Abstract
It has been demonstrated that the dorsal CA1 subregion of the hippocampus mediates temporal processing of information, that dorsal CA3 participates in the spatiotemporal processing of memory, and the dorsal dentate gyrus mediates spatial pattern separation. A temporal ordering of spatial locations task was developed to test the role of the dorsal dentate gyrus, CA3, and CA1 for the temporal processing of spatial information with either high or low levels of spatial interference. The results indicate that animals with dentate gyrus lesions showed difficulty performing the task at high levels of spatial interference, but were able to perform the task well when there was low spatial interference. Animals with lesions to CA3 did not show a preference for either spatial location presented during the study phase during the preference test, suggesting impaired spatiotemporal processing. Animals with lesions to CA1 showed a preference for a later presented spatial location over the earlier, the opposite preference to that shown by control animals.
Keywords: Dorsal CA3, Dorsal CA1, Dorsal Dentate Gyrus, Temporal Processing, Episodic Memory, Spatial Pattern Separation, Spatial Memory, Associative Memory
Introduction
It has been proposed that the CA1 subregion of the hippocampus mediates temporal processing of information (Fortin, Agster, & Eichenbaum, 2002; Hoge & Kesner, 2007; Hunsaker, Thorup, Welch, & Kesner, 2006; Hunsaker, Fieldsted, Rosenberg, & Kesner 2008b in press; Kesner, Lee, & Gilbert, 2004; Kesner, Hunsaker, & Gilbert, 2005; Manns, Howard, & Eichenbaum, 2007; Rogers, Hunsaker, & Kesner, 2006; Rolls & Treves, 1998; Vago, Bevan, & Kesner, 2007). The dentate gyrus and CA3 have been proposed to mediate pattern separation and pattern completion processes, respectively (Gilbert & Kesner, 2006; Gilbert, Kesner, & Lee, 2001; Gold & Kesner, 2005; Leutgeb & Leutgeb, 2007; Leutgeb, Leutgeb, Moser, & Moser, 2007; Kesner et al., 2004; McHugh et al., 2007; Rolls & Kesner, 2006; Rolls & Treves, 1998; Treves, 2004).
To properly study the mnemonic functions of hippocampal subregions, one has to find a task, or small set of very similar tasks, that can be used to tease apart the different processes underlying the formation of spatial and/or temporal memory (cf. Kesner et al., 2004). Previous work has succeeded in dissociating the roles of the dorsal dentate gyrus, dorsal CA3, and dorsal CA1 for numerous tasks (Gilbert & Kesner, 2003, 2006; Gilbert et al., 2001; Kesner et al., 2005; Lee, Hunsaker & Kesner, 2005), but it has proven difficult to tease apart the contributions of all three subregions using a single task (cf. Gilbert & Kesner, 2006; Gilbert et al., 2001; Kesner et al., 2004). A temporal order for spatial locations task (Hunsaker et al., 2008b in press) has been modified to include a spatial interference component. The strength of this paradigm is that we were able to study the contributions of the dorsal dentate gyrus, dorsal CA3, and dorsal CA1 for spatiotemporal processing by making subtle parametric changes to a single temporal ordering for spatial locations paradigm that required no pre-training prior to experimentation.
The present experiment was designed to test the roles of each of the dorsal hippocampal subregions for spatiotemporal processing and to expand upon the findings of Gilbert et al. (2001), Gilbert & Kesner (2006), and Goodrich-Hunsaker, Hunsaker, & Kesner (2008). The present experiment tested animals with dorsal dentate gyrus, dorsal CA3, or dorsal CA1 lesions on a task that emphasizes spatiotemporal processing. It has previously been shown that ventral CA1 lesions do not affect performance on the present temporal order for spatial locations task (Hunsaker et al., 2008 in press). In short, the results of the present experiment demonstrate that lesions of the dorsal dentate gyrus, dorsal CA3, or dorsal CA1 all result in distinct patterns of behavioral deficits. Animals with lesions to the dentate gyrus have difficulty in processing the temporal order of spatial locations, as reflected by selective re-exploration of one location over another location, when the spatial locations were 54 cm apart, but showed no difficulty when the spatial locations were 108 cm apart. Animals with lesion to CA3 showed difficulty in processing the temporal order of spatial locations at both the 54 and 108 cm spatial separation conditions. Animals with lesions to CA1 were able to process the temporal order of the spatial locations. These animals showed a clear preference for the spatial location presented most recently, the opposite preference to that shown by control animals. These data are not sufficient to infer a triple dissociation of hippocampal function, but they do support computational models of hippocampal function that emphasize subregional specificity of hippocampal function (cf. Rolls & Kesner, 2006; Treves, 2004).
Material and Methods
Animals
Twenty-six Long-Evans rats (Simonsen Laboratories, Inc., Gilroy, CA), approximately 4 months of age and weighing 300-400 g at the start of experimentation served as subjects. The rats were housed individually in plastic tubs located in a colony with a 12 h light-dark cycle. All testing was conducted during the light portion the light-dark cycle. All rats were free fed and had ad libitum access to water. Animals were not food deprived during experimentation. All experiments were conducted according to the NIH Guide for the Care and Use of Laboratory Animals and the University of Utah Institutional Animal Care and Use Committee
Surgical Methods
The surgical method was identical to that reported by Goodrich-Hunsaker et al. (2008). Experimentally naive rats were randomly assigned to a surgery group (dorsal dentate gyrus n=6; dorsal CA3 n=6; dorsal CA1 n=6; control n=8). Rats were anesthetized and maintained with isoflurane (2-4% (vol/vol) in 2 L/m medical air) and given atropine sulfate (0.2 mg/kg i.m.) as a prophylactic. Rats were placed in a stereotaxic apparatus (David Kopf Instruments, Tunjana, CA). Lesions of dorsal dentate gyrus granule cells were made by infusion of 0.8 μL colchicine (Sigma-Aldrich; St. Louis, MO; 2.5 mg/mL phosphate buffer solution (PBS)) into each of two sites bilaterally at a 20 μL/h flow rate. Excitotoxic lesions of dorsal CA3 and dorsal CA1 were made by infusing ibotenic acid (Ascent Scientific; Bristol, UK; 6 mg/mL PBS), at a rate of 6 μL/h and a volume of 0.05 to 0.15 μL depending on the site, bilaterally into three sites. After infusion, the injection cannula remained in each site for 1 m to allow diffusion of the injected excitotoxin prior to infusion cannula retraction.
The coordinates for dorsal dentate gyrus lesions (n=6) were 1) 2.7 mm posterior to bregma, 2.1 mm lateral to midline, and 3.4 mm ventral to the dura mater (0.8 μL colchicine infused), and 2) 3.7 mm posterior to bregma, 2.3 mm lateral to the midline, and 3.0 mm ventral to the dura mater (0.8 μL colchicine infused). Dorsal CA3 lesions (n = 6) were made using ibotenic acid, which was infused into three sites bilaterally located 1) 2.5 mm posterior to bregma, 2.6 mm lateral to midline, and 3.2 mm ventral to dura (0.05 μL of ibotenic acid injected); 2) 3.3 mm posterior to bregma, 3.3 mm lateral to midline, and 3.2 mm ventral to dura (0.08 μL infused); 3) 4.2 mm posterior to bregma, 4.2 mm lateral to the midline, and 3.1 mm ventral to dura (0.15 μL infused). Dorsal CA1 lesions (n=6), were made using ibotenic acid infused into three sited bilaterally located 1) 3.6 mm posterior to bregma, 1.0 mm lateral to the midline, 2.4 mm ventral to dura (0.1 μL of ibotenic acid injected); 2) 3.6 mm posterior to bregma, 2.0 mm lateral to the midline, 2.1 mm ventral to dura (0.1 μL infused); 3) 3.6 mm posterior to bregma, 3.0 mm lateral to the midline, 2.3 mm ventral to dura (0.15 μL infused). Vehicle control lesions (dentate gyrus vehicle n = 2, CA3 vehicle n=2, CA1 vehicle n=2, sham lesion n=2) were made using the same coordinates and procedures; however, for the vehicle control lesions an equivalent volume of PBS vehicle were infused instead of the neurotoxin. Sham lesion animals were anesthetized and were given the initial incisions. The choice of the low number of each type of vehicle control lesion (2 vehicle controls for each subregional lesion) was based on experience that the different vehicle control groups have never shown behavioral differences in our lab.
Following surgery, the incision was sutured, 1.5 mL of saline was injected into each thigh subcutaneously to hydrate the animal, and the rats were allowed to recover on a heating pad before returning to their home cage. In addition, rats received acetaminophen (Children's Tylenol; 200 mg/100 mL water) in their drinking water as an analgesic and were provided with powdered food for 3 d following surgery. The behavior of all animals was visually monitored for epileptiform activity for 7 d post-surgery; no behavioral seizures were observed in any of the lesioned animals.
Behavioral Methods
The same animals were run in Experiments 1 and 2. The order in which animals ran Experiments 1 and 2 were counterbalanced, and the two experiments were in separate rooms. That is to say, the objects used during experimentation as well as the experiments themselves (e.g. low and high spatial interference) were randomized. All experimenters were blind to the experimental hypotheses as well as to the surgical manipulation of the animals. All experiments were videotaped and scored offline by an experimenter blind to the experimental manipulations.
Experiment 1: Temporal Order and Novelty Detection of Spatial Locations with Low Spatial Interference
The temporal order and novelty detection of spatial locations task with low spatial interference was run on a modified open field cheeseboard covered with a thick vinyl sheet to obscure the holes (cf. Hunsaker, Mooy, Swift, & Kesner, 2007; Lee et al., 2005). Three identical objects were prepared. These objects were large transparent carafes (30 cm in height and 10 cm in width) with rainbow colored party streamers placed in the bottom to provide a contrast with the distal environment (it must be noted that the order of the objects used was not the same for different animals, so the order of objects given in this section are examples of those given to a single animal). These objects were designed to be large and attract the attention of the animal, but transparent to allow the animal to see the distal environment when close to the object. Seven spatial locations were defined on the maze by marking their locations on a television monitor in the adjacent room. This allowed the experimenter to use the precise spatial locations intended. One day prior to experimentation, the animals were placed on the open field with no objects present to habituate to the open field and to explore the spatial layout of the room. This allowed the animal to learn the spatial layout of the room and to habituate to the board prior to experimentation.
For the temporal ordering of spatial locations task with low spatial interference, the animal was placed on the maze facing due east with an object in a particular spatial location and allowed to explore for 5 m (cf. Figure 1A). After this initial exploration, the animal was removed to a cage outside the running room for a 3 m intersession interval. After this interval, the animal was placed on the maze again with the object moved to a second spatial location. The animal was given 5 m to explore. After another 3 m intersession interval, the animal was placed on the maze with the object moved to a third spatial location and allowed to explore for 5 m. Following a 30 m intersession interval to allow for sufficient consolidation of the spatial information acquired during the study phase, the rat was placed on the maze with identical objects in the first and last experienced spatial locations and allowed to explore for 5 m. The first and third spatial locations were separated by 108 cm to reduce spatial interference (based on the findings reported by Gilbert et al., 2001; Goodrich-Hunsaker, Hunsaker, & Kesner, 2005, 2008). This tested the rat's preference for an earlier experienced spatial location relative to one experienced later.
Figure 1.
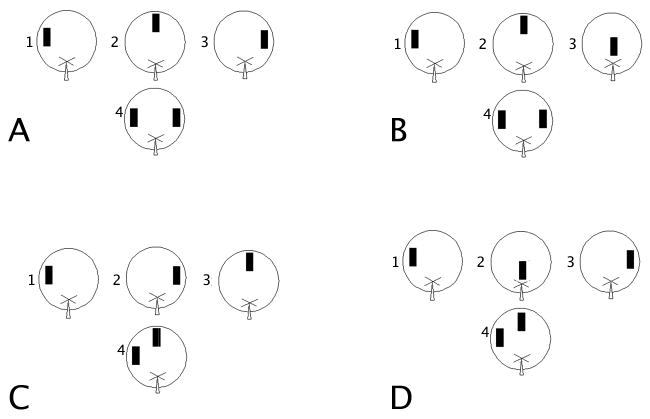
Behavioral Paradigms. A. Temporal order for spatial locations with low spatial interference. B. Novelty detection for spatial location with low spatial interference. C. Temporal order for spatial locations with high spatial interference. D. Novelty detection for spatial locations with high spatial interference. Each diagram shows three typical spatial locations presented for 5 m each (1-3) and the test phase (4) presented after 30 m.
As a control for the temporal ordering task, a novelty detection of spatial locations task with low spatial interference (Figure 1B) was given 48 h later in a different room except the objects during the test were 108 cm. apart to decrease spatial interference and a new set of objects were used (e.g. 5 cm × 10 cm white PVC T-joints). For the novelty detection of spatial locations task with low spatial interference, the animal was placed on the maze facing due east with an object in a particular spatial location and allowed to explore for 5 m. After this initial exploration, the animal was removed to a cage outside the running room for a 3 m intersession interval. After this interval, the animal was placed on the maze again with the object moved to a second spatial location. The animal was given 5 m to explore. After another 3 m intersession interval, the animal was placed on the maze with the object moved to a third spatial location and allowed to explore for 5 m. Following a 30 m intersession interval to allow for sufficient consolidation of the spatial information acquired during the study phase, the two identical objects covered the first experienced spatial location and a novel spatial location never before occupied by the object. The animal explored for 5 m. This tested the animal's preference for a novel spatial location over a familiar spatial location. The first and novel spatial locations in the novelty test were always 108 cm apart to minimize spatial interference.
Experiment 2: Temporal Order and Novelty Detection of Spatial Locations with High Spatial Interference
The temporal order for spatial locations task with high spatial interference was again run on a different modified open field cheeseboard as that used in Experiment 1. During this temporal ordering of spatial locations task, everything was maintained identical to Experiment 1, except that during the test the spatial locations were located 54 cm apart to increase spatial interference (Figure 1C), and large candlestick holders (10 cm wide and deep × 30 cm tall) were used for objects. The results of Experiment 2 were compared to the results from Experiment 1 to assess the role of spatial interference in temporal ordering for spatial locations.
As a control for the temporal ordering task, novelty detection for spatial locations task with high spatial interference (Figure 1D) was given 48 h later in a different room, following the same protocol as the novelty detection of spatial locations task during Experiment 1, except the objects during the test were 54 cm. apart to increase spatial interference and a new set of objects were used (e.g. 10 cm × 20 cm ceramic fish). The animal explored for 5 m. This tested the animal's preference for a novel spatial location over a familiar spatial location.
Data Collection and Statistical Analysis
In both experiments, time spent exploring the objects was the dependent variable and was recorded by an experimenter blind to the experimental manipulations of each animal from videotapes. Object exploration was recorded when an animal was facing the object and had its snout within 1 cm of the object for greater than 0.5 seconds in 0.5 second increments. Exploration totals were used to calculate a ratio score to normalize for total exploration time, which differed between individual animals but not between experimental groups. The ratio was calculated as follows. For temporal ordering tasks, the time spent in seconds exploring the object in the spatial location that came later in the sequence subtracted from the time spent exploring the object in the spatial location that came earlier in the sequence. This was divided by the total exploration time of the objects in the two spatial locations (e.g. [(earlier - later) / (earlier + later)]). This ratio was designed such that a score of zero indicated that there was no preferential re-exploration of one location over the other, as well as to facilitate visual interpretation of the plotted data since a positive number indicates that the animal explored the first spatial location presented over the last.
For the novelty detection paradigm, the ratio was the time spent exploring the familiar spatial location was subtracted from the time spent exploring the novel spatial location, divided by the total exploration time in seconds of the two spatial locations (e.g. [(novel - familiar) / (novel + familiar)]). A positive result of this calculation means that the animal explored the novel spatial location more than the familiar one presented during the study phase. For each task, the performance of each lesion group was compared to the null hypothesis that there would be no preference for one object over the other in control and lesion groups. The temporal ordering of spatial locations with low or high spatial interference was analyzed similarly. A significant deviation from no preference implied that the animals preferentially directed their exploration toward one spatial location over the other.
The object exploration ratio scores were calculated and input into a matrix that was used to calculate a one-way ANOVA with lesion group (control, dentate gyrus, CA3, and CA1) as the grouping factor. Tukey's HSD post hoc paired comparison tests were run on all significant effects and results were considered significant at p<0.05. To characterize the effect of spatial interference on the temporal ordering of spatial locations, two way repeated measures ANOVA were performed with lesion group as the between and the spatial distance (54 cm, 108 cm) as the repeated within factor. A priori planned one tailed paired t-tests were run on the lesion group × distance interaction for both temporal ordering and novelty detection for spatial locations (SPSS 15.0; SPSS, Inc; Chicago, IL).
Locomotor activity was also monitored to control for differences in locomotor activity. For the spatial locations temporal ordering task, a three by three grid was drawn on the monitor and every time a rat crossed a grid it was recorded (cf. Lee et al., 2005; Hunsaker, Rogers, & Kesner, 2007).
Histological Methods
After all behavioral testing was completed, rats were euthanized with a lethal dose of sodium pentobarbital (70 mg/mL i.p.) and perfused intracardially with 0.9% PBS (pH 6.0) for 2 m followed by 10% (wt/vol) formalin (pH 7.0) for another 5 m. The brains were then extracted and stored in 30% (wt/vol) sucrose formalin at 4 degrees Celsius for 72 h before being flash frozen and sliced into 40 μm sections with a freezing-stage microtome. Every third section from the tissue block containing the hippocampus was mounted on microscopic slides and Nissl stained with cresyl violet for microscopic verification of the lesions.
Sections were photographed and imported into ImageJ (v1.35j National Institute of Health; Bethesda, MD) for quantitative lesion analysis. Briefly, the anatomical region in question (dorsal dentate gyrus, dorsal CA3, and dorsal CA1) was traced using the freehand selection tool on Image J and the area contained within the tracing was calculated. The number of pixels contained within the traced region that were darker than the background (e.g. showed staining) were counted and compared to the total volume of the region of interest traced. This was performed on every section collected from each animal (there was ∼ 120 μm separation between analyzed sections). The damage was calculated in the dorsal hippocampus, as defined by Bannerman et al. (2002) and Moser &Moser (1998) as the anterior half of the hippocampus. The spared portion of the anatomical region was then traced and the percent damage was calculated based upon those two values. The percent damage for each animal was calculated from the sections and then averaged across animals.
Results
Histological Results
Axon-sparing, subregion-specific lesions of the dorsal hippocampus were made with specific neurotoxins. Figure 2 shows representative lesions of each hippocampal subregion and a diagram depicting the maximal and minimal damage in each subregion after each lesion. A quantitative analysis revealed that lesions to the dorsal dentate gyrus (Figure 2A) were >95% complete with no visible damage to dorsal CA3a,b; 10% damage to dorsal CA3c, and in one case <5% damage to the overlying dorsal CA1. Lesions to dorsal CA3 (Figure 2B) were approximately 90% complete with sparing in dorsal CA3c and no cortical damage. There were no instances of damage to dorsal CA1 or to the dorsal dentate gyrus. Lesions to dorsal CA1 (Figure 2C) were approximately 80-85% complete with sparing in the aspect closest to dorsal CA3a,b. No dorsal CA3c damage was observed in dorsal CA1 lesion animals and in one case, there was less than 10% damage to the upper blade of the dorsal dentate gyrus. There was also minimal damage to the overlying cortex. In all lesions, there was some sparing mostly at the septal pole of the hippocampus, but the lesion could be verified from 2.3 to 4.3 mm posterior to bregma. There was no observed damage to the ventral 50% of the hippocampus, nor was there damage to postrhinal, perirhinal, entorhinal, or posterior parietal cortices. Figure 2D shows sections from a control rat brain. Figure 2E shows the maximal and minimal lesion extents of each lesion on plates from Paxinos & Watson (1997).
Figure 2.
Histology A. Photomicrograph of a typical dentate gyrus lesion. B. Photomicrograph of a typical CA3 lesion. C. Photomicrograph of a typical CA1 lesion. D. Photomicrograph of a typical control lesion. Next to each photomicrograph is a corresponding diagram of maximal (grey) and minimal (black) lesion extents on plates from Paxinos and Watson (1997).
Behavioral Results
Locomotor Activity
In no cases did locomotor activity significantly differ among groups (data not shown). There were also no significant differences for spatial location exploration as a function of session (e.g. all animals explored the first, second, and third spatial locations presented for a similar number of seconds). This was the case for all temporal ordering and novelty detection tasks (all ps>0.2).
Temporal Order for Spatial Locations with Low Spatial Interference
The ratio scores calculated for the dentate gyrus, CA3, CA1 lesioned, and control animals were compared with a one-way ANOVA with lesion as the grouping factor (Figure 3Ai). There was a significant lesion effect (F(3,25)=8.010, p=0.001). A subsequent Tukey's HSD post hoc paired comparisons test revealed that control animals and dentate gyrus lesioned animals did not significantly differ (p>0.05). Both control animals and dorsal dentate gyrus lesioned animals explored the first spatial location presented during the test more than dorsal CA3 (p<0.05) and dorsal CA1 (p<0.01) lesioned animals. Dorsal CA3 lesioned animals explored the first spatial location more than dorsal CA1 lesioned animals (p<0.01). To further characterize the effects of the lesions, t-tests were performed for each group against a no preference for one spatial location over the other spatial location (no preference corresponds to a zero value on the plotted data). Control animals showed a significant preference for the first experienced spatial location over the latter (t(7)=4.22, p=0.002). Dorsal dentate gyrus lesioned animals showed a significant preference for the first experienced spatial location over the latter (t(5)=3.79, p=0.006). Dorsal CA3 lesioned animals showed no preference for one spatial location over the other (t(5)=1.12, p=0.16). For the CA3 lesioned animals, the no preference was indicative of low levels of exploration for both of the spatial locations. Dorsal CA1 lesioned animals showed a significant preference for the latter experienced spatial location over the first spatial location (t(5)=-4.73, p=0.003).
Figure 3.
Behavioral Results. A. Temporal ordering for spatial locations with low (Ai) and high (Aii) spatial interference. At 108 cm separation, both dorsal CA1 lesioned and dorsal CA3 lesioned animals show deficits. At 54 cm separation, dorsal CA1, dorsal CA3, and the dorsal dentate gyrus lesioned animals show deficits. B. Novelty detection for spatial locations with low (Bi) and high (Bii) spatial interference. Only the dorsal dentate gyrus lesion groups show a deficit for detection of a novel spatial location at 54 cm separation.
For the novelty detection of spatial locations task (Figure 3Bi), the ratios were similarly compared with a one-way ANOVA. There was no effect for lesion (F(3,25)=0.64, p=0.598). Control animals showed a significant preference for the novel experienced spatial location over the familiar one (t(7)=5.51, p=0.0004). Dorsal dentate gyrus lesioned animals showed a significant preference for the novel spatial location over the familiar spatial location (t(5)=4.12, p=0.005). Dorsal CA3 lesioned animals showed a significant preference for the novel spatial location over the familiar one (t(5)=3.97, p=0.005). Dorsal CA1 lesioned animals showed a significant preference for the novel spatial location over the familiar one (t(5)=4.09, p=0.005).
Temporal Order for Spatial Locations with High Spatial Interference
The ratio scores calculated for the dorsal dentate gyrus, dorsal CA3, dorsal CA1 lesioned, and control animals were compared with a one-way ANOVA with lesion as the grouping factor (Figure 3Aii). There was a significant lesion effect (F(3,25)=15.88, p<0.0001). A subsequent Tukey's HSD post hoc paired comparisons test revealed that control animals explored the first spatial location presented during the test more than dorsal dentate gyrus (p<0.05), dorsal CA3 (p<0.05) and dorsal CA1 (p<0.01) lesioned animals. Dorsal dentate gyrus and dorsal CA3 lesioned animals did not differentially explore the spatial locations (p>0.05). Dorsal CA1 lesioned animals explored the last spatial location more than all other groups (p<0.05). Control animals showed a significant preference for the first experienced spatial location over the latter (t(7)=3.87, p=0.003). Dorsal dentate gyrus lesioned animals showed no significant preference for one spatial location over the other (t(5)=0.94, p=0.20). Dorsal CA3 lesioned animals showed no preference for one spatial location over the other (t(5)=1.22, p=0.14). Dorsal CA1 lesioned animals showed a significant preference for the latter experienced spatial location (t(5)=-6.07, p=0.0009).
For the novelty detection for spatial locations task (Figure 3Bii) there was a lesion effect (F(3,25)=11.99, p<0.0001). A subsequent Tukey's HSD post hoc paired comparisons test revealed that only the dorsal dentate gyrus lesion group differed from all other groups (all ps<0.01). Control, dorsal CA3, and dorsal CA1 lesion groups did not differ (all ps>0.05). Control animals showed a significant preference for the novel spatial location over the familiar one (t(7)=5.14, p=0.0007). Dorsal dentate gyrus lesioned animals showed no preference for one spatial location over the other (t(5)=1.17, p=0.15). Dorsal CA3 lesioned animals showed a significant preference for the novel spatial location over the familiar one (t(5)=3.70, p=0.007). Dorsal CA1 lesioned animals showed a significant preference for the novel spatial location over the familiar one (t(5)=3.99, p=0.005).
A Comparison between Temporal Ordering with Low and High Spatial Interference
From the plotted data, it appears that only the dorsal dentate gyrus lesions group showed differential performance as a function of spatial interference. To determine the role of spatial pattern separation in the temporal ordering for spatial locations as a function of spatial interference (e.g. comparing the 108 cm vs. 54 cm separation conditions), a two way repeated-measures ANOVA was performed with lesion as the grouping factor and distance (108 cm, 54 cm) as the repeated within factor. There was a significant effect of lesion (F(3,22)=13.52, p<0.0001), a trend toward a significant effect for distance (F(1,22)=4.00, p=0.06), and a significant lesion group × distance interaction (F(3,22)=3.04, p=0.05). Since the interaction contains the important information, a priori planned one-tailed paired t-tests were run on lesion group within distance. Dorsal dentate gyrus lesioned animals showed a significant difference in their performance of the high and low spatial interference condition (i.e. dorsal dentate gyrus lesioned animals explored the first spatial location more than the latter in the 108 cm condition, but showed no preference during the 54 cm condition; t(5)=-2.64, p=0.046). No other groups showed any significant differences in their exploration preferences as a function of spatial separations [(Control (t(7)=-1.127, p=0.30), dorsal CA3 (t(5)=0.508, p=0.63), dorsal CA1 (t(5)=0.620, p=0.56)]. These data verify that only the dorsal dentate gyrus lesioned animals showed any differential performance on the temporal ordering of spatial locations task as a function of spatial interference.
To determine the role of spatial pattern separation in the novelty detection for spatial locations task as a function of spatial interference (e.g. 108 cm vs. 54 cm), a two way repeated-measures ANOVA was performed with lesion group and distance (108 cm and 54 cm) as the repeated within factor was performed. There was a significant effect of lesion (F(3,22)=3.953, p=0.021), an effect for distance (F(1,22)=75.76, p<0.0001), but no significant lesion group × distance interaction (F(3,22)=2.18, p=0.119). Since the plotted data suggest that only the dorsal dentate gyrus lesioned animals show differential performance as a function of distance, a priori planned one tailed paired t-tests were run on the lesion group × distance interaction. Dorsal dentate gyrus lesioned animals showed a significant difference in their performance of the high and low spatial interference condition (i.e. animals with dorsal dentate gyrus lesions preferred the novel spatial location over the familiar in the 108 cm condition, but showed no preference in the 54 cm condition; t(5)=-2.81, p=0.02). No other groups showed any significant differences in preference for the novel spatial location relative to the familiar as a function of spatial separation [(Control (t(7)=-0.97, p=0.19), dorsal CA3 (t(5)=0.21, p=0.42), dorsal CA1 (t(5)=0.71, p=0.26)]. These data verify that only the dorsal dentate gyrus lesioned animals showed any differential performance on the novelty detection of spatial locations task as a function of spatial interference.
Discussion
The present experiment suggests that each dorsal hippocampal subregion may play a different role in the processing of spatiotemporal information. To test the roles of hippocampal subregions for spatiotemporal processing, we made small parametric changes to a simple temporal ordering for spatial locations task. The results of the present task can be interpreted in a number of ways. The experimental design of the temporal order for spatial locations task did not test memory for a sequence, and did not discriminate between temporal ordering per se and judgment of recency. We assume that the present task could, theoretically, be solved either by temporal ordering or sequential processing of the spatial location information or by weighting the relative recency of the memory traces of experienced spatial locations. The present task was designed to evaluate the role of each hippocampal subregion for temporal ordering (or judgment of recency tasks), not to elucidate the fundamental processes underlying the temporal processing. Further research is required to determine whether the hippocampal subregions play differential roles in tasks emphasizing sequential processing, temporal ordering, or recency judgments.
CA1
It has been demonstrated that the dorsal (and ventral) CA1 subregion of the hippocampus mediates temporal processing in lesion (Hoge & Kesner, 2007; Hunsaker et al., 2008 in press; Kesner et al., 2005; Lee & Kesner, 2002, 2003), genetic (Huerta, Sun, Wilson, & Tonegawa, 2000), and neurophysiological studies (McEchron, Tseng, & Disterhoft, 2003). In the present study, the dorsal CA1 lesion group appeared to prefer the later spatial location presented, the opposite preference to that shown by control animals. This effect parallels that seen for temporal ordering of visual objects (Hoge & Kesner, 2007; Hunsaker et al., 2008 in press).
As postulated by Hunsaker et al. (2008 in press), the role of the dorsal CA1 for temporal ordering tasks such as the present experiment may be to bias recall toward primacy and away from recency (i.e. weigh the relative differences in recency of the two spatial locations and bias the selective re-exploration toward the less recent). In short, the information from the first spatial location is preferentially recalled over information about the last spatial location. This means that, when dorsal CA1 retrieves the information from the cortex, the stronger memory trace is for the first spatial location presented. The animal reflects this consolidation by preferentially re-exploring the first spatial location since the last object is not novel, just not as well consolidated—this may be what underlies the preference for primacy observed in animals with an intact hippocampus. An animal with a lesion to dorsal CA1 showed a clear preference for the latter spatial location presented, suggesting the animal favored recency over primacy (i.e. the most recent over the least recent). It should be noted that similar recency processing has been attributed to the perirhinal and postrhinal cortices in the rat (Brown & Aggleton, 2001). This interpretation is consistent with the observation that rats with small dorsal hippocampal lesions display an impairment for remembering the first item (primacy), but have no difficulty remembering the last item (recency) of list of five spatial locations (Di Mattia & Kesner, 1984). Also, patients that have suffered from hypoxia and have hippocampal damage display an impairment of the first item (primacy), but not the last item (recency) of a list of six spatial locations (Hopkins, Kesner, & Goldstein. 1995).
Despite the altered temporal ordering, dorsal CA1 lesioned animals were able to efficiently show a novelty preference for a spatial location never before encountered over a familiar one. Animals with dorsal CA1 lesions did show a significant preference for the spatial location experienced later over the first experienced spatial location, suggesting that the altered preference for the latter over the former spatial location was not due to impaired memory of the former spatial location. Furthermore, intact novelty detection suggests that animals with dorsal CA1 lesions were able to discriminate between the spatial locations; they showed an altered preference for the latter spatial location over the former. These findings rule out any sensory deficits or impaired novelty detection mechanisms in the dorsal CA1 lesioned animals.
Dentate Gyrus
Gilbert et al. (2001) demonstrated that the dentate gyrus mediates spatial pattern separation or orthogonalization (cf. Costa, Bueno, & Xavier, 2005; Xavier, Oliviera-Filho, & Santos, 1999 for further dentate gyrus lesion studies that arrive at similar conclusions). The authors were able to uncover this deficit via a delayed matching to sample task with varying levels of spatial interference. Only the dorsal dentate gyrus lesion group (not dorsal CA1 or dorsal CA3 lesion groups; cf. Gilbert & Kesner, 2006) showed a near linear improvement in performance as a function of decreasing spatial interference. Although the results of the present experiment are suggestive of a similar effect, there are too few spatial separations to make a clear conclusion. Animals with lesions to the dorsal dentate gyrus did show an inability to perform a temporal ordering task for spatial locations and a spatial location novelty detection task with high levels of spatial interference, but did not display a problem performing the same task when spatial interference is minimized. Neither dorsal CA3 nor dorsal CA1 lesioned animals showed differential performance of either task as a function of spatial interference under the present experimental conditions.
It is critical to note that the impaired performance of the dentate gyrus lesioned group did not occur only during the temporal ordering task, but also during the novelty detection task. This pattern suggests that the dorsal dentate gyrus lesioned animals were unable to discriminate the two spatial locations during the test when they were separated by only 54 cm (e.g. high spatial interference), whereas they were able to discriminate the two when separated by 108 cm (e.g. low spatial interference). These results match those found by Gilbert et al. (2001) and Goodrich-Hunsaker et al. (2008) who found that animals with lesions to the dentate gyrus have difficulties discriminating spatial locations when separated by less than 68 cm. Additionally, the dentate gyrus lesioned animals showed no preference for one spatial location over the other when presented 54 cm apart. The most parsimonious explanation of the dorsal dentate gyrus lesion effect is that animals with lesions of the dorsal dentate gyrus are unable to discriminate spatial locations when interference is high, but are able to do so when spatial interference is low.
CA3
It has been demonstrated that CA3 lesioned animals show spatial memory deficits (Gilbert & Kesner, 2003; 2006; Jerman, Kesner, & Hunsaker, 2006; Lee & Kesner, 2003), as well as pattern completion deficits for spatial information (e.g. they are unable to take partial information and use that information to recall the previous experience; cf. Gold & Kesner, 2005;Nakazawa, Sun, Quirk, Rondi-Reig, Wilson, & Tonegawa, 2003). CA3 lesions and inactivations also result in deficits for spatial memory (Gilbert & Kesner, 2003; Hunsaker et al., 2006; Jerman et al., 2006; Lee & Kesner, 2002, 2003). The present results are suggestive of a spatiotemporal memory deficit in rats that have received a CA3 lesion, since CA3 lesioned animals do not show a preference for the first spatial location presented or for the last spatial location presented, but they do show intact novelty detection. This effect suggests that the animals knew which spatial locations were presented in the study phases, but that they did not encode this information well enough to make a judgment of temporal order (cf. Jerman, et al., 2006; Hunsaker et al., 2008b in press; Vago et al., 2007).
The CA3 lesioned animals do not show a preference for the first or last experienced spatial location without regard to the level of spatial interference (e.g. there was no preference for one spatial location during either the 54 and 108 cm separations). These data also differ from the dentate gyrus lesioned animals that showed no preference for one spatial location over the other when the locations were separated by 54 cm, but did show a preference for the first spatial location over the latter when the two were separated by 108 cm. It is important to note that this effect was not affected by spatial interference; there was no difference between performance under high spatial interference and low spatial interference conditions. The CA3 lesioned animals were able to discern spatial novelty. These results suggest that neither a general inability to recognize spatial locations nor a novelty detection deficit (e.g. a reference memory deficit) underlies this effect.
An alternative interpretation of this data is that animals with lesions to dorsal CA3 have difficulties in recalling object-location associations. These animals appear to be capable of identifying novel spatial locations when encountered, but are unable to discriminate between previously encountered spatial locations. In other words, these animals are able to discriminate spatial novelty, but they may lack the ability to process associative novelty as proposed by Kumaran and Maguire (2007) for human subjects. The dorsal CA3 lesioned animals' pattern of deficits suggest that the animals can report (via directed exploration) when a spatial location was previously unoccupied by an object, but they are unable to discriminate between two previously learned object and spatial location pairings (also cf. Eichenbaum, Yonelinas, & Ranganath, 2007; Nyberg, 2005 for a similar analysis applied to the hippocampus as a whole). These results suggest a role for CA3 in spatiotemporal processing
General Remarks
The present report, to the author's knowledge, is the first report to concurrently demonstrate different roles for all three dorsal hippocampal subregions during the same task. The benefit of the present task over that reported by Gilbert et al. (2001), Gilbert & Kesner (2006), is, primarily, that the present task does not require training of any kind. The animals are simply required to explore and their patterns of exploration are analyzed to tease apart the role of each subregion for processing the spatiotemporal information.
The present experiment revealed that the dentate gyrus may play a role in spatial information processing, validating previous neurophysiological reports that suggested the same (Leutgeb, et al., 2007; McHugh, et al., 2007). The role of the dorsal dentate gyrus in the present experiment appears to be one of discriminating the spatial locations from one another to facilitate efficient coding of the spatial locations so that spatiotemporal processing may be more efficient.
The data support a role for dorsal CA3 in spatiotemporal memory processes. It has been shown neurophysiologically that dorsal CA3 is able to show both pattern separation as well as pattern completion mechanisms, depending upon the task contingencies (Lee, Rao, & Knierim, 2004, Lee, Yoganarasimha, Rao, & Knierim, 2004; Leutgeb & Leutgeb, 2007; cf. Treves, 2004). It has been suggested that interactions between pattern separation and pattern completion can lead to effective spatial memory processing (Rolls & Kesner, 2006; Rolls & Treves, 1998). It has also been suggested that dorsal CA3 is uniquely set up as a sort of bottleneck within the hippocampus that primes it to be a spatial memory buffer (due to the recurrent circuitry; Rolls & Kesner, 2006; Treves, 2004). The dorsal CA3 lesioned animals do not show a preference for one spatial location over another at even very low levels of spatial interference (e.g. 108 cm). The animals' performance at 54 cm does not change from their performance at 108 cm. This pattern suggests it is not spatial interference, per se, but a deficit in efficiently encoding or retrieving the spatiotemporal information presented during the study phase of the task. This is not due to a complete deficit in spatial processing since the dorsal CA3 lesioned animals are capable of identifying and reacting to spatial novelty at both low and high levels of spatial interference. These types of spatial memory deficits have been seen in numerous tasks after dorsal CA3 lesions (Gilbert & Kesner, 2006; Lee & Kesner, 2003; cf. Kesner, 2007a,b; Kesner et al., 2004 for a thorough review of dorsal CA3 function).
The results of the dorsal CA1 lesions support models that suggest dorsal CA1 mediates temporal processing (Hoge & Kesner, 2007; Rolls & Kesner, 2006; Rolls & Treves, 1998; Treves, 2004). The temporal ordering deficits demonstrated by animals with dorsal CA1 lesions were not dependent upon levels of spatial interference. The animals with dorsal CA1 lesions consistently showed a preference for the later spatial location presented when given a preference test between the first and last location presented. As postulated by Hunsaker et al. (2008 in press), dorsal CA1 may mediate this temporal ordering effect via biasing an animal to act upon primacy judgments as opposed to recency judgments mediated by the cortex. The animals with dorsal CA1 lesions appeared to have lost the natural preference for primacy but strongly favor recency in judgments of temporal order.
Acknowledgments
This research was supported by NSF Grant IBN-0135273 and NIH Grant RO1MH065314 awarded to RPK. The authors wish to thank Jenna Hall, Jenna Rosenberg, and Ryan Thierolf for assistance with data collection.
References
- Bannerman DM, Deacon RM, Offen S, Friswell J, Grubb M, Rawlins JN. Double dissociation of function within the hippocampus: spatial memory and hyponeophagia. Behav Neurosci. 2002;116:884–901. doi: 10.1037//0735-7044.116.5.884. [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus. Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Costa VC, Bueno JL, Xavier GF. Dentate gyrus-selective colchicines lesion and performance in temporal and spatial tasks. Behav Brain Res. 2005;160:286–303. doi: 10.1016/j.bbr.2004.12.011. [DOI] [PubMed] [Google Scholar]
- DiMattia BV, Kesner RP. Serial position curves in rats: Automatic vs effortful information processing. J of Exp Psychol: Animal Behav Proc. 1984;10:557–563. [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial tenmporal lobe and recognition memory. Ann Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin NJ, Agster KL, Eichenbaum HB. Critical role of the hippocampus in memory for sequences of events. Nat Neurosci. 2002;5:458–462. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP. Localization of function within the dorsal hippocampus: the role of the CA3 subregion in paired-associate learning. Behav Neurosci. 2003;117:1385–1394. doi: 10.1037/0735-7044.117.6.1385. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP. The role of the dorsal CA3 hippocampal subregion in spatial spatial memory and pattern separation. Behav Brain Res. 2006;169:142–149. doi: 10.1016/j.bbr.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- Gold AE, Kesner RP. The role of the CA3 subregion of the dorsal hippocampus in spatial pattern completion in the rat. Hippocampus. 2005;15:808–814. doi: 10.1002/hipo.20103. [DOI] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Hunsaker MR, Kesner RP. Dissociating the role of parietal cortex and dorsal hippocampus for spatial information processing. Behav Neurosci. 2005;119:1307–1315. doi: 10.1037/0735-7044.119.5.1307. [DOI] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Hunsaker MR, Kesner RP. The interaction and dissociations of the dorsal hippocampus subregions: How the dentate gyrus, CA3, and CA1 process spatial information. Behav Neurosci. 2008;122 doi: 10.1037/0735-7044.122.1.16. in press. [DOI] [PubMed] [Google Scholar]
- Hoge J, Kesner RP. Role of CA3 and CA1 subregions of the dorsal hippocampus on temporal processing of objects. Neurobiol Learn Mem. 2007;88:225–231. doi: 10.1016/j.nlm.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins RO, Kesner RP, Goldstein M. Item and order recognition memory in subjects with hypoxic brain injury. Brain and Cognition. 1995;27:180–201. doi: 10.1006/brcg.1995.1016. [DOI] [PubMed] [Google Scholar]
- Huerta PT, Sun LD, Wilson MA, Tonegawa S. Formation of temporal memory requires NMDA receptors within CA1 pyramidal neurons. Neuron. 2000;25:473–480. doi: 10.1016/s0896-6273(00)80909-5. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Fieldsted PM, Rosenberg JS, Kesner RP. Dissociating the roles of dorsal CA1 and ventral CA1 for processing he temporal order of spatial locations. Behav Neurosci. 2008;122 doi: 10.1037/0735-7044.122.3.643. in press. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Mooy GG, Swift JS, Kesner RP. Dissociations of the medial and lateral perforant path projections into dorsal DG, CA3, and CA1 for spatial and nonspatial information processing. Behav Neurosci. 2007;121:742–750. doi: 10.1037/0735-7044.121.4.742. [DOI] [PubMed] [Google Scholar]
- Hunsaker MR, Rogers JL, Kesner RP. Behavioral characterization of a transection of dorsal CA3 subcortical efferents: comparison with scopolamine and physostigmine infusions into dorsal CA3. Neurobiol Learn Mem. 2007;88:127–136. doi: 10.1016/j.nlm.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, Thorup JA, Welch T, Kesner RP. The role of CA3 and CA1 in the acquisition of an object-trace place paired-associate task. Behav Neurosci. 2006;120:1252–1256. doi: 10.1037/0735-7044.120.6.1252. [DOI] [PubMed] [Google Scholar]
- Jerman T, Kesner RP, Hunsaker MR. Disconnection analysis of CA3 and DG in mediating encoding but not retrieval in a spatial maze learning task. Learning and Mem. 2006;13:458–464. doi: 10.1101/lm.246906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP. A behavioral analysis of dentate gyrus function. Prog Brain Res. 2007a;163:567–576. doi: 10.1016/S0079-6123(07)63030-1. [DOI] [PubMed] [Google Scholar]
- Kesner RP. Behavioral functions of the CA3 subregion of the hippocampus. Learn Mem. 2007b;14:771–781. doi: 10.1101/lm.688207. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Lee I, Gilbert PE. A behavioral assessment of hippocampal function based on a subregional analysis. Rev Neurosci. 2004;15:333–351. doi: 10.1515/revneuro.2004.15.5.333. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Hunsaker MR, Gilbert PE. The role of CA1 in the acquisition of an object-trace-odor paired associate task. Behav Neurosci. 2005;119:781–786. doi: 10.1037/0735-7044.119.3.781. [DOI] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA. Match mismatch processes underlie human hippocampal responses to associative novelty. J Neurosci. 2007;27:5817–8524. doi: 10.1523/JNEUROSCI.1677-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Hunsaker MR, Kesner RP. The role of hippocampal subregions in detecting spatial novelty. Behav Neurosci. 2005;119:145–153. doi: 10.1037/0735-7044.119.1.145. [DOI] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Diffferential contribution of NMDA receptors in hippocampal subregions to spatial spatial memory. Nat Neurosci. 2002;5:162–168. doi: 10.1038/nn790. [DOI] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Differential roles of dorsal hippocampal subregions in spatial memory with short versus intermediate delay. Behav Neurosci. 2003;117:1044–1053. doi: 10.1037/0735-7044.117.5.1044. [DOI] [PubMed] [Google Scholar]
- Lee I, Rao G, Knierim JJ. A double dissociation between hippocampal subfields: differential time course of CA3 and CA1 place cells for processing changed environments. Neuron. 2004;42:803–815. doi: 10.1016/j.neuron.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Lee I, Yoganarasimha D, Rao G, Knierim JJ. Comparison of population coherence of place cells in hippocampal subfields CA1 and CA3. Nature. 2004;430:456–459. doi: 10.1038/nature02739. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Leugteb S, Leutgeb JK. Pattren separation, pattern completion, and new neuronal codes within a continuous CA3 map. Learn Mem. 2007;14:745–757. doi: 10.1101/lm.703907. [DOI] [PubMed] [Google Scholar]
- Manns JR, Howard MW, Eichenbaum H. Gradual changes in hippocampal activity support remembering the order of events. Neuron. 2007;53:530–540. doi: 10.1016/j.neuron.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEchron MD, Tseng W, Disterhoft JE. Single neurons in CA1 hippocampus encode trace interval duration during trace heart rate (fear) conditioning in rabbit. J Neurosci. 2003;23:1535–1547. doi: 10.1523/JNEUROSCI.23-04-01535.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, et al. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8:608–619. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Sun LD, Quirk MC, Rondi-Reig L, Wilson MA, Tonegawa S. Hippocampal CA3 NMDA receptors are crucial for memory acquisition of one-time experience. Neuron. 2003;38:305–315. doi: 10.1016/s0896-6273(03)00165-x. [DOI] [PubMed] [Google Scholar]
- Nyberg L. Any novelty in hippocampal formation and memory? Curr Opin Neurobiol. 2005;18:424–428. doi: 10.1097/01.wco.0000168080.99730.1c. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain: In stereotaxic coordinates. Academic Press; San Diego, CA: 1997. [Google Scholar]
- Rogers JL, Hunsaker MR, Kesner RP. Effects of ventral and dorsal CA1 subregional lesions on trace fear conditioning. Neurobiol Learn Mem. 2006;86:72–81. doi: 10.1016/j.nlm.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Prog Neurobiol. 2006;79:1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Treves A. Neural networks and brain function. Oxford University Press; Oxford; 1998. [Google Scholar]
- Treves A. Computational constraints between retrieving the past and predicting the future, and the CA30CA1 differentiation. Hippocampus. 2004;14:539–556. doi: 10.1002/hipo.10187. [DOI] [PubMed] [Google Scholar]
- Vago DR, Bevan AD, Kesner RP. The role of the direct perforant path input to the CA1 subregion of the dorsal hippocampus in memory retention and retrieval. Hippocampus. 2007;17:977–987. doi: 10.1002/hipo.20329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier GF, Oliviera-Filho FJ, Santos AM. Dentate gyrus-selective colchicine lesion and disruption of performance in spatial tasks: difficulties in “place strategy” because of a lack of flexibility in the use of environmental cues? Hippocampus. 1999;9:668–681. doi: 10.1002/(SICI)1098-1063(1999)9:6<668::AID-HIPO8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]




