Abstract
Mucosal immune responses induced by HIV-1 vaccines are likely critical for prevention. We report a Phase 1 safety and immunogenicity trial in 8 participants using the vaccinia-based TBC-3B vaccine given subcutaneously to determine the relationship between HIV-1 specific systemic and gastrointestinal mucosal responses. Across all subjects, detectable levels of blood vaccinia- and HIV-1-specific antibodies were elicited but none were seen mucosally. While the vaccinia component was immunogenic for CD8+ T lymphocyte (CTL) responses in both blood and mucosa, it was greater in blood. The HIV-1 component of the vaccine was poorly immunogenic in both blood and mucosa. Although only 8 volunteers were studied intensively, the discordance between mucosal and blood responses may highlight mechanisms contributing to recent vaccine failures.
Keywords: mucosal immunity, HIV, vaccine
INTRODUCTION
Worldwide, sexual transmission is the leading cause of HIV-1 infection. As HIV-1 primarily infects across sexually-exposed mucosae, preventive vaccines must provoke protective immune responses at these tissue frontiers that are rich in vulnerable cellular targets [1–6;35]. Given past experience in human vaccine trials, as well as mucosal studies of highly-exposed yet persistently seronegative (HEPS) individuals, it is anticipated that having mucosal HIV-1-neutralizing antibody and cytotoxic T lymphocyte (CTL) responses would be preferable features of HIV-1 vaccines [11;13;15;16;20–25]. Human studies have suggested that induction of systemic (blood) immune responses do not always translate into comparable mucosal responses, while inducing mucosal responses often, but not always, predict detectable and stable systemic, peripheral blood responses [7;8].
While the window of opportunity for vaccine-generated immune responses to prevent establishment of infection following exposure remains unknown, having functionally effective and locally active mucosal immune responses seems intuitively essential. Inducing mucosal immune responses has been the focus of many efforts over the past 5–10 years, including comparisons of different immunization routes, often alternating prime-boost strategies mucosally and systemically, as well as comparisons of different immunization sites, with subsequent evaluation of induced mucosal immune responses [7–19]. While this human, Phase 1 trial aimed to secondarily assess the differential impact on induced mucosal responses with two systemic immunization sites (deltoid and inguinal)[10], but was halted after enrollment of 8 subjects in favor of a larger trial to address the question using a canarypox vaccine. While not sufficiently powered to clarify the original question regarding route of administration, this report sheds critical first insights on discordances in detected mucosal and systemic immune responses with an HIV-1 vaccine that utilizes a replicating vector.
MATERIALS AND METHODS
Study subjects
Eight subjects were enrolled on the basis of being vaccinia naïve by age (born after 1970) and history (travel, military service), HIV-1-seronegative, and at low risk for HIV-1 infection (6 males, 2 females; mean age 29.5 years with a range from 23–32 years). They were fully briefed on the infectious risks of vaccinia and TBC-3B vaccine safety in previous vaccine trials [5;26;27] as well as the potential for induced false positive HIV-1 serology [28]. Persons with immunological or gastrointestinal disorders were excluded. All subjects provided signed informed consent under University of California, Los Angeles (UCLA) IRB-approved protocols.
Vaccine
The vaccine used for these studies was a live recombinant vaccinia virus containing HIV-1IIIB env/gag/pol, TBC-3B (Therion Biologics Corporation, Cambridge, MA). This vaccine was produced under GMP conditions and provided, with IND support through the FDA, by Therion Biologics. Wild-type vaccinia (NYCBH) for laboratory studies was also provided by Therion Biologics.
Vaccination protocol
Participants were randomized (blinded to laboratory research personnel) to receive three SC deltoid (n=4) or inguinal (n=4) immunizations at weeks 0, 6, and 20, with clinical follow-up to week 72; all subjects received vaccine. The initial dose at week 0 was 106 PFU, followed by doses of 108 PFU at weeks 6 and 20. Inguinal vaccinations were administered as a modification of a previously described targeted iliac lymph node (TILN) protocol [10], by injection medial to the femoral vein to optimize delivery to the superficial inguinal, deep inguinal and external iliac lymph nodes. Both deltoid and inguinal vaccinations alternated between left and right extremities. While the data from the two sites is unique (meriting an IND with FDA for new site administration) and may be useful for others in the field, due to the small number of subjects studied, results are generally reported as “systemic”, not ‘deltoid’ or inguinal’. However, in Figures, different legends clarify immunization sites.
Clinical laboratory safety monitoring
Routine clinical laboratory testing of complete blood counts, chemistries, HIV-1 ELISA, and plasma HIV RNA PCR (Roche Amplicor kit, Roche Diagnostics, Indianapolis, IN) were performed by the UCLA Medical Center clinical laboratories.
Blood sampling
Blood was obtained by standard venipuncture for plasma, serum separation and isolation of peripheral blood mononuclear cells (PBMC) by Ficoll-Hypaque gradient centrifugation.
Mucosal sampling
Mucosal sampling was performed as previously described [2;29;30] during two baseline visits (two weeks prior, and immediately pre-vaccination at week 0), followed by two weeks after each vaccination (weeks 2, 8, and 22), and then again at 32 and 72 weeks after the first vaccination. During each sampling, anoscopy was first performed for placement of two, premoistened surgical sponges (Ultracell® Medical Technologies, North Stonington, CT) for 5 minutes to collect mucosal secretions for antibody quantification [31]. Flexible sigmoidoscopy was then performed with 20 biopsies acquired at approximately 30 cm from the anal verge as previously described [2;20;30], for isolation of mucosal mononuclear cells. Briefly, biopsies (8×2×1mm from large-cup, endoscopic biopsy forceps [Microvasive Radial Jaw #1589, outside diameter 3.3 mm] were taken and immediately placed into 15ml of tissue culture medium (RPMI 1640, Irvine Scientific).
Elution of mucosal antibodies from surgical sponges
Elution of antibody-containing fluid from the surgical sponges was performed with a protocol modified from previous reports [31]. Briefly, sponge samples for antibody quantification were immediately transported to the laboratory on ice and frozen at −80°C for later batch processing. Absorbed rectal secretions were eluted twice with 250 µl cold PBS containing 0.25% BSA (Sigma Chemicals, St Louis, MO), 1% Igepal (Sigma Chemicals, St Louis, MO) and 1x protease inhibitor cocktail (Sigma Chemicals, St Louis, MO) from the sponges by centrifugation (10,000rpm for 30 minutes at 4°C). The recovered volume of secretion was calculated by subtracting the recovered volume from that recovered from negative control sponges that were run in parallel. Duplicate samples were pooled, frozen, and retrieved in batches for further analysis.
Evaluation of HIV-1-specific antibody responses
Total HIV-1 specific immunoglobulin was quantified in plasma and eluted rectal secretion samples from concurrent visits throughout the trial (weeks 0, 2, 8, 22). Quantification of HIV-1-specific antibodies was performed with a modification of previously reported protocols using the Vironostika ®HIV-1 MICROELISA system (Organon Teknika Corp, Durham, NC) [20;32;37;38]. Samples were run according to the manufacturer’s instructions with the addition of a standard curve generated using serial dilutions (10–3000ng/ml) of human anti-gp-120/160 HIV-1 IgG (ImmunoDiagnostics, Inc Woburn, MA). Total IgG and total IgA were quantified in the eluted rectal secretions or plasma by ELISA previously reported [20;31]. In brief, 96-well plates (Corning Inc, Corning, NY) were coated overnight at 4°C with rabbit anti-human IgG or IgA (Dako Corp, Carpenteria, CA) diluted 1/6000 in bicarbonate buffer (ph 9.6). Serially diluted standard curves utilized purified human immunoglobulin (IgG or IgA) ranging from 7.8–500 ng/mL (Jackson Immunoresearch Laboratories, West Grove, PA). Samples were run in duplicate, along with a positive control sample, for which performance characteristics and acceptable ranges had been previously established. Plates were incubated for 60 minutes at 37°C, and washed five times in wash buffer prior to the addition of 100 µl of peroxidase conjugated rabbit anti-human IgG or IgA (Dako Corp, Carpenteria, CA). Absorbance was read at 492 nm using a Benchmark Plus ELISA plate reader (Biorad, Hercules, CA) equipped with Microplate Manger® software. Values were expressed in ng/mL as extrapolated from standard curves, and the means were calculated for each sample. Final results were expressed in units of anti-HIV-1/µg of total IgG + IgA.
Evaluation of vaccinia-specific antibody responses
Vaccinia-specific antibodies in blood and rectal secretions were detected by ELISA at the same timepoints. Wells were coated with 50 µl of inactivated vaccinia virus (2×105 pfu/ml in 0.1% Tween 20 in PBS) for 60 minutes at 37°C. After blocking for 60 minutes at room temperature with 1% BSA in PBS, wells were layered with serial dilutions of plasma. Plates were incubated for 90 minutes at 37°C. Bound vaccinia-specific antibodies were detected with specific peroxidase-conjugated anti-human IgG, anti-human IgA, and antihuman IgM. Standard serial dilutions of human IgG, IgA, and IgM were used to enable comparisons of plasma and rectal secretion readings. Plasma and rectal fluids from weeks 2, 8, and 22 were compared to baseline levels from week 0.
Isolation of mucosal mononuclear cells
Colonic mucosal mononuclear cells (MMC) were isolated from the sigmoid colon biopsies as previously reported [2;30]. Briefly, the biopsy fragments were washed, collagenase digested, and disrupted into single cell suspensions in medium containing piperacillin-tazobactam antibiotic (Zosyn, Wyeth Co, Philadelphia, PA) and amphotericin B (Fungizone, GIBCO Invitrogen, Carlsbad, CA). Typically, this procedure yielded between 2 and 5 million viable CD3+ T lymphocytes per 17 biopsies. Cell yield and phenotypes were quantified with Multitest staining and TRUCount counting beads (Becton Dickinson Immunocytometry Systems, San Jose, CA.) respectively, following the manufacturer’s instructions. The remaining biopsies were used for histology and banking.
Polyclonal expansion of CD8± T lymphocytes from PBMC and MMC
To obtain adequate numbers of CD8+ T lymphocytes for measurements of vaccine responses, CD8+ T lymphocytes from MMC and PBMC preparations were polyclonally-expanded using a CD3:CD4 bi-specific monoclonal antibody as previously described [20]. Briefly, the cells were cultured for 14 days with the antibody and IL-2, which inhibits CD4+ T lymphocyte growth and stimulates CD8+ T lymphocyte growth. This procedure has been shown to produce polyclonally expanded CD8+ T lymphocytes allowing quantitative measurement of antigen-specific cells reasonably approximating those in non-expanded lymphocytes [20;33;34]. Average yield of expanded CD3+ T lymphocytes was roughly 20 million expanded cells from 1 million fresh MMC [20], providing sufficient CD8+ T lymphocytes to use in the 53-pool ELISpot assays. Verification of expanded CD8+ T lymphocyte numbers was confirmed using 3-color flow cytometry (CD3/CD4/CD8) and routinely demonstrated >85% purity of expanded CD8+ T lymphocytes from MMC and >95% from PBMC.
Evaluation of vaccinia- and HIV-1-specific CD8± T lymphocyte responses
Standard IFN-γ ELISpot assays using the expanded CD8+ T lymphocytes from MMC and PBMC were utilized to measure both vaccinia- and HIV-1-specific CD8+ T lymphocyte responses as previously reported [20;30;34;35]. For vaccinia-specific responses, autologous PBMC were infected with wild type vaccinia virus (NYCBH) at a multiplicity of 3 PFU/cell for 16 hours. These cells were then washed and utilized as antigen presenting cells at a ratio of 3 × 105 expanded MMC or PBMC CD8+ T lymphocytes with 3 × 104 vaccinia-infected PBMC. For HIV-1-specific responses, a library of HIV-1 peptides (consecutive 15-mers overlapping by 11 amino acids) spanning all HIV-1 proteins was added directly to the expanded MMC or PBMC. These were obtained from the NIH AIDS Research and Reference Reagent Repository (Gag catalog # 8116, Pol #6208, Env #9487, Nef #5189, Tat #5138, Rev #6445, Vpr #6447, Vpu #6444, Vif #6446, all Clade B consensus sequences with the exception of Env). The peptides were screened in 53 pools of 12 to 16 peptides each. Triplicate negative controls included expanded CD8+ T lymphocytes alone, and a positive control included expanded CD8+ T lymphocytes with anti-CD2/CD2R and anti-CD28 monoclonal antibodies (Becton Dickinson, San Jose, CA). After counting using an automated ELISpot counting system (Cellular Technologies Limited, Cleveland, OH), results were expressed as spot-forming cells (SFC) per million cells after subtracting the background mean of the negative controls (generally <50 SFC/well, usually <20 SFC/well).
Statistical analysis
As this Phase 1 trial was not powered for statistically significant endpoints, formal statistical inference was de-emphasized. Means and standard deviations of available data are reported for antibody and CTL data with estimated means and standard errors at each time point, computed using a mixed effects linear model. Specifically, the log-transformed values were modeled using a separate mean for each time period (baseline, 8, 22, 32, and 72 weeks) and a random subject effect. To confirm these observations, more complex models were also fit and similar results obtained. The resulting time trajectories are intended to visually convey the average response each week, and are not used for formal inference.
RESULTS
Systemic (Inguinal and deltoid) vaccinations with a recombinant vaccinia virus and mucosal biopsies were well tolerated in the study subjects
The eight individuals examined in this study were HIV-1-uninfected, vaccinia-naïve volunteers. These participants were vaccinated via either deltoid or inguinal SC inoculations of the TCB-3B vaccine at weeks 0, 6, and 20, with the rationale that inguinal delivery might better access deep inguinal lymph nodes, antigenically stimulating lymphocytes that would preferentially home to the colonic mucosa [10]. All eight were vaccinated; six of eight subjects completed the full vaccination series, and two had incomplete vaccination schedules due to mild adverse events (AEs). All eight subjects completed the follow-up biopsy protocol. There were no Grade 3 or 4 AEs, procedure-related events or HIV-1 infections during the trial. There were a total of 107 Grade 1 and 2 events (58 Grade 1 AEs for inguinal versus 42 in deltoid group; 5 Grade 2 AEs in inguinal versus 2 in deltoid group). Almost half of these Grade 1 and 2 events were mild vaccinia-related injection site related events (27 for inguinal and 27 for deltoid group). Of the reported AEs, 26 were Grade 1 constitutional symptoms post-vaccination such as malaise, myalgia, arthralgia, and headache. Overall, vaccination by both routes was well-tolerated, with mild AEs as expected for vaccinia exposure.
Significant peripheral blood antibody responses to both HIV-1 and vaccinia vaccine components were observed.
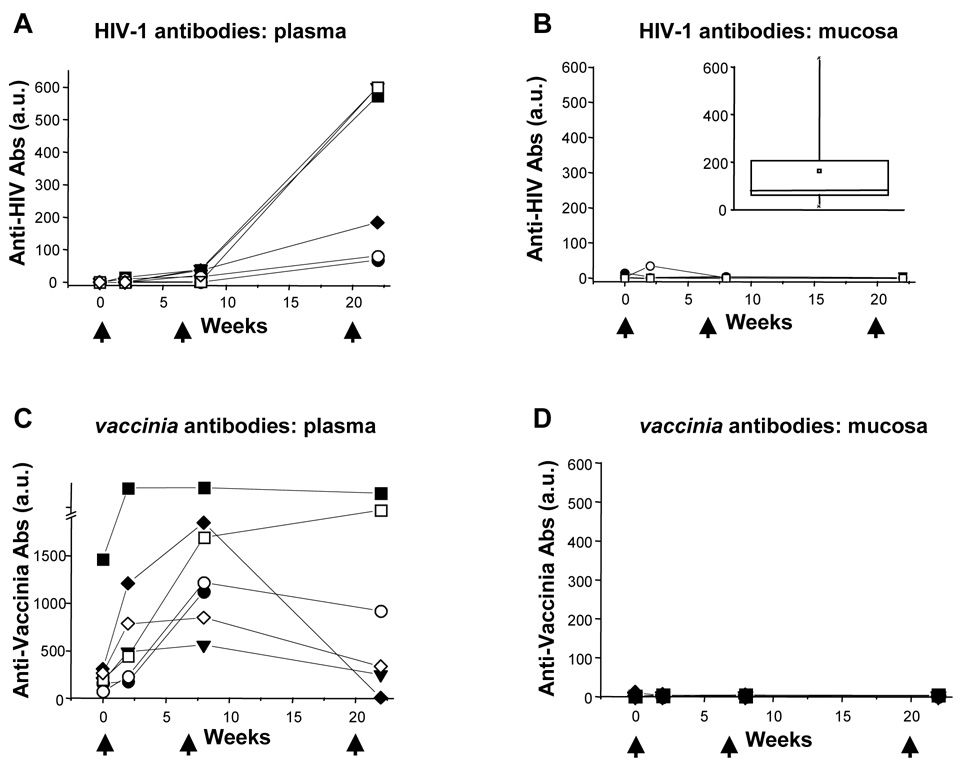
The vaccinees were assessed for their HIV- and vaccinia-specific peripheral blood antibody responses by ELISA (Figure 1A, 1C) at multiple time points after vaccinations with TCB-3B. HIV-1-specific antibody responses developed in all vaccinees’ plasma, becoming detectable after the second or third vaccination (Figure 1A). Vigorous vaccinia-specific antibody responses were observed in most vaccinees after the first vaccination (Figure 1C), peaking earlier than to the HIV-1 component (compare Figure 1A to 1C). Of note, one subject (B01) had detectable vaccinia antibodies at baseline suggesting this individual had been exposed/vaccinated despite screening questions (all results were batch-processed at trial’s end). The blood HIV-1-specific antibody responses remained detectable out to 72 weeks, and peaked around week 22 at 351 ± 266 units/µg IgG + IgA. This level was lower than that observed in chronically HIV-1-infected individuals on antiretroviral drug treatment, who demonstrated mean blood levels of 17,256 ± 8838 units/µg IgG + IgA [20]. Thus, humoral responses against the HIV-1 component of the vaccine appeared to be less vigorous than natural responses against HIV-1 infection or the vaccinia component of the vaccine [20;32;35].
Figure 1. HIV-1-specific and Vaccinia-specific antibody responses to immunization in blood and gut mucosal secretions.
Antibody (IgG + IgA) levels against HIV-1 (panels A/B) and vaccinia (panels C/D) in blood (panels A/C) and gut mucosal (panels B/D) compartments are plotted over time (the value plotted at week 0 is the mean of two baseline pre-vaccination evaluations). Subject B00 was excluded from analysis due to lack of serum samples. The solid and open symbols indicate participants who received deltoid and inguinal immunization respectively; arrowheads indicate the timing of vaccinations. The inset box plot in panel B reflects prior results of comparable mucosal HIV-1-specific antibody measurements in 10 HIV-1-infected individuals [20].
No mucosal antibody responses against either vaccinia or HIV-1 were observed
Mucosal antibody responses to vaccinia and HIV-1 were evaluated in the vaccinees at the same time points. In contrast to the clear blood humoral responses against both vaccinia and HIV-1 after TCB-3B vaccination, mucosal antibodies against HIV-1 and vaccinia were essentially absent in all participants (Figure 1B and 1D). By comparison, we previously measured significant HIV-1-specific antibody levels in the mucosa of chronically HIV-1-infected individuals on antiretroviral drug treatment [20], with average values of 38,464 ± 44,441 units/µg IgG + IgA (Figure 1B inset). Thus, this vaccine failed to induce mucosal vaccinia-or HIV-1-specific antibodies comparable to natural infection.
Vaccinia-specific CTL responses were detected in blood and mucosa
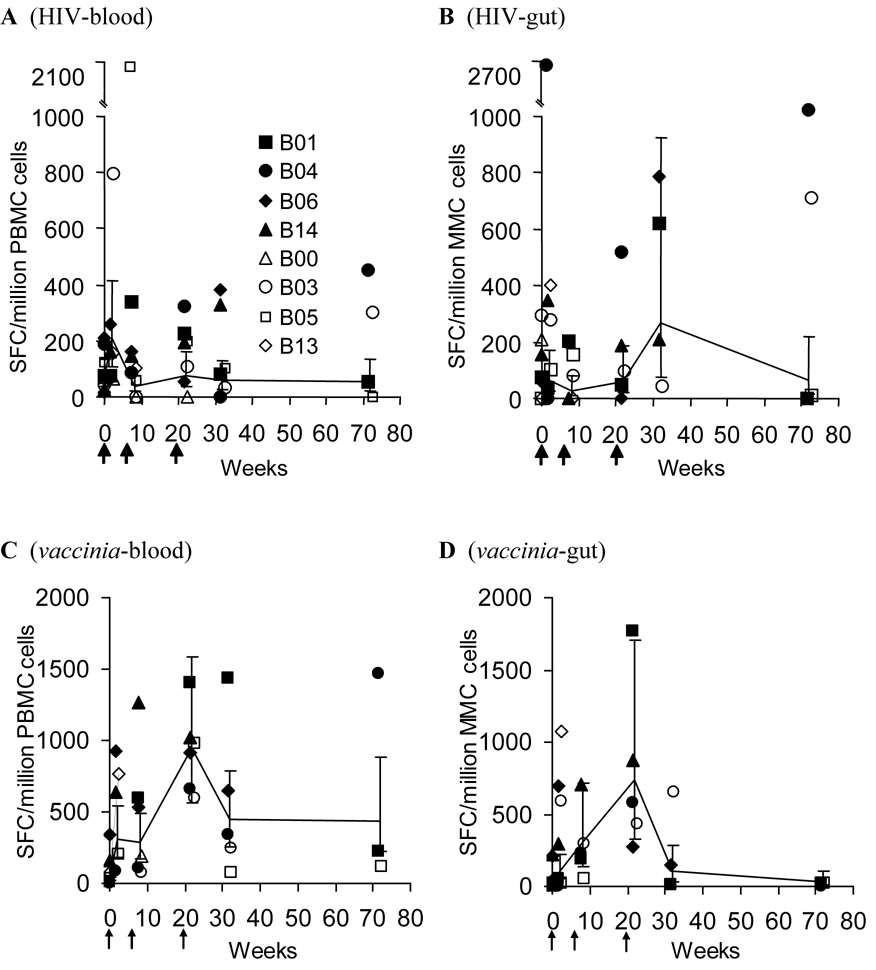
Given the global CD8+ T lymphocyte activation observed after vaccination with TCB-3B and the known immunogenic potency of vaccinia as a smallpox vaccine, the blood (Figure 2C) and mucosa (Figure 2D) compartments were assessed for CTL responses against the vaccinia component of TCB-3B. There was early evidence of a vaccinia-specific CTL response in both compartments. Across all vaccinees, a rapid rise in blood vaccinia-specific CTL was noted after the first vaccination, and this response appeared to peak after the third vaccination (Figure 2C). There was a similar pattern in the mucosa, although overall frequencies appeared lower than the blood (Figure 2D). These results suggest that vaccinia did promote CTL responses that trafficked through both blood and mucosa compartments.
Figure 2. Blood and mucosal CTL responses to HIV-1 and vaccinia components of the TBC-3B vaccine.
The CTL response (by IFN-γ ELISpot) against HIV-1 (panels A/B) and vaccinia (panels C/D) in blood (panels A/C) and gut mucosa (panels B/D) compartments are plotted over time (the value plotted at week 0 is the mean of two baseline pre-vaccination evaluations). The solid and open symbols indicate participants who received deltoid and inguinal immunization respectively; arrowheads indicate the timing of vaccinations. The lines and error bars represent the means and SE across all participants.
Minimal HIV-1-specific CTL responses were detected in blood and mucosa
At the same timepoints, CTL responses against the HIV-1 component of TCB-3B were evaluated in both blood (Figure 2A) and mucosa (Figure 2B) of the vaccinees. HIV-1-targeted CTL responses were modest or absent in the blood, with a few individuals showing possible low level responses but the majority demonstrating no detectable HIV-1-specific CTL (Figure 2A). Within the mucosal compartment, there were no clearly discernable patterns of reactivity, and the majority of vaccinees had no detectable HIV-1-specific CTL (Figure 2B). Background levels were much higher in mucosal assays, and it was thus unclear whether these represented true specific activity. Overall, these data suggested that vaccinia was immunogenic in both compartments, with higher reactivity in the blood, while the HIV-1 component of the vaccine was minimally immunogenic in either compartment.
DISCUSSION
The recent failure of the STEP trial, which tested a promising candidate for generating HIV-1-specific cellular immunity, raises important questions about the mechanisms of HIV vaccine protection. One potential explanation for recent observed failures would be that mucosal humoral and CTL responses were lacking at the site where HIV-1 transmission occurs. Mucosal immunity in response to vaccines remains a poorly understood area.
This is the first Phase 1 trial investigating mucosal immune responses to the previously studied HIV-1 vaccine TBC-3B, a live recombinant vaccinia virus containing HIV-1IIIB env/gag/pol. Low risk HIV-1 seronegative subjects were immunized via deltoid versus inguinal routes to evaluate human response correlates of previously reported macaque data using targeted inguinal lymph node vaccination [1;10]. Eight subjects completed the trial. This number was too few to provide initial insights into the question of whether deltoid versus inguinal site of systemic immunization induces more pronounced mucosal immune response, which will be addressed in a recently completed, larger, similarly designed trial using a non-replicating, canarypox recombinant vaccine (Anton, et al; manuscript in preparation). This small vaccinia trial demonstrated safety for both routes of vaccination; the safety and blood immune responses are similar to other reported vaccinia-based HIV-1 vaccine trials [26–28;36].
As expected, the vaccinees demonstrated detectable blood humoral responses to vaccinia and HIV-1 antigens after vaccination. These blood responses were durable, providing evidence that both vaccinia and HIV-1 vaccine components were antigenically available to generate immune responses. However, it was observed that the peak levels of vaccine-induced blood HIV-1-specific antibodies were significantly lower than in natural HIV-1 infection [20].
Disappointingly, there were no detectable mucosal antibodies against either vaccinia or HIV-1. These data, using an assay that consistently detects HIV-1-specific mucosal antibodies in HIV-1-infected subjects [20], suggest that vaccine access to the mucosal immune compartment might be a limiting factor. During pilot studies, we compared rectal lavage collection methods, as reported by others [37;38], but found tremendous variability in recovery, often relating to the participant’s state of hydration. Using the same method with surgical sponges, we’ve reported vigorous mucosally secreted HIV-1 specific antibodies in HIV-1 seropositive subjects, indicating the ability of this technique to detect such responses [20;39]. However, comparative data on mucosal antibody responses against other vaccines that successfully protect against other mucosally-acquired infections, such as Hepatitis B virus, are lacking.
CTL responses to the both vaccinia and HIV-1 components were contrasting. Consistent with its known immunogenicity and efficacy as a vaccine, vaccinia induced a CTL response in most vaccinees. Blood frequencies of vaccinia-specific CTL appeared to be somewhat higher than in mucosa. In contrast, HIV-1-specific CTL responses were sporadic and generally absent in both compartments. This finding may correlate, in part, with the lesser immunogenicity of the HIV-1 component of the vaccine (subdominance) compared to the replicating vaccinia portion.
The discordance of responses between blood and mucosal compartments may be instructive. It has long been known that adaptive immunity is compartmentalized between the mucosa and systemic circulation, and that systemic immunization does not ensure mucosal protective responses, while the converse is more often true [7;8]. Insufficient immunogenicity, particularly of the vaccine’s HIV-1 component, may have contributed to the observed greater response in blood versus gut mucosa; the same was observed for responses against the vaccinia component. However, while vaccinia is an historically effective vaccine against smallpox, the usual CTL responses against wild type vaccinia in the mucosae and their contribution to vaccine efficacy against smallpox are unknown. In the absence of vigorous CTL responses against either vaccinia or HIV-1 detected in the mucosal compartment, it is difficult to know whether immunodominance of one vaccine component over the other could play a role in the observed paucity of HIV-specific incurred CTL responses.
In summary, this first attempt to quantify mucosal immune responses to a systemically delivered HIV-1 vaccine demonstrated the safety and feasibility of targeted inguinal delivery of this vaccine. The highly immunogenic vaccinia component of the vaccine generated blood but not mucosal antibody responses, and elicited vaccinia-specific CTL responses in both blood and mucosa compartments. The HIV-1 portion of the vaccine was more weakly immunogenic, generating delayed blood antibody responses and no mucosal antibody responses, with low or absent CTL responses in both blood and mucosa compartments. The results suggest that generating mucosal HIV-specific, humoral and CTL responses via systemic immunization with this vaccine is difficult. These observations underscore the need to investigate mucosal responses to future HIV-1 vaccine candidates at an early timepoint in product pipeline development.
ACKNOWLEDGMENTS
This paper is dedicated to Dr. Janis Giorgi, UCLA, whose vigor, insight and passionate commitment to understand why, and how, some individuals remain well in the setting of HIV infection inspired this study. These efforts toward a protective vaccine with effective mucosal responses owe much to her focused energy. We would like to thank the sustained dedication of the subjects as well as the nursing and support staff in the endoscopy unit and the NIH General Clinical Research Center at UCLA (M01RR00865). We would also like to thank Dr. Thomas Lehner, Guys and St. Thomas Hospital, London who, with Dr. Janis Giorgi, helped inspire the concept of this trial.
This study was supported by Therion, Inc for IND assistance, vaccine and vaccinia wild type and technical assistance in vaccinia antibody assays. Pallotta Teamworks™ and the riders of the Vaccine Rides as well as American Foundation for AIDS Research (amFAR) (#02620) provided initial seed funding. National Institutes of Health (NIH) funding provided the following support: NIH R01 (#AI50467); the UCLA Center for AIDS Research (CFAR) grant (P30 #AI028697), including the Cores of Mucosal Immunology, Flow Cytometry, Virology, ELISPOTs and Biostatistics; NIH K24 AI01610 (PAA); NIH T32 AI07370. Ying Zhou and Xin Huang provided assistance with data management and analysis. Additional support was provided by the MACY*s Foundation and the Oppenheimer Brother’s Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Lehner T, Anton PA. Mucosal immunity and vaccination against HIV. AIDS. 2002;16 Suppl 4:S125–S132. doi: 10.1097/00002030-200216004-00017. [DOI] [PubMed] [Google Scholar]
- 2.Anton PA, Elliott J, Poles MA, et al. Enhanced levels of functional HIV-1 co-receptors on human mucosal T cells demonstrated using intestinal biopsy tissue. AIDS. 2000 Aug 18;14(12):1761–1765. doi: 10.1097/00002030-200008180-00011. [DOI] [PubMed] [Google Scholar]
- 3.Gurney KB, Elliott J, Nassanian H, et al. Binding and transfer of human immunodeficiency virus by DC-SIGN+ cells in human rectal mucosa. J Virol. 2005 May;79(9):5762–5773. doi: 10.1128/JVI.79.9.5762-5773.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Letvin NL. Progress and obstacles in the development of an AIDS vaccine. Nat Rev Immunol. 2006 Dec;6(12):930–939. doi: 10.1038/nri1959. [DOI] [PubMed] [Google Scholar]
- 5.Gherardi MM, Esteban M. Recombinant poxviruses as mucosal vaccine vectors. J Gen Virol. 2005 Nov;86(Pt 11):2925–2936. doi: 10.1099/vir.0.81181-0. [DOI] [PubMed] [Google Scholar]
- 6.Belyakov IM, Ahlers JD, Berzofsky JA. Mucosal AIDS vaccines: current status and future directions. Expert Rev Vaccines. 2004 Aug;3(4 Suppl):S65–S73. doi: 10.1586/14760584.3.4.s65. [DOI] [PubMed] [Google Scholar]
- 7.Ranasinghe C, Medveczky JC, Woltring D, et al. Evaluation of fowlpox-vaccinia virus prime-boost vaccine strategies for high-level mucosal and systemic immunity against HIV-1. Vaccine. 2006 Jul 26;24(31–32):5881–5895. doi: 10.1016/j.vaccine.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 8.Sharpe S, Hanke T, Tinsley-Bown A, et al. Mucosal immunization with PLGA-microencapsulated DNA primes a SIV-specific CTL response revealed by boosting with cognate recombinant modified vaccinia virus Ankara. Virology. 2003 Aug 15;313(1):13–21. doi: 10.1016/s0042-6822(03)00282-4. [DOI] [PubMed] [Google Scholar]
- 9.Peacock JW, Nordone SK, Jackson SS, et al. Gender differences in human immunodeficiency virus type 1-specific CD8 responses in the reproductive tract and colon following nasal peptide priming and modified vaccinia virus Ankara boosting. J Virol. 2004 Dec;78(23):13163–13172. doi: 10.1128/JVI.78.23.13163-13172.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehner T, Wang Y, Cranage M, et al. Protective mucosal immunity elicited by targeted iliac lymph node immunization with a subunit SIV envelope and core vaccine in macaques. Nat Med. 1996 Jul;2(7):767–775. doi: 10.1038/nm0796-767. [DOI] [PubMed] [Google Scholar]
- 11.Neeson P, Boyer J, Kumar S, et al. A DNA prime-oral Listeria boost vaccine in rhesus macaques induces a SIV-specific CD8 T cell mucosal response characterized by high levels of alpha4beta7 integrin and an effector memory phenotype. Virology. 2006 Oct 25;354(2):299–315. doi: 10.1016/j.virol.2006.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellenberger D, Otten RA, Li B, et al. HIV-1 DNA/MVA vaccination reduces the per exposure probability of infection during repeated mucosal SHIV challenges. Virology. 2006 Aug 15;352(1):216–225. doi: 10.1016/j.virol.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Belyakov IM, Kuznetsov VA, Kelsall B, et al. Impact of vaccine-induced mucosal high-avidity CD8+ CTLs in delay of AIDS viral dissemination from mucosa. Blood. 2006 Apr 15;107(8):3258–3264. doi: 10.1182/blood-2005-11-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buonaguro L, Visciano ML, Tornesello ML, Tagliamonte M, Biryahwaho B, Buonaguro FM. Induction of systemic and mucosal cross-clade neutralizing antibodies in BALB/c mice immunized with human immunodeficiency virus type 1 lade A virus-like particles administered by different routes of inoculation. J Virol. 2005 Jun;79(11):7059–7067. doi: 10.1128/JVI.79.11.7059-7067.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vajdy M, Singh M, Kazzaz J, et al. Mucosal and systemic anti-HIV responses in rhesus macaques following combinations of intranasal and parenteral immunizations. AIDS Res Hum Retroviruses. 2004 Nov;20(11):1269–1281. doi: 10.1089/aid.2004.20.1269. [DOI] [PubMed] [Google Scholar]
- 16.Devito C, Zuber B, Schroder U, et al. Intranasal HIV-1-gp160-DNA/gp41 peptide prime-boost immunization regimen in mice results in long-term HIV-1 neutralizing humoral mucosal and systemic immunity. J Immunol. 2004 Dec 1;173(11):7078–7089. doi: 10.4049/jimmunol.173.11.7078. [DOI] [PubMed] [Google Scholar]
- 17.Egan MA, Chong SY, Hagen M, et al. A comparative evaluation of nasal and parenteral vaccine adjuvants to elicit systemic and mucosal HIV-1 peptide-specific humoral immune responses in cynomolgus macaques. Vaccine. 2004 Sep 9;22(27–28):3774–3788. doi: 10.1016/j.vaccine.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Kutteh WH, Kantele A, Moldoveanu Z, Crowley-Nowick PA, Mestecky J. Induction of specific immune responses in the genital tract of women after oral or rectal immunization and rectal boosting with Salmonella typhi Ty 21a vaccine. J Reprod Immunol. 2001 Oct;52(1–2):61–75. doi: 10.1016/s0165-0378(01)00109-7. [DOI] [PubMed] [Google Scholar]
- 19.Moldoveanu Z, Clements ML, Prince SJ, Murphy BR, Mestecky J. Human immune responses to influenza virus vaccines administered by systemic or mucosal routes. Vaccine. 1995 Aug;13(11):1006–1012. doi: 10.1016/0264-410x(95)00016-t. [DOI] [PubMed] [Google Scholar]
- 20.Ibarrondo FJ, Anton PA, Fuerst M, et al. Parallel Human Immunodeficiency Virus Type 1-Specific CD8+ T-Lymphocyte Responses in Blood and Mucosa during Chronic Infection. J Virol. 2005 Apr;79(7):4289–4297. doi: 10.1128/JVI.79.7.4289-4297.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Broliden K, Hinkula J, Devito C, et al. Functional HIV-1 specific IgA antibodies in HIV-1 exposed, persistently IgG seronegative female sex workers. Immunol Lett. 2001 Nov 1;79(1–2):29–36. doi: 10.1016/s0165-2478(01)00263-2. [DOI] [PubMed] [Google Scholar]
- 22.Devito C, Hinkula J, Kaul R, et al. Mucosal and plasma IgA from HIV-exposed seronegative individuals neutralize a primary HIV-1 isolate. AIDS. 2000 Sep 8;14(13):1917–1920. doi: 10.1097/00002030-200009080-00006. [DOI] [PubMed] [Google Scholar]
- 23.Kaul R, Dong T, Plummer FA, et al. CD8(+) lymphocytes respond to different HIV epitopes in seronegative and infected subjects. J Clin Invest. 2001 May;107(10):1303–1310. doi: 10.1172/JCI12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rowland-Jones SL, Pinheiro S, Kaul R, et al. How important is the 'quality' of the cytotoxic T lymphocyte (CTL) response in protection against HIV infection? Immunol Lett. 2001 Nov 1;79(1–2):15–20. doi: 10.1016/s0165-2478(01)00261-9. [DOI] [PubMed] [Google Scholar]
- 25.McNicholl JM, Promadej N. Insights into the role of host genetic and T-cell factors in resistance to HIV transmission from studies of highly HIV-exposed Thais. Immunol Res. 2004;29(1–3):161–174. doi: 10.1385/IR:29:1-3:161. [DOI] [PubMed] [Google Scholar]
- 26.Goonetilleke N, Moore S, Dally L, et al. Induction of multifunctional human immunodeficiency virus type 1 (HIV-1)-specific T cells capable of proliferation in healthy subjects by using a prime-boost regimen of DNA- and modified vaccinia virus Ankara-vectored vaccines expressing HIV-1 Gag coupled to CD8+ T-cell epitopes. J Virol. 2006 May;80(10):4717–4728. doi: 10.1128/JVI.80.10.4717-4728.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cebere I, Dorrell L, McShane H, et al. Phase I clinical trial safety of DNA- and modified virus Ankara-vectored human immunodeficiency virus type 1 (HIV-1) vaccines administered alone and in a prime-boost regime to healthy HIV-1-uninfected volunteers. Vaccine. 2006 Jan 23;24(4):417–425. doi: 10.1016/j.vaccine.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 28.Ackers ML, Parekh B, Evans TG, et al. Human immunodeficiency virus (HIV) seropositivity among uninfected HIV vaccine recipients. J Infect Dis. 2003 Mar 15;187(6):879–886. doi: 10.1086/368169. [DOI] [PubMed] [Google Scholar]
- 29.Anton PA, Poles MA, Elliott J, et al. Sensitive and reproducible quantitation of mucosal HIV-1 RNA and DNA viral burden in patients with detectable and undetectable plasma viral HIV-1 RNA using endoscopic biopsies. J Virol Methods. 2001 Jun;95(1–2):65–79. doi: 10.1016/s0166-0934(01)00295-6. [DOI] [PubMed] [Google Scholar]
- 30.Shacklett BL, Yang OO, Hausner MA, et al. Optimization of methods to assess human mucosal T-cell responses to HIV infection and vaccination. J Immunol Methods. 2003;279:17–31. doi: 10.1016/s0022-1759(03)00255-2. [DOI] [PubMed] [Google Scholar]
- 31.Kozlowski PA, Lynch RM, Patterson RR, Cu-Uvin S, Flanigan TP, Neutra MR. Modified wick method using Weck-Cel sponges for collection of human rectal secretions and analysis of mucosal HIV antibody. J Acquir Immune Defic Syndr. 2000 Aug 1;24(4):297–309. doi: 10.1097/00126334-200008010-00001. [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Maza O, Crabb E, Mitsuyasu RT, Fahey JL, Giorgi JV. Infection with the human immunodeficiency virus (HIV) is associated with an in vivo increase in B lymphocyte activation and immaturity. J Immunol. 1987 Jun 1;138(11):3720–3724. [PubMed] [Google Scholar]
- 33.Jones N, Agrawal D, Elrefaei M, et al. Evaluation of antigen-specific responses using in vitro enriched T cells. J Immunol Methods. 2003 Mar 1;274(1–2):139–147. doi: 10.1016/s0022-1759(02)00510-0. [DOI] [PubMed] [Google Scholar]
- 34.Yang OO, Kalams SA, Trocha A, et al. Suppression of human immunodeficiency virus type 1 replication by CD8+ cells: evidence for HLA class I-restricted triggering of cytolytic and noncytolytic mechanisms. J Virol. 1997 Apr;71(4):3120–3128. doi: 10.1128/jvi.71.4.3120-3128.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jamieson BD, Ibarrondo FJ, Wong JT, et al. Transience of vaccine-induced HIV-1-specific CTL and definition of vaccine "response". Vaccine. 2006 Apr 24;24(17):3426–3431. doi: 10.1016/j.vaccine.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 36.Slobod KS, Lockey TD, Howlett N, et al. Subcutaneous administration of a recombinant vaccinia virus vaccine expressing multiple envelopes of HIV-1. Eur J Clin Microbiol Infect Dis. 2004 Feb;23(2):106–110. doi: 10.1007/s10096-003-1075-3. [DOI] [PubMed] [Google Scholar]
- 37.Grewal HM, Hemming KT, Vetvik H, et al. Measurement of specific IgA in faecal extracts and intestinal lavage fluid for monitoring of mucosal immune responses. J Immunol Methods. 2000 May 26;239(1–2):53–62. doi: 10.1016/s0022-1759(00)00171-x. [DOI] [PubMed] [Google Scholar]
- 38.Mucosal Immunology. Third Ed. Burlington, MA USA: Elsevier Academic Press; 2005. [Google Scholar]
- 39.McGowan I, Elliot J, Cortina G, et al. Characterization of baseline intestinal mucosal indices of injury and inflammation in men for use in rectal microbicide Trials (HIV Prevention Trials Network-056) J Acquir Immune Defic Syndr. 2007 doi: 10.1097/QAI.0b013e318156ef16. In Press. [DOI] [PubMed] [Google Scholar]