Abstract
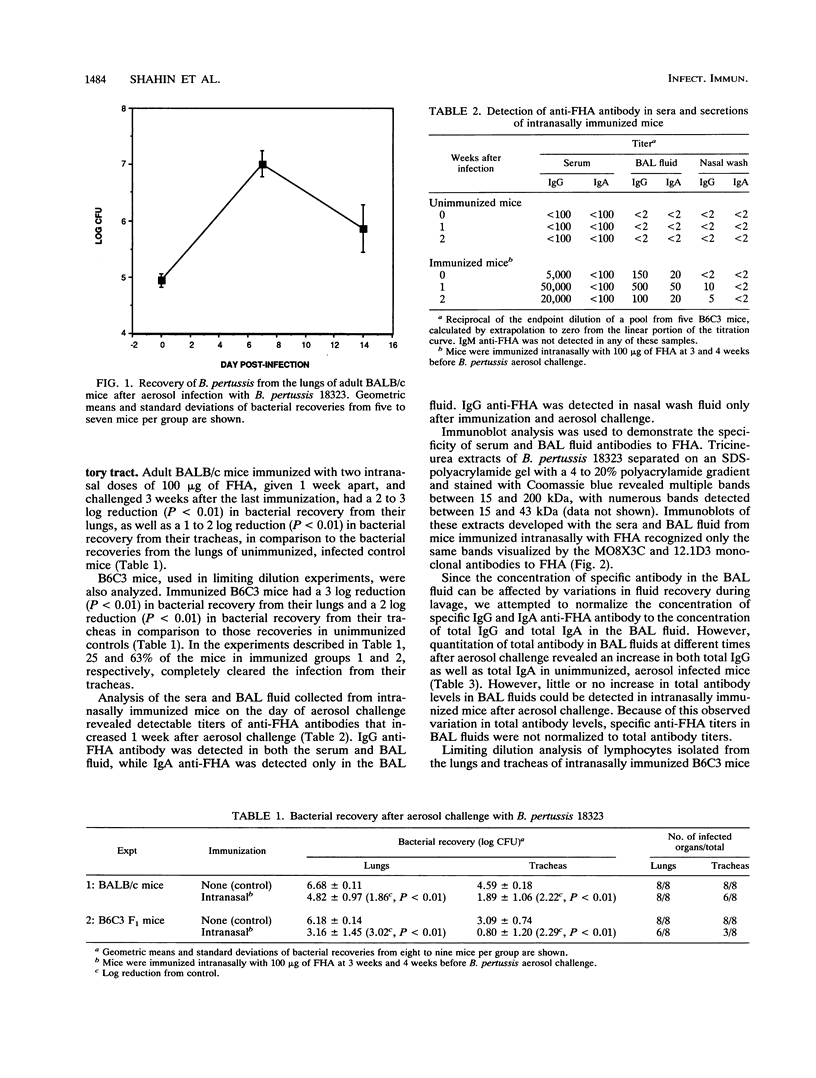
Mucosal immunization of mice with purified Bordetella pertussis filamentous hemagglutinin (FHA), by either the respiratory or the gut route, was found to protect against B. pertussis infection of the trachea and lungs. Intranasal immunization of BALB/c and (C57BL/6 x C3H/HeN)F1 adult female mice with FHA prior to B. pertussis aerosol challenge resulted in a 2 to 3 log reduction in number of bacteria recovered from the lungs and the tracheas of immunized mice in comparison to unimmunized controls. Intraduodenal immunization of adult mice with FHA before infection also resulted in approximately a 2 log reduction in the recovery of bacteria from the lungs and the tracheas of immunized mice in comparison to unimmunized controls. Immunoglobulin A and immunoglobulin G anti-FHA were both detected in bronchoalveolar lavage fluids of mucosally immunized mice. Limiting dilution analysis revealed a 60-fold increase in the frequency of FHA-specific B cells isolated from the lungs of mice immunized intranasally with FHA in comparison to unimmunized control mice. These data suggest that both gut and respiratory mucosal immunization with a major adhesin of B. pertussis generates a specific immune response in the respiratory tract that may serve as one means of mitigating subsequent B. pertussis respiratory infection.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann E., Binder B. R., Falk W., Huber E. G., Kurz R., Rosanelli K. Development and clinical use of an oral heat-inactivated whole cell pertussis vaccine. Dev Biol Stand. 1985;61:511–516. [PubMed] [Google Scholar]
- Chen K. S., Burlington D. B., Quinnan G. V., Jr Active synthesis of hemagglutinin-specific immunoglobulin A by lung cells of mice that were immunized intragastrically with inactivated influenza virus vaccine. J Virol. 1987 Jul;61(7):2150–2154. doi: 10.1128/jvi.61.7.2150-2154.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Magistris M. T., Romano M., Nuti S., Rappuoli R., Tagliabue A. Dissecting human T cell responses against Bordetella species. J Exp Med. 1988 Oct 1;168(4):1351–1362. doi: 10.1084/jem.168.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delisse-Gathoye A. M., Locht C., Jacob F., Raaschou-Nielsen M., Heron I., Ruelle J. L., de Wilde M., Cabezon T. Cloning, partial sequence, expression, and antigenic analysis of the filamentous hemagglutinin gene of Bordetella pertussis. Infect Immun. 1990 Sep;58(9):2895–2905. doi: 10.1128/iai.58.9.2895-2905.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge J. H., Gilley R. M., Staas J. K., Moldoveanu Z., Meulbroek J. A., Tice T. R. Biodegradable microspheres: vaccine delivery system for oral immunization. Curr Top Microbiol Immunol. 1989;146:59–66. doi: 10.1007/978-3-642-74529-4_6. [DOI] [PubMed] [Google Scholar]
- Finn T. M., Shahin R., Mekalanos J. J. Characterization of vir-activated TnphoA gene fusions in Bordetella pertussis. Infect Immun. 1991 Sep;59(9):3273–3279. doi: 10.1128/iai.59.9.3273-3279.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freihorst J., Merrick J. M., Ogra P. L. Effect of oral immunization with Pseudomonas aeruginosa on the development of specific antibacterial immunity in the lungs. Infect Immun. 1989 Jan;57(1):235–238. doi: 10.1128/iai.57.1.235-238.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman J. A., Cebra J. J. Special features of the priming process for a secretory IgA response. B cell priming with cholera toxin. J Exp Med. 1981 Mar 1;153(3):534–544. doi: 10.1084/jem.153.3.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmsen A. G., Muggenburg B. A., Snipes M. B., Bice D. E. The role of macrophages in particle translocation from lungs to lymph nodes. Science. 1985 Dec 13;230(4731):1277–1280. doi: 10.1126/science.4071052. [DOI] [PubMed] [Google Scholar]
- Kimura A., Mountzouros K. T., Relman D. A., Falkow S., Cowell J. L. Bordetella pertussis filamentous hemagglutinin: evaluation as a protective antigen and colonization factor in a mouse respiratory infection model. Infect Immun. 1990 Jan;58(1):7–16. doi: 10.1128/iai.58.1.7-16.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMBERT H. J. EPIDEMIOLOGY OF A SMALL PERTUSSIS OUTBREAK IN KENT COUNTY, MICHIGAN. Public Health Rep. 1965 Apr;80:365–369. [PMC free article] [PubMed] [Google Scholar]
- Leininger E., Roberts M., Kenimer J. G., Charles I. G., Fairweather N., Novotny P., Brennan M. J. Pertactin, an Arg-Gly-Asp-containing Bordetella pertussis surface protein that promotes adherence of mammalian cells. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):345–349. doi: 10.1073/pnas.88.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S. S., Welkon C. J., Clark J. L. Widespread silent transmission of pertussis in families: antibody correlates of infection and symptomatology. J Infect Dis. 1990 Mar;161(3):480–486. doi: 10.1093/infdis/161.3.480. [DOI] [PubMed] [Google Scholar]
- Mason M. J., Gillett N. A., Bice D. E. Comparison of systemic and local immune responses after multiple pulmonary antigen exposures. Reg Immunol. 1989 May-Jun;2(3):149–157. [PubMed] [Google Scholar]
- Molina N. C., Parker C. D. Murine antibody response to oral infection with live aroA recombinant Salmonella dublin vaccine strains expressing filamentous hemagglutinin antigen from Bordetella pertussis. Infect Immun. 1990 Aug;58(8):2523–2528. doi: 10.1128/iai.58.8.2523-2528.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedrud J. G., Liang X. P., Hague N., Lamm M. E. Combined oral/nasal immunization protects mice from Sendai virus infection. J Immunol. 1987 Nov 15;139(10):3484–3492. [PubMed] [Google Scholar]
- Neutra M. R., Phillips T. L., Mayer E. L., Fishkind D. J. Transport of membrane-bound macromolecules by M cells in follicle-associated epithelium of rabbit Peyer's patch. Cell Tissue Res. 1987 Mar;247(3):537–546. doi: 10.1007/BF00215747. [DOI] [PubMed] [Google Scholar]
- Owen R. L., Pierce N. F., Apple R. T., Cray W. C., Jr M cell transport of Vibrio cholerae from the intestinal lumen into Peyer's patches: a mechanism for antigen sampling and for microbial transepithelial migration. J Infect Dis. 1986 Jun;153(6):1108–1118. doi: 10.1093/infdis/153.6.1108. [DOI] [PubMed] [Google Scholar]
- Relman D. A., Domenighini M., Tuomanen E., Rappuoli R., Falkow S. Filamentous hemagglutinin of Bordetella pertussis: nucleotide sequence and crucial role in adherence. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2637–2641. doi: 10.1073/pnas.86.8.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relman D., Tuomanen E., Falkow S., Golenbock D. T., Saukkonen K., Wright S. D. Recognition of a bacterial adhesion by an integrin: macrophage CR3 (alpha M beta 2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell. 1990 Jun 29;61(7):1375–1382. doi: 10.1016/0092-8674(90)90701-f. [DOI] [PubMed] [Google Scholar]
- Renegar K. B., Small P. A., Jr Passive transfer of local immunity to influenza virus infection by IgA antibody. J Immunol. 1991 Mar 15;146(6):1972–1978. [PubMed] [Google Scholar]
- Robinson A., Ashworth L. A., Baskerville A., Irons L. I. Protection against intranasal infection of mice with Bordetella pertussis. Dev Biol Stand. 1985;61:165–172. [PubMed] [Google Scholar]
- Rose F. V., Cebra J. J. Isotype commitment of B cells and dissemination of the primed state after mucosal stimulation with Mycoplasma pulmonis. Infect Immun. 1985 Aug;49(2):428–434. doi: 10.1128/iai.49.2.428-434.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Izumiya K., Sato H., Cowell J. L., Manclark C. R. Aerosol infection of mice with Bordetella pertussis. Infect Immun. 1980 Jul;29(1):261–266. doi: 10.1128/iai.29.1.261-266.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader C. E., George A., Kerlin R. L., Cebra J. J. Dendritic cells support production of IgA and other non-IgM isotypes in clonal microculture. Int Immunol. 1990;2(6):563–570. doi: 10.1093/intimm/2.6.563. [DOI] [PubMed] [Google Scholar]
- Shahin R. D., Brennan M. J., Li Z. M., Meade B. D., Manclark C. R. Characterization of the protective capacity and immunogenicity of the 69-kD outer membrane protein of Bordetella pertussis. J Exp Med. 1990 Jan 1;171(1):63–73. doi: 10.1084/jem.171.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storsaeter J., Hallander H., Farrington C. P., Olin P., Möllby R., Miller E. Secondary analyses of the efficacy of two acellular pertussis vaccines evaluated in a Swedish phase III trial. Vaccine. 1990 Oct;8(5):457–461. doi: 10.1016/0264-410x(90)90246-i. [DOI] [PubMed] [Google Scholar]
- Thomas G. Respiratory and humoral immune response to aerosol and intramuscular pertussis vaccine. J Hyg (Lond) 1975 Apr;74(2):233–237. doi: 10.1017/s0022172400024293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. G., Ashworth L. A., Miller E., Lambert H. P. Serum IgG, IgA, and IgM responses to pertussis toxin, filamentous hemagglutinin, and agglutinogens 2 and 3 after infection with Bordetella pertussis and immunization with whole-cell pertussis vaccine. J Infect Dis. 1989 Nov;160(5):838–845. doi: 10.1093/infdis/160.5.838. [DOI] [PubMed] [Google Scholar]
- Toews G. B., Hart D. A., Hansen E. J. Effect of systemic immunization on pulmonary clearance of Haemophilus influenzae type b. Infect Immun. 1985 May;48(2):343–349. doi: 10.1128/iai.48.2.343-349.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomanen E., Weiss A. Characterization of two adhesins of Bordetella pertussis for human ciliated respiratory-epithelial cells. J Infect Dis. 1985 Jul;152(1):118–125. doi: 10.1093/infdis/152.1.118. [DOI] [PubMed] [Google Scholar]
- VACCINATION against whooping-cough; relation between protection in children and results of laboratory tests; a report to the Whooping-cough Immunization Committee of the Medical Research Council and to the medical officers of health for Cardiff, Leeds, Leyton, Manchester, Middlesex, Oxford, Poole, Tottenham, Walthamstow, and Wembley. Br Med J. 1956 Aug 25;2(4990):454–462. [PMC free article] [PubMed] [Google Scholar]
- Weissman D. N., Bice D. E., Siegel D. W., Schuyler M. R. Murine lung immunity to a soluble antigen. Am J Respir Cell Mol Biol. 1990 Apr;2(4):327–333. doi: 10.1165/ajrcmb/2.4.327. [DOI] [PubMed] [Google Scholar]
- Weisz-Carrington P., Grimes S. R., Jr, Lamm M. E. Gut-associated lymphoid tissue as source of an IgA immune response in respiratory tissues after oral immunization and intrabronchial challenge. Cell Immunol. 1987 Apr 15;106(1):132–138. doi: 10.1016/0008-8749(87)90156-0. [DOI] [PubMed] [Google Scholar]
- de Aizpurua H. J., Russell-Jones G. J. Oral vaccination. Identification of classes of proteins that provoke an immune response upon oral feeding. J Exp Med. 1988 Feb 1;167(2):440–451. doi: 10.1084/jem.167.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]



