated that the BM microvasculature is comprised of functional domains characterized by the expression of specific vascular cell surface adhesion molecules and chemoattractants crucial for malignant cell homing to these areas. In the benign setting, these distinct domains appear to define sites at which circulating hematopoietic stem/progenitor cells and lymphocytes enter the BM. Of note, other molecules that are more diffusely expressed may still contribute to the localized homing and engraftment processes that we have observed. Our in vivo imaging and flow cytometry approaches should be widely applicable to the study of these and other molecules (see Supplementary Figs. 8 and 9).
Whether these patterns of molecular expression represent a response to ongoing signals from adjacent stromal or hematopoietic elements or reflect embryologic patterning is not yet known. While we have focused our studies on the calvarial flat bone, the molecular and functional regionalization of BM vasculature and the capability of these domains to sustain cell engraftment may not be identical in all marrow spaces.29 Since flat and long bones form via different processes (intramembranous vs. endochondral ossification), it will be particularly interesting to investigate whether marrow cavities that arise through different developmental pathways show unique vascular molecular patterning.
Since these BM vascular gateways also appear to denote favorable environments for tumor engraftment, their targeted blockade could protect the marrow from continued metastasis in settings where residual disease is suspected. A further understanding of how these regions might foster cell growth and whether malignant vs. benign cells compete for entry or survival within these BM microdomains could thus provide novel points of intervention for the treatment of metastatic disease.
METHODS
BALB/c or SCID mice were anesthetized and a small incision made in the scalp so as to expose the underlying dorsal skull surface. For cell tracking, 5 £ 106 leukaemic cells labelled with the fluorescent lipophilic tracers DiD or DiR (Molecular Probes) were injected into the tail vein. Leukaemic cell homing to bone marrow vasculature of the skull was then imaged using a custom-built fluorescence confocal/multiphoton microscope while the mice were under anaesthesia on a warmed microscope stage. For in vivo immunofluorescence imaging, antibodies were either purchased as fluorescent conjugates or were conjugated to fluorescent cyanine compounds (Amersham) or to AlexaFluor750 (Molecular Probes) according to the manufacturers’ protocols. Labelled antivascular cell adhesion or isotype control antibodies were injected into mice through the tail vein at doses of 0.5–1 mg kg21. Approximately 24 h were allowed to elapse before imaging in order to maximize antibody binding and clearance of unbound label from the circulation. High-resolution images with cellular details were obtained through the intact mouse skull at depths of up to 250 mm from the surface of the skull using an £ 30 0.9NA water immersion objective lens. Quantitative evaluation was made by dividing the bone marrow into pre-determined quadrants and counting the number of fluorescent cells per field. Please refer to the Supplementary Methods for descriptions of the imaging system and the in vivo flow cytometer, and for additional information on cell purification, cell labelling, vascular CAM immunostaining, serial imaging, colocalization, quantitative PCR, chemotaxis assays, CXCR4 desensitization by SDF-1 and CXCR4 blockade by AMD3100.
Supplementary Material
Acknowledgments
We thank I. Alley, G. Adams, R. Klein and D. Worhunsky for help with stem cell harvesting and purification and C. Pitsillides for assistance with T cell purification techniques. We are grateful to D. Dombkowski for expert assistance with cell sorting. Special thanks to S. Harvey for aid with illustrations and to A. Chenn for his careful review of the manuscript. This work was supported by National Institute of Health (NIH) grants EB000664 (C.L.) and 5 P50 CA86355-04 (D.T.S.), NIH training grant 5 T32 CA071345-07 (D.A.S.) and a Whitaker Foundation Graduate Fellowship (J.W.)
Footnotes
Supplementary Information accompanies the paper on www.nature.com/nature.
Competing interests statement The authors declare that they have no competing financial interests.
References
- 1.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: Stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 2.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–72. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 3.Honn KV, Tang DG. Adhesion molecules and tumor cell interaction with endothelium and endothelial matrix. Cancer and Metastasis Reviews. 1992;11:353–375. doi: 10.1007/BF01307187. [DOI] [PubMed] [Google Scholar]
- 4.Burger JA, Kipps TJ. Chemokine receptors and stromal cells in the homing and homeostasis of chronic lymphocytic leukemia B cells. Leukemia & Lymphoma. 2002;43:461–466. doi: 10.1080/10428190290011921. [DOI] [PubMed] [Google Scholar]
- 5.Reuss-Borst MA, Klein G, Waller HD, Muller CA. Differential expression of adhesion molecules in acute leukemia. Leukemia. 1995;9:869–874. [PubMed] [Google Scholar]
- 6.Mazo IB, et al. Hematopoietic progenitor cell rolling in bone marrow microvessels: Parallel contributions by endothelial selectins and vascular cell adhesion molecule 1. Journal of Experimental Medicine. 1998;188:465–474. doi: 10.1084/jem.188.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cristy M. Active bone marrow distribution as a function of age in humans. Phys Med Biol. 1981:26389–400. doi: 10.1088/0031-9155/26/3/003. [DOI] [PubMed] [Google Scholar]
- 8.Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–6. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 9.Muller A, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 10.Murphy PM. Chemokines and the molecular basis of cancer metastasis. New England Journal of Medicine. 2001;345:833–5. doi: 10.1056/NEJM200109133451113. [DOI] [PubMed] [Google Scholar]
- 11.Shen W, Bendall LJ, Gottlieb DJ, Bradstock KF. The chemokine receptor CXCR4 enhances integrin-mediated in vitro adhesion and facilitates engraftment of leukemic precursor-B cells in the bone marrow. Experimental Hematology. 2001;29:1439–1447. doi: 10.1016/s0301-472x(01)00741-x. [DOI] [PubMed] [Google Scholar]
- 12.Tavor S, et al. CXCR4 regulates migration and development of human acute myelogenous leukemia stem cells in transplanted NOD/SCID mice. Cancer Research. 2004;64:2817–24. doi: 10.1158/0008-5472.can-03-3693. [DOI] [PubMed] [Google Scholar]
- 13.Spiegel A, et al. Unique SDF-1-induced activation of human precursor-B All cells as a result of altered CXCR4 expression and signaling. Blood. 2004;103:2900–7. doi: 10.1182/blood-2003-06-1891. [DOI] [PubMed] [Google Scholar]
- 14.Darash-Yahana M, et al. Role of high expression levels of CXCR4 in tumor growth, vascularization, and metastasis. FASEB J. 2004 doi: 10.1096/fj.03-0935fje. [DOI] [PubMed] [Google Scholar]
- 15.Spano JP, et al. Chemokine receptor CXCR4 and early-stage non-small cell lung cancer: pattern of expression and correlation with outcome. Ann Oncol. 2004;15:613–7. doi: 10.1093/annonc/mdh136. [DOI] [PubMed] [Google Scholar]
- 16.Ma Q, et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci USA. 1998;95:9448–53. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peled A, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–8. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 18.Mazo IB, von Andrian UH. Adhesion and homing of blood-borne cells in bone marrow microvessels. J Leukoc Biol. 1999;66:25–32. doi: 10.1002/jlb.66.1.25. [DOI] [PubMed] [Google Scholar]
- 19.Hatse S, Princen K, Bridger G, De Clercq E, Schols D. Chemokine receptor inhibition by AMD3100 is strictly confined to CXCR4. FEBS Lett. 2002;527:255–62. doi: 10.1016/s0014-5793(02)03143-5. [DOI] [PubMed] [Google Scholar]
- 20.Liles WC, et al. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood. 2003;102:2728–30. doi: 10.1182/blood-2003-02-0663. [DOI] [PubMed] [Google Scholar]
- 21.Peled A, et al. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34+ cells on vascular endothelium under shear flow. JCI. 1999;104:1199–1211. doi: 10.1172/JCI7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jo DY, Hwang JH, Kim JM, Yun HJ, Kim S. Human bone marrow endothelial cells elaborate non-stromal-cell-derived factor-1 (SDF-1)-dependent chemoattraction and SDF-1-dependent transmigration of hematopoietic progenitors. Br J Haematol. 2003;121:649–52. doi: 10.1046/j.1365-2141.2003.04326.x. [DOI] [PubMed] [Google Scholar]
- 23.Novak J, Georgakoudi I, Wei X, Prossin A, Lin CP. In vivo flow cytometer for real-time detection and quantification of circulating cells. Opt Lett. 2004;29:77–79. doi: 10.1364/ol.29.000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hendrix CW, et al. Pharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteers. Antimicrobial Agents and Chemotherapy. 2000;44:1667–73. doi: 10.1128/aac.44.6.1667-1673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–30. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christopherson KW, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305:1000–3. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- 28.Mastro AM, Gay CV, Welch DR. The skeleton as a unique environment for breast cancer cells. Clin Exp Metastasis. 2003;20:275–284. doi: 10.1023/a:1022995403081. [DOI] [PubMed] [Google Scholar]
- 29.Cao YA, et al. Shifting foci of hematopoiesis during reconstitution from single stem cells. Proc Natl Acad Sci USA. 2004;101:221–6. doi: 10.1073/pnas.2637010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen H, et al. CXCR-4 desensitization is associated with tissue localization of hemopoietic progenitor cells. J Immunol. 2001;166:5027–33. doi: 10.4049/jimmunol.166.8.5027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


