Abstract
Human research has shown that lesions of the parietal cortex disrupt spatial information processing, specifically topological information. Similar findings have been found in nonhumans. It has been difficult to determine homologies between human and non-human mnemonic mechanisms for spatial information processing because methodologies and neuropathology differ. The first objective of the present study was to adapt a previously established human task for rats. The second objective was to better characterize the role of parietal cortex (PC) and dorsal hippocampus (dHPC) for topological spatial information processing. Rats had to distinguish whether a ball inside a ring or a ball outside a ring was the correct, rewarded object. After rats reached criterion on the task (>95%) they were randomly assigned to a lesion group (control, PC, dHPC). Animals were then re-tested. Post-surgery data show that controls were 94% correct on average, dHPC rats were 89% correct on average, and PC rats were 56% correct on average. The results from the present study suggest that the parietal cortex, but not the dHPC processes topological spatial information. The present data are the first to support comparable topological spatial information processes of the parietal cortex in humans and rats.
Keywords: Parietal Cortex, Hippocampus, Topological
INTRODUCTION
Topological spatial information is defined as relationships of connectedness, neighborhood, and enclosure among stimuli (Gallistel, 1990; Poucet, 1993; Kuipers and Levitt, 1998; Poucet and Herrmann, 2001; Goodrich-Hunsaker et al., 2005) and is important for the development of spatial maps (Poucet, 1993; Thinus-Blanc et al., 1998). Topological transformations involve either stretching on contracting the entire environment as a whole or disrupting particular relationships of enclosure or connectivity (Gallistel, 1990; Goodrich- Hunsaker et al., 2005). For the present study, we investigated the topological relationship between a ring and a ball; rats were trained to distinguish whether a ball was located inside a ring or outside a ring (see Figure 1).
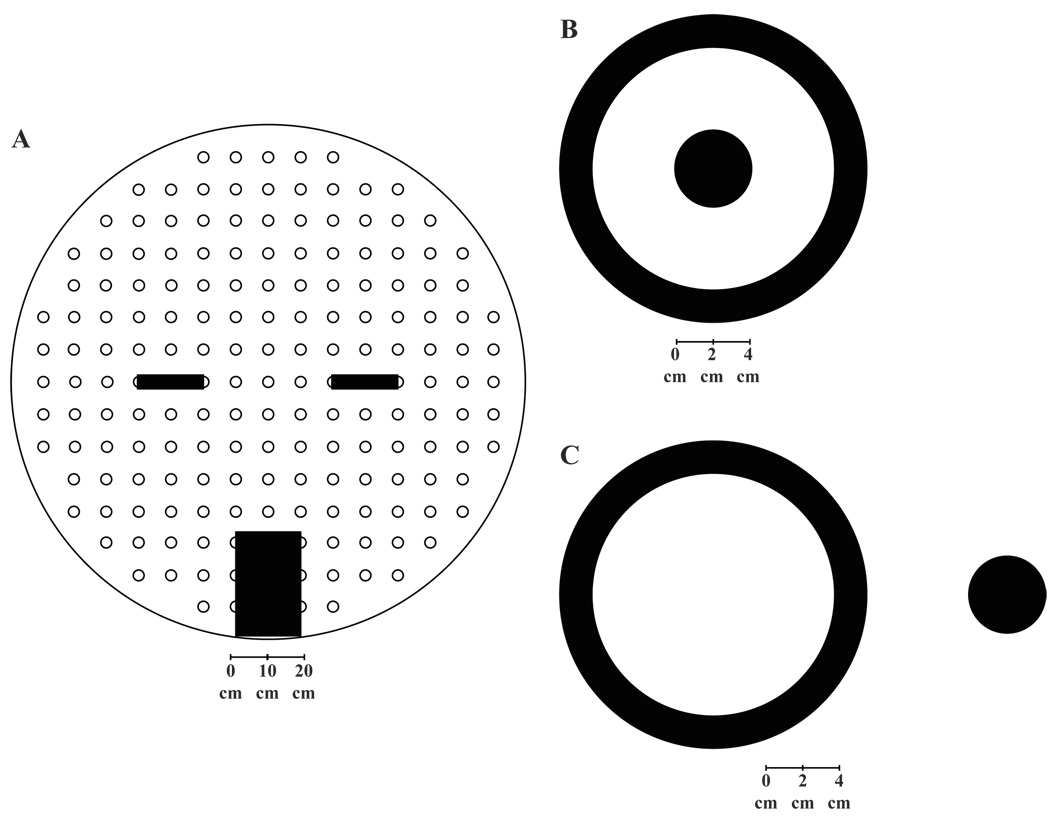
Figure 1.
(a) Apparatus. Displayed are a schematic representation of the white cheese board and the positions of the two objects and black box. A black start box was constructed to house the rat between trials. The black box was positioned on top of the round board perpendicular to the rows and parallel to the columns with the posterior edge of the box at the edge. The black box was on the center vertical row with the back edge of the box in line with the edge of the cheeseboard. The two objects were positioned on the center horizontal row of the cheeseboard covering the 5th hole from the left and the right, leaving 5 holes between the left and the right objects. (b) Ball Inside Ring. Displayed is a pictorial representation of the black ball positioned inside the black ring. The black ball is attached to the ring with a white toothpick. (c) Ball Outside Ring. Displayed is a pictorial representation of the black ball positioned outside the black ring. Again, the black ball is attached to the ring with a white toothpick.
Previous research suggests that the neuroanatomical correlate of topological spatial information processing is primarily the parietal cortex, but that the hippocampus may also play a role. In humans, the parietal cortex (PC) is important for processing spatial relationships among objects in large-scale virtual environments (Maguire et al., 1998) and relationships among objects (Robertson et al., 1997).
Study of the non-human parietal cortex also suggests a significant role in processing topological information. Goodrich-Hunsaker et al. (2005) showed that only PC lesioned rats had a deficit on a topological novelty-detection paradigm, but not a metric novelty-detection paradigm compared to dorsal hippocampus (dHPC) lesioned and control rats. The topological novelty-detection task consisted of four objects in a square orientation and the topological shift occurred when two of the objects were transposed with each other. The square configuration remained intact, but the relationships of the objects shifted topologically (Goodrich-Hunsaker et al., 2005). However, there are several limitations to the topological novelty-detection paradigm. First, it is difficult to measure whether performance was based upon topological spatial information processing or some other mnemonic mechanisms (e.g. object-place paired association). Second, novelty-detection was based upon exploration time (in sec) with the objects. There is a great deal of indiscretion over habituation to objects and what object exploration entails (i.e. sniffing the objects, biting the objects, pawing at the objects, or crawling all over the objects).
Due to aforementioned limitations of novelty-detection paradigms, a new topological task is needed to better characterize the role of the PC and dHPC for processing topological spatial information. The purpose of the present study was to continue our investigation of topological spatial information processes by using a new paradigm and to determine whether the PC in rats and humans may similarly mediate topological spatial information processing. To date human and non-human studies have differed (e.g. methodologies and PC pathology), thereby making comparisons of human and non-human PC function more difficult. The present study mimics the bilateral parietal cortex lesions seen in a human patient and the task demands are quite similar. Therefore, the results of the present study will provide a novel insight into the neuroanatomical correlates of topological spatial information processing in rats and humans.
In the present study, a previous human task (Robertson et al., 1997) was modified for rats. Robertson et al. (1997) presented to patient R. M., who had bilateral parietal cortex damage, a picture in which a smaller circle was located either inside or outside a larger circle. The smaller circle was either touching or not touching the larger circle. Patient R. M. performed at 49% correct (18 out of 37 trials) when asked to describe the location of the small circle in relation to the larger circle (Robertson et al., 1997). Patient R. M. appeared not to be able to process and describe the spatial (topological) relationship of the ball to the ring. For the present study, rats had to distinguish whether a ball inside a ring or a ball outside a ring was the correct, rewarded object. We hypothesized that PC, but not dHPC, lesions would disrupt previously learned discrimination of the correct, rewarded object.
METHODS
Subjects
Twenty male, Long Evans rats initially weighing ~350 g were used as subjects. At the beginning of the study, all rats were food-deprived to 80% of their free-feed weight and allowed access to water ad libitum. The rats were housed independently in standard plastic rodent cages and maintained on a 12-hour light/dark cycle. All testing was conducted in the light portion of the light/dark cycle.
Apparatus
A white cheese board served as the testing apparatus for the experiment. The surface of the apparatus stood 65 cm above the floor, was 119 cm in diameter, and was 3.5 cm in thickness. One-hundred seventy-seven food wells (2.5 cm in diameter and 1.5 cm in depth) were drilled into surface of the round board in evenly spaced parallel rows and columns, which were 5 cm apart. White poster board (22 cm high) surrounded the white cheese board. White Velcro was used to attach the white poster board to the white cheese board. The white poster board was used to reduce spatial cues and to increase the saliency of the objects.
A black start box (24 cm long, 15 cm wide and 17 cm high) was constructed to house the rat between trials. The black box was positioned on top of the round board perpendicular to the rows and parallel to the columns with the posterior edge of the box at the edge of the cheeseboard. The box had a hinged top for easily transferring animals into and out of the box. The front of the box had a guillotine door that could only be raised and lowered by the experimenter. See Figure 1a for a pictorial depiction of the apparatus.
Two objects were constructed as the stimuli. Both objects consisted of a black ring (15.25 cm diameter and 3.175 cm thick) mounted upright on washers. The objects were easily moveable along the surface of the cheeseboard. For one of the objects, a black ball, 3.81 cm in diameter, was positioned outside the ring and parallel to the white cheese board. The edge of the black ball was 5.08 cm away from the edge of the black ring (see Figure 1b). The black ball was attached to the ring with a white toothpick. For the second object, another black ball, 3.81 cm in diameter, was positioned inside the ring and parallel to the white cheese board. The edge of the black ball was 5.08 cm away from the edge of the black ring (see Figure 1c). The black ball was again attached to the ring with a white toothpick. The two objects were positioned on the center row of the cheeseboard covering the 5th hole from the left and the right, leaving 5 holes between the left and the right object.
Procedures
Shaping
During the first week of training, rats were handled fifteen minutes daily. During the second week of training, rats were introduced to the apparatus. Rats were given fifteen minutes to explore the white cheese board with the white poster board surrounding the cheese board and black box present. Froot Loops (Kellogg, Battle Creek, MI) were randomly distributed over the maze to induce exploration and the guillotine door on the black box remained open. Beginning with the third week of training, rats were trained to displace salient objects covering a food well to receive a ½ Froot Loop reward. Objects included such things as: a rubber duck, an inverted plastic cup, a blue electrical box, etc.
Rats were presented with two objects placed on the middle row of the white cheese board and 45 cm apart. Initially, the two objects were placed behind a food well and not covering the food well. Across trials and days, the objects were placed increasingly further over the food well. For every trial, each food well was baited with ½ of a Froot Loop. The rat began every trial in the black box, and then the guillotine door was opened. Rats were given time to exit the black box, retrieve the Froot Loops, and then return to the black box. After the rat was able to retrieve the Froot Loops in less than 3 minutes, the two objects were placed further over the food well. The experiment began when rats were able to retrieve the Froot Loops from completely covered food wells.
Experiment Pre-Surgery
A recognition task was used to assess topological spatial information processing in rats. Each rat received 20 trials daily. The two objects (one with the ball inside the ring and one with the ball outside the ring) were placed on the middle row of the round board (45 cm apart) each covering a food well. The direction of the black ball (left or right) was randomly assigned for each trial. Furthermore, the two objects (one with the ball inside the ring and one with the ball outside) left and right placements were randomly counterbalanced. Lastly, rats were randomly assigned to one of the two objects (one with the ball inside the ring and one with the ball outside) as the rewarded object. The food well covered by the reward object contained ½ of a Froot Loop. Rats began each trial in the black box, and then the guillotine door was opened. Rats were given time to exit the black box, retrieve the Froot Loop under the rewarded object, and then return to the black box. If a rat displaced the incorrect object (non-rewarded object), it was immediately returned to the black box with no reward. There was ~5–7 sec between each trial. Once a rat reached a criterion of 95% on 20 trials across 2 consecutive days (i.e. 40 consecutive trials), testing was terminated and the rat was scheduled for surgery. The latency (in sec) to displace the object, as well as which object was displaced were recorded as the dependent variables.
Surgery
Once criterion was achieved, rats were randomly assigned to a surgery group (n = 6 for parietal cortex, n = 6 for dorsal hippocampus, n = 8 for control). Rats were anesthetized with isoflurane. Each rat was placed in a stereotaxic apparatus (David Kopf Instruments) with an isothermal heating pad to maintain body temperature at 37°C. With its head level, the scalp was incised and retracted to expose bregma and lambda and positioned them in the same horizontal plane. Parietal cortex lesions (PC) were made via aspiration. As an alternative, ibotenic acid can be used to lesion the PC (Burcham, Corwin, Stoll, & Reep, 1997; Bucci & Chess, 2005). The aspiration lesions were 1 mm posterior to bregma to 4.5 mm posterior to bregma, 2 mm lateral to midline to approximately 1 mm above the rhinal sulcus in the medial-lateral plane, and 2 mm ventral to dura. Half of the control animals received dorsal hippocampus vehicle injections and the other half received sham surgery. Following surgery, the incisions were sutured.
Dorsal hippocampal lesions (dHPC) were made using 6mg/mL of ibotenic acid mixed in PBS. .2 µL ibotenic acid was injected at 6 µL/hour bilaterally into 3 sites within the dHPC (2.8 mm posterior to bregma, 1.6 mm lateral to the midline, 3.0 mm ventral to dura; 3.3 mm posterior to bregma, 1.8 mm lateral to the midline, 2.8 ventral to dura, 4.1 mm posterior to bregma, 2.6 mm lateral to the midline, 2.8 ventral to dura). All injections were made with a 10- µL Hamilton (Reno, NV) syringe with a microinjection pump (Cole Parmer Instrument Company, Vernon Hill, IL) through a 26-gauge cannula needle. The cannula needle was left in the brain for 1-minute after each drug injections.
Experiment Post-Surgery
After a 7–10 day recovery period from surgery, each rat was retested on the task following the same task procedures used before surgery. Each rat was given 20 trials daily for 5 days (100 trials total). The latency (in sec) to displace the object, as well as which object (i.e. left object or right object) was displaced were recorded as the dependent variables.
Histology
At the end of the experiments, each rat was given a lethal intraperitoneal injection of sodium pentobarbital. The rat was perfused intracardially with 10% (wt/vol) Formalin in 0.1 M phosphate buffer. The brain was then removed and stored in 30% (vol/vol) sucrose-Formalin for 1 week. Transverse sections (24 µm) were cut with a cryostat through the lesioned area and stained with cresyl violet. A program, ImageJ 1.31 (NIH), was used to quantify the extent of the lesion.
RESULTS
Histology
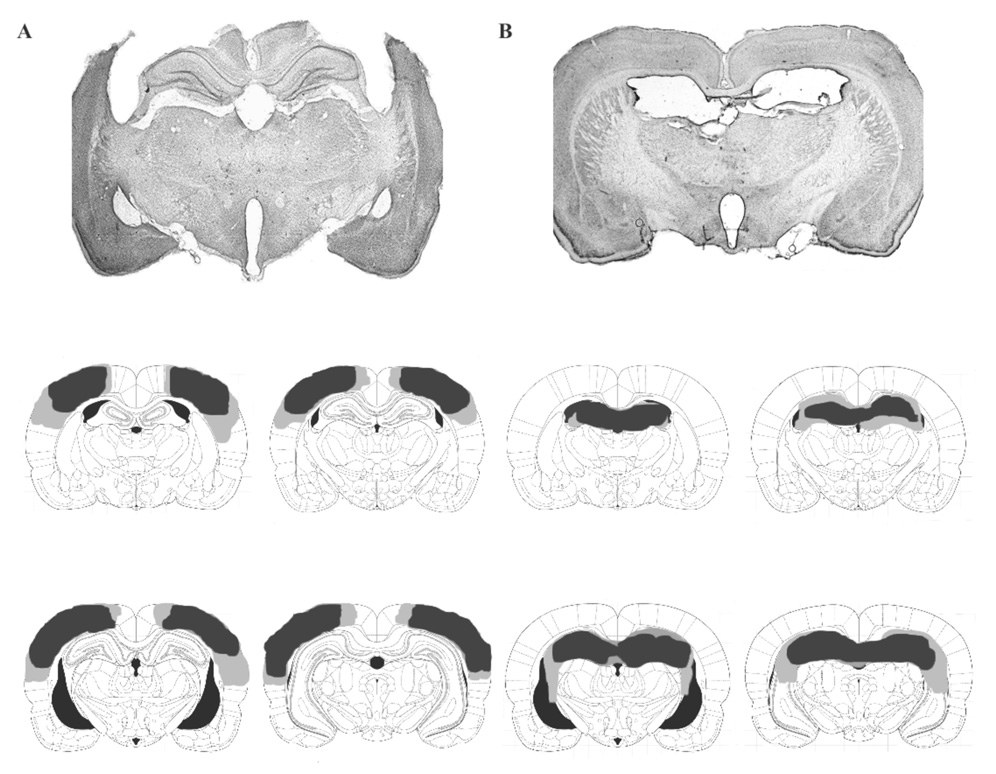
Aspiration was used to lesion the parietal cortex (PC). The lesions extended from 1 mm posterior to bregma to 4.5 mm posterior to bregma, and 2 mm lateral to midline to approximately 1 mm above the rhinal sulcus in the medial-lateral plane (Figure 2b). There was some sparing of the PC at the ventrolateral aspect adjacent to the temporal association cortex (TeA) as well as some sparing between 1 and 2 mm lateral to midline, but these sparings accounted for < 10%. The PC lesions generally did not result in damage to the dorsal or ventral hippocampus, fimbria / fornix, or temporal cortices. In one case, there was unilateral damage to the lateral aspect of area CA1, but this accounted for < 10% of area CA1. There was some anatomical deformation of the hippocampus into the empty space created by removal of the PC, but the hippocampus remained intact and showed no signs of damage.
Figure 2.
Serial sections were taken along the septotemporal axis from top to bottom. (a) Photomicrographs (12.5 X) and schematic drawing of the largest (light gray) and the smallest (dark gray) of a parietal cortex lesioned rat brain. (b) Photomicrographs (12.5 X) and schematic drawing of the largest (light gray) and the smallest (dark gray) of a dorsal hippocampus lesioned rat brain. Lesions were limited to the anterior 50% of the hippocampus.
Ibotenic acid was used to produce lesions of the dorsal hippocampus (dHPC). Although it is difficult to define the exact boundary that separates the dorsal from the ventral component of the hippocampus, the dorsal region is defined as the anterior 50% of the hippocampus (Moser & Moser, 1998). A quantitative analysis showed that a dHPC lesion resulted in > 95% damage to the dHPC with < 5% damage to the ventral hippocampus and < 5% damage to the overlying cortex (Figure 2a). The > 5% sparing of the hippocampus was usually in the subiculum or small remnants of the lower blade of the dentate gyrus. In one case, there was unilateral sparing of CA3 pyramidal cells at the most lateral aspect adjacent to the fimbria, but this accounted for < 2% of the dorsal hippocampus; area CA1, the dentate gyrus, and the hilus were ablated. Based on microscopic observation of cresyl violet stained sections, ibotenic acid lesions produced little damage, if any, in other extrahippocampal areas of the brain including the entorhinal cortex.
Experiment
On average, control rats took 495 ± 177.84 trials, PC lesioned rats took 476.67 ± 185.85 trials, and dPHC lesioned rats took 426.67 ± 142.36 trials to reach criterion. Once criterion was achieved, each rat was randomly assigned to a surgery group. At 20 trials per day, it took animals approximately 23 days to reach criterion.
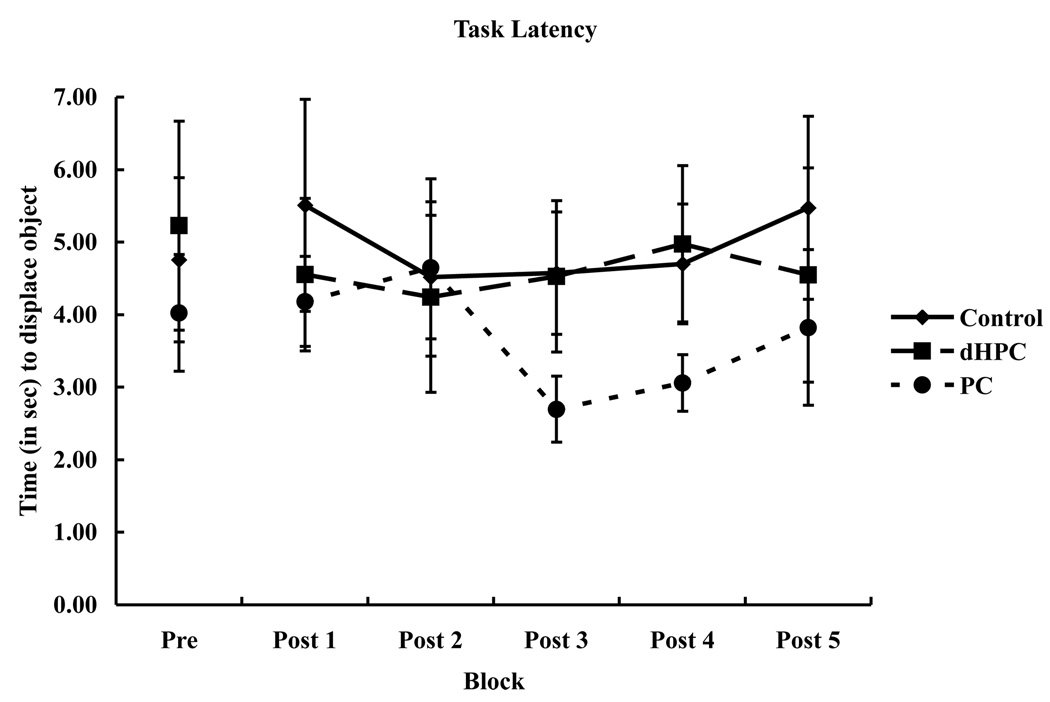
There were no significant differences between latencies among the lesion groups (see Figure 3). A two-way repeated measures ANOVA with lesion groups (control, PC, and dHPC) as the between-factor and blocks of trials (Pre, Post 1, Post 2, Post 3, Post 4, Post 5) as the within-factor found no significant lesion effect (F (2, 17) = .48, p = .63), no significant blocks of trials effect (F (5, 85) = .91, p = .48), and no lesion×blocks of trials interaction (F (10, 85) = .44, p = .92). Additionally, no lesion group displayed significant place preference (F (2, 17) = 1.72, p = .21).
Figure 3.
Graphed is the mean latency (in sec), ± SEM, to displace the first object for each lesion group (controls, parietal cortex (PC), dorsal hippocampus (dHPC)) across blocks (Pre, Post 1, Post 2, Post 3, Post 4, Post 5). There were no significant differences between groups or blocks.
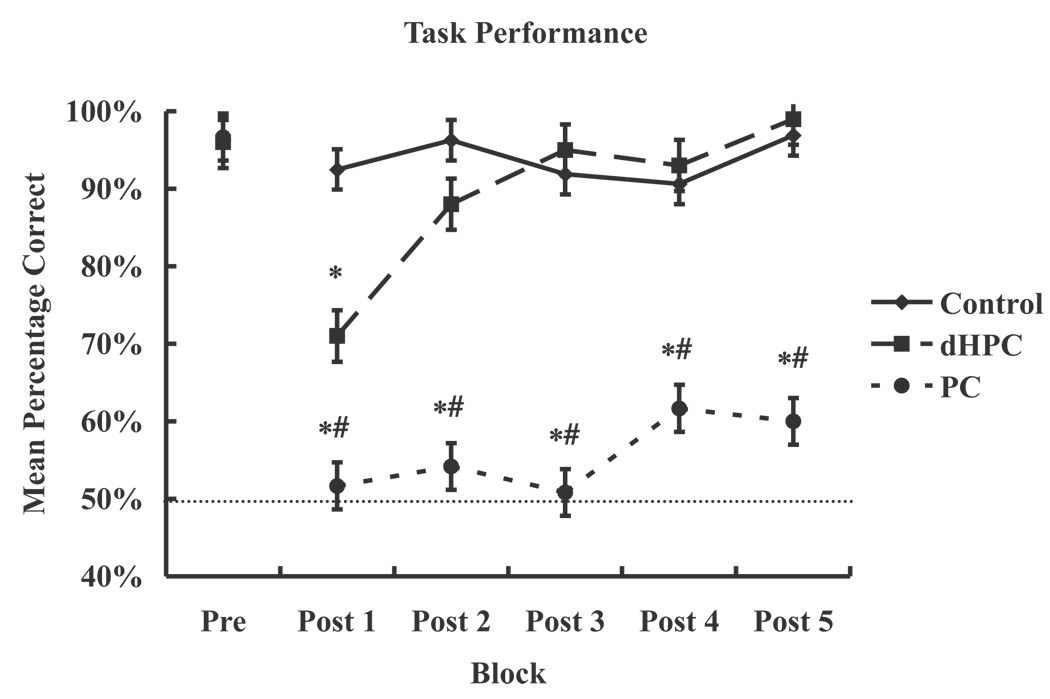
Figure 4 displays the average percentage correct across blocks of 20 trials (Pre, Post 1, Post 2, Post 3, Post 4, Post 5) for each lesion group (control, PC, and dHPC). A two-way repeated measures ANOVA with lesion groups (control, PC, and dHPC) as the between-factor and blocks of trials (Pre, Post 1, Post 2, Post 3, Post 4, and Post 5) as the within-factor found a significant lesion effect (F (2, 17) = 87.28, p < .0001), a significant blocks of trials effect (F (5, 85) = 26.12, p < .0001), and a significant lesion x blocks of trials interaction (F (10, 85) = 13.08, p < .0001).
Figure 4.
Graphed is the mean percentage correct, ± SEM, for each lesion group (controls, parietal cortex (PC), and dorsal hippocampus (dHPC)) across blocks (Pre, Post 1, Post 2, Post 3, Post 4, Post 5). The line at 50% represents chance performance. PC lesioned rats were impaired compared to controls and dorsal hippocampus lesioned rats.
A Fisher’s LSD post hoc on the lesion effect found that the PC lesioned rats made a greater number of errors in recognizing the correct, rewarded object compared to controls (p < .0001) and dHPC lesioned rats (p < .0001).
A Fisher’s LSD post hoc on the lesion×session interaction found that, within controls, there was no significant difference among the Pre, Post 1, Post 2, Post 3, and Post 4 sessions. PC lesioned rats had better performance during the Pre session then Post 1 (p < .0001), Post 2 (p < .0001), Post 3 (p < .0001), Post 4 (p < .0001), and Post 5 (p < .0001). These data suggest the PC lesioned rats did not have access to topological spatial information as Post 1, Post 2, Post 3, Post 4, and Post 5 performance was significantly lower then Pre performance. As stated previously, topological deficits are not likely due to place preference or lack of motivation. The bilateral PC lesions did not result in hemineglect, as indicated by the lack of a place preference. PC lesioned rats had similar latencies as dorsal HPC lesioned and control rats, suggesting the PC lesion effect was not due to a decrease in motivation. Dorsal HPC lesioned rats had impaired performance for Post 1 session compared to Pre (p < .0001), Post 2 (p < .05), Post 3 (p < .0001), Post 4 (p < .0001), and Post 5 (p < .0001). In addition, dorsal HPC lesioned rats performance on Post 2 was less then their performance on Pre (p < .05), Post 3 (p < .05), and Post 5 (p < .005). Post 3, Post 4, and Post 5 sessions were equal and not significantly different (p > .05) within dHPC lesioned rats. These data suggest that dHPC lesioned rats had disrupted memory initially, but the topological representation of the rewarded object remained intact.
DISCUSSION
The current study was based upon a previous human task that was modified for rats (Robertson et al., 1997). Robertson et al. (1997) presented to patient R. M., who had bilateral parietal cortex damage, a picture in which a smaller circle was located either inside or outside a larger circle. The smaller circle was either touching or not touching the larger circle. Patient R. M. performed at 49% correct (18 out of 37 trials) when asked to describe the location of the small circle in relation to the larger circle (Robertson et al., 1997). Patient R. M. appeared not to be able to process and describe the spatial relationship of the small circle to the larger circle. For the present study, we presented rats with similar stimuli (e.g., a ball located either inside or outside a larger ring). A parietal cortex lesioned group with similar bilateral parietal cortex lesions to patient R. M.’s neuropathology was run on this task along with a control group and a dorsal hippocampus (dHPC) lesioned group.
The first objective of the present study was to adapt a previously established human task for rats in order to compare possible similarities of the PC in rats and humans. Therefore, for the present experiment, a new, robust paradigm was adapted from a previous human task to test topological spatial information processing in control, parietal cortex (PC) lesioned, and dorsal hippocampus (dHPC) lesioned rats (Robertson et al., 1997). The second objective of the present study was to more clearly characterize the role of the PC and dHPC for topological spatial information processing. The results from the present study suggest analogous topological spatial information processing by the parietal cortex in rats and humans, as well as support previous human and non-human research that suggests that the parietal cortex (PC) mediates and stores topological spatial information.
PC lesioned rats were impaired across all sessions (Post 1, Post 2, Post 3, Post 4, and Post 5). This result suggests that PC lesioned rats did not have access to topological representations. Dorsal hippocampus lesioned rats were only impaired on Post 1 and Post 2 session, possibly due to temporary memory impairments. Topological representations remained intact for Post 3, Post 4, and Post 5 sessions. Even though dHPC lesioned rats had an initial disruption of memory, the topological representation of the rewarded object remained intact. It is thought that the transient effects of the dHPC lesions are either due to temporary memory loss (i.e. there was a week delay between surgery and post-surgery testing) or the hippocampus may also support topological spatial information (Goodrich-Hunsaker et al., 2008). These data are supported by previous research that showed only PC lesioned rats had a deficit on a topological novelty-detection paradigm, but not a metric novelty-detection paradigm as compared to dorsal hippocampus (dHPC) lesioned and control rats (Goodrich-Hunsaker et al., 2005). Thus, these data suggest that the PC, but not the dHPC, mediates topological spatial information.
Besides task performance, latencies to displace the first object and place preference were measured in the present study. The latencies among the rats were not different; rats also did not spend time exploring around the board. The task was not a navigational task. Rats took only enough time to exit the black box, displace an object, and then return back to the black box. Therefore, we did not record trajectories (e.g. because the animals went straight to the objects and displaced the object of their choice quickly and directly). We concluded that the rats had similar motivation. Also, since the animals made rapid decisions and stayed on task, we concluded that there were no attentional deficits after the PC lesions, at least not as far as task performance was concerned. Place preference was also recorded. Hemineglect is a common neuropathology of PC lesions and it is expected that hemineglect would lead to place preferences. However, that was not the case in the present study. The above mentioned evidence suggest that the deficits seen in PC lesioned animals were due to deficits in topological information processing, not by attentional or motivational confounds resulting from the lesion.
It is possible that PC lesioned rats were impaired in object discrimination. However, previous research suggests that PC lesioned rats are not impaired in object discrimination (Long, Mellem, & Kesner, 1998). For each trial, Long et al. (1998) presented PC lesioned rats with one of two possible objects located in various spatial locations. One object was always rewarded, the other object never rewarded. Location of the object never mattered. PC lesioned rats were able to learn the go / no-go paradigm by only displacing the rewarded object (Long et al., 1998). However, this paradigm only involved simple object discrimination. In another study, PC lesioned rats were also able to detect object changes in a multiple object scene discrimination task (DeCoteau and Kesner, 1998). For this object discrimination task, rats were presented four objects in a square configuration. There were a total of 8 objects. Rats were rewarded when the correct four objects were presented. Rats were not rewarded when one of the correct objects was replaced with a non-rewarded object. This was a go / no-go paradigm. PC lesioned rats were able to learn when to displace the four rewarded objects (i.e. go) and when not to displace the objects if there was a non-rewarded object present (i.e. no go; DeCoteau & Kesner, 1998). Although the present study does not directly address the issue of object discrimination, the results of previous research support that the PC does not subserve object discrimination (DeCoteau & Kesner, 1998; Long et al., 1998). What the present task aims to test is the ability of rats to determine which of two objects is rewarded based on the topological relationships of the elements making up the object. Therefore, object discrimination impairments do not account for the observed PC lesioned rats topological deficits in the present study.
Another factor that may contribute to the PC lesioned rat topological deficits is that PC lesioned rats may be unable to discriminate the distances between objects. Long & Kesner (1996) found that rats with PC lesions similar to those in the present study were able to perform a go / no-go successive match-to-sample task. For the study phase, rats were presented with two different objects at either a separation of 2 cm or 7 cm. For the test phase, the same two objects were presented again. If the distance between the two objects (2 cm or 7 cm) matched the study phase distance, the objects were rewarded. If the distance between the two objects (2 cm or 7 cm) did not match the study phase distance, the objects were not rewarded. Similarly, PC lesioned rats were not impaired on a metric novelty-detection paradigm (Goodrich-Hunsaker et al., 2005). After habituation to two different objects, PC lesioned rats would re-explore the two objects when the distance was either increased or decreased between them. Altogether, these data suggest that the PC does not support distance discrimination (i.e. metric spatial information).
A potential limitation of this study is that our parietal cortex lesions were extensive and not limited to any specific region of the PC (e.g., anteromedial or posterior). Such ablation may disconnect axonal projections from regions outside the PC (Reep, Chandler, King, & Corwin, 1994) and thus lead to attentional deficits (Burcham, Corwin, Stoll, & Reep, 1997; Bucci & Chess, 2005). However, similar extensive PC lesions have in many cases not resulted in attentional deficits (Goodrich-Hunsaker, Hunsaker, & Kesner, 2005; Long & Kesner, 1996; DeCoteau & Kesner, 1998; Long et al., 1998). The possibility of attention deficits cannot be entirely disregarded in the current study. Additionally, Pinto-Hamuy et al. (2004) found that the degree of impairment was positively correlated with the size of the PC lesion. Since our ablations were extensive, topological spatial deficits were possibly at the most extreme. We would hypothesize that smaller lesions of the PC may result in decreased topological spatial processing deficits.
In conclusion, the results of the present study demonstrate analogous functions of the rat and human parietal cortex. The methods of the present experiment were similar to a previously established human paradigm (Robertson et al., 1997) and the neuropathology was similar between patient R. M. and the rats. Patient R. M. with bilateral parietal cortex lesions and the bilateral PC lesioned rats showed similar impairments on a nearly identical topological spatial information task. These data also support the hypothesis that the parietal cortex mediates and stores topological spatial information.
Acknowledgements
This research was supported by NSF IBN-0135273 and NIH R01-MH065314.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bucci DJ, Chess AC. Specific changes in conditioned responding following neurotoxic damage to the posterior parietal cortex. Behavorial Neuroscience. 2005;119(6):580–587. doi: 10.1037/0735-7044.119.6.1580. [DOI] [PubMed] [Google Scholar]
- Burcham KJ, Corwin JV, Stoll ML, Reep RL. Disconnection of medial agranular and posterior parietal cortex produces multimodal neglect in rats. Behavorial Brain Research. 1997;86(1):41–47. doi: 10.1016/s0166-4328(96)02241-3. [DOI] [PubMed] [Google Scholar]
- DeCoteau WE, Kesner RP. Effects of hippocampal and parietal cortex lesions on the processing of multiple object scenes. Behavioral Neuroscience. 1998;112:68–82. doi: 10.1037//0735-7044.112.1.68. [DOI] [PubMed] [Google Scholar]
- Gallistel CR. The organization of learning. Cambridge: MIT Press; 1990. [Google Scholar]
- Goodrich-Hunsaker NJ, Hunsaker MR, Kesner RP. Dissociating the role of the parietal cortex and dorsal hippocampus for spatial information processing. Behavioural Neuroscience. 2005;119:1607–1315. doi: 10.1037/0735-7044.119.5.1307. [DOI] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Hunsaker MR, Kesner RP. The interactions and dissociations of the dorsal hippocampus subregions: how the dentate gyrus, CA3, and CA1 process spatial information. Behavioural Neuroscience. 2008;122(1):16–26. doi: 10.1037/0735-7044.122.1.16. [DOI] [PubMed] [Google Scholar]
- Kuipers BJ, Levitt TS. Navigation and mapping in large-scale space. AI Magazine, summer. 1988:25–42. [Google Scholar]
- Long JM, Kesner RP. The effects of dorsal vs. ventral hippocampal, total hippocampal, and parietal cortex lesions on memory for allocentric distance in rats. Behavioral Neuroscience. 1996;110:922–932. doi: 10.1037//0735-7044.110.5.922. [DOI] [PubMed] [Google Scholar]
- Long JM, Mellem ME, Kesner RP. The effects of parietal cortex lesions on an object/spatial location paired-associate task in rats. Psychobiology. 1998;26:128–133. [Google Scholar]
- Maguire EA, Burgess N, Donnett JG, O’Keefe J, Frith CD. Knowing where things are: parahippocampal involvement in encoding object locations in virtual large-scale space. Journal of Cognitive Neuroscience. 1998;10:61–76. doi: 10.1162/089892998563789. [DOI] [PubMed] [Google Scholar]
- Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8:608–619. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Pinto-Hamuy T, Montero VM, Torrealba F. Neurotoxic lesion of anteromedial/posterior parietal cortex disrupts spatial maze memory in blind rats. Behavorial Brain Research. 2004;153(2):465–470. doi: 10.1016/j.bbr.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Poucet B. Spatial cognitive maps in animals: new hypotheses on their structure and neural mechanisms. Psychological Review. 1993;100:163–182. doi: 10.1037/0033-295x.100.2.163. [DOI] [PubMed] [Google Scholar]
- Poucet B, Herrmann T. Exploratory patterns of rats on a complex maze provide evidence for topological coding. Behavioral Processes. 2001;54:155–162. doi: 10.1016/s0376-6357(00)00151-0. [DOI] [PubMed] [Google Scholar]
- Reep RL, Chandler HC, King V, Corwin JW. Rat posterior parietal cortex: topography of corticocortical and thalamic connections. Experimental Brain Research. 1994;100(1):67–84. doi: 10.1007/BF00227280. [DOI] [PubMed] [Google Scholar]
- Robertson L, Triesman A, Friedman-Hill S, Grabowecky M. The interaction of spatial and object pathways: evidence from Balint’s Syndrome. Journal of Cognitive Neuroscience. 1997;9:295–317. doi: 10.1162/jocn.1997.9.3.295. [DOI] [PubMed] [Google Scholar]
- Thinus-Blanc C, Poucet B, Save E. Animal spatial cognition and exploration. In: Foreman EP, Gillett R R, editors. Handbook of spatial research paradigms and methodologies, volume 2: clinical and comparative issues. Hove: Psychology Press; 1998. pp. 59–85. [Google Scholar]