Abstract
The basic structural organization of axonal projections from the small but distinct magnocellular and ventral nuclei (of the bed nuclei of the stria terminalis) were analyzed with the PHAL anterograde tract tracing method in adult male rats. The former's overall projection pattern is complex, with over 80 distinct terminal fields ipsilateral to injection sites. Innervated regions in the cerebral hemisphere and brainstem fall into 9 general functional categories: cerebral nuclei, behavior control column, orofacial motor-related, humorosensory/thirst-related, brainstem autonomic control network, neuroendocrine, hypothalamic visceromotor pattern generator network, thalamocortical feedback loops, and behavioral state control. The most novel findings indicate that the magnocellular nucleus projects to virtually all known major parts of the brain network that controls pelvic functions including micturition, defecation, and penile erection—as well as to brain networks controlling nutrient and body water homeostasis. This and other evidence suggests that the magnocellular nucleus is part of a cortico-striatopallidal differentiation modulating and coordinating pelvic functions with the maintenance of nutrient and body water homeostasis. Projections of the ventral nucleus are a subset of those generated by the magnocellular nucleus, with the obvious difference that the ventral nucleus does not project detectably to Barrington's nucleus, the subfornical organ, the median preoptic and parastrial nuclei, the neuroendocrine system, and midbrain orofacial motor-related regions.
Keywords: amygdala; autonomic; Barrington's nucleus; bed nucleus of the stria terminalis; hypothalamus; paraventricular nucleus, pelvic nerve
INTRODUCTION
As part of a systematic, high-resolution analysis of how axonal projections from the various cell groups making up the bed nuclei of the stria terminalis (BST) are organized and distributed spatially to the rest of the brain, we have already described experiments dealing with the posterior division (Dong and Swanson, 2004b), as well as with the lateral group of the anterior division, also known as the anterolateral group (Dong et al., 2000, 2001b; Dong and Swanson, 2003, 2004a). This and the accompanying two papers (Dong and Swanson, 2005a,b) deal with the third major component of the BST, the medial group of the anterior division (the BST anteromedial group; BSTamg).
Here we focus on a distinct cell group buried deep in the BST anteromedial group, the BST magnocellular nucleus (BSTmg), and also provide information on the tiny, subjacent BST ventral nucleus (BSTv). The BSTmg is readily distinguished from other BST components by its large, darkly stained neurons as viewed in transverse, Nissl-stained sections of the rat brain (Ju and Swanson, 1989). A number of peptides—including corticotropin-releasing hormone, neurotensin, and galanin (Ju et al., 1989), as well as vasopressin (van Leeuwen and Caffe, 1983)—are expressed by neurons in the BSTmg. Furthermore, neurons in this vicinity concentrate gonadal steroid hormones (Pfaff and Keiner, 1973; Sar and Stumpf, 1975; Stumpf et al., 1975), and express estrogen and androgen receptor mRNAs (Simerly et al., 1990; Shughrue et al., 1997).
Recent PHAL pathway tracing analyses indicate that the BSTmg receives substantial inputs from both the central and medial amygdalar nuclei, which are associated with autonomic control and pheromonal sensory systems, respectively; from the posterior basomedial amygdalar nucleus (which receives major inputs from the main olfactory system); from the infralimbic area of the medial prefrontal region; and from the ventral subiculum of the hippocampal formation (see Dong et al., 2001a). The BSTmg is thus positioned to integrate neural inputs from the central autonomic control system, main and accessory olfactory systems, medial prefrontal cortex, and hippocampal formation with the effects of circulating gonadal steroid hormones.
The pattern of axonal projections specifically from the BSTmg has never been examined, although general projections of the “ventral” part of the BST were analyzed recently in lactating rats with PHAL and wheat germ agglutinin (Numan and Numan, 1996, 1997). (BSTdm; Dong and Swanson, 2005a). Of particular interest, a number of major terminal fields shared by the BSTdm, BSTmg, and BSTv in turn send descending projections to the region of sympathetic and parasympathetic preganglionic neurons in the spinal intermediolateral column, as well as lumbosacral somatic motor neuron pools, that in turn innervate pelvic organs. The overall projection pattern of the BSTmg suggests that it coordinates specific neuroendocrine, autonomic, and somatomotor responses, components of which include micturition, defecation, and penile erection.
MATERIALS AND METHODS
They were identical to those described earlier (Dong and Swanson, 2003). Experiments were performed according to NIH Guidelines for the Care and Use of Laboratory Animals, and all protocols were approved by the University of Southern California Institutional Animal Care and Use Committee. The experiments described here were chosen from a collection of over 200 PHAL injections in all parts of the BST. Adult male Harlan Sprague-Dawley rats (300-350 g) received a single, stereotaxically placed iontophoretic injection of a 2.5% solution of PHAL (Vector Laboratories, Burlingame, CA), prepared in 0.1 M sodium phosphate-buffered saline (NaPBS), pH 7.4, into various regions of the BST through a glass micropipette (15 μm tip diameter) by applying a positive current (5 μA, 7 sec on/off intervals) for 7-10 min. Animals were anesthetized for stereotaxic surgery with an equal mixture of ketamine and xylazine solutions (50 mg/ml ketamine, 10 mg xylazine/ml; 1 ml/kg body weight).
After surviving 14-16 days, the rats were deeply anesthetized with pentobarbital (40 mg/kg body weight, intraperitoneal) and perfused transcardially with 150 ml of 0.9% NaCl followed by 300 ml of ice-cold 4% paraformaldehyde in 0.1 M borate buffer (pH 9.5). Brains were removed, post-fixed overnight at 4°C in the same fixative containing 10% sucrose, and frozen. Then serial 30 μm-thick sections (1-in-4) were cut in the transverse plane on a sliding microtome. One complete series of sections was processed to detect PHAL using the immunohistochemical procedure described elsewhere (Gerfen and Sawchenko, 1984; Petrovich and Swanson, 1997). PHAL-containing cells (in the injection sites) and fibers were plotted with the aid of a camera lucida onto cytoarchitectonic drawings of adjacent thionin-stained sections, and then transferred onto a series of standard drawings of the rat brain (Swanson, 2004) with the aid of a computer (Apple, Mac PowerPC G4, Adobe Illustrator CS). Photomicrographs were taken with a CCD camera (Diagnostics Instruments, Sterling Heights, MI) or with a Wild Leitz 35 mm camera mounted on a Wild M3Z stereozoom microscope. Film was digitized with a Nikon scanner (LS-1000), and all digital files were composed, and adjusted for brightness and contrast, in Adobe Photoshop 5 using a Mac PowerPC G4. Parceling of the rat brain, the terminology for describing morphological features of PHAL-labeled axons, and mapping strategies and procedures follow Swanson (2004), unless indicated otherwise.
RESULTS
Nomenclature
Parceling of the rat BST is based on the original work of Ju and colleagues (Ju and Swanson, 1989; Ju et al., 1989), refined as a result of later architectonic and connectional evidence. The boundaries of the BSTmg have stayed the same, but the lateral half of the original BSTv (Atlas Level 21; Swanson 1998-1999) has been assigned to the BST anteromedial area (BSTam), the third, relatively undifferentiated, component of the BSTamg (along with the BSTmg and BSTv). This contraction of the BSTv is based first on a great deal of additional cytoarchitectonic analysis (in some 200 brains), from which it is clear that the lateral half of the original BSTv actually resembles more closely the BSTam (with a preponderance of small rather than medium-sized neurons; Ju and Swanson, 1989); and second on evidence that the connections of this lateral region of the original BSTv generates connections resembling those of the BSTam (e.g., experiments BST112 and ACB12 in Dong and Swanson, 2005b) rather than the definitive BSTv (below). This interpretation of BST parceling is adopted in Swanson (2004).
Injection sites
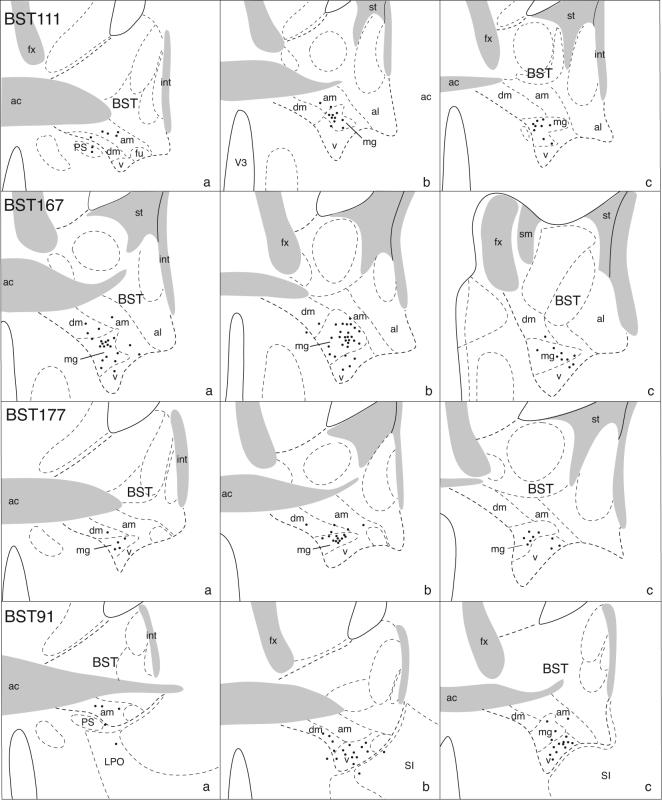
In three experiments (BST111, 167, and 171) the PHAL injection site is centered in the BSTmg. Figures 1 and 2 show that these small injection sites are restricted almost entirely to the nucleus, as judged by the distribution of PHAL-labeled neuronal somata. However, partly because of the BSTmg's tiny volume and irregular shape and partly because dendrites of neurons in adjacent regions may spread into the BSTmg and incorporate the tracer, all three injection sites labeled a few neurons in adjacent regions, particularly the BSTv, BSTdm, and BST anteromedial area—although no labeled neurons were found in the medially adjacent medial preoptic area. The projections labeled in experiment BST167 are illustrated in detail because its injection site is the largest of the three, and because its overall projection pattern is indistinguishable from the other two.
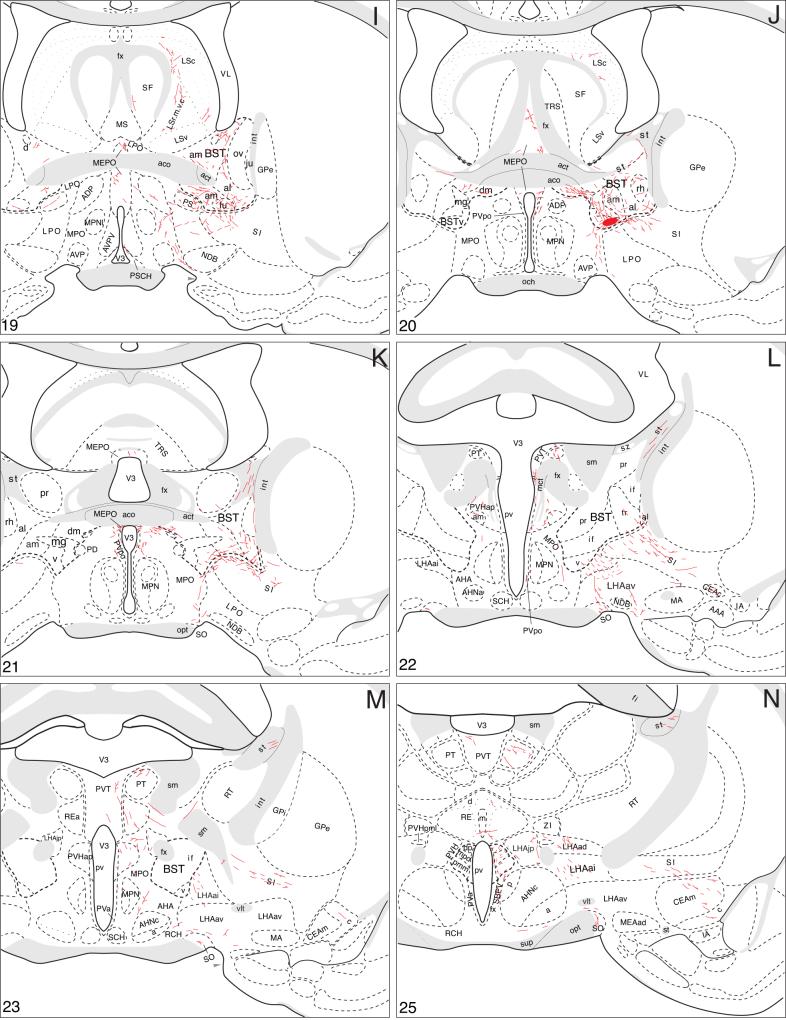
Fig. 1.
Camera lucida plots to show the distribution of PHAL-labeled neurons (black dots) in the BST of transverse histological sections through the injection site in 4 experiments. The injections are centered in the BSTmg (experiments BST111, 167, and 177), and the BSTv (experiment BST91). In each row, drawings are arranged from rostral (left) to caudal (right).
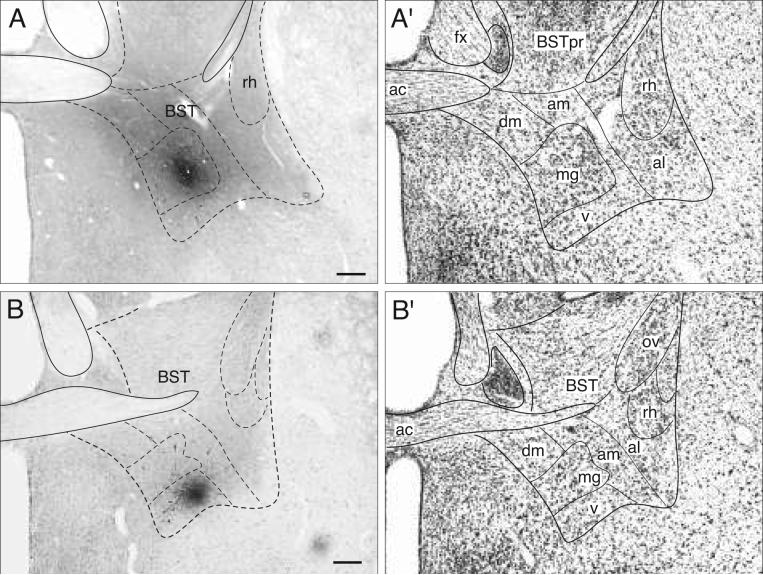
Fig. 2.
Brightfield photomicrographs to show the appearance of a PHAL injection site centered in the BSTmg (A, experiment BST167), and BSTv (B, experiment BST91). For cytoarchitectonic comparison an adjacent Nissl-stained section is shown to the right (A′ and B′; both at about level I in Fig. 4). All scale bars = 200 μm.
As a control for projections labeled by PHAL uptake outside the BSTmg borders, detailed comparisons are made with projections labeled by PHAL injections centered in ventral regions of the BST anteromedial area (e.g., experiment ACB12), BSTdm (e.g., experiment BST116), and BSTv (experiment BST91). Detailed analyses of projection patterns from the former two BST cell groups are dealt with in the accompanying papers (Dong and Swanson, 2005a,b), whereas the latter will be considered here.
Organization of projections from the BSTmg
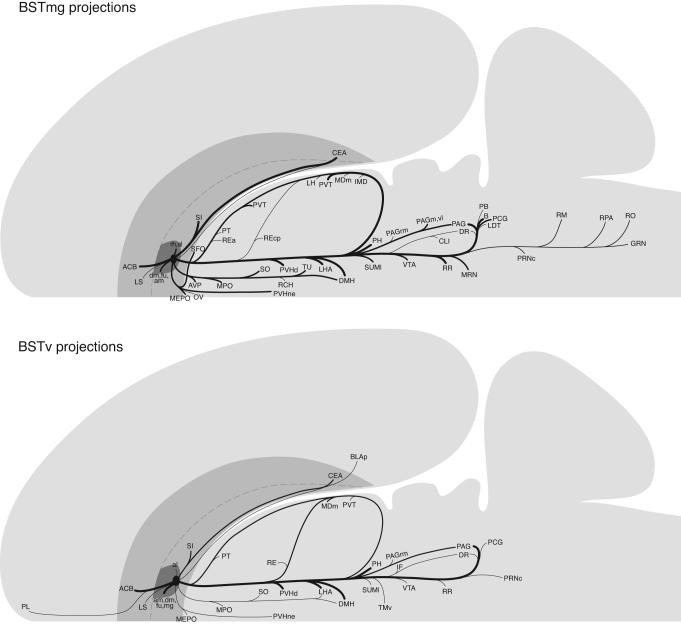
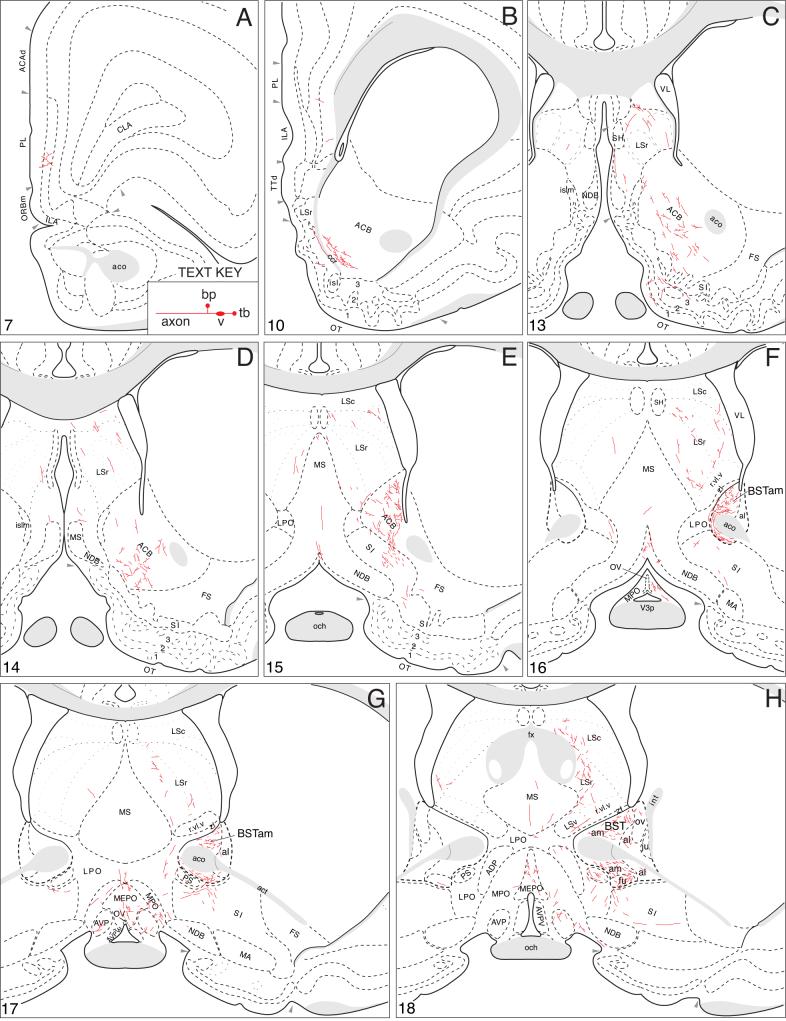
The axonal projections of the BSTmg follow at least seven pathways that are illustrated schematically in Figure 3. A more precise rendering of the pathways and their associated terminal fields, along with the distribution of local connections within the BST, is presented in a series of transverse atlas levels (Fig. 4).
Fig. 3.
Summary diagrams indicating the general organization of projections from the BSTmg and BSTv. The relative size of each pathway is roughly proportional to the thickness of the line representing it. The flatmap is based on Swanson (2004).
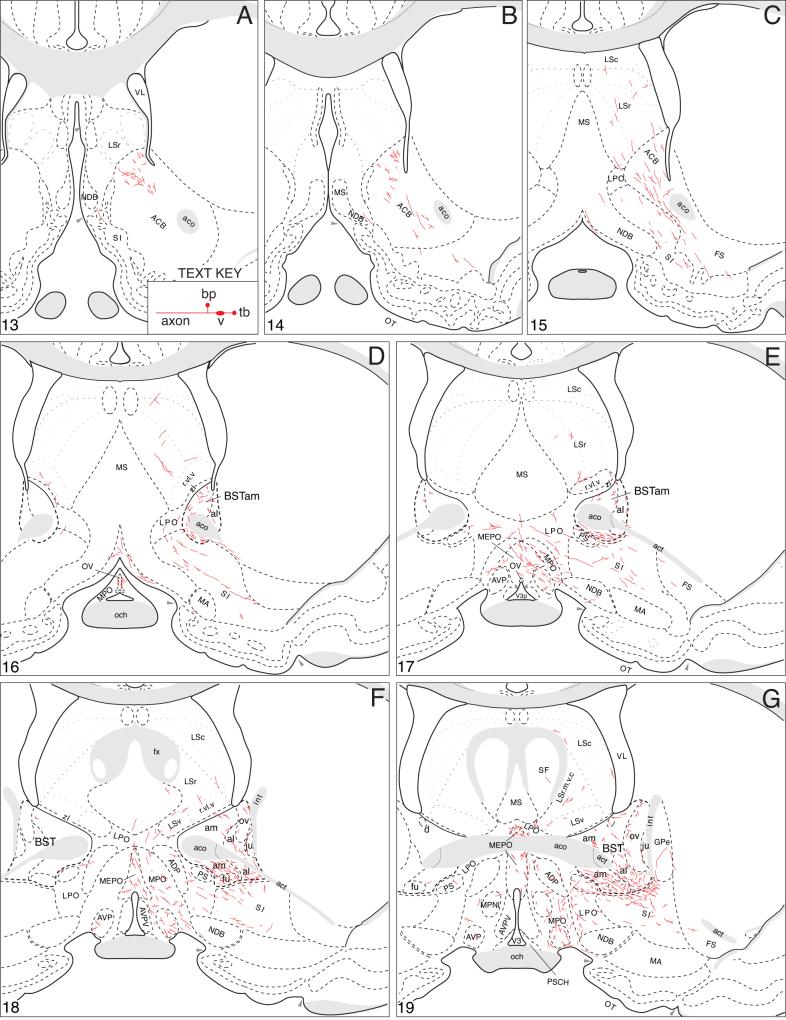
Fig. 4.
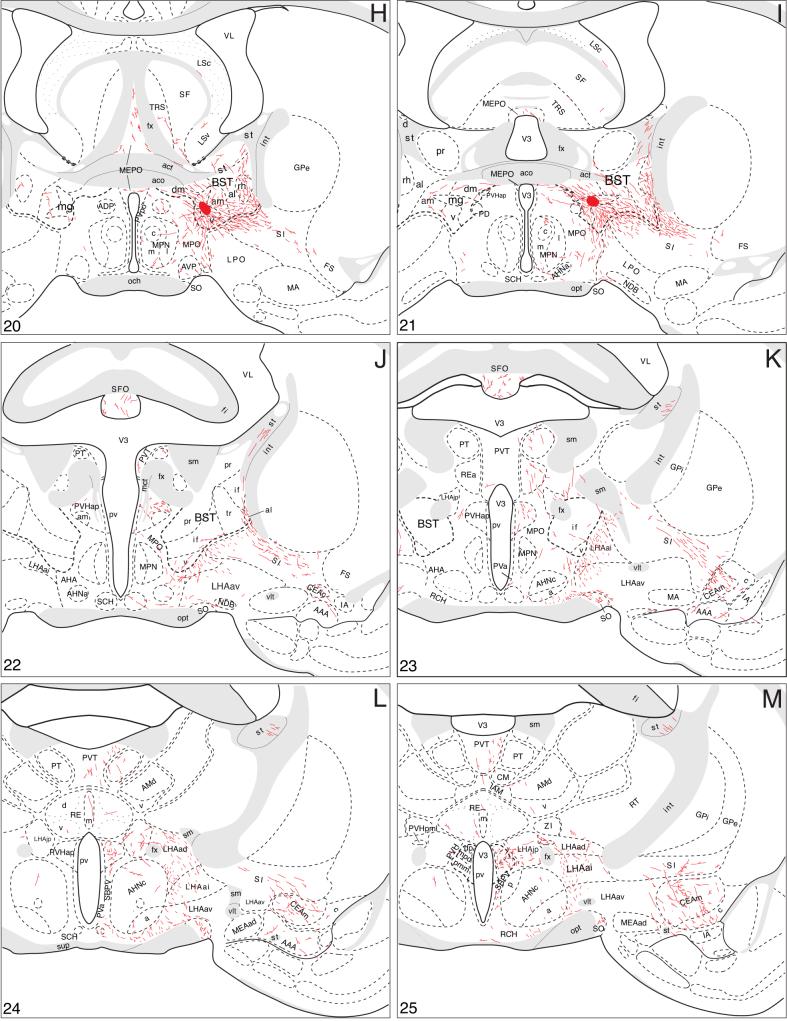
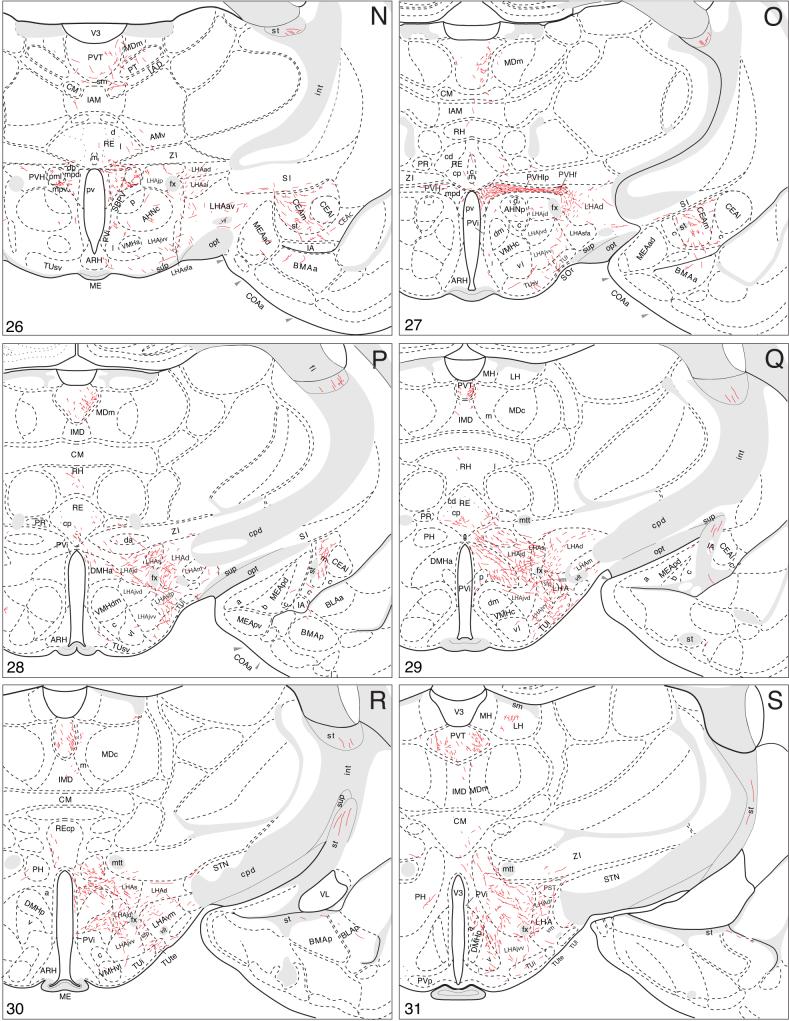
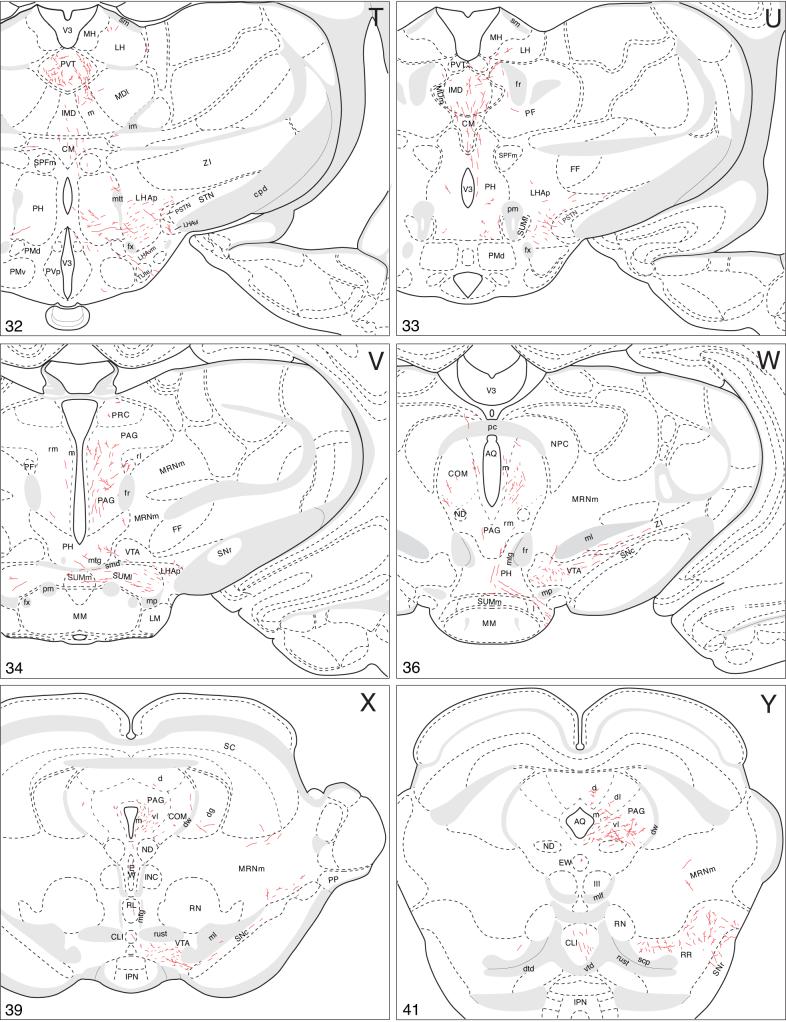
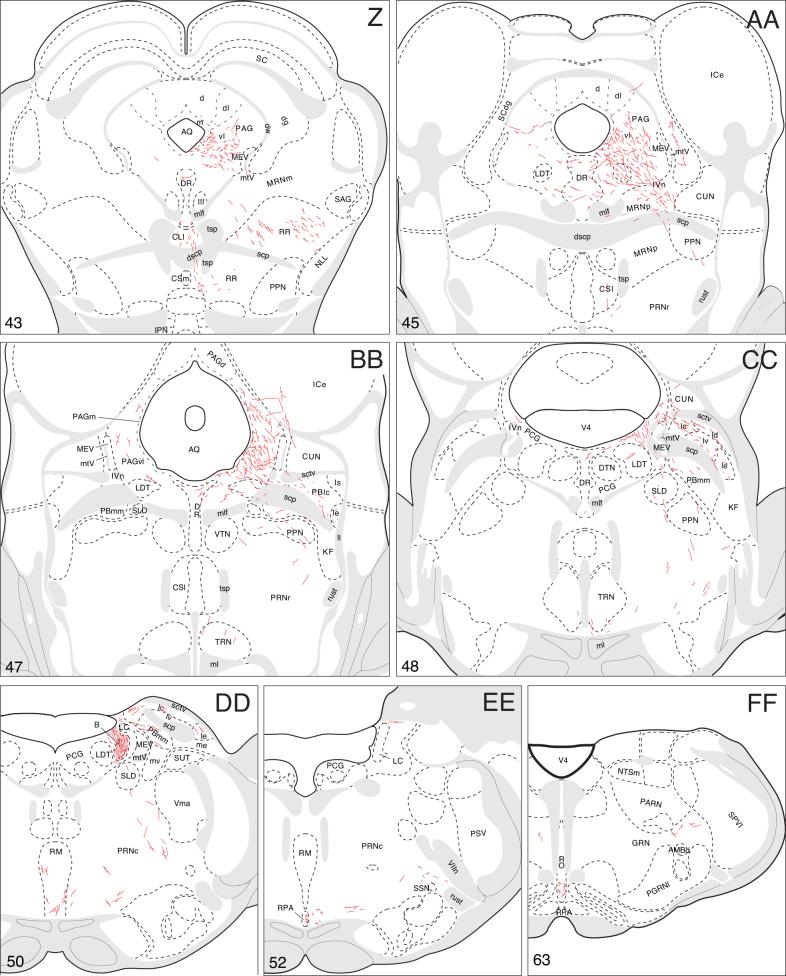
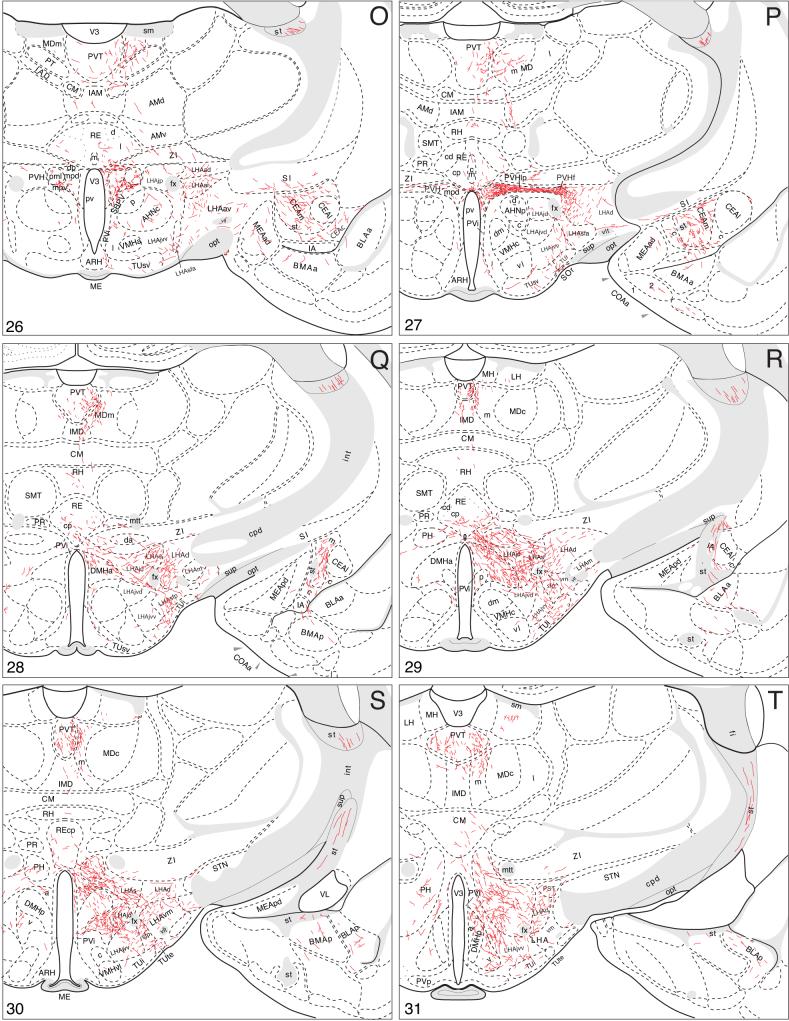
A summary of BSTmg projections. The distribution of PHAL-labeled axons (thin red lines) in experiment BST167 was plotted onto a series of standard or reference drawings of the rat brain derived from an atlas (Swanson, 2004), and arranged from rostral (A) to caudal (FF). The red area centered in the BSTmg at levels H and I indicate the injection site (see Figs. 1 and 2). The key in the lower right corner of level A illustrates the meaning of terms used in the text to describe PHAL-labeled axons. Boutons-of-passage (bp) and terminal boutons (tb) are also referred to simply as terminals, whereas varicosities (v) may or may not form synapses (see Swanson, 2004). The scale of the drawings themselves is too small to illustrate these features. The number in the lower left corner of each drawing refers to the corresponding Atlas Level.
Local projections
Within the BST itself, the BSTmg provides its densest terminal fields ventral to the anterior commissure, in the ventral, dorsomedial, fusiform, and rhomboid nuclei, and in the anteromedial area and caudoventral regions of the anterolateral area. Dorsal to the anterior commissure the oval nucleus and anteromedial area receive light inputs. The rest of the BST is not innervated significantly in these PHAL experiments.
These intra-BST terminal fields arise from several pathways that extend to other regions of the brain (Fig. 3). First, a large group of PHAL-labeled axons extends dorsally, medially, and rostrally—along the way generating abundant boutons in the BST dorsomedial and fusiform nuclei and the BST anterolateral area (Fig. 4D-H). At the rostral end of the BST (Fig. 4D) the vast majority of such fibers continue rostrally into the nucleus accumbens (see below), whereas a few arch dorsally around the medial aspect of the anterior commissure to end in the rostral tip of the dorsal BST anterodorsal area (Fig. 4D,E). A small number of PHAL-labeled axons with terminal boutons are also observed in circumscribed regions of the BST oval nucleus, anterolateral area, and caudal two-thirds of the dorsal BST anteromedial area (Fig. 4F,G).
A second group of PHAL-labeled axons from the BSTmg injection site extends laterally through the BSTv and ventral BST anteromedial area to enter the BST anterolateral area (Fig. 4H,I), where they split into two bundles, a smaller one extending dorsally through the BST rhomboid nucleus to join the stria terminalis, and a larger one coursing ventrally into the substantia innominata to continue on in the ansa peduncularis. Before leaving the BST, the second pathway generates abundant terminal boutons and boutons of passage in the ventral BST anteromedial area, rhomboid nucleus, and caudoventral corner of the anterolateral area (Fig. 4H,I).
The third group of BSTmg axons courses medially through and to the BST dorsomedial nucleus (which receives a moderately dense input; Fig. 4H,I) before entering the median preoptic nucleus ventral to the anterior commissure (Fig. 4H,I). From there, these axons project through the periventricular propriohypothalamic pathway of Thompson and colleagues (Thompson et al., 1996; Thompson and Swanson, 2003) (Fig. 3; see below).
The fourth and final group of PHAL-labeled axons from BSTmg injection sites descends into the subjacent BSTv, which appears to receive the densest intra-BST projection from the BSTmg (Fig. 4H,I). These axons then split into two projections, with one small group of fibers coursing ventromedially through the medial preoptic and anterior hypothalamic areas before descending through the ventral propriohypothalamic pathway of Thompson and colleagues (Thompson et al., 1996; Thompson and Swanson, 2003) (see below) and the other larger group of fibers courses ventrally through dorsomedial regions of the substantia innominata and adjacent regions of the lateral preoptic and lateral hypothalamic areas (Fig. 4H,I) to enter the medial forebrain bundle (Fig. 3; see below).
Rostral projections from the BSTmg
Rostrally directed fibers enter the adjacent caudal end of the nucleus accumbens, where they branch and generate boutons (Fig. 4C) before extending into, and innervating, dorsomedial regions of the nucleus, in dorsal “shell” regions” (Fig. 4A,B). A number of other rather scattered PHAL-labeled axons with boutons of passage also extend rostrally in the rostral third of the substantia innominata (Fig. 4C-F). Some of these axons appear to extend laterally into the striatal fundus (Fig. 4B-H). And finally, a small number of BSTmg axons appear to enter the lateral septal nucleus, where they are found without any obvious concentration in all three parts (Fig. 4C-H).
Ascending stria terminalis and ansa peduncularis pathways to temporal region
Few BSTmg axons follow the stria terminalis to the amygdalar region (Fig. 3). They are in ventral regions of the stria and after entering the amygdalar region are impossible in this material to distinguish from those arriving via the ansa peduncularis (Fig. 4I-S).
Most BSTmg axons use the ventral, ansa peduncularis pathway to reach the amygdalar region (Fig. 3). From the beginning, on leaving the BST to enter the substantia innominata, these axons begin to generate branches and abundant boutons, and continue to do so uninterrupted in dorsomedial regions of the caudal substantia innominata (Fig. 4G-M), until reaching the amygdalar region where most of them establish a rich terminal field in the central nucleus that is densest in its medial part (Fig. 4K-P). The ventral capsular part of the central nucleus receives a relatively moderate input (Fig. 4L-O), and the lateral part is completely avoided by PHAL labeling from the BSTmg (Fig. 4L-O). A few axons from the injection site generate terminal boutons in the anterior amygdalar area (Fig. 4K,L) and anterior basomedial nucleus (Fig. 4N,), and the rest of the amygdalar region receives no detectible inputs from the BSTmg.
Descending periventricular propriohypothalamic pathway
As already mentioned, medially directed BSTmg axons enter the periventricular hypothalamic region near the median preoptic nucleus, just ventral to the crossing of the anterior commissure (Fig. 4H,I). These axons branch little but generate abundant boutons in the median preoptic nucleus itself (Fig. 4E-I) and rostroventrally some of them extend along the border, and perhaps enter, the vascular organ of the lamina terminalis (Fig. 4D). Caudodorsally, some of these axons extend through the dorsal end of the median preoptic nucleus to end in the subfornical organ, where many terminal boutons are observed (Fig. 4H-K).
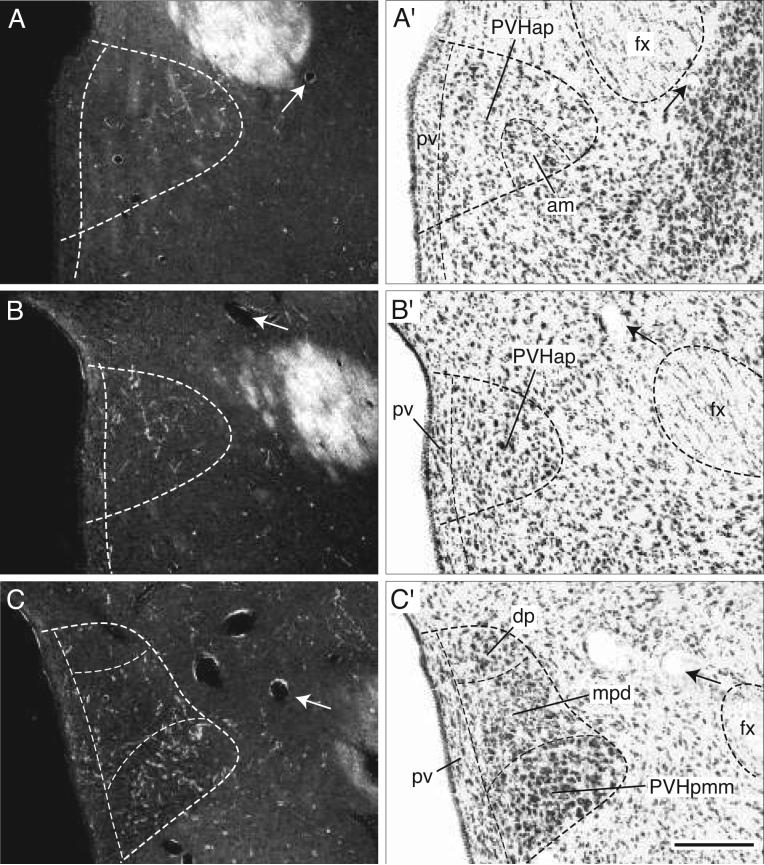
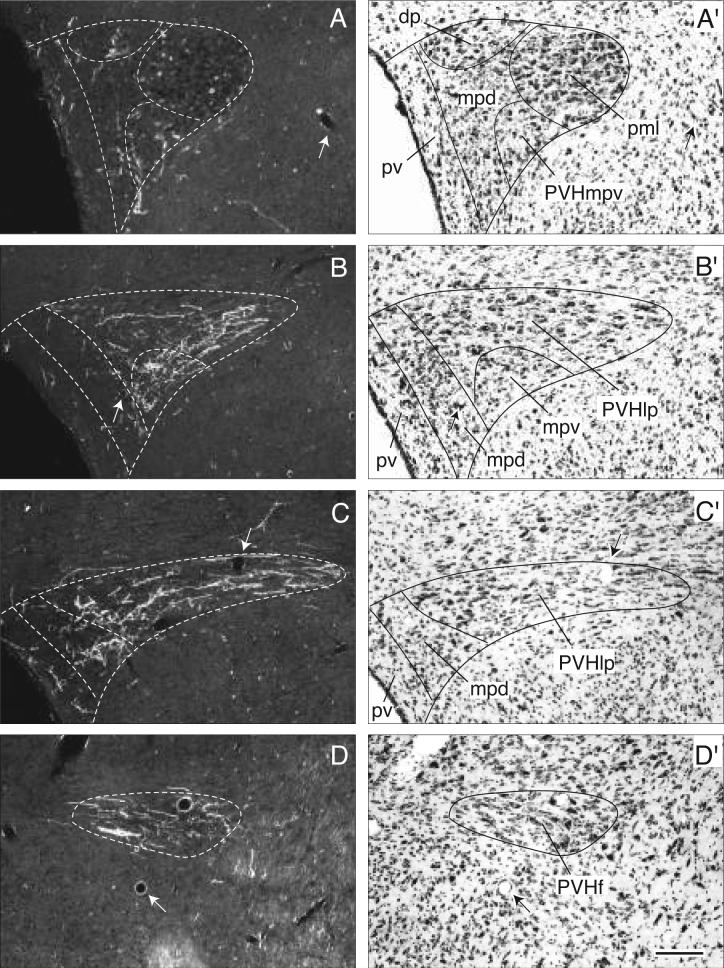
Most periventricular axons end in the paraventricular hypothalamic nucleus (Figs. 3, 4J-O, and 6A-G). Initially they generate a small number of boutons in the anterior parvicellular part (Figs. 4H-L and 6A,B), but avoid the anterior magnocellular part (Fig. 4J). More caudally BSTmg fibers generate a considerable terminal plexus in the medial zone (PVHpmm) of the posterior magnocellular part (Figs. 4M and 5C), whereas in contrast, very few axons and terminals are found in the lateral zone (Figs. 4N and 5D). At this level the dorsal medial parvicellular part and the medially subjacent periventricular part also contain only scattered fibers with terminal boutons (Figs. 4M,N and 5C,D). In contrast, a much denser terminal field is observed in the caudal end of the dorsal medial parvicellular part (Figs. 4O and 5E,F), a region immediately rostral to the paraventricular nucleus lateral parvicellular part. The latter, as well as the rest of the descending division, receive the densest inputs from the BSTmg of any parts of the paraventricular nucleus. However, a substantial component of these terminal fields may arrive via the medial forebrain bundle, and will be described below.
Fig. 6.
Darkfield photomicrographs showing the appearance of PHAL-labeled axons from the BSTmg (experiment BST167) in transverse histological sections through the hypothalamic paraventricular nucleus from rostral to caudal (A-G). For subdivisions of the paraventricular nucleus, see the corresponding caudally adjacent thionin-stained transverse sections (A′-G′; about levels J-O in Fig. 4). Scale bar = 200 μm.
Fig. 5.
A summary of BSTv projections. The distribution of PHAL-labeled axons (thin red lines) in experiment BST91 was plotted onto a series of standard or reference drawings of the rat brain as in Figure 4. The gray area centered in the BSTv at level J indicates the injection site (see Figs. 1 and 2).
The periventricular and arcuate hypothalamic nuclei contain at most very scattered axons from the BSTmg.
Descending ventral propriohypothalamic pathway
Many axons arch ventromedially from the BSTmg into the medial preoptic and anterior hypothalamic areas (Fig. 4G-I) before descending along the base of the hypothalamus in the ventral propriohypothalamic tract (of Thompson and Swanson, 2003).
PHAL-labeled axons generate an extensive terminal plexus in the medial preoptic area, especially in the lateral half and rostral end (Fig. 4E-I). These ventrally directed axons also contribute a relatively light to moderate terminal plexus to the anteroventral preoptic and parastrial nuclei in the hypothalamic preoptic region. A very few scattered axons with terminal boutons are also observed in the medial preoptic nucleus itself (Fig. 4G,I).
Most of the axons turn caudally and descend near the ventral surface of the hypothalamus in the ventral propriohypothalamic pathway (Fig. 4J-M). At anterior hypothalamic levels the fibers branch a little and generate a few boutons in the retrochiasmatic area and suprachiasmatic nucleus (Fig. 4J-M), and a number of them turn laterally to generate a distinct cluster of terminals in the supraoptic nucleus, and in the anterior region of the lateral hypothalamic area just dorsal to it (Fig. 4K-M).
On reaching tuberal hypothalamic levels these fibers display moderate branching and generate moderate numbers of boutons in the tuberal nucleus itself, mostly in its intermediate part (Fig. 4N-S). Most of the fibers arch dorsally to generate a terminal plexus in the posterior zone of the lateral hypothalamic area's subfornical region (Fig. 4P-R), and this terminal field extends medially to merge with terminal fields (derived from the medial forebrain bundle; see below) in and around the dorsomedial hypothalamic nucleus (Fig. 4R,S).
A few axons in the ventral pathway continue to descend along the base of the hypothalamus into the fibrous capsule surrounding the cellular part of the mammillary body, and then they arch dorsomedially through the posterior hypothalamic nucleus to enter the periaqueductal gray (Fig. 4W).
Descending medial forebrain bundle in the hypothalamus
This is the largest descending pathway from the BSTmg. At preoptic and anterior hypothalamic levels many axons travel mainly through dorsal regions of the substantia innominata and the intermediate zone of the lateral hypothalamic area's anterior region, where they are cut in cross section and most of them appear to be fibers of passage, although meager branching and boutons of passage are observed (Fig. 4K-N). Throughout the length of the medial forebrain bundle in the hypothalamus a few BSTmg axons arch dorsally into the thalamus (see below).
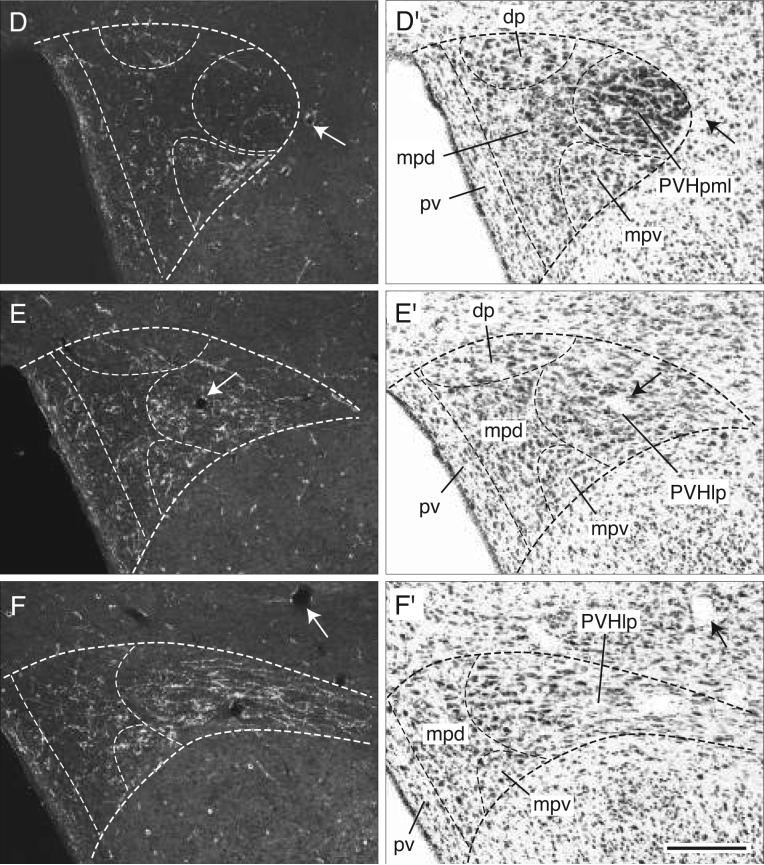
When this major descending bundle reaches the level of the caudal end of the anterior hypothalamic nucleus many fibers extend medially through the lateral parvicellular (lateral wing) of the paraventricular nucleus (Figs. 4O and 5G). These transverse fibers display boutons of passage and generate a clear terminal plexus in the tiny forniceal part, and the entire length of the lateral parvicellular part, of the paraventricular nucleus (Figs. 4O and 5E-G). Then they continue medially to innervate the dorsal parvicellular part and ventral zone of the medial parvicellular part of the paraventricular nucleus (Figs. 4N,O and 5D-F). The caudal end of the dorsal zone of the paraventricular nucleus medial parvicellular part also receives a dense input from the BSTmg, although this terminal field may arise primarily from fibers in the periventricular pathway described above (Fig. 4N).
Just caudal to the paraventricular nucleus many BSTmg axons extend medially from the medial forebrain bundle to innervate densely the lateral hypothalamic area's suprafornical region (Fig. 4P,Q). A few of these axons extend dorsally into the zona incerta, and ventrally the plexus caps the fornix (Fig. 4P,Q). These fibers are crinkled, very richly branched, and bouton-laden. Medially, this dense terminal field extends through and to the lateral hypothalamic area's juxtadorsomedial region to enter the anterior part of the dorsomedial nucleus, although its rostral end is avoided (Fig. 4P,Q). Toward the caudal end of the dorsomedial nucleus its ventral part is also moderately innervated, although BSTmg axons tend to avoid the posterior part of the dorsomedial nucleus (Fig. 4Q-S). The terminal field just described that spreads to include the suprafornical region, juxtadorsomedial region, and dorsomedial nucleus itself extends ventrally, as described above, to merge with the terminal field that includes the tuberal nucleus and posterior subfornical region and is derived mostly from the ventral propriohypothalamic pathway (Fig. 4P-S).
Many axons in and around the suprafornical region arch medially through the posterior hypothalamic nucleus and then arch dorsally into the thalamus (Fig. 4P-T). In the posterior hypothalamic nucleus the arching fibers branch extensively and generate many boutons (Fig. 4Q-S), although caudal to the level of the dorsomedial hypothalamic nucleus the plexus fades considerably (Fig. 4W).
PHAL-labeled BSTmg axons display very little branching and few boutons in the posterior lateral hypothalamic area (Fig. 4T-V), although some of them turn medially to generate a terminal field in the lateral supramammillary nucleus (Fig. 4V). Before BSTmg axons in the medial forebrain bundle enter the midbrain, a contingent leaves to extend medially and dorsally through the posterior hypothalamic nucleus to enter the periaqueductal gray, whereas the majority continues on into the ventral tegmental area of the midbrain (Fig. 4T-W). The distribution of BSTmg axons to the midbrain and beyond will be described after considering its inputs to the thalamus.
Projections to thalamus
Along the length of the interbrain segment of the medial forebrain bundle, some BSTmg axons arch medially and then dorsally into the thalamus (Figs. 3 and 4J-U). Most rostrally these fibers display some branching and boutons in the anterior part of the nucleus reuniens rostral division, and in the paratenial and paraventricular thalamic nuclei (Fig. 4K,L). These fibers tend to extend caudally in the dorsal periventricular bundle centered in the paraventricular thalamic nucleus. Then, throughout anterior and tuberal levels of the hypothalamus scattered fibers leave the medial forebrain bundle to arch medially through the zona incerta, and more caudally the posterior hypothalamic nucleus, to enter the midline thalamic nuclei (Fig. 4M-S). These fibers initially branch and generate boutons in the nucleus reuniens, especially in the posterior part of the caudal division (Fig. 4Q). More dorsally these fibers course through the rhomboid, interanteromedial, central medial, and intermediodorsal nuclei, eventually to reach the paraventricular thalamic nucleus (Fig. 4M-S). These PHAL-labeled axons from the BSTmg injection sites generate very few terminal boutons and boutons of passage in these cell groups (see Fig. 4O,P,S).
The largest number of mediodorsally arching fibers leave the medial forebrain bundle near the caudal end of the hypothalamus, coursing initially through and to the posterior hypothalamic nucleus (Fig. 4R-T). These fibers present obvious branching and boutons in the central medial, intermediodorsal, and medial mediodorsal nuclei, eventually ending in the paraventricular thalamic nucleus. In the thalamus, the BSTmg innervates most densely the paraventricular nucleus, especially in its caudal half. PHAL-labeled axons generate branches and boutons through all but the rostral tip of the paraventricular nucleus (Fig. 4K-T), although their density increases steadily in the caudal dimension. In the caudal half of the nucleus the terminal field is relatively denser and bilateral, with an ipsilateral predominance (Fig. 4R-T).
For completeness, a small terminal field in the caudal half of the ipsilateral lateral habenula should be noted (Fig. 4S).
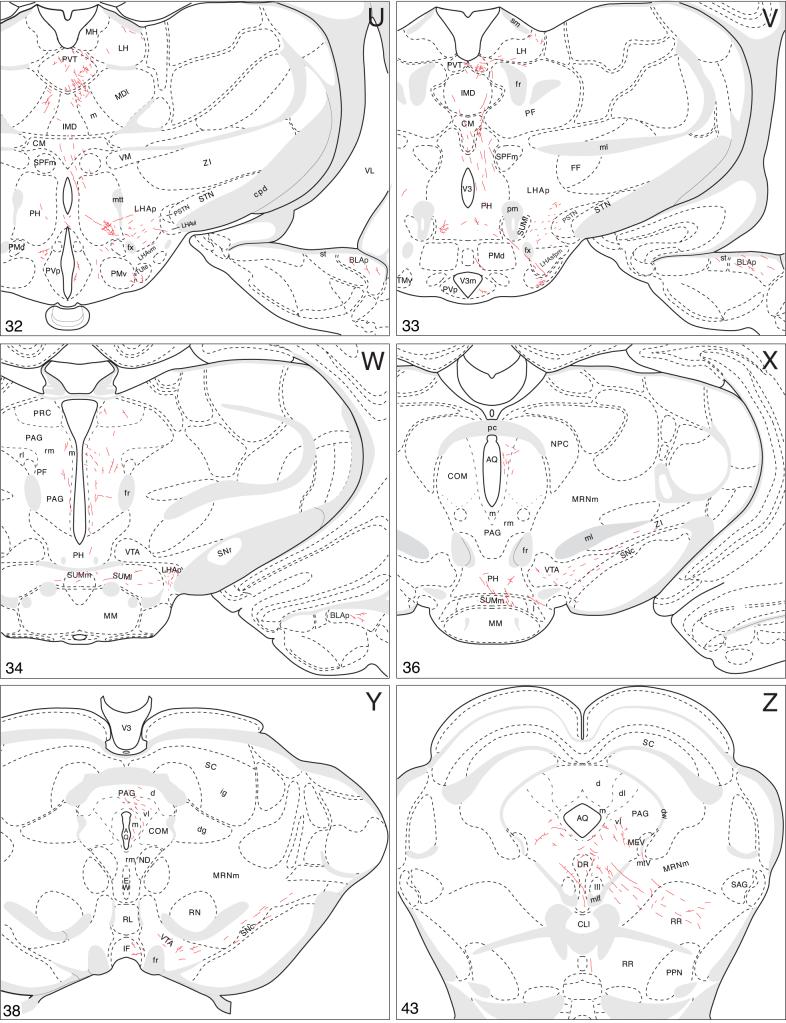
Projections to midbrain and hindbrain
Caudal to the interbrain (diencephalon), most BSTmg axons continue in the medial forebrain bundle, which generates a small median branch to the raphé, whereas a small contingent extends dorsally into the periaqueductal gray (Fig. 3).
Rostrally in the periaqueductal gray, labeled axons from the PHAL injection site branch and generate moderate numbers of boutons in an intermediate zone of the rostromedial division (Fig. 4V), although most descending periaqueductal gray fibers seem to course through and immediately adjacent to the medial division. A few terminal boutons and boutons of passage are found along the length of the medial division (Fig. 4V-BB), and there are scattered fibers and boutons in the commissural nucleus, and the dorsolateral and dorsal divisions, of the periaqueductal gray (Fig. 4W-Y). Eventually the descending fibers generate a dense terminal field in the periaqueductal gray ventrolateral division and immediately adjacent regions of the medial division (Fig. 4Y-BB), where axons from other pathways described below merge. A few axons labeled from the BSTmg-centered injection site extend dorsally from the periaqueductal gray into the deep gray layer of the overlying superior colliculus (Fig. 4X,AA) and external nucleus of the inferior colliculus (Fig. 4BB).
The majority of BSTmg axons descending through the medial forebrain bundle enter the ventral tegmental area where they branch little but do generate considerable numbers of boutons (Fig. 4W,X). Then, one small group of fibers turns medially to enter the central linear nucleus of the raphé, where they generate a few boutons (Fig. 4X-Z), and then descend through a median pathway to the hindbrain (Fig. 3). Along the way a very small dorsal branch extends into a terminal field in the dorsal nucleus of the raphé (Fig. 4Z,AA), although most of the median axons continue through the lateral part of the superior central nucleus of the raphé and tegmental reticular nucleus to the medullary raphé nuclei (Fig. 4Z-FF). In the nucleus raphé magnus and nucleus raphé pallidus (Fig. 4DD,EE) one observes little branching but considerable boutons, whereas, finally, in the nucleus raphé obscurus a few labeled axons form a ring or basket-like terminal field (Fig. 4FF).
The major descending pathway from the BSTmg leaves the ventral tegmental area and extends laterally and somewhat caudally through the region between the substantia nigra and medial lemniscus, including the caudal end of the zona incerta—where there is little evidence of branching or bouton generation (Fig. 4W). Eventually the PHAL-labeled axons arch dorsally and then medially through the midbrain reticular nucleus and its retrorubral area to enter the periaqueductal gray at about the level of the pedunculopontine nucleus (Fig. 4X-AA). Along the way they generate conspicuous terminal fields in the retrorubral area (Fig. 4Y,Z) and dorsal regions of the midbrain reticular nucleus, parvicellular part (Fig. 4AA).
Axons from the BSTmg that course through the medial forebrain bundle and the periaqueductal gray converge in the ventrolateral division of the latter (Figs. 3 and 4Z-BB), where they generate a very dense terminal plexus. A few axons with boutons invade the laterodorsal tegmental nucleus and dorsal nucleus of the raphé (Fig. 4Z-BB).
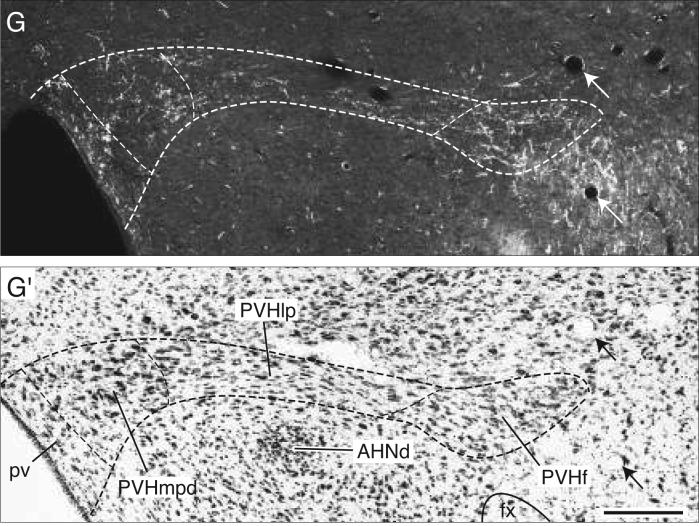
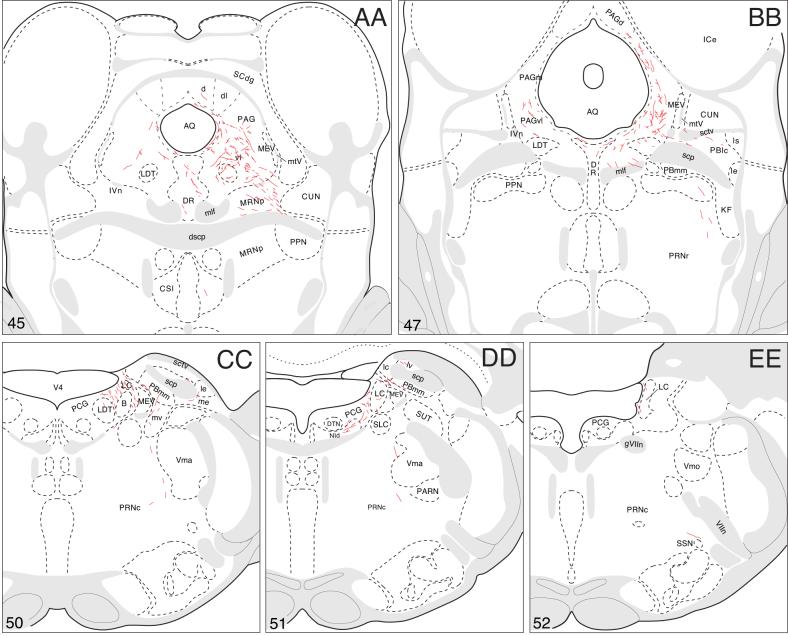
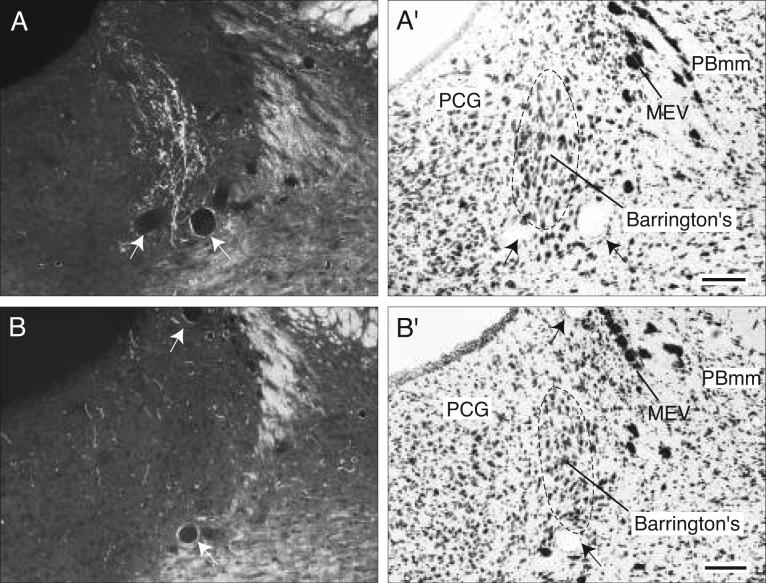
The remaining periaqueductal gray fibers extend caudally into the pontine central gray (Fig. 4CC), where they tend to funnel into a restricted region that lies immediately medial to the root of the trochlear nerve, and more caudally just medial to the locus ceruleus (Fig. 4CC,DD). After generating a few boutons in the pontine central gray, these axons dive ventrally to end in Barrington's nucleus, which is very densely innervated (Figs. 4DD and 8A). This terminal plexus is confined within the cellular limits of Barrington's nucleus, essentially avoiding other pontine central gray components.
Fig. 8.
Photomicrographs (darkfield illumination) comparing the distribution of PHAL-labeled axons in Barrington's nucleus following a PHAL injection centered in the BSTmg (A, experiment BST167), and in the BSTv (B, experiment BST91), along with caudally adjacent thionin-stained transverse sections (A′, B′; about level CC in Fig. 5). All scale bars = 100 μm.
Finally, a small number of scattered fibers labeled by the BSTmg-centered PHAL injection extend laterally from the central gray into the parabrachial nucleus, where a few boutons are found (Fig. 4CC,DD). Some fibers also extend ventrally through the sublaterodorsal nucleus and pedunculopontine nucleus into the pontine reticular nucleus (Fig. 4BB-DD). These fibers display scattered branching and boutons in ventral regions of the caudal pontine reticular nucleus, and a few of them may also contribute terminals to the medullary raphé nuclei (Fig. 4CC-EE). The few remaining axons end in the gigantocellular reticular nucleus (Fig. 4FF).
Contralateral projections
As noted above, the BSTmg generates moderate bilateral projections to a number of midline structures, including the median preoptic nucleus, subfornical organ, caudal third of the paraventricular thalamic nucleus, and various raphé nuclei. In these instances the contralateral projection is only slightly lighter than the ipsilateral, whereas all other contralateral projections are considerably lighter, tend to be associated with the contralateral medial forebrain bundle, and where present faintly mirror the ipsilateral pattern.
Projections from the BSTv
Because each BSTmg injection site labeled a few neurons in adjacent BST cell groups (Figs. 1 and 2) some of the lightly labeled projections in our BSTmg experiments may arise from the “contamination”. To clarify this possibility we examined projections from the subjacent BSTv, which contains the most contamination in each BSTmg experiment. Because of its tiny volume and irregular shape it is probably not possible to obtain a PHAL injection site confined entirely to the BSTv. Nevertheless, we were fortunate to obtain one experiment (BST91; Figs. 1 and 2) where the very small injection site labeled neurons confined almost entirely to the BSTv, except for a few in the BSTmg.
The results of this experiment (Figs. 3 and 5) show that the projections of the BSTv follow the same general course as those from the BSTmg, but are not as extensive and show some striking differences. For example, the BSTv does not innervate Barrington's nucleus, or the median preoptic nucleus and subfornical organ—and its inputs to the substantia innominata and central amygdalar nucleus are considerably less than those from the BSTmg. Projections from other regions adjacent to the BSTmg have already been published (see section on Injection sites, above).
Local projections
First, the BSTv injection labels a substantial input to the adjacent rostral end of the BSTmg (Fig. 5J), but leaves the caudal end of the BSTmg free of input (Fig. 5K). Like the BSTmg, the BSTv innervates substantially the BST fusiform and dorsomedial nuclei, and ventral regions of the anteromedial area (Fig. 5F-J). The BSTv provides much less input to the BST rhomboid nucleus and caudal anterolateral area (Fig. 5H-K) than the BSTmg, but a much greater input to the dorsal anteromedial area (Fig. 5F-I). Other BST cell groups contain only scattered fibers at most (Fig. 5F-L).
Rostral projections
As with the BSTmg, rostrally directed axons from the BSTv end primarily in the nucleus accumbens (Fig. 3). This terminal field is most dense in the caudal pole, adjacent to the rostral pole of the BST (Fig. 5E), and becomes somewhat lighter more rostrally, where it is centered ventrally in medial (shell) regions of the nucleus accumbens (Fig. 5B-D). The BSTv appears to innervate more densely than the BSTmg the lateral septal nucleus, where significant numbers of axons with boutons are distributed through both the rostral and caudal parts (Fig. 5C-I). A few BSTv axons extend rostrally from the lateral septal nucleus to end in the prelimbic cortical area (Fig. 5A,B), which appears to receive little or no input from the BSTmg.
Ascending pathways to the amygdalar region
Like the BSTmg, BSTv axons use both the stria terminalis and ansa peduncularis to reach primarily the medial part of the central amygdalar nucleus (Figs. 3 and 5K-Q), although the posterior basolateral nucleus also receives a light input (Fig. 5S-W). The BSTv input to the substantia innominata, along the ansa peduncularis pathway to the amygdalar region (Fig. 5L-O), is much lighter than that from the BSTmg.
Descending periventricular pathway to hypothalamus
A small number of PHAL-labeled axons from the BSTv injection site course dorsomedially through the BSTdm to enter the periventricular hypothalamic region just ventral to the crossing of the anterior commissure (Fig. 5J,K) and generate a few boutons in the median preoptic nucleus (Fig. 5G-K). Whether these fibers arise in the BSTv or BSTmg needs to be determined with retrograde tracer experiments. No PHAL labeling is observed in the vascular organ of the lamina terminalis or subfornical organ, both of which are innervated by the BSTmg.
This pathway also generates a few branches and boutons in the anterior parvicellular, dorsal medial parvicellular, and periventricular parts of the paraventricular hypothalamic nucleus (Figs. 5L-P and 7A-C). The paraventricular magnocellular neuroendocrine division is not innervated in this experiment, unlike those centered in the BSTmg.
The ventral propriohypothalamic pathway
This pathway (Fig. 3) is much more lightly labeled by the BSTv injection site than by those involving the BSTmg. A small number of labeled axons leave the BSTv region in a medial and ventral direction to enter the medial preoptic area, where scattered branches and boutons are found laterally (Fig. 5J,K). A few axons also enter the medial preoptic nucleus at more rostral levels, after generating boutons in the parastrial nucleus (Fig. 5H,I). Only very scattered fibers with boutons are observed in the anteroventral preoptic nucleus (Fig. 5H), which receives more labeled axons from injection sites centered in the BSTmg.
At the level of the anterior hypothalamic nucleus (Fig. 5K-O), axons in this pathway turn caudally to run near the ventral surface of the hypothalamus near the supraoptic nucleus (which contains a few terminal boutons from the pathway; Fig. 5L-N). More caudally these fibers course through the tuberal nucleus, where few boutons are displayed (Fig. 5O-T). Most of these fibers turn dorsomedially to branch and generate boutons in a terminal field that is centered in the posterior zone of the lateral hypothalamic area's subfornical region and that extends medially into the nearby ventral part of the dorsomedial hypothalamic nucleus (Fig. 5P-T).
A few axons labeled from the BSTv injection site extend even more caudally to generate terminal boutons in the ventral premammillary and ventral tuberomammillary nuclei (Fig. 5U,V).
The descending medial forebrain bundle
This is the major descending pathway of the BSTv (Fig. 3). The vast majority of PHAL-labeled axons turn caudally and ventrally through the caudal end of the BSTv to enter the lateral hypothalamic area's anterior region (Fig. 5J-M). At anterior hypothalamic levels this fascicle courses through a region of the lateral hypothalamic area just ventrolateral to the fascicle labeled from the BSTmg (compare Fig. 5N-P with Fig. 4M-O). A few of these axons consistently turn dorsally to course through the zona incerta (which contains a few boutons) to the dorsal thalamus (Fig. 5O,P).
At the level of the caudal tip of the anterior hypothalamic nucleus, many fibers in the medial forebrain bundle turn dorsomedially to generate an extremely dense terminal field in the forniceal part of the paraventricular hypothalamic nucleus (Figs. 5P and 7D). These fibers then extend medially through and to the lateral parvicellular part (lateral wing) of the paraventricular nucleus where they generate numerous boutons of passage and terminal boutons (Figs. 5P and 7B,C). Most of these fibers appear to end in medial regions of the lateral parvicellular part, which is the densest terminal field labeled in this experiment. Then, this terminal field extends medially and rostrally into the ventral medial parvicellular part of the paraventricular nucleus, which also contains substantial branching and terminal boutons (Figs. 5O and 7A,B), whereas the dorsal parvicellular part (dorsal cap) of the paraventricular nucleus contains only a few boutons (Figs. 5O and 7A,B). Compared to the BSTmg, the BSTv generates only a small number of boutons in caudal regions of the dorsal medial parvicellular part of the paraventricular nucleus immediately adjacent to the lateral parvicellular part (Figs. 5P and 7A-C).
Fig. 7.
Darkfield photomicrographs showing the appearance of PHAL-labeled axons from the BSTv (experiment BST91) in transverse histological sections through the hypothalamic paraventricular nucleus from rostral to caudal (A-D). For subdivisions of the paraventricular nucleus, see the corresponding caudally adjacent thionin-stained transverse sections (A′-D′; about levels O and P in Fig. 5). Scale bar = 150 μm.
Caudal to the paraventricular nucleus, many descending fibers in the medial forebrain bundle generate a rather broad terminal plexus that extends from medial regions of the dorsal lateral hypothalamic area, through the suprafornical and juxtadorsomedial regions of the lateral hypothalamic area, to the dorsomedial and posterior hypothalamic nuclei (Fig. 5Q-T). Compared to a similar terminal field generated by the BSTmg, this terminal field is less dense rostrally (Fig. 5Q,R) and more dense caudally (Fig. 5S). The anterior and ventral parts of the dorsomedial nucleus are clearly innervated, whereas in contrast, the posterior part contains only a few labeled axons (Fig. 5Q-T).
At tuberal hypothalamic levels, many BSTv axons turn medially and dorsally to innervate heavily the rostral end of the posterior hypothalamic nucleus (Fig. 5R-T). A number of these fibers continue on medially and dorsally into the thalamus (Fig. 5U,V), whereas others enter the midbrain periaqueductal gray (Fig. 5W). The terminal field in the posterior hypothalamic area extends caudally as a thin column just dorsal to the dorsal premammillary nucleus (Fig. 5U,V), and a few axons with terminal boutons are found in both parts of the supramammillary nucleus (Fig. 5W,X).
BSTv projections to thalamus
Rostrally, some BSTv axons enter directly the dorsal thalamus (Fig. 5L,M). Then, at anterior and tuberal hypothalamic levels, labeled fibers in the medial forebrain bundle extend dorsally through the zona incerta (ventral thalamus) and posterior hypothalamic nucleus to enter the dorsal thalamus. Finally, most fibers enter the dorsal thalamus through the posterior hypothalamic nucleus at mammillary levels of the hypothalamus.
In contrast to the BSTmg, the BSTv provides only a few axons with boutons to most of the paraventricular thalamic nucleus (Fig. 5L-T), except in the caudal end, where PHAL-labeled axons display considerable terminal boutons (Fig. 5U,V). Instead, the densest BSTv thalamic terminal field is in the medial part of the mediodorsal nucleus, which is lightly innervated by the BSTmg. PHAL-labeled axons from the BSTv branch profusely and display abundant terminal boutons in a restricted region of the medial mediodorsal nucleus that lies immediately adjacent to the paraventricular nucleus throughout its length (Fig. 5O-U), and this terminal field appears to spread into adjacent regions of the intermediodorsal nucleus (Fig. 5QV). A combined retrograde tracer-in situ hybridization study indicates that the “ventral region” of the BST (which probably includes the BSTv) sends a GABAergic projection to the mediodorsal nucleus (Churchill et al., 1996).
PHAL-labeled axons from the BSTv injection also generate considerable boutons of passage and terminal boutons in a restricted medial region of the dorsal part of the anteromedial thalamic nucleus (Fig. 5P) that does not appear to be innervated by the BSTmg. The BSTv injection site also labels small numbers of branches and boutons in the paratenial, rhomboid, and central medial nuclei, and in several components of the nucleus reuniens (Fig. 5M-V). No PHAL labeling is observed in the lateral habenula, which is innervated by the BSTmg.
BSTv projections to midbrain and hindbrain
Like the BSTmg, the BSTv uses a dorsal, periaqueductal gray pathway, as well as median and lateral branches of the medial forebrain bundle (Fig. 3).
Only a few BSTv axons follow the dorsal pathway, and they branch and generate boutons in the rostromedial division of the periaqueductal gray, as well as descend through the medial division to the pontine central gray. Even fewer BSTv axons course through the median branch of the medial forebrain bundle, generating boutons in the interfascicular nucleus of the raphé and ending in the dorsal nucleus of the raphé (Fig. 5V-AA).
Most BSTv descending axons follow the lateral branch of the medial forebrain bundle. They display very few boutons in the ventral tegmental area (Fig. 5X,Y) and caudodorsal regions of the midbrain reticular nucleus (Fig. 5AA), and virtually none in the retrorubral area (Fig. 5Z), which is innervated densely by the BSTmg.
These axons eventually reach the periaqueductal gray, where they establish a dense terminal plexus of branches and boutons in the ventrolateral division (Fig. 5AA,BB). A few axons with terminal boutons are observed in the laterodorsal tegmental nucleus (Fig. 5AA), and in the pontine central gray, where they extend into the rostral end of the locus ceruleus and lateral regions of the diffuse part of the nucleus incertus (Fig. 5CC,DD). No BSTv labeling is observed in Barrington's nucleus (Figs. 5CC and 8B), which is very heavily and specifically innervated by the BSTmg. Occasional fibers from the BSTv injection site are also found in the parabrachial and pontine reticular nuclei (Fig. 5BB-EE).
Contralateral projections
The BSTv generates a very sparse contralateral projection, most of which is associated with the medial forebrain bundle and mirrors faintly the ipsilateral pattern.
DISCUSSION
The results of the present experiments indicate that the overall projection patterns of the BSTmg and BSTv are similar in many ways to those of the dorsally adjacent BSTdm (Dong and Swanson, 2005a). Nevertheless, there are distinct features of each of the three projection patterns—and they in turn are quite distinct from the adjacent BST anteromedial area (Dong and Swanson, 2005b), the BST anterolateral group (Dong et al., 2000, 2001b; Dong and Swanson, 2003, 2004a), and the BST posterior division (Dong and Swanson, 2004b). After considering the very meager previous literature on BSTmg and BSTv projections, the Discussion turns to evidence suggesting that they play an important role in coordinating neuroendocrine, autonomic, and somatomotor responses, most uniquely with regard to pelvic organ functions including micturition, defecation, and penile erection.
Previous literature
Numan and Numan (1996) recently examined with PHAL neural projections from the “ventral part” of the BST in lactating rats. Their injection sites appear to include at least part of our BSTmg and BSTv, as well as the BST principal nucleus of the posterior division (see their injection site 1). The general pattern of labeled projections is similar to, though obviously considerably more dense than, that described here. This difference may be due in part to their use of larger diameter injection pipettes and longer injection times, but it is possible that the region of the BSTmg is sexually dimorphic because neurons here concentrate gonadal steroid hormones (Pfaff and Keiner, 1973; Sar and Stumpf, 1975; Stumpf et al., 1975) and express estrogen and androgen receptor mRNAs (Simerly et al., 1990; Shughrue et al., 1997), and it is also possible that the extreme physiological demands of lactation may change the apparent strength of PHAL-labeled projections. Obviously, more detailed work is needed to address these intriguing possibilities.
Numan and Numan (1996) showed dense projections to the medial preoptic area, paraventricular hypothalamic nucleus, lateral hypothalamic area, posterior hypothalamic nucleus, retrorubral area, periaqueductal gray, and Barrington's nucleus—areas shown here to be innervated by the BSTmg and/or BSTv. However, they also reported major projections to the lateral septal; medial, cortical, and posterior amygdalar; ventromedial hypothalamic; and dorsal and ventral premammillary nuclei—which are not demonstrated here, but are known to arise from the BST principal nucleus (Gu et al., 2003; Dong and Swanson, 2004b).
Comparison of BSTmg, BSTv, and BSTdm projections
These three nuclei lie stacked on one another in caudomedial regions of the BST anteromedial group. Before comparing long projections of the BSTmg and BSTv with the BSTdm (the latter described in the accompanying paper, Dong and Swanson, 2005a) it is important to note that all three are highly interconnected—each of the three projects to the other two—and there are several features common to their projection patterns that distinguish them readily from the BST anteromedial region (described in the accompanying paper, Dong and Swanson, 2005b), the BST anterolateral group, and the BST posterior division. First, like all parts of the BST anterior division, the BSTmg, BSTdm, and BSTv project densely to the medial part of the central amygdalar nucleus, but only sparsely to the medial amygdalar nucleus. In contrast, all BST posterior division components project heavily to the medial amygdalar nucleus and relatively lightly to the central amygdalar nucleus. Second, like the BST anteromedial region the BSTdm and BSTmg provide substantial inputs to the region of multiple hypothalamic neuroendocrine motoneuron pools in the hypothalamus, although they do not project to the arcuate nucleus, which receives a dense input from the BST anteromedial region. Third, the BSTdm, BSTmg, and BSTv send no detectable projections to the dorsal vagal complex, which instead receives dense inputs from the BST anterolateral group (Dong and Swanson, 2004a). Rather, the three BST nuclei send dense projections to all regions of the paraventricular hypothalamic nucleus descending division, and the BSTdm and BSTmg also project to Barrington's nucleus, which is not innervated by other BST anterior division cell groups. In contrast, the principal nucleus of the BST posterior division also projects to Barrington's nucleus, but not to the paraventricular nucleus descending division (Dong and Swanson, 2004b).
Thus, the BSTmg, BSTdm, and BSTv together are positioned to coordinate neuroendocrine, sympathetic, and sacral parasympathetic responses (see below), whereas the BST anterolateral group projects heavily to the dorsal vagal complex.
Major terminal fields established by the BSTmg, BSTv, and BSTdm are compared in Table 1. Clearly, projections from the BSTmg are essentially a subset of those arising in the BSTdm (although projection strength differs qualitatively in many target regions), and projections from the BSTv are a subset of those arising in the BSTmg—with several unique features that readily distinguish projection patterns generated by the BSTmg and BSTv.
TABLE 1.
Comparison of Major Projections from Three Adjacent Regions of the Anteromedial Group (BSTamg).
| to/from | BSTv | BSTmg | BSTdm |
|---|---|---|---|
| Cerebral nuclei | |||
| LS | • | ||
| ACB | • | • | • |
| CEA | • | • | • |
| SI | • | • | • |
| Behavior control column | |||
| PVHd | • | • | • |
| MPNl | • | ||
| AHN | • | ||
| VTA | • | • | |
| Orofacial motor-related | |||
| RR | • | • | |
| MRN | • | • | |
| Humorosensory, thirst-related | |||
| SFO | • | • | |
| MEPO | • | • | |
| Brainstem autonomic control network | |||
| PVHd | • | • | • |
| PAGvl | • | • | • |
| B | • | • | |
| Neuroendocrine | |||
| SO | • | • | |
| PVHm | • | • | |
| PVHp | • | • | |
| PVa | • | • | |
| Hypothalamic visceromotor pattern generator network, periventricular region | |||
| DMH | • | • | • |
| PS | • | • | |
| MEPO | • | • | |
| AVP | • | • | |
| ADP | • | ||
| MPO | • | • | |
| AHA | • | ||
| Thalamocortical feedback loops | |||
| PVT | • | • | |
| MDm | • | ||
| IMD | • | ||
| CM | • | ||
| RE | • | ||
| Behavioral state control | |||
| SBPV | • | ||
| LPO | • | ||
| RCH | • | ||
| LHAjd | • | • | • |
| LHAs | • | • | |
| LHAd | • | • | • |
| LHAsfp | • | • | • |
| TU | • | • | |
| SUM | • | • | |
| PH | • | • | • |
| LH | • | ||
| PRC | • | ||
| LDT | • | ||
| RM | • | ||
| Miscellaneous | |||
| PRNc | • | ||
| MARN | • | ||
Major projections are defined as relatively moderate to dense terminal fields based on qualitative assessments. Bolded cell groups highlight those targeted by all three BST regions.
Like the BSTdm, the BSTmg and/or BSTv send projections to brain regions can be assigned at least provisionally to a number of functional systems, including the cerebral nuclei, behavioral control column, orofacial motor-related, humorosensory and thirst-related, brainstem autonomic control network, neuroendocrine system, hypothalamic visceromotor pattern generator network, thalamocortical feedback loops, and behavioral state control (Table 1; also see Fig. 11 in Dong and Swanson, 2005a). The functional evidence for most of these assignments is reviewed in the accompanying paper (Dong and Swanson, 2005a) and needn't be repeated here. There is very limited supporting evidence (see below) for our results based on retrograde pathway tracing because earlier authors usually did not employ detailed BST parceling schemes. The following connections deserve special consideration.
Direct inputs to the neuroendocrine system
The BSTdm provides the densest known inputs from the BST to regions containing groups of magnocellular and parvicellular neuroendocrine neurons—although, interestingly, it does not project to the arcuate nucleus where dopamine (prolactin-inhibiting) and growth hormone-releasing hormone neurons are centered (Dong and Swanson, 2005a). In sharp contrast, the only significant direct neuroendocrine projection from the BSTv is to the supraoptic nucleus. Overall, the pattern of BSTmg projections to regions associated with the neuroendocrine system is similar to that of the BSTdm. However, for the magnocellular neuroendocrine system (for reviews see Swanson, 1986; Thompson and Swanson, 2003) the oxytocinergic region (medial posterior magnocellular zone of the paraventricular nucleus and ventral/rostral regions of the supraoptic nucleus) receives substantial inputs from the BSTmg, which sends only light projections to the vasopressinergic region (lateral posterior magnocellular zone of the paraventricular nucleus and dorsal/caudal regions of the supraoptic nucleus).
With reference to the parvicellular neuroendocrine system, the BSTmg provides substantial inputs to the zone that contains corticotropin-releasing hormone neurons, and light inputs to the vicinity of somatostatin and thyrotropin-releasing hormone neurons (in and near the paraventricular nucleus). Previous anterograde (Risold et al., 1997) and retrograde (Spencer et al., 2005) tracer studies with relatively large injections pointed toward this projection.
Inputs to the brainstem autonomic control network
In contrast to their differentiated inputs to neuroendocrine regions of the paraventricular nucleus, the BSTdm, BSTmg, and BSTv send dense projections to the lateral parvicellular/forniceal and ventral medial parvicellular parts of the paraventricular nucleus descending division, which project to the dorsal vagal complex and spinal intermediolateral column (Saper et al., 1976; Swanson and Kuypers, 1980). Fluorogold injection in the paraventricular nucleus lateral parvicellular part retrogradely labels many neurons in the BSTmg, BSTv, and BSTdm (Lori Gorton and Alan G. Watts, personal communication). However, the “dorsal cap” (dorsal parvicellular part) of the paraventricular nucleus, which projects preferentially to the spinal cord (Swanson and Kuypers, 1980), is differentially innervated, with a relatively dense input from the BSTdm, a relatively moderate input from the BSTmg, and a relatively sparse input from the BSTv.
The paraventricular nucleus descending division (especially its ventral medial and lateral parvicellular parts) also projects directly to Barrington's nucleus (Valentino et al., 1994), which contains preautonomic neurons in turn projecting to the sacral parasympathetic preganglionic pool (see Loewy et al., 1979). In addition, Barrington's nucleus receives substantial direct inputs from both the BSTdm and BSTmg, but not from the BSTv. And furthermore, the caudal raphé and adjacent reticular formation, which contains preautonomic neurons (see Loewy, 1991), also receive clear inputs from the BSTdm and BSTmg, but not from the BSTv, as confirmed by retrograde tract tracing experiments (Hermann et al., 1997; Nogueira et al., 2000).
Several other regions with neurons participating in central autonomic control—including the medial part of the central amygdalar nucleus, dorsal lateral hypothalamic area, posterior hypothalamic nucleus, and ventrolateral division of the periaqueductal gray—receive inputs from the BSTdm, BSTmg, and BSTv.
Inputs to the hypothalamic visceromotor pattern generator network
The BSTdm, BSTmg, and BSTv all project densely to the dorsomedial hypothalamic nucleus, the most important component of a recently identified hypothalamic visceromotor pattern generator network (Thompson and Swanson, 2003; Dong and Swanson, 2005a), as demonstrate originally with retrograde tracing experiments (Thompson and Swanson, 1998). The BSTmg and BSTdm (but not the BSTv) also project substantially to the median preoptic, anteroventral preoptic, and parastrial nuclei, three other nodes in the network.
Neural inputs to the BSTmg and BSTv
The origin of neural inputs to the BSTmg and BSTv has not been examined systematically. Available evidence suggests that they overlap considerably with those summarized for the adjacent BSTdm (Dong and Swanson, 2005a). They fall into three broad categories: the cerebral hemisphere (cortex and nuclei), brainstem regions processing viscerosensory information, and brainstem regions involved in modulating behavioral state.
Cerebral hemisphere (cortico-striatopallidal) inputs
The BSTmg receives direct inputs from both the central and medial amygdalar nuclei (Dong et al., 2001a). The central nucleus was discussed above. The medial nucleus can be regarded as a putative striatal or medial ventricular ridge component of the accessory olfactory system that processes pheromonal information (see Swanson and Petrovich, 1998). These inputs are complemented by others from the BST (a pallidal or lateral ventricular ridge differentiation): from the rhomboid nucleus (Dong and Swanson, 2003), which receives a dense input from the central amygdalar nucleus (Dong et al., 2001a), and from the posterior division principal and interfascicular nuclei, which receive topographic inputs from the medial amygdalar nucleus (Dong et al., 2001a). The central nucleus receives direct inputs from the primary gustatory and visceral cortical areas, secondary main olfactory cortical areas (mostly in the amygdalar region), medial prefrontal cortex, and the ventral subiculum of the hippocampal formation (see McDonald, 1998; Swanson and Petrovich, 1998), in addition to direct inputs from the hindbrain nucleus of the solitary tract (Ricardo and Koh, 1978) and parabrachial nucleus (Bernard et al., 1993). The central nucleus plays a key role in modulating autonomic activity following various physiological and emotional stimuli, including stress, fear, and anxiety (e.g., Fendt and Fanselow, 1999; Van de Kar and Blair, 1999; LeDoux, 2000; Davis et al., 2003). The central nucleus, together with the BST anterolateral group, is also strategically positioned to modulate ingestive behavior (e.g., Watts et al., 1995; Holland et al., 2002; Dong and Swanson, 2003, 2004a).
In contrast, the medial amygdalar nucleus and BST posterior division are part of a “striatopallidal” component of the cerebral hemisphere critically positioned to influence the expression of the two basic classes of social behavior, reproductive and defensive (see Dong and Swanson, 2004b). Specifically, the posterodorsal part of the medial nucleus and BST principal nucleus are components of the sexually dimorphic circuit that integrates pheromonal, main olfactory, and genital sensory information with the influences of gonadal steroid hormones on coordinated neuroendocrine, autonomic, and somatomotor activities during sexual behavior (e.g., ejaculation; see Kollack-Walker and Newman, 1997; Coolen et al., 1998; Simmons and Yahr, 2002).
The BSTmg and BSTv also receive direct cortical inputs from the infralimbic area of the medial prefrontal region, ventral subiculum, and main olfactory part of the amygdalar region (Vertes and Todorova, 1999; Dong et al., 2001a). Specifically, the BSTmg receives dense inputs from the posterior basomedial amygdalar nucleus, whereas the anterior basomedial amygdalar nucleus projects preferentially to the BSTv (Dong et al., 2001a). In addition to its massive inputs from main olfactory cortical areas, the basomedial amygdalar nucleus also receives massive inputs from the lateral amygdalar nucleus, which in turn receives widespread inputs from the frontal, parietal, and temporal regions that process visual, auditory, and somatosensory information, and many of these cortical inputs may also reach directly the basomedial amygdalar nucleus (Pitkanen et al., 1997; McDonald, 1998; Swanson and Petrovich, 1998).
In summary, there are clearly defined neuroanatomical pathways for highly processed cognitive information from the cerebral cortex to reach the BSTmg and BSTv, directly and via the central and medial amygdalar nuclei, which may be viewed as functional differentiations of the striatum broadly defined (McDonald, 1992; Swanson and Petrovich, 1998, Swanson, 2003).
Viscerosensory and humoral inputs
It remains unclear whether the BSTmg and BSTv themselves receive direct inputs from the subfornical organ, parabrachial nucleus, and nucleus of the solitary tract (Ricardo and Koh, 1978; Swanson and Lind, 1986; Alden et al., 1994). However, both cell groups do receive inputs from the median preoptic, parastrial, and dorsomedial hypothalamic nuclei, all of which in turn receive inputs from the subfornical organ (Swanson and Lind, 1986), parabrachial nucleus (Bester et al., 1997), and nucleus of the solitary tract (Ricardo and Koh, 1978; ter Horst et al., 1989). In addition, many neurons in the vicinity of the BSTmg concentrate gonadal steroid hormones (Pfaff and Keiner, 1973; Sar and Stumpf, 1975; Stumpf et al., 1975), and express estrogen and androgen receptor mRNAs (Simerly et al., 1990; Shughrue et al., 1997; Yokosuka et al., 1997); and several hypothalamic cell groups that concentrate gonadal steroid hormones, including the ventral premammillary and ventromedial hypothalamic nuclei, also send projections to the BSTmg (Canteras et al., 1992; Canteras et al, 1994).
Inputs from the behavioral state control system
Finally, the BSTmg and BSTv may also receive inputs related to behavioral state control from brain structures that project to the BSTdm as well (see Dong and Swanson, 2005a).
Functional implications
Pelvic region
BSTmg and BSTv connections suggest they play important roles in coordinating neuroendocrine, autonomic, and somatomotor responses that help maintain homeostasis of nutrients and body water. The functional evidence for this has been reviewed in accompanying papers (Dong et al., 2001; Dong and Swanson 2003, 2004a, 2005a,b). Here we discuss important functions that have received inadequate attention for many years—cerebral hemisphere regulation of pelvic activities that include micturition, defecation, and penile erection.
To clarify possible BST involvement, it is helpful to note that the pelvic organs are controlled mainly by three sets of peripheral nerves: lumbosacral preganglionic parasympathetic (pelvic nerve), thoracolumbar sympathetic (sympathetic chain and hypogastric nerve), and lumbosacral somatic (pudendal nerve). Lumbosacral parasympathetic neurons (centered in L6-S4) provide major excitatory inputs to the urinary bladder, colon-rectum, and penis (Satoh et al., 1978; Loewy et al., 1979; de Groat and Steers, 1990; Masson et al., 1991; Blok and Holstege, 1996; Steers, 2000; Giuliano and Rampin, 2000). Thoracolumbar sympathetic nerves (arising mainly from T11-L2) provide inhibitory inputs to bladder neck smooth muscle and the colon-rectum, and excitatory inputs to the urethra and anal canal. They also innervate penile and clitoral erectile tissue, and vascular and non-vascular smooth muscle—and glandular tissue—throughout the reproductive organ system. Sacral somatic motoneurons (“Onuf's nucleus” in most species) send axons via the pudendal nerves to innervate (1) periurethral and external urethral striated muscles (for micturition), and external anal sphincter muscles (for defecation). In rat, motoneurons innervating the anal and urethral sphincters lie in two separate nuclei (the dorsolateral and dorsomedial nuclei, respectively) at the L5-L6 level; and (2) the bulbocavernosus and ischiocavernosus muscles, responsible for ejaculation in males and contributing to rhythmic perineal contractions during orgasm in females. Coordination of these three classes of inputs is critical for normal pelvic functions including micturition, defecation, and penile erection (Appenzeller, 1990).
Many supraspinal regions modulate pelvic function via descending projections. Two brain structures most densely innervated by the BSTmg and BSTdm—Barrington's nucleus and the paraventricular hypothalamic nucleus descending division—play critical roles in pelvic function modulation. Barrington's nucleus has long been viewed as the supraspinal switching center regulating storage and elimination of urine via direct projections to sacral parasympathetic preganglionic neurons (Barrington, 1925; Satoh et al., 1978; Loewy et al., 1979; Holstege et al., 1986; de Groat and Steers, 1990; Masson et al., 1991; Blok and Holstege, 1996).
In contrast, the paraventricular nucleus descending division sends descending projections to sympathetic and parasympathetic preganglionic neurons throughout the length of the spinal intermediolateral column, and some of the projection neurons use oxytocin as a neurotransmitter (Swanson and McKellar, 1979; Swanson and Kuypers, 1980; Schwanzel-Fukuda et al., 1984; Tang et al., 1998; Veronneau-Longueville et al., 1999). The paraventricular nucleus descending division also projects to somatic motoneurons of Onuf's nucleus that contribute to the pudental nerve (Holstege and Tan, 1987; Shen et al., 1990; Monaghan and Breedlove, 1991; Tang et al., 1998).
Abundant evidence implicates Barrington's nucleus and the paraventricular nucleus in control of micturition (for reviews see Appenzeller, 1990; de Groat and Steers, 1990; Blok and Holstege, 1996; de Groat, 1998; Holstege, 1998) and penile erection (e.g., Chen and Chang, 2002; Giuliano and Rampin, 2000; Rampin and Giuliano, 2000; Sachs, 2000; Steers, 2000; Melis et al., 2001). Recently, these two nuclei also have been implicated in the coordination of colonic functions like defecation (Pavcovich et al., 1998; Vizzard et al., 2000).
Several other brain regions innervated by the BSTmg, BSTdm, and BSTv also have been implicated in micturition and penile erection. They include the caudal raphé nuclei and adjacent reticular formation, ventrolateral division of the periaqueductal gray, posterior hypothalamic nucleus, and lateral hypothalamic area, all of which have been shown to send direct inputs to the lumbosacral spinal cord (Saper et al., 1976; Kausz, 1990; Masson et al., 1991; Schwanzel-Fukuda et al., 1984), as well as to Barrington's nucleus (Valentino et al., 1994).
The raphé magnus, raphé pallidus, and raphé pallidus—and adjacent reticular formation (e.g., the nucleus paragigantocellularis)—send both serotonergic and non-serotonergic projections to the thoracic intermediolateral column and lumbar ventral horn (Loewy and McKellar, 1981; Holstege, 1991; Allen and Cechetto, 1994; Tang et al., 1998; Giuliano and Rampin, 2000; de Groat, 2002).
The caudal central gray receives bladder fullness information from the sacral spinal cord, and in turn projects to Barrington's nucleus, completing a “sensory-motor” reflex for micturition (Blok et al., 1995; Holstege, 1998; de Groat, 2002), although Barrington's nucleus also receives direct inputs from sacral spinal cord in rat (Ding et al., 1997). The caudal central gray appears to be important for integrating autonomic and somatomotor responses in a variety of motivated behaviors involving ingestion, mating, and defense. In particular, the caudal central gray sends massive projections to the medullary reticular formation, which in turn projects to distinct lumbosacral somatomotor neurons innervating specific hindlimb and axial muscles (Vanderhorst and Holstege, 1995). For example, these pathways have been proposed as a final common pathway for lordosis in the cat (Vanderhorst and Holstege, 1995; Holstege, 1998). Obviously, the same muscles might be used to prepare appropriate body posture for micturition and defecation.
The dorsal lateral hypothalamic area and posterior hypothalamic nucleus send descending projections to Barrington's nucleus, the dorsal vagal complex, and the lumbosacral spinal cord (Swanson and Kuypers, 1980; Schwanzel-Fukuda et al., 1984; Allen and Cechetto, 1992; Valentino et al., 1994), suggesting a role in the integration of vagal parasympathetic, sacral parasympathetic, and somatic motor responses.
Finally, the region including the medial preoptic area and nucleus, which is densely innervated by the BSTmg and BSTdm and lightly innervated by the BSTv, also has been implicated in penile erection and micturition (Blok and Holstege, 1996; Giuliano et al., 1996; de Groat, 1998; Holstege, 1998; Giuliano and Rampin, 2000; Rampin and Giuliano, 2000; Steers, 2000). This region sends no direct projections to the sacral spinal cord (Schwanzel-Fukuda et al., 1984; Simerly and Swanson, 1988), but does innervate densely Barrington's nucleus (Rizvi et al., 1994; Ding et al., 1999), and the caudal raphé and adjacent reticular formation (Hermann et al., 1997).
Classic studies indicated that stimulating various regions of the hypothalamus, including the preoptic region, lateral hypothalamic area, and posterior hypothalamus, could increase or decrease bladder contraction and provoke urination (see Appenzeller, 1990); and more recent functional imaging studies also suggest that the hypothalamus, including the preoptic region, is activated in humans during micturition (Blok et al., 1997). The medial preoptic region also has been implicated in many aspects of sexual behavior, including non-contact penile erection (Liu et al., 1997) and urogenital reflexes associated with ejaculation (Marson and McKenna, 1994). Electrical stimulation of this general region elicits penile erection, whereas lesions suppress sexual behavior and mating (see de Groat and Steers, 1990; Giuliano and Rampin, 2000; Rampin and Giuliano, 2000; Steers, 2000).
Transneuronally labeled neurons are observed in all brain regions just mentioned following pseudorabies virus injections into pelvic organs of the rat, including the urinary bladder (Nadelhaft and Vera, 1995), urethra (Vizzard et al., 1995), external urethral sphincter (Nadelhaft and Vera, 1996), ischiocavernosus and bulbospongiosus muscles (Marson and McKenna, 1996; Tang et al., 1999), penis (Marson et al., 1993), and colon (Valentino et al., 2000; Vizzard et al., 2000). In some of these analyses, transneuronally labeled neurons were reported in the vicinity of the BSTmg (Tang et al., 1999).
In summary, brain regions innervated densely by three adjacent BST cell groups (BSTmg, BSTdm, and BSTv) have been implicated in the control of micturition, penile erection, and defecation. Thus, it is easy to propose that these three BST nuclei play a critical role in coordinating pelvic functions in general. Classic studies performed decades ago indicated that stimulation of many forebrain and midbrain sites induces bladder contraction, urination, and defecation (Ranson et al., 1935; Kabat et al., 1936; Hess, 1949; Gjone, 1966; Monnier, 1968; Appenzeller, 1990). These regions included many hypothalamic and lower brainstem structures innervated by the BSTmg, BSTdm and BSTv, as discussed above.
Interestingly, the most sensitive area found in older studies to elicit urination and defecation lies in the “ventral portion” or “posterior margin” of the septum and preoptic region around the anterior commissure, in the vicinity at least of the BSTmg and BSTdm. Furthermore, Hess (1949) showed that stimulation of the “septal pallium” (including the BST) induces the appropriate posture (somatomotor response) for defecation and micturition. In view of BSTmg and BSTdm projections directly to brain regions involved in somatomotor responses (see above), these BST nuclei appear positioned to help coordinate autonomic and somatic motor responses. More recent work also implicates “ventral” regions of the BST in non-contact penile erection (see Valcourt, 1979; Liu et al., 1997).
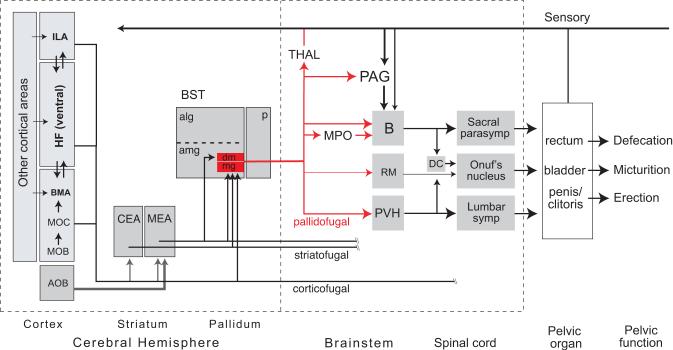
In summary, the cerebral hemispheres have a profound influence on pelvic functions (see (Appenzeller, 1990; de Groat and Steers, 1990; Blok and Holstege, 1996; de Groat, 1998; Holstege, 1998; Giuliano and Rampin, 2000; Rampin and Giuliano, 2000; Sachs, 2000; Steers, 2000), although exact neural mechanism remain poorly understood. The contiguous BSTmg, BSTdm, and BSTv may form part of a cortico-striatopallidal differentiation concerned in part with voluntary or learned influences on pelvic function (Fig. 9). Evidence for the hypothesis that a differentiation of the cortico-striatopallidal system is involved rests on a combination of extensive developmental, connectional, fast neurotransmitter utilization, and functional evidence (McDonald, 1992; Swanson and Petrovich, 1998; Swanson, 2000, 2003).
Fig. 9.
Neural network model of cerebral hemisphere involvement in pelvic organ control. The BSTdm and BSTmg can be viewed as pallidal components of a cortico-striatopallidal differentiation that coordinates pelvic visceral and somatic responses from brainstem and spinal cord, along with a thalamocortical feedback loop (Swanson & Petrovich, 1998; Dong et al., 2001a). As reviewed in the text, the BSTdm/mg project directly to all known major nodes in the brainstem pelvic motor control neuronal network. The BSTdm/mg receive GABAergic inputs from two striatal amygdalar components, the central (CEA) and medial (MEA) nuclei that in turn receive massive cortical inputs from amygdalar region, hippocampal formation (HF), and medial prefrontal cortex (infralimbic area, ILA)—all of which in turn receive inputs from other cortical areas involved in processing all major classes of sensory information (McDonald, 1998; Swanson & Petrovich, 1998). The accessory olfactory bulb (AOB) sends particularly massive inputs to MEA, and the BSTmg receives direct cortical inputs (Dong et al., 2001a). Diagram adapted from Figure 32C in Swanson (2000).
Maternal behavior and parturition
Numan and colleagues have documented that the “ventral part” of the BST and the adjacent medial preoptic region are critical for the neural control of maternal behavior in the rat (Numan and Numan, 1996, 1997; Numan and Sheehan, 1997; Stack et al., 2002). Using PHAL they also described axonal projection patterns similar to those described here in the male. In the female rat, they showed that the “ventral part” of the BST densely innervates the magnocellular neuroendocrine system of the paraventricular nucleus (preferentially oxytocinergic), paraventricular descending division (which projects to spinal preganglionic neurons), and Barrington's nucleus (specifically for sacral parasympathetic preganglionic neurons). In females, the sacral parasympathetic outflow and thoracolumbar sympathetic outflow innervate the uterus and external genitalia. Some work reports c-fos activation associated with parturition in the medial preoptic region and paraventricular nucleus (Fenelon et al., 1993; Lin et al., 1998; Douglas et al., 2002), but a role for the BST remains to be clarified experimentally.
OVERVIEW
Micturition and defecation are basic aspects of body waste storage and periodic elimination. In contrast to “pure” autonomic activities, these pelvic functions are controlled by involuntary reflexes and by voluntary or cognitive mechanisms. Involuntary reflex control is readily apparent in human infants and anesthetized animals, whereas voluntary initiation and interruption of micturition and defecation occurs in humans and other mammals. High-level cognitive inputs apparently are required for the voluntary elimination of body waste at advantageous times and places, and this requires considerable learning in children. Penile erection also is controlled by “reflexogenic” and “psychogenic” mechanisms, with the latter requiring highly processed olfactory, visual, auditory, somatosensory, and other types of information (see de Groat and Steers, 1990; Sachs, 2000). Emotion and/or motivation also profoundly influence these pelvic functions. Fear, stress, and depression all contribute to pelvic clinic disorders, including bladder, bowl, and erectile dysfunctions (Blanchard et al., 1990; Pavcovich et al., 1998; Sachs, 2000; Blomhoff et al., 2001; Rouzade-Dominguez et al., 2001).
The three adjacent BST nuclei discussed here (BSTmg, BSTv, and BSTdm) are the only known cerebral nuclei that project simultaneously to Barrington's nucleus and the paraventricular nucleus descending division. Massive axonal inputs from parts of the cerebral cortex and striatum that process information related to affect/emotion and motivation to these three BST nuclei suggest they are a pallidal medial ventricular ridge differentiation of the embryonic cerebral hemisphere (Bayer, 1987; Swanson and Alvarez-Bolado, 1996; Swanson and Petrovich, 1998; Swanson, 2000, 2003), involved differentially in the cerebral control of pelvic functions including, micturition, defection, and penile erection. However, the functional organization of this putative cortico-striatopallidal differentiation remains to be examined experimentally (Holstege, 2004).
Acknowledgments
Grant sponsor: National Institutes of Health; Grant number: 2R01NS16686
ABBREVIATIONS
- AAA
anterior amygdalar area
- ac
anterior commissure
- ACAd
anterior cingulate area, dorsal part
- ACB
nucleus accumbens
- aco
anterior commissure, olfactory limb
- act
anterior commissure, temporal limb
- AD
anterodorsal nucleus
- ADP
anterodorsal preoptic nucleus
- AHA
anterior hypothalamic area
- AHN
anterior hypothalamic nucleus
- AHNa
anterior hypothalamic nucleus, anterior part
- AHNc
anterior hypothalamic nucleus, central part
- AHNd
anterior hypothalamic nucleus, dorsal part
- AHNp
anterior hypothalamic nucleus, posterior part
- AI
agranular insular area
- AId
agranular insular area, dorsal part
- AIp
agranular insular area, posterior part
- AMBd
nucleus ambiguus, dorsal part
- AMBv
nucleus ambiguus, ventral part
- amc
amygdalar capsule
- AMd
anteromedial nucleus thalamus, dorsal part
- AMv
anteromedial nucleus thalamus, ventral part
- AOB
accessory olfactory bulb
- AQ
cerebral aqueduct
- ARH
arcuate nucleus hypothalamus
- AV
anteroventral nucleus thalamus
- AVP
anteroventral preoptic nucleus
- AVPV
anteroventral periventricular nucleus
- B
Barrington's nucleus
- BA
bed nucleus accessory olfactory tract
- BAC
bed nucleus anterior commissure
- BLAa
basolateral nucleus amygdala, anterior part
- BLAp
basolateral nucleus amygdala, posterior part
- BMAa
basomedial nucleus amygdala, anterior part
- BMAp
basomedial nucleus amygdala, posterior part
- bp
bouton of passage
- BST
bed nuclei of the stria terminalis
- BSTal
bed nuclei of the stria terminalis, anterior division, anterolateral area
- BSTalg
bed nuclei of the stria terminalis, anterior division, anterolateral group
- BSTam
bed nuclei of the stria terminalis, anterior division, anteromedial area
- BSTamg
bed nuclei of the stria terminalis, anterior division, anteromedial group
- BSTd
bed nuclei of the stria terminalis, posterior division, dorsal nucleus
- BSTdl
bed nuclei of the stria terminalis, anterior division, dorsolateral nucleus
- BSTdm
bed nuclei of the stria terminalis, anterior division, dorsomedial nucleus
- BSTfu
bed nuclei of the stria terminalis, anterior division, fusiform nucleus
- BSTif
bed nuclei of the stria terminalis, posterior division, interfascicular nucleus
- BSTju
bed nuclei of the stria terminalis, anterior division, juxtacapsular nucleus
- BSTmg
bed nuclei of the stria terminalis, anterior division, magnocellular nucleus
- BSTov
bed nuclei of the stria terminalis, anterior division, oval nucleus
- BSTp
bed nuclei of the stria terminalis, posterior division
- BSTpr
bed nuclei of the stria terminalis, posterior division, principal nucleus
- BSTrh
bed nuclei of the stria terminalis, anterior division, rhomboid nucleus
- BSTsc
bed nuclei of the stria terminalis, anterior division, subcommissural zone
- BSTse
bed nuclei of the stria terminalis, posterior division, stria extension
- BSTsz
bed nuclei of the stria terminalis, posterior division, cell-sparse zone
- BSTtr
bed nuclei of the stria terminalis, posterior division, transverse nucleus
- BSTv
bed nuclei of the stria terminalis, anterior division, ventral nucleus
- CA3
field CA3, Ammon's horn
- ccr
corpus callosum, rostrum
- CEA
central nucleus amygdala
- CEAc
central nucleus amygdala, capsular part
- CEAl
central nucleus amygdala, lateral part
- CEAm
central nucleus amygdala, medial part
- CLA
claustrum
- CLI
central linear nucleus raphé
- CM
central medial nucleus thalamus
- COAa1,2
cortical nucleus amygdala, anterior part, layers 1, 2
- COApl
cortical nucleus amygdala, posterior part, lateral zone
- COApm
cortical nucleus amygdala, posterior part, medial zone
- COM
commissural nucleus, periaqueductal gray
- CP
caudoputamen
- cpd
cerebral peduncle
- CSl
superior central nucleus raphé, lateral part
- CSm
superior central nucleus raphé, medial part
- CUN
cuneiform nucleus
- DMH
dorsomedial nucleus hypothalamus
- DMHa
dorsomedial nucleus hypothalamus, anterior part
- DMHp
dorsomedial nucleus hypothalamus, posterior part
- DMHv
dorsomedial nucleus hypothalamus, ventral part
- DR
dorsal nucleus raphé
- dscp
decussation superior cerebellar peduncle
- DTN
dorsal tegmental nucleus
- ec
external capsule
- em
external medullary lamina
- EPd
endopiriform nucleus, dorsal part
- EPv
endopiriform nucleus, ventral part
- EW
Edinger-Westphal nucleus
- FF
fields of Forel
- fi
fimbria
- fr
fasciculus retroflexus
- FS
fundus of the striatum
- fx
columns of the fornix
- GPe
globus pallidus, external segment
- GPi
globus pallidus, internal segment
- GRN
gigantocellular reticular nucleus
- GU
gustatory area
- gVIIn
genu of the facial nerve
- HF
hippocampal formation
- I
internuclear area
- IA
intercalated nuclei amygdala
- IAD
interanterodorsal nucleus thalamus
- IAM
interanteromedial nucleus thalamus
- ICe
inferior colliculus, external nucleus
- IF
interfascicular nucleus raphé
- III
oculomotor nucleus
- ILA
infralimbic cortical area
- im
internal medullary lamina thalamus
- IMD
intermediodorsal nucleus thalamus
- INC
interstitial nucleus of Cajal
- int
internal capsule
- IPN
interpeduncular nucleus
- isl
islands of Calleja
- islm
major islands of Calleja
- IVn
trochlear nerve
- KF
Kölliker-Fuse subnucleus (of PB)
- LA
lateral amygdalar nucleus
- LC
locus ceruleus
- LDT
laterodorsal tegmental nucleus
- LH
lateral habenula
- LHA
lateral hypothalamic area
- LHAad
lateral hypothalamic area, anterior region, dorsal zone
- LHAai
lateral hypothalamic area, anterior region, intermediate zone
- LHAav
lateral hypothalamic area, anterior region, ventral zone
- LHAd
lateral hypothalamic area, dorsal region
- LHAjd
lateral hypothalamic area, juxtadorsomedial region
- LHAjp
lateral hypothalamic area, juxtaparaventricular region
- LHAjvd
lateral hypothalamic area, juxtaventromedial region, dorsal zone
- LHAjvv
lateral hypothalamic area, juxtaventromedial region, ventral zone
- LHAm
lateral hypothalamic area, magnocellular nucleus
- LHAp
lateral hypothalamic area, posterior region
- LHApc
lateral hypothalamic area, parvicellular region
- LHAs
lateral hypothalamic area, suprafornical region
- LHAsfa
lateral hypothalamic area, subfornical region, anterior zone
- LHAsfp
lateral hypothalamic area, subfornical region, posterior zone
- LHAsfpm
lateral hypothalamic area, subfornical region, premammillary zone
- LHAvl
lateral hypothalamic area, ventral region, lateral zone
- LHAvm
lateral hypothalamic area, ventral region, medial zone
- LM
lateral mammillary nucleus
- lot
lateral olfactory tract
- LPO
lateral preoptic area
- LS
lateral septal nucleus
- LSc
lateral septal nucleus, caudal part
- LSc.d
lateral septal nucleus, caudal part, dorsal zone
- LSc.d.r
lateral septal nucleus, caudal part, dorsal zone, rostral region
- LSc.v.i
lateral septal nucleus, caudal part, ventral zone, intermediate region
- LSc.v.l.d/v
lateral septal nucleus, caudal part, ventral zone, lateral region, dorsal/ventral domains
- LSc.v.m.d/v
lateral septal nucleus, caudal part, ventral zone, medial region, dorsal/ventral domains
- LSr
lateral septal nucleus, rostral part
- LSr.dl.l.d/v
lateral septal nucleus, rostral part, dorsolateral zone, lateral region, dorsal/ventral domains
- LSr.dl.m.d/v
lateral septal nucleus, rostral part, dorsolateral zone, medial region, dorsal/ventral domains
- LSr.m.
lateral septal nucleus, rostral part, medial zone
- LSr.m.d
lateral septal nucleus, rostral part, medial zone, ventral region, caudal domain
- LSr.m.v.c/d/r
lateral septal nucleus, rostral part, medial zone, ventral region, caudal/dorsal/rostral domains
- LSr.vl.d.l/m
lateral septal nucleus, rostral part ventrolateral zone, dorsal region, lateral/medial domains
- LSr.vl.v
lateral septal nucleus, rostral part, ventrolateral zone, ventral region
- LSv
lateral septal nucleus, ventral part
- LTN
lateral tegmental nucleus
- MA
magnocellular preoptic nucleus
- MARN
magnocellular reticular nucleus
- mct
medial corticohypothalamic tract
- MDc
mediodorsal nucleus thalamus, central part
- MDl
mediodorsal nucleus thalamus, lateral part
- MDm
mediodorsal nucleus thalamus, medial part
- MDRNv
medullary reticular nucleus, ventral part
- ME
median eminence
- MEAad
medial nucleus amygdala, anterodorsal part
- MEAav
medial nucleus amygdala, anteroventral part
- MEApd-a,b,c
medial nucleus amygdala, posterodorsal part, sublayers a-c
- MEApv
medial nucleus amygdala, posteroventral part
- MEPO
median preoptic nucleus
- MEV
midbrain nucleus of the trigeminal
- MH
medial habenula
- ml
medial lemniscus
- mlf
medial longitudinal fascicle
- MM
medial mammillary nucleus
- MOB
main olfactory bulb
- MOC
main olfactory cortex
- mp
mammillary peduncle
- MPN
medial preoptic nucleus
- MPNc
medial preoptic nucleus, central part
- MPNl
medial preoptic nucleus, lateral part
- MPNm
medial preoptic nucleus, medial part
- MPO
medial preoptic area
- MRNm
midbrain reticular nucleus, magnocellular part
- MRNp
midbrain reticular nucleus, parvicellular part
- MS
medial septal nucleus
- mtg
mammillotegmental tract
- mtt
mammillothalamic tract
- mtV
midbrain tract of the trigeminal nerve
- NC
nucleus circularis
- ND
nucleus of Darkschewitsch
- NDB
nucleus of the diagonal band
- NId
nucleus incertus, dorsal part
- NLL
nucleus of the lateral lemniscus
- NLOT
nucleus of the lateral olfactory tract
- NLOT1
nucleus of the lateral olfactory tract, molecular layer
- NLOT2
nucleus of the lateral olfactory tract, pyramidal layer
- NLOT3
nucleus of the lateral olfactory tract, dorsal cap
- NPC
nucleus of the posterior commissure
- NTSm
nucleus of the solitary tract, medial part
- och
optic chiasm
- opt
optic tract
- ORBm
orbital area, medial part
- ORBv2,3
orbital area, ventral part, layers 2, 3
- OT
olfactory tubercle
- OT1
olfactory tubercle, molecular layer
- OT2
olfactory tubercle, pyramidal layer
- OT3
olfactory tubercle, polymorph layer
- OV
vascular organ lamina terminalis
- PA
posterior nucleus amygdala
- PAG
periaqueductal gray
- PAGd
periaqueductal gray, dorsal division
- PAGdl
periaqueductal gray, dorsolateral division
- PAGm
periaqueductal gray, medial division
- PAGrl
periaqueductal gray, rostrolateral division
- PAGrm
periaqueductal gray, rostromedial division
- PAGvl
periaqueductal gray, ventrolateral division
- PARN
parvicellular reticular nucleus
- PB
parabrachial nucleus
- PBlc
parabrachial nucleus, central lateral part
- PBle
parabrachial nucleus, external lateral part
- PBls
parabrachial nucleus, superior lateral part
- PBlv
parabrachial nucleus, ventral lateral part
- PBme
parabrachial nucleus, external medial part
- PBmm
parabrachial nucleus, medial medial part
- PBmv
parabrachial nucleus, ventral medial part
- pc
posterior commissure
- PCG
pontine central gray
- PD
posterodorsal preoptic nucleus
- PF
parafascicular nucleus
- PGRN
paragigantocellular reticular nucleus
- PGRNl
paragigantocellular reticular nucleus, lateral part
- PH
posterior hypothalamic nucleus
- PIR
piriform area
- PIR1
piriform area, molecular layer
- PIR2
piriform area, pyramidal layer
- PIR3
piriform area, polymorph layer
- PL
prelimbic cortical area
- pm
principal mammillary tract
- PMd
dorsal premammillary nucleus
- PMv
ventral premammillary nucleus
- PP
peripeduncular nucleus
- PPN
pedunculopontine nucleus
- PR
perireuniens nucleus
- PRC
precommissural nucleus, periaqueductal gray
- PRNc
pontine reticular nucleus, caudal part
- PRNr
pontine reticular nucleus, rostral part
- PS
parastrial nucleus
- PSCH
suprachiasmatic preoptic nucleus
- PST
preparasubthalamic nucleus
- PSTN
parasubthalamic nucleus of the lateral hypothalamic area
- PSV
principal sensory nucleus of the trigeminal
- PT
paratenial nucleus
- PVa
anterior periventricular nucleus hypothalamus
- PVH
paraventricular nucleus hypothalamus
- PVHam
paraventricular nucleus hypothalamus, anterior magnocellular part
- PVHap
paraventricular nucleus hypothalamus, anterior parvicellular part
- PVHd
paraventricular nucleus hypothalamus, descending division
- PVHdp
paraventricular nucleus hypothalamus, dorsal parvicellular part
- PVHf
paraventricular nucleus hypothalamus, forniceal part
- PVHlp
paraventricular nucleus hypothalamus, lateral parvicellular part
- PVHmpd
paraventricular nucleus hypothalamus, medial parvicellular part, dorsal zone
- PVHmpv
paraventricular nucleus hypothalamus, medial parvicellular part, ventral zone
- PVHne
paraventricular nucleus hypothalamus, neuroendocrine division
- PVHpml
paraventricular nucleus hypothalamus, posterior magnocellular part, lateral zone
- PVHpmm
paraventricular nucleus hypothalamus, posterior magnocellular part, medial zone
- PVHpv
paraventricular nucleus hypothalamus, periventricular part
- PVi
intermediate periventricular nucleus hypothalamus
- PVp
posterior periventricular nucleus hypothalamus
- PVpo
preoptic periventricular nucleus
- PVT
paraventricular nucleus thalamus
- RCH
retrochiasmatic area
- RE
nucleus reuniens
- REa
nucleus reuniens, rostral division, anterior part
- REc
nucleus reuniens, caudal division, caudal part
- REcd
nucleus reuniens, caudal division, dorsal part
- REcm
nucleus reuniens, caudal division, median part
- REcp
nucleus reuniens, caudal division, posterior part
- REd
nucleus reuniens, rostral division, dorsal part
- REl
nucleus reuniens, rostral division, lateral part
- REm
nucleus reuniens, rostral division, medial part
- REv
nucleus reuniens, rostral division, ventral part
- RH
rhomboid nucleus
- RL
rostral linear nucleus raphé
- RM
nucleus raphé magnus
- RN
red nucleus
- RO
nucleus raphé obscurus
- RPA
nucleus raphé pallidus
- RR
midbrain reticular nucleus, retrorubral area
- RT
reticular nucleus thalamus
- rust
rubrospinal tract
- SAG
nucleus sagulum
- SBPV
subparaventricular zone hypothalamus
- SC
superior colliculus
- SCdg
superior colliculus, deep gray layer
- SCdw
superior colliculus, deep white layer
- SCH
suprachiasmatic nucleus
- SCig-a,b,c
superior colliculus, intermediate gray layer, sublayers a-c
- scp
superior cerebellar peduncle
- sctv
ventral spinocerebellar tract
- SF
septofimbrial nucleus
- SFO
subfornical organ
- SH
septohippocampal nucleus
- SI
substantia innominata
- SLC
subceruleus nucleus
- SLD
sublaterodorsal nucleus
- sm
stria medullaris
- smd
supramammillary decussation
- SMT
submedial nucleus thalamus
- SNc
substantia nigra, compact part
- SNr
substantia nigra, reticular part
- SO
supraoptic nucleus
- SOr
supraoptic nucleus, retrochiasmatic part
- SPFm
subparafascicular nucleus thalamus, magnocellular part
- SPVI
spinal nucleus of the trigeminal, interpolar part
- SSN
superior salivatory nucleus
- st
stria terminalis
- STN
subthalamic nucleus
- SUBv
subiculum, ventral part
- SUM
supramammillary nucleus
- SUMl
supramammillary nucleus, lateral part
- SUMm
supramammillary nucleus, medial part
- sup
supraoptic commissures
- SUT
supratrigeminal nucleus
- tb
terminal bouton
- THAL
dorsal thalamus
- TMd
tuberomammillary nucleus, dorsal part
- TMv
tuberomammillary nucleus, ventral part
- TRS
triangular nucleus septum
- tsp
tectospinal pathway
- TTd1-4
tenia tecta, dorsal part, layers 1-4
- TU
tuberal nucleus
- TUi
tuberal nucleus, intermediate part
- TUl
tuberal nucleus, lateral part
- TUsv
tuberal nucleus, subventromedial part
- TUte
tuberal nucleus, terete subnucleus
- v
varicosity
- V3
third ventricle
- V3m
third ventricle, mammillary recess
- V3p
third ventricle, preoptic recess
- V4
fourth ventricle
- vhc
ventral hippocampal commissure
- VIIn
facial nerve
- VISC
visceral area
- VL
lateral ventricle
- VLP
ventrolateral preoptic nucleus
- vlt
ventrolateral hypothalamic tract
- VM
ventral medial nucleus thalamus
- Vma
motor nucleus of the trigeminal, magnocellular part
- VMHa
ventromedial nucleus hypothalamus, anterior part
- VMHc
ventromedial nucleus hypothalamus, central part
- VMHdm
ventromedial nucleus hypothalamus, dorsomedial part
- VMHvl
ventromedial nucleus hypothalamus, ventrolateral part
- VPMpc
ventral posteromedial nucleus thalamus, parvicellular part
- VTA
ventral tegmental area
- vtd
ventral tegmental decussation
- ZI
zona incerta
- ZIda
zona incerta, dopaminergic group
- zl
zona limitans
LITERATURE CITED
- Alden M, Besson JM, Bernard JF. Organization of the efferent projections from the pontine parabrachial area to the bed nucleus of the stria terminalis and neighboring regions: a PHA-L study in the rat. J Comp Neurol. 1994;341:289–314. doi: 10.1002/cne.903410302. [DOI] [PubMed] [Google Scholar]
- Allen GV, Cechetto DF. Functional and anatomical organization of cardiovascular pressor and depressor sites in the lateral hypothalamic area. I. Descending projections. J Comp Neurol. 1992;315:313–332. doi: 10.1002/cne.903150307. [DOI] [PubMed] [Google Scholar]
- Allen GV, Cechetto DF. Serotoninergic and nonserotoninergic neurons in the medullary raphe system have axon collateral projections to autonomic and somatic cell groups in the medulla and spinal cord. J Comp Neurol. 1994;350:357–366. doi: 10.1002/cne.903500303. [DOI] [PubMed] [Google Scholar]
- Alvarez-Bolado G, Swanson LW. Developmental brain maps: structure of the embryonic rat brain. Elsevier; Amsterdam: 1996. [Google Scholar]
- Appenzeller O. The autonomic nervous system: an introduction to basic and clinical concepts. Elsevier; Amsterdam: 1990. Neurogenic control and disorders of micturition; pp. 443–489. [Google Scholar]
- Barrington FJF. The effect of lesions of the hind-and mid-brain on micturition in the cat. Quart J Exp Physiol. 1925;15:81–102. [Google Scholar]
- Bayer SA. Neurogenetic and morphogenetic heterogeneity in the bed nucleus of the stria terminalis. J Comp Neurol. 1987;265:47–64. doi: 10.1002/cne.902650105. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Alden M, Besson JM. The organization of the efferent projections from the pontine parabrachial area to the amygdaloid complex: a Phaseolus vulgaris leucoagglutinin (PHA-L) study in the rat. J Comp Neurol. 1993;329:201–229. doi: 10.1002/cne.903290205. [DOI] [PubMed] [Google Scholar]
- Bester H, Besson JM, Bernard JF. Organization of efferent projections from the parabrachial area to the hypothalamus: a Phaseolus vulgaris leucoagglutinin study in the rat. J Comp Neurol. 1997;383:245–281. doi: 10.1002/(sici)1096-9861(19970707)383:3<245::aid-cne1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Scharff L, Schwarz SP, Suls JM, Barlow DH. The role of anxiety and depression in the irritable bowel syndrome. Behav Res Ther. 1990;28:401–405. doi: 10.1016/0005-7967(90)90159-g. [DOI] [PubMed] [Google Scholar]
- Blok BF, De Weerd H, Holstege G. Ultrastructural evidence for a paucity of projections from the lumbosacral cord to the pontine micturition center or M-region in the cat: a new concept for the organization of the micturition reflex with the periaqueductal gray as central relay. J Comp Neurol. 1995;359:300–309. doi: 10.1002/cne.903590208. [DOI] [PubMed] [Google Scholar]
- Blok BF, Holstege G. The neuronal control of micturition and its relation to the emotional motor system. Prog Brain Res. 1996;107:113–126. doi: 10.1016/s0079-6123(08)61861-0. [DOI] [PubMed] [Google Scholar]
- Blok BF, Willemsen AT, Holstege G. A PET study on brain control of micturition in humans. Brain. 1997;120:111–121. doi: 10.1093/brain/120.1.111. [DOI] [PubMed] [Google Scholar]
- Blomhoff S, Spetalen S, Jacobsen MB, Malt UF. Phobic anxiety changes the function of brain-gut axis in irritable bowel syndrome. Psychosom Med. 2001;63:959–965. doi: 10.1097/00006842-200111000-00015. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Projections of the ventral premammillary nucleus. J Comp Neurol. 1992;324:195–212. doi: 10.1002/cne.903240205. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Organization of the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol. 1994;348:41–79. doi: 10.1002/cne.903480103. [DOI] [PubMed] [Google Scholar]
- Chen KK, Chang LS. Involvement of L-arginine/nitric oxide pathway at the paraventricular nucleus of hypothalamus in central neural regulation of penile erection in the rat. Int J Impot Res. 2002;14:139–145. doi: 10.1038/sj.ijir.3900825. [DOI] [PubMed] [Google Scholar]
- Churchill L, Zahm DS, Kalivas PW. The mediodorsal nucleus of the thalamus in rats. I. Forebrain gabaergic innervation. Neurosci. 1996;70:93–102. doi: 10.1016/0306-4522(95)00351-i. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Peters HJ, Veening JG. Anatomical interrelationships of the medial preoptic area and other brain regions activated following male sexual behavior: a combined fos and tract-tracing study. J Comp Neurol. 1998;397:421–435. doi: 10.1002/(sici)1096-9861(19980803)397:3<421::aid-cne8>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Myers KM. Role of the amygdala in fear extinction measured with potentiated startle. Ann N Y Acad Sci. 2003;985:218–232. doi: 10.1111/j.1749-6632.2003.tb07084.x. [DOI] [PubMed] [Google Scholar]
- de Groat WC. Anatomy of the central neural pathways controlling the lower urinary tract. Eur Urol. 1998;34(Suppl 1):2–5. doi: 10.1159/000052265. [DOI] [PubMed] [Google Scholar]
- de Groat WC. Influence of central serotonergic mechanisms on lower urinary tract function. Urology. 2002;59:30–36. doi: 10.1016/s0090-4295(01)01636-3. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Steers WD. Autonomic regulation of the urinary bladder and sexual organs. In: Loewy AD, Spyer KM, editors. Central regulation of autonomic functions. Oxford University Press; New York: 1990. pp. 310–333. [Google Scholar]
- Ding YQ, Wang D, Xu JQ, Ju G. Direct projections from the medial preoptic area to spinally-projecting neurons in Barrington's nucleus: an electron microscope study in the rat. Neurosci Lett. 1999;271:175–178. doi: 10.1016/s0304-3940(99)00562-5. [DOI] [PubMed] [Google Scholar]
- Ding YQ, Zheng HX, Gong LW, Lu Y, Zhao H, Qin BZ. Direct projections from the lumbosacral spinal cord to Barrington's nucleus in the rat: a special reference to micturition reflex. J Comp Neurol. 1997;389:149–160. doi: 10.1002/(sici)1096-9861(19971208)389:1<149::aid-cne11>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Dong H-W, Petrovich GD, Swanson LW. Organization of projections from the juxtacapsular nucleus of the BST: a PHAL study in the rat. Brain Res. 2000;859:1–14. doi: 10.1016/s0006-8993(99)02246-5. [DOI] [PubMed] [Google Scholar]
- Dong H-W, Petrovich GD, Swanson LW. Topography of projections from amygdala to the bed nuclei of the stria terminalis. Brain Res Rev. 2001a;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Dong H-W, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol. 2001b;436:430–455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- Dong H-W, Swanson LW. Projections from the rhomboid nucleus of the BST: implications for cerebral hemisphere modulation of ingestive behaviors. J Comp Neurol. 2003;463:434–472. doi: 10.1002/cne.10758. [DOI] [PubMed] [Google Scholar]
- Dong H-W, Swanson LW. Organization of axonal projections from the anterolateral area of the bed nuclei of the stria terminalis. J Comp Neurol. 2004a;468:277–298. doi: 10.1002/cne.10949. [DOI] [PubMed] [Google Scholar]
- Dong H-W, Swanson LW. Projections from bed nuclei of the stria terminalis, posterior division: implications for cerebral hemisphere regulation of defensive and reproductive behaviors. J Comp Neurol. 2004b;471:396–433. doi: 10.1002/cne.20002. [DOI] [PubMed] [Google Scholar]
- Dong H-W, Swanson LW. Projections from bed nuclei of the stria terminalis, dorsomedial nucleus: implications for cerebral hemisphere integration of neuroendocrine, autonomic, and drinking responses. J Comp Neurol. 2005a doi: 10.1002/cne.20790. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H-W, Swanson LW. Projections from bed nuclei of the stria terminalis, anteromedial area: cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. J Comp Neurol. 2005b doi: 10.1002/cne.20788. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas AJ, Leng G, Russell JA. The importance of oxytocin mechanisms in the control of mouse parturition. Reproduction. 2002;123:543–552. doi: 10.1530/rep.0.1230543. [DOI] [PubMed] [Google Scholar]
- Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Fenelon VS, Poulain DA, Theodosis DT. Oxytocin neuron activation and Fos expression: a quantitative immunocytochemical analysis of the effect of lactation, parturition, osmotic and cardiovascular stimulation. Neurosci. 1993;53:77–89. doi: 10.1016/0306-4522(93)90286-o. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Sawchenko PE. An anterograde neuroanatomical tracing method that shows the detailed morphology of neurons, their axons and terminals: immunohistochemical localization of an axonally transported plant lectin, Phaseolus vulgaris-leucoagglutinin (PHA-L) Brain Res. 1984;290:219–238. doi: 10.1016/0006-8993(84)90940-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano F, Rampin O. Central neural regulation of penile erection. Neurosci Biobehav Rev. 2000;24:517–533. doi: 10.1016/s0149-7634(00)00020-8. [DOI] [PubMed] [Google Scholar]
- Giuliano F, Rampin O, Brown K, Courtois F, Benoit G, Jardin A. Stimulation of the medial preoptic area of the hypothalamus in the rat elicits increases in intracavernous pressure. Neurosci Lett. 1996;209:1–4. doi: 10.1016/0304-3940(96)12594-5. [DOI] [PubMed] [Google Scholar]
- Gjone R. Excitatory and inhibitory bladder responses to stimulation of ‘limbic’, diencephalic and mesencephalic structures in the cat. Acta Physiol Scand. 1966;66:91–102. doi: 10.1111/j.1748-1716.1966.tb03171.x. [DOI] [PubMed] [Google Scholar]
- Gu GB, Cornea A, Simerly RB. Sexual differentiation of projections from the principal nucleus of the bed nuclei of the stria terminalis. J Comp Neurol. 2003;460:542–562. doi: 10.1002/cne.10677. [DOI] [PubMed] [Google Scholar]
- Hermann DM, Luppi PH, Peyron C, Hinckel P, Jouvet M. Afferent projections to the rat nuclei raphe magnus, raphe pallidus and reticularis gigantocellularis pars alpha demonstrated by iontophoretic application of choleratoxin (subunit b) J Chem Neuroanat. 1997;13:1–21. doi: 10.1016/s0891-0618(97)00019-7. [DOI] [PubMed] [Google Scholar]
- Hess WR. Das Zwischenhirn. Benno Schwabes; Basel: 1949. [Google Scholar]
- Holland PC, Petrovich GD, Gallagher M. The effects of amygdala lesions on conditioned stimulus-potentiated eating in rats. Physiol Behav. 2002;76:117–129. doi: 10.1016/s0031-9384(02)00688-1. [DOI] [PubMed] [Google Scholar]
- Holstege G. Descending motor pathways and the spinal motor system: limbic and non-limbic components. Prog Brain Res. 1991;87:307–421. doi: 10.1016/s0079-6123(08)63057-5. [DOI] [PubMed] [Google Scholar]
- Holstege G. The emotional motor system in relation to the supraspinal control of micturition and mating behavior. Behav Brain Res. 1998;92:103–109. doi: 10.1016/s0166-4328(97)00182-4. [DOI] [PubMed] [Google Scholar]
- Holstege G. Central nervous system control of micturition. In: Paxinos G, editor. The rat nervous system. 2nd ed Elsevier; Amsterdam: 2004. pp. 321–331. [Google Scholar]
- Holstege G, Griffiths D, de Wall H, Dalm E. Anatomical and physiological observations on supraspinal control of bladder and urethral sphincter muscles in the cat. J Comp Neurol. 1986;250:449–461. doi: 10.1002/cne.902500404. [DOI] [PubMed] [Google Scholar]
- Holstege G, Tan J. Supraspinal control of motoneurons innervating the striated muscles of the pelvic floor including urethral and anal sphincters in the cat. Brain. 1987;110:1323–1344. doi: 10.1093/brain/110.5.1323. [DOI] [PubMed] [Google Scholar]
- Ju G, Swanson LW. Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat. I. Cytoarchitecture. J Comp Neurol. 1989;280:587–602. doi: 10.1002/cne.902800409. [DOI] [PubMed] [Google Scholar]
- Ju G, Swanson LW, Simerly RB. Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat. II. Chemoarchitecture. J Comp Neurol. 1989;280:603–621. doi: 10.1002/cne.902800410. [DOI] [PubMed] [Google Scholar]
- Kabat H, Magoun HW, Ranson SW. Reaction of the bladder to stimulation of points in the forebrain and midbrain. J Comp Neurol. 1936;63:211–239. [Google Scholar]
- Kausz M. Distribution of hypothalamic neurons projecting to the thoracic and sacral spinal segments in the cat. J Hirnforsch. 1990;31:697–703. [PubMed] [Google Scholar]
- Kollack-Walker S, Newman S. Mating-induced expression of c-fos in the male Syrian hamster brain: role of experience, pheromones, and ejaculations. J Neurobiol. 1997;32:481–501. doi: 10.1002/(sici)1097-4695(199705)32:5<481::aid-neu4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Ann Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lin SH, Miyata S, Weng W, Matsunaga W, Ichikawa J, Furuya K, Nakashima T, Kiyohara T. Comparison of the expression of two immediate early gene proteins, FosB and Fos in the rat preoptic area, hypothalamus and brainstem during pregnancy, parturition and lactation. Neurosci Res. 1998;32:333–341. doi: 10.1016/s0168-0102(98)00100-x. [DOI] [PubMed] [Google Scholar]
- Liu YC, Salamone JD, Sachs BD. Lesions in medial preoptic area and bed nucleus of stria terminalis: differential effects on copulatory behavior and noncontact erection in male rats. J Neurosci. 1997;17:5245–5253. doi: 10.1523/JNEUROSCI.17-13-05245.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewy AD. Forebrain nuclei involved in autonomic control. Prog Brain Res. 1991;87:253–268. doi: 10.1016/s0079-6123(08)63055-1. [DOI] [PubMed] [Google Scholar]
- Loewy AD, McKellar S. Serotonergic projections from the ventral medulla to the intermediolateral cell column in the rat. Brain Res. 1981;211:146–152. doi: 10.1016/0006-8993(81)90074-3. [DOI] [PubMed] [Google Scholar]
- Loewy AD, Saper CB, Baker RP. Descending projections from the pontine micturition center. Brain Res. 1979;172:533–538. doi: 10.1016/0006-8993(79)90584-5. [DOI] [PubMed] [Google Scholar]
- Marson L, McKenna KE. Stimulation of the hypothalamus initiates the urethrogenital reflex in male rats. Brain Res. 1994;638:103–108. doi: 10.1016/0006-8993(94)90638-6. [DOI] [PubMed] [Google Scholar]
- Marson L, McKenna KE. CNS cell groups involved in the control of the ischiocavernosus and bulbospongiosus muscles: a transneuronal tracing study using pseudorabies virus. J Comp Neurol. 1996;374:161–179. doi: 10.1002/(SICI)1096-9861(19961014)374:2<161::AID-CNE1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Marson L, Platt KB, McKenna KE. Central nervous system innervation of the penis as revealed by the transneuronal transport of pseudorabies virus. Neuroscience. 1993;55:263–280. doi: 10.1016/0306-4522(93)90471-q. [DOI] [PubMed] [Google Scholar]
- Masson RL, Sparkes ML, Ritz LA. Descending projections to the rat sacrocaudal spinal cord. J Comp Neurol. 1991;307:120–130. doi: 10.1002/cne.903070111. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cell types and intrinsic connections of the amygdala. In: Aggleton JP, editor. The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. Wiley-Liss; New York: 1992. pp. 67–99. [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- Melis MR, Succu S, Mascia MS, Argiolas A. The activation of gamma aminobutyric acid(A) receptors in the paraventricular nucleus of the hypothalamus reduces non-contact penile erections in male rats. Neurosci Lett. 2001;314:123–126. doi: 10.1016/s0304-3940(01)02287-x. [DOI] [PubMed] [Google Scholar]
- Monaghan EP, Breedlove M. Brain sites projecting to the spinal nucleus of the bulbocavernosus. J Comp Neurol. 1991;307:370–374. doi: 10.1002/cne.903070303. [DOI] [PubMed] [Google Scholar]
- Monnier M. Functions of the nervous system. Elsevier; Amsterdam: 1968. [Google Scholar]
- Nadelhaft I, Vera PL. Central nervous system neurons infected by pseudorabies virus injected into the rat urinary bladder following unilateral transection of the pelvic nerve. J Comp Neurol. 1995;359:443–456. doi: 10.1002/cne.903590307. [DOI] [PubMed] [Google Scholar]
- Nadelhaft I, Vera PL. Neurons in the rat brain and spinal cord labeled after pseudorabies virus injected into the external urethral sphincter. J Comp Neurol. 1996;375:502–517. doi: 10.1002/(SICI)1096-9861(19961118)375:3<502::AID-CNE11>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Nogueira MI, de Rezende BD, do Vale LE, Bittencourt JC. Afferent connections of the caudal raphe pallidus nucleus in rats: a study using the fluorescent retrograde tracers fluorogold and true-blue. Anat Anz. 2000;182:35–45. doi: 10.1016/s0940-9602(00)80118-1. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ. A lesion and neuroanatomical tract-tracing analysis of the role of the bed nucleus of the stria terminalis in retrieval behavior and other aspects of maternal responsiveness in rats. Dev Psychobiol. 1996;29:23–51. doi: 10.1002/(SICI)1098-2302(199601)29:1<23::AID-DEV2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ. Projection sites of medial preoptic area and ventral bed nucleus of the stria terminalis neurons that express Fos during maternal behavior in female rats. J Neuroendocrinol. 1997;9:369–384. doi: 10.1046/j.1365-2826.1997.t01-1-00597.x. [DOI] [PubMed] [Google Scholar]
- Numan M, Sheehan TP. Neuroanatomical circuitry for mammalian maternal behavior. Ann N Y Acad Sci. 1997;807:101–125. doi: 10.1111/j.1749-6632.1997.tb51915.x. [DOI] [PubMed] [Google Scholar]
- Pavcovich LA, Yang M, Miselis RR, Valentino RJ. Novel role for the pontine micturition center, Barrington's nucleus: evidence for coordination of colonic and forebrain activity. Brain Res. 1998;784:355–361. doi: 10.1016/s0006-8993(97)01178-5. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Swanson LW. Projections from the lateral part of the central amygdalar nucleus to the postulated fear conditioning circuit. Brain Res. 1997;763:247–254. doi: 10.1016/s0006-8993(96)01361-3. [DOI] [PubMed] [Google Scholar]
- Pfaff D, Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J Comp Neurol. 1973;151:121–158. doi: 10.1002/cne.901510204. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Rampin O, Giuliano F. Central control of the cardiovascular and erection systems: possible mechanisms and interactions. Am J Cardiol. 2000;86:19F–22F. doi: 10.1016/s0002-9149(00)00886-9. [DOI] [PubMed] [Google Scholar]
- Ranson SW, Kabat H, Magoun HW. Autonomic responses to electrical stimulation of hypothalamus, preoptic region and septum. Arch Neurol Psychiat. 1935;33:474–477. [Google Scholar]
- Ricardo JA, Koh ET. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res. 1978;153:1–26. doi: 10.1016/0006-8993(78)91125-3. [DOI] [PubMed] [Google Scholar]
- Risold PY, Thompson RH, Swanson LW. The structural organization of connections between hypothalamus and cerebral cortex. Brain Res Rev. 1997;24:197–154. doi: 10.1016/s0165-0173(97)00007-6. [DOI] [PubMed] [Google Scholar]
- Rizvi TA, Ennis M, Aston-Jones G, Jiang M, Liu WL, Behbehani MM, Shipley MT. Preoptic projections to Barrington's nucleus and the pericoerulear region: architecture and terminal organization. J Comp Neurol. 1994;347:1–24. doi: 10.1002/cne.903470102. [DOI] [PubMed] [Google Scholar]
- Rouzade-Dominguez ML, Curtis AL, Valentino RJ. Role of Barrington's nucleus in the activation of rat locus coeruleus neurons by colonic distension. Brain Res. 2001;917:206–218. doi: 10.1016/s0006-8993(01)02917-1. [DOI] [PubMed] [Google Scholar]
- Sachs BD. Contextual approaches to the physiology and classification of erectile function, erectile dysfunction, and sexual arousal. Neurosci Biobehav Rev. 2000;24:541–560. doi: 10.1016/s0149-7634(00)00022-1. [DOI] [PubMed] [Google Scholar]
- Saper CB, Loewy AD, Swanson LW, Cowan WM. Direct hypothalamo-autonomic connections. Brain Res. 1976;117:305–312. doi: 10.1016/0006-8993(76)90738-1. [DOI] [PubMed] [Google Scholar]
- Sar M, Stumpf WE. Distribution of androgen-concentrating neurons in rat brain. In: Stumpf WE, Grant LD, editors. Anatomical neuroendocrinology. Karger; Basel: 1975. pp. 120–133. [Google Scholar]
- Satoh K, Shimizu M, Tohyama M, Maeda T. Localization of the micturition reflex center at dorsolateral pontine tegmentum of the rat. Neurosci Lett. 1978;8:27–33. doi: 10.1016/0304-3940(78)90092-7. [DOI] [PubMed] [Google Scholar]
- Schwanzel-Fukuda M, Morrell JI, Pfaff DW. Localization of forebrain neurons which project directly to the medulla and spinal cord of the rat by retrograde tracing with wheat germ agglutinin. J Comp Neurol. 1984;226:1–20. doi: 10.1002/cne.902260102. [DOI] [PubMed] [Google Scholar]
- Shen P, Arnold AP, Micevych PE. Supraspinal projections to the ventromedial lumbar spinal cord in adult male rats. J Comp Neurol. 1990;300:263–272. doi: 10.1002/cne.903000209. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. Projections of the medial preoptic nucleus: a Phaseolus vulgaris leucoagglutinin anterograde tract-tracing study in the rat. J Comp Neurol. 1988;270:209–242. doi: 10.1002/cne.902700205. [DOI] [PubMed] [Google Scholar]
- Simmons DA, Yahr P. Projections of the posterodorsal preoptic nucleus and the lateral part of the posterodorsal medial amygdala in male gerbils, with emphasis on cells activated with ejaculation. J Comp Neurol. 2002;444:75–94. doi: 10.1002/cne.10128. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Buller KM, Day TA. Medial prefrontal cortex control of the paraventricular hypothalamic nucleus response to psychological stress: possible role of the bed nucleus of the stria terminalis. J Comp Neurol. 2005;481:363–376. doi: 10.1002/cne.20376. [DOI] [PubMed] [Google Scholar]
- Stack EC, Balakrishnan R, Numan MJ, Numan M. A functional neuroanatomical investigation of the role of the medial preoptic area in neural circuits regulating maternal behavior. Behav Brain Res. 2002;131:17–36. doi: 10.1016/s0166-4328(01)00370-9. [DOI] [PubMed] [Google Scholar]
- Steers WD. Neural pathways and central sites involved in penile erection: neuroanatomy and clinical implications. Neurosci Biobehav Rev. 2000;24:507–516. doi: 10.1016/s0149-7634(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Stumpf WE, Sar M, Keefer DA. Atlas of estrogen target cells in rat brain. In: Stumpf WE, Grant LD, editors. Anatomical neuroendocrinology. Karger; Basel: 1975. pp. 104–119. [Google Scholar]
- Swanson LW. Organization of mammalian neuroendocrine system. In: Bloom FE, editor. Handbook of physiology, the nervous system, IV. Waverly Press; Baltimore: 1986. pp. 317–363. [Google Scholar]
- Swanson LW. Brain maps: structure of the rat brain. Elsevier; Amsterdam: 1992. [Google Scholar]
- Swanson LW. Brain maps: structure of the rat brain. 2nd ed Elsevier; Amsterdam: 1998-1999. [Google Scholar]
- Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The amygdala and its place in the cerebral hemisphere. Ann NY Acad Sci. 2003;985:174–184. doi: 10.1111/j.1749-6632.2003.tb07081.x. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: structure of the rat brain. 3rd ed Elsevier; Amsterdam: 2004. [Google Scholar]
- Swanson LW, Kuypers HGJM. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Lind RW. Neural projections subserving the initiation of a specific motivated behavior in the rat: new projections from the subfornical organ. Brain Res. 1986;79:399–403. doi: 10.1016/0006-8993(86)90799-7. [DOI] [PubMed] [Google Scholar]
- Swanson LW, McKellar S. The distribution of oxytocin- and neurophysin-stained fibers in the spinal cord of the rat and monkey. J Comp Neurol. 1979;188:87–106. doi: 10.1002/cne.901880108. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Tang Y, Rampin O, Calas A, Facchinetti P, Giuliano F. Oxytocinergic and serotonergic innervation of identified lumbosacral nuclei controlling penile erection in the male rat. Neurosci. 1998;82:241–254. doi: 10.1016/s0306-4522(97)00290-x. [DOI] [PubMed] [Google Scholar]
- Tang Y, Rampin O, Giuliano F, Ugolini G. Spinal and brain circuits to motoneurons of the bulbospongiosus muscle: retrograde transneuronal tracing with rabies virus. J Comp Neurol. 1999;414:167–192. [PubMed] [Google Scholar]
- ter Horst GJ, de Boer P, Luiten PG, van Willigen JD. Ascending projections from the solitary tract nucleus to the hypothalamus. A Phaseolus vulgaris lectin tracing study in the rat. Neurosci. 1989;31:785–797. doi: 10.1016/0306-4522(89)90441-7. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Canteras NS, Swanson LW. Organization of projections from the dorsomedial nucleus of the hypothalamus: a PHA-L study in the rat. J Comp Neurol. 1996;376:143–73. doi: 10.1002/(SICI)1096-9861(19961202)376:1<143::AID-CNE9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Swanson LW. Organization of inputs to the dorsomedial nucleus of the hypothalamus: a reexamination with fluorogold and PHAL in the rat. Brain Res Rev. 1998;27:89–118. doi: 10.1016/s0165-0173(98)00010-1. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Swanson LW. Structural characterization of a hypothalamic visceromotor pattern generator network. Brain Res Rev. 2003;41:153–202. doi: 10.1016/s0165-0173(02)00232-1. [DOI] [PubMed] [Google Scholar]
- Valcourt RJ, Sachs BD. Penile reflexes and copulatory behavior in male rats following lesions in the bed nucleus of the stria terminalis. Brain Res Bull. 1979;4:131–133. doi: 10.1016/0361-9230(79)90068-6. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Kosboth M, Colflesh M, Miselis RR. Transneuronal labeling from the rat distal colon: anatomic evidence for regulation of distal colon function by a pontine corticotropin-releasing factor system. J Comp Neurol. 2000;417:399–414. [PubMed] [Google Scholar]
- Valentino RJ, Page ME, Luppi PH, Zhu Y, Van Bockstaele E, Aston-Jones G. Evidence for widespread afferents to Barrington's nucleus, a brainstem region rich in corticotropinreleasing hormone neurons. Neurosci. 1994;62:125–143. doi: 10.1016/0306-4522(94)90320-4. [DOI] [PubMed] [Google Scholar]
- Van de Kar L, Blair ML. Forebrain pathways mediating stress-induced hormone secretion. Front Neuroendocrinol. 1999;20:1–48. doi: 10.1006/frne.1998.0172. [DOI] [PubMed] [Google Scholar]
- van Leeuwen F, Caffe R. Vasopressin-immunoreactive cell bodies in the bed nucleus of the stria terminalis of the rat. Cell Tiss Res. 1983;228:525–534. doi: 10.1007/BF00211473. [DOI] [PubMed] [Google Scholar]
- Vanderhorst VG, Holstege G. Caudal medullary pathways to lumbosacral motoneuronal cell groups in the cat: evidence for direct projections possibly representing the final common pathway for lordosis. J Comp Neurol. 1995;359:457–475. doi: 10.1002/cne.903590308. [DOI] [PubMed] [Google Scholar]
- Veronneau-Longueville F, Rampin O, Freund-Mercier MJ, Tang Y, Calas A, Marson L, McKenna KE, Stoeckel ME, Benoit G, Giuliano F. Oxytocinergic innervation of autonomic nuclei controlling penile erection in the rat. Neurosci. 1999;93:1437–1447. doi: 10.1016/s0306-4522(99)00262-6. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Todorova NV. Comparison of efferent projections from the prelimbic and infralimbic prefrontal cortices in the rat. Abstr Soc Neurosci. 1999;25:383. [Google Scholar]
- Vizzard MA, Brisson M, de Groat WC. Transneuronal labeling of neurons in the adult rat central nervous system following inoculation of pseudorabies virus into the colon. Cell Tiss Res. 2000;299:9–26. doi: 10.1007/s004419900128. [DOI] [PubMed] [Google Scholar]
- Vizzard MA, Erickson VL, Card JP, Roppolo JR, de Groat WC. Transneuronal labeling of neurons in the adult rat brainstem and spinal cord after injection of pseudorabies virus into the urethra. J Comp Neurol. 1995;355:629–640. doi: 10.1002/cne.903550411. [DOI] [PubMed] [Google Scholar]
- Watts AG, Kelly BA, Sanchez-Watts G. Neuropeptides and thirst: the temporal responses of corticotropin-releasing hormone and neurotensin/neuromedin N gene expression in rat limbic forebrain neurons to drinking hypertonic saline. Behav Neurosci. 1995;109:1146–1157. doi: 10.1037//0735-7044.109.6.1146. [DOI] [PubMed] [Google Scholar]
- Yokosuka M, Okamura H, Hayashi S. Postnatal development and sex difference in neurons containing estrogen receptor-alpha immunoreactivity in the preoptic brain, the diencephalon, and the amygdala in the rat. J Comp Neurol. 1997;389:81–93. doi: 10.1002/(sici)1096-9861(19971208)389:1<81::aid-cne6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]