Abstract
Objective
FLRF (Rnf41) gene was identified through screening of subtracted cDNA libraries form murine hematopoietic stem cells and progenitors. Subsequent work has revealed that FLRF acts as E3 ubiquitin ligase, and that it regulates steady-state levels of neuregulin receptor ErbB3, and participates in degradation of IAP protein BRUCE and parkin. The objective of this study was to start exploring the role of FLRF during hematopoiesis.
Methods
FLRF was over-expressed in a murine multipotent hematopoietic progenitor cell line EML, which can differentiate into almost all blood cell lineages, and in pro-B progenitor cell line BaF3. The impact of FLRF over-expression on EML cell differentiation into myelo-erythroid lineages was studied using hematopoietic colony-forming assays. The interaction of FLRF with cytokine receptors and receptor levels in control cells and EML and BaF3 cells over-expressing FLRF were examined with Western and immunoprecipitation.
Results
Remarkably, over-expression of FLRF significantly attenuated erythroid and myeloid differentiation of EML cells in response to cytokines Epo and IL-3, and retinoic acid (RA), and resulted in significant and constitutive decrease of steady-state levels of IL-3, Epo and RA receptor RARα in EML and BaF3 cells. Immunoprecipitation has revealed that FLRF interacts with IL-3, Epo and RARα receptors in EML and BaF3 cells, and that FLRF-mediated down-regulation of these receptors is ligand binding-independent.
Conclusions
The results of this study have revealed new FLRF-mediated pathway for ligand-independent receptor level regulation, and support the notion that through maintaining basal levels of cytokine receptors, FLRF is involved in the control of hematopoietic progenitor cell differentiation into myelo-erythroid lineages.
Keywords: Multipotent hematopoietic progenitors, Cytokine receptors, Retinoic acid, Myeloid and erythroid differentiation
Introduction
Differentiation of hematopoietic stem cells (HSC), multipotent progenitors (MPPs) and lineage-committed progenitors is regulated by diverse cytokines, including interleukins (ILs) and colony stimulating factors (CSFs) [1-5], which exert their biological effects on target cells through interaction with corresponding receptors. Regulation of the duration and level of cytokine signaling occurs at multiple levels, including regulation of the amount of receptors present before ligand binding. In general, ligand stimulation results in receptor internalization and degradation. Ligand binding-dependent degradation of receptors through ubiquitin system is believed to be a critical step in modulating their activity and plays a critical role in controlling receptor signaling [6].
Ubiquitin (Ub)-dependent proteolysis of cellular proteins impacts a broad range of cell functions (proliferation, differentiation, cell cycle progression, transcription, antigen presentation, receptor maintenance, signal transduction etc.) [7-10]. Defects in ubiquitin-dependent proteolysis are involved in etiology of developmental abnormalities, autoimmunity, neurodegenerative diseases, metabolic disorders, and cancer [11, 12].
Covalent attachment of ubiquitin to substrate proteins is a multistep process orchestrated by ubiquitin-activating enzymes (E1), ubiquitin-conjugating enzymes (E2), and ubiquitin protein ligases (E3). The high efficiency and exquisite selectivity of ubiquitination reactions depend on E3 ligases. Generally, E3 ligases contain protein–protein interaction domains that recognize substrate proteins, as well as catalytic domains that interact with E2 enzymes, thereby promoting ubiquitin transfer from the E2 to the substrate. The E3 ligases are classified into three groups: HECT domain E3 enzymes (e.g. Smurf, Nedd4), multisubunit RING finger protein complexes (e.g. SCF ubiquitin ligases, the VBC ubiquitin ligase and the anaphase-promoting (APC) complexes), and single RING-finger E3 enzymes (e.g. Cbl, Mdm2, and BRCA1) [7-9, 13-15].
Despite numerous advances in understanding of the ligand binding-dependent receptor down-regulation and degradation, relatively little is known about the mechanisms controlling ligand-independent down-regulation and degradation of receptors [16-18].
Through screening of cDNA libraries prepared from fetal liver HSC and progenitors, and differential screening of subtracted cDNA libraries prepared from mouse Lin-Sca-1+ and Lin-Sca-1- bone marrow (BM) cells, enriched for HSC and progenitors, we have identified new RING finger gene, named FLRF (Fetal Liver Ring Finger) (Rnf41) [19]. Mouse FLRF cDNA (Accession Nos. AF305730 and NM_026259) encodes a protein of 317 amino acids (Mw: ∼37 kDa) with N-terminal C3HC4 RING finger domain, a TRAF-type zinc finger domain, and a coiled-coil domain (19). Human FLRF cDNA encodes a RING finger protein identical to mouse FLRF protein [19].
Two subsequent studies have reported that human FLRF (Nrdp1) acts as E3 ubiquitin ligase, and that it interacts with and regulates steady-state levels of neuregulin ErbB3 and ErbB4 receptors by mediating their ubiquitination, thus influencing the neuregulin signaling [20, 21]. Additional studies have reported that FLRF (a) mediates degradation of the inhibitor of apoptosis gigantic protein BRUCE, and (b) accelerates degradation of parkin (whose loss of function leads to death of dopaminergic neurons in Parkinson disease), and regulates parkin stability and activity [22, 23].
To study its role in hematopoiesis FLRF was over-expressed in a murine multipotent hematopoietic progenitor cell line EML, which can differentiate into almost all blood cell lineages [24]. Over-expression of FLRF significantly attenuated erythroid and myeloid differentiation of EML cells in response to cytokines erythropoietin (Epo) and interleukin 3 (IL-3), and retinoic acid (RA). The excess amount of FLRF protein also led to a significant and constitutive decrease in the steady-state levels of IL-3, Epo and RA receptor RARα in EML cells and mouse pro-B progenitor cell line BaF3. Immunoprecipitation and Western analysis of proteins from EML and BaF3 cells have shown that FLRF protein associates with IL-3, Epo and RA receptors, and that FLRF-mediated down-regulation of IL-3, Epo and RA receptors is independent of the ligand binding. Our findings have revealed a new role of FLRF in regulating optimal steady-state levels of IL-3, Epo and RA receptors, necessary for proper differentiation of hematopoietic progenitors.
Materials and methods
Cells and Expression Vectors
Stem cell factor (SCF)-dependent EML cell line was maintained in IMDM medium (Gibco) with 20% equine serum (HyClone) and 10% SCF-conditioned medium from BHK/MKL cell line [24, 25]. Mouse pro-B cell line BaF3 was grown in RPMI medium supplemented with 10% FCS (HyClone, Logan, UT) and 10% WEHI-3B conditioned medium. Mouse pre-B cell line 70Z3 was grown in RPMI 1640 medium supplemented with 10% FCS and 0.05 mM 2-mercaptoethanol. Mouse FLRF cDNA [19] was subcloned into XhoI digested pcDNA4/TO vector (Invitrogen). The orientation of the insert was identified with NotI digest and automated DNA sequencing. To achieve stable transfection EML cells (107 cells) were electroporated with 20 μg of NruI linearized pcDNA4/TO or pcDNA4/TO/FLRF vectors. Stable EML transfectants were selected with Zeocin (Invitrogen) and maintained continuously under selection.
Hematopoietic Colony-forming Assays
To assess their myelo-erythroid differentiation, EML cells were cultured in 2 ml of 0.3% low melting temperature agarose (SeaKem) with 2X IMDM, 20% heat inactivated horse serum and 10% SCF-conditioned medium in replicate 6-well plates (103 cells/well). The media was supplemented with 0.1 ng/ml of recombinant mouse interleukin 3 (IL-3) (R&D Systems), 8U/ml of recombinant human erythropoietin (Epo) (Ortho Biotech), or 3ng/ml of recombinant mouse thrombopoietin (Tpo) (R&D Systems). The granulocyte/macrophage (CFU-GM), burst-forming unit erythroid (BFU-E) and megakaryocytic (CFU-Meg) colonies were counted 7-10 days after plating [1].
Western blotting and Immunoprecipitation
Antibodies used for Western blotting were: IL-3Rα (V-18), IL-3Rβ (K-19; detects the unique mouse IL-3R b chain and the b chain common to IL-3R, IL-5R and GM-CSFR), GM-CSFRα (M-20), EpoR (M-20) (Santa Cruz Biotechnology, Inc.), α-actin (Sigma), and α-ubiquitin (Calbiochem). The protein for SCF receptor c-kit was detected using goat α-c-kit (M-14, Santa Cruz) antibody. Polyclonal rabbit α-FLRF Abs 1123 and 1124 were obtained from Bethyl Inc. The expression of proteins for retinoic acid (RA) receptors RARα and RXR was examined by Western using RARα (C-20) and RXR (D20) Abs (Santa Cruz). The cells were lysed with buffer (2 mM Tris.Cl pH 8.0, 0.14 M NaCl, 1 mM PMSF, 0.1% Triton X-100 and protease inhibitors) at 4°C for 1 hour. Protein concentrations were determined by Pc protein Assay kit (Bio-Rad). Up to 50 μg of protein per lane was resolved on 12% Tris-glycine ready gel (Bio-Rad) and electro-transferred to Immuno-Blot PVDF membrane (Bio-Rad). The PVDF membranes were blocked with 5% dry milk in TBS-T buffer at RT for 1 hour, and incubated overnight at 4°C with primary antibodies at recommended dilutions. After washing with TBS-T the membranes were incubated with secondary antibodies HRP-Goat-anti-rabbit IgG or HRP-rabbit-anti-goat IgG (Zymed). The proteins were detected with ECL Western blotting analysis detection system (Amersham) or SuperSignal West Chemiluminescent subtrate for detection of HRP (Pierce). Where indicated, the membranes were stripped and re-probed with α-actin antibody (Sigma). For immunoprecipitation, the cells were resuspended in lysis buffer, and cell lysates precleared with Protein G or Protein A. About 1 mg of protein from equal cell numbers in lysis buffer was incubated with EpoR, IL-3Rβ, RARα or FLRF antibodies, and Protein A or G, and than washed with lysis buffer. The samples were resolved and processed identically to the Western blot procedure described above.
Results
FLRF over-expression attenuates differentiation of multipotent hematopoietic progenitors
To study its function during hematopoiesis we have over-expressed FLRF in a stem cell factor (SCF)-dependent multipotent hematopoietic cell line EML [24, 25]. EML cell line shares phenotypic and functional features with murine Lin- c-kit+ Sca-1+ (LKS) and LKS Flk2- bone marrow (BM) cells, which are enriched for hematopoietic stem cells (HSC) and multipotent progenitors (MPPs) [24, 25]. Similar to HSC and MPPs, almost all EML cells are Sca-1+c-kit+ Flk2-, and express all functionally relevant hematopoietic genes (e.g. Hoxb4, Meis1, Rae28, SCL, GATA-2, AML1, AML2, Ikaros, PU.1, Notch1, Bmi-1 etc.) [25-27].
In the presence of SCF EML cells undergo proliferative self-renewal and remain undifferentiated, whereas in the presence of cytokines and/or stroma EML cells differentiate into erythroid, myeloid and lymphoid lineages (Fig. 1) [24, 25, 28, 29]. For example, in colony-forming assays EML cells generate granulocyte/macrophage (CFU-GM), burst-forming unit erythroid (BFU-E) and megakaryocytic (CFU-Meg) colonies in the presence of IL-3 or GM-CSF, Epo, and Tpo, respectively (Fig. 1). In co-culture with stromal cell line W20 and IL-7, EML cells give rise to the pro-B cells that express RAG-1 and undergo D-J rearrangements [24]. In addition, we have discovered that in co-culture with OP9 stromal cells, expressing Notch ligand Delta-like 1[30], EML cells undergo T cell lineage commitment and differentiate into immature and mature T lymphocytes (manuscript in preparation).
Figure 1.
The model of the maintenance and multilineage differentiation of a multipotent hematopoietic cell line EML. In the presence of SCF EML cells undergo proliferative self-renewal and remain undifferentiated, whereas in the presence of cytokines and/or stroma EML cells differentiate into erythroid, myeloid and lymphoid lineages.
Thus, unlike other hematopoietic progenitor cell lines, EML cells are a unique model to study mechanisms of lineage commitment and differentiation of multipotent progenitors into most hematopoietic lineages [31-35].
In collaboration with Dr. McIntush from Bethyl Inc. we tested two α-FLRF antibodies (Abs) that recognize epitopes in the C-terminal part of mouse and human FLRF proteins. Anti-FLRF Abs 1123 and 1124 have detected a 37 kDa FLRF protein in the lysates from EML cells, human myeloid cell line KG-1, and mouse pre-B progenitor cell line 70Z3, which transcribe FLRF [19] (Fig. 2A). Moreover, reciprocal immunoprecipitation (IP) and Western analysis have shown that both FLRF Abs recognize the protein of the correct size in EML cells and a mouse pro-B cell line BaF3 (Fig. 2B).
Figure 2.
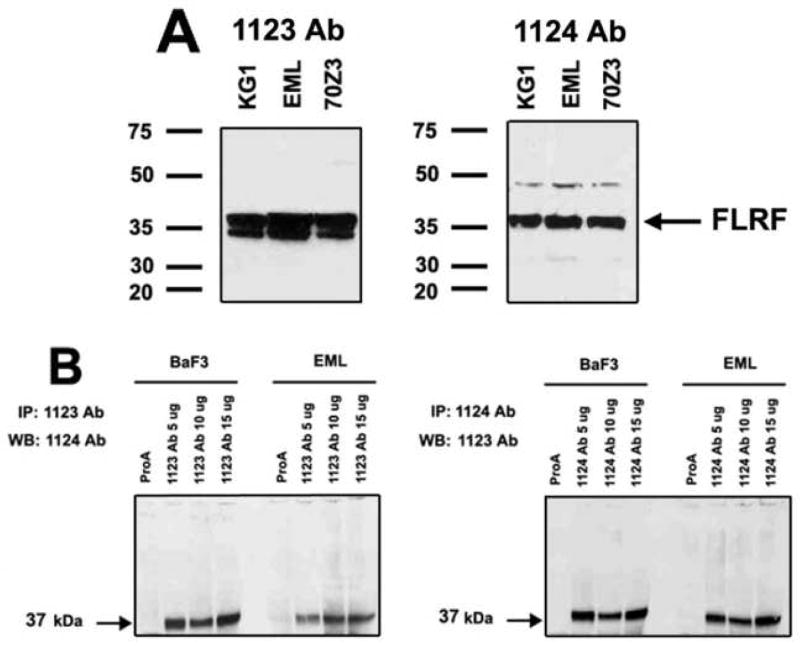
The expression of FLRF protein in murine and human hematopoietic cell lines. A. Detection of mouse and human FLRF protein in EML cells, human myeloid cell line KG-1, and mouse pre-B cell line 70Z3 with rabbit α-FLRF Abs 1123 and 1124. B. Protein immunoprecipitation (IP) with α-FLRF Abs 1123 and 1124 and subsequent reciprocal Western analysis have shown that both α-FLRF Abs recognize the 37 kDa protein in EML cells and mouse pro-B cell line BaF3.
EML cells were transfected with pcDNA4 control vector or pcDNA4/FLRF vector (containing the full-length mouse FLRF cDNA), and selected with Zeocin. Two EML cell clones stably transfected with control vector (EML/pcDNA cells), and three EML cell clones stably transfected with pcDNA4/FLRF vector (EML/FLRF cells), were established independently. Figure 3A shows Western analysis of FLRF in two EML/FLRF cell clones, which express 3-fold higher level of FLRF protein than wild type (wt EML) and vector control EML/pcDNA cells. No difference in the proliferation rate or cell death during culture was observed between wt, vector control or EML/FLRF cells (data not shown). One vector control and two EML/FLRF cell clones were studied in parallel, and have yielded identical results in the experiments described below.
Figure 3.

Over-expression of FLRF attenuates myelo-erythroid differenmtiation of EMl cells. A. Western analysis of FLRF protein in wt EML cells, vector control EML/pcDNA cells, and two EML cell clones over-expressing FLRF (EML/FLRF). Fifty μg of protein from each cell sample were loaded per lane. The blots were stripped and re-probed with α-actin Ab. B. Comparison of the differentiation capacity and generation of granulocyte/macrophage (CFU-GM), erythroid (BFU-E) and megakaryocytic (CFU-Meg) colonies by wild type EML, vector control EML/pcDNA cells and EML/FLRF cells. C. Comparison of the CFU-GM, BFU-E and CFU-Meg colony size generated by wild type EML, EML/pcDNA and EML/FLRF cells.
Myelo-erythroid differentiation of wt EML, EML/pcDNA and EML/FLRF cells was examined using hematopoietic colony-forming assays [1, 36, 37]. EML cells were resuspended in low melting temperature agarose supplemented with IL-3, Epo or Tpo, and plated into triplicate 6-well plates (103 cells/well). The colonies, originating from single progenitors, were enumerated 7-10 days after plating. Figure 3B shows the representative results from four independent experiments with two different EML/FLRF clones. Wild type EML and vector control EML/pcDNA cells have consistently generated very reproducible and similar number of CFU-GM, BFU-E and CFU-Meg colonies (Fig. 3B). In contrast, EML/FLRF cells have reproducibly generated 3 to 5- fold lower numbers of CFU-GM, BFU-E and CFU-Meg colonies than wt and EML/pcDNA cells (Fig. 3B). The same results were obtained even after using increasing concentrations of cytokines in CFC assays. In addition, the colonies produced by EML/FLRF cells were smaller in size, and contained fewer cells per colony than colonies derived from wt EML and EML/pcDNA cells (Fig. 3C). These data indicate that FLRF over-expression attenuates myelo-erythroid differentiation of EML cells.
Over-expression of FLFR leads to constitutively decreased levels of Epo and IL-3 receptors
To determine the cause for attenuated differentiation of EML/FLRF cells we first analyzed the levels of Epo and IL-3 receptors in the wt EML, EML/pcDNA and EML/FLRF cells by Western. To assess the receptor levels before and after ligand binding, the proteins were isolated from control EML cells cultured with SCF only (time point 0′), and EML cells cultured in the presence of IL-3 or Epo for 10, 20, 30 and 60 minutes in liquid culture. In comparison to wt EML and EML/pcDNA cells, EML/FLRF cells have exhibited markedly reduced levels of EpoR, IL-3Rα and IL-3β receptors, both prior to and after exposure to Epo and IL-3 (Fig. 4A). These observations indicate that gain of FLRF function leads to a constitutive decrease of Epo and IL-3 receptor levels, and suggest that FLRF regulates ligand binding-independent, steady-state basal levels of Epo and IL-3 receptors.
Figure 4.
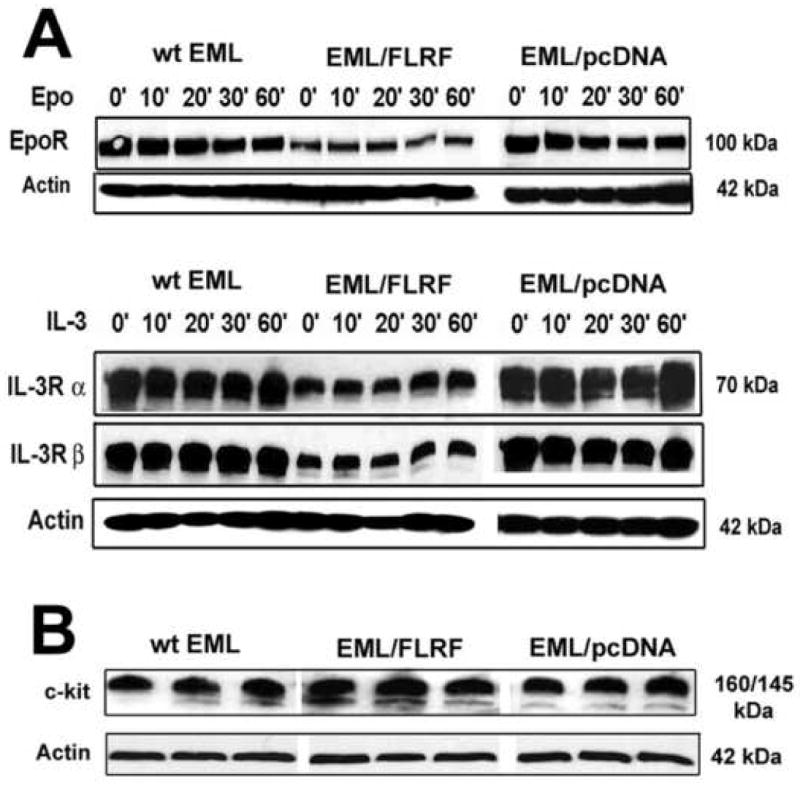

FLRF associates with and regulates basal levels of cytokine receptors in EML cells. A. Western analysis of Epo and IL-3 receptor levels in wt EML, EML/pcDNA and EML/FLRF cells. Over-expression of FLRF leads to constitutively reduced steady-state levels of Epo, IL-3Rα and IL-3β receptors in EML/FLRF cells, independent of the treatment with Epo or IL-3. Equal amounts (50 μg) of protein from each cell sample were loaded per lane. The blots were stripped and re-probed with α-actin Ab. B. Western analysis of basal levels of c-kit receptor protein in undifferentiated wt EML, EML/pcDNA and EML/FLRF cells has revealed that over-expression of FLRF does not have any effect on the levels of c-kit protein in EML cells. Protein extracts from triplicate cultures of wt EML, EML/pcDNA and EML/FLRF cells were analyzed with goat α-c-kit M-14 antibody. The blots were stripped and re-probed with α-actin Ab. C. Protein IP with IL-3R and EpoR Abs has shown that FLRF associates with EpoR and both IL-3Rα and IL-3Rβ receptor subunits in wt EML, EML/pcDNA and EML/FLRF cells, both prior to and after exposure to cytokines. D. Epo and IL-3 receptors in EML/FLRF cells are associated with increased levels of poly-ubiquitin chains prior to and after the treatment with Epo or IL-3.
The survival and proliferation of EML cells absolutely depend on the presence of SCF, and ≥95% of EML cells express SCF receptor c-kit on their cell surface, as shown by flow cytometry analysis [24, 25]. Since SCF/c-kit signaling has synergistic effect on Epo and IL-3-induced differentiation of EML cells as well, we sought to determine whether attenuated differentiation of EML/FLRF cells could be due also to altered steady-state levels of c-kit receptor protein. Western analysis of multiple protein extracts has shown that the levels of c-kit protein were almost identical among undifferentiated wt EML, EML/pcDNA and EML/FLRF cells cultured in the presence of SCF alone (Fig. 4B), indicating that over-expression of FLRF does not have any effect on the levels of c-kit protein in EML cells. In addition, flow cytometry analysis of c-kit expression did not reveal any changes either, with 95-98% of wt EML, EML/pcDNA and EML/FLRF cells being c-kit+ (data not shown). These findings were in agreement with observation that undifferentiated wt EML, EML/pcDNA and EML/FLRF cells, cultured with SCF alone, did not show any differences in survival, proliferation rate and doubling time.
FLRF protein associates with IL-3 and Epo receptors prior to ligand binding
To determine whether FLRF associates with Epo and IL-3 receptors, equal amount of proteins from untreated wt EML, EML/pcDNA and EML/FLRF cells (time point 0′), and cells treated with Epo or IL-3 for 10, 20, 30 and 60 minutes were immuno-precipitated with α-EpoR and α-IL-3Rb Abs. Subsequent Western blotting with FLRF and receptor Abs has shown that FLRF protein indeed associates with EpoR and IL-3Rα and IL-3Rβ receptor subunits in EML cells (Fig. 4C). More importantly, the results in Fig. 4C show that Epo and IL-3 receptors associate with FLRF protein in wt EML, EML/pcDNA and EML/FLRF cells in the absence of cytokines Epo or IL-3 (time point 0′), as well as in EML cells treated with Epo or IL-3. These observations were corroborated by IP of proteins with FLRF Abs and detection of FLRF and receptor proteins by Western (data not shown).
To analyze whether FLRF over-expression affected poly-ubiquitination of Epo and IL-3 receptors, equal amounts of proteins from untreated (time point 0′) and cytokine treated wt EML and EML/FLRF cells were immunoprecipitated with EpoR and IL-3Rb Abs, and analyzed with α-Ubiquitin Ab. Figure 4D shows that in EML/FLRF cells Epo and IL-3 receptors are associated with increased levels of poly-ubiquitin chains both prior to and after exposure to Epo or IL-3.
In summary, the over-expression of FLRF suppresses myelo-erythroid differentiation of EML cells, which correlates with constitutive decrease in the levels and increased ubiquitination of Epo and IL-3 receptors. More importantly, the observation that FLRF associates with receptors prior to ligand binding suggests that FLRF is regulating basal levels of Epo and IL-3 receptors in a ligand binding-independent manner.
FLRF associates with and regulates Epo and IL-3 receptor levels in BaF3 cell line
To determine whether FLRF associates with Epo and IL-3 receptors in other growth factor-dependent progenitor cell lines, we have used a panel of mouse pro-B BaF3 progenitor cell lines [38, 39]. The original BaF3 cell line is IL-3-dependent, and is maintained in RPMI medium supplemented with murine recombinant IL-3. BaF3/EpoR cells are stably transfected with the human Epo receptor cDNA [38], and can be maintained either with IL-3 or human recombinant Epo. The BaF3/Mpl cells were engineered to express the murine thrombopoietin (Tpo) receptor (c-Mpl) [39], and can be maintained with murine recombinant IL-3 or Tpo (ZymoGenetics).
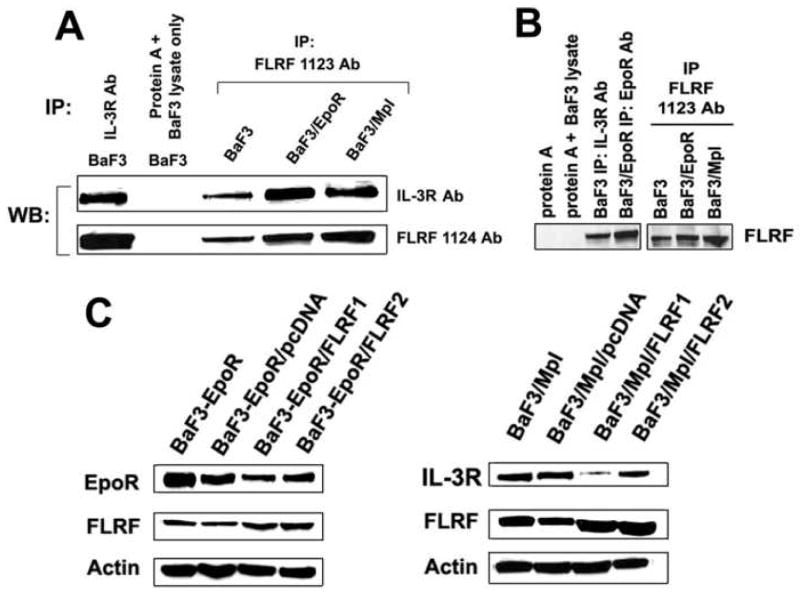
Reciprocal IP experiments have shown that FLRF associates with IL-3 receptor in BaF3, BaF3/EpoR and BaF3/Mpl cells, maintained with IL-3, Epo and Tpo, respectively (Fig. 5A). In addition, IP with both IL-3R and EpoR Abs has shown that endogenous FLRF protein associates with endogenous IL-3 receptor in BaF3 cells, and with ectopically expressed Epo receptor in BaF3/EpoR cells (Fig. 5B).
Figure 5.
FLRF associates with and regulates basal levels of cytokine receptors in a panel of BaF3 pro-B progenitor cell lines. Protein IP with IL-3R, EpoR and FLRF Abs has shown that FLRF associates with IL-3 receptor in BaF3, BaF3/EpoR and BaF3/Mpl cells (A and B), and with Epo receptor in BaF3/EpoR cells (B). C. Transient over-expression of FLRF in BaF3/EpoR/FLRF and BaF3/Mpl/FLRF cells leads to reduced levels of Epo and IL-3 receptor proteins. BaF3/EpoR and BaF3/Mpl cells are mock controls. BaF3/EpoR/pcDNA and BaF3/Mpl/pcDNA are cells transfected with control vector. The blots were stripped and re-probed with α-actin Ab.
To examine how FLRF over-expression affects steady state EpoR and IL-3R levels in BaF3 cells, the BaF3/EpoR cells maintained with rIL-3 and BaF3/Mpl cells maintained with rTpo, were transfected with pcDNA4 or pcDNA4/FLRF plasmids. The transient over-expression (72 hours) of FLRF resulted in decreased levels of Epo and IL-3 receptors in two independent BaF3/EpoR and one of the BaF3/Mpl transfectants (Fig. 5C), indicating that FLRF exerts the same effect on steady-state levels of IL-3R and EpoR in EML and BaF3 cells.
Treatment with retinoic acid (RA) further attenuates myeloid differentiation of EML cells over-expressing FLRF
Retinoic acid (RA) is an important regulator of myeloid progenitor differentiation [40]. The RA functions through RARα and RXR receptors, which act as ligand-inducible transcription factors, and interact with specific DNA targets as heterodimers (RAR-RXR) or homodimers (RXR-RXR) [40-42]. We have confirmed previously published observation [24] that treatment with RA enhances IL-3 or GM-SCF-induced differentiation of wt EML cells into CFU-GM progenitors (Fig. 6A). Hence, we wanted to determine whether the treatment of EML/FLRF cells with RA will improve their impaired differentiation into CFU-GM progenitors.
Figure 6. Retinoic acid further attenuates myeloid differentiation of EML cells over-expressing FLRF.

Wild type EML, EML/pcDNA and EML/FLRF cells were cultured for 20 hours with SCF alone (control), or in the presence of SCF and RA (10-5 M), or SCF, RA and IL-3. The cells were than plated into agarose cultures supplemented with IL-3 to examine their differentiation into granulocyte/macrophage (CFU-GM) progenitors. A. Generation of CFU-GM colonies by control wt EML, EML/pcDNA and EML/FLRF cells (SCF), and cells cultured for 20 hours with SCF and RA, or with SCF, RA and IL-3. Treatment with RA increases the number of CFU-GM colonies produced by wt EML and EML/pcDNA cells. In contrast, RA treatment further decreased the number of CFU-GM colonies produced by EML/FLRF cells. B. Western analysis of RARα and RXR receptor proteins in untreated control wt EML, EML/pcDNA and EML/FLRF cells (SCF), and cells cultured for 20 hours with SCF and RA, or with SCF, RA and IL-3. C. Western analysis of RARα and RXR receptor proteins in control wt EML, EML/pcDNA and EML/FLRF cells, and cells cultured for 20 hours with IL-3. D. Transient over-expression of FLRF in four BaF3 cell clones (BaF3/FLRF 1-4) decreased the amount of RARα receptor protein, whereas the levels of RXR receptor remained unchanged. The blots were stripped and re-probed with α-actin Ab. E. Reciprocal IP of proteins from EML and BaF3 cells with α-FLRF, α-RARα and α-RXR Abs has shown that endogenous FLRF protein associates with endogenous RARα and RXR receptor proteins in EML and BaF3 cells.
Wild type EML, EML/pcDNA and EML/FLRF cells were cultured for 20 hours with SCF alone (control), or in the presence of SCF and RA (10-5 M), or SCF, RA and IL-3. The cells were than plated into agarose cultures supplemented with IL-3 to examine their differentiation into CFU-GM progenitors. Figure 6A shows representative results from three independent experiments with two different EML/FLRF clones. As shown before [24], pretreatment with RA augmented differentiation of wt EML and EML/pcDNA cells into CFU-GM progenitors in comparison to control cells cultured with SCF alone (Fig. 6A). Surprisingly however, RA treatment resulted in even further decline in the number of CFU-GM progenitors generated by EML/FLRF (Fig. 6A). Furthermore, while the pretreatment with SCF, RA and IL-3 further enhanced CFU-GM colony production by wt EML and EML/pcDNA cells, it had very little effect on generation of CFU-GM colonies by EML/FLRF cells (Fig. 6A).
To determine the mechanism behind RA-mediated decline in CFU-GM progenitors generated by EML/FLRF cells, we examined the expression of RARα and RXR receptor proteins in control wt EML, EML/pcDNA and EML/FLRF cells (cultured with SCF alone), and cells cultured for 20 hours with SCF and RA or with SCF, RA and IL-3 (Fig. 6B).
Remarkably, the control EML/FLRF cells (SCF) exhibited reduced steady-state levels of RARα receptors as compared to control wt EML and EML/pcDNA cells (Fig. 6B). Moreover, the culture of EML/FLRF cells with SCF and RA for 20 hours decreased RARα receptor levels even further (Fig. 6B). However, the treatment of wt EML, EML/pcDNA and EML/FLRF cells with SCF, RA and IL-3 for 20 hours did not change significantly the levels of RARα receptors in comparison to control cells (Fig. 6B). On the other hand, control wt EML, EML/pcDNA and EML/FLRF cells, and cells cultured with SCF/RA or SCF/RA/IL-3 expressed similar levels of RXR receptors (Fig. 6B). Also, the culture of wt EML, EML/pcDNA and EML/FLRF cells with IL-3 alone for 20 hours did not significantly change the levels of RARα and RXR receptors in comparison to control cells (Fig. 6C).
Notably, the transient over-expression of FLRF in BaF3 cells resulted in significantly decreased steady-state levels of RARα receptors, whereas levels of RXR receptors remained unchanged (Fig. 6D), indicating that FLRF exerts the same effect on RARα in EML and BaF3 cells. Moreover, IP and Western blotting of proteins with α-FLRF, α-RARα or α-RXR Abs, have shown that endogenous FLRF protein associates with RARα and RXR receptors in EML and BαF3 cells (Fig. 6E).
Taken together, these results support the notion that over-expression of FLRF negatively affects RA-mediated differentiation of EML cells into CFU-GM progenitors by down-regulating RARα levels, and indicate that FLRF regulates basal levels of RARα receptors in hematopoietic progenitors in a ligand-independent fashion.
Discussion
Multiple lines of evidence support the notion that quantitative changes in receptor/ligand signaling complex and the thresholds in receptor expression are important in regulating blood cell differentiation. According to the signaling-threshold model the pre-determined amount of cytokine receptors expressed by cells determines the level of formed ligand-receptor complexes, the signaling threshold, and the cell fate [43, 44]. Thus, the precise control of receptor levels prior to ligand binding is important to ensure optimal cellular proliferation and differentiation.
The basal levels of cytokine receptors are thought to be maintained through the balance between synthesis, delivery, retention, and degradation [45]. The accumulating evidence shows that besides the well-characterized ligand-induced pathways, there are ligand-independent pathways of receptor degradation. However, the molecular mechanisms that maintain the basal levels of receptors, and are underlying ligand-independent receptor degradation are not well understood [46, 47].
The FLRF over-expression experiments have revealed that the excess amount of FLRF protein is constitutively decreasing Epo and IL-3 receptor levels in EML and BaF3 progenitor cell lines, and is attenuating differentiation of multipotent EML cells into myeloid and erythroid lineages. More importantly, these data support the notions that FLRF protein regulates steady-state, basal levels of Epo and IL-3 receptors in a ligand binding-independent manner, and that it could be maintaining optimal receptor levels for proper differentiation of EML cells into myeloid and erythroid lineages. Thus, the function of FLRF in hematopoietic progenitors is similar to its previously reported regulation of steady-state levels of neuregulin ErbB3 and ErbB4 receptors through their ligand-independent degradation [20, 21, 48]. Moreover, the finding that over-expression of FLRF in EML cells affects the levels of Epo and IL-3 receptors but not c-kit, provides additional insight into the specificity of FLRF in regulating levels of cytokine receptors in hematopoietic cells.
Retinoic acid (RA) is known to augment IL-3-induced differentiation of BM-derived hematopoietic progenitors and EML cell line into granulocyte and macrophage lineages [24, 40]. Several studies have also reported that IL-3 and GM-CSF are enhancing RARα transcription in EML cells, myeloid progenitor cell line 32D, and mouse Lin- Sca-1 + c-kit+ bone marrow cells through activation of the Jak2/Stat5 pathway [49, 50]. These findings have revealed a previously unknown cytokine-RAR interaction during myelopoiesis, and suggested that RAR activation could be a critical downstream event following IL-3 and GM-CSF signaling during myeloid differentiation [49, 50].
Surprisingly, the RA treatment further suppressed IL-3-induced myeloid differentiation of EML cells over-expressing FRLF. Interestingly, protein IP and Western analysis have shown that FLRF protein associates with RARα and RXR receptors in EML and BaF3 cell lines, and that EML cells and BaF3 cells over-expressing FLRF exhibit significantly reduced steady-state levels of RARα protein, whereas the levels of RXR protein remained unchanged. Moreover, the treatment of EML/FLRF cells with RA resulted in even further decrease of RARα protein levels. The observation that the treatment of wt EML, EML/pcDNA and EML/FLRF cells with IL-3 alone did not change significantly the levels of RARα protein in comparison to untreated cells, is in agreement with the previous report that IL-3-mediated enhancement of RARα transcription in EML cells is not associated with changes in the RARα protein levels [49]. These results show that elevated levels of FLRF protein attenuate IL-3 and RA-mediated EML cell differentiation into CFU-GM progenitors by down-regulating the steady-state levels of IL-3R and RARα levels. Cumulatively, our results support the conclusion that FLRF (a) regulates steady-state, basal levels of RARα receptors in hematopoietic progenitors in a ligand binding-independent manner, and (b) participates in regulation of hematopoietic progenitor differentiation into myeloid lineages by independently governing steady state levels of IL-3 and RAR receptors, thus impacting both IL-3 and RA signaling.
The studies in progress are aimed at determining (a) what other hematopoietic cytokine receptors are regulated by FLRF, (b) whether human FLRF regulates basal levels of cytokine and RA receptors in human hematopoietic cells, and (c) whether dysregulation of FLRF and aberrant regulation of steady-state receptor levels play a role in development and progression of hematopoietic malignancies.
In that regard, it will be also important to analyze whether hFLRF associates with and regulates the levels of mutant RARα receptor in acute promyelocytic leukemia (APL) cells, in which the RARα gene is fused to the promyelocytic leukemia (PML) gene, coding for a fusion PML/RARα protein [51-54]. Notably, the query of the ONCOMINE database has revealed that FLRF transcription was down-regulated in human hematopoietic cell line U937 engineered to express the PML/RAR and PLZF/RAR fusion proteins [55], suggesting that the expression of human FLRF could be dysregulated in primary APL cells and APL-derived cell lines.
Importantly, a recent study reported that FLRF expression levels inversely correlated with the levels of ErbB3 receptor (commonly overexpressed with ErbB2 in breast cancer) in primary human breast cancers and in a mouse model of ErbB2 mammary tumorigenesis, suggesting that FLRF loss could be promoting breast tumor progression by augmenting ErbB2/ErbB3 signaling [56].
In summary, this study has discovered new FLRF-mediated pathway for ligand-independent receptor level regulation, and the results support the notion that through governing and maintaining the optimal steady-state, basal levels of Epo, IL-3 and RAR receptors, FLRF is involved in the control of hematopoietic progenitor cell differentiation into myelo-erythroid lineages.
Further functional analysis of FLRF will improve our understanding of the mechanisms that govern steady-state levels of hematopoietic cytokine receptors, and how the increased and decreased maintenance of specific cytokine receptors affect and regulate differentiation of HSC and progenitors into particular lineages.
Acknowledgments
We thank Dr. McIntush (Bethyl Inc.) for generously generating and sharing two α-FLRF Abs, and Dr. Sytkowski (Harvard Medical School) and Dr. Kaushansky (UC San Diego School of Medicine) for sharing with us BaF3/EpoR and BaF3/Mpl cell lines. This work was supported by NIH RO1 RR15242 and American Cancer Society Institutional Grants (RJ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Metcalf D. Lineage commitment of hemopoietic progenitor cells in developing blast cell colonies: influence of colony-stimulating factors. Proc Natl Acad Sci USA. 1991;88:11310–11314. doi: 10.1073/pnas.88.24.11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smithgall TE. Signal transduction pathways regulating hematopoietic differentiation. Pharmacol Rev. 1998;50:1–19. [PubMed] [Google Scholar]
- 3.Thomas D, Vadas M, Lopez A. Regulation of haematopoiesis by growth factors - emerging insights and therapies. Expert Opin Biol Ther. 2004;4:869–879. doi: 10.1517/14712598.4.6.869. [DOI] [PubMed] [Google Scholar]
- 4.Barreda DR, Hanington PC, Belosevic M. Regulation of myeloid development and function by colony stimulating factors. Dev Comp Immunol. 2004;28:509–554. doi: 10.1016/j.dci.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Richmond TD, Chohan M, Barber DL. Turning cells red: signal transduction mediated by erythropoietin. Trends Cell Biol. 2005;15:146–155. doi: 10.1016/j.tcb.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Yasukawa H, Sasaki A, Yoshimura A. Negative regulation of cytokine signaling pathways. Annu Rev Immunol. 2000;18:143–164. doi: 10.1146/annurev.immunol.18.1.143. [DOI] [PubMed] [Google Scholar]
- 7.Jackson PK, Eldridge AG, Freed E, et al. The lore of the RINGs: substrate recognition and catalysis by ubiquitin ligases. Trends Cell Biol. 2000;10:429–439. doi: 10.1016/s0962-8924(00)01834-1. [DOI] [PubMed] [Google Scholar]
- 8.Joazeiro CA, Weissman AM. RING finger proteins: mediators of ubiquitin ligase activity. Cell. 2000;102:549–552. doi: 10.1016/s0092-8674(00)00077-5. [DOI] [PubMed] [Google Scholar]
- 9.Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2000;70:503–533. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- 10.Ben-Neriah Y. Regulatory functions of ubiquitination in the immune system. Nat Immunol. 2002;3:20–26. doi: 10.1038/ni0102-20. [DOI] [PubMed] [Google Scholar]
- 11.Sakamoto KM. Ubiquitin-dependent proteolysis: its role in human diseases and the design of therapeutic strategies. Mol Genet Metab. 2002;77:44–56. doi: 10.1016/s1096-7192(02)00146-4. [DOI] [PubMed] [Google Scholar]
- 12.Pagano M, Benmaamar R. When protein destruction runs amok, malignancy is on the loose. Cancer Cell. 2003;4:251–256. doi: 10.1016/s1535-6108(03)00243-5. [DOI] [PubMed] [Google Scholar]
- 13.Thien CB, Langdon WY. Cbl: many adaptations to regulate protein tyrosine kinases. Nature Rev Mol Cell Biol. 2001;2:294–307. doi: 10.1038/35067100. [DOI] [PubMed] [Google Scholar]
- 14.Fang D, Wang HY, Fang N, Altman Y, Elly C, Liu YC. Cbl-b, a RING-type E3 ubiquitin ligase, targets phosphatidylinositol 3- kinase for ubiquitination in T cells. J Biol Chem. 2001;276:4872–4878. doi: 10.1074/jbc.M008901200. [DOI] [PubMed] [Google Scholar]
- 15.Fang D, Liu YC. Proteolysis-independent regulation of PI3K by Cbl-b-mediated ubiquitination in T cells. Nature Immunol. 2001;2:870–875. doi: 10.1038/ni0901-870. [DOI] [PubMed] [Google Scholar]
- 16.Fujimoto M, Naka T. Regulation of cytokine signaling by SOCS family molecules. Trends Immunol. 2003;24:659–666. doi: 10.1016/j.it.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Kubo M, Hanada T, Yoshimura A. Suppressors of cytokine signaling and immunity. Nat Immunol. 2003;4:1169–1176. doi: 10.1038/ni1012. [DOI] [PubMed] [Google Scholar]
- 18.Alexander WS, Hilton DJ. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu Rev Immunol. 2004;22:503–529. doi: 10.1146/annurev.immunol.22.091003.090312. [DOI] [PubMed] [Google Scholar]
- 19.Abdullah JM, Li X, Nachtman RG, Jurecic R. FLRF, a Novel Evolutionarily Conserved RING Finger Gene, Differentially Expressed in Mouse Fetal and Adult Hematopoietic Stem Cells and Progenitors. Blood Cells, Molecules and Diseases. 2001;27:320–333. doi: 10.1006/bcmd.2001.0390. [DOI] [PubMed] [Google Scholar]
- 20.Diamonti AJ, Guy PM, Ivanof C, Wong K, Sweeney C, Carraway KL., 3rd An RBCC protein implicated in maintenance of steady-state neuregulin receptor levels. Proc Natl Acad Sci USA. 2002;99:2866–2871. doi: 10.1073/pnas.052709799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiu XB, Goldberg AL. Nrdp1/FLRF is a ubiquitin ligase promoting ubiquitination and degradation of the epidermal growth factor receptor family member, ErbB3. Proc Natl Acad Sci USA. 2002;99:14843–14848. doi: 10.1073/pnas.232580999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiu XB, Markant SL, Yuan J, Goldberg AL. Nrdp1-mediated degradation of the gigantic IAP, BRUCE, is a novel pathway for triggering apoptosis. EMBO J. 2004;23:800–810. doi: 10.1038/sj.emboj.7600075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong L, Tan Y, Zhou A, Yu Q, Zhou J. RING finger ubiquitin-protein isopeptide ligase Nrdp1/FLRF regulates parkin stability and activity. J Biol Chem. 2005;280:9425–9430. doi: 10.1074/jbc.M408955200. [DOI] [PubMed] [Google Scholar]
- 24.Tsai S, Bartelmez S, Sitnicka E, Collins S. Lymphohematopoietic progenitors immortalized by a retroviral vector harboring a dominant-negative retinoic acid receptor can recapitulate lymphoid, myeloid, and erythroid development. Genes Dev. 1994;8:2831–2841. doi: 10.1101/gad.8.23.2831. [DOI] [PubMed] [Google Scholar]
- 25.Zayas J, Spassov DS, Nachtman RG, Jurecic R. Hematopoietic stem cells and multipotent progenitors express new truncated intracellular form of c-kit receptor. Stem Cells and Development. 2008;17 doi: 10.1089/scd.2007.0101. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc Natl Acad Sci USA. 2001;98:14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terskikh AV, Miyamoto T, Chang C, Diatchenko L, Weissman IL. Gene expression analysis of purified hematopoietic stem cells and committed progenitors. Blood. 2003;102:94–101. doi: 10.1182/blood-2002-08-2509. [DOI] [PubMed] [Google Scholar]
- 28.Tsai S. Differential effects of c-fms and c-kit ligands on the lineage development of the lymphohematopoietic cell line EML C1. Cancer Chemother Pharmacol. 1996;38:S58–63. doi: 10.1007/s002800051040. [DOI] [PubMed] [Google Scholar]
- 29.Lawson ND, Krause DS, Berliner N. Normal neutrophil differentiation and secondary granule gene expression in the EML and MPRO cell lines. Exp Hematol. 1998;26:1178–1185. [PubMed] [Google Scholar]
- 30.Schmitt TM, Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 31.Chiang MY, Monroe JG. BSAP/Pax5A expression blocks survival and expansion of early myeloid cells implicating its involvement in maintaining commitment to the B-lymphocyte lineage. Blood. 1999;94:3621–3632. [PubMed] [Google Scholar]
- 32.Pawlak G, Grasset MF, Arnaud S, Blanchet JP, Mouchiroud G. Receptor for macrophage colony-stimulating factor transduces a signal decreasing erythroid potential in the multipotent hematopoietic EML cell line. Exp Hematol. 2000;28:1164–1173. doi: 10.1016/s0301-472x(00)00522-1. [DOI] [PubMed] [Google Scholar]
- 33.Bourette RP, De Sepulveda P, Arnaud S, Dubreuil P, Rottapel R, Mouchiroud G. Suppressor of cytokine signaling 1 interacts with the macrophage colony-stimulating factor receptor and negatively regulates its proliferation signal. J Biol Chem. 2001;276:22133–22139. doi: 10.1074/jbc.M101878200. [DOI] [PubMed] [Google Scholar]
- 34.Du Y, Campbell JL, Nalbant D, et al. Mapping gene expression patterns during myeloid differentiation using the EML hematopoietic progenitor cell line. Exp Hematol. 2002;30:649–658. doi: 10.1016/s0301-472x(02)00817-2. [DOI] [PubMed] [Google Scholar]
- 35.Kirito K, Fox N, Kaushansky K. Thrombopoietin stimulates Hoxb4 expression: an explanation for the favorable effects of TPO on hematopoietic stem cells. Blood. 2003;102:3172–3178. doi: 10.1182/blood-2003-03-0944. [DOI] [PubMed] [Google Scholar]
- 36.Metcalf D. Clonal culture of hematopoietic cells: techniques and applications. Elsevier Press; 1984. [Google Scholar]
- 37.Testa NG, Molineux G, editors. Haemopoiesis: A Practical Approach. IRL Press; 1993. [Google Scholar]
- 38.Chen C, Sytkowski AJ. Erythropoietin regulation of Raf-1 and MEK: evidence for a Ras-independent mechanism. Blood. 2004;104:73–80. doi: 10.1182/blood-2003-04-1340. [DOI] [PubMed] [Google Scholar]
- 39.Miyakawa Y, Rojnuckarin P, Habib T, Kaushansky K. Thrombopoietin induces phosphoinositol 3-kinase activation through SHP2, Gab, and insulin receptor substrate proteins in BAF3 cells and primary murine megakaryocytes. J Biol Chem. 2000;276:2494–2502. doi: 10.1074/jbc.M002633200. [DOI] [PubMed] [Google Scholar]
- 40.Collins SJ. The role of retinoids and retinoic acid receptors in normal hematopoiesis. Leukemia. 2002;16:1896–1905. doi: 10.1038/sj.leu.2402718. [DOI] [PubMed] [Google Scholar]
- 41.Klein ES, Wang JW, Khalifa B, Gavigan SA, Chandraratna RA. Recruitment of nuclear receptor corepressor and coactivator to the retinoic acid receptor by retinoid ligands. Influence of DNA-heterodimer interactions. J Biol Chem. 2000;275:19401–19408. doi: 10.1074/jbc.M002472200. [DOI] [PubMed] [Google Scholar]
- 42.Zhu J, Gianni M, Kopf E, et al. Retinoic acid induces proteasome-dependent degradation of retinoic acid receptor alpha (RAR alpha) and oncogenic RAR alpha fusion proteins. Proc Natl Acad Sci USA. 1999;96:14807–14812. doi: 10.1073/pnas.96.26.14807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zandstra PW, Lauffenburger DA, Eaves CJ. A ligand-receptor signaling threshold model of stem cell differentiation control: a biologically conserved mechanism applicable to hematopoiesis. Blood. 2000;96:1215–1222. [PubMed] [Google Scholar]
- 44.Viswanathan S, Benatar T, Rose-John S, Lauffenburger DA, Zandstra PW. Ligand/receptor signaling threshold (LIST) model accounts for gp130-mediated embryonic stem cell self-renewal responses to LIF and HIL-6. Stem Cells. 2002;20:119–138. doi: 10.1634/stemcells.20-2-119. [DOI] [PubMed] [Google Scholar]
- 45.Rubin C, Gur G, Yarden Y. Negative regulation of receptor tyrosine kinases: unexpected links to c-Cbl and receptor ubiquitylation. Cell Res. 2005;15:66–71. doi: 10.1038/sj.cr.7290268. [DOI] [PubMed] [Google Scholar]
- 46.Katz M, Shtiegman K, Tal-Or P, et al. Ligand-independent degradation of epidermal growth factor receptor involves receptor ubiquitylation and Hgs, an adaptor whose ubiquitin-interacting motif targets ubiquitylation by Nedd4. Traffic. 2002;3:740–751. doi: 10.1034/j.1600-0854.2002.31006.x. [DOI] [PubMed] [Google Scholar]
- 47.Liu J, Plotnikov A, Banerjee A, et al. Ligand-independent pathway that controls stability of interferon alpha receptor. Biochem Biophys Res Commun. 2008;367:388–393. doi: 10.1016/j.bbrc.2007.12.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao Z, Wu X, Yen L, Sweeney C, Carraway KL., 3rd Neuregulin-induced ErbB3 downregulation is mediated by a protein stability cascade involving the E3 ubiquitin ligase Nrdp1. Mol Cell Biol. 2007;27:2180–2188. doi: 10.1128/MCB.01245-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnson BS, Mueller L, Si J, Collins SJ. The cytokines IL-3 and GM-CSF regulate the transcriptional activity of retinoic acid receptors in different in vitro models of myeloid differentiation. Blood. 2002;99:746–753. doi: 10.1182/blood.v99.3.746. [DOI] [PubMed] [Google Scholar]
- 50.Jutong S, Collins SJ. IL-3–induced enhancement of retinoic acid receptor activity is mediated through Stat5, which physically associates with retinoic acid receptors in an IL-3–dependent manner. Blood. 2003;100:4401–4409. doi: 10.1182/blood-2001-12-0374. [DOI] [PubMed] [Google Scholar]
- 51.Ferrucci PF, Grignani F, Pearson M, Fagioli M, Nicoletti I, Pelicci PG. Cell death induction by the acute promyelocytic leukemia-specific PML/RARalpha fusion protein. Proc Natl Acad Sci USA. 1997;94:10901–10906. doi: 10.1073/pnas.94.20.10901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fanelli M, Minucci S, Gelmetti V, Nervi C, Gambacorti-Passerini C, Pelicci PG. Constitutive degradation of PML/RARalpha through the proteasome pathway mediates retinoic acid resistance. Blood. 1999;93:1477–1481. [PubMed] [Google Scholar]
- 53.Fenaux P, Wang ZZ, Degos L. Treatment of acute promyelocytic leukemia by retinoids. Curr Top Microbiol Immunol. 2007;313:101–128. doi: 10.1007/978-3-540-34594-7_7. [DOI] [PubMed] [Google Scholar]
- 54.Scaglioni PP, Pandolfi PP. The theory of APL revisited. Curr Top Microbiol Immunol. 2007;313:85–100. doi: 10.1007/978-3-540-34594-7_6. [DOI] [PubMed] [Google Scholar]
- 55.Alcalay M, Meani N, Gelmetti V, et al. Acute myeloid leukemia fusion proteins deregulate genes involved in stem cell maintenance and DNA repair. J Clin Invest. 2003;112:1751–1761. doi: 10.1172/JCI17595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yen L, Cao Z, Wu X, et al. Loss of Nrdp1 enhances ErbB2/ErbB3-dependent breast tumor cell growth. Cancer Res. 2006;66:11279–11286. doi: 10.1158/0008-5472.CAN-06-2319. [DOI] [PubMed] [Google Scholar]