Abstract
Apelin, a novel peptide with significant cardioactive properties, is upregulated by insulin in adipocytes. However, the mechanism by which insulin promotes apelin production is unknown. Hypoxia-inducible factor-1 (HIF-1), a heterodimeric transcription factor involved in the angiogenic and metabolic responses to tissue hypoxia, has been shown to be activated by insulin in various settings. We therefore hypothesized that HIF-1 regulates insulin-mediated apelin expression in adipocytes. 3T3L1 cells were differentiated into adipocytes in culture. For experiments, serum starved 3T3L1 cells were exposed to insulin and/or a 1% O2 environment. Apelin expression was assessed using quantitative real-time PCR and ELISA. To directly assess the role of HIF-1 in apelin production, we differentiated mouse embryonic fibroblasts (MEFs) containing a targeted deletion of the HIF-1α gene into adipocytes and measured their response to insulin and hypoxia. Apelin expression in mature 3T3L1 adipocytes was increased significantly by insulin, and was attenuated by pharmacologic inhibition of insulin signaling. Exposure of cells to either hypoxia or the chemical HIF activators cobalt chloride (CoCl2) and dimethyloxaloylglycine (DMOG), resulted in significant upregulation of apelin, consistent with a role for HIF in apelin induction. Moreover, hypoxia-, CoCl2-, DMOG-, and insulin-induced apelin expression were all attenuated in differentiated HIF-1α-deficient MEFs. In summary, in cultured 3T3L1 adipocytes and differentiated MEFs, HIF-1 appears to be involved in hypoxia- and insulin-induced apelin expression.
Keywords: Apelin, hypoxia inducible factor, adipocyte, insulin, obesity
INTRODUCTION
Obesity has become increasingly recognized as a major health care problem in the Western world, with epidemic proportions and significant medical sequlae (29). In the search for the mechanistic basis of obesity, the investigational focus into its pathogenesis has shifted in recent times from the organismic to the tissue and cellular level. Though traditionally believed to function predominantly as storage depots, adipocytes in fact have highly complex biological actions and are known to secrete a variety of factors affecting remote organs in a paracrine fashion (23). Among these “adipokines” is the 36-amino acid peptide apelin, which was originally discovered in 1998 as an endogenous ligand for what was then an orphan G-protein coupled receptor designated APJ (30). Although it was initially isolated from bovine stomach extracts (42), it has become appreciated that apelin is present in multiple tissues and, in particular, is secreted by adipocytes (4).
Apelin exerts multiple unique physiologic actions in various organs. To date, the effects of apelin/APJ signaling on the cardiovascular system have been the most widely reported. Apelin has been demonstrated to increase cardiac contractility, decrease mean arterial blood pressure, and increase heart rate (3, 24, 41, 43). Furthermore, apelin functions as a positive regulator of angiogenesis (9, 21).
By contrast, apelin's exact role(s) in adipose tissue remain unclear. Despite this lack of certainty, the prevailing evidence suggests that apelin is upregulated in states of adipocyte excess (i.e. obesity). Indeed, adipocyte apelin mRNA expression is elevated in obese mice compared to their lean controls (4). Moreover, a direct correlation between serum apelin levels and BMI in humans has been identified (4, 14, 25). Overall these data strongly suggest an association between apelin production and obesity.
Unfortunately, little is known about how (or even whether) obesity affects the upstream regulation of apelin. A recent study showed that apelin expression is increased by insulin, and that this effect is dependent, to varying extents, on mitogen activated protein kinase (MAPK), phosphatidylinositol 3-kinase (PI3K), and protein kinase C (PKC) (4). That said, the signal transduction distal to these intermediates is currently unknown.
Hypoxia inducible factor (HIF) is an oxygen-sensitive heterodimeric transcription factor that promotes the expression of genes containing hypoxia response element (HRE) in their promoter (38). These genes generally function to facilitate cell survival in a low oxygen environment by promoting glycolytic metabolism, angiogenesis, or maintenance of redox status. The heterodimer is composed of an α-subunit (of which HIF-1α, HIF-2α [EPAS], and HIF-3α family members have been described) and a β-subunit (also known as aryl hydrocarbon nuclear translocator). The transcriptional activity of this complex is tightly regulated by oxygen tension. That apelin expression in adipocytes may be dependent on HIF is supported by multiple lines of evidence. It has recently been reported that introduction of enhancers and inhibitors of HIF-mediated transcription respectively induce or repress apelin expression in cultured cardiomyocytes (9, 33). Furthermore, it has been speculated that apelin is modulated by HIF based on the presence of putative HREs in the apelin promoter (9). Finally, a pathologic hallmark of obesity is adipocyte hypertrophy. As such, hypertrophic adipocytes frequently expand beyond the limits of effective oxygen diffusion, and adipose tissue hypoxia has been observed in obese individuals (15, 20). In light of these observations, we sought to determine whether HIF modulates hypoxic expression of apelin in cultured adipocytes. Additionally, because insulin stimulates apelin expression and has been shown to stabilize HIF-1α in normoxic conditions, we examined the role of HIF in insulin-induced apelin expression.
METHODS
Cell culture
3T3L1 preadipocytes (American Type Culture Collection, Manassas, VA) were grown in Dulbecco's Modified Eagle's Medium (DMEM) containing 25 mM glucose, 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% fetal bovine serum (FBS) at 37° C in 5% CO2. Cells were differentiated into adipocytes by the method of Student, et al (40) using a cocktail of 0.5 mM isobutylmethylxanthine, 0.25 μM dexamethasone, and 100 nM insulin (heretofore referred to as MDI). Confluent cells were allowed to grow in DMEM alone for 2 days, exposed to MDI media for 2 days, treated with insulin (100 nM) for an additional 2 days, then finally incubated with DMEM alone until full differentiation (defined as > 80% surface area coverage by fat droplets) was achieved. All adipocytes used for these experiments were harvested between 7−9 days after initial exposure to MDI media.
To further investigate the involvement of HIF in apelin induction, mouse embryonic fibroblasts (MEFs) derived from mouse embryos with a targeted deletion of the HIF-1α gene (35, 36) [MEF HIF-1(−/−); HIF-KO] harvested at day E 9.5 were used for these experiments. Immortalized wild type and HIF-KO MEFs were differentiated using the MDI induction protocol described above, with the addition of the thiazolidinedione compound rosiglitazone (1 μM in DMSO; (GlaxoSmithKline, Middlesex, UK) from the beginning to the end of the differentiation process (this protocol has been used and validated in multiple studies to produce a recognizable adipocyte phenotype in MEFs (6, 31, 32)).
Fully differentiated cells were serum starved for 18 hours in DMEM with 1% FBS. Following this, cells were exposed to one of several experimental conditions. In addition to control (DMEM without serum), various plates were incubated in the presence of insulin (100 nM), a hypoxic chamber (Billups-Rothenberg, Del Mar, CA) containing a 1% oxygen environment, and/or the HIF-1α stabilizers cobalt chloride (CoCl2; 150 μM) and dimethyloxaloylglycine (DMOG; 500 μM). Each plate was allowed to incubate for 6 hours prior to cell harvest. For selected experiments, pharmacologic inhibitors of the phosphatidylinositol 3-kinase (wortmannin; 100 nM; Sigma-Aldrich, St. Louis, MO), Akt (Akt Inhibitor IV; EMD Bioscicences, San Diego, CA; 1 μM), and mitochondrial complex I (rotenone; 2.5 μM) were added immediately prior to either insulin or hypoxic exposure.
At the conclusion of each experimental exposure, cells were washed once with phosphate-buffered saline (PBS), harvested with a cell scraper, and suspended in Trizol reagent (Invitrogen, Carlsbad, CA). The samples were then used for mRNA extraction and real-time quantitative polymerase chain reaction as described below.
Oil Red O staining
To provide confirmatory evidence of differentiation in wild type and HIF-KO MEFs, Oil Red O staining was performed. Selected culture dishes were washed with PBS, then incubated with 10% formalin (Sigma-Aldrich) for 1 hour at 4° C. After washing with 60% isopropanol, fixed cells were incubated with Oil Red O (Sigma-Aldrich) for 10 minutes. Afterward, excess stain was cleared with 4 washes in distilled water. Finally, the Oil Red O was eluted from the cells with 100% isopropanol, and the amount was quantified by measuring the absorbance of the eluent at 500 nm with a spectrophotometer.
Real time quantitative PCR
Tissue and culture-derived samples were suspended in Trizol as described above. Genomic DNA was sheared using the QIAshredder system (Qiagen, Valencia, CA). RNA was then extracted using an RNeasy Mini Kit (Qiagen), and cDNA conversion was performed using a SuperScript™ First-Strand cDNA synthesis kit (Invitrogen). The cDNA was then used as a template in a TaqMan® real time polymerase chain reaction (qRT-PCR) assay using the ABI Prism 7700 Sequence Detection System (Applied Biosystems, Foster City, CA). All samples were run in triplicate. Proprietary gene expression assay reagents for apelin (catalog number Mm00443562_m1; Applied Biosystems), HIF-1α, (Mm00468875_m1; Applied Biosystems), and 18S ribosomal RNA (4319413E; Applied Biosystems) were used for these experiments.
Threshold cycles were placed in the logarithmic portion of the amplification curve, and each sample was referenced to the 18S RNA amplification to control for the total amount of RNA. Fold difference between two samples (relative quantification) was determined using the delta-delta method [S1/S2 = 2−(T1−T2)], where S1 and S2 represent samples 1 and 2 and T1 and T2 represent the threshold cycles of samples 1 and 2.
Nuclear localization of HIF
3T3L1 preadipocytes were differentiated as described above. Following 18 hours of serum deprivation, cells were incubated in control conditions, insulin, DMOG, or hypoxia for 6 hours as described above. At the conclusion of the experimental exposure, cells were washed and harvested in PBS. The nuclear fraction of each sample was isolated using a nuclear extraction kit (NE-PER; Pierce Biotechnology, Rockford, IL), and the concentration of protein was determined by the BCA method (Sigma-Aldrich). Nuclear extracts were denatured at 98° C for 5 minutes in sample buffer, loaded onto a precast 7.5% polyacrylamide gel (Bio-Rad), and subjected to electrophoresis at 200 V for 40 minutes. The protein was then transferred onto a polyvinylidene fluoride membrane (Hybond-P; Amersham, Piscataway, NJ) at 100 V for 75 minutes and probed with a polyclonal antibody for HIF-1α (Novus Biologicals, Littleton, CO; catalog number NB100−449) according to the vendor's recommendations. Finally, HIF-1α binding was visualized using a horseradish peroxidase-based chemiluminescent system (ECL Plus, Amersham). The relative intensity of each band was quantified by ImageJ software (NIH).
Cellular apelin secretion
3T3L1 and MEF cells were differentiated as described previously. Following 18 hours of serum starvation, cells were subjected to the indicated conditions. After 24 hours of incubation, the conditioned media from each sample were collected. The concentration of apelin in each sample was determined using an enzyme-linked immunosorbent assay (ELISA) directed against apelin-12 (Phoenix Pharmaceuticals, Belmont, CA) and normalized to the total protein concentration as measured by the BCA method.
Statistics
Unless otherwise stated, pairwise comparisons between control and experimental samples were performed using a two-way ANOVA (GB-Stat 10.0, Dynamic Microsystems, Silver Spring, MD). A p value less than 0.05 was considered statistically significant.
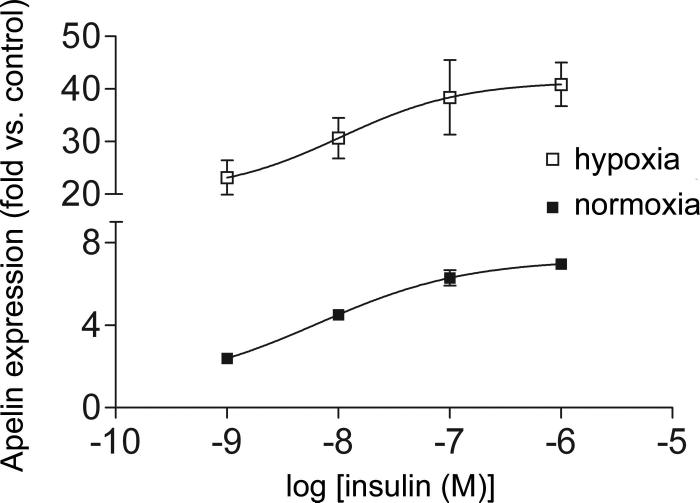
For the dose response curves highlighted in Figure 3, the results were analyzed with Prism 4 software (GraphPad Software, Inc. San Diego, CA). The data were used to fit a nonlinear regression curve with the following equation: Y = Emin + (Emax − Emin) / (1 + 10^((Log[EC50] − X) * HillSlope)) where X represents the insulin concentration, Y represents the mRNA expression of apelin, and Emin and Emax represent the baseline and maximal effects, respectively. Statistical comparison between the curves was accomplished with an F test.
Figure 3.
Dose-response curves for apelin induction in the presence of increasing doses of insulin (lower curve) and insulin plus 1% oxygen (upper curve). Though the EC50s are not different, the addition of hypoxia to insulin significantly increases apelin expression, consistent with an additive effect of the two stimuli. Values represent the mean of 4 separate experiments. Values are ± standard error.
RESULTS
Apelin expression is induced by hypoxia and insulin in cultured adipocytes
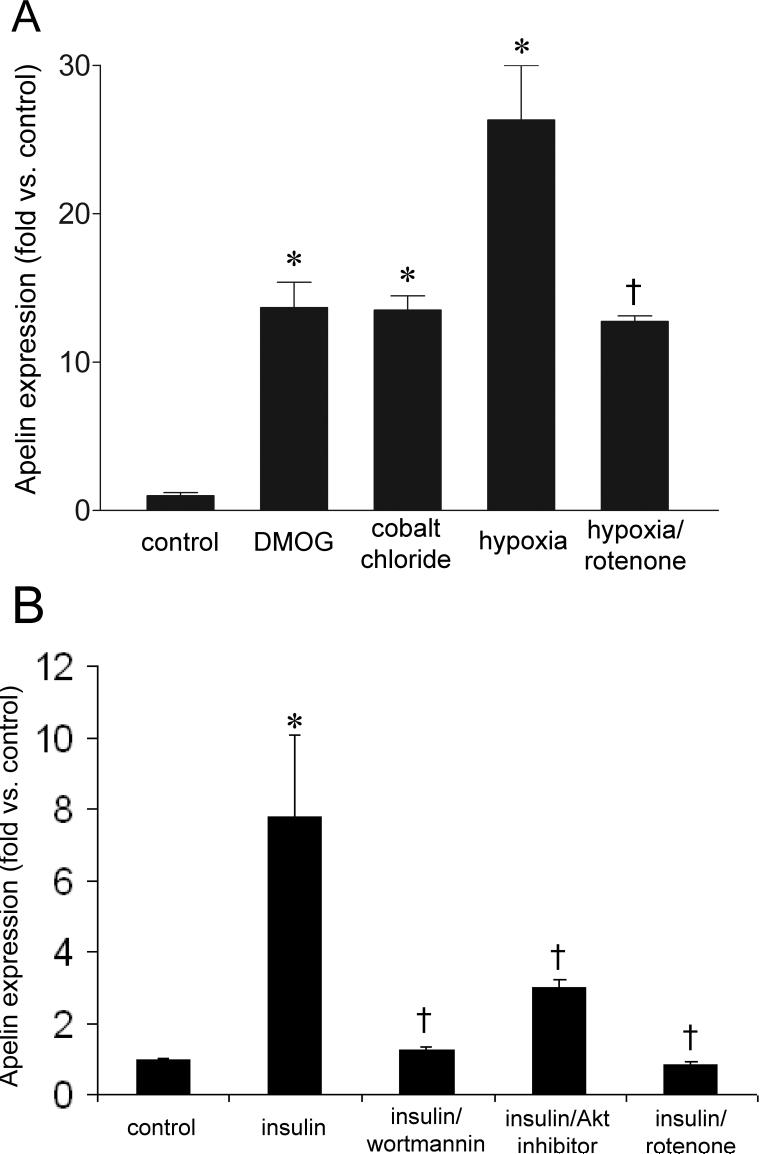
Because apelin is known to be angiogenic (9, 21), and its promoter contains putative HREs (9), we hypothesized that apelin expression was inducible by hypoxic conditions. To test this, we placed 3T3L1 adipocytes in a 1% oxygen environment for 6 hours. As assessed by qRT-PCR, apelin mRNA expression from these samples was increased over 25-fold compared to normoxic control (Figure 1 A). This increase was significantly inhibited by the mitochondrial complex I inhibitor rotenone, a well-described inhibitor of hypoxic HIF-1α stabilization (1, 37). No significant changes compared to control were seen with rotenone alone. Additionally, significant upreguation of apelin was observed when cells were exposed to the HIF-inducers CoCl2 and DMOG, in the absence of hypoxia.
Figure 1.
Apelin mRNA expression in 3T3L1 adipocytes, as assessed by qRT-PCR, after 6 hours of exposure to the listed experimental conditions. (A) Apelin transcription is increased by hypoxia, as well as the HIF-mimetics cobalt chloride and dimethyloxaloylglycine (DMOG). In addition, apelin induction is attenuated by the complex I inhibitor rotenone. (B) Apelin expression is increased in the setting of insulin (100 nM), and this effect is significantly abrograted with the addition of wortmannin, Akt inhibitor IV, and rotenone. Values represent the mean of 4 separate experiments. Values are ± standard error. * p < 0.05 vs. control. † p < 0.05 vs. hypoxia.
Incubation with insulin (100 nM) for 6 hours also increased apelin mRNA expression in 3T3L1 adipocytes nearly 8-fold (Figure 1 B), consistent with prior reports (4). Moreover, this increase was attenuated when the cells were pretreated with inhibitors of PI3K (wortmannin) and Akt (Akt inhibitor IV). Interestingly, incubation with rotenone essentially abolished apelin expression, suggesting the possible involvement of HIF-1 in insulin-induced apelin transcription. No significant changes in expression were observed in the presence of PI3K, Akt, or HIF-1 inhibition alone.
Hypoxia results in significantly increased apelin protein expression
Though increased apelin protein secretion in response to insulin has been observed in cultured adipocytes (4), this finding has not been recapitulated in hypoxia-stimulated cells. We therefore measured apelin secretion using an ELISA for apelin-12. Serum-starved 3T3L1 cells were exposed to 1% oxygen for 24 hours; the conditioned media was then collected for the assay. The amount of apelin in response to hypoxia, normalized to the total amount of protein in the media, was increased over 4-fold compared to normoxic control (Figure 2). Apelin production was also increased by DMOG and CoCl2, though the magnitude of induction was lower compared to hypoxia. Finally, hypoxia-induced apelin protein secretion was significantly attenuated by rotenone.
Figure 2.
Apelin protein expression in 3T3L1 adipocytes after exposure to the listed experimental conditions. Apelin protein production was upregulated by hypoxia, cobalt chloride, and DMOG, while rotenone nearly abolished the hypoxia-mediated increase in apelin expression. Data are normalized to the protein concentration for each sample. N = 4 each group. Values represent the mean of 4 separate experiments. * p < 0.05 vs. control. † p < 0.05 vs. hypoxia.
Taken together, these data indicate that 3T3L1 adipocytes express apelin in response to hypoxia and insulin, and suggest that HIF-1α is involved in apelin induction in these conditions.
The effects of hypoxia and insulin on apelin induction are additive
To determine whether hypoxia and insulin interact with respect to apelin stimulation, we exposed differentiated 3T3L1 cells to varying doses of insulin in the presence or absence of hypoxia for 6 hours (Figure 3). The EC50s for apelin induction were not significantly different with or without hypoxia (6.6 nM [95% CI: 392 pM to 110 nM] vs. 10.5 nM [95% CI: 70.4 pM to 1.58 μM] for insulin alone vs. hypoxia plus insulin, respectively). However, the combined effect of hypoxia and insulin was significantly greater than that of insulin alone. These data thus suggest that, based on the magnitude of effect, the individual influences of hypoxia and insulin are additive.
Hypoxia and insulin increase the nuclear translocation of HIF-1α
To provide evidence of activation of HIF-1 in the setting of hypoxia, we assessed the nuclear translocation of HIF-1α by isolating the nuclear fraction from 3T3L1 adipocytes exposed to hypoxia and DMOG. Compared to control, there was clear evidence of HIF-1α stabilization as determined by nuclear translocation (Figure 4). Similarly, 3T3L1 adipocytes treated with insulin also demonstrated increased nuclear accumulation of the α-subunit, consistent with greater HIF-1 activity in this setting.
Figure 4.
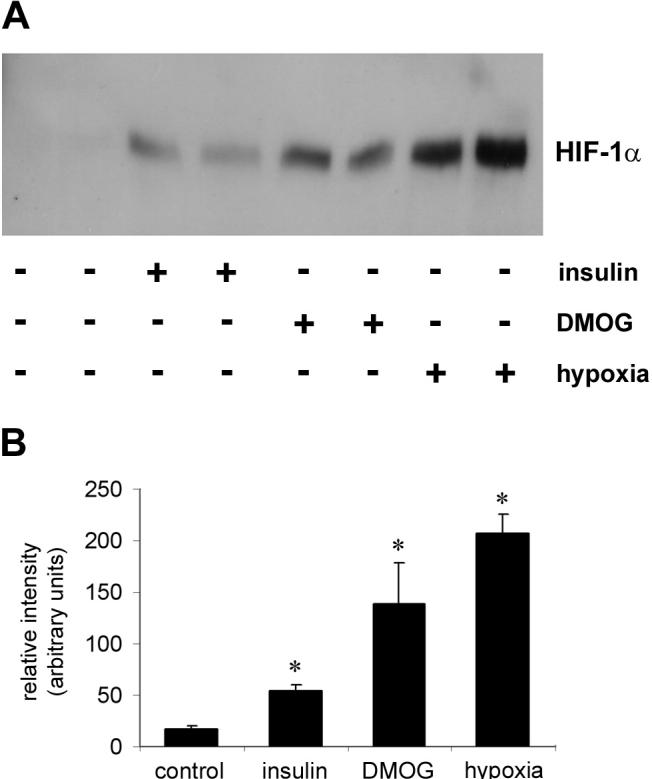
(A) Representative Western blot with a HIF-1α antibody of nuclear isolates from 3T3L1 adipocytes exposed to insulin, DMOG, and hypoxia for 6 hours. Relative to control, all 3 inducers significantly increase HIF-1α activation. (B) Densitometry of gel bands derived from Western blots for HIF-1α subject to the same conditions as in (A). Values represent the mean of 3 separate experiments. Values are ± standard error. * p < 0.05 vs. control.
Hypoxia-inducible factor-1α is involved in the transcriptional response to hypoxia and insulin
Though the data presented above are consistent with a significant role for HIF-1α in apelin expression, they do not provide direct evidence for its involvement. We therefore obtained mouse embryonic fibroblasts (MEFs) with a targeted deletion of the HIF-1α gene (HIF-KO) and differentiated them into adipocytes. Quantitative Oil Red O staining demonstrated that both wild type and HIF-KO MEFs had increased staining compared to undifferentiated controls (500 nm absorbance: 0.240 vs. 0.071 for wild type, 0.146 vs. 0.063 for HIF-KO; p < 0.05 for both comparisons; n = 7 all groups). Additionally, the expression of the adipocyte differentiation markers adiponectin and CEBPβ was increased in both wild type (3.91-fold for adiponectin, 4.43-fold for CEBPβ; p < 0.05; n = 4) and HIF-KO (4.55-fold for adiponectin, 2.81-fold for CEBPβ; p < 0.05; n = 4) MEFs.
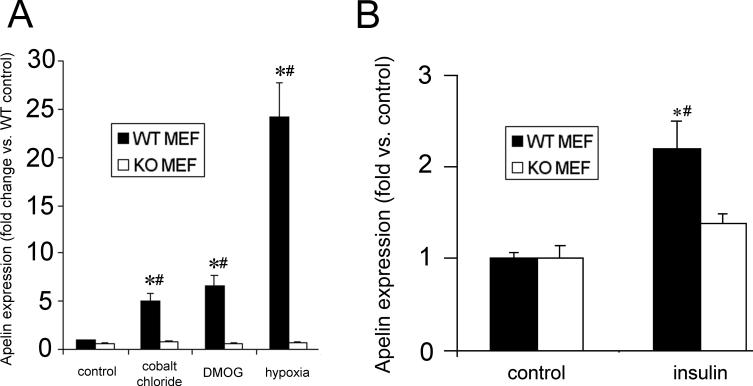
In the wild type MEFs, apelin was significantly upregulated by hypoxia (Figure 5 A). Additionally, apelin transcription in response to the HIF-1α stabilizers CoCl2 and DMOG was increased in the wild type MEFs. However, no induction was observed in response to any of the above stimuli in the HIF-KO cells.
Figure 5.
Apelin gene expression, as measured by qRT-PCR, in wild type (WT) differentiated mouse embryonic fibroblasts (MEF) and MEFs with a targeted deletion of HIF-1α (KO). (A) HIF activation via cobalt chloride, DMOG, and hypoxia all significantly increase apelin expression in WT, but not KO MEFs. (B) Similarly, insulin-induced apelin gene expression in HIF-KO MEFs is significantly attenuated compared to wild type. Values represent the mean of 4 separate experiments. Values are ± standard error. * p < 0.05 vs. control WT. # p < 0.05 vs. matched KO.
Given the evidence of HIF-1α involvement in hypoxia-induced apelin expression, we next sought to determine whether HIF-1α participated in insulin-induced expression as well. Accordingly we incubated serum-starved WT and HIF-KO MEFs with insulin (100 nM) for 6 and 24 hours to evaluate the mRNA and protein expression, respectively, of apelin. In the WT MEFs, a 2-fold increase in mRNA expression occurred after insulin exposure. However, no significant changes were observed in the HIF-KO MEFs (Figure 5 B).
Hypoxia-inducible factor-1α is involved in apelin protein secretion secondary to hypoxia and insulin
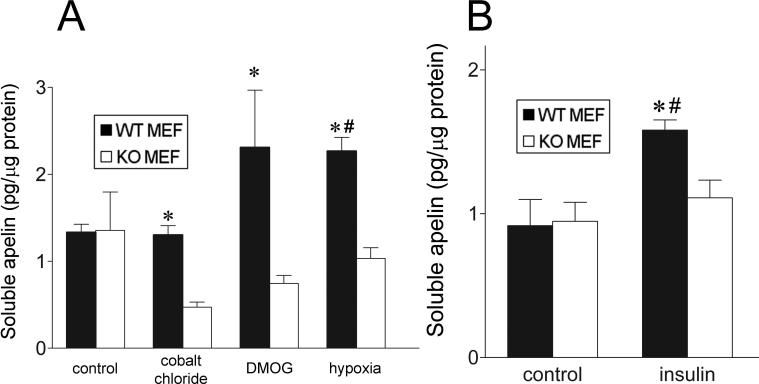
To extend our results beyond the transcriptional level, we exposed differentiated, serum-starved wild type and HIF-KO MEFs to a 1% oxygen environment for 24 hours (Figure 6). We then assessed apelin secretion into the conditioned media using an apelin ELISA. Hypoxia resulted in a significant 70% increase in apelin protein in the media from the wild type MEFs, though a similar increase was not observed in the knockout MEFs (Figure 6 A). Incubation with DMOG also enhanced apelin secretion, though this increase did not quite achieve statistical significance compared to untreated wild type cells (p = 0.096). Interestingly, CoCl2 significantly reduced apelin secretion in HIF-KO MEFs compared to unstimulated control cells. Finally, while insulin significantly increased apelin secretion in the WT MEFs by 72%, no such increase was seen in the knockouts (Figure 6 B).
Figure 6.
Deletion of the HIF-1α gene results in decreased hypoxia- and insulin-stimulated apelin protein expression in MEFs. (A) Hypoxia increases apelin protein production in WT but not in KO cells, as assessed by ELISA. Apelin expression in the presence of cobalt chloride and DMOG are not significantly elevated vs. untreated WT cells, though a significant increase is observed when compared to similarly treated knockout cells. (B) Insulin significantly increases apelin protein secretion in the WT group but not in the KO cells. Values represent the mean of 4 separate experiments. Values are ± standard error. * p < 0.05 vs. matched KO. # p < 0.05 vs. untreated WT.
Overall, the data in Figure 5 and Figure 6 show that ablation of the HIF-1α gene attenuates the increase in apelin expression observed in the setting of diminished oxygen tension and/or insulin administration. These findings therefore strongly suggest that HIF-1α is involved in the signaling events leading to apelin production in response to these stimuli.
DISCUSSION
The data presented here demonstrate that HIF-1 is a mediator of apelin transcription in cultured adipocytes, and appears to participate in insulin induction of the expression of this gene. Hypoxia strongly induced apelin expression in differentiated 3T3-L1 adipocytes, an effect that was duplicated by CoCl2, a prototypic chemical inducer of normoxic HIF-1α stabilization, and DMOG, a well-described inhibitor of prolyl hydroxylase activity. Furthermore, insulin-activated apelin expression was abolished by rotenone, a well-known inhibitor of HIF activation. Extension of these experiments in mouse embryonic fibroblasts containing a targeted deletion of the HIF-1α gene showed clear involvement of HIF in the hypoxic and insulin-mediated induction of apelin.
Our results are complementary to those of a recently published paper reporting hypoxia-induced apelin expression in cultured cardiomyocytes (33). In this study, Ronkainen et al demonstrated that expression of a constitutively active form of HIF-1α increased apelin expression in the absence of other stimuli, whereas expression of IPAS, an inhibitor of HIF-mediated transcription, decreased apelin induction in the setting of hypoxia. Our findings extend these observations by showing that (1) HIF is involved in apelin upregulation in response to insulin, and (2) ablation of HIF-1α diminishes both hypoxia- and insulin-induced apelin expression.
Of note, apelin elaboration in the HIF- KO MEFs was significantly reduced by CoCl2, and to a lesser extent, by DMOG and hypoxia. This effect was not apparent at the transcriptional level. While we are unable to provide a definitive explanation for this phenomenon, it is possible that these treatments may exert an effect on post-translational processing and/or secretion of the apelin peptide. In any event, it remains apparent that none of these stimuli induce apelin expression in cells lacking the HIF-1α gene. Apelin possesses functionality consistent with a response to hypoxia. Recruitment of vasculature to a hypoxic or ischemic region is critical to establishing an environment in which the metabolic demands of that tissue can be met. A number of HIF-inducible factors, most notably VEGF, have been demonstrated to play integral roles in the proliferation, migration, and tube-formation of endothelial cells that is characteristic of angiogenesis (39). Apelin demonstrates similar properties. For example, Masri et al demonstrated that apelin stimulates the proliferation of human umbilical vein endothelial cells in a p70 S6 protein kinase-sensitive fashion (27). Additionally, Kasai et al determined that apelin not only is a mitogenic stimulus to retinal endothelial cells, but also promotes migration and tube formation in these cells (21).
In the in vivo setting, it has been reported that apelin is crucial for the normal development of the Xenopus embryonic vasculature. Anti-sense silencing of either the apelin mRNA transcript, or that of its receptor, APJ, results in absent or disrupted formation of intersegmental vessels (9). Furthermore, implantation of apelin soaked beads produces ectopic vascularization of the implanted area. Finally, apelin-APJ signaling has also been implicated in normal cardiac development of the Xenopus embryo (17). Whether apelin modulates cardiomyocyte development directly, or whether this effect occurs via malformation of the cardiac vasculature remains to be elucidated. Taken together, these studies, coupled with the data presented here, are consistent with a central role for apelin as an effector of HIF mediated angiogenesis. In addition to its angiogenic actions, HIF is known to modulate vascular tone via transactivation of several factors, including endothelial nitric oxide synthase (eNOS), endothelin, and adrenomedullin (8, 11, 16) . Interestingly, apelin is also involved in the regulation of vascular tone, though its precise role is controversial. A decrease in mean arterial blood pressure following apelin administration in anesthetized rats was first demonstrated by Tatemoto et al (43). This effect was abolished by of NOS inhibition, and was accompanied by an increase in plasma nitrate/nitrite, suggesting that apelin modulates eNOS activity. However, other authors have reported that apelin has vasoconstrictive properties in certain circumstances (12, 22). Nevertheless, the predominant systemic effect in vivo appears to be arterial and venous vasodilation (7, 18, 24). Dilatation of collateral vessels is an important means of increasing perfusion to hypoxic regions of the myocardium; thus the vasoactive effects of apelin are also consistent with a response to tissue hypoxia.
Given its identity as an adipokine, the expression of apelin in adipose tissue is induced by insulin in culture (4, 44), in animals (4), and in human subjects (4, 25). Our data demonstrate that insulin induction of apelin is significantly reduced in cells lacking a functioning HIF-1α subunit. Furthermore, we show that insulin stimulates HIF-1α stabilization and nuclear translocation in differentiated 3T3-L1 cells, and that insulin stimulation of apelin in these cells is inhibited by rotenone. These data indicate that insulin stimulates apelin in adipocytes, at least in part, via its ability stabilize HIF-1α and promote HIF-1 transcriptional activity. Further analysis of the apelin promoter is required to fully elucidate the relative contributions of individual HREs to the transcriptional response of apelin to insulin signaling.
Our results suggest a potential novel role for HIF in the insulin-mediated induction of apelin expression in the adipocytes of obese subjects. Obesity is associated with hyperinsulinemia, and HIF-1α expression in adipose tissue has also been shown to be increased in preoperative gastric bypass patients (5). A number of HIF-responsive genes are upregulated in the adipose tissue of this population, including VEGF, PAI-1, leptin, and visfatin (2, 10, 26, 28), and data suggests that apelin exhibits a similar pattern of expression (4). It is therefore possible that insulin mediates the expression of these genes in adipose tissue via a HIF-dependent mechanism.
How, then, do we reconcile the response-to-hypoxia role of HIF-1 with its expression in adipose tissue? HIF-1 is essential for normal vascular development in embryogenesis; targeted deletion of HIF-1α severely restricts vascularization of the developing embryo, resulting in lethality by day E11 (19, 35). Given the absolute requirement for HIF-mediated angiogenesis in embryonic organ development, it is not unreasonable to hypothesize that the same mechanism is involved in adipogenesis throughout the life of the organism. In ontogeny, putative fetal fat depots exist as vascular beds prior to the appearance of fat cells (13). Angiogenesis is also implicated in the maintenance and expansion of adipose tissue in adult animals. Rupnick, et al, demonstrated that in genetically obese db/db mice, weight gain, weight maintenance, and adipose tissue mass are all inhibited by the antiangiogenic agents TNP-490, angiostatin, and endostatin (34). In this regard, the function of HIF as a central mediator of angiogenesis is consistent with a role in adipogenesis. We speculate that, as a pro-angiogenic target of HIF-1, apelin may promote the development of new vasculature to a developing or expanding fat depot, augmenting VEGF and PAI-1 function in this process.
Obesity and its attendant complications represent a burgeoning public health crisis in the Western world. Despite this urgency, very little is known regarding the normal and pathophysiologic molecular processes governing adipose tissue development, expansion, and progression to insulin resistance. Similarly, it is only recently that the endocrine functionalities of this organ have come to be appreciated. It is certainly possible that the expression of apelin, as well as that of other adipokines that demonstrate similar patterns of expression in the adipose tissue of obese individuals, are integral regulators of the molecular events that govern development and expansion of fat depots. It is also conceivable that some of these adipokines may be implicated in the progression from obesity to insulin resistance, either as contributors, mitigating factors, or merely as passive markers. Further examination of the roles of apelin, HIF, and other HIF-mediated adipokines in adipose tissue angiogenesis, as well as preadipocyte proliferation, migration, and differentiation, may provide valuable insight into the regulation and dysregulation of this tissue as it applies to the pathogenesis of obesity and insulin resistance.
Support
This study was supported by funds from a Stanford University Dean's Fellowship Award (PY) and National institutes of Health grants 5F32HL078108 (PY) and R01 HL073084 (PST).
Footnotes
Publisher's Disclaimer: This PDF receipt will only be used as the basis for generating PubMed Central (PMC) documents. PMC documents will be made available for review after conversion (approx. 2−3 weeks time). Any corrections that need to be made will be done at that time. No materials will be released to PMC without the approval of an author. Only the PMC documents will appear on PubMed Central -- this PDF Receipt will not appear on PubMed Central.
Disclosure information
The authors have nothing to disclose.
REFERENCES
- 1.Agani FH, Pichiule P, Chavez JC, LaManna JC. The role of mitochondria in the regulation of hypoxia-inducible factor 1 expression during hypoxia. J Biol Chem. 2000;275:35863–35867. doi: 10.1074/jbc.M005643200. [DOI] [PubMed] [Google Scholar]
- 2.Berndt J, Kloting N, Kralisch S, Kovacs P, Fasshauer M, Schon MR, Stumvoll M, Bluher M. Plasma visfatin concentrations and fat depot-specific mRNA expression in humans. Diabetes. 2005;54:2911–2916. doi: 10.2337/diabetes.54.10.2911. [DOI] [PubMed] [Google Scholar]
- 3.Berry MF, Pirolli TJ, Jayasankar V, Burdick J, Morine KJ, Gardner TJ, Woo YJ. Apelin has in vivo inotropic effects on normal and failing hearts. Circulation. 2004;110:II187–193. doi: 10.1161/01.CIR.0000138382.57325.5c. [DOI] [PubMed] [Google Scholar]
- 4.Boucher J, Masri B, Daviaud D, Gesta S, Guigne C, Mazzucotelli A, Castan-Laurell I, Tack I, Knibiehler B, Carpene C, Audigier Y, Saulnier-Blache JS, Valet P. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology. 2005;146:1764–1771. doi: 10.1210/en.2004-1427. [DOI] [PubMed] [Google Scholar]
- 5.Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, Bouloumie A, Barbatelli G, Cinti S, Svensson PA, Barsh GS, Zucker JD, Basdevant A, Langin D, Clement K. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–2286. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 6.Cariou B, van Harmelen K, Duran-Sandoval D, van Dijk TH, Grefhorst A, Abdelkarim M, Caron S, Torpier G, Fruchart JC, Gonzalez FJ, Kuipers F, Staels B. The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. J Biol Chem. 2006;281:11039–11049. doi: 10.1074/jbc.M510258200. [DOI] [PubMed] [Google Scholar]
- 7.Cheng X, Cheng XS, Pang CC. Venous dilator effect of apelin, an endogenous peptide ligand for the orphan APJ receptor, in conscious rats. Eur J Pharmacol. 2003;470:171–175. doi: 10.1016/s0014-2999(03)01821-1. [DOI] [PubMed] [Google Scholar]
- 8.Coulet F, Nadaud S, Agrapart M, Soubrier F. Identification of hypoxia-response element in the human endothelial nitric-oxide synthase gene promoter. J Biol Chem. 2003;278:46230–46240. doi: 10.1074/jbc.M305420200. [DOI] [PubMed] [Google Scholar]
- 9.Cox CM, D'Agostino SL, Miller MK, Heimark RL, Krieg PA. Apelin, the ligand for the endothelial G-protein-coupled receptor, APJ, is a potent angiogenic factor required for normal vascular development of the frog embryo. Dev Biol. 2006;296:177–189. doi: 10.1016/j.ydbio.2006.04.452. [DOI] [PubMed] [Google Scholar]
- 10.Eriksson P, Reynisdottir S, Lonnqvist F, Stemme V, Hamsten A, Arner P. Adipose tissue secretion of plasminogen activator inhibitor-1 in non-obese and obese individuals. Diabetologia. 1998;41:65–71. doi: 10.1007/s001250050868. [DOI] [PubMed] [Google Scholar]
- 11.Garayoa M, Martinez A, Lee S, Pio R, An WG, Neckers L, Trepel J, Montuenga LM, Ryan H, Johnson R, Gassmann M, Cuttitta F. Hypoxia-inducible factor-1 (HIF-1) up-regulates adrenomedullin expression in human tumor cell lines during oxygen deprivation: a possible promotion mechanism of carcinogenesis. Mol Endocrinol. 2000;14:848–862. doi: 10.1210/mend.14.6.0473. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto T, Kihara M, Ishida J, Imai N, Yoshida S, Toya Y, Fukamizu A, Kitamura H, Umemura S. Apelin stimulates myosin light chain phosphorylation in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2006;26:1267–1272. doi: 10.1161/01.ATV.0000218841.39828.91. [DOI] [PubMed] [Google Scholar]
- 13.Hausman GJ, Richardson RL. Adipose tissue angiogenesis. J Anim Sci. 2004;82:925–934. doi: 10.2527/2004.823925x. [DOI] [PubMed] [Google Scholar]
- 14.Heinonen MV, Purhonen AK, Miettinen P, Paakkonen M, Pirinen E, Alhava E, Akerman K, Herzig KH. Apelin, orexin-A and leptin plasma levels in morbid obesity and effect of gastric banding. Regul Pept. 2005;130:7–13. doi: 10.1016/j.regpep.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Helmlinger G, Yuan F, Dellian M, Jain RK. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat Med. 1997;3:177–182. doi: 10.1038/nm0297-177. [DOI] [PubMed] [Google Scholar]
- 16.Hu J, Discher DJ, Bishopric NH, Webster KA. Hypoxia regulates expression of the endothelin-1 gene through a proximal hypoxia-inducible factor-1 binding site on the antisense strand. Biochem Biophys Res Commun. 1998;245:894–899. doi: 10.1006/bbrc.1998.8543. [DOI] [PubMed] [Google Scholar]
- 17.Inui M, Fukui A, Ito Y, Asashima M. Xapelin and Xmsr are required for cardiovascular development in Xenopus laevis. Dev Biol. 2006;298:188–200. doi: 10.1016/j.ydbio.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 18.Ishida J, Hashimoto T, Hashimoto Y, Nishiwaki S, Iguchi T, Harada S, Sugaya T, Matsuzaki H, Yamamoto R, Shiota N, Okunishi H, Kihara M, Umemura S, Sugiyama F, Yagami K, Kasuya Y, Mochizuki N, Fukamizu A. Regulatory roles for APJ, a seven-transmembrane receptor related to angiotensin-type 1 receptor in blood pressure in vivo. J Biol Chem. 2004;279:26274–26279. doi: 10.1074/jbc.M404149200. [DOI] [PubMed] [Google Scholar]
- 19.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kabon B, Nagele A, Reddy D, Eagon C, Fleshman JW, Sessler DI, Kurz A. Obesity decreases perioperative tissue oxygenation. Anesthesiology. 2004;100:274–280. doi: 10.1097/00000542-200402000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasai A, Shintani N, Oda M, Kakuda M, Hashimoto H, Matsuda T, Hinuma S, Baba A. Apelin is a novel angiogenic factor in retinal endothelial cells. Biochem Biophys Res Commun. 2004;325:395–400. doi: 10.1016/j.bbrc.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 22.Katugampola SD, Maguire JJ, Matthewson SR, Davenport AP. [(125)I]-(Pyr(1))Apelin-13 is a novel radioligand for localizing the APJ orphan receptor in human and rat tissues with evidence for a vasoconstrictor role in man. Br J Pharmacol. 2001;132:1255–1260. doi: 10.1038/sj.bjp.0703939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 24.Lee DK, Cheng R, Nguyen T, Fan T, Kariyawasam AP, Liu Y, Osmond DH, George SR, O'Dowd BF. Characterization of apelin, the ligand for the APJ receptor. J Neurochem. 2000;74:34–41. doi: 10.1046/j.1471-4159.2000.0740034.x. [DOI] [PubMed] [Google Scholar]
- 25.Li L, Yang G, Li Q, Tang Y, Yang M, Yang H, Li K. Changes and relations of circulating visfatin, apelin, and resistin levels in normal, impaired glucose tolerance, and type 2 diabetic subjects. Exp Clin Endocrinol Diabetes. 2006;114:544–548. doi: 10.1055/s-2006-948309. [DOI] [PubMed] [Google Scholar]
- 26.Lonnqvist F, Arner P, Nordfors L, Schalling M. Overexpression of the obese (ob) gene in adipose tissue of human obese subjects. Nat Med. 1995;1:950–953. doi: 10.1038/nm0995-950. [DOI] [PubMed] [Google Scholar]
- 27.Masri B, Morin N, Cornu M, Knibiehler B, Audigier Y. Apelin (65−77) activates p70 S6 kinase and is mitogenic for umbilical endothelial cells. Faseb J. 2004;18:1909–1911. doi: 10.1096/fj.04-1930fje. [DOI] [PubMed] [Google Scholar]
- 28.Miyazawa-Hoshimoto S, Takahashi K, Bujo H, Hashimoto N, Yagui K, Saito Y. Roles of degree of fat deposition and its localization on VEGF expression in adipocytes. Am J Physiol Endocrinol Metab. 2005;288:E1128–1136. doi: 10.1152/ajpendo.00003.2004. [DOI] [PubMed] [Google Scholar]
- 29.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. Jama. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 30.O'Dowd BF, Heiber M, Chan A, Heng HH, Tsui LC, Kennedy JL, Shi X, Petronis A, George SR, Nguyen T. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene. 1993;136:355–360. doi: 10.1016/0378-1119(93)90495-o. [DOI] [PubMed] [Google Scholar]
- 31.Oishi Y, Manabe I, Tobe K, Tsushima K, Shindo T, Fujiu K, Nishimura G, Maemura K, Yamauchi T, Kubota N, Suzuki R, Kitamura T, Akira S, Kadowaki T, Nagai R. Kruppel-like transcription factor KLF5 is a key regulator of adipocyte differentiation. Cell Metab. 2005;1:27–39. doi: 10.1016/j.cmet.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Phan J, Peterfy M, Reue K. Lipin expression preceding peroxisome proliferator-activated receptor-gamma is critical for adipogenesis in vivo and in vitro. J Biol Chem. 2004;279:29558–29564. doi: 10.1074/jbc.M403506200. [DOI] [PubMed] [Google Scholar]
- 33.Ronkainen VP, Ronkainen JJ, Hanninen SL, Leskinen H, Ruas JL, Pereira T, Poellinger L, Vuolteenaho O, Tavi P. Hypoxia inducible factor regulates the cardiac expression and secretion of apelin. Faseb J. 2007;21:1821–1830. doi: 10.1096/fj.06-7294com. [DOI] [PubMed] [Google Scholar]
- 34.Rupnick MA, Panigrahy D, Zhang CY, Dallabrida SM, Lowell BB, Langer R, Folkman MJ. Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci U S A. 2002;99:10730–10735. doi: 10.1073/pnas.162349799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryan HE, Lo J, Johnson RS. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. Embo J. 1998;17:3005–3015. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryan HE, Poloni M, McNulty W, Elson D, Gassmann M, Arbeit JM, Johnson RS. Hypoxia-inducible factor-1alpha is a positive factor in solid tumor growth. Cancer Res. 2000;60:4010–4015. [PubMed] [Google Scholar]
- 37.Schroedl C, McClintock DS, Budinger GR, Chandel NS. Hypoxic but not anoxic stabilization of HIF-1alpha requires mitochondrial reactive oxygen species. Am J Physiol Lung Cell Mol Physiol. 2002;283:L922–931. doi: 10.1152/ajplung.00014.2002. [DOI] [PubMed] [Google Scholar]
- 38.Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88:1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- 39.Semenza GL, Agani F, Iyer N, Kotch L, Laughner E, Leung S, Yu A. Regulation of cardiovascular development and physiology by hypoxia-inducible factor 1. Ann N Y Acad Sci. 1999;874:262–268. doi: 10.1111/j.1749-6632.1999.tb09241.x. [DOI] [PubMed] [Google Scholar]
- 40.Student AK, Hsu RY, Lane MD. Induction of fatty acid synthetase synthesis in differentiating 3T3-L1 preadipocytes. J Biol Chem. 1980;255:4745–4750. [PubMed] [Google Scholar]
- 41.Szokodi I, Tavi P, Foldes G, Voutilainen-Myllyla S, Ilves M, Tokola H, Pikkarainen S, Piuhola J, Rysa J, Toth M, Ruskoaho H. Apelin, the novel endogenous ligand of the orphan receptor APJ, regulates cardiac contractility. Circ Res. 2002;91:434–440. doi: 10.1161/01.res.0000033522.37861.69. [DOI] [PubMed] [Google Scholar]
- 42.Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, Kurokawa T, Onda H, Fujino M. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251:471–476. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- 43.Tatemoto K, Takayama K, Zou MX, Kumaki I, Zhang W, Kumano K, Fujimiya M. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul Pept. 2001;99:87–92. doi: 10.1016/s0167-0115(01)00236-1. [DOI] [PubMed] [Google Scholar]
- 44.Wei L, Hou X, Tatemoto K. Regulation of apelin mRNA expression by insulin and glucocorticoids in mouse 3T3-L1 adipocytes. Regul Pept. 2005;132:27–32. doi: 10.1016/j.regpep.2005.08.003. [DOI] [PubMed] [Google Scholar]