Summary
Objectives
Hypothermia improves outcomes after cardiac arrest (CA), while hyperthermia worsens injury. EEG recovers through periodic bursting from isoelectricity after CA, the duration of which is associated with outcome in normothermia. We quantified burst frequency to study the effect of temperature on early EEG recovery after CA.
Methods
Twenty-four rats were divided into 3 groups, based on 6 hours of hypothermia (T=33°C), normothermia (T=37°C), or hyperthermia (T=39°C) immediately post-resuscitation from 7-minute asphyxial CA. Temperature was maintained using surface cooling and rewarming. Neurological recovery was defined by 72-hour Neurological Deficit Score (NDS).
Results
Burst frequency was higher during the first 90 minutes in rats treated with hypothermia (25.6±12.2/min) and hyperthermia (22.6±8.3/min) compared to normothermia (16.9±8.5/min) (p<0.001). Burst frequency correlated strongly with 72-hour NDS in normothermic rats (p<0.05) but not in hypothermic or hyperthermic rats. The 72-hour NDS of the hypothermia group (74, 61–74; median, 25th–75th percentile) was significantly higher than the normothermia (49, 47–61) and hyperthermia (43, 0–50) groups (p<0.001).
Conclusions
In normothermic rats resuscitated from CA, early EEG burst frequency is strongly associated with neurological recovery. Increased bursting followed by earlier restitution of continuous EEG activity with hypothermia may represent enhanced recovery, while heightened metabolic rate and worsening secondary injury is likely in the hyperthermia group. These factors may confound use of early burst frequency for outcome prediction.
Keywords: Cardiac arrest, Hypothermia, Hyperthermia, EEG, Brain, ischemia, Functional outcome
Introduction
Approximately 166,200 out-of-hospital cardiac arrests (CA) occur in the United States each year1. Of these initial 5–8% out-of-hospital CA survivors, approximately 40,000 patients are admitted to an intensive care unit2, where 80% remain comatose in the immediate post-resuscitative period3. Half of patients survive the hospitalization, but less than half of those recover without significant neurologic deficits2. Among survivors, neurological complications represent the leading cause of disability 4.
Electroencephalography (EEG) is a specific indicator of poor neurological outcome in comatose survivors of CA when characteristic changes occur5. Among potential predictors reviewed by the American Academy of Neurology, presence of burst-suppression on EEG at 24 hours was regarded as a specific predictor for poor functional outcome6. Temperature manipulation critically influences neuropathological outcomes7 with hyperthermia worsening brain injury after ischemia in animal models7, 8 and clinical studies9. Mild hypothermia is recommended for comatose survivors of CA and significantly mitigates brain injury in animal models10 and clinical trials11–13.
Previous studies have shown that EEG recovers from generalized suppression through periodic bursting (burst-suppression) to restitution of continuous EEG patterns after CA under normothermic conditions14, 15. EEG recovery precedes neurological recovery and early burst frequency is associated with functional outcome14–18. In this study, we sought to examine the effect of mild hypothermia and hyperthermia on the evolution of EEG bursting. We hypothesized that EEG burst frequency would be increased by hypothermia and decreased by hyperthermia. In addition, we hypothesized that higher early burst frequency would be strongly associated with good neurological outcomes across the temperature range.
Material and Methods
Twenty- four adult male Wistar rats (300–350g, Charles River, Wilmington, MA) were assigned to 7-minute asphyxial CA and resuscitation with hypothermia (Hypo group), normothermia (Normo group), and hyperthermia (Hyper group) (n=8 per group). The experimental protocol was approved by the Johns Hopkins Animal Care and Use Committee and all procedures were compliant with NIH guidelines. The rats had free access to food and water before and after the experiments and were housed in a quiet environment with 12-hour day-night cycles.
Experimental asphyxia-CA model
This rat model has been previously validated to study multiple aspects of calibrated brain injury after asphyxial CA, including CA physiologic parameters, short-term and long term neurobehavioral outcome, EEG recovery, and histology16, 17, 19–21. In brief, rats were endotracheally intubated and mechanically ventilated at 50 breaths per minute (Harvard Apparatus model 683, South Natick, MA) with 1.0% Halothane in N2/O2 (50%/50%). Ventilation was adjusted to maintain physiologic pH, pO2, and pCO2. Body temperature of 37.0±0.5°C was maintained throughout the experiment, except where noted. Venous and arterial catheters were inserted into the femoral vessels to continuously monitor mean arterial pressure (MAP), intermittently sample arterial blood gas (ABG), and administer fluid and drugs. After 5 minutes of baseline recording, halothane was discontinued for 5 minutes to ensure no significant residual effect on qEEG 22 and vecuronium 2 mg/kg was infused. No sedative or anesthetic agents were subsequently administered throughout the remainder of the experiment to avoid confounding effects on EEG15. CA was initiated via asphyxia with cessation of mechanical ventilation after neuromuscular blockade for a period of 7 minutes. CA was defined by pulse pressure <10 mmHg and asystole. Cardiopulmonary resuscitation (CPR) was performed with resumption of ventilation and oxygenation (100% FIO2), infusion of epinephrine (0.005 mg/kg), NaHCO3 (1 mmol/kg), and sternal chest compressions (200/min) until return of spontaneous circulation (ROSC) (MAP >60 mmHg and pulse waveform). Ventilator adjustments were made to normalize ABG findings. The animals were allowed to recover spontaneously after resuscitation and subsequently extubated along with discontinuation of all invasive catheters.
Immediate temperature manipulation after CA
An intraperitoneal temperature sensor (G2 E-mitter 870-0010-01, Mini Mitter, Oregon, USA) implanted 1 week prior to experiments was used to monitor the core temperature 17. Hypothermia or hyperthermias were induced immediately after ROSC. Hypothermia was achieved through evaporative surface cooling with misted cold water and alcohol aided by an electric fan to achieve the target temperature of 33°C within 15 minutes. The core temperature was maintained between 32–34°C for 6 hours. A warming blanket was used to prevent precipitous temperature decline. Re-warming was initiated after hypothermia was completed and rats were gradually re-warmed from 33.0° to 37.0° C over 2 hours using a warming blanket.
Hyperthermia was achieved using a warming blanket and an automatic warming lamp (Thermalet TH-5, model 6333, Phyritemp, NJ, USA) to the target temperature of 39 °C in 15 minutes and the core temperature was maintained at 38.5–39.5°C for 6 hours. Re-cooling was initiated using surface cooling techniques described above after hyperthermia was complete and rats were gradually re-cooled from 39.0° to 37.0° C over 2 hours.
The normothermia group was maintained at 36.5–37.5°C for 8 hours after ROSC. To ensure that no temperature fluctuation occurred after the resuscitation, such as the spontaneous hypothermia previously reported23, all animals were then kept inside a neonatal incubator (Isolette infant incubator model C-86, Air-shields Inc, Pennsylvania, USA) for the first 24 hours post-ROSC.
EEG recording and burst analysis
Two channels of EEG were recorded using epidural screw electrodes (Plastics One, Roanoke, VA) in the right and left parietal areas starting from baseline throughout the temperature manipulation and recovery periods using DI700 Windaq system17. Serial 30-minute EEG recordings were then performed at 24-, 48-, and 72-hours after ROSC in each group. The signals were digitized using the data acquisition package CODAS (DATAQ Instruments INC., Akron OH). A sampling frequency of 250 Hz and 12 bit A/D conversion were used. Raw EEG was reviewed for movement artifact and signal quality and artifact-ridden epochs were removed prior to analysis.
The raw EEG was visually evaluated from the initial burst period, which was measured from the start of CA to the appearance of the first EEG burst by 2 researchers blinded to experimental group assignments. The first burst was defined by the following criteria: sharply contoured morphology, after-going slow wave, bilateral electrical field, and conspicuity from baseline16, 17. The frequency of bursting was determined by burst number per minute averaged over serial 10-minute periods.
Neurological evaluation
We have previously established and validated a rat model for global ischemic brain injury following CA16, 17, 19, 20, 22 using a standardized Neurological Deficit Scale (NDS) that was adapted from human and animal scales10, 13, 24, 25. The NDS was determined after the temperature manipulation recovery period on the first day (8 hours post-ROSC), and then repeated at 24-, 48-, and 72-hours after ROSC. The NDS measures level of arousal, cranial nerve reflexes, motor function, and simple behavioral responses and has a range of 0–80 (Normal = 80; Brain dead = 0) 17, 20. The standardized NDS examination was performed by a trained examiner blinded to temperature group assignment and the primary outcome measure of this experiment was defined as the 72-hour NDS score.
Statistical methods
Statistical analysis was performed using a standard computerized statistical package (Statistics Program for the Social Sciences version 16.0, Chicago IL). Group values that are parametric (i.e. temperature, ABG results, and burst frequency) are reported as mean±STD and non-parametric variables (i.e. NDS) are reported as median (25th – 75th percentile). Univariate analysis was performed for parametric data with the use of the Student’s t-test for continuous variables, the chi-square test for categorical variables and least significant difference (LSD) analysis used for multiple comparisons. The Multivariate General Linear Model was used for advanced comparison of aggregate data to account for influencing factors such as temperature group. Non-parametric analysis of variance was used to test for differences in rank order NDS as a repeated measure. Pearson’s correlation of bivariate analysis was used to determine the correlation between 72-hour NDS score and serial burst frequency. An alpha level <0.05 was selected to consider the differences significant.
Results
Physiologic data: temperature and ABG monitoring
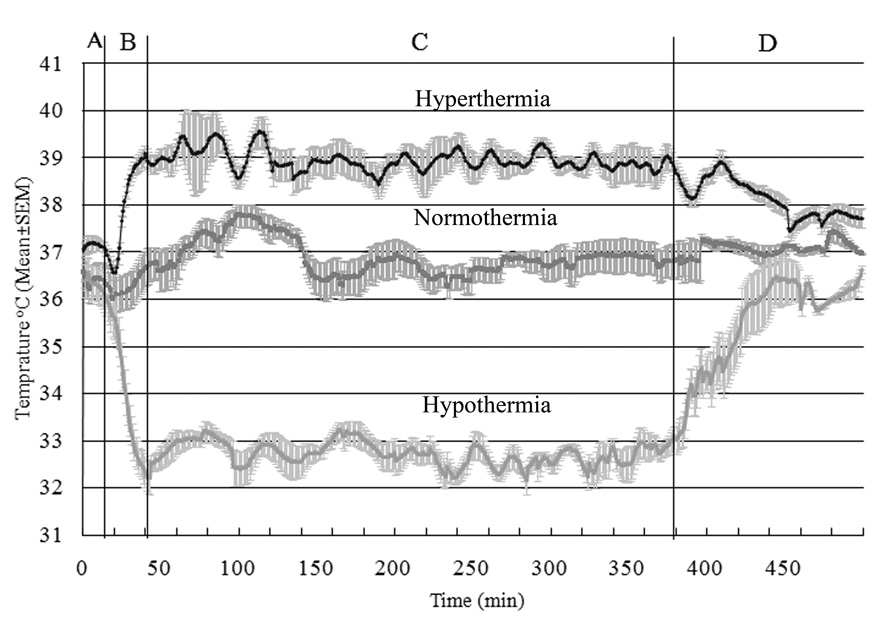
The target temperature was readily achieved and maintained for the defined duration for each of the 3 group, as shown in Figure 1. ABG data at baseline and after CA (10, 20, and 40 minutes after ROSC) including arterial pH, HCO3−, PCO2, PO2 and O2 sat were similar in hypothermic, normothermic and hyperthermic animals.
Figure 1. Temperature recording of 7-minute asphyxial CA rats.
The different cohorts received hyperthermia (upper), normothermia (middle), and hypothermia (bottom). The plots show temperatures during four different phases of the experiment. A: baseline and asphyxial CA period, B: temperature manipulation induction period, C: temperature manipulation maintenance period, and D: temperature manipulation recovery period.
Functional outcome with post-resuscitation temperature manipulation
There was significantly decreased mortality rate (p<0.05) in rats treated with hypothermia (0/8, 0%) compared to the hyperthermia group (4/8, 50%), while the differences between the normothermia (1/8, 12.5%) and other groups were non-significant. All rats who died prior to completion of the 72-hour study were assigned an NDS of 0 on subsequent analyses. There were significant differences (p<0.001) between the 3 temperature groups in median NDS at each measurement point. The hypothermia group had consistently higher NDS scores than normothermia and hyperthermia groups at each time point (Table 1) and in composite measurements during the 72-hour experiment (p<0.001).
Table 1.
NDS Results with post-resuscitation temperature manipulation (Median (25th – 75th percentile))
| Group | 8hour***, ## | 24hour***,#,& | 48hour***,# | 72hour***, ## |
|---|---|---|---|---|
| Hypothermia | 55(51.25–59.5) | 73(68.5–74) | 74(72.5–74) | 74(74–77.25) |
| Normothermia | 46(39.25–49) | 50 (49–69.25) | 54 (49–70.75) | 54.5 (46.75–72.25) |
| Hyperthermia | 43 (0–46) | 45 (0–49) | 43(0–55) | 24.5(0–55) |
Hypothermia/hyperthermia
p<0.001
hypothermia/normothermia
p<0.05
p<0.01
normothermia/hyperthermia
p<0.05
p<0.01
Early burst changes post-resuscitation
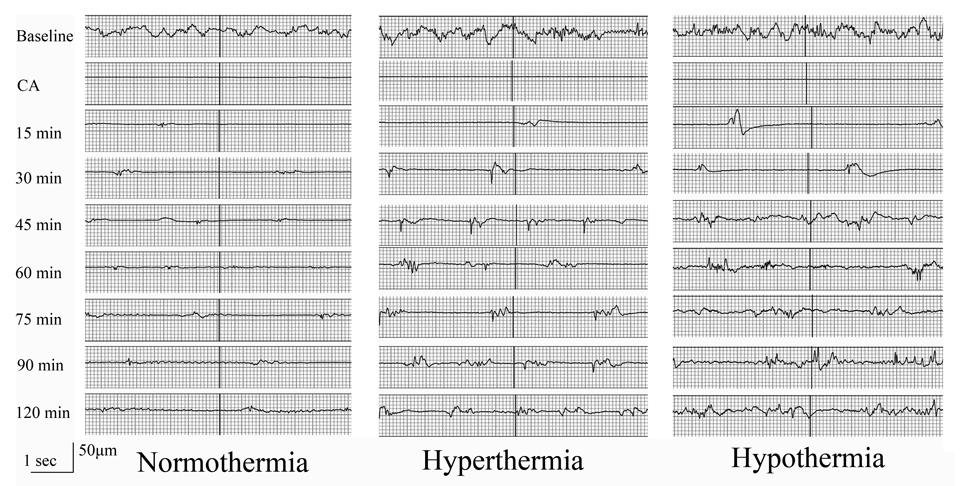
There was higher mean burst frequency during the first 90 minutes post-CA in rats treated with hypothermia (25.6±12.2/min) and hyperthermia (22.6±8.3/min) compared to normothermia (16.9±8.5/min) (p<0.001). Different patterns of burst frequency were noted in each temperature group. Raw EEG data of representative rats showing burst-suppression after CA are shown in Figure 2.
Figure 2. Raw EEG data of representative rats in the 3 temperature groups.
Burst-suppression after CA recovered slower in normothermic rats than hypothermic and hypothermic rats.
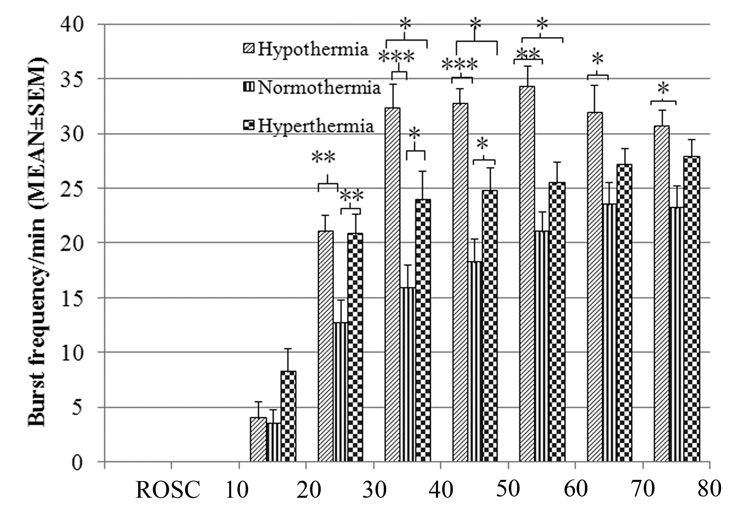
Starting 30 minutes post-resuscitation, the hypothermia group had significantly higher burst frequency than the hyperthermia and normothermia groups, which was maintained throughout the subsequent 1-hour period (p<0.05) (Figure 3). In addition, the hyperthermia group had a significantly higher burst frequency than the normothermia group during the first 60 minutes after resuscitation (Figure 3). After 60 minutes, the burst frequency began to converge in all 3 temperature groups. This convergence occurred due to fusion of bursts into a continuous EEG pattern beginning during this time period which decreased the number of bursts, as previously described20.
Figure 3. Early burst frequency post-resuscitation in the 3 temperature groups.
(*p<0.05, **p<0.01, ***p<0.001) The hypothermia group had a significantly higher burst frequency than both the normothermia and hyperthermia groups between 30 and 60 minutes post-resuscitation.
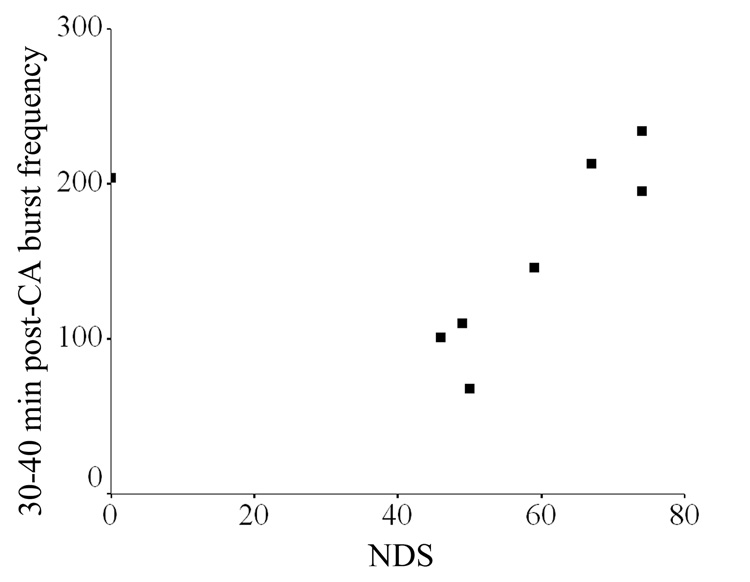
Burst frequency during each 10-minute post-resuscitation period correlated strongly with 72-hour NDS in normothermic rats beginning with 20–30min (Pearson correlation 0.920) and continuing through 30–40min (Pearson correlation 0.930), 40–50min (Pearson correlation 0.885), 50–60min (Pearson correlation 0.812), 60–70min (Pearson correlation 0.61), and 70–80min (Pearson correlation 0.857) (all p<0.05) with the highest correlation noted during the 30–40min period (Pearson correlation 0.930, p=0.002) (Figure 4). No significant correlation between early burst frequency and 72-hour NDS existed in the hypothermic and hyperthermic animals.
Figure 4.
Early burst frequency correlated strongly with 72-hour NDS in normothermic rats (p<0.01)
Most animals had resolution of burst-suppression and restitution of continuous EEG activity at 90 minutes post-resuscitation. Restitution of continuous EEG activity occurred significantly earlier in the hypothermia group compared to the normothermia and hyperthermia groups (p=0.025).
No significant differences were noted in the interval (minutes) between CA and the first burst among the hyperthermic (18.4±2.4), normothermic (18.0±1.9) and hypothermic (16.2±2.4) animals (p=0.143).
Discussion
This study demonstrates that temperature influences early EEG bursting after resuscitation from CA. In normothermic rats, EEG burst frequency in the first hour after resuscitation was strongly associated with 72-hour NDS, which reaffirms previous observations in animals 14 and humans26. Because hyperthermia is known to increase brain injury after CA, we hypothesized that early burst frequency – a marker of neurological outcome – would be lower in the hyperthermia group. Surprisingly, we found that burst frequency was increased during this period by both hypothermia and hyperthermia, despite the predicted opposite effects on outcome. Additionally, burst frequency was not predictive of outcome in either hypothermic or hyperthermic rats. As predicted, hypothermic rats had increased burst frequency, earlier restitution of continuous EEG activity, and better neurological outcomes. With hyperthermia, however, we found a paradoxical increase in burst frequency during the first hour but later restitution of continuous EEG activity and an increase in mortality and poor neurological outcomes. Use of early burst frequency as a marker of neurological recovery outside the normal physiological range may, therefore, lead to inaccurate outcome prediction.
Burst-suppression is a highly stereotyped pattern of EEG activity that is characterized by spontaneous alternation of periodic high amplitude, low frequency bursts followed by intervals of generalized EEG suppression27. This pattern of activity is frequently seen among comatose survivors of CA28, 29. The presence of persistent burst-suppression >24 hours after resuscitation from CA has high specificity for poor neurological outcomes (death or persistent vegetative state)30, 31, as was recently highlighted by an American Academy of Neurology consensus statement on prognostication after CA6. Despite its prognostic infamy, burst-suppression is part of the natural evolution of EEG activity after CA, representing an intermediate stage between generalized suppression and continuous activity32, 33. This finding has been demonstrated in humans32 as well as in dog34, monkey35, rat36, and pig25 models of CA.
We have previously demonstrated that a shorter duration of EEG burst-suppression and higher burst frequency soon after ROSC is strongly associated with good neurological recovery in normothermic rats14, 15, 20 and pigs25. The human translation of this finding is implicit in the conclusion that the presence of burst-suppression has excellent specificity for poor outcome after 24 hours but lower accuracy at earlier timepoints6, 37, 38.
Over the past decade, an increasing amount of clinical and basic research has focused on manipulation of temperature to improve or worsen neurological outcomes and neuronal injury after global ischemia. This body of literature has consistently shown worsening neuronal injury with spontaneous or induced hyperthermia9 and a neuroprotective effect of mild hypothermia11, 12. The interaction between temperature and neuronal activity is complex and non-linear. Brain slice and in vivo preparations have consistently shown a transient increase in neuronal firing within the range of mild hypothermia (30–34°C) and a relatively linear decrease in activity below this range39–41. Similarly, mild hyperthermia transiently increases evoked and spontaneous neuronal spikes39, 41.
The current experiment was designed to explore the effects of different temperature ranges on the evolution of EEG bursting after CA. Based on the correlation between outcome and burst frequency in this setting and the known protective effect of hypothermia and injurious effect of hyperthermia, we anticipated that early burst frequency would decrease with increasing temperature. As described above, however, a non-linear relationship between temperature and burst frequency was demonstrated with higher frequency in both the hyperthermia and hypothermia groups compared to the normothermic animals. The reason for this nonlinear relationship is not known, but these results closely parallel the relationship between temperature and spontaneous neuronal activity described above. Increased burst frequency in the hyperthermia group may reflect an overall increase in the spontaneous neuronal depolarization rate, lower threshold for depolarization, and higher basal metabolic rate associated with increased temperature. On the other hand, early burst frequency may be an inaccurate measure of outcome because the detrimental effects of hyperthermia on neuronal injury are delayed beyond this period which presumably due to overheightened metabolic rate7–9.
As previously demonstrated in normothermic rats after CA14, 15, 20, earlier EEG recovery was associated with significant improvement in NDS. Burst frequency in the first 90 minutes after resuscitation was strongly associated with neurological outcome. This strong linear relationship between early burst frequency and 72-hour NDS, however, was not present in the hypothermic and hyperthermic groups. The reason for this finding most likely relates to confounding influences of temperature on burst frequency. These findings may invalidate use of early burst frequency as an outcome predictor among CA survivors treated with induced hypothermia and in those with spontaneous hyperthemia. Simple burst counting methods, however, may not account for alterations in burst complexity caused by temperature manipulation and quantitative EEG methods may be required to capture the discriminative information contained within EEG signal outside the normal physiological temperature range.
Limitations of this study include a small number of animals. This limitation was especially evident in the hyperthermia group, in which 3/7 animals did not survive to the conclusion of the 72-hour experiment. The small number of animals and the assignment of an NDS score of 0 for non-survivors may have influenced the predictive accuracy of burst counting in the hyperthermia group. In addition, the major limitation of burst counting is that it does not account for the burst-suppression ratio and burst duration or complexity, which may contain discriminative information.
Conclusions
In conclusion, in normothermic rats resuscitated from CA, early EEG burst frequency is strongly associated with neurological recovery. Increased bursting followed with earlier restitution of continuous EEG activity in hypothermia group may represent enhanced recovery, while heightened metabolic rate and worsening secondary injury is likely in the hyperthermia group. These factors may confound use of early burst frequency for outcome prediction.
Supplementary Material
Acknowledgments
This research was supported by NIH Grants R01 HL071568 and R21 NS054146.
Footnotes
Conflict of interest statement
There are no conflicts of interest in this study.
Portions of this work were previously oral presented in 5th Annual Meeting of the Neurocritical Care Society in Las Vegas, NV (November 2007).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Rosamond W, Flegal K, Furie K, et al. Heart Disease and Stroke Statistics 2008 Update. A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007 doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Eisenberg MS, Mengert TJ. Cardiac resuscitation. N Engl J Med. 2001;344:1304–1313. doi: 10.1056/NEJM200104263441707. [DOI] [PubMed] [Google Scholar]
- 3.Safar P. Cerebral resuscitation after cardiac arrest: a review. Circulation. 1986;74:IV138–IV153. [PubMed] [Google Scholar]
- 4.Vaagenes P, Ginsberg M, Ebmeyer U, et al. Cerebral resuscitation from cardiac arrest: pathophysiologic mechanisms. Crit Care Med. 1996;24:S57–S68. [PubMed] [Google Scholar]
- 5.Jorgensen EO, Malchow-Moller A. Natural history of global and critical brain ischaemia. Part II: EEG and neurological signs in patients remaining unconscious after cardiopulmonary resuscitation. Resuscitation. 1981;9:155–174. doi: 10.1016/0300-9572(81)90024-1. [DOI] [PubMed] [Google Scholar]
- 6.Wijdicks EF, Hijdra A, Young GB, Bassetti CL, Wiebe S. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;67:203–210. doi: 10.1212/01.wnl.0000227183.21314.cd. [DOI] [PubMed] [Google Scholar]
- 7.Kim Y, Busto R, Dietrich WD, Kraydieh S, Ginsberg MD. Delayed postischemic hyperthermia in awake rats worsens the histopathological outcome of transient focal cerebral ischemia. Stroke; a journal of cerebral circulation. 1996;27:2274–2280. doi: 10.1161/01.str.27.12.2274. discussion 2281. [DOI] [PubMed] [Google Scholar]
- 8.Hickey RW, Kochanek PM, Ferimer H, Alexander HL, Garman RH, Graham SH. Induced hyperthermia exacerbates neurologic neuronal histologic damage after asphyxial cardiac arrest in rats. Critical care medicine. 2003;31:531–535. doi: 10.1097/01.CCM.0000050323.84293.11. [DOI] [PubMed] [Google Scholar]
- 9.Zeiner A, Holzer M, Sterz F, et al. Hyperthermia after cardiac arrest is associated with an unfavorable neurologic outcome. Arch Intern Med. 2001;161:2007–2012. doi: 10.1001/archinte.161.16.2007. [DOI] [PubMed] [Google Scholar]
- 10.Xiao F, Safar P, Radovsky A. Mild protective and resuscitative hypothermia for asphyxial cardiac arrest in rats. Am J Emerg Med. 1998;16:17–25. doi: 10.1016/s0735-6757(98)90059-6. [DOI] [PubMed] [Google Scholar]
- 11.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 12.Hypothermia After Cardiac Arrest (HACA) Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 13.Zeiner A, Holzer M, Sterz F, et al. Mild resuscitative hypothermia to improve neurological outcome after cardiac arrest. A clinical feasibility trial. Hypothermia After Cardiac Arrest (HACA) Study Group. Stroke. 2000;31:86–94. doi: 10.1161/01.str.31.1.86. [DOI] [PubMed] [Google Scholar]
- 14.Geocadin RG, Sherman DL, Christian Hansen H, et al. Neurological recovery by EEG bursting after resuscitation from cardiac arrest in rats. Resuscitation. 2002;55:193–200. doi: 10.1016/s0300-9572(02)00196-x. [DOI] [PubMed] [Google Scholar]
- 15.Luft AR, Buitrago MM, Paul JS, et al. Early restitution of electrocorticogram predicts subsequent behavioral recovery from cardiac arrest. J Clin Neurophysiol. 2002;19:540–546. doi: 10.1097/00004691-200212000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Jia X, Koenig MA, Shin HC, et al. Improving neurological outcomes post-cardiac arrest in a rat model: Immediate hypothermia and quantitative EEG monitoring. Resuscitation. 2008;76:431–442. doi: 10.1016/j.resuscitation.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jia X, Koenig MA, Shin HC, et al. Quantitative EEG and neurological recovery with therapeutic hypothermia after asphyxial cardiac arrest in rats. Brain Res. 2006;1111:166–175. doi: 10.1016/j.brainres.2006.04.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin HC, Tong S, Yamashita S, Jia X, Geocadin RG, Thakor NV. Quantitative EEG and effect of hypothermia on brain recovery after cardiac arrest. IEEE Trans Biomed Eng. 2006;53:1016–1023. doi: 10.1109/TBME.2006.873394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jia X, Koenig MA, Nickl R, Zhen G, Thakor NV, Geocadin RG. Early Electrophysiologic Markers Predict Functional Outcome Associated with Temperature Manipulation after Cardiac Arrest in Rats. Critical Care Medicine. 2008 doi: 10.1097/CCM.0b013e3181760eb5. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geocadin RG, Muthuswamy J, Sherman DL, Thakor NV, Hanley DF. Early electrophysiological and histologic changes after global cerebral ischemia in rats. Mov Disord. 2000;15 Suppl 1:14–21. doi: 10.1002/mds.870150704. [DOI] [PubMed] [Google Scholar]
- 21.Schreckinger M, Geocadin RG, Savonenko A, et al. Long-lasting cognitive injury in rats with apparent full gross neurological recovery after short-term cardiac arrest. Resuscitation. 2007;75:105–113. doi: 10.1016/j.resuscitation.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 22.Geocadin RG, Ghodadra R, Kimura T, et al. A novel quantitative EEG injury measure of global cerebral ischemia. Clin Neurophysiol. 2000;111:1779–1787. doi: 10.1016/s1388-2457(00)00379-5. [DOI] [PubMed] [Google Scholar]
- 23.Hickey RW, Kochanek PM, Ferimer H, Alexander HL, Garman RH, Graham SH. Induced hyperthermia exacerbates neurologic neuronal histologic damage after asphyxial cardiac arrest in rats. Crit Care Med. 2003;31:531–535. doi: 10.1097/01.CCM.0000050323.84293.11. [DOI] [PubMed] [Google Scholar]
- 24.Katz L, Ebmeyer U, Safar P, Radovsky A, Neumar R. Outcome model of asphyxial cardiac arrest in rats. J Cereb Blood Flow Metab. 1995;15:1032–1039. doi: 10.1038/jcbfm.1995.129. [DOI] [PubMed] [Google Scholar]
- 25.Sherman DL, Brambrink AM, Ichord RN, et al. Quantitative EEG during early recovery from hypoxic-ischemic injury in immature piglets: burst occurrence and duration. Clin Electroencephalogr. 1999;30:175–183. doi: 10.1177/155005949903000410. [DOI] [PubMed] [Google Scholar]
- 26.Jorgensen EO, Holm S. The natural course of neurological recovery following cardiopulmonary resuscitation. Resuscitation. 1998;36:111–122. doi: 10.1016/s0300-9572(97)00094-4. [DOI] [PubMed] [Google Scholar]
- 27.Niedermeyer E, Sherman DL, Geocadin RJ, Hansen HC, Hanley DF. The burst-suppression electroencephalogram. Clin Electroencephalogr. 1999;30:99–105. doi: 10.1177/155005949903000305. [DOI] [PubMed] [Google Scholar]
- 28.Chen R, Bolton CF, Young B. Prediction of outcome in patients with anoxic coma: a clinical and electrophysiologic study. Crit Care Med. 1996;24:672–678. doi: 10.1097/00003246-199604000-00020. [DOI] [PubMed] [Google Scholar]
- 29.Scollo-Lavizzari G, Bassetti C. Prognostic value of EEG in post-anoxic coma after cardiac arrest. Eur Neurol. 1987;26:161–170. doi: 10.1159/000116329. [DOI] [PubMed] [Google Scholar]
- 30.Young GB. The EEG in coma. J Clin Neurophysiol. 2000;17:473–485. doi: 10.1097/00004691-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Pampiglione G. Electroencephalographic studies after cardiorespiratory resuscitation. Proc R Soc Med. 1962;55:653–657. [PMC free article] [PubMed] [Google Scholar]
- 32.Jorgensen EO, Malchow-Moller A. Natural history of global and critical brain ischaemia. Part I: EEG and neurological signs during the first year after cardiopulmonary resuscitation in patients subsequently regaining consciousness. Resuscitation. 1981;9:133–153. doi: 10.1016/0300-9572(81)90023-x. [DOI] [PubMed] [Google Scholar]
- 33.Koenig MA, Kaplan PW, Thakor NV. Clinical neurophysiologic monitoring and brain injury from cardiac arrest. Neurol Clin. 2006;24:89–106. doi: 10.1016/j.ncl.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 34.Gurvitch AM, Mutuskina EA, Novoderzhkina IS. Quantitative evaluation of brain damage in dogs resulting from circulatory arrest to the central nervous system or the whole animal. 2. Electroencephalographic evaluation during early recovery of the gravity and reversibility of post-ischaemic cerebral damage. Resuscitation. 1972;1:219–228. doi: 10.1016/0300-9572(72)90051-2. [DOI] [PubMed] [Google Scholar]
- 35.Myers RE, Yamaguchi S. Nervous system effects of cardiac arrest in monkeys. Preservation of vision. Arch Neurol. 1977;34:65–74. doi: 10.1001/archneur.1977.00500140019003. [DOI] [PubMed] [Google Scholar]
- 36.Radovsky A, Katz L, Ebmeyer U, Safar P. Ischemic neurons in rat brains after 6, 8, or 10 minutes of transient hypoxic ischemia. Toxicol Pathol. 1997;25:500–505. doi: 10.1177/019262339702500512. [DOI] [PubMed] [Google Scholar]
- 37.Moss J, Rockoff M. EEG monitoring during cardiac arrest and resuscitation. Jama. 1980;244:2750–2751. [PubMed] [Google Scholar]
- 38.Losasso TJ, Muzzi DA, Meyer FB, Sharbrough FW. Electroencephalographic monitoring of cerebral function during asystole and successful cardiopulmonary resuscitation. Anesth Analg. 1992;75:1021–1024. doi: 10.1213/00000539-199212000-00025. [DOI] [PubMed] [Google Scholar]
- 39.Buldakova S, Dutova E, Ivlev S, Weiss M. Temperature change-induced potentiation: a comparative study of facilitatory mechanisms in aged and young rat hippocampal slices. Neuroscience. 1995;68:395–397. doi: 10.1016/0306-4522(95)00135-6. [DOI] [PubMed] [Google Scholar]
- 40.Volgushev M, Vidyasagar TR, Chistiakova M, Eysel UT. Synaptic transmission in the neocortex during reversible cooling. Neuroscience. 2000;98:9–22. doi: 10.1016/s0306-4522(00)00109-3. [DOI] [PubMed] [Google Scholar]
- 41.Shen KF, Schwartzkroin PA. Effects of temperature alterations on population and cellular activities in hippocampal slices from mature and immature rabbit. Brain Res. 1988;475:305–316. doi: 10.1016/0006-8993(88)90619-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.