Abstract
Dimethyl sulfoxide (DMSO) is commonly used in biological studies to dissolve drugs and enzyme inhibitors with low solubility. While DMSO is generally thought of as being relatively inert, it can induce biological effects that are often overlooked. An example highlighting this potential problem is found in the recent report demonstrating a pathogenic role for NKT and NK cells in acetaminophen-induced liver injury (AILI) in C57Bl/6 mice in which DMSO was used to facilitate APAP dissolution. We report here that NKT and NK cells do not play a pathologic role in AILI in C57Bl/6 mice in the absence of DMSO. While AILI was significantly attenuated in mice depleted of NKT and NK cells prior to APAP treatment in the presence of DMSO, no such effect was observed when APAP was dissolved in saline. Due to this unexpected finding, the effects of DMSO on hepatic NKT and NK cells were subsequently investigated. When given alone, DMSO activated hepatic NKT and NK cells in vivo as evidenced by increased NKT cell numbers and higher intracellular levels of the cytotoxic effector molecules, interferon (IFN)-γ and granzyme B, in both cell types. Similarly, when used as a solvent for APAP, DMSO again increased NKT cell numbers and induced IFN-γ and granzyme B expression in both cell types. In conclusion, these data demonstrate a previously unappreciated effect of DMSO on hepatic NKT and NK cells, suggesting that DMSO should be used cautiously in experiments involving these cells.
Keywords: Drug-induced liver injury, innate immune system, dimethyl sulfoxide, IFN-γ, granzyme B
Introduction
Drug-induced liver injury (DILI) is a serious, often fatal, side effect of drug treatment representing a major impediment to drug development (1, 2). Attempts to identify hepatotoxic drugs early in development have been hindered in part by a poor understanding of the underlying mechanism of DILI. Current evidence suggests that reactive metabolites, drug-protein adducts, and glutathione depletion might be common events involved in the initiation of DILI; however, it is currently not possible to predict hepatotoxicity based on these criteria alone (3). Therefore, there is a great deal of interest in studying the downstream events of these initiating factors in hope of identifying potential markers of hepatotoxicity and pathways leading to DILI (4, 5).
The most extensively used model for uncovering fundamental mechanisms of DILI has been acetaminophen (APAP)-induced liver injury (AILI) in mice (4, 6). In this model, the preponderance of evidence indicates that N-acetyl-p-benzoquinone imine (NAPQI), the reactive metabolite of APAP; NAPQI-protein adducts; glutathione depletion; oxidative stress; and mitochondria damage all play a role in AILI (7). More recently, evidence suggests that the innate immune system can contribute to the severity of AILI through the production of pro-inflammatory cytokines and other pro-toxicant factors subsequent to the early metabolic events initiated by NAPQI formation (8). However, there are conflicting views over the contribution of some innate immune system factors to the pathology of AILI (9-13). For example, Kupffer cells have been shown to increase or decrease the extent of AILI depending on the method used to inactivate these cells (14, 15). Another example involves the role of neutrophils in AILI. Initial studies reported a significant decrease in liver injury in C57BL/6 mice following antibody-induced depletion of neutrophils 24 hours prior to APAP treatment (12, 13). These studies have since been disputed based on the possibility that the protective effects of anti-neutrophil antibodies might not be due to neutrophil depletion, but rather to hepatoprotective factors secreted by activated Kupffer cells (14) following the binding and engulfing of antibody-coated neutrophils (11).
Herein, we report another controversy involving the role of innate immune cells, specifically natural killer (NK)T and NK cells, in AILI. A recent study demonstrated a pathogenic role for NKT and NK cells in AILI in C57Bl/6 mice (16). In this study, DMSO was used to facilitate APAP dissolution (Liu, Z.X., personal communication). Unexpectedly, we found that NKT and NK cells only contributed to liver injury when DMSO was used to facilitate APAP dissolution, thus prompting us to study the effects of this solvent on NKT and NK cells in vivo. Initial experiments revealed that DMSO, both alone and within the APAP model, led to the activation of hepatic NKT and NK cells as evidenced by higher NKT cell numbers and increased expression of the cytotoxic effector molecules interferon (IFN)-γ and granzyme B in both NKT and NK cells. These findings demonstrate a previously unappreciated effect of DMSO on NKT and NK cells in the liver and illustrate the potential danger of using this solvent in experiments involving the hepatic immune system.
Materials and Methods
Mice and Treatment
Male and female C57BL/6 (8 weeks; Jackson Laboratory) mice were acclimatized for one week at NIH facilities. Experiments were conducted with the approval of the NHLBI Animal Use and Care Committee, and all animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication 86-23 revised 1985). For all experiments, food was withheld overnight (16 h) as described previously (17) to equally deplete hepatic glutathione stores. Food supplies were restored upon treatment the following morning. To investigate the effects of DMSO, mice were injected intraperitoneally (20 μl/g) with 0%, 3.5%, 5%, or 10% DMSO (v/v) in saline, corresponding to 0, 0.7, 1, and 2 mL/kg respectively. In other experiments, mice were injected intraperitoneally (20 μl/g) with APAP (500 mg/kg) dissolved in 3.5% DMSO (v/v) in saline or with APAP (250 or 500 mg/kg) dissolved in warm saline. Where indicated, hepatic NKT and NK cells were depleted by intraperitoneal injections of 300 μg mouse anti-NK1.1 antibody (PK136, purified from cell culture supernatant) at 48, 24, and 0 h prior to APAP treatment (16). Flow cytometry confirmed that 98% of NKT and 96% of NK cells were depleted in the liver prior to APAP treatment. Control mice received equivalent amounts of mouse IgG2a antibody (BD Biosciences, San Jose, CA) prior to APAP treatment.
Assessment of Liver Injury
Liver injury was evaluated by measuring serum levels of alanine transaminase (ALT) with a diagnostic kit (Teco Diagnostics, Anaheim, CA).
IFN-γ mRNA Expression
RNA was isolated from total liver homogenates using RNeasy Plus Mini Kit (Qiagen, Valencia, CA). cDNA was generated by reverse transcription using High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). Real time polymerase chain reaction was performed using commercially available probes and primers for mouse IFN-γ and β-actin (Applied Biosystems) and a 7500 Fast Real Time PCR System (Applied Biosciences). The levels of gene expression were calculated relative to the housekeeping gene β-actin.
Flow Cytometry
Hepatic lymphocyte populations were enumerated by flow cytometry as previously described with some minor modifications (18). Briefly, livers were perfused with Hank's Buffered Salt Solutions, passed through 100 μm mesh strainers, and centrifuged at 300 × g for 5 min. The resulting pellet from each liver was resuspended in 15 mL of 35% Percoll in RPMI containing 100 IU/mL heparin, and centrifuged at 500 × g for 15 min. The pelleted leukocytes were incubated in ammonium chloride (ACK) lysis buffer for 2 min. After washing with 5% fetal calf serum in PBS, viable cells were counted by Trypan Blue exclusion. Non-specific binding was prevented by a pre-incubation with anti-FcγR II/III (0.1 μg; BD Biosciences, San Jose, CA) for 10 min at 4°C. NKT and NK cell populations were characterized by staining with anti-NK1.1 (PK 136, PE or APC; BD Biosciences) and anti-CD3 (145-2C11, FITC or PE; BD Biosciences) for 30 min at 4°C Dead cells were excluded from analysis by adding 5 μl of 7-amino-actinomycin D (7-AAD; BD Biosciences) 10 minutes prior to flow cytometry. The frequency of positive cells was determined using a CyAnTM LX flow cytometer (DakoCytomation, Carpintaria, CA) and FlowJo software (TreeStar, Ashland, OR). The number of NKT and NK cells in each liver was calculated by multiplying their respective percentages, as determined by flow cytometry, by total hepatic leukocyte counts.
Intracellular Staining of IFN-γ and Granzyme B
The frequency of NKT and NK cells expressing IFN-γ and granzyme B was determined by intracellular flow cytometry. For IFN-γ expression, isolated hepatic leukocytes (106 cells/mL RPMI) were stimulated with PMA/ionomycin (5 ng/mL and 500 ng/mL, respectively) in the presence of GolgiStop (1μl/mL; BD Biosciences) for 4 hours at 37°C followed by cell-surface staining for NKT and NK cells as described above. For granzyme B expression, cells were stained without stimulation. At this point, cells were fixed, permeabilized and stained using BD Cytofix/Cytoperm kit as per manufacturer's instructions (BD Biosciences). Cells were incubated with anti-IFN-γ APC (BD Biosciences) or anti-granzyme B FITC (Biosource, San Diego, CA) for 30 min at 4°C in perm/wash solution and then washed twice. Following data acquisition and analysis (as described above), the frequency of IFN-γ+ and granzyme B+ cells within NKT and NK cell populations was determined by gating on the respective cell types.
Statistics
Statistical analyses comparing means were performed using one-way Analysis of Variance (ANOVA) with Bonferroni's Multiple Comparison Test. All analyses were performed with Prism 4 software (GraphPad Software, San Diego, CA). Differences were considered significant when P < 0.05.
Results
Pathogenic Role of NKT and NK Cells in AILI is Dependent upon the Presence of DMSO
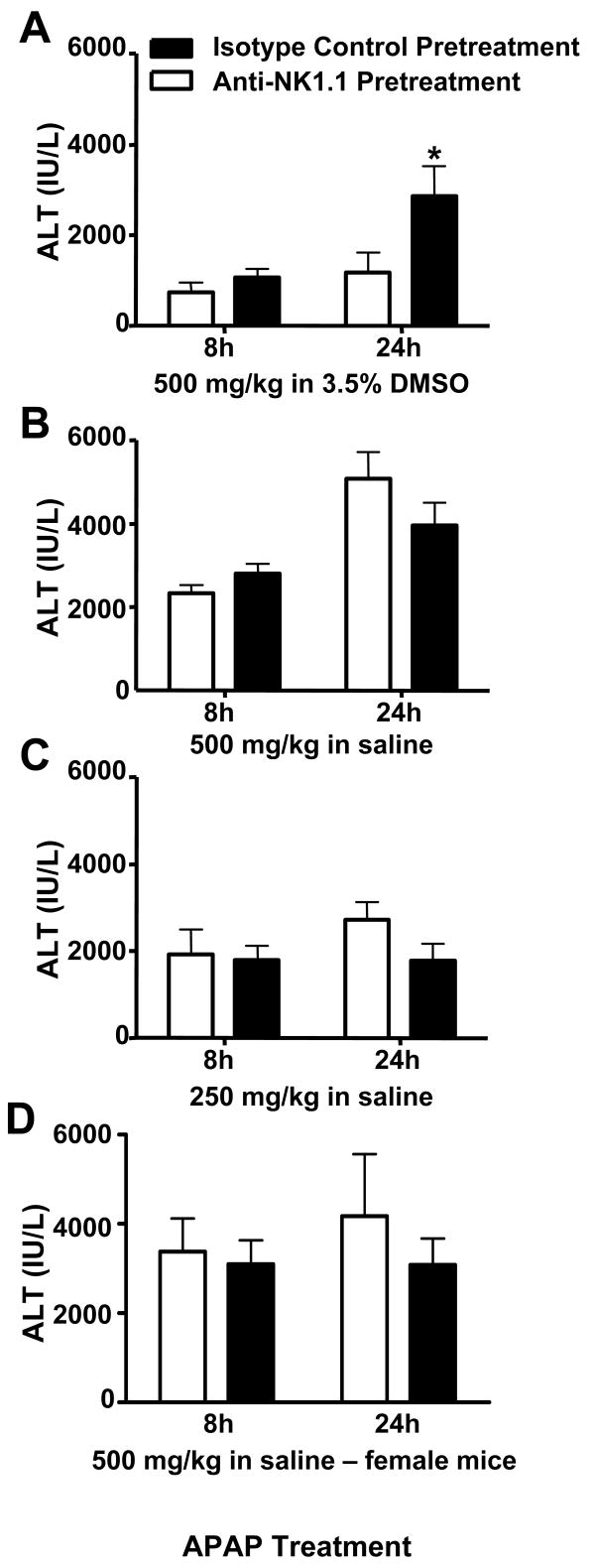
In a recent study reporting a pathogenic role for NKT and NK cells in AILI (16), 3.5 - 5% DMSO was used to facilitate APAP dissolution (Liu, Z.X., personal communication). As we were unable to reproduce these findings in a preliminary study using warm saline to dissolve APAP instead of DMSO, we decided to assess the role of NKT and NK cells in AILI more thoroughly in male C57Bl/6 mice. AILI, as measured by ALT activity in the serum, was indeed attenuated when NKT and NK cells were depleted prior to APAP dosing at 500 mg/kg in the presence of 3.5 % DMSO (Fig. 1A), as previously reported (16). In contrast, depleting these cells had no effect on AILI when APAP was administered in saline at 500 or 250 mg/kg (Fig. 1B and C, respectively). In addition, the use of female, instead of male, C57Bl/6 mice did not alter these results as AILI in female C57Bl/6 mice was not affected by NKT and NK cell-depletion when saline was used to dissolve 500 mg/kg APAP (Fig. 1D).
Figure 1.
Effect of DMSO on the role of NKT and NK cells in acetaminophen-induced liver injury. Following pretreatment with anti-NK1.1 (NKT and NK cell-depleting antibody) or mouse IgG2a (control antibody), male C57Bl/6 mice were treated with (A) APAP (500 mg/kg) in 3.5% DMSO, (B) APAP (500 mg/kg) in saline, or (C) APAP (250 mg/kg) in saline. In (D), female C57Bl/6 mice pretreated with anti-NK1.1 or mouse IgG2a prior to treatment with APAP (500 mg/kg) in saline. Serum ALT activity was measured at 8 h and 24 h as an indicator of liver injury. Results shown represent mean ± SEM of 8-12 mice per group. (*) P < 0.05 versus all other groups.
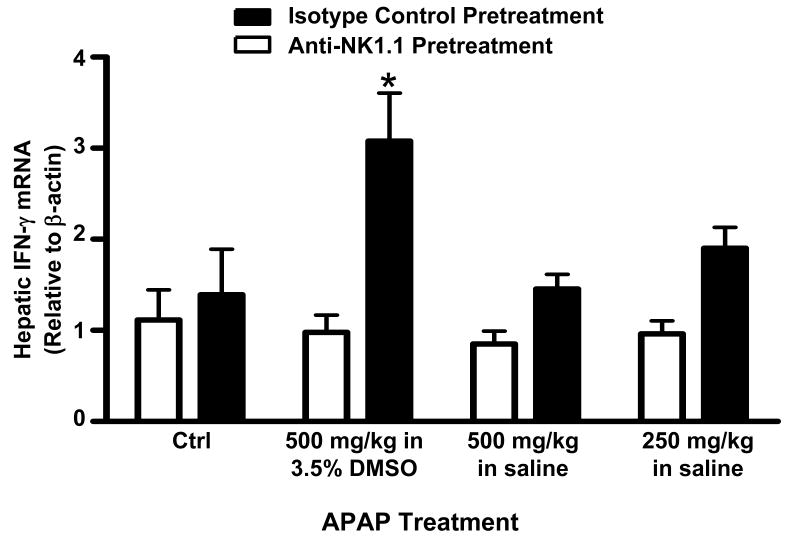
It was also reported that NKT and NK cells might promote AILI through the production of IFN-γ, a pro-inflammatory cytokine that contributes to AILI (19), as significantly less IFN-γ mRNA was found in the livers of NKT and NK cell-depleted mice following APAP treatment (16). When we repeated this experiment, we also observed decreased hepatic IFN-γ mRNA in NKT and NK cell-depleted mice 24h following APAP treatment; however, this decrease was only significant in the presence of DMSO (Fig. 2). Surprisingly, the use of DMSO to facilitate APAP dissolution appeared to enhance IFN-γ production as evidenced by the observation that hepatic IFN-γ mRNA levels were only elevated in non-depleted mice relative to control mice at 24h in the presence of DMSO (Fig. 2). Taken together, these findings suggest that NKT and NK cells can have a pathologic role in AILI when activated by DMSO.
Figure 2.
Effect of DMSO on hepatic IFN-γ mRNA expression following acetaminophen treatment. Following pretreatment with anti-NK1.1 (NKT and NK cell-depleting antibody) or mouse IgG2a (control antibody), mice were treated with saline (controls), APAP (500 mg/kg) in 3.5% DMSO, APAP (500 mg/kg) in saline, or APAP (250 mg/kg) in saline. Relative IFN-γ mRNA levels were measured in the liver at 24 h. Results shown represent mean ± SEM of 8 mice per group. (*) P < 0.05 versus control mice.
DMSO Activates Hepatic NKT and NK cells in the Absence of APAP In Vivo
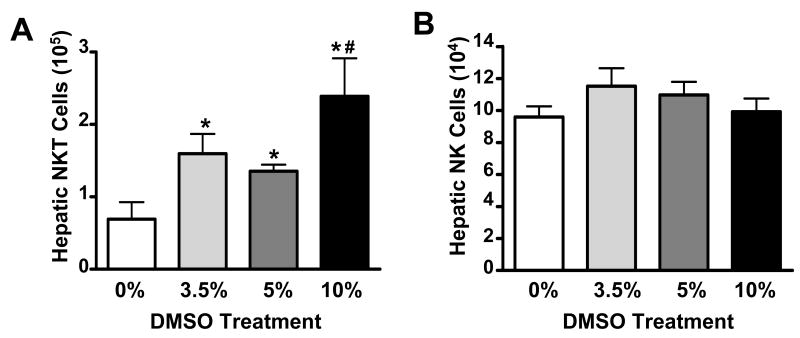
We first investigated the in vivo effects of DMSO on hepatic NKT and NK cells by administering DMSO in the absence of APAP at doses previously reported in AILI studies (0%, 3.5%, 5%, and 10% solutions corresponding to 0, 0.7, 1, and 2 mL/kg, respectively) (20-22). After 24 hours, the effects of DMSO were evaluated. While DMSO did not cause liver injury at these doses (data not shown), it did have an effect on hepatic NKT cells as evidenced by increased numbers of NKT cells in the livers of DMSO-treated mice (Fig. 3A). This rise in NKT cell numbers was due to an increase in the frequency of NKT cells rather than an increase in the number of total leukocytes (Table 1). In contrast, the absolute numbers of hepatic NK cells remained unchanged (Fig. 3B).
Figure 3.
DMSO increases NKT cells in the liver. Total number of hepatic (A) NKT cells (NK1.1+CD3+) and (B) NK cells (NK1.1+CD3-) in the liver 24 h following treatment with DMSO in saline (0%), 3.5%, 5%, or 10%. Data shown are representative of three independent experiments. Values represent the mean ± SEM of 5 mice per group. (*) P < 0.05 versus saline treatment; (#) P < 0.05 versus 5% DMSO treatment.
Table 1.
Frequencies of NKT and NK Cells in the Liver After DMSO Treatment
| % DMSO | % NKT Cells | % NK Cells | Total Hepatic Leukocytes |
|---|---|---|---|
| 0 | 7.1 ± 1.3 | 12.4 ± 0.9 | 9.4×105 ± 1.4×105 |
| 3.5 | 16.9 ± 3.5* | 12.2 ± 0.5 | 9.8×105 ± 1.7×105 |
| 5 | 13.7 ± 1.6* | 12.3 ± 0.7 | 1.0×106 ± 1.4×105 |
| 10 | 27.7 ± 4.6* | 10.5 ± 0.8 | 9.7×105 ± 9.1×104 |
p < 0.05 compared to 0% DMSO
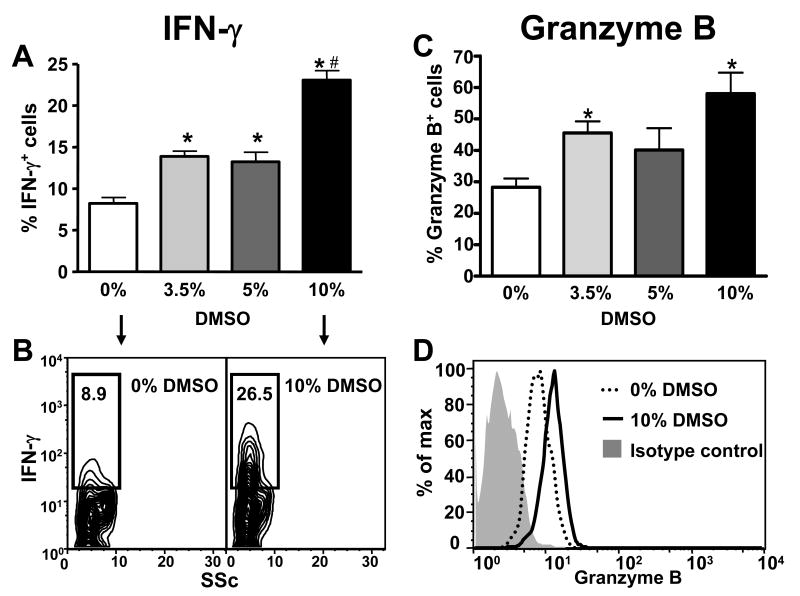
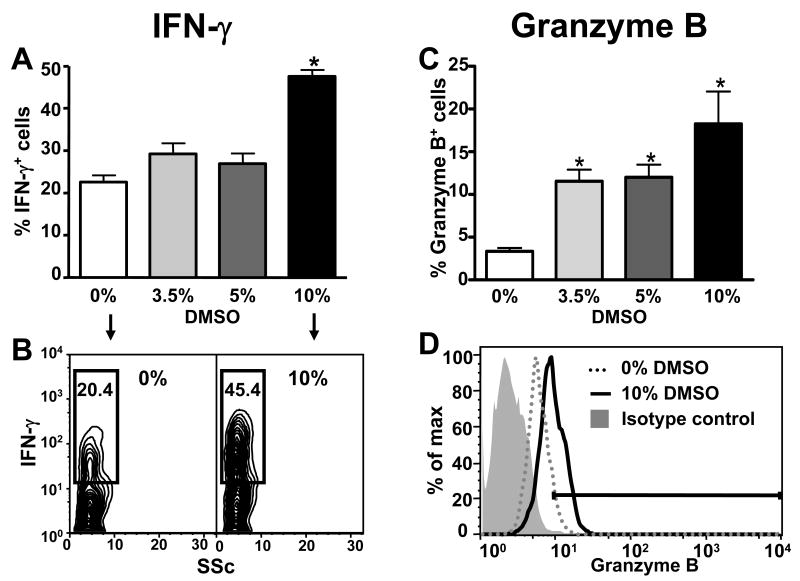
The effects of DMSO on the NKT and NK effector molecules, IFN-γ and granzyme B (23-25), were also studied 24 hours after treatment. The frequency of IFN-γ-expressing hepatic NKT cells was increased at all doses of DMSO (Fig. 4A and B) while the frequency of IFN-γ-expressing NK cells was only increased after 10% DMSO (Fig. 5A and B), suggesting that NKT cells might be more sensitive to the effects of DMSO on IFN-γ expression than NK cells. DMSO also increased the frequency of granzyme B-expressing NKT and NK cells in the liver (Fig. 4C and D and Fig. 5C and D, respectively). In this case, NKT and NK cells appeared equally sensitive to the effects of DMSO as a significant increase in the frequency of granzyme B-positive cells was detected at 3.5% DMSO in both cell types.
Figure 4.
DMSO induces IFN-γ and granzyme B expression in hepatic NKT cells. Frequencies of hepatic NKT cells expressing IFN-γ (A and B) and granzyme B (C and D) 24 h following treatment with DMSO (v/v) in saline (0%), 3.5%, 5%, or 10%. The frequencies of IFN-γ+ and granzyme B+ NKT cells were determined by gating on the NKT (NK1.1+CD3+) cell population. Data shown are representative of two independent experiments. Values represent the mean ± SEM of 5 mice per group. (*) P < 0.05 versus saline control. (#) P < 0.05 versus 5% DMSO. Also shown are representative flow cytometry data of IFN-γ (B) and granzyme B (D) expression by NKT cells in mice treated with (0%) or 10%DMSO in saline.
Figure 5.
DMSO induces IFN-γ and granzyme B expression in hepatic NK cells. Frequencies of hepatic NK cells expressing IFN-γ (A and B) and granzyme B (C and D) 24 h following treatment with DMSO in saline (0%), 3.5%, 5%, or 10%. The frequencies of IFN-γ+ and granzyme B+ NK cells were determined by gating on the NK (NK1.1+CD3-) cell population. Data shown are representative of two independent experiments. Values represent the mean ± SEM of 5 mice per group. (*) P < 0.05 versus all other groups. Also shown are representative flow cytometry data of IFN-γ (B) and granzyme B (D) expression by NK cells in mice treated with (0%) or 10% DMSO in saline.
DMSO Activates Hepatic NKT and NK Cells in the Presence of APAP In Vivo
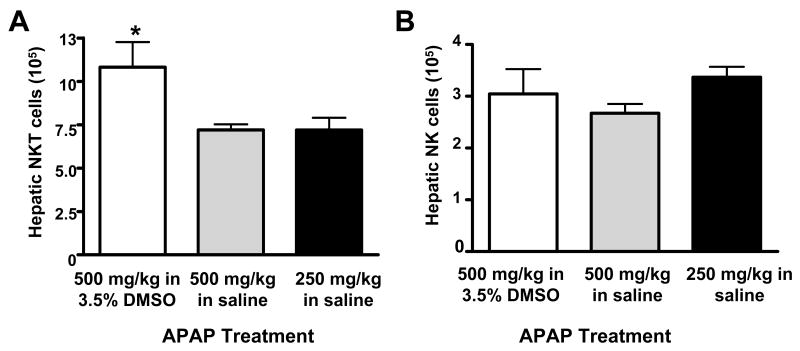
The effects of DMSO on hepatic NKT and NK cells were also evaluated within the context of AILI. Absolute numbers of NKT cells, but not NK cells, were highest in mice treated with APAP in DMSO (Fig. 6). This effect was again due to an increase in the frequency of NKT cells in the liver rather than an increase in the number of total leukocytes (Table 2).
Figure 6.
DMSO increases NKT cells in the livers of APAP-treated mice. Total number of hepatic (A) NKT (NK1.1+CD3+) and (B) NK (NK1.1+CD3-) cells in the liver 24 h following treatment with APAP (500 mg/kg) in 3.5% DMSO, APAP (500 mg/kg) in saline, or APAP (250 mg/kg) in saline. The total number of hepatic NKT and NK cells in control mice were 1.38 × 105 ± 1.71 × 104 and 1.22 × 105 ± 1.26 × 104, respectively. Data shown are representative of three independent experiments. Values represent the mean ± SEM of 5 mice per group. (*) P < 0.05 versus all other groups.
Table 2.
Frequencies of NKT and NK Cells in the Liver After APAP Treatment
| APAP | % NKT Cells | % NK Cells | Total Hepatic Leukocytes |
|---|---|---|---|
| 500 mg/kg in 3.5% DMSO | 27.5 ± 5.0 | 5.9 ± 0.6 | 5.7×106 ± 1.5×106 |
| 500 mg/kg in saline | 10.7 ± 0.3* | 4.0 ± 0.3 | 6.8×106 ± 7.5×105 |
| 250 mg/kg in saline | 11.6 ± 1.3* | 6.2 ± 0.8 | 5.6×106 ± 7.5×105 |
p < 0.05 compared to 500 mg/kg APAP in 3.5% DMSO
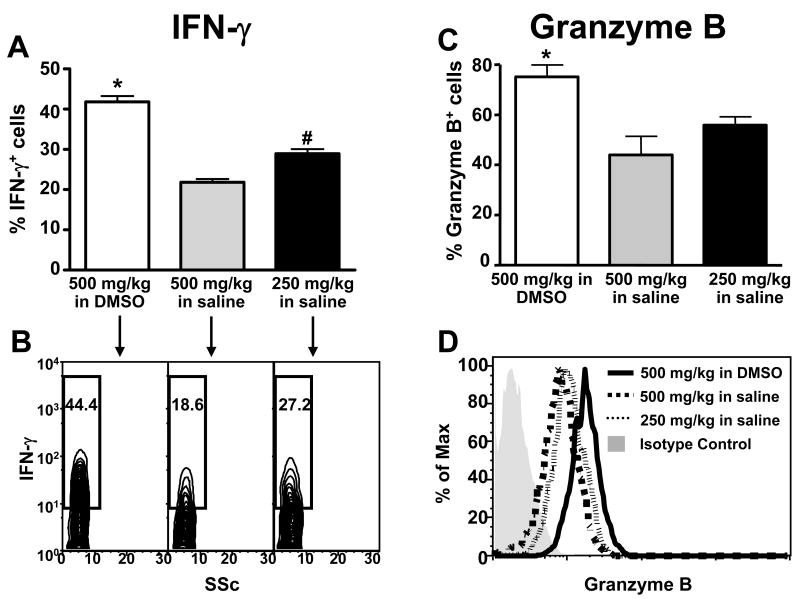
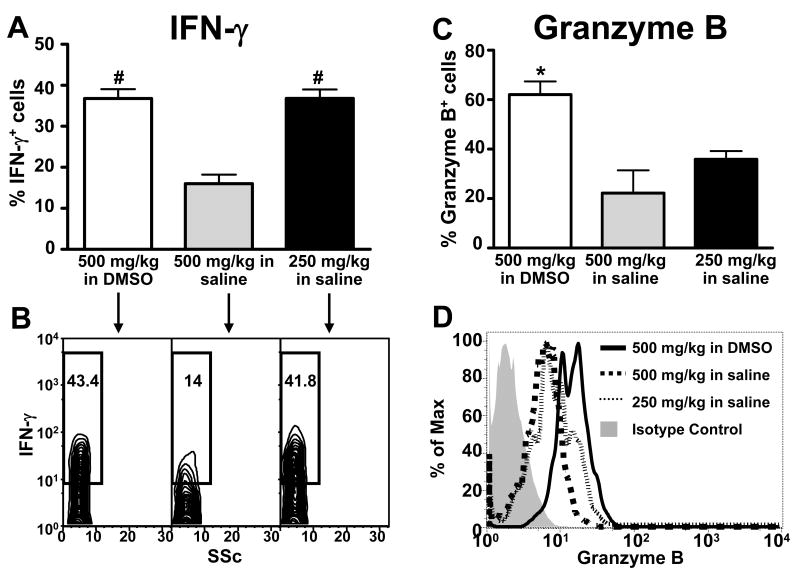
The frequency of IFN-γ-expressing hepatic NKT cells was elevated in mice treated with 500 mg/kg APAP in 3.5% DMSO as compared to mice treated with either 500 or 250 mg/kg APAP in saline (Fig. 7A and B). In addition, the frequency of IFN-γ-expressing NKT cells was significantly higher in mice treated with 250 mg/kg APAP in saline than in those treated with 500 mg/kg of APAP in saline (Fig. 7A and B). The effects of DMSO on IFN-γ expression within hepatic NK cells were somewhat different. Specifically, the frequency of IFN-γ-expressing NK cells was only elevated in mice treated with APAP in DMSO when compared to mice treated with 500 mg/kg of APAP in saline (Fig. 8A and B). The frequencies of IFN-γ-expressing hepatic NK cells were similar in mice treated with either 250 mg/kg APAP in saline or 500 mg/kg APAP in DMSO (Fig. 8A and B). In contrast, DMSO induced granzyme B expression in both hepatic NKT and NK cells. The frequencies of granzyme B-positive hepatic NKT (Fig. 7C and D) and NK cells (Fig. 8C and D) were significantly higher in mice treated with APAP in DMSO than in mice treated with either 500 mg/kg or 250 mg/kg APAP in saline. Taken together, these data suggest that DMSO could enhance the pathogenic role of NKT and NK cells in AILI by increasing the number of hepatic NKT cells and inducing the expression of cytotoxic effector molecules in both cell types.
Figure 7.
DMSO induces IFN-γ and granzyme B expression in hepatic NKT cells following APAP treatment. Frequencies of hepatic NKT cells expressing IFN-γ (A and B) and granzyme B (C and D) 24 h following treatment with APAP (500 mg/kg) in 3.5% DMSO, APAP (500 mg/kg) in saline, or APAP (250 mg/kg) in saline. The frequencies of IFN-γ+ and granzymeB+ NKT cells were determined by gating on the NKT (NK1.1+CD3+) cell population. The frequencies of hepatic NKT cells expressing IFN-γ and granzyme B in control mice were 5.72% ± 0.89% and 32.84% ± 2.27%, respectively. Data shown are representative of two independent experiments. Values represent the mean ± SEM of 5 mice per group. (*) P < 0.05 versus all other groups. (#) P < 0.05 versus APAP (500 mg/kg) in saline. Also shown are representative flow cytometry data of IFN-γ (B) and granzymeB (D) expression in NKT cells.
Figure 8.
DMSO induces IFN-γ and granzyme B expression in hepatic NK cells following APAP treatment. Frequencies of hepatic NK cells expressing IFN-γ (A and B) and granzyme B (C and D) 24 h following treatment with APAP (500 mg/kg) in 3.5% DMSO, APAP (500 mg/kg) in saline, or APAP (250 mg/kg) in saline. The frequencies of IFN-γ+ and granzyme B+ NK cells were determined by gating on the NK (NK1.1+CD3-) cell population. The frequencies of hepatic NK cells expressing IFN-γ and granzyme B in control mice were 5.96% ± 1.62% and 5.96% ± 0.88%, respectively. Data shown are representative of two independent experiments. Values represent the mean ± SEM of 5 mice per group. (*) P < 0.05 versus all other groups. (#) P < 0.05 versus APAP (500 mg/kg) in saline. Also shown are representative flow cytometry data of IFN-γ (B) and granzymeB (D) expression in NK cells.
Discussion
While DMSO is widely used in experimental systems due to its extraordinary solvent properties, it also exerts a number of biological effects that might not always be considered during experimental design. These effects include inhibition of APAP hepatic drug metabolism (26, 27), anti-oxidative activity (26-28), as well as adjuvant-like actions on the immune system (29-32). A recent example highlighting an unanticipated effect of DMSO was observed in a study testing the involvement of caspases, and possibly apoptosis, in AILI (20). In this study, a pancaspase inhibitor solubilized in 2.5% DMSO was administered to mice just prior to APAP treatment. The observed inhibition of AILI following treatment with this compound was subsequently discovered to be due apparently to the inhibition of APAP metabolism by DMSO rather than to the inhibition of caspases by the pancaspase inhibitor (20, 21).
Our findings here illustrate another example where the use of DMSO has confounded the interpretation of experimental results in AILI. The authors of a recent report concluded that NKT and NK cells of the innate immune system play a pathologic role in AILI (16). Our failure to reproduce these findings in either male (Fig. 1A and B) or female (Fig. 1D) C57Bl/6 mice led to the discovery that DMSO (3.5 – 5%) was used to facilitate the dissolution of APAP in these studies (Liu Z.X., personal communication). By comparing the degree of AILI between control mice and NKT and NK cell-depleted mice in the absence or presence of DMSO, we found that NKT and NK cells did not play a role in the pathology of AILI in C57Bl/6 male mice in the absence of DMSO (Fig. 1). In addition to affecting the role NKT and NK cells in AILI, the presence of DMSO also decreased the severity of liver injury at equivalent doses of APAP (Fig. 1A and B). This effect is likely due to the inhibition of APAP bioactivation by DMSO (26, 27) or to the anti-oxidative activity of DMSO (26-28) as discussed earlier. As a result of this decreased liver injury, mice were also treated with 250 mg/kg in saline to approximate the extent of hepatotoxicity in mice treated with 500 mg/kg of APAP in DMSO (Fig. 1C). Even at this lower dose of APAP, NKT and NK did not play a role in AILI. In contrast to these findings, depleting NKT and NK cells in C57Bl/6 mice deficient in IL-13 (33) has been reported to decrease susceptibility to AILI thereby indicating that NKT and NK cells may play a role in AILI under certain conditions.
We next explored possible ways DMSO might be affecting NKT and NK cells to enhance their pathogenic role in AILI. It was found DMSO, both alone and in the presence of APAP, increased the number of potentially cytotoxic NKT cells in the liver (Figs. 3A and 6A, respectively) while not affecting the number of hepatic NK cells (Figs. 3B and 6B, respectively). The mechanism(s) by which DMSO increased the number of NKT in liver is unclear at this time. However, it is possible that the anti-oxidative effects of DMSO might selectively extend the half-life of NKT cells in the liver by inhibiting activation-induced cell death as N-acetyl-cysteine, an antioxidant, has been shown to extend the lifetime of T cells (34). DMSO may also increase the number of NKT (NK1.1+CD3+) cells in the liver by up-regulating NK1.1 expression on the surface of hepatic NK1.1- NKT cells (35, 36) or by facilitating the mobilization of NKT cells from the blood to the liver (37-39).
We also demonstrated that both NKT and NK cells became activated following treatment with DMSO alone or together with APAP as indicated by increased expression of the cytotoxic effector molecules, IFN-γ and granzyme B (23-25) (Figs. 4 and 5 and Figs. 7 and 8, respectively). Although IFN-γ has been shown to play a pathogenic role in AILI (19), the role of granzyme B is unknown; as a result, granzyme B expression was only measured to assess the degree of hepatic NKT and NK cell activation. In addition, it was also discovered that the reported increase in hepatic IFN-γ mRNA levels 24h following APAP treatment (16) was only observed in our studies when animals were treated with APAP in 3.5% DMSO (Fig. 2). In the absence of DMSO, IFN-γ levels did not differ from control values at 24h (Fig. 2). This observation is consistent with the findings of Ishida et al (19) who first demonstrated a pathogenic role for IFN-γ in APAP-induced liver injury and did not use DMSO as a vehicle for APAP. They reported that although hepatic IFN-γ mRNA levels were significantly elevated 10h after APAP, IFN-γ mRNA levels returned to baseline control values by 24h. These results suggest that DMSO may sustain elevated levels of IFN-γ mRNA by increasing the number of NKT cells in the liver and by activating hepatic NKT and NK cells to produce more IFN-γ. The mechanism by which DMSO induces hepatic NKT and NK cell activation in our studies remains to be determined. To our knowledge, there are only three in vivo studies reporting such immune-stimulatory actions of DMSO. The first study reported increased IL-2 and IL-2 receptor expression in MRL/lpr mice after 1 month of continuous DMSO treatment (40). In the second study, a single injection of DMSO was reported to augment the immune response to Sindbis virus infection in a mouse model by increasing antibody titers and serum IFN-γ levels (30). The third reported that when combined with DMSO, bacterial antigen given intranasally to rabbits, induced higher titres of agglutinizing antibodies than antigen alone (41).
As IFN-γ has been clearly shown to play a pro-inflammatory role in AILI (19), it is logical to speculate that IFN-γ might also be produced outside the liver. In fact, we have observed an increase in splenic IFN-γ mRNA following acetaminophen treatment (unpublished observations). Currently, we do not know what stimulates the production of IFN-γ outside the liver or what cells could make IFN-γ. One possibility is that factors such as macrophage migration inhibitory factor (MIF), which are released from hepatocytes following acetaminophen (42), could signal the production of IFN-γ by extra-hepatic T cells.
We did not examine the effects of DMSO on hepatic NKT vs. NK cells in AILI using CD1-/- mice or anti-asialo antibodies, respectively, as it was previously found that both NKT and NK cells must be depleted in order to attenuate AILI when DMSO was used as a solvent for APAP (16). This finding suggested that DMSO acts on both hepatic NKT and NK cells, and in keeping with this observation, DMSO was found to activate both cell types in the absence of APAP (Fig. 3 to 5). While DMSO activates both cell types, it affects hepatic NKT and NK cells differentially. Specifically, DMSO increased the number of NKT cells, but had no effect on NK cell numbers (Fig. 3). In addition, although DMSO appeared to increase IFN-γ expression in both cell types, the frequency of IFN-γ-expressing hepatic NKT cells was increased at all doses (3.5 – 10%) of DMSO, while the frequency of IFN-γ-expressing hepatic NK cells was only increased after 10% DMSO (Figures 4 and 5). Currently, we do not know the mechanism by which DMSO activates hepatic NKT and NK cells in AILI. Future studies should examine whether DMSO affects these cells directly by analyzing the activation status of hepatic NKT and NK cells following incubation with DMSO in vitro.
In conclusion, these findings indicate that the previously reported pathogenic role of NKT and NK cells in AILI is dependent on the use of DMSO to facilitate APAP dissolution. The ability of DMSO to increase the number of hepatic NKT cells and activate both NKT and NK cells to produce cytotoxic products might be relevant to a wide variety of other liver disease models involving NKT and NK cells, including those mediated by concanavalin A (43), alpha-galactosylceramide (44), endotoxin (45), viral hepatitis (46), and NKT and NK cell-mediated rejection of liver metastases (47, 48). Therefore, the use of DMSO to facilitate the dissolution of insoluble compounds must be carefully considered in studies involving the hepatic immune system and possibly other organ systems.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH and the NHLBI.
Funding Source: This research was supported by the Intramural Research Program of the NIH and the NHLBI.
Abbreviations
- APAP
acetaminophen
- DMSO
dimethyl sulfoxide
- DILI
drug-induced liver injury
- ALT
alanine transaminase
- IFN
interferon
- NK
natural killer
Contributor Information
Mary Jane Masson, Email: massonm@nhlbi.nih.gov.
Leah D. Carpenter, Email: carpenterld@gmail.com.
Mary L. Graf, Email: graft@nhbli.nih.gov.
Lance R. Pohl, Email: pohll@nhlbi.nih.gov.
References
- 1.Classen DC, Pestotnik SL, Evans RS, Lloyd JF, Burke JP. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. Jama. 1997;277:301–306. [PubMed] [Google Scholar]
- 2.Abboud G, Kaplowitz N. Drug-induced liver injury. Drug Saf. 2007;30:277–294. doi: 10.2165/00002018-200730040-00001. [DOI] [PubMed] [Google Scholar]
- 3.Jaeschke H, Bajt ML. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol Sci. 2006;89:31–41. doi: 10.1093/toxsci/kfi336. [DOI] [PubMed] [Google Scholar]
- 4.Gunawan BK, Kaplowitz N. Mechanisms of drug-induced liver disease. Clin Liver Dis. 2007;11:459–475. v. doi: 10.1016/j.cld.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Ju C. Immunological mechanisms of drug-induced liver injury. Curr Opin Drug Discov Devel. 2005;8:38–43. [PubMed] [Google Scholar]
- 6.Cohen SD, Pumford NR, Khairallah EA, Boekelheide K, Pohl LR, Amouzadeh HR, Hinson JA. Selective protein covalent binding and target organ toxicity. Toxicol Appl Pharmacol. 1997;143:1–12. doi: 10.1006/taap.1996.8074. [DOI] [PubMed] [Google Scholar]
- 7.Larson AM. Acetaminophen hepatotoxicity. Clin Liver Dis. 2007;11:525–548. vi. doi: 10.1016/j.cld.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Liu ZX, Kaplowitz N. Role of innate immunity in acetaminophen-induced hepatotoxicity. Expert Opin Drug Metab Toxicol. 2006;2:493–503. doi: 10.1517/17425255.2.4.493. [DOI] [PubMed] [Google Scholar]
- 9.Dambach DM, Watson LM, Gray KR, Durham SK, Laskin DL. Role of CCR2 in macrophage migration into the liver during acetaminophen-induced hepatotoxicity in the mouse. Hepatology. 2002;35:1093–1103. doi: 10.1053/jhep.2002.33162. [DOI] [PubMed] [Google Scholar]
- 10.Hogaboam CM, Bone-Larson CL, Steinhauser ML, Matsukawa A, Gosling J, Boring L, Charo IF, et al. Exaggerated hepatic injury due to acetaminophen challenge in mice lacking C-C chemokine receptor 2. Am J Pathol. 2000;156:1245–1252. doi: 10.1016/S0002-9440(10)64995-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaeschke H. How relevant are neutrophils for acetaminophen hepatotoxicity? Hepatology. 2006;43:1191–1194. doi: 10.1002/hep.21246. [DOI] [PubMed] [Google Scholar]
- 12.Liu ZX, Han D, Gunawan B, Kaplowitz N. Neutrophil depletion protects against murine acetaminophen hepatotoxicity. Hepatology. 2006;43:1220–1230. doi: 10.1002/hep.21175. [DOI] [PubMed] [Google Scholar]
- 13.Ishida Y, Kondo T, Kimura A, Tsuneyama K, Takayasu T, Mukaida N. Opposite roles of neutrophils and macrophages in the pathogenesis of acetaminophen-induced acute liver injury. Eur J Immunol. 2006;36:1028–1038. doi: 10.1002/eji.200535261. [DOI] [PubMed] [Google Scholar]
- 14.Ju C, Reilly TP, Bourdi M, Radonovich MF, Brady JN, George JW, Pohl LR. Protective role of Kupffer cells in acetaminophen-induced hepatic injury in mice. Chem Res Toxicol. 2002;15:1504–1513. doi: 10.1021/tx0255976. [DOI] [PubMed] [Google Scholar]
- 15.Laskin DL, Gardner CR, Price VF, Jollow DJ. Modulation of macrophage functioning abrogates the acute hepatotoxicity of acetaminophen. Hepatology. 1995;21:1045–1050. [PubMed] [Google Scholar]
- 16.Liu ZX, Govindarajan S, Kaplowitz N. Innate immune system plays a critical role in determining the progression and severity of acetaminophen hepatotoxicity. Gastroenterology. 2004;127:1760–1774. doi: 10.1053/j.gastro.2004.08.053. [DOI] [PubMed] [Google Scholar]
- 17.Bartolone JB, Sparks K, Cohen SD, Khairallah EA. Immunochemical detection of acetaminophen-bound liver proteins. Biochem Pharmacol. 1987;36:1193–1196. doi: 10.1016/0006-2952(87)90069-4. [DOI] [PubMed] [Google Scholar]
- 18.You Q, Cheng L, Reilly TP, Wegmann D, Ju C. Role of neutrophils in a mouse model of halothane-induced liver injury. Hepatology. 2006;44:1421–1431. doi: 10.1002/hep.21425. [DOI] [PubMed] [Google Scholar]
- 19.Ishida Y, Kondo T, Ohshima T, Fujiwara H, Iwakura Y, Mukaida N. A pivotal involvement of IFN-gamma in the pathogenesis of acetaminophen-induced acute liver injury. Faseb J. 2002;16:1227–1236. doi: 10.1096/fj.02-0046com. [DOI] [PubMed] [Google Scholar]
- 20.El-Hassan H, Anwar K, Macanas-Pirard P, Crabtree M, Chow SC, Johnson VL, Lee PC, et al. Involvement of mitochondria in acetaminophen-induced apoptosis and hepatic injury: roles of cytochrome c, Bax, Bid, and caspases. Toxicol Appl Pharmacol. 2003;191:118–129. doi: 10.1016/s0041-008x(03)00240-0. [DOI] [PubMed] [Google Scholar]
- 21.Jaeschke H, Cover C, Bajt ML. Role of caspases in acetaminophen-induced liver injury. Life Sci. 2006;78:1670–1676. doi: 10.1016/j.lfs.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165–178. doi: 10.1053/j.gastro.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 23.Kaneko Y, Harada M, Kawano T, Yamashita M, Shibata Y, Gejyo F, Nakayama T, et al. Augmentation of Valpha14 NKT cell-mediated cytotoxicity by interleukin 4 in an autocrine mechanism resulting in the development of concanavalin A-induced hepatitis. J Exp Med. 2000;191:105–114. doi: 10.1084/jem.191.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi L, Kraut RP, Aebersold R, Greenberg AH. A natural killer cell granule protein that induces DNA fragmentation and apoptosis. J Exp Med. 1992;175:553–566. doi: 10.1084/jem.175.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim S, Iizuka K, Aguila HL, Weissman IL, Yokoyama WM. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc Natl Acad Sci U S A. 2000;97:2731–2736. doi: 10.1073/pnas.050588297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeffery EH, Arndt K, Haschek WM. Mechanism of inhibition of hepatic bioactivation of paracetamol by dimethyl sulfoxide. Drug Metabol Drug Interact. 1988;6:413–424. doi: 10.1515/dmdi.1988.6.3-4.413. [DOI] [PubMed] [Google Scholar]
- 27.Park Y, Smith RD, Combs AB, Kehrer JP. Prevention of acetaminophen-induced hepatotoxicity by dimethyl sulfoxide. Toxicology. 1988;52:165–175. doi: 10.1016/0300-483x(88)90202-8. [DOI] [PubMed] [Google Scholar]
- 28.Dunphy GB, Chen G, Webster JM. The antioxidants dimethylsulfoxide and dimethylthiourea affect the immediate adhesion responses of larval haemocytes from 3 lepidopteran insect species. Can J Microbiol. 2007;53:1330–1347. doi: 10.1139/W07-096. [DOI] [PubMed] [Google Scholar]
- 29.Yamasaki T, Klein G, Ljunggren HG, Hoglund P, Ohlen C, Petersson MG, Karre K. Effects of dimethyl sulfoxide treatment on H-2 expression and susceptibility to NK- or cytotoxic T-lymphocyte-mediated lysis of the YAC-1 lymphoma and its beta 2-microglobulin-deficient variant. J Natl Cancer Inst. 1988;80:263–269. doi: 10.1093/jnci/80.4.263. [DOI] [PubMed] [Google Scholar]
- 30.Kunze M. Production of interferon in the white mouse by dimethyl sulfoxide. Ann N Y Acad Sci. 1975;243:308–310. doi: 10.1111/j.1749-6632.1975.tb25370.x. [DOI] [PubMed] [Google Scholar]
- 31.Bartfeld H, Goldstein A. Cell-mediated immunity: its modulation by dimethyl sulfoxide. Ann N Y Acad Sci. 1975;243:81–90. doi: 10.1111/j.1749-6632.1975.tb25346.x. [DOI] [PubMed] [Google Scholar]
- 32.Dennis AJ, Wilson HE. Altered mitogenic responsiveness of chronic leukemic lymphocytes and normal human lymphocytes treated with dimethyl sulfoxide. Ann N Y Acad Sci. 1975;243:73–80. doi: 10.1111/j.1749-6632.1975.tb25345.x. [DOI] [PubMed] [Google Scholar]
- 33.Yee SB, Bourdi M, Masson MJ, Pohl LR. Hepatoprotective role of endogenous interleukin-13 in a murine model of acetaminophen-induced liver disease. Chem Res Toxicol. 2007;20:734–744. doi: 10.1021/tx600349f. [DOI] [PubMed] [Google Scholar]
- 34.Sandstrom PA, Mannie MD, Buttke TM. Inhibition of activation-induced death in T cell hybridomas by thiol antioxidants: oxidative stress as a mediator of apoptosis. J Leukoc Biol. 1994;55:221–226. doi: 10.1002/jlb.55.2.221. [DOI] [PubMed] [Google Scholar]
- 35.Stenstrom M, Skold M, Andersson A, Cardell SL. Natural killer T-cell populations in C57BL/6 and NK1.1 congenic BALB.NK mice-a novel thymic subset defined in BALB.NK mice. Immunology. 2005;114:336–345. doi: 10.1111/j.1365-2567.2004.02111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benlagha K, Kyin T, Beavis A, Teyton L, Bendelac A. A thymic precursor to the NK T cell lineage. Science. 2002;296:553–555. doi: 10.1126/science.1069017. [DOI] [PubMed] [Google Scholar]
- 37.Eberl G, MacDonald HR. Rapid death and regeneration of NKT cells in anti-CD3epsilon-or IL-12-treated mice: a major role for bone marrow in NKT cell homeostasis. Immunity. 1998;9:345–353. doi: 10.1016/s1074-7613(00)80617-2. [DOI] [PubMed] [Google Scholar]
- 38.Halder RC, Aguilera C, Maricic I, Kumar V. Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease. J Clin Invest. 2007;117:2302–2312. doi: 10.1172/JCI31602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnston B, Kim CH, Soler D, Emoto M, Butcher EC. Differential chemokine responses and homing patterns of murine TCR alpha beta NKT cell subsets. J Immunol. 2003;171:2960–2969. doi: 10.4049/jimmunol.171.6.2960. [DOI] [PubMed] [Google Scholar]
- 40.Wysenbeek AJ, Lourie S, Weinberger A, Schoenfeld N, Pick AI, Pecht M. Immunologic alterations in MRL/lpr mice after chronic dimethyl sulfoxide administration. Int J Immunopharmacol. 1987;9:769–773. doi: 10.1016/0192-0561(87)90072-5. [DOI] [PubMed] [Google Scholar]
- 41.Tschope M. The Potential of DMSO in Experimental Immunology. In: Laudahn G, G K, editors. Dimethylsuloxyl; Internationales Symposium in Wien; Germany: Saladruck; 1966. [Google Scholar]
- 42.Bourdi M, Reilly TP, Elkahloun AG, George JW, Pohl LR. Macrophage migration inhibitory factor in drug-induced liver injury: a role in susceptibility and stress responsiveness. Biochem Biophys Res Commun. 2002;294:225–230. doi: 10.1016/S0006-291X(02)00466-7. [DOI] [PubMed] [Google Scholar]
- 43.Takeda K, Hayakawa Y, Van Kaer L, Matsuda H, Yagita H, Okumura K. Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc Natl Acad Sci U S A. 2000;97:5498–5503. doi: 10.1073/pnas.040566697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Osman Y, Kawamura T, Naito T, Takeda K, Van Kaer L, Okumura K, Abo T. Activation of hepatic NKT cells and subsequent liver injury following administration of alpha-galactosylceramide. Eur J Immunol. 2000;30:1919–1928. doi: 10.1002/1521-4141(200007)30:7<1919::AID-IMMU1919>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 45.Li Z, Oben JA, Yang S, Lin H, Stafford EA, Soloski MJ, Thomas SA, et al. Norepinephrine regulates hepatic innate immune system in leptin-deficient mice with nonalcoholic steatohepatitis. Hepatology. 2004;40:434–441. doi: 10.1002/hep.20320. [DOI] [PubMed] [Google Scholar]
- 46.Baron JL, Gardiner L, Nishimura S, Shinkai K, Locksley R, Ganem D. Activation of a nonclassical NKT cell subset in a transgenic mouse model of hepatitis B virus infection. Immunity. 2002;16:583–594. doi: 10.1016/s1074-7613(02)00305-9. [DOI] [PubMed] [Google Scholar]
- 47.Park SH, Kyin T, Bendelac A, Carnaud C. The contribution of NKT cells, NK cells, and other gamma-chain-dependent non-T non-B cells to IL-12-mediated rejection of tumors. J Immunol. 2003;170:1197–1201. doi: 10.4049/jimmunol.170.3.1197. [DOI] [PubMed] [Google Scholar]
- 48.Smyth MJ, Thia KY, Street SE, Cretney E, Trapani JA, Taniguchi M, Kawano T, et al. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med. 2000;191:661–668. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]