Abstract
Vascular calcification is associated with increased cardiovascular risk and occurs by osteochondrogenic differentiation of vascular cells. Many of the same regulatory factors that control skeletal mineralization, including the complex metabolic pathway controlling levels of the activator, inorganic phosphate, and the potent inhibitor, pyrophosphate, also govern vascular calcification. We previously found that cAMP/PKA signaling pathway mediates in vitro vascular cell calcification induced by inflammatory factors including tumor necrosis factor-alpha 1 and oxidized phospholipids. In this report, we tested whether this signaling pathway modulates phosphate and pyrophosphate metabolism. Treatment of primary murine aortic cells with the PKA activator, forskolin, significantly induced osteoblastic differentiation markers, including alkaline phosphatase (ALP), osteopontin, osteocalcin as well as the pyrophosphate generator, ectonucleotide-pyrophosphatase/phosphodiesterase-1 (Enpp1) and the pyrophosphate transporter, ankylosis protein, but not the sodium/phosphate cotransporter, Pit-1. In the presence of a substrate for ALP, beta-glycerophosphate, which generates inorganic phosphate, forskolin also enhanced matrix mineralization. Inhibitors of ALP or Pit-1 abrogated forskolin-induced osteopontin expression and mineralization but not forskolin-induced osteocalcin or ALP. These results suggest that phosphate is necessary for PKA-induced calcification of vascular cells and that the extent of PKA-induced calcification is controlled by feedback induction of the inhibitor, pyrophosphate.
Keywords: Calcification, phosphate, pyrophosphate, Enpp1, Pit-1, alkaline phosphatase, PKA
Introduction
In clinical calcific vasculopathy and valvulopathy, calcium mineral is deposited in the artery wall in the form of amorphous mineralized matrix or as cartilage or bone-like tissue within atherosclerotic plaque, vessel medial layers, and/or cardiac valves [1–3]. It is widespread in patients with atherosclerosis, type II diabetes, and/or end-stage renal disease, although the mechanism(s) may differ in each of these disease contexts [4–6]. Coronary artery calcification correlates with coronary atherosclerotic burden and is an independent predictor of cardiovascular morbidity and mortality [7]. As in skeletal mineralization, vascular calcification is governed by both positive and negative regulators, and, under normal conditions, constitutive inhibition prevails. Some of the key regulatory factors include alkaline phosphatase (ALP), osteopontin, and levels of extracellular inorganic phosphate (Pi) and pyrophosphate (PPi) [8–12].
The balance between levels of Pi and PPi determines whether hydroxyapatite mineral crystals form and grow in cartilage and bone. Pi transport via type III sodium-phosphate cotransporters/receptor for gibbon ape leukemia virus/solute carrier family 20A1 (Pit-1/Glvr-1/SLC20A1) is a critical step in calcification [13, 14]. However, under physiological conditions, calcification is inhibited by extracellular PPi, elaborated by cellular export via the multipass transmembrane protein, ankylosis (Ank), as well as by cleavage of nucleotide triphosphates by ectonucleotide pyrophosphatase/phosphodiesterase 1 (Enpp1/Npp1/PC-1)[8, 15]. Enpp1, one of the seven members of NPP family, is localized both intracellularly on endoplasmic reticulum and extracellularly on the plasma membrane of cells and matrix vesicles [16]. Under certain pathological conditions, the extracellular PPi level is reduced, allowing for calcification to proceed. In elegant studies, the group led by Terkeltaub showed that the serious human disorder, idiopathic infantile arterial calcinosis, results from Enpp1 deficiency [17]. They further demonstrated ectopic mineralization and cartilaginous metaplasia in Enpp1−/− mice and inhibition of in vitro chondrogenesis by exogenous PPi, thus demonstrating the critical role of PPi production in prevention of ectopic mineralization [18, 19]. Ectopic mineralization was also found in ank/ank mice, demonstrating the role of transmembrane PPi transport in this process [20]. Upregulation of ALP and reduced pyrophosphate levels have also been postulated as a potential mechanism for uremic vascular calcification [21].
Both in vitro and in vivo studies have established that protein kinase A (PKA) activators induce vascular calcification. One PKA activator, parathyroid hormone-related protein (PTHrP), has been demonstrated in calcified atherosclerotic lesions [22]. Another PKA activator, PTH, when present at continuously high levels, induces vascular calcification in rats independently of calcium and phosphorus levels or renal function [23, 24]. Both primary and secondary hyperparathyroidism induce aortic valve calcification [25, 26]. Conversely, vascular calcification resolves in parallel with normalization of PTH levels in these models [27]. Consistent with these findings, Chen et al. showed that the PKA pathway is involved in uremic serum-induced vascular calcification [28]. Our previous work also supports a critical role for the PKA pathway in vascular calcification, since PKA activation with tumor necrosis factor-alpha (TNF-α) or directly with cAMP analogs, induces osteoblastic differentiation and mineralization of bovine vascular cells in vitro [29, 30]. However, it is not clear by what mechanism the PKA pathway leads to osteogenic differentiation and mineralization.
In this report, we tested the hypothesis that hyperparathyroidism-associated vascular calcification involves the interaction of PKA activation with pyrophosphate and phosphate metabolism. We modeled a hyperparathyroid state in the vasculature by prolonged exposure of murine aortic cells to forskolin, an activator of adenylate cyclase, which activates PKA through cAMP production. The findings support the hypothesis that PKA activation drives vascular calcification via effects on phosphate transport proteins and pyrophosphate generating enzymes.
Methods
Materials
Forskolin from Calbiochem, and levamisole hydrochloride, probenecid, H89, phosphonoformic acid (PFA) and porcine kidney alkaline phosphatase were from Sigma.
Cell isolation
Primary aortic cells were isolated from the thoracic aortas of C57BL/6 mice by the explant technique, as described previously [31]. Briefly, the thoracic aorta was gently cleaned of periadventitial fat and connective tissues and cut into rings 3 mm in length. The aortic segments of each mouse were placed in DMEM medium supplemented with 20% fetal bovine serum and 1% penicillin/streptomycin. The vessel segments were removed once cell outgrowth was achieved. Passages 4–8 were used for experiments. Eighty-five to ninety percent of the cells were positively immunoreactive for smooth muscle α-actin, (Dako) but none of the cells were positively immunoreactive for the endothelial marker, von Willebrand factor (Dako).
Culture
Cells (10,000/cm2) were treated with the indicated reagents in α-minimal essential medium supplemented with 10% fetal bovine serum. Media with fresh agents was changed every 3–4 days.
Matrix Calcification
Ten days after the treatment, cells were incubated overnight in 0.6N HCl. Calcium levels were analyzed by the o-cresolphthalein complexone method (Teco Diagnostics) in quintuplicate. Values were normalized to total protein, as determined by the Bradford method.
Alkaline Phosphatase Activity
Four days after the treatment, whole cell lysate was incubated with Sigma 104 Phosphatase Substrate (Sigma), and alkaline phosphatase activity was assayed in quintuplicate. Values were normalized to total protein, as determined by the Bradford method.
One Step Real-time RT-PCR
After 10–11 days of treatment with the indicated reagents, total RNA was isolated using TRIzol reagent (Invitrogen). Real-time RT-PCR was performed on 50 ng RNA using Qcell-Eva One-step qRT-PCR SuperMix Kit (Biochain Institute, Inc.) in the Mx3005P (Stratagene). Sequences for the primers are as follows: OPN: (forward) 5'-CCCGGTGAAAGTGACTGATT- 3' (reverse) 5' - TTCTTCAGAGGACACAGCATTC- 3'; Cbfa1: (forward) 5'-CTACCAGCCTCACCATAC-3' (reverse) 5'-AGGACAGCGACTTCATTC-3'; TAZ: (forward) 5'-ATGAATCACCAACACCAG-3' (reverse) 5'-CTTGACGCATCCTAATCC-3'; ALP (forward) 5'-TGAATCGGAACAACCTGAC-3' (reverse) 5'-CCACCAGCAAGAAGAAGC-3'; ANK (forward) 5'-GATGGCACTAGAGCGAGAAG-3' (reverse) 5'-TCAGAAGTTACGAGACAAGACC-3'; OCN (forward) 5'-CCGGGAGCAGTGTGAGCTTA3' (reverse) 5'-TAGATGCGTTTGTAGGCGGTC-3'; Pit-1 (forward) 5'-ACGAGTGGGTAGAGAGTC-3' (reverse) 5'-ATGGCGGATTAGAGAAAGG-3'; Enpp-1 (Forward) 5'-GCTAATCATCAGGAGGTCAAG-3' (Reverse) 5'-CTGGTAGAATCCCGTCAATC-3'. β-actin was used as a normalizing gene.
Data Analysis
Each experiment was performed in at least triplicate wells. Data are expressed as mean ± SEM from all experiments where noted. Means were compared using one-way ANOVA, with comparison of different groups by Fisher’s protected least significant difference test. A value of p < 0.05 was considered significant.
Results
Effects of forskolin on osteoblastic differentiation and calcification
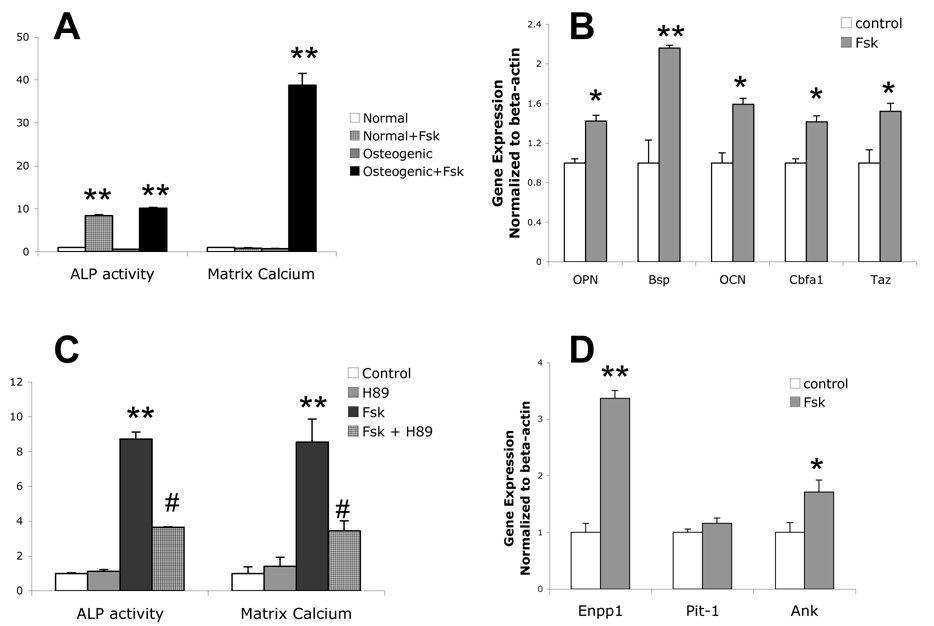
Consistent with our previous findings in bovine aortic calcifying vascular cells [30, 32], treatment of murine aortic cells with forskolin for 4 days induced ALP activity (Figure 1A). This forskolin-induced ALP activity did not require the presence of βGP (Figure 1A). In contrast to bovine calcifying vascular cells, murine aortic cells require βGP for forskolin-induced matrix calcification (Figure 1A). Forskolin treatment also induced expression of osteoblastic differentiation markers, osteopontin, bone sialoprotein, and osteocalcin (Figure 1B), suggesting that this mineralization occurs as a result of osteoblastic differentiation. In addition, forskolin induced expression of core binding factor alpha 1 (Cbfa1) and its coactivator, transcription adapter putative Zinc finger (TAZ) [33], upstream factors known to drive osteogenic differentiation (Figure 1B). The mineralization induced by forskolin was specific to PKA activation since both forskolin-induced ALP activity and calcification were attenuated by the PKA inhibitor, H89 (Figure 1C).
Figure 1. Effects of forskolin on osteoblastic differentiation and calcification.
A) ALP activity and matrix calcium from mouse aortic cells treated for 4 days and 10 days, respectively with or without forskolin (Fsk; 25 µM) in normal or osteogenic medium (normal medium supplemented with 5 mM βGP). Both ALP activity and matrix calcium were normalized to total protein, as described in Methods. B) RT-qPCR analysis of osteopontin (OPN), bone sialoprotein (BSP), osteocalcin (OCN), Cbfa1 and Taz expression from cells treated with control or Fsk (25 µM) for 7 days in osteogenic medium. C) ALP activity and matrix calcification of cells cotreated with control (DMSO) or Fsk (25 µM) and/or H89 (10 µM), as indicated, in the osteogenic medium. D) RT-qPCR analysis of Enpp1, Pit-1 and Ank expression from cells treated with control or Fsk (25 µM) for 7 days in osteogenic medium. *p < 0.05 compared to control; **p < 0.001 compared to control; #p < 0.0001 compared to Fsk-treated.
Since the balance between extracellular levels of the activator, inorganic phosphate (Pi), and the potent inhibitor, pyrophosphate (PPi), is a key determinant of mineralization, we next assessed the effects of forskolin on expression of Pit-1, Enpp1 and Ank, which govern phosphate intake, pyrophosphate generation, and pyrophosphate output to the extracellular milieu, respectively. Treatment of cells with forskolin upregulated expression of Enpp1 and Ank but not Pit-1 (Figure 1D), suggesting that forskolin induces extracellular PPi concentration as a negative feedback mechanism to limit PKA-mediated calcification. Induction of Enpp1 by PKA has been reported previously in osteosarcoma cells [34].
Effects of phosphate transport on forskolin-induced osteoblastic differentiation and mineralization
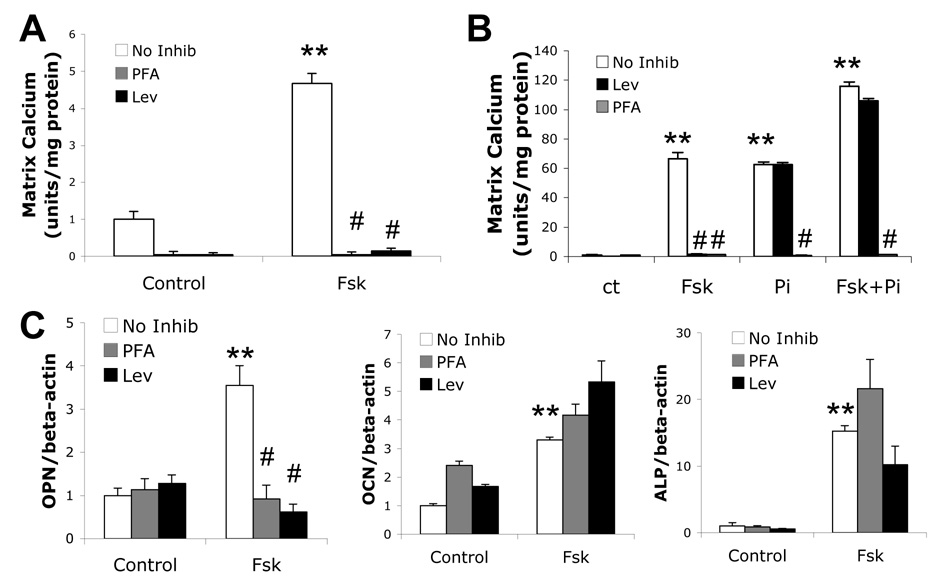
To assess whether forskolin-induced osteogenic differentiation and mineralization depends on cellular phosphate intake via Pit-1, cells were cotreated with forskolin and the Pit-1 inhibitor, PFA. As shown in Figure 2A, PFA completely abrogated forskolin-induced mineralization, suggesting that Pi transport is a necessary mediator of mineralization downstream of the PKA pathway. Consistent with this finding, blocking of Pi generation by ALP using levamisole, an ALP inhibitor, also abrogated forskolin-induced mineralization (Figure 2A). In contrast, levamisole did not inhibit calcification induced by exogenous Pi (Figure 2B), suggesting that Pi transport from extracellular to intracellular is downstream of ALP activity. Consistent with this finding, exogenous Pi rescued the inhibitory effect of levamisole on calcification but not that of PFA (Figure 2B). Cotreatment of cells with PFA or levamisole inhibited forskolin-induced OPN expression, but not ALP or osteocalcin, suggesting that forskolin acts via both phosphate dependent and independent mechanisms (Figure 2C).
Figure 2. Effects of phosphate transport on forskolin-induced osteoblastic differentiation and mineralization.
A) Matrix calcification of cells cotreated with control, Fsk (25 µM), PFA (0.5 mM) and/or Lev (10−4 M), as indicated, for 11 days in osteogenic medium. B) Matrix calcification of cells cotreated with control, Pi (3 mM), PFA (0.5 mM), and/or Lev (10−4 M), as indicated, for 11 days in osteogenic medium. C) RT-qPCR analysis of ALP, OPN and OCN expression from cells treated with control or Fsk (25 µM), PFA (0.5 mM), and/or Lev (10−4 M) for 12 days in osteogenic medium. **p < 0.001 compared to non-Fsk-treated control; #p < 0.0001 compared to Fsk-alone-treated.
Forskolin-induced osteoblastic differentiation and mineralization depends on pyrophosphate status
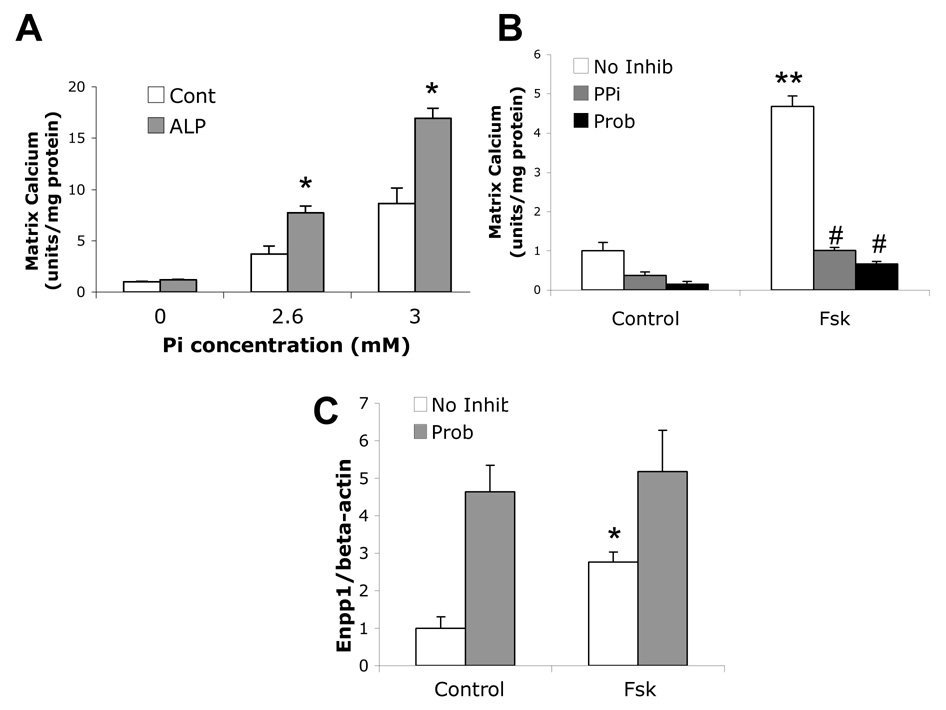
To assess the role of ALP in cleaving the inhibitor, PPi, purified porcine kidney ALP was added to mouse aortic cells, in the absence of βGP. As shown in Figure 3A, addition of exogenous ALP further promoted calcification induced by Pi, suggesting that ALP cleavage of pyrophosphate enhanced calcification. Addition of exogenous PPi to the cultures inhibited forskolin-induced calcification (Figure 3B). Interestingly, inhibition of Ank by probenecid attenuated forskolin-induced mineralization (Figure 3B). RT-qPCR analysis showed that in the presence of probenecid, Enpp1 expression was further increased compared to forskolin treatment alone (Figure 3C).
Figure 3. Effects of pyrophosphate on forskolin-induced osteoblastic differentiation and mineralization.
Matrix calcification of cells treated with control, Pi (2.6 or 3.0 mM), and/or purified porcine ALP (1 unit/mL), as indicated, for 10 days in normal medium. B) Matrix calcification of cells cotreated with control, Fsk (25 µM), PPi (0.1 mM), probenecid (Prob, 1 mM) as indicated in osteogenic medium. C) RT-qPCR analysis of Enpp1 expression from cells treated with control or Fsk (25 µM), or probenecid (1 mM) for 12 days in osteogenic medium. *p < 0.05 compared to control; **p < 0.001 compared to control; #p < 0.0001 compared to Fsk-treated.
Discussion
Chronic hyperparathyroidism is known to induce medial calcification in animal models [23, 24, 27]. Since PTH acts through its receptor to generate cAMP, we modeled hyperparathyroidism by prolonged in vitro exposure of murine aortic cells to the PKA activator, forskolin and found that PKA-induced matrix calcification of murine aortic cells appears to depend on modulation of phosphate and pyrophosphate metabolism.
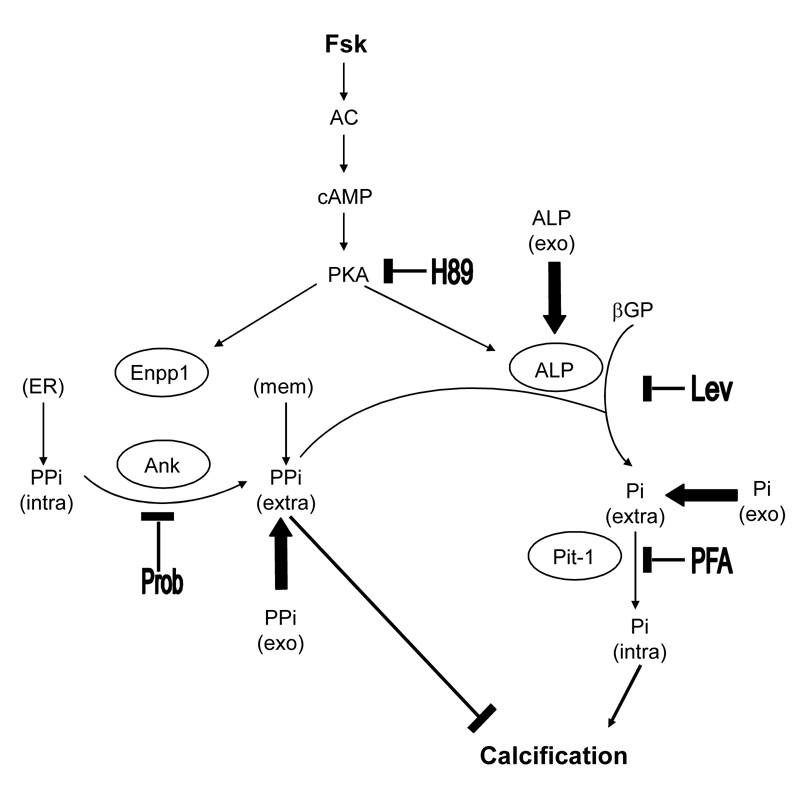
Our working model for the mechanism of forskolin-induced matrix calcification in vascular cells is shown in Figure 4. Forskolin induces both positive and negative regulators of calcification, ALP and Enpp1. The former cleaves the inhibitor PPi to generate the activator Pi while the latter generates the inhibitor PPi. Clearly, this system features tight feedback regulatory mechanisms. When βGP is added to cells, ALP cleaves it to provide extracellular inorganic phosphate (Pi-extra), which is transported by Pit-1 to the cell interior where it may drive gene expression [35]. It is also postulated that intracellular Pi (Pi-intra) accumulates in matrix vesicles, which bud off from the plasma membrane and initiate extracellular nucleation of hydroxyapatite crystals [36]. Intracellular inorganic pyrophosphate PPi (PPi-intra), is produced by endoplasmic reticulum-bound Enpp1 and is transported to the extracellular milieu by Ank protein, where PPi acts as a matrix calcification inhibitor. The extracellular PPi (PPi-extra) pool is also enriched by plasma membrane- and matrix vesicle-bound Enpp1. Altering the balance of PPi to Pi concentrations pathologically or with inhibitors regulates matrix calcification.
Figure 4. Schematic illustration of forskolin-induced mineralization in murine aortic cells.
cAMP/PKA activation by Fsk induces expression of ALP and Enpp1, generating activator Pi and inhibitor PPi, respectively. In vitro, ALP cleaves βGP to provide extracellular inorganic phosphate (Pi-extra), which is transported by Pit-1 to the cell interior where it influences gene expression. It has been postulated that intracellular Pi (Pi-intra) ultimately accumulates in matrix vesicles that bud off and serve as initiators of hydroxyapatite crystals. Intracellular inorganic PPi (PPi-intra), produced by endoplasmic reticulum (ER)-bound Enpp1, is transported to the extracellular milieu by Ank protein, where it acts as a potent calcification inhibitor. Extracellular PPi (PPi-extra) is also produced by plasma membrane-bound (memb)-Enpp1. Upregulation of ALP reduces the extracellular PPi level, causing calcification. The exogenously added Pi, PPi, ALP as well as inhibitors at each step are shown; H89, levamisole (Lev), probenecid (Prob) and PFA.
Although ALP in matrix calcification produces Pi necessary for calcification, its key in vivo regulatory action appears to be cleavage of extracellular PPi [37]. Poorly mineralized bones in ALP-deficient mice are corrected by deletion of Enpp1 or Ank genes. The double knockout mice (Enpp1−/−/alp−/− or ank/ank/alp−/−) show normalization of extracellular PPi concentrations and corrections at the skeletal sites [37]. This may be due to availability of Pi from other sources for matrix calcification [37]. Interestingly, our findings show that, in the absence of βGP, forskolin-induced ALP was not able to induce matrix calcification by cleaving PPi, suggesting that matrix calcification in the in vitro cultures is also dependent on ALP generation of Pi. It is important to note that PPi inhibits calcification in the micromolar range whereas Pi needed for matrix calcification is in the millimolar range, suggesting that PPi appears to be more potent than Pi in regulating calcification. In addition, Pi released from the cleavage of PPi by ALP alone did not promote mineralization in cultures (Figure 3A), as also demonstrated previously in aortic ring cultures [9, 38]. The results also suggest that in addition to regulating mineral deposition, Pi and PPi contribute to regulation of osteogenic gene expression. Forskolin-induced expression of osteopontin is dependent on cell-mediated Pi levels, consistent with the findings of Beck and colleagues[39, 40], whereas ALP and osteocalcin are not. Interestingly, inhibition of Ank-mediated PPi transport by probenecid inhibited forskolin-induced calcification. This finding appears paradoxical since inhibition of PPi export would likely reduce PPi levels in the extracellular milieu, and permit greater mineralization. However the findings are consistent with reports by Wang et al. in skeletal cells [41]. Our findings suggest that the inhibitory effects may likely result from increased Enpp1 expression by probenecid/forskolin compared to forskolin treatment alone. It is likely that increased expression of Enpp1 may be to compensate for the drop in extracellular PPi caused by probenecid.
Altogether, these findings suggest that vascular calcification induced by chronically elevated PTH levels may result directly from persistent activation of the PKA pathway in vascular cells, and that PKA drives calcification through changes in cell-mediated phosphate and pyrophosphate metabolism.
Acknowledgment
This research was supported by grants from the National Institutes of Health (DK076009-01 and HL081202) and American Heart Association (AHA Postdoctoral Fellowship 0825033F to A.P.S).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Michael S. Huang, Division of Cardiology, Department of Medicine, David Geffen School of Medicine at UCLA
Andrew P. Sage, Division of Cardiology, Departments of Medicine, David Geffen School of Medicine at UCLA
Jinxiu Lu, Division of Cardiology, Department of Physiology, David Geffen School of Medicine at UCLA.
Linda L. Demer, Division of Cardiology, Department of Physiology, David Geffen School of Medicine at UCLA Division of Cardiology, Department of Biomedical Engineering, David Geffen School of Medicine at UCLA.
Yin Tintut, Division of Cardiology, Department of Medicine, David Geffen School of Medicine at UCLA.
References
- 1.Jeziorska M, Woolley DE. Neovascularization in early atherosclerotic lesions of human carotid arteries: its potential contribution to plaque development. Hum Pathol. 1999;30:919–925. doi: 10.1016/s0046-8177(99)90245-9. [DOI] [PubMed] [Google Scholar]
- 2.Jeziorska M, Woolley DE. Local neovascularization and cellular composition within vulnerable regions of atherosclerotic plaques of human carotid arteries. J Pathol. 1999;188:189–196. doi: 10.1002/(SICI)1096-9896(199906)188:2<189::AID-PATH336>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 3.Stary HC. Natural history of calcium deposits in atherosclerosis progression and regression. Z Kardiol. 2000;89 Suppl 2:28–35. doi: 10.1007/s003920070097. [DOI] [PubMed] [Google Scholar]
- 4.Abedin M, Lim J, Tang TB, Park D, Demer LL, Tintut Y. N-3 fatty acids inhibit vascular calcification via the p38-mitogen-activated protein kinase and peroxisome proliferator-activated receptor-gamma pathways. Circ Res. 2006;98:727–729. doi: 10.1161/01.RES.0000216009.68958.e6. [DOI] [PubMed] [Google Scholar]
- 5.Moe SM, Chen NX. Pathophysiology of vascular calcification in chronic kidney disease. Circ Res. 2004;95:560–567. doi: 10.1161/01.RES.0000141775.67189.98. [DOI] [PubMed] [Google Scholar]
- 6.Shao JS, Cai J, Towler DA. Molecular mechanisms of vascular calcification: lessons learned from the aorta. Arterioscler Thromb Vasc Biol. 2006;26:1423–1430. doi: 10.1161/01.ATV.0000220441.42041.20. [DOI] [PubMed] [Google Scholar]
- 7.Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, Flores FR, Callister TQ, Raggi P, Berman DS. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49:1860–1870. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 8.Harmey D, Hessle L, Narisawa S, Johnson KA, Terkeltaub R, Millan JL. Concerted regulation of inorganic pyrophosphate and osteopontin by akp2, enpp1, and ank: an integrated model of the pathogenesis of mineralization disorders. Am J Pathol. 2004;164:1199–1209. doi: 10.1016/S0002-9440(10)63208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lomashvili KA, Cobbs S, Hennigar RA, Hardcastle KI, O'Neill WC. Phosphate-induced vascular calcification: role of pyrophosphate and osteopontin. J Am Soc Nephrol. 2004;15:1392–1401. doi: 10.1097/01.asn.0000128955.83129.9c. [DOI] [PubMed] [Google Scholar]
- 10.Shanahan CM, Cary NR, Metcalfe JC, Weissberg PL. High expression of genes for calcification-regulating proteins in human atherosclerotic plaques. J Clin Invest. 1994;93:2393–2402. doi: 10.1172/JCI117246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tyson KL, Reynolds JL, McNair R, Zhang Q, Weissberg PL, Shanahan CM. Osteo/chondrocytic transcription factors and their target genes exhibit distinct patterns of expression in human arterial calcification. Arterioscler Thromb Vasc Biol. 2003;23:489–494. doi: 10.1161/01.ATV.0000059406.92165.31. [DOI] [PubMed] [Google Scholar]
- 12.Shao JS, Cheng SL, Charlton-Kachigian N, Loewy AP, Towler DA. Teriparatide (human parathyroid hormone (1–34)) inhibits osteogenic vascular calcification in diabetic low density lipoprotein receptor-deficient mice. J Biol Chem. 2003;278:50195–50202. doi: 10.1074/jbc.M308825200. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Yang HY, Giachelli CM. Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circ Res. 2006;98:905–912. doi: 10.1161/01.RES.0000216409.20863.e7. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Yang HY, Giachelli CM. BMP-2 promotes phosphate uptake, phenotypic modulation, and calcification of human vascular smooth muscle cells. Atherosclerosis. 2008 doi: 10.1016/j.atherosclerosis.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terkeltaub R. Physiologic and pathologic functions of the NPP nucleotide pyrophosphatase/phosphodiesterase family focusing on NPP1 in calcification. Purinergic Signal. 2006;2:371–377. doi: 10.1007/s11302-005-5304-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaingankar SM, Fitzpatrick TA, Johnson K, Goding JW, Maurice M, Terkeltaub R. Subcellular targeting and function of osteoblast nucleotide pyrophosphatase phosphodiesterase 1. Am J Physiol Cell Physiol. 2004;286:C1177–C1187. doi: 10.1152/ajpcell.00320.2003. [DOI] [PubMed] [Google Scholar]
- 17.Rutsch F, Vaingankar S, Johnson K, Goldfine I, Maddux B, Schauerte P, Kalhoff H, Sano K, Boisvert WA, Superti-Furga A, Terkeltaub R. PC-1 nucleoside triphosphate pyrophosphohydrolase deficiency in idiopathic infantile arterial calcification. Am J Pathol. 2001;158:543–554. doi: 10.1016/S0002-9440(10)63996-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson K, Goding J, Van Etten D, Sali A, Hu SI, Farley D, Krug H, Hessle L, Millan JL, Terkeltaub R. Linked deficiencies in extracellular PP(i) and osteopontin mediate pathologic calcification associated with defective PC-1 and ANK expression. J Bone Miner Res. 2003;18:994–1004. doi: 10.1359/jbmr.2003.18.6.994. [DOI] [PubMed] [Google Scholar]
- 19.Johnson K, Polewski M, van Etten D, Terkeltaub R. Chondrogenesis mediated by PPi depletion promotes spontaneous aortic calcification in NPP1−/− mice. Arterioscler Thromb Vasc Biol. 2005;25:686–691. doi: 10.1161/01.ATV.0000154774.71187.f0. [DOI] [PubMed] [Google Scholar]
- 20.Ho AM, Johnson MD, Kingsley DM. Role of the mouse ank gene in control of tissue calcification and arthritis. Science. 2000;289:265–270. doi: 10.1126/science.289.5477.265. [DOI] [PubMed] [Google Scholar]
- 21.Lomashvili KA, Garg P, Narisawa S, Millan JL, O'Neill WC. Upregulation of alkaline phosphatase and pyrophosphate hydrolysis: potential mechanism for uremic vascular calcification. Kidney Int. 2008;73:1024–1030. doi: 10.1038/ki.2008.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rattazzi M, Bennett BJ, Bea F, Kirk EA, Ricks JL, Speer M, Schwartz SM, Giachelli CM, Rosenfeld ME. Calcification of advanced atherosclerotic lesions in the innominate arteries of ApoE-deficient mice: potential role of chondrocyte-like cells. Arterioscler Thromb Vasc Biol. 2005;25:1420–1425. doi: 10.1161/01.ATV.0000166600.58468.1b. [DOI] [PubMed] [Google Scholar]
- 23.Mizobuchi M, Ogata H, Hatamura I, Koiwa F, Saji F, Shiizaki K, Negi S, Kinugasa E, Ooshima A, Koshikawa S, Akizawa T. Up-regulation of Cbfa1 and Pit-1 in calcified artery of uraemic rats with severe hyperphosphataemia and secondary hyperparathyroidism. Nephrol Dial Transplant. 2006;21:911–916. doi: 10.1093/ndt/gfk008. [DOI] [PubMed] [Google Scholar]
- 24.Neves KR, Graciolli FG, dos Reis LM, Graciolli RG, Neves CL, Magalhaes AO, Custodio MR, Batista DG, Jorgetti V, Moyses RM. Vascular calcification: contribution of parathyroid hormone in renal failure. Kidney Int. 2007;71:1262–1270. doi: 10.1038/sj.ki.5002241. [DOI] [PubMed] [Google Scholar]
- 25.Horl WH. The clinical consequences of secondary hyperparathyroidism: focus on clinical outcomes. Nephrol Dial Transplant. 2004;19 Suppl 5:V2–V8. doi: 10.1093/ndt/gfh1049. [DOI] [PubMed] [Google Scholar]
- 26.Linhartova K, Veselka J, Sterbakova G, Racek J, Topolcan O, Cerbak R. Parathyroid hormone and vitamin D levels are independently associated with calcific aortic stenosis. Circ J. 2008;72:245–250. doi: 10.1253/circj.72.245. [DOI] [PubMed] [Google Scholar]
- 27.Shuvy M, Abedat S, Beeri R, Danenberg HD, Planer D, Ben-Dov IZ, Meir K, Sosna J, Lotan C. Uraemic hyperparathyroidism causes a reversible inflammatory process of aortic valve calcification in rats. Cardiovasc Res. 2008 doi: 10.1093/cvr/cvn088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen NX, Duan D, O'Neill KD, Wolisi GO, Koczman JJ, Laclair R, Moe SM. The mechanisms of uremic serum-induced expression of bone matrix proteins in bovine vascular smooth muscle cells. Kidney Int. 2006;70:1046–1053. doi: 10.1038/sj.ki.5001663. [DOI] [PubMed] [Google Scholar]
- 29.Tintut Y, Parhami F, Tsingotjidou A, Tetradis S, Territo M, Demer LL. 8-Isoprostaglandin E2 enhances receptor-activated NFkappa B ligand (RANKL)-dependent osteoclastic potential of marrow hematopoietic precursors via the cAMP pathway. J Biol Chem. 2002;277:14221–14226. doi: 10.1074/jbc.M111551200. [DOI] [PubMed] [Google Scholar]
- 30.Tintut Y, Patel J, Parhami F, Demer LL. Tumor necrosis factor-alpha promotes in vitro calcification of vascular cells via the cAMP pathway. Circulation. 2000;102:2636–2642. doi: 10.1161/01.cir.102.21.2636. [DOI] [PubMed] [Google Scholar]
- 31.Miyoshi T, Tian J, Matsumoto AH, Shi W. Differential response of vascular smooth muscle cells to oxidized LDL in mouse strains with different atherosclerosis susceptibility. Atherosclerosis. 2006;189:99–105. doi: 10.1016/j.atherosclerosis.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Tintut Y, Parhami F, Bostrom K, Jackson SM, Demer LL. cAMP stimulates osteoblastlike differentiation of calcifying vascular cells. Potential signaling pathway for vascular calcification. J Biol Chem. 1998;273:7547–7553. doi: 10.1074/jbc.273.13.7547. [DOI] [PubMed] [Google Scholar]
- 33.Hong JH, Hwang ES, McManus MT, Amsterdam A, Tian Y, Kalmukova R, Mueller E, Benjamin T, Spiegelman BM, Sharp PA, Hopkins N, Yaffe MB. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science. 2005;309:1074–1078. doi: 10.1126/science.1110955. [DOI] [PubMed] [Google Scholar]
- 34.Solan JL, Deftos LJ, Goding JW, Terkeltaub RA. Expression of the nucleoside triphosphate pyrophosphohydrolase PC-1 is induced by basic fibroblast growth factor (bFGF) and modulated by activation of the protein kinase A and C pathways in osteoblast-like osteosarcoma cells. J Bone Miner Res. 1996;11:183–192. doi: 10.1002/jbmr.5650110207. [DOI] [PubMed] [Google Scholar]
- 35.Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, Morii H, Giachelli CM. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87:E10–E17. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 36.Giachelli CM, Jono S, Shioi A, Nishizawa Y, Mori K, Morii H. Vascular calcification and inorganic phosphate. Am J Kidney Dis. 2001;38:S34–S37. doi: 10.1053/ajkd.2001.27394. [DOI] [PubMed] [Google Scholar]
- 37.Hessle L, Johnson KA, Anderson HC, Narisawa S, Sali A, Goding JW, Terkeltaub R, Millan JL. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci U S A. 2002;99:9445–9449. doi: 10.1073/pnas.142063399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson KA, Yao W, Lane NE, Naquet P, Terkeltaub RA. Vanin-1 pantetheinase drives increased chondrogenic potential of mesenchymal precursors in ank/ank mice. Am J Pathol. 2008;172:440–453. doi: 10.2353/ajpath.2008.070753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beck GR, Jr, Moran E, Knecht N. Inorganic phosphate regulates multiple genes during osteoblast differentiation, including Nrf2. Exp Cell Res. 2003;288:288–300. doi: 10.1016/s0014-4827(03)00213-1. [DOI] [PubMed] [Google Scholar]
- 40.Beck GR, Jr, Zerler B, Moran E. Phosphate is a specific signal for induction of osteopontin gene expression. Proc Natl Acad Sci U S A. 2000;97:8352–8357. doi: 10.1073/pnas.140021997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang W, Xu J, Du B, Kirsch T. Role of the progressive ankylosis gene (ank) in cartilage mineralization. Mol Cell Biol. 2005;25:312–323. doi: 10.1128/MCB.25.1.312-323.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]