Abstract
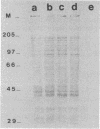
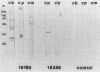
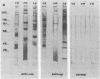
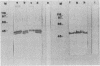
A comparison was made of the antigenic composition of oocyst walls and sporozoites from Cryptosporidium baileyi from turkeys, C. muris from rodents, and C. parvum from ruminants, employing immunoblotting and immunofluorescence. In immunoblotting, oocyst antigens were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting (immunoblotting) and detected with rabbit polyclonal anti-C. muris or -C. parvum antibodies or murine monoclonal antibodies developed against C. parvum. Immunofluorescence was used to investigate the reactivity of these monoclonal antibodies with air-dried excystation mixtures of sporozoites and oocysts of the different species. The results from both types of experiment indicated that the three Cryptosporidium species could be differentiated immunologically. In comparison, few antigenic differences were found between a number of isolates of C. parvum in immunoblotting. There was also evidence to suggest that C. parvum and C. baileyi were more closely related antigenically to one another than to C. muris.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anusz K. Z., Mason P. H., Riggs M. W., Perryman L. E. Detection of Cryptosporidium parvum oocysts in bovine feces by monoclonal antibody capture enzyme-linked immunosorbent assay. J Clin Microbiol. 1990 Dec;28(12):2770–2774. doi: 10.1128/jcm.28.12.2770-2774.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Current W. L., Bick P. H. Immunobiology of Cryptosporidium spp. Pathol Immunopathol Res. 1989;8(3-4):141–160. doi: 10.1159/000157146. [DOI] [PubMed] [Google Scholar]
- Current W. L., Upton S. J., Haynes T. B. The life cycle of Cryptosporidium baileyi n. sp. (Apicomplexa, Cryptosporidiidae) infecting chickens. J Protozool. 1986 May;33(2):289–296. doi: 10.1111/j.1550-7408.1986.tb05608.x. [DOI] [PubMed] [Google Scholar]
- Ditrich O., Palkovic L., Sterba J., Prokopic J., Loudová J., Giboda M. The first finding of Cryptosporidium baileyi in man. Parasitol Res. 1991;77(1):44–47. doi: 10.1007/BF00934383. [DOI] [PubMed] [Google Scholar]
- Fayer R., Ungar B. L. Cryptosporidium spp. and cryptosporidiosis. Microbiol Rev. 1986 Dec;50(4):458–483. doi: 10.1128/mr.50.4.458-483.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iseki M., Maekawa T., Moriya K., Uni S., Takada S. Infectivity of Cryptosporidium muris (strain RN 66) in various laboratory animals. Parasitol Res. 1989;75(3):218–222. doi: 10.1007/BF00931279. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazo A., Barriga O. O., Redman D. R., Bech-Nielsen S. Identification by transfer blot of antigens reactive in the enzyme-linked immunosorbent assay (ELISA) in rabbits immunized and a calf infected with Cryptosporidium sp. Vet Parasitol. 1986 Aug;21(3):151–163. doi: 10.1016/0304-4017(86)90062-2. [DOI] [PubMed] [Google Scholar]
- Lindsay D. S., Sundermann C. A., Blagburn B. L. Cultivation of Cryptosporidium baileyi: studies with cell cultures, avian embryos, and pathogenicity of chicken embryo-passaged oocysts. J Parasitol. 1988 Apr;74(2):288–293. [PubMed] [Google Scholar]
- Lumb R., Lanser J. A., O'Donoghue P. J. Electrophoretic and immunoblot analysis of Cryptosporidium oocysts. Immunol Cell Biol. 1988 Oct-Dec;66(Pt 5-6):369–376. doi: 10.1038/icb.1988.48. [DOI] [PubMed] [Google Scholar]
- McDonald V., Deer R. M., Nina J. M., Wright S., Chiodini P. L., McAdam K. P. Characteristics and specificity of hybridoma antibodies against oocyst antigens of Cryptosporidium parvum from man. Parasite Immunol. 1991 May;13(3):251–259. doi: 10.1111/j.1365-3024.1991.tb00280.x. [DOI] [PubMed] [Google Scholar]
- McDonald V., Stables R., Warhurst D. C., Barer M. R., Blewett D. A., Chapman H. D., Connolly G. M., Chiodini P. L., McAdam K. P. In vitro cultivation of Cryptosporidium parvum and screening for anticryptosporidial drugs. Antimicrob Agents Chemother. 1990 Aug;34(8):1498–1500. doi: 10.1128/aac.34.8.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead J. R., Arrowood M. J., Current W. L., Sterling C. R. Field inversion gel electrophoretic separation of Cryptosporidium spp. chromosome-sized DNA. J Parasitol. 1988 Jun;74(3):366–369. [PubMed] [Google Scholar]
- Mead J. R., Arrowood M. J., Sterling C. R. Antigens of Cryptosporidium sporozoites recognized by immune sera of infected animals and humans. J Parasitol. 1988 Feb;74(1):135–143. [PubMed] [Google Scholar]
- Tilley M., Upton S. J., Blagburn B. L., Anderson B. C. Identification of outer oocyst wall proteins of three Cryptosporidium (Apicomplexa: Cryptosporidiidae) species by 125I surface labeling. Infect Immun. 1990 Jan;58(1):252–253. doi: 10.1128/iai.58.1.252-253.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzipori S. Cryptosporidiosis in perspective. Adv Parasitol. 1988;27:63–129. doi: 10.1016/S0065-308X(08)60353-X. [DOI] [PMC free article] [PubMed] [Google Scholar]