Abstract
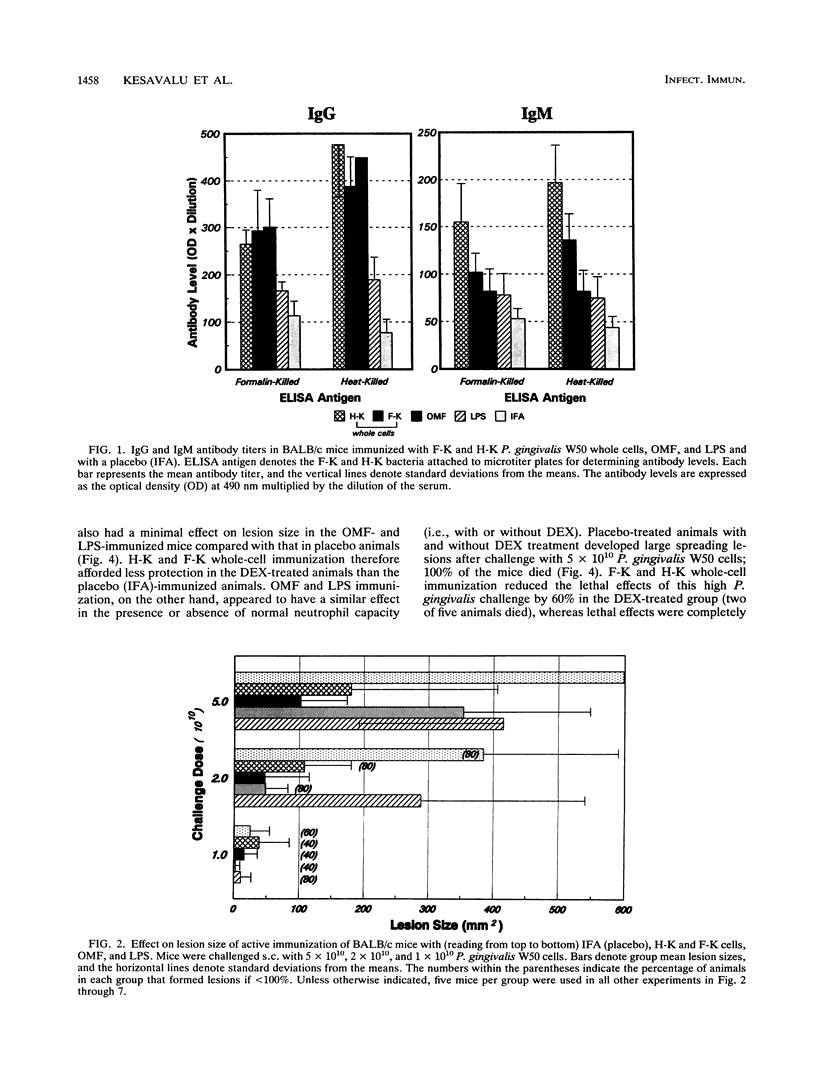
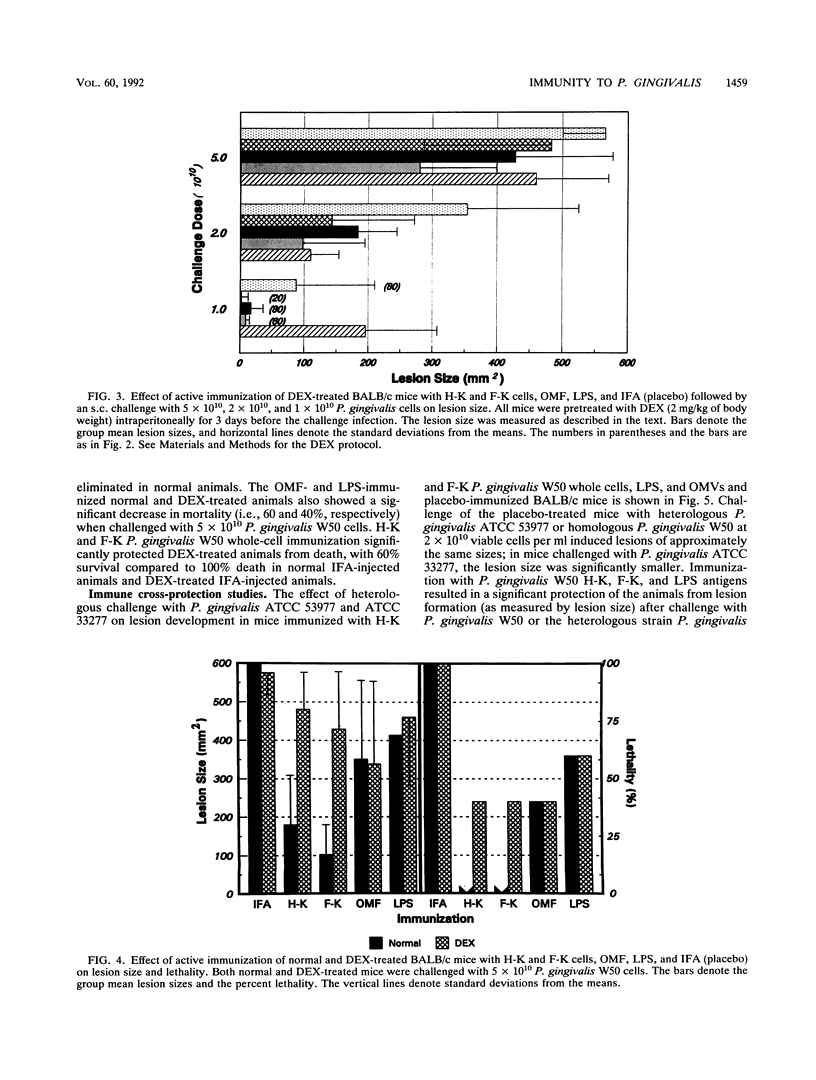
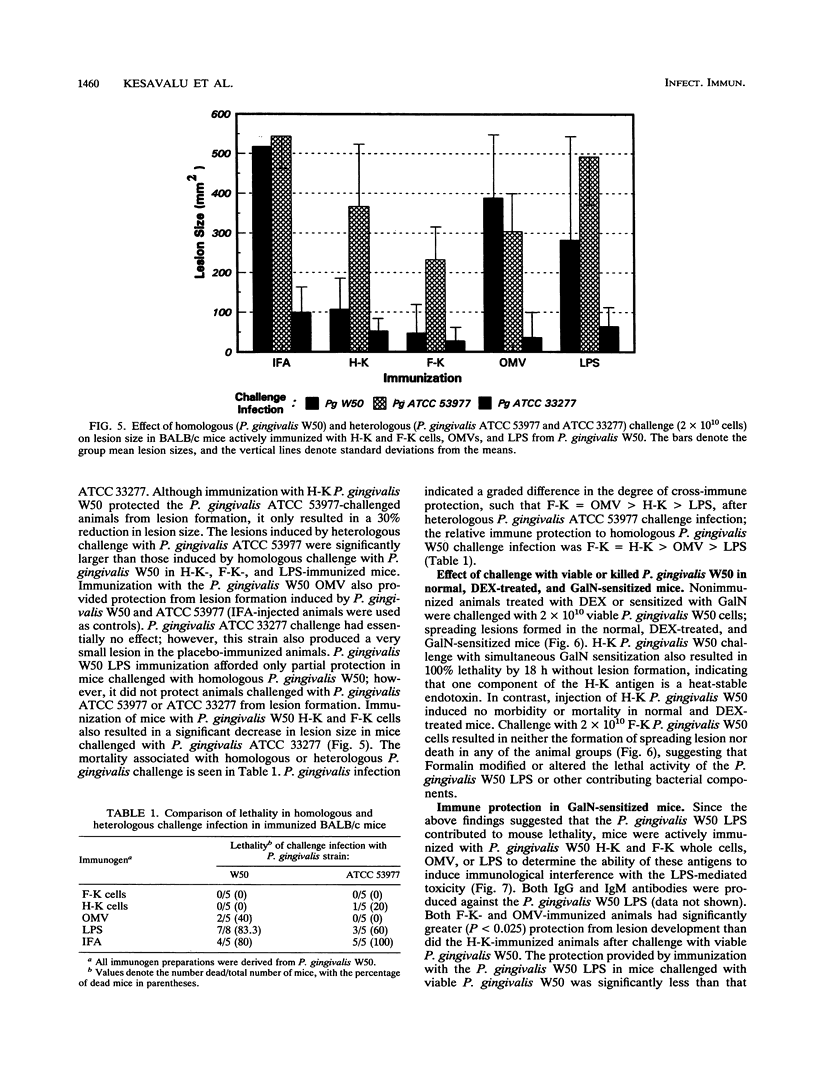
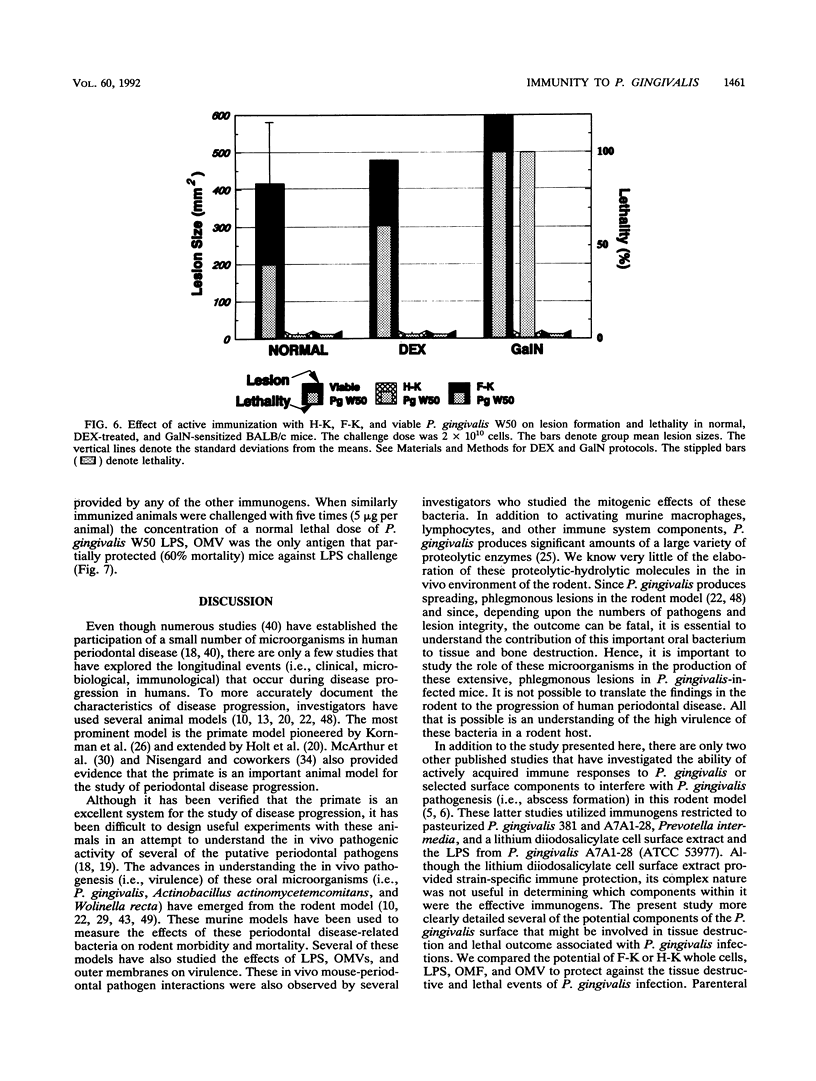
Selected cell envelope components of Porphyromonas gingivalis were tested in a BALB/c mouse model in an attempt to elucidate further the outer membrane components of this putative oral pathogen that might be considered as virulence factors in host tissue destruction. Lipopolysaccharide (LPS), outer membrane, and outer membrane vesicles of P. gingivalis W50, ATCC 53977, and ATCC 33277 were selected to examine an immunological approach for interference with progressing tissue destruction. Mice were actively immunized with heat-killed (H-K) or Formalin-killed (F-K) whole cells or with the outer membrane fraction, LPS, or outer membrane vesicles of the invasive strain P. gingivalis W50. The induction of invasive spreading lesions with tissue destruction and lethality were compared among different immunization groups in normal, dexamethasone-treated (dexamethasone alters neutrophil function at the inflammatory site), and galactosamine-sensitized (galactosamine sensitization increases endotoxin sensitivity) mice after challenge infection with the homologous strain (W50) and heterologous strains (ATCC 53977 and ATCC 33277). Enzyme-linked immunosorbent assay analyses revealed significantly elevated immunoglobulin G and M antibody responses after immunization with H-K or F-K cells or the outer membrane fraction compared with those of nonimmunized mice. The killed whole-cell vaccines provided significantly greater protection against challenge infection in normal mice (decreased lesion size and death) than did either the outer membrane fraction or LPS immunization. The lesion development observed in dexamethasone-pretreated mice was significantly enhanced compared with that of normal mice after challenge with P. gingivalis. Immunization with P. gingivalis W50 provided less protection against heterologous challenge infection with P. gingivalis ATCC 53977; however, some species-specific antigens were recognized and induced protective immunity. Only viable P. gingivalis induced a spreading lesion in normal, dexamethasone-treated, or galactosamine-sensitized mice; F-K or H-K bacteria did not induce lesions. The F-K and outer membrane vesicle immunization offered greater protection from lesion induction than did the H-K immunogen after challenge infection simultaneous with galactosamine sensitization. The H-K cell challenge with galactosamine sensitization produced 100% mortality without lesion induction, suggesting that LPS or LPS-associated outer membrane molecules were functioning like endotoxin. Likewise, P. gingivalis W50 LPS (1 micrograms per animal) administered intravenously produced 80% mortality in galactosamine-sensitized mice. In contrast to the effects of immunization on lesion development, immunization with H-K or F-K cells or LPS provided no protection against intravenous challenge with LPS; 100% of the mice died from acute endotoxin toxicity.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baranowska H. I., Palmer R. M., Wilson R. F. A comparison of antibody levels to Bacteroides gingivalis in serum and crevicular fluid from patients with untreated periodontitis. Oral Microbiol Immunol. 1989 Sep;4(3):173–175. doi: 10.1111/j.1399-302x.1989.tb00247.x. [DOI] [PubMed] [Google Scholar]
- Bosseray N. Immunity to Brucella in mice vaccinated with a fraction (F8) or a killed vaccine (H38) with or without adjuvant. Level and duration of immunity in relation to dose of vaccine, recall injection and age of mice. Br J Exp Pathol. 1978 Aug;59(4):354–365. [PMC free article] [PubMed] [Google Scholar]
- Bramanti T. E., Holt S. C. Iron-regulated outer membrane proteins in the periodontopathic bacterium, Bacteroides gingivalis. Biochem Biophys Res Commun. 1990 Feb 14;166(3):1146–1154. doi: 10.1016/0006-291x(90)90986-w. [DOI] [PubMed] [Google Scholar]
- Chen P. B., Davern L. B., Schifferle R., Zambon J. J. Protective immunization against experimental Bacteroides (Porphyromonas) gingivalis infection. Infect Immun. 1990 Oct;58(10):3394–3400. doi: 10.1128/iai.58.10.3394-3400.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P. B., Neiders M. E., Millar S. J., Reynolds H. S., Zambon J. J. Effect of immunization on experimental Bacteroides gingivalis infection in a murine model. Infect Immun. 1987 Oct;55(10):2534–2537. doi: 10.1128/iai.55.10.2534-2537.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzink J. L., Gibbons R. J., Childs W. C., 3rd, Socransky S. S. The predominant cultivable microbiota of crevicular epithelial cells. Oral Microbiol Immunol. 1989 Mar;4(1):1–5. doi: 10.1111/j.1399-302x.1989.tb00398.x. [DOI] [PubMed] [Google Scholar]
- Ebersole J. L., Frey D. E., Taubman M. A., Smith D. J. An ELISA for measuring serum antibodies to Actinobacillus actinomycetemcomitans. J Periodontal Res. 1980 Nov;15(6):621–632. doi: 10.1111/j.1600-0765.1980.tb00321.x. [DOI] [PubMed] [Google Scholar]
- Ebersole J. L., Taubman M. A., Smith D. J., Frey D. E. Human immune responses to oral microorganisms: patterns of systemic antibody levels to Bacteroides species. Infect Immun. 1986 Feb;51(2):507–513. doi: 10.1128/iai.51.2.507-513.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errasfa M., Russo-Marie F. A purified lipocortin shares the anti-inflammatory effect of glucocorticosteroids in vivo in mice. Br J Pharmacol. 1989 Aug;97(4):1051–1058. doi: 10.1111/j.1476-5381.1989.tb12561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T., Ogawa T., Sobue S., Hamada S. Chemical, immunobiological and antigenic characterizations of lipopolysaccharides from Bacteroides gingivalis strains. J Gen Microbiol. 1990 Feb;136(2):319–326. doi: 10.1099/00221287-136-2-319. [DOI] [PubMed] [Google Scholar]
- Galanos C., Freudenberg M. A., Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier D., Mayrand D. Functional characterization of extracellular vesicles produced by Bacteroides gingivalis. Infect Immun. 1987 Jan;55(1):111–117. doi: 10.1128/iai.55.1.111-117.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier D., Mayrand D. Selected characteristics of pathogenic and nonpathogenic strains of Bacteroides gingivalis. J Clin Microbiol. 1987 Apr;25(4):738–740. doi: 10.1128/jcm.25.4.738-740.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C., Bramanti T. E. Factors in virulence expression and their role in periodontal disease pathogenesis. Crit Rev Oral Biol Med. 1991;2(2):177–281. doi: 10.1177/10454411910020020301. [DOI] [PubMed] [Google Scholar]
- Holt S. C., Ebersole J., Felton J., Brunsvold M., Kornman K. S. Implantation of Bacteroides gingivalis in nonhuman primates initiates progression of periodontitis. Science. 1988 Jan 1;239(4835):55–57. doi: 10.1126/science.3336774. [DOI] [PubMed] [Google Scholar]
- Kastelein P., van Steenbergen T. J., Bras J. M., de Graaff J. An experimentally induced phlegmonous abscess by a strain of Bacteroides gingivalis in guinea pigs and mice. Antonie Van Leeuwenhoek. 1981 Mar;47(1):1–9. doi: 10.1007/BF00399062. [DOI] [PubMed] [Google Scholar]
- Kennell W., Holt S. C. Comparative studies of the outer membranes of Bacteroides gingivalis, strains ATCC 33277, W50, W83, 381. Oral Microbiol Immunol. 1990 Jun;5(3):121–130. doi: 10.1111/j.1399-302x.1990.tb00409.x. [DOI] [PubMed] [Google Scholar]
- Keppler D., Decker K. Studies on the mechanism of galactosamine-1-phosphate and its inhibition of UDP-glucose pyrophosphorylase. Eur J Biochem. 1969 Sep;10(2):219–225. doi: 10.1111/j.1432-1033.1969.tb00677.x. [DOI] [PubMed] [Google Scholar]
- Kornman K. S., Holt S. C., Robertson P. B. The microbiology of ligature-induced periodontitis in the cynomolgus monkey. J Periodontal Res. 1981 Jul;16(4):363–371. doi: 10.1111/j.1600-0765.1981.tb00987.x. [DOI] [PubMed] [Google Scholar]
- Mansheim B. J., Stenstrom M. L., Low S. B., Clark W. B. Measurement of serum and salivary antibodies to the oral pathogen Bacteroides asaccharolyticus in human subjects. Arch Oral Biol. 1980;25(8-9):553–557. doi: 10.1016/0003-9969(80)90067-9. [DOI] [PubMed] [Google Scholar]
- Mayrand D., Grenier D. Biological activities of outer membrane vesicles. Can J Microbiol. 1989 Jun;35(6):607–613. doi: 10.1139/m89-097. [DOI] [PubMed] [Google Scholar]
- Mayrand D., Holt S. C. Biology of asaccharolytic black-pigmented Bacteroides species. Microbiol Rev. 1988 Mar;52(1):134–152. doi: 10.1128/mr.52.1.134-152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur W. P., Magnusson I., Marks R. G., Clark W. B. Modulation of colonization by black-pigmented Bacteroides species in squirrel monkeys by immunization with Bacteroides gingivalis. Infect Immun. 1989 Aug;57(8):2313–2317. doi: 10.1128/iai.57.8.2313-2317.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee A. S., McDermid A. S., Baskerville A., Dowsett A. B., Ellwood D. C., Marsh P. D. Effect of hemin on the physiology and virulence of Bacteroides gingivalis W50. Infect Immun. 1986 May;52(2):349–355. doi: 10.1128/iai.52.2.349-355.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton C., Hammond P. G., Slots J., Genco R. J. Serum antibodies to oral Bacteroides asaccharolyticus (Bacteroides gingivalis): relationship to age and periondontal disease. Infect Immun. 1981 Jan;31(1):182–192. doi: 10.1128/iai.31.1.182-192.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiders M. E., Chen P. B., Suido H., Reynolds H. S., Zambon J. J., Shlossman M., Genco R. J. Heterogeneity of virulence among strains of Bacteroides gingivalis. J Periodontal Res. 1989 May;24(3):192–198. doi: 10.1111/j.1600-0765.1989.tb02005.x. [DOI] [PubMed] [Google Scholar]
- Nisengard R., Blann D., Zelonis L., McHenry K., Reynolds H., Zambon J. Effects of immunization with B. macacae on induced periodontitis--preliminary findings. Immunol Invest. 1989 Jan-May;18(1-4):225–237. doi: 10.3109/08820138909112239. [DOI] [PubMed] [Google Scholar]
- Remick D. G., Strieter R. M., Eskandari M. K., Nguyen D. T., Genord M. A., Raiford C. L., Kunkel S. L. Role of tumor necrosis factor-alpha in lipopolysaccharide-induced pathologic alterations. Am J Pathol. 1990 Jan;136(1):49–60. [PMC free article] [PubMed] [Google Scholar]
- Roeterink C. H., van Steenbergen T. J., de Jong W. F., de Graaff J. Histopathological effects in the palate of the rat induced by injection with different black-pigmented Bacteroides strains. J Periodontal Res. 1984 May;19(3):292–302. doi: 10.1111/j.1600-0765.1984.tb00820.x. [DOI] [PubMed] [Google Scholar]
- Saito H., Watanabe T., Horikawa Y. Effects of Lactobacillus casei on Pseudomonas aeruginosa infection in normal and dexamethasone-treated mice. Microbiol Immunol. 1986;30(3):249–259. doi: 10.1111/j.1348-0421.1986.tb00940.x. [DOI] [PubMed] [Google Scholar]
- Schenck K., Helgeland K., Tollefsen T. Antibodies against lipopolysaccharide from Bacteroides gingivalis before and after periodontal treatment. Scand J Dent Res. 1987 Apr;95(2):112–118. doi: 10.1111/j.1600-0722.1987.tb01816.x. [DOI] [PubMed] [Google Scholar]
- Schifferle R. E., Reddy M. S., Zambon J. J., Genco R. J., Levine M. J. Characterization of a polysaccharide antigen from Bacteroides gingivalis. J Immunol. 1989 Nov 1;143(9):3035–3042. [PubMed] [Google Scholar]
- Slots J., Listgarten M. A. Bacteroides gingivalis, Bacteroides intermedius and Actinobacillus actinomycetemcomitans in human periodontal diseases. J Clin Periodontol. 1988 Feb;15(2):85–93. doi: 10.1111/j.1600-051x.1988.tb00999.x. [DOI] [PubMed] [Google Scholar]
- Sundqvist G., Figdor D., Hänström L., Sörlin S., Sandström G. Phagocytosis and virulence of different strains of Porphyromonas gingivalis. Scand J Dent Res. 1991 Apr;99(2):117–129. doi: 10.1111/j.1600-0722.1991.tb01874.x. [DOI] [PubMed] [Google Scholar]
- Syed S. A., Loesche W. J. Survival of human dental plaque flora in various transport media. Appl Microbiol. 1972 Oct;24(4):638–644. doi: 10.1128/am.24.4.638-644.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada H., Hirai H., Fujiwara T., Koga T., Ogawa T., Hamada S. Bacteroides lipopolysaccharides (LPS) induce anaphylactoid and lethal reactions in LPS-responsive and -nonresponsive mice primed with muramyl dipeptide. J Infect Dis. 1990 Aug;162(2):428–434. doi: 10.1093/infdis/162.2.428. [DOI] [PubMed] [Google Scholar]
- Taubman M. A., Yoshie H., Ebersole J. L., Smith D. J., Olson C. L. Host response in experimental periodontal disease. J Dent Res. 1984 Mar;63(3):455–460. doi: 10.1177/00220345840630031801. [DOI] [PubMed] [Google Scholar]
- Tiegs G., Wolter M., Wendel A. Tumor necrosis factor is a terminal mediator in galactosamine/endotoxin-induced hepatitis in mice. Biochem Pharmacol. 1989 Feb 15;38(4):627–631. doi: 10.1016/0006-2952(89)90208-6. [DOI] [PubMed] [Google Scholar]
- Van Dyke T. E., Levine M. J., Genco R. J. Neutrophil function and oral disease. J Oral Pathol. 1985 Feb;14(2):95–120. doi: 10.1111/j.1600-0714.1985.tb00474.x. [DOI] [PubMed] [Google Scholar]
- Van Steenbergen T. J., Delemarre F. G., Namavar F., De Graaff J. Differences in virulence within the species Bacteroides gingivalis. Antonie Van Leeuwenhoek. 1987;53(4):233–244. doi: 10.1007/BF00393930. [DOI] [PubMed] [Google Scholar]
- Wyss C., Guggenheim B. Effects of the association of conventional rats with Actinomyces viscosus Nyl and Bacteroides gingivalis W83. J Periodontal Res. 1984 Nov;19(6):574–577. doi: 10.1111/j.1600-0765.1984.tb01317.x. [DOI] [PubMed] [Google Scholar]
- Yoshimura F., Sugano T., Kawanami M., Kato H., Suzuki T. Detection of specific antibodies against fimbriae and membrane proteins from the oral anaerobe Bacteroides gingivalis in patients with periodontal diseases. Microbiol Immunol. 1987;31(9):935–941. doi: 10.1111/j.1348-0421.1987.tb03154.x. [DOI] [PubMed] [Google Scholar]
- van Steenbergen T. J., Kastelein P., Touw J. J., de Graaff J. Virulence of black-pigmented Bacteroides strains from periodontal pockets and other sites in experimentally induced skin lesions in mice. J Periodontal Res. 1982 Jan;17(1):41–49. doi: 10.1111/j.1600-0765.1982.tb01129.x. [DOI] [PubMed] [Google Scholar]




