Abstract
Colonization of the respiratory tract with Pseudomonas aeruginosa is a serious problem in cystic fibrosis and seriously ill hospitalized patients. Human tracheobronchial mucin (HTBM), the major glycoprotein of human tracheobronchial secretions, is known to interact with this pathogen, which may then be cleared by mucociliary action. However, the mechanism of interaction is not known. To understand this process, pure HTBM was isolated from tracheobronchial secretions of a laryngectomee. Following initial fractionation on Sepharose CL-2B, the HTBM-containing fraction was subjected to reductive methylation and then gel filtration. Pure HTBM was employed in an overlay binding assay to identify the bacterial adhesin(s) and mucin receptors that participate in mucin-P. aeruginosa interactions. An approximately 16-kDa nonpilus protein component(s) of P. aeruginosa was found to be the adhesin(s) for HTBM. The mucin receptor for the 16-kDa component(s) was found in the peptide moiety. This study confirms that P. aeruginosa utilizes the nonpilus adhesin(s) to bind to HTBM. Identification of the specificity of the HTBM-P. aeruginosa interactions can lead to a better understanding of the predominance of P. aeruginosa colonization in individuals with cystic fibrosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker N., Hansson G. C., Leffler H., Riise G., Svanborg-Edén C. Glycosphingolipid receptors for Pseudomonas aeruginosa. Infect Immun. 1990 Jul;58(7):2361–2366. doi: 10.1128/iai.58.7.2361-2366.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar K. R., Reid L. Application of density gradient methods for the study of mucus glycoprotein and other macromolecular components of the sol and gel phases of asthmatic sputa. J Biol Chem. 1981 Jul 25;256(14):7583–7589. [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Byrd J. C., Lamport D. T., Siddiqui B., Kuan S. F., Erickson R., Itzkowitz S. H., Kim Y. S. Deglycosylation of mucin from LS174T colon cancer cells by hydrogen fluoride treatment. Biochem J. 1989 Jul 15;261(2):617–625. doi: 10.1042/bj2610617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doig P., Todd T., Sastry P. A., Lee K. K., Hodges R. S., Paranchych W., Irvin R. T. Role of pili in adhesion of Pseudomonas aeruginosa to human respiratory epithelial cells. Infect Immun. 1988 Jun;56(6):1641–1646. doi: 10.1128/iai.56.6.1641-1646.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
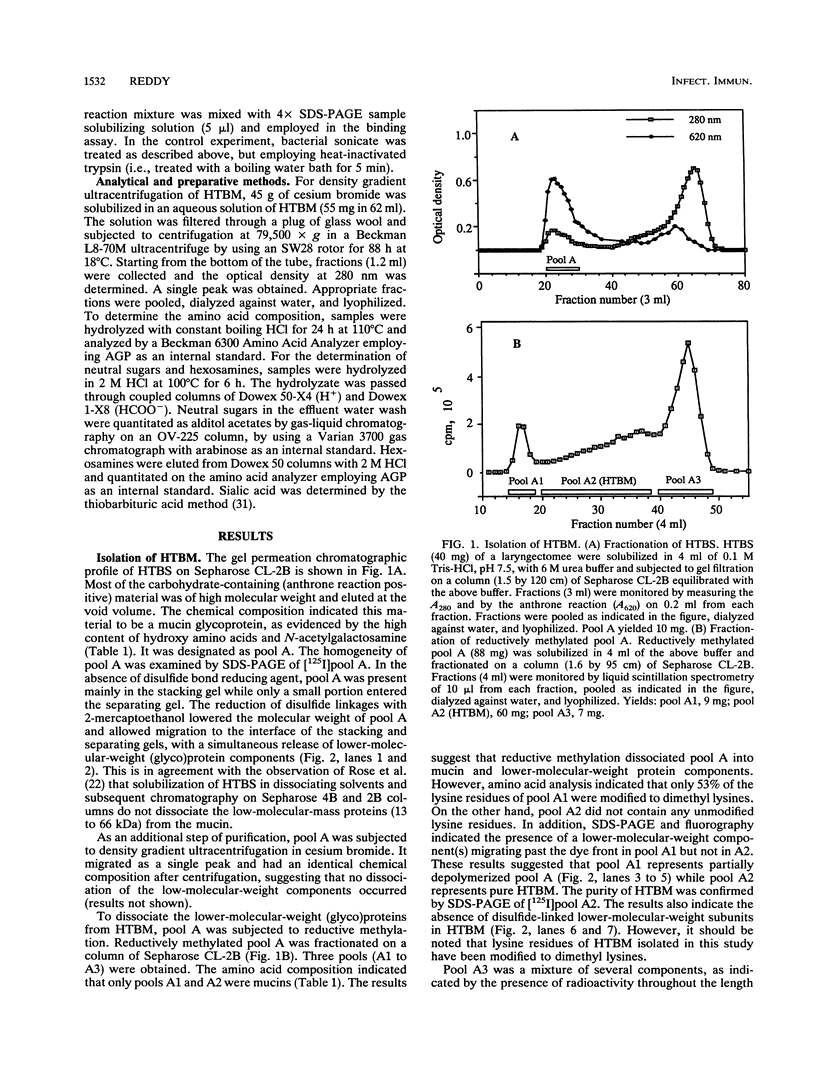
- Gupta R., Jentoft N., Jamieson A. M., Blackwell J. Structural analysis of purified human tracheobronchial mucins. Biopolymers. 1990 Feb 5;29(2):347–355. doi: 10.1002/bip.360290207. [DOI] [PubMed] [Google Scholar]
- Jentoft N., Dearborn D. G. Labeling of proteins by reductive methylation using sodium cyanoborohydride. J Biol Chem. 1979 Jun 10;254(11):4359–4365. [PubMed] [Google Scholar]
- Krivan H. C., Roberts D. D., Ginsburg V. Many pulmonary pathogenic bacteria bind specifically to the carbohydrate sequence GalNAc beta 1-4Gal found in some glycolipids. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6157–6161. doi: 10.1073/pnas.85.16.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Naziruddin B., Shankar V., Reyes de la Rocha S., Sachdev G. P. Polymeric structure of human respiratory mucin: studies on two protein components released upon reduction of disulfide bonds. Biochim Biophys Acta. 1990 Nov 15;1041(2):164–171. doi: 10.1016/0167-4838(90)90061-j. [DOI] [PubMed] [Google Scholar]
- Ramasubbu N., Reddy M. S., Bergey E. J., Haraszthy G. G., Soni S. D., Levine M. J. Large-scale purification and characterization of the major phosphoproteins and mucins of human submandibular-sublingual saliva. Biochem J. 1991 Dec 1;280(Pt 2):341–352. doi: 10.1042/bj2800341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., Carnoy C., Fievre S., Michalski J. C., Houdret N., Lamblin G., Strecker G., Roussel P. Pseudomonas aeruginosa recognizes carbohydrate chains containing type 1 (Gal beta 1-3GlcNAc) or type 2 (Gal beta 1-4GlcNAc) disaccharide units. Infect Immun. 1991 Feb;59(2):700–704. doi: 10.1128/iai.59.2.700-704.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
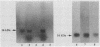
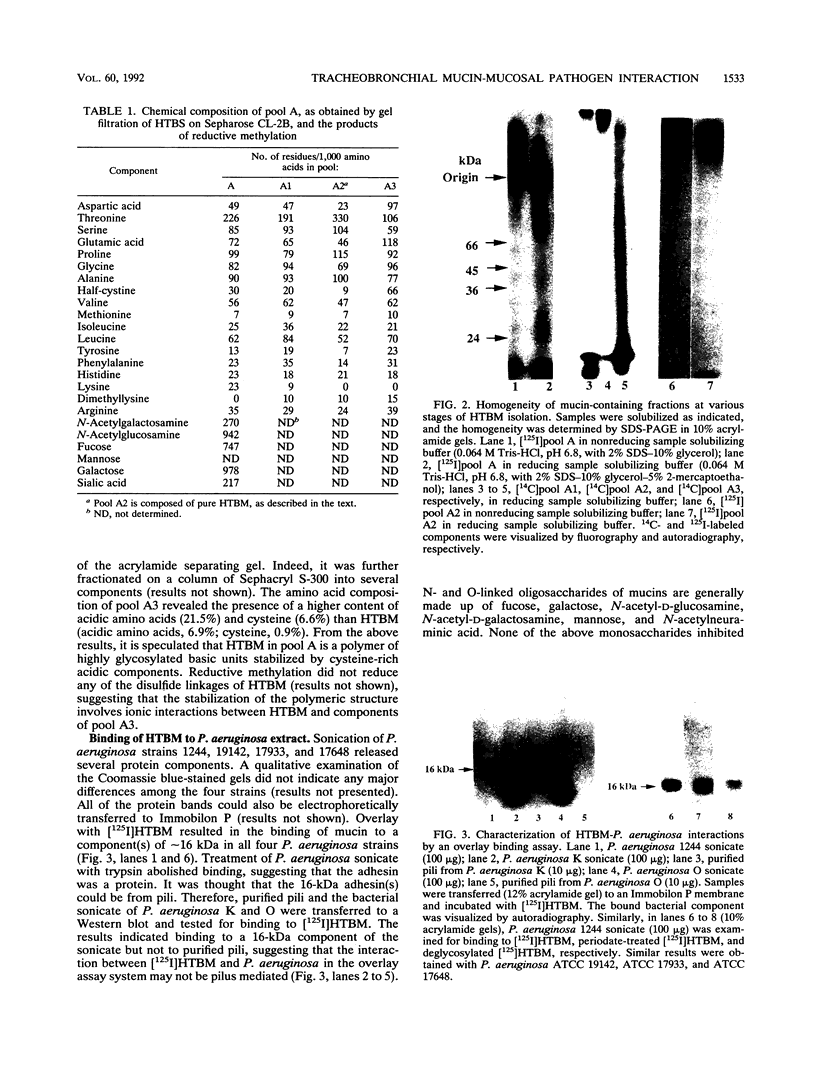
- Ramphal R., Guay C., Pier G. B. Pseudomonas aeruginosa adhesins for tracheobronchial mucin. Infect Immun. 1987 Mar;55(3):600–603. doi: 10.1128/iai.55.3.600-603.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., Houdret N., Koo L., Lamblin G., Roussel P. Differences in adhesion of Pseudomonas aeruginosa to mucin glycopeptides from sputa of patients with cystic fibrosis and chronic bronchitis. Infect Immun. 1989 Oct;57(10):3066–3071. doi: 10.1128/iai.57.10.3066-3071.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., Koo L., Ishimoto K. S., Totten P. A., Lara J. C., Lory S. Adhesion of Pseudomonas aeruginosa pilin-deficient mutants to mucin. Infect Immun. 1991 Apr;59(4):1307–1311. doi: 10.1128/iai.59.4.1307-1311.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., Pyle M. Evidence for mucins and sialic acid as receptors for Pseudomonas aeruginosa in the lower respiratory tract. Infect Immun. 1983 Jul;41(1):339–344. doi: 10.1128/iai.41.1.339-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., Sadoff J. C., Pyle M., Silipigni J. D. Role of pili in the adherence of Pseudomonas aeruginosa to injured tracheal epithelium. Infect Immun. 1984 Apr;44(1):38–40. doi: 10.1128/iai.44.1.38-40.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
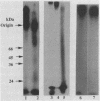
- Ringler N. J., Selvakumar R., Woodward H. D., Bhavanandan V. P., Davidson E. A. Protein components of human tracheobronchial mucin: partial characterization of a closely associated 65-kilodalton protein. Biochemistry. 1988 Oct 18;27(21):8056–8063. doi: 10.1021/bi00421a013. [DOI] [PubMed] [Google Scholar]
- Roberton A. M., Mantle M., Fahim R. E., Specian R. D., Bennick A., Kawagishi S., Sherman P., Forstner J. F. The putative 'link' glycopeptide associated with mucus glycoproteins. Composition and properties of preparations from the gastrointestinal tracts of several mammals. Biochem J. 1989 Jul 15;261(2):637–647. doi: 10.1042/bj2610637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. C., Lynn W. S., Kaufman B. Resolution of the major components of human lung mucosal gel and their capabilities for reaggregation and gel formation. Biochemistry. 1979 Sep 4;18(18):4030–4037. doi: 10.1021/bi00585a029. [DOI] [PubMed] [Google Scholar]
- Roussel P., Lamblin G., Lhermitte M., Houdret N., Lafitte J. J., Perini J. M., Klein A., Scharfman A. The complexity of mucins. Biochimie. 1988 Nov;70(11):1471–1482. doi: 10.1016/0300-9084(88)90284-2. [DOI] [PubMed] [Google Scholar]
- Sajjan S. U., Forstner J. F. Role of the putative "link" glycopeptide of intestinal mucin in binding of piliated Escherichia coli serotype O157:H7 strain CL-49. Infect Immun. 1990 Apr;58(4):868–873. doi: 10.1128/iai.58.4.868-873.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder C. E., Nadziejko C. E., Herp A. Binding of basic proteins to glycoproteins in human bronchial secretions. Int J Biochem. 1982;14(10):895–898. doi: 10.1016/0020-711x(82)90072-6. [DOI] [PubMed] [Google Scholar]
- Snyder C. E., Nadziejko C. E., Herp A. Isolation of bronchial mucins from cystic fibrosis sputum by use of citraconic anhydride. Carbohydr Res. 1982 Jul 1;105(1):87–93. doi: 10.1016/s0008-6215(00)81856-x. [DOI] [PubMed] [Google Scholar]
- Tabachnik N. F., Blackburn P., Cerami A. Biochemical and rheological characterization of sputum mucins from a patient with cystic fibrosis. J Biol Chem. 1981 Jul 25;256(14):7161–7165. [PubMed] [Google Scholar]
- Tabak L. A., Reddy M. S., Monte L. D., Levine M. J. Isolation and characterization of tracheobronchial mucin from a laryngectomee. Carbohydr Res. 1984 Dec 15;135(1):117–128. doi: 10.1016/0008-6215(84)85009-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishwanath S., Ramphal R. Tracheobronchial mucin receptor for Pseudomonas aeruginosa: predominance of amino sugars in binding sites. Infect Immun. 1985 May;48(2):331–335. doi: 10.1128/iai.48.2.331-335.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Woods D. E., Straus D. C., Johanson W. G., Jr, Berry V. K., Bass J. A. Role of pili in adherence of Pseudomonas aeruginosa to mammalian buccal epithelial cells. Infect Immun. 1980 Sep;29(3):1146–1151. doi: 10.1128/iai.29.3.1146-1151.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]